Abstract
Purpose
Anti-proliferative, antiviral, and immunomodulatory activities of endogenous type I interferons (IFN1) prompt the design of recombinant IFN1 for therapeutic purposes. However, most of designed interferons exhibited suboptimal therapeutic efficacies against solid tumors. Here we report evaluation of the in vitro and in vivo anti-tumorigenic activities of a novel recombinant interferon termed sIFN-I.
Experimental Design
We compared primary and tertiary structures of sIFN-I with its parental human IFNα-2b, as well as affinities of these ligands for IFN1 receptor chains and pharmacokinetics. These IFN1 species were also compared for their ability to induce JAK-STAT signaling and expression of the IFN1-stimulated genes and to elicit anti-tumorigenic effects. Effects of sIFN-I on tumor angiogenesis and immune infiltration were also tested in transplanted and genetically engineered immunocompetent mouse models.
Results
sIFN-I displayed greater affinity for IFNAR1 (over IFNAR2) chain of the IFN1 receptor and elicited a greater extent of IFN1 signaling and expression of IFN-inducible genes in human cells. Unlike IFNα-2b, sIFN-I induced JAK-STAT signaling in mouse cells and exhibited an extended half-life in mice. Treatment with sIFN-I inhibited intratumoral angiogenesis, increased CD8+ T cell infiltration, and robustly suppressed growth of transplantable and genetically engineered tumors in immune-deficient and immune-competent mice.
Conclusions
These findings define sIFN-I as a novel recombinant IFN1 with potent preclinical anti-tumorigenic effects against solid tumor thereby prompting the assessment of sIFN-I clinical efficacy in humans.
Keywords: Type I interferon, cancer, IFNAR1, recombinant interferon, anti-tumor effects
INTRODUCTION
Type I interferons (IFN1) family of anti-viral cytokines comprises thirteen different subtypes of IFNα, as well as IFNβ, IFNε, IFNκ, IFNω etc (1–3). Potent anti-proliferative, pro-apoptotic, anti-angiogenic and immunomodulatory effects of IFN1 prompted their use for anti-cancer treatment (reviewed in (4, 5)). However, after more than 40 years of trials, the use of IFN1 against tumors is limited by the suboptimal ratio between clinical efficacy and the severity of its side effects (6), as well as limited response rate, which is often attributed to the downregulation of IFN1 receptor (7). This heterodimeric receptor complex encompassing the IFNAR1 and IFNAR2 chains mediates all effects of IFN1 on cells (8–10). Levels of IFN1 receptor were indeed shown to correlate with IFN1-induced growth arrest (11) and apoptosis in the tumor samples (12, 13).
The levels of IFN1 receptor on cell surface are largely regulated by the ubiquitin-mediated internalization and degradation of IFNAR1 (10, 14–18). Downregulation of IFNAR1 can be accelerated in some cancers (19–22) thereby limiting the anti-tumorigenic effects of IFN1. Remarkably, although activation of the JAK-STAT pathway is required for both anti-viral and anti-tumor effects of IFN1, lower receptor density still allows efficient antiviral responses while impeding ability of IFN1 to suppress cell proliferation (23). Schreiber and colleagues have proposed that responses to IFN1 could be classified as “robust” (such as anti-viral effects) or “tunable” (such as anti-proliferative or pro-inflammatory), the latter being much more sensitive to receptor density (24). Indeed, high cell surface receptor density and maximal receptor occupancy by relatively high doses of ligands are required to mount an efficient anti-proliferative effect (24, 25).
Furthermore, the affinity of IFN1 subtypes for the extracellular domain of IFNAR1 correlates with ability of these subtypes to elicit specific anti-proliferative effect (26–29). Thus, anti-tumorigenic efficacy of IFN1 may be optimized by increasing cell surface receptor density and/or by designing novel recombinant IFN1 species that display a greater affinity for IFNAR1. A number of IFN1 variants were generated and shown to be effective against tumor cells. For example, a mutant derivative of IFNα-2, IFNα-YNS exhibited tight binding to IFNAR1 and elicited potent pro-apoptotic activity and anti-proliferative/anti-angiogenesis effects in vivo; this mutant surpassed IFNα-2 in anti-tumorigenic activity in a breast cancer xenograft (28, 30).
Yet, another approach to increase efficacy of IFN1 treatment is to improve its pharmacokinetics and biological activities. Various efforts in this direction including the use of IFNα-2b-albumin fusion protein (31), antibody armed with IFN1 (32), and peggylation of IFN1 (33). Furthermore, given that many of anti-tumorigenic effects of IFN1 are mediated by the stromal cells, generation of an elegant transgenic mouse model that expresses human IFNAR1 and IFNAR2 subunits, and can be used for transplantation of human tumors resulted in improved ability to test the anti-tumorigenic effects of IFN1 (34).
Here, we characterized anti-tumorigenic properties of a novel recombinant IFN1 derived from human IFNα-2b and other IFN1 subtypes by mutagenesis and termed “super-compound interferon-I” (sIFN-I). Compared to IFNα-2b, sIFN-I exhibited higher anti-HIV activity in severe combined immunodeficient mice reconstituted with human peripheral blood leukocytes (35). Current studies revealed that sIFN-I exhibits increased affinity for IFNAR1 and has greatly improved pharmacokinetics and signaling in human and mouse cells. sIFN-I robustly inhibits intratumoral angiogenesis and suppresses growth of transplantable and genetically engineered tumors in immune deficient and immunocompetent mice. We discuss the direct and indirect mechanisms of potent anti-tumorigenic effects of sIFN-I and potential perspectives of its use in human cancer treatment.
METHODS AND MATERIALS
Cytokines
The novel recombinant super-compound interferon (sIFN-I) and interferon IFNα-2b were provided by Sichuan Huiyang Life Sci & Tech Corporation and Shanghai Huaxin Biotech, respectively. Human IFNβ (#: 10704-HNAS) and murine IFNβ (#: 50708-M02H), M-CSF (#: 11792-H08H), recombinant type I interferon receptor subunit extracellular domain IFNAR1-EC (#: 13222-H08H) and IFNAR2-EC (#: 10359-H08H) were purchased from Sino Biological Inc, Beijing. Recombinant B18R protein (Vaccinia Virus-Encoded Neutralizing Type I Interferon Receptor) was purchased from eBioscience (#: 14-8185).
Protein crystallization, data collection and structure determination
Crystals of super interferon (sIFN-I) were grown by the hanging-drop vapor diffusion method (3 mg/mL protein concentration) at 20 °C with, in the buffer of 1.2 M Li2SO4, 0.1 M 3-(cyclohexylamino)-1-propanesulfonic acid, pH 11.1, 0.02 M MgCL2. Before data collection, the crystals were equilibrated in a solution containing paraffin oil for a few seconds, and then flash-cooled in a liquid nitrogen stream at -173 °C. Original Data collection to 2.6 Å resolutions was conducted by using the synchrotron radiation from beamline BL5A at a photon factory in Tsukuba, Japan. Primary structural determination was achieved by a combination of molecular replacement method. The position of the sIFN-I was found by molecular replacement using PHASER with the crystal structure of IFNα (Protein Data Bank name: IB5L) used as the search model. The final sIFN-I structure was refined by using molecular modeling techniques and a computerized optimization program, CNS1.1.
Surface Plasmon Resonance assay
Based on surface plasmon resonance technology, binding affinities of both IFNα-2b and super interferon (sIFN-I) toward recombinant extracellular (EC) domain of type I interferon receptor subunit IFNAR1-EC or IFNAR2-EC were measured using the Biacore T100 Protein Interaction Array system (General Electric HealthCare Co.). For immobilization of the receptor subunit via binding the carboxylated dextran surface of the chip via amino groups in protein, a CM5 sensor chip was incubated with the IFNAR1-EC subunit and IFNAR2-EC subunit, at 20 and 50 μg/mL, respectively. The two tested Interferons were then injected perpendicularly to ligands at different concentrations within the range of 100–3000 nM for IFNα-2b/IFNAR1 binding, 50–1000 nM for sIFN-I/IFNAR1 binding and 3.125–80 nM for both of them on IFNAR2 binding. During IFNs/IFNAR2 binding, a five-second regeneration procedure with 2 M NaCl was added between each step of concentration. Data was analyzed by using Biacore T100 software. Dissociation constants KD were determined from the rate constants according to the Equation KD= kd/ka (d: dissociation; a: association).
