Abstract
Objective
Variation in taste perception and exposure to risk factors of taste alterations have been independently linked with elevated adiposity. Using a laboratory database, we modeled taste-adiposity associations and examined whether taste functioning mediates the association between taste-related risk factors and adiposity.
Methods
Healthy women (n=407, 35.5±16.9 years) self-reported histories of risk factors of altered taste functioning (tonsillectomy, multiple ear infections, head trauma) and were assessed for taste functioning (tongue-tip and whole-mouth intensities of quinine and salt) and density of taste papillae. Twenty-four percent had elevated waist circumferences; 39% were overweight or had obesity. Using structural equation modeling, we tested direct and indirect associations between taste-related risk factors, taste functioning, and adiposity.
Results
In models with good fit, elevated central adiposity was explained directly by history of risk factors (tonsillectomy, multiple ear infections) and directly by lower taste functioning (lower tongue-tip taste function, lower papillae density). Risk factors of taste alterations were significantly associated with lower taste functioning, with taste mediating the association between head trauma and reduced adiposity.
Conclusion
This large laboratory-based study supports associations between taste-related risk factors, taste functioning and adiposity. Our findings need to be confirmed with other population-based studies, including the NHANES 2013–2014 taste data.
Keywords: taste, weight, NHANES, modeling, waist circumference
Introduction
Variation in taste perception, from normal genetic variation to taste alterations, is associated with differences in dietary behaviors and adiposity status.1–3 Taste variation has been linked with differences in preferences for dietary sources that can influence total energy consumption, including sweets, high-fat foods, vegetables, and alcoholic-beverages.1–3 Growing evidence suggests that taste receptors expressed along the gut regulate neurotransmitters and metabolic hormones involved in appetite, energy intake and metabolism, further supporting taste-adiposity linkages.4 Some variation in taste perception has been explained by polymorphisms in taste receptor genes5,6 and density of taste receptors on the tongue.1,2 Environmental insults to taste-related cranial nerves also may cause taste alterations, including diminished, intensified, or phantom (i.e. dysgeusia) sensations.2 Exposures to these environmental insults have been directly associated with adiposity.1–3,7–10 Here, we employed structural equation modeling (SEM) to simultaneously examine direct and indirect associations between reported risk factors of taste alterations, taste functioning, and adiposity in an existing database of laboratory-based studies. Secondly, we aimed to develop a framework for analyzing taste data from available population-based studies, including the first taste protocol11 included in the National Health and Nutrition Examination Survey (NHANES).
Taste functioning can be assessed by measuring the perceived intensity of tastants, sampled with the whole-mouth or regionally-applied to areas of taste innervation, and the number of fungiform papillae (FP) on the anterior tongue, a proxy for taste receptor density.2,12 Complete or severe loss of taste is rare, as multiple cranial nerves throughout the oral cavity and throat are involved in taste perception.2 Regional loss of taste sensation, particularly from the anterior tongue, is more common, related to damage to the taste fibers of the chorda tympani branch of cranial nerve VII by viral infections, middle-ear surgeries, oral infections, head trauma and some dental procedures.2 Diminished taste sensations on the tongue-tip may be unnoticed due to the release of inhibition from other taste-related cranial nerves for the preservation of whole-mouth taste functioning2 With chorda tympani taste damage, this disinhibitory feedback may alter oral sensations from foods and beverages, and in the extreme, cause dysgeusia.2 Diminished taste intensity on the tongue-tip, whether examined alone or relative to whole-mouth intensity, has been associated with differences in preference for and intake of vegetables,13 alcoholic beverages14 and sweet/fatty foods.3 Whole-mouth to tongue-tip ratios, in particular, could be useful measure of taste functioning as they associate with dietary behaviors,13,14 and can diminish individual variability in intensity scale usage.
Studies investigating associations between taste functioning and weight in the literature are few and inconsistent. Some have found that, compared to normal-weight individuals, individuals with obesity have lower taste functioning,15–18 whereas others observed no such differences.19,20 Taste phenotype and reduced FP density have been linked with excess adiposity and with dietary behaviors that may influence weight and adiposity.1–3,21,22 Exposure to risk factors of taste alteration, including tonsillectomy and otitis media, have been associated with regional taste losses3, and separately linked to elevated adiposity.3,7–9 In contrast, weight loss also has been linked to dysgeusia resulting from tonsillectomy or other causes.23,24 There are no reports examining whether taste functioning mediates the relationship between taste-related risk factors and adiposity.