Cells, cell culture and reagents
Human amnion epithelium WISH cells, all human (A549, HeLa, HT-29, SMMC-7721) and murine (MC38, LLC, B16F10) cancer cell lines were cultured in their complete conditional medium, primary murine melanoma cell line YUMM was cultured as reported previously (36). Lentiviral shRNA targeting sequences were used for knocking down expression of IFNAR1 in WISH cells. For the construction of A549-IFNAR1-KO cells, the IFNAR1 gRNA targeting sequences were inserted into the Cas9/gRNA target vector LentiCRISPR (37). Lentivirus was packaged and used to infect parental A549 cells. The IFNAR1-negative cell clones were selected with 0.2 μg/mL puromycin and then confirmed by FACS assay and immunoblot. Detailed information about the cell lines and cell culture, shRNA and sgRNA sequences are provided in Supplementary materials and methods and Supplementary Table 1.
Preparation of cell suspensions from murine spleen, lymph nodes, liver and small intestinal epithelial tissues
Spleen, lymph nodes (including inguinal, brachial, axillary, bilateral superficial cervical, and mesenteric lymph nodes), liver and small intestinal epithelial tissue were isolated from C57BL/6 mice. Briefly after organs were mechanically disaggregated, primary splenocytes and liver cells were obtained and resuspended in PBS after depletion of red blood cells. For isolation of small intestinal epithelial tissue cells (IEC), the intestinal tube of 3 cm length distant from the connection with stomach was cut out and the interior side was washed from one end by using syringe and sterile PBS. Cells were scraped off with the edge of a cover glass, counted and collected for further cell culture or mRNA extraction by Trizol.
Preparation of murine bone marrow-derived macrophages
Bone marrow cells were flushed from the femurs and tibias of sacrificed C57BL/6 mice and then depleted for red blood cells using red cell lysing solution. The cells (1 × 107 cells/well) were cultured in 6-well plates in medium supplemented with 20 ng/mL macrophage colony-stimulating factor (M-CSF). Nonadherent cells were carefully removed, and fresh conditional medium was added every 2 days. On day 5, the adherent murine BMM cells were collected for further treatment.
Mice
Female nude mice (6–8 weeks old) and female C57BL/6 mice (8 weeks old) were purchased from Shanghai SLAC Company. C57BL/6 Ifnar1+/+ or Ifnar1−/− mouse (strain: B6.129S2-Ifnar1tm1Agt/Mmjax) were purchased from The Jackson Laboratory. More detailed information for nude mice models and syngenic transplantable model is provided in Supplementary Materials and Methods. The experiments and animal procedures conducted at Shanghai Institute of Biochemistry and Cell Biology were approved by the Institution Animal Care and Use Committee (IACUC, protocol recording code: IBCB0029REV1). Experiments and all animal procedures conducted at the University of Pennsylvania were approved by the IACUC (protocols # 803995). Female C57BL/6 mice harboring Tyr::CreERT2; Braf CA/+; Ptenf/f alleles (which, upon tamoxifen treatment, were converted into BrafV600E/+, PtenΔ/Δ specifically in melanocytes) were kindly provided by Drs. McMahon and Bosenberg. Induction of malignant melanoma by tamoxifen treatment was carried out as previously described (22, 38).
Pharmacokinetics (PK) animal experiments
For PK studies, female C57BL/6 mice (8-week old; Shanghai SLAC Co.) were injected intraperitoneally with sIFN-I or IFNα-2b. All mice in each IFN treated group (n= 9, further divided into three subgroups) simultaneously received a dose of 50 μg/kg in PBS. Blood samples from each group were collected after 5 min, 15 min, 30 min, 1 h, 1.5 h, 2 h, 4 h, 6 h, 12 h and 24 h from the retro-orbital sinus (subgroup I: 5 min, 1 h, 4 h and 24 h; subgroup II: 15 min, 1.5 h and 6 h; subgroup III: 30 min, 2 h and 12 h). Serum was obtained by centrifugation at 10,000 rpm for 10 min at 4°C, and was stored at −80°C. Untreated mice (n=3) served as negative control. To determine half-life of the two interferons in serum, the concentration values, determined from ELISA measurements (VeriKine TM Human IFN Alpha ELISA Kit, #: 41100, PBL Assay Science Inc.), were plotted against time post injection and numerically fitted using WinNonlin version 6.2 software (Pharsight, St Louis, MO, USA) as described elsewhere (39). Non-compartmental models were assumed. Data (including standard deviations) and curve fits were finally plotted with Graphpad Prism 5.
FACS assays
A549 IFNAR1−/− cells (3×105) were seeded into 6-well plates. After 24 h, the cells were dissociated with cell dissociation buffer (#: 13151-014, Life Technologies) and 1,500 rpm centrifugation for 5 min in FACS tube, washed with 1x PBS for one time. Then, cells were stained with the self-made mouse anti-human IFNAR1 antibody (1:1000 diluted in 1% BSA-PBS) for 30 min at room temperature. After washing with PBS, cells were stained with AF488 conjugated goat anti-mouse IgG (1:1000 diluted in 1% BSA-PBS) for 30 min. Cells stained with IgG isotype and secondary Ab only were used as negative control were then analyzed.
For detection of cell populations in spleen from tumor bearing mice (BrafV600E/+, PtenΔ/Δ), splenocytes were suspended after red cell lysis. Then, cells were incubated with Fc Blocker antibody for 15 min at room temperature. Subsequently, specific antibodies (listed in Supplementary Table 2) were added and staining was continued for 20 min on ice. After a washing step, cells were stained with 0.5 μg/mL DAPI and were then analyzed immediately. Flow cytometry data acquisition was performed by LSRFortessa machine (BD, NJ, USA), and analysis was performed using FlowJo software.
Immunological and other techniques
Immunoblots, immunofluorescent analysis and other immunological techniques using antibodies listed in the Supplementary information and have been described in our previous publications (15–17). For details on the methods for RNA extraction, cDNA synthesis, quantitative PCR, the information of the synthesized primers, H&E staining, cellular senescence detection of paraffin sections, immunofluorescent analysis of frozen sections, for cell viability assay on human and mouse cells and illustrator image processing, data analyzing, and statistics were described in Supplementary Materials and Methods and our previous publications (22).
Statistical analysis
Comparisons between experimental groups were performed using the Student’s t-test and GraphPad Prism 5 software (GraphPad Prism software Inc.). All data were shown as Mean ± SEM. Statistically siginificant differences are indicated in figures by single (p<0.05), double (p<0.01) or triple (p<0.001) symbols (such as * or #).
RESULTS
sIFN-I differs from IFNα-2b in spatial structure and receptor binding affinity
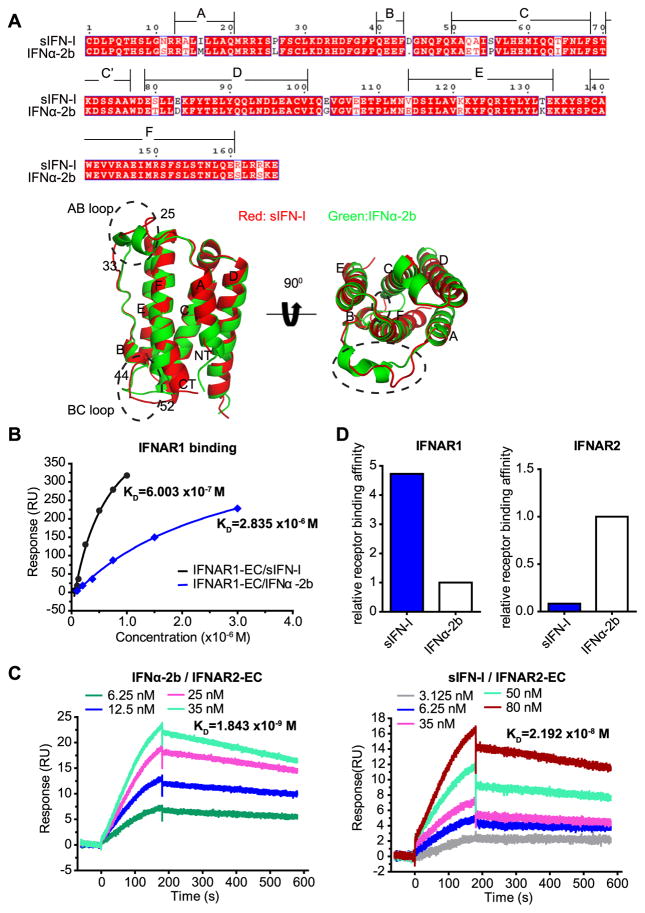
Primary structural analysis showed that sIFN-I has 89% amino acid sequence homology with IFNα-2b (Figure 1A). The crystal structure of sIFN-I was solved at 2.6 Å resolution; the resulting structure showed that sIFN-I is mainly composed of 6 helixes (A, B, C, D, E, F as shown in Figure 1A) and two distinct loops (AB and BC). This structure was generally comparable to the one previously reported for IFNα-2b (40). Nevertheless, a difference between these proteins was noted in the structure of AB loop (residues 25-33: SPFSCLKDR) and BC loop (residues 44-52: DGNQFQKAQ) (Figure 1A and Supplementary Figure 1A). Given previously published data regarding putative role of these loops in the interaction with the ligands (30), we next sought to determine relative affinities of sIFN-I for the receptor chains IFNAR1 and IFNAR2.
Figure 1. sIFN-I exibits altered binding affinities towards the receptor subunits compared to IFNα-2b.
A. Protein sequence and structure comparison between sIFN-I (red) and IFNα-2b (green). Top, amino acid sequence alignment between IFNα-2b and sIFN-I. Bottom, the secondary structures on monomer including side view (left) and vertical view (right). Each monomer consists of 6 main segments of the helices (A, B, C, D, E, F) and the connecting peptide segments. Broken ellipses represent the AB or BC loop.
B. Comparison of the dissociation constants for sIFN-I (black) and IFNα-2b (blue) to immobilized IFNAR1-EC. The constants were determined by steady-analysis model.
C. Binding curves of IFNα-2b or sIFN-I to immobilized IFNAR2-EC. The constants were determined by dynamic-analysis model.
D. Quantification of the binding affinities toward the two receptor subunits between sIFN-I and IFNα-2b.
Surface Plasmon Resonance assay indeed demonstrated different receptor binding affinities for sIFN-I and IFNα-2b. Under the condition of steady-analysis model used in this experiment, sIFN-I exhibited greater affinity for the extracellular domain of IFNAR1 (KD 6.003×10−7 mol/L (0.6 μM)) than IFNα-2b (KD 2.835×10−6 mol/L (2.8 μM)) (Figures 1B and 1D). Affinity constant of the extracellular domain of IFNAR2 chain analyzed by the dynamic-analysis model exhibited KD for sIFN-I of 2.192×10−8 mol/L (21.9 nM) and KD for IFNα-2b of 1.843×10−9 mol/L (1.84 nM). Compared to IFNα-2b, sIFN-I displayed a higher affinity to IFNAR1 (4.72 folds) but lower affinity for IFNAR2 (11.9-fold) (Figure 1C–D). These properties distinguish sIFN-I from other IFN1 variants such as IFN-YNS and IFN-YNS-α8tail which exhibit increased affinities to both IFNAR1 and IFNAR2 (24). In fact, with weaker binding towards IFNAR2 but stronger binding to IFNAR1, sIFN-I is mostly reminiscent of properties reported for IFNα-21 (29) that shared 95 % homology with sIFN-I (Supplementary Figure 1B)
sIFN-I requires IFNAR1/IFNAR2 for activating the JAK-STATs pathway
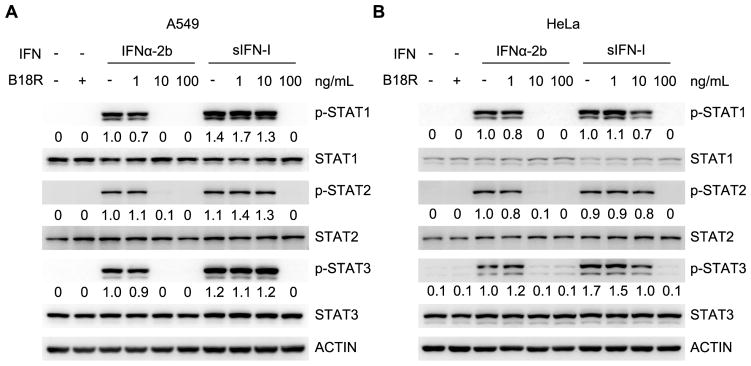
We next compared signaling elicited by sIFN-I and IFNα-2b in human A549 or HeLa cells. A similar extent of STAT1, STAT2 and STAT3 tyrosine phosphorylation was detected after administering both IFN1 types. However, IFNα-2b-induced signaling was more sensitive to inhibition by the vaccinia virus-derived B18R protein mimicking soluble IFN1 receptor and known to inhibit IFN1 pathway via ligand squelching (41) in both cell lines (Figures 2A–B). This result suggests that sIFN-I may exhibit an enhanced signaling capacity under signaling limiting conditions.
Figure 2. sIFN-I is capable of an increased signaling under limiting conditions.
A. IFN signaling in A549 cells: 10-fold serial dilutions of recombinant B18R protein (1 to 100 ng/mL, final assay concentration) were prepared in media and combined with a constant amount (1 ng/mL, final assay concentration) of each IFN protein (sIFN-I or IFNα-2b) for 1 h at room temperature. The B18R/IFN complexes were transferred to cells and then incubated for 30 min. The phosphorylation and total signal of STAT1, STAT2, and STAT3 were detected by immunoblot and the p-STATs level were quantified compared to their corresponding total STAT proteins, +, means treatment with 100 ng/mL B18R protein.
B. IFN signaling in HeLa cells was analyzed as in Panel A.
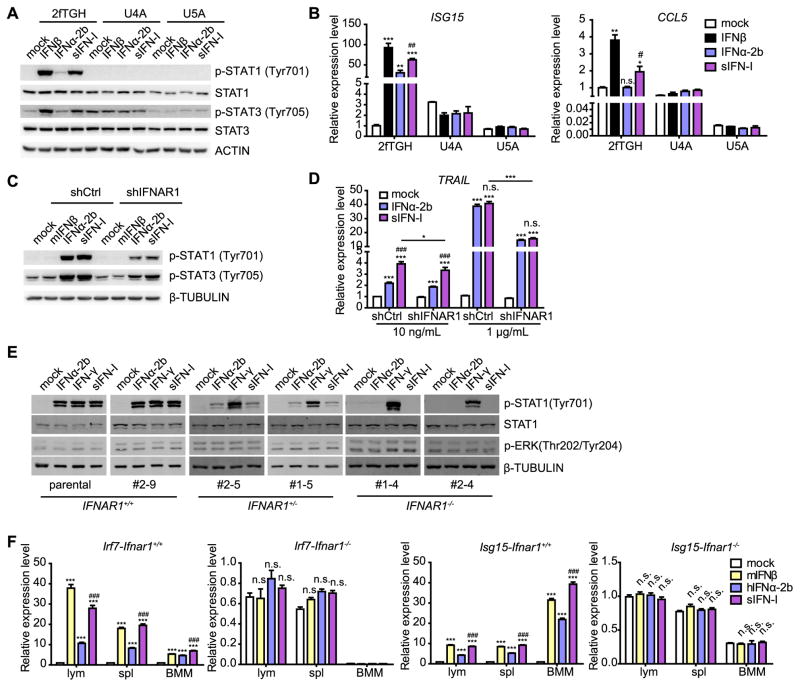
Recombinant IFN1 proteins were shown to opportunistically bind other receptors besides IFNAR1/2 such as the opioid receptors (42–44). Thus, we sought to determine whether signaling by sIFN-I depends on canonical IFNAR1/2-JAK-STAT pathway. Experiments in human fibrosarcoma 2fTGH cells (sensitive to IFN1) and derivative clones lacking IFNAR2 (U5A) or JAK1 (U4A) revealed that both IFNAR2 and JAK1 are required for sIFN-I-induced phosphorylation of STAT1 and STAT3 (Figure 3A).