Similar to the NHANES 2013–2014 taste protocol, the present study assessed regional and whole-mouth taste intensities of concentrated quinine (bitter) and NaCl solutions as measures of taste functioning. The rationale for selection of these taste measures in NHANES have been described in detail elsewhere.11 Briefly, the NHANES taste measures were selected on their ability to capture genetic and environmentally mediated variation in taste as well as relevance to diet and health outcomes. Here, we analyzed an existing database of similar taste measures, health history and anthropometric data to model taste-adiposity associations and provide an analytical framework for future population-based studies. Specifically, we aimed to identify key taste measures that predicted variability in adiposity and were explained by exposures to taste-related risk factors. We also investigated the ability of taste intensity measures to predict adiposity relative to FP density, another presumed marker of variation in oral sensation that has been associated with dietary behaviors and adiposity.1–3,21,22 Using a latent variable approach under structural equation modeling (SEM), we examined direct and indirect associations between taste-related risk factors, taste measures, and adiposity in adult females. We hypothesized that taste-related risk factors (multiple ear infections, tonsillectomy, head trauma) will be associated with diminished taste functioning, with both showing direct associations with increased adiposity.
Methods
Participants
A convenience sample of 407 non-smoking, ostensibly healthy women (mean age 35.5 ± 16.9 years) was recruited via posters in the areas surrounding the university campus. Exclusion criteria included pregnancy, severe food allergies, and dietary restrictions. The study had University of Connecticut-Storrs IRB approval. All participating women gave written consent and received monetary compensation.
Risk factors of taste alterations
Women answered questions on history (yes/no) and frequency (once, twice, 3–5 times, 6+ times) of middle ear infections, if ever received treatment with antibiotics (yes/no) for ear infections, and if ever had tympanostomy tubes inserted for treatment (yes/no). History of multiple ear infections was dichotomized into ‘absent’ or ‘present’ (having ear infections ≥3 times or being treated with tympanostomy tubes). History of head trauma also was dichotomized; participants who answered “no,” or “yes, not serious” to the question “Have you ever suffered from a head injury?” were classified as ‘absent’ for head trauma, whereas those who answered “yes, had either concussion or loss of consciousness” were classified as having a history of head trauma. Participants who answered affirmatively to the yes/no question of “Did you ever have a tonsillectomy?” were indicated as having a history of tonsillectomy.
Taste functioning
Taste assessment
Regional and whole-mouth taste intensity was measured using the validated general Labeled Magnitude Scale (gLMS).25 The gLMS was vertically-oriented, labeled from the bottom ‘no sensation’ (scale score=0) to the top ‘strongest sensation of any kind’ (100), with intermediate labels, ‘barely detectable’ (1.4), ‘weak’ (6), ‘moderate’ (17), ‘strong’ (34), and ‘very strong’ (53). Prior to use, the women received verbal gLMS orientation/training with instructions to treat the top of the scale as generalized across all sensory domains. The participants then practiced rating intensities of recalled sound and light experiences (e.g., loudness of a conversation, brightness of the sun) and five, 1000-Hz tones ranging in 12-dB hearing level (HL) steps from 50 to 98 dB.
Participants used the gLMS to report intensities for 1M NaCl and 1mM quinine hydrochloride drawn along the tongue-tip with a cotton-tipped applicator.12,26 Next, they sampled three solutions with the whole-mouth (0.32M NaCl, 1mM quinine, 1M NaCl) using a sip-and-spit procedure. The taste test concentrations were selected to be matched in intensity across quality12 and were consistent with the NHANES taste protocol.11 After each taste stimulus, the participants rinsed their mouth with water to remove any residual stimulus.
Fungiform Papillae (FP) assessment
Number of FP, the tissue structures containing taste buds on the anterior tongue, was measured in a 6 mm circular area on the anterior tongue with color videomicrosopy using a procedure described previously.14
Measures of Adiposity
Trained technicians measured the women’s height and weight using a standard balance-beam scale that included a stadiometer for height measurement (Health-o-meter, Bridgeview, IL). Body mass index (BMI, kg/m2) was calculated and classified as underweight (<18.5), normal (18.50–24.99), overweight (25.00–29.99), obese (30.00–34.99), severely obese (35.00–39.99), and morbidly obese (≥40.00). Waist circumference was measured to the nearest 0.5 inch at the height of the iliac crest using a tape measure.