Figure 3. sIFN-I elicits its signaling in an IFNAR1/2 dependent manner.
A. Human fibrosarcoma 2fTGH cells (sensitive to IFN1) and derivative clones deficient in either JAK1 (U4A) or IFNAR2 (U5A) were treated with human IFNβ, IFNα-2b or sIFN-I (10 ng/mL). The phosphorylation and total signal of STATs were detected by Western blot after 15-min treatment.
B. The induction of indicated IFN-stimulated genes in cells described in Panel A was detected by qPCR after 24-h treatment.
C. WISH cells with stable IFNAR1 knockdown expression were treated with human IFNα-2b or sIFN-I (10 ng/mL) or mouse IFNβ (negative control) for 15 min. The phosphorylation of STAT proteins was detected by immunoblot.
D. Cells described in panel C were treated with indicated IFN for 24h and the induction of TRAIL mRNA was detected by qPCR.
E. A549 cells harboring various IFNAR1 status expression were treated with indicated IFNs (10 ng/mL) for 30 min. The phosphorylation of STAT1 and ERK were detected by immunoblot.
F. Lymphocytes (lym), splenocytes (spl) and bone marrow-derived macrophages (BMM) were obtained from Ifnar1 knockout (Ifnar1−/−) or wild type (Ifnar1+/+) mice. The similar numbers (6–10×106) of these primary cells were cultured and treated with murine IFNβ (10 ng/mL), hIFNα-2b (1 μg/mL) or sIFN-I (1 μg/mL) for 24 h, then the induction of Irf7 and Isg15 were quantified by qPCR. *, P<0.05, **, P<0.01, ***, P<0.001, vs mock group; #, P<0.05, ##, P<0.01, ###, P<0.001, vs IFNα-2b group.
Consistent with these results, sIFN-I did not induce the expression of interferon-stimulated genes (ISG) such as ISG15 or CCL5 in either U4A or U5A cells (Figure 3B). Furthermore, RNAi-mediated knockdown of IFNAR1 attenuated sIFN-I-induced phosphorylation of STAT1/STAT3 and expression of TRAIL in WISH cells (Figure 3C–D, Supplementary Figure 2) suggesting an important role of IFNAR1 in sIFN-I signaling. To corroborate these data, we used CRISPR/Cas9 approach to knock out IFNAR1 in human A549 cells (Supplementary Figure 3). A robust phosphorylation of STAT1 observed in response to IFNγ (which utilizes Type II IFN receptor (45)) in selected IFNAR1+/− or IFNAR1−/− clones demonstrated that these cells do not harbor defects in JAK signaling. Importantly, STAT1 phosphorylation in IFNAR1 deficient clones was not induced by sIFN-I (Figure 3E). These data suggest that sIFN-I signals through the IFNAR1/IFNAR2-JAK pathway in human cells.
sIFN-I can act on mouse cells and exhibits distinct pharmacokinetics and tissues responses in vivo
Poor sensitivity of mouse IFN1 receptor to human IFN1 species and suboptimal pharmacokinetics of IFN1-based agents pose a challenge for efficient testing of biological effects of human IFN1 (34). Notably, treatment of primary mouse cells with sIFN-I revealed that activity of this ligand in induction of ISGs (Irf7 and Isg15) is superior to that of human IFNα-2b. All these effects were dependent on IFN1 receptor status as evident from the lack of sIFN-I-induced gene expression increase in Ifnar1 knockout mice (Figure 3F).
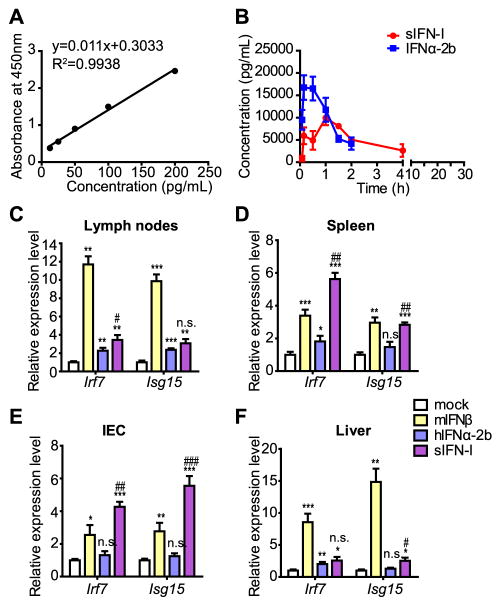
We further compared pharmacokinetics of sIFN-I and IFNα-2b in mice after intraperitoneal injection of these agents. To this end, blood samples were taken at fixed time points after IFN administration and IFN concentrations in serum were assessed by ELISA followed by numerical analysis using WinNonlin6.2 software (Figure 4A–B). The pharmacokinetic parameters of sIFN-I and IFNα-2b after administration at the same dose are summarized in Supplementary Table 3. At 15 minutes after injection, the mean serum peak concentration (Cmax) for IFNα-2b was 16730 pg/mL. However, the Cmax of sIFN-I with 9915 pg/mL was delayed to 1 hour after administration. Despite the Cmax differences between sIFN-I and IFNα-2b, the area under concentration versus time curve [AUC (0-ι)] for sIFN-I and IFNα-2b exhibited comparable value (27425 and 24648 pg per hour/mL, respectively) at the same dosage. Such PK data suggested volume distribution (Vz-F) of sIFN-I (4384 mg/kg) is more extensive than that of IFNα-2b (2055 mL/kg) at steady state. In other words, the tissue concentrations of sIFN-I were higher than that of IFNα-2b. Consistent with these data, the induction of expression of IFN-induced genes Irf7 and Isg15 in mouse lymph nodes, spleen, liver and intestinal epithelial cells was notably greater after treatment of mice with sIFN-I compared to IFNα-2b (Figure 4C–F) treatment. In all, these data suggest that, compared to IFNα-2b, sIFN-I exhibits a greater distribution in mouse tissues and accordingly elicits a greater IFN-stimulated genes induction in these tissues.
Figure 4. sIFN-I displays different pharmacokinetics and distinct tissues responsiveness in vivo.
A. Calibration curve of human IFNα ELISA used for pharmacokinetics assay.
B. C57BL/6 wild type mice were subjected to intraperitoneal injection in each group (n=3) with hIFNα-2b or sIFN-I. Serum was obtained at the indicated time point and the concentrations of serum IFNs were detected by ELISA assay. Comparison of the pharmacokinetic curves of hIFNα-2b or sIFN-I administered as in Panel A. Additional information is provided in the Supplementary Table 3.
C. C57BL/6 wild type mice were intraperitoneal injected with murine IFNβ (1 μg/mL), human IFNα-2b (1 μg/mL) or sIFN-I (1 μg/mL), respectively. Primary tissues were collected for gene expression detection after 6-h treatment. The induction of Irf7 and Isg15 mRNA in lymph nodes was quantified by qPCR.
D. Analysis of Irf7 and Isg15 mRNA was quantified by qPCR in spleen tissues of mice described in Panel C.
E. Analysis of Irf7 and Isg15 mRNA was quantified by qPCR in intestinal epithelial tissues of mice described in Panel C.
F. Analysis of Irf7 and Isg15 mRNA was quantified by qPCR in liver tissues of mice described in Panel C. *, P<0.05, **, P<0.01, ***, P<0.001, vs mock group; #, P<0.05, ##, P<0.01, ###, P<0.001, vs IFNα-2b group.
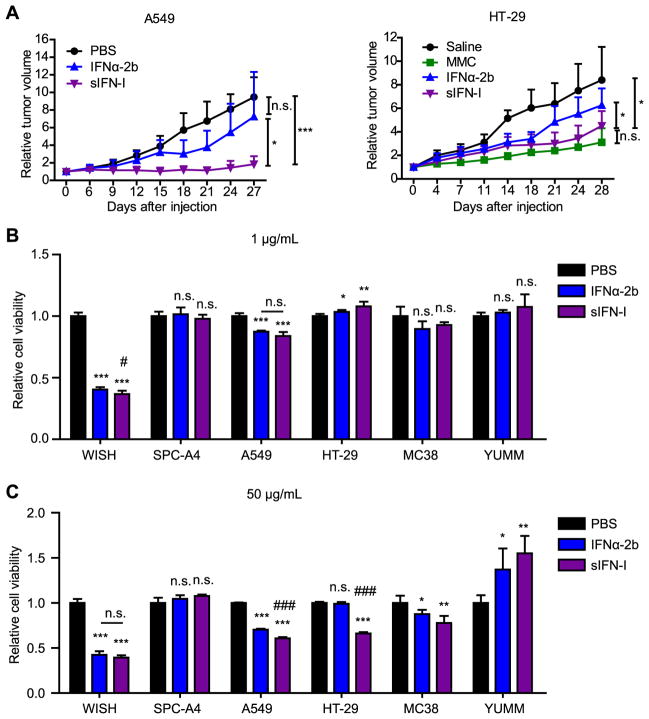
sIFN-I inhibits growth of solid tumors
We next compared the anti-tumorigenic properties of sIFN-I and of IFNα-2b. These agents administered at the doses of 50 μg-150 μg/mice were reasonably well tolerated by the A549 or HT-29 tumor-bearing immunocompromised mice; these mice did not exhibited body weight loss during the course of treatment (Supplemental Figure 4). Whereas a modest inhibition of tumor growth was elicited by IFNα-2b, administration of sIFN-I robustly suppressed this growth and led to a stable disease (Figure 5A and Supplementary Figure 4). Analysis of tumor tissues revealed that sIFN-I treatment increased cell senescence markers (senescence-associated β-galactosidase) and dramatically decreased the rate of cell proliferation (assessed by Ki67 staining). Accordingly, an increased expression of p53 tumor suppressor protein as well as cyclin-dependent kinase inhibitors p21 and p27 was found in tumor tissues from mice treated with sIFN-I (Supplemental Figure 5).
Figure 5. sIFN-I exhibits potent anti-solid tumor effects in xenotransplanted tumor models.
(A) A549 or HT-29 xenograft tumors were treated with intratumoral injection of sIFN-I or IFNα-2b (5 mg/kg) every other day for the indicated days; in the HT-29 model, 5 mg/kg Mitomycin (MMC) treatment as a positive control. The tumor volume was measured and calculated as follows: tumor volume (mm3) = (length × width2)/2. (B-C) Indicated cancer cell lines (human lung cancer cells SPC-A4, A549, human colon adenocarcinoma cell HT-29, murine colorectal cancer cell line MC38 and murine primary melanoma cell line YUMM) and WISH cells were treated with 1 μg/mL (B) or 50 μg/mL (C) interferons for four days. Cell viability and proliferation was assessed by WST1 assay. *, P<0.05, **, P<0.01, ***, P<0.001, vs mock group; #, P<0.05, ##, P<0.01, ###, P<0.001, vs IFNα-2b group.
When tested for growth inhibition in vitro, both IFNα-2b and sIFN-I exhibited robust effects on human WISH cells at the dose of 1 μg/mL (Figure 5B). A greater dose (50 μg/mL) was required to detect modest inhibitory effect of either of these IFN1 agents on growth of A549, HT-29 human cancer cells and MC38 mouse cancer cell line. Under these conditions, sIFN-I was slightly more efficient than IFNα-2b while growth of some of human (SPC-A4) or mouse (YUMM) cancer cell lines (MC38, YUMM) in vitro was not inhibited by IFN1 even at 50 μg/mL (Figure 5C). Given that IFN1 can act on tumor vascularization and anti-tumor immunity (45), it is plausible that these indirect mechanisms may contribute to potent anti-tumorigenic effects of sIFN-I observed in vivo.
sIFN-I suppresses angiogenesis and stimulates anti-tumor immunity
Treatment of C57BL/6 mice bearing a syngeneic B16F10 melanoma with sIFN-I but not IFNα-2b resulted in suppression of tumor growth (Supplementary Figure 6A). sIFN-I also suppressed tumor growth in mice burdened with syngeneic colorectal (MC38) or lung (LLC) adenocarcinomas (Supplementary Figure 6B–C). These results suggest that sIFN-I can elicit its anti-tumorigenic activities in immunocompetent hosts.
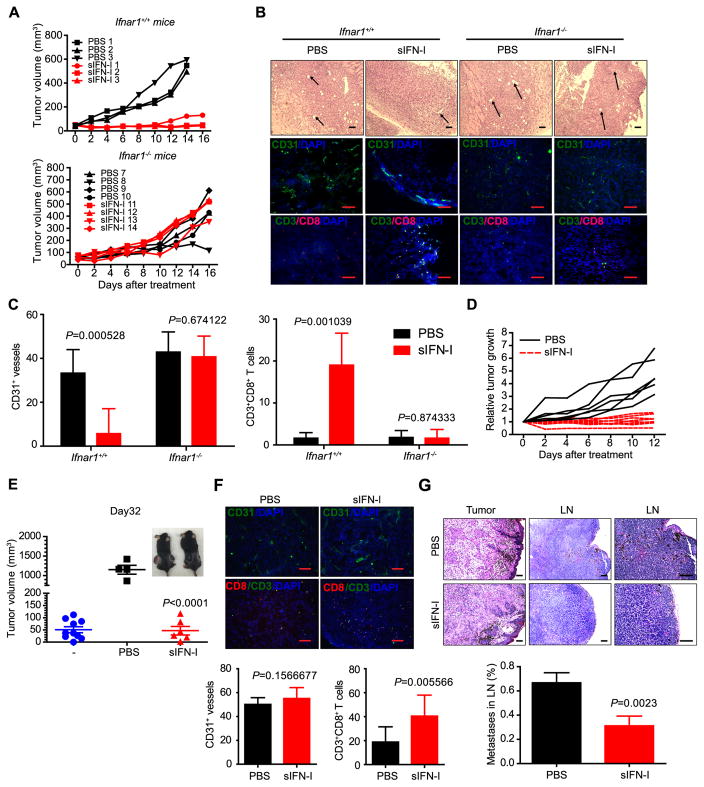
To further understand the antitumor effects of sIFN-I on tumor host, we tested its action in immunocompetent C57BL/6 mice inoculated with syngeneic murine melanoma cell line YUMM (Braf V600E/+/PtenΔ/Δ/Cdkn2aΔ/Δ). Administration of sIFN-I into tumor bearing Ifnar1+/+ mice led to a dramatic suppression of growth of transplanted tumor. Importantly, when Ifnar1 knockout animals were chosen as hosts, tumors grew more aggressively and did not respond to treatment with sIFN-I (Figure 6A). Given this Ifnar1-dependent difference in responses to sIFN-I and the fact that YUMM cells were poorly sensitive to growth inhibition by sIFN-I in vitro (Figure 5B–C), these results suggest that sIFN-I can suppress tumor growth through affecting tumor stromal compartment.