Statistical Analyses
Statistical analyses were accomplished using SPSS 20.0 (Chicago, IL) and Mplus 7.0 (Los Angeles, CA). Statistical significance was set at P≤0.05; multiple comparisons were adjusted for using the Bonferroni correction. Missing data (14%) were imputed using the Markov Chain Monte Carlo method. Linear relationships between taste intensities and adiposity measures were evaluated with Pearson’s correlations (r); t-tests examined the mean differences in adiposity and taste intensities between individuals with and without exposure to taste-related risk factors (multiple ear infections, head trauma and tonsillectomy).
The theoretical model that taste-related risk factors influence taste functioning, and that both of these associate with adiposity measures, was tested using a latent variable approach under SEM. First, we used confirmatory factor analysis measurement models to determine which of the taste measures could effectively define the latent construct of taste functioning. Taste measures for this model were taste ratios, obtained by dividing the whole-mouth taste intensity by tongue-tip intensity. Intensities of 1M NaCl and 1mM quinine on the tongue-tip were expressed as ratios relative to the corresponding whole-mouth intensities of these stimuli as well as relative to the whole-mouth intensity of 0.32M NaCl.
Next, a series of SEM models were tested with direct and indirect pathways of associations between taste-related risk factors, latent variable taste functioning, and either BMI or waist circumference. Age, FP count and race/ethnicity were included as covariates. The models were estimated using the default Maximum Likelihood Estimation method. The model fit was evaluated using four measures: Chi-square (non-significance desirable), Comparative Fit Index (CFI), Root Mean Squared Error of approximation (RMSEA), and Tucker-Lewis Index (TLI). For RMSEA, values ≤0.05 were considered a good fit; values between 0.05–0.08 were interpreted as an acceptable fit. For CFI and TLI, values >0.95 were considered a good fit; values between 0.90–0.95 were considered an acceptable fit. We used normalizing transformations for variables with skewed or elongated distributions, but analyses with transformed and untransformed data produced similar results; the latter are reported.
Results
Table 1 shows the descriptive and anthropometric measures of the sample. The majority of the women were Non-Hispanic white (80.6%). Risk factors for taste alterations were well represented, with frequencies consistent with those observed among women respondents in the NHANES 2011–2012. There was variability in adiposity measures—27.3% of the women were classified as overweight, 12% of the women had obesity and 24% had elevated waist circumference (>88 cm) according to the National Heart, Blood and Lung Institute criteria.27
Table 1.
Characteristics of the study sample (N=407)
| Variables | Mean ± SD |
|---|---|
| Age (years) | 35.5 ± 16.9 |
| BMI (kg/m2) | 24.5 ± 4.3 |
| Waist Circumference (inches) | 31.9 ± 4.5 |
| Fungiform Papillae (per cm2) | 99.81 ±35.56 |
| N (%) | |
| Ethnicity | |
| Asian | 38 (9.3%) |
| Hispanic | 11 (2.7%) |
| Non-Hispanic white | 328 (80.6%) |
| Non-Hispanic black | 19 (4.7%) |
| Other race | 11 (2.7%) |
| BMI classification | |
| Underweight | 7 (1.7%) |
| Normal | 240 (59.0%) |
| Overweight | 111 (27.3%) |
| Obese | 36 (8.8%) |
| Severely or Morbidly obese | 13 (3.2%) |
| Histories of Taste-related Risk Factors | |
| Tonsillectomy | 101 (24.8%) |
| Multiple Ear Infections | 140 (34.4%) |
| Head trauma | 72 (17.7%) |
As shown in Supporting Information Table S1, perceived intensities for 1mM quinine and 1M NaCl on the tongue-tip were significantly lower in women with histories of tonsillectomy and multiple ear infections. In contrast, taste intensities for 1M NaCl on the tongue-tip were greater in women with history of head trauma. Whole-mouth intensities of quinine and 0.32M NaCl were significantly lower in women with histories of tonsillectomy and multiple ear infections, whereas no whole-mouth intensity differences were observed with history of head trauma.