Figure 6. Anti-tumorigenic, anti-angiogenic and immunostimulating effects of sIFN-I in immunocompetent mouse models.
A. YUMM (BrafV600E/+; PtenΔ/Δ; CDKN2A−/−) cells were injected subcutaneously into Ifnar1+/+ and Ifnar1−/− mice to establish transplantable tumor model. sIFN-I or IFNα-2b (5 mg/kg) were injected intraperitoneally every other day for the indicated days. The tumor volume was measured and calculated.
B. H&E and immunofluorescence staining of YUMM tumors isolated from mice after sIFN-I treatment. Arrows indicate the vessels in tumor tissue. Scale bar, 100 μm.
C. Quantification on the positive CD31+ vessels number in the fields (n=7) and CD3+CD8+ T cells infiltrated in YUMM allograft tumor microenvironment (n=10).
D. Melanocyte-specific Cre activity was induced in adult mice (BrafCA/+Ptenf/f) by topical application of 4-HT to shaved back skin. Melanoma growth was measured after intraperitoneal injection with sIFN-I every other day.
E. Volume of melanoma tumors that grew in BrafV600E/+; PtenΔ/Δ mice to initial volume (“-”, blue circles). After that, mice were randomly assigned to two groups treated with PBS (black squares, left mouse in the inset) or sIFN-I (red triangles, right mouse at inset) for 32 days. p<0.001 between PBS and sIFN-I group.
F. Immunofluorescence staining of the tumor isolated from BrafV600E/+; PtenΔ/Δ mice after sIFN-I treatment. Bottom, quantification on the average positive CD31+ vessels number in the fields (n=10) and the double positive CD3+CD8+ cells in the fields (n=10) presenting infiltrated effector T cells in tumors from BrafV600E; PtenΔ/Δ mice. Scale bar: 100 μm.
G. H&E staining of the tumors and superficial lymph nodes (n=12) isolated from BrafV600E/+; PtenΔ/Δ mice after sIFN-I treatment. Bottom, quantification on the number of metastatic tumors in lymph node (LN). Scale bar, 100 μm.
Consistent with this possibility, compared to untreated animals or treated Ifnar1−/− mice, tumors from sIFN-I-treated Ifnar1+/+ mice contained fewer blood vessels and were less positive for endothelial marker CD31 (Figure 6B–C). Furthermore, these tumors contained a greater number of CD3+CD8+ cytotoxic lymphocytes (Figure 6B–C). These results support a notion that sIFN acts on tumor stromal compartment and may impede tumor growth via inhibiting tumor angiogenesis and increasing tumor infiltration by CD3+CD8+ cytotoxic lymphocytes (indicative of reversing tumor immunosuppression) in the IFNAR1-dependent manner.
Having observed a robust therapeutic effect of sIFN-I in transplanted tumors, we sought to determine whether this agent can also be active in genetically engineered models. To this end, we induced melanoma tumors in BrafV600E/+; PtenΔ/Δ mice and started the treatment after establishing tumors with the average size of 51 mm3 in both groups. Administration of sIFN-I to notably suppressed growth of these tumors (Figure 6D). When all control mice receiving vehicle had to be sacrificed for humane reasons (i.e. tumor size reaching the limit required by IACUC), animals receiving sIFN-I exhibited either stable disease or partial/complete tumor regression (Figure 6D–E).
In this model, sIFN-I did not noticeably affect infiltration of tumors with CD31-positive cells. However, consistent with tumor regression, we observed significant increase of infiltrating cytotoxic lymphocytes in tumors treated with sIFN-I in genetically engineered mouse melanomas (Figure 6F and Supplemental Figure 7). Furthermore, sIFN-I notably suppressed metastases of genetically engineered melanoma into the lymph nodes (Figure 6G). These results strongly suggest that sIFN-I exhibit a potent anti-tumorigenic effect against primary tumors and metastatic disease.
DISCUSSION
Endogenous IFN1 plays an important role in protection against tumors due to their anti-proliferative, anti-angiogenic and immunostimulating activities (2). The response rate and therapeutic efficacy of IFN1-based pharmaceutical agents is limited, especially in solid tumors (4, 6) because oncogene signaling, tumor microenvironment stress, unfolded protein response and inflammation can decrease the levels of IFNAR1 available for ligand interaction (19–21, 46, 47). Besides developing means to reverse downregulation of IFNAR1, additional solutions for optimizing IFN1 therapy can be based on the observation that anti-tumorigenic efficacy of diverse IFN1 subtypes parallels affinity of these types for IFNAR1 (48, 49). Here we describe sIFN-I, a novel recombinant IFN1 exhibiting increased affinity for IFNAR1 and potent anti-tumorigenic properties.
Intriguingly, while tightly binds to IFNAR1, sIFN-I exhibits a lesser affinity for IFNAR2 (normally a chain with greater affinity for endogenous ligands (48) compared to its “parental” molecule IFNα-2b (Figure 1), which is different from the other reported interferon variants such as IFN-YNS and IFN-YNS-α8tail (24). The latter variants displayed enhanced ligand binding affinity to both IFNAR1/2, and also showed enhanced anti-proliferation activity for cancer cells in vitro (28). Whereas in vitro activities of sIFN-I are relatively underwhelming, sIFN-I exerts its potent anti-tumor effect in vivo (Figure 5A and 6, Supplementary Figure 4A).
sIFN-I elicits notable activation of STAT proteins and ensuing induction of ISGs (Figures 2 and 3F); importantly, all these effects of sIFN-I depend on integrity of the IFNAR1/IFNAR2-JAK pathway (Figure 3). Furthermore, tumor-bearing mice lacking Ifnar1 are poorly responsive to anti-tumorigenic activities of sIFN-I (Figure 6). These results suggest that despite (or because of) potentially altered ligand-IFNAR1-IFNAR2 complex, sIFN-I robustly activates this receptor and downstream IFN1 signaling pathway.
Remarkably, compared to human IFNα-2b, the effects of sIFN-I appear to transcend the species differences. Data presented here reveal that sIFN-I elicits the IFN1-stimulated gene induction responses in primary mouse cells and mice in vivo (Figures 4–5). Furthermore, in terms of pharmacokinetics in mouse, sIFN-I exhibited longer half-life and lower peak drug concentration in serum compared to IFNα-2b (Figure 4). Intriguingly, there was a two-step serum increase for sIFN-I; this phenomenon was not observed for IFNα-2b injected into mice. These differences could be attributed to the different binding model for sIFN-I towards plasma protein or lipoprotein in blood which lead to re-release of sIFN-I from the sIFN-I/plasma protein or sIFN-I/lipoprotein dynamic binding complex (50). Such possibility would be consistent with two peaks in concentration-time curve for serum concentration of 2′5-OAS (a well-known downstream markers of the parmacodynamic activity of interferon) observed in blood after sIFN-I subcutaneous injection for the healthy volunteers (51). Altered pharmacokinetic characteristics of sIFN-I may contribute to greater ISG induction and improved anti-tumorigenic activities in vivo (Figure 5) and, furthermore, may potentially cause lesser side effects. These possibilities in humans will be revealed by clinical trials of sIFN-I in Singapore (CTC1300056) and USA (NCT02464007) that are currently conducted in patients with solid tumors.
Previous published data suggested that sIFN-I can suppress the tumor growth in some isolated clinical cases in human patients (52). Our current data demonstrate greater efficacy of sIFN-I over IFNα-2b against human tumors xenotransplanted into immunocompromised mice (Figure 5A). Given a robust response of mouse tissues to sIFN-I, this response may at least in part be attributed to the effects of sIFN-I on mouse stromal cells. Indeed, in immunocompetent syngeneic transplantation or genetically engineered mouse melanoma models, sIFN-I notably suppressed angiogenesis and/or increased tumor infiltration with cytotoxic lymphocytes. These anti-angiogenic and immunostimulatory effects of sIFN-I are likely to contribute to robust anti-tumorigenic efficacy of sIFN-I that elicit stable disease or/and tumor regression in very aggressive melanoma tumors (Figure 6). Detailed studies of the mechanisms underlying immunostimulatory and other effects of sIFN-I are ongoing. These studies will be instrumental in designing clinical trials in humans that will address clinical efficacy of sIFN-I alone or in combination with traditional, molecularly targeted or immune targeted therapies.
Supplementary Material
Statement of translational relevance.
Despite potent anti-tumorigenic properties of natural and pharmacological Type I interferons (IFN1), these agents achieved only a limited success in cancer therapy. This manuscript describes the molecular and biological characterization of de novo engineered and highly potent recombinant interferon (sIFN-I), which has evoked massive clinical interest and is currently undergoing clinical trials in patients with solid tumors in Singapore (CTC1300056) and USA (NCT02464007), as well as patients with HBV in China (2009L04155). Here we present data obtained both in vitro and in vivo settings; these data demonstrate that sIFN-I exhibits superior pharmacodynamics and pharmacokinetics characteristics compared to its parental human IFNα-2b species. Furthermore, studies conducted in cells and in animals harboring transplantable and genetically engineered tumor models reveal that sIFN-I evokes potent anti-tumorigenic effects at least in part by inhibiting stromal angiogenesis and by stimulating anti-tumor immunity.
Acknowledgments
We are grateful to Drs. McMahon (USCF), Bosenberg (Yale University), Jiang (Peking University), Melissa Wong (Oregon Health and Science University) and Stark (Cleveland Clinics) for sharing the reagents and to members of Fuchs and Liu labs for insightful comments. This work was supported by NIH/NCI grant CA092900 (to S.Y.F.), Sichuan Science and Technology project 2013ZZ0004 (to K-J.Z.), Shanghai Institutes for Biological Science, Chinese Academy of Sciences & Sichuan Huiyang Life Science and Technology Corp. research program Y363S21763 (to X-Y.L.), National Basic Research Program of China 973 Program, No. 2011CB510104 (to X-Y.L.), Zhejiang Sci-Tech University grant 1204807-Y (to X-Y.L.), Chinese Ministry of Science and Technology fund 2014CB964704 (to X-Y.L.), Grant from the Sino-American joint laboratory between Conba Group and Zhejiang Sci-Tech University (to X-Y.L.).
Footnotes
Conflict of interest
Guang-Wen Wei is the Chairman of the Board, Xin-Yuan Liu is a Board member, and Rong-Bing Guo is a senior scientist of Sichuan Huiyang Life Science and Technology Corp. Chengdu, Sichuan, China that produces sIFN-I. Kang-Jian Zhang’s travel was in part supported by the Sichuan Huiyang Life Science and Technology Corp. Other authors claim no conflict of interest.
References
- 1.Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nature reviews Immunology. 2015;15:405–14. doi: 10.1038/nri3845. [DOI] [PubMed] [Google Scholar]
- 2.Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR, et al. Interferons at age 50: past, current and future impact on biomedicine. Nature reviews Drug discovery. 2007;6:975–90. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunological reviews. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 4.Kirkwood JM, Ernstoff MS. Interferons in the treatment of human cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1984;2:336–52. doi: 10.1200/JCO.1984.2.4.336. [DOI] [PubMed] [Google Scholar]
- 5.Borden EC, Lindner D, Dreicer R, Hussein M, Peereboom D. Second-generation interferons for cancer: clinical targets. Semin Cancer Biol. 2000;10:125–44. doi: 10.1006/scbi.2000.0315. [DOI] [PubMed] [Google Scholar]
- 6.Bracarda S, Eggermont AM, Samuelsson J. Redefining the role of interferon in the treatment of malignant diseases. Eur J Cancer. 2010;46:284–97. doi: 10.1016/j.ejca.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs SY. Hope and fear for interferon: the receptor-centric outlook on the future of interferon therapy. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2013;33:211–25. doi: 10.1089/jir.2012.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uze G, Schreiber G, Piehler J, Pellegrini S. The receptor of the type I interferon family. Current topics in microbiology and immunology. 2007;316:71–95. doi: 10.1007/978-3-540-71329-6_5. [DOI] [PubMed] [Google Scholar]
- 9.Carbone CJ, Fuchs SY. Eliminative signaling by Janus kinases: role in the downregulation of associated receptors. J Cell Biochem. 2014;115:8–16. doi: 10.1002/jcb.24647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchs SY. Ubiquitination-mediated regulation of interferon responses. Growth Factors. 2012;30:141–8. doi: 10.3109/08977194.2012.669382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eguchi H, Nagano H, Yamamoto H, Miyamoto A, Kondo M, Dono K, et al. Augmentation of antitumor activity of 5-fluorouracil by interferon alpha is associated with up-regulation of p27Kip1 in human hepatocellular carcinoma cells. Clin Cancer Res. 2000;6:2881–90. [PubMed] [Google Scholar]
- 12.Mejia C, Navarro S, Colamonici OR, Pellin A, Castel V, Llombart-Bosch A. Expression of type I interferon receptor and its relation with other prognostic factors in human neuroblastoma. Oncol Rep. 1999;6:149–53. [PubMed] [Google Scholar]
- 13.Vitale G, van Eijck CH, van Koetsveld Ing PM, Erdmann JI, Speel EJ, van der Wansem Ing K, et al. Type I interferons in the treatment of pancreatic cancer: mechanisms of action and role of related receptors. Annals of surgery. 2007;246:259–68. doi: 10.1097/01.sla.0000261460.07110.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huangfu WC, Fuchs SY. Ubiquitination-dependent regulation of signaling receptors in cancer. Genes Cancer. 2010;1:725–34. doi: 10.1177/1947601910382901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar KG, Tang W, Ravindranath AK, Clark WA, Croze E, Fuchs SY. SCF(HOS) ubiquitin ligase mediates the ligand-induced down-regulation of the interferon-alpha receptor. EMBO J. 2003;22:5480–90. doi: 10.1093/emboj/cdg524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar KG, Krolewski JJ, Fuchs SY. Phosphorylation and specific ubiquitin acceptor sites are required for ubiquitination and degradation of the IFNAR1 subunit of type I interferon receptor. The Journal of biological chemistry. 2004;279:46614–20. doi: 10.1074/jbc.M407082200. [DOI] [PubMed] [Google Scholar]
- 17.Kumar KG, Barriere H, Carbone CJ, Liu J, Swaminathan G, Xu P, et al. Site-specific ubiquitination exposes a linear motif to promote interferon-alpha receptor endocytosis. J Cell Biol. 2007;179:935–50. doi: 10.1083/jcb.200706034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, HuangFu WC, Kumar KG, Qian J, Casey JP, Hamanaka RB, et al. Virus-induced unfolded protein response attenuates antiviral defenses via phosphorylation-dependent degradation of the type I interferon receptor. Cell Host Microbe. 2009;5:72–83. doi: 10.1016/j.chom.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhattacharya S, HuangFu WC, Dong G, Qian J, Baker DP, Karar J, et al. Anti-tumorigenic effects of Type 1 interferon are subdued by integrated stress responses. Oncogene. 2013;32:4214–21. doi: 10.1038/onc.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huangfu WC, Qian J, Liu C, Liu J, Lokshin AE, Baker DP, et al. Inflammatory signaling compromises cell responses to interferon alpha. Oncogene. 2012;31:161–72. doi: 10.1038/onc.2011.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.HuangFu WC, Qian J, Liu C, Rui H, Fuchs SY. Melanoma cell-secreted soluble factor that stimulates ubiquitination and degradation of the interferon alpha receptor and attenuates its signaling. Pigment Cell Melanoma Res. 2010;23:838–40. doi: 10.1111/j.1755-148x.2010.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katlinskaya YV, Katlinski KV, Yu Q, Ortiz A, Beiting DP, Brice A, et al. Suppression of Type I Interferon Signaling Overcomes Oncogene-Induced Senescence and Mediates Melanoma Development and Progression. Cell Rep. 2016;15:171–80. doi: 10.1016/j.celrep.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalie E, Jaitin DA, Podoplelova Y, Piehler J, Schreiber G. The stability of the ternary interferon-receptor complex rather than the affinity to the individual subunits dictates differential biological activities. The Journal of biological chemistry. 2008;283:32925–36. doi: 10.1074/jbc.M806019200. [DOI] [PubMed] [Google Scholar]
- 24.Levin D, Harari D, Schreiber G. Stochastic receptor expression determines cell fate upon interferon treatment. Molecular and cellular biology. 2011;31:3252–66. doi: 10.1128/MCB.05251-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moraga I, Harari D, Schreiber G, Uze G, Pellegrini S. Receptor density is key to the alpha2/beta interferon differential activities. Molecular and cellular biology. 2009;29:4778–87. doi: 10.1128/MCB.01808-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaks E, Gavutis M, Uze G, Martal J, Piehler J. Differential receptor subunit affinities of type I interferons govern differential signal activation. Journal of molecular biology. 2007;366:525–39. doi: 10.1016/j.jmb.2006.11.053. [DOI] [PubMed] [Google Scholar]
- 27.Jaitin DA, Roisman LC, Jaks E, Gavutis M, Piehler J, Van der Heyden J, et al. Inquiring into the differential action of interferons (IFNs): an IFN-alpha2 mutant with enhanced affinity to IFNAR1 is functionally similar to IFN-beta. Molecular and cellular biology. 2006;26:1888–97. doi: 10.1128/MCB.26.5.1888-1897.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalie E, Jaitin DA, Abramovich R, Schreiber G. An interferon alpha2 mutant optimized by phage display for IFNAR1 binding confers specifically enhanced antitumor activities. The Journal of biological chemistry. 2007;282:11602–11. doi: 10.1074/jbc.M610115200. [DOI] [PubMed] [Google Scholar]
- 29.Lavoie TB, Kalie E, Crisafulli-Cabatu S, Abramovich R, DiGioia G, Moolchan K, et al. Binding and activity of all human alpha interferon subtypes. Cytokine. 2011;56:282–9. doi: 10.1016/j.cyto.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Thomas C, Moraga I, Levin D, Krutzik PO, Podoplelova Y, Trejo A, et al. Structural linkage between ligand discrimination and receptor activation by type I interferons. Cell. 2011;146:621–32. doi: 10.1016/j.cell.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subramanian GM, Fiscella M, Lamouse-Smith A, Zeuzem S, McHutchison JG. Albinterferon alpha-2b: a genetic fusion protein for the treatment of chronic hepatitis C. Nature biotechnology. 2007;25:1411–9. doi: 10.1038/nbt1364. [DOI] [PubMed] [Google Scholar]
- 32.Yang X, Zhang X, Fu ML, Weichselbaum RR, Gajewski TF, Guo Y, et al. Targeting the tumor microenvironment with interferon-beta bridges innate and adaptive immune responses. Cancer cell. 2014;25:37–48. doi: 10.1016/j.ccr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ceaglio N, Etcheverrigaray M, Conradt HS, Grammel N, Kratje R, Oggero M. Highly glycosylated human alpha interferon: An insight into a new therapeutic candidate. Journal of biotechnology. 2010;146:74–83. doi: 10.1016/j.jbiotec.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 34.Harari D, Abramovich R, Zozulya A, Smith P, Pouly S, Koster M, et al. Bridging the species divide: transgenic mice humanized for type-I interferon response. PLoS One. 2014;9:e84259. doi: 10.1371/journal.pone.0084259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang W, Tong X, Nakasone T, Yue XT, Yamamoto N, Liu XY, et al. Activity of superior interferon alpha against HIV-1 in severe combined immunodeficient mice reconstituted with human peripheral blood leukocytes. Chinese medical journal. 2011;124:396–400. [PubMed] [Google Scholar]
- 36.Pencheva N, Buss CG, Posada J, Merghoub T, Tavazoie SF. Broad-spectrum therapeutic suppression of metastatic melanoma through nuclear hormone receptor activation. Cell. 2014;156:986–1001. doi: 10.1016/j.cell.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 37.Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–7. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr, et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nature genetics. 2009;41:544–52. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Czyrski A, Kondys K, Szalek E, Karbownik A, Grzeskowiak E. The pharmacokinetic interaction between levofloxacin and sunitinib. Pharmacol Rep. 2015;67:542–4. doi: 10.1016/j.pharep.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 40.Radhakrishnan R, Walter LJ, Hruza A, Reichert P, Trotta PP, Nagabhushan TL, et al. Zinc mediated dimer of human interferon-alpha 2b revealed by X-ray crystallography. Structure. 1996;4:1453–63. doi: 10.1016/s0969-2126(96)00152-9. [DOI] [PubMed] [Google Scholar]
- 41.Symons JA, Alcami A, Smith GL. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell. 1995;81:551–60. doi: 10.1016/0092-8674(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 42.Jiang CL, Son LX, Lu CL, You ZD, Wang YX, Sun LY, et al. Analgesic effect of interferon-alpha via mu opioid receptor in the rat. Neurochem Int. 2000;36:193–6. doi: 10.1016/s0197-0186(99)00124-2. [DOI] [PubMed] [Google Scholar]
- 43.Wang YX, Jiang CL, Lu CL, Song LX, You ZD, Shao XY, et al. Distinct domains of IFNalpha mediate immune and analgesic effects respectively. J Neuroimmunol. 2000;108:64–7. doi: 10.1016/s0165-5728(00)00271-x. [DOI] [PubMed] [Google Scholar]
- 44.Wang YX, Xu WG, Sun XJ, Chen YZ, Liu XY, Tang H, et al. Fever of recombinant human interferon-alpha is mediated by opioid domain interaction with opioid receptor inducing prostaglandin E2. J Neuroimmunol. 2004;156:107–12. doi: 10.1016/j.jneuroim.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 45.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nature reviews Immunology. 2005;5:375–86. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 46.Kumar KG, Liu J, Li Y, Yu D, Thomas-Tikhonenko A, Herlyn M, et al. Raf inhibitor stabilizes receptor for the type I interferon but inhibits its anti-proliferative effects in human malignant melanoma cells. Cancer Biol Ther. 2007;6:1437–41. doi: 10.4161/cbt.6.9.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng H, Qian J, Carbone CJ, Leu NA, Baker DP, Fuchs SY. Vascular endothelial growth factor-induced elimination of the type 1 interferon receptor is required for efficient angiogenesis. Blood. 2011;118:4003–6. doi: 10.1182/blood-2011-06-359745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schreiber G, Piehler J. The molecular basis for functional plasticity in type I interferon signaling. Trends Immunol. 2015;36:139–49. doi: 10.1016/j.it.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Piehler J, Thomas C, Garcia KC, Schreiber G. Structural and dynamic determinants of type I interferon receptor assembly and their functional interpretation. Immunological reviews. 2012;250:317–34. doi: 10.1111/imr.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wasan KM, Brocks DR, Lee SD, Sachs-Barrable K, Thornton SJ. Impact of lipoproteins on the biological activity and disposition of hydrophobic drugs: implications for drug discovery. Nat Rev Drug Discov. 2008;7:84–99. doi: 10.1038/nrd2353. [DOI] [PubMed] [Google Scholar]
- 51.Zeng J, Yu Q, Liang M, Duan J, Zheng Y. Study on pharmacokinetics and bioequivalence of rSIFN-co in healthy volunteers. Modern Preventive Medicine. 2008;35:982–4. [Google Scholar]
- 52.Liu X-Y, Wei G-W, Yang D-Q, Liu L-X, Ma L, Li X, et al. Possibility to Win the War against Cancer. In: Liu X-Y, Pestka S, Shi Y-F, editors. Recent Advance in Cancer Research and Therapy. Tsinghua University press limited: Elsevier; 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.