Table 2 shows the mean differences in constructed taste intensity ratios and adiposity measures between women with and without reported exposures to taste-related risk factors. History of tonsillectomy was associated with higher whole-mouth to tongue-tip ratios, significant for all four constructed taste ratios. Head trauma, conversely, was associated negatively with all taste ratios. History of multiple ear infections only was associated with higher whole-mouth to tongue-tip ratio for quinine. In terms of adiposity measures, history of tonsillectomy was significantly associated with greater waist circumference and greater BMI, whereas history of head trauma significantly was associated with lower waist circumference. Although women with multiple ear infections had greater waist circumference and BMI, the differences failed to reach statistical significance.
Table 2.
Mean differences in constructed taste intensity ratios and adiposity measures between women with and without reported exposures to taste-related risk factors
| Characteristic | Tonsillectomy | Multiple Ear Infections | Head Trauma | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | P- value |
Yes | No | P- value |
Yes | No | P- value |
|
| Constructed taste ratios | |||||||||
| 1 mM quinine WM/quinine tongue-tip ratio | 3.9±0.3 | 2.9±0.1 | 0.001 | 3.4±0.2 | 3.0±0.1 | 0.08 | 2.7±0.2 | 3.3±0.2 | 0.13 |
| 0.32M NaCl WM/quinine tongue-tip ratio | 2.7±0.2 | 1.9±0.1 | 0.001 | 2.2±0.2 | 2.1±0.1 | 0.70 | 1.6±0.1 | 2.2±0.1 | 0.02 |
| 1 M NaCl WM/ NaCl tongue-tip ratio | 3.2±0.4 | 2.2±0.1 | 0.001 | 2.4±0.2 | 2.5±0.1 | 0.85 | 2.0±0.2 | 2.5±0.1 | 0.10 |
| 0.32M NaCl WM/ NaCl tongue-tip ratio | 2.2 ±0.2 | 1.6±0.1 | 0.002 | 1.7±0.1 | 1.8±0.1 | 0.89 | 1.3±0.1 | 1.9±0.1 | 0.001 |
| Adiposity measures | |||||||||
| Waist Circumference (inches) | 33.9±0.4 | 31.2±0.2 | 0.001 | 32.3±0.3 | 31.4±0.3 | 0.06 | 30.9±0.4 | 32.1±0.2 | 0.03 |
| Body Mass Index (kg/m2) | 26.0±0.4 | 24.0±0.2 | 0.001 | 24.9±0.3 | 24.0±0.3 | 0.07 | 24.0±0.4 | 24.6±0.2 | 0.34 |
Data are presented as mean ± standard error of the mean
Abbreviations: WM, whole mouth.
Among the five taste measures, only the quinine tongue-tip intensity was associated significantly with waist circumference and BMI, with lower intensity associating with higher adiposity (Table 3). Among the four constructed taste ratios, whole-mouth to tongue-tip ratio for quinine, and the ratio of 0.32M NaCl whole-mouth intensity to quinine tongue-tip were positively and significantly correlated with waist circumference and BMI.
Table 3.
Correlationsa among variables used in structural models.
| Variable # | Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Age | 1 | |||||||||
| 2 | Quinine tongue-tip |
−.29 | 1 | ||||||||
| 3 | 1 M NaCl tongue-tip |
−.26 | .56 | 1 | |||||||
| 4 | Quinine WM/quinine tongue-tip | .21 | −.57 | −.29 | 1 | ||||||
| 5 | 0.32 M NaCl WM/quinine tongue-tip |
.18 | −.54 | −.19 | .71 | 1 | |||||
| 6 | 1 M NaCl WM/ NaCl tongue-tip | .24 | −.23 | −.46 | .44 | .32 | 1 | ||||
| 7 | 0.32 M NaCl WM/NaCl tongue-tip | .20 | −.16 | −.54 | .29 | .45 | .72 | 1 | |||
| 8 | FP count |
−.10 | .08 | −.05 | −.05 | −.01 | .09 | .10 | 1 | ||
| 9 | Waist circumference | .34 | −.17 | −.11 | .17 | .22 | .12 | .12 | −.15 | 1 | |
| 10 | BMI | .33 | −.15 | −.13 | .17 | .16 | .17 | .12 | −.16 | .83 | 1 |
Abbreviations: WM, whole-mouth; FP, fungiform papillae; BMI, body mass index.
Correlations above .14 were significant at P<.005
Structural equation modeling with taste-related risk factors, taste functioning and adiposity measures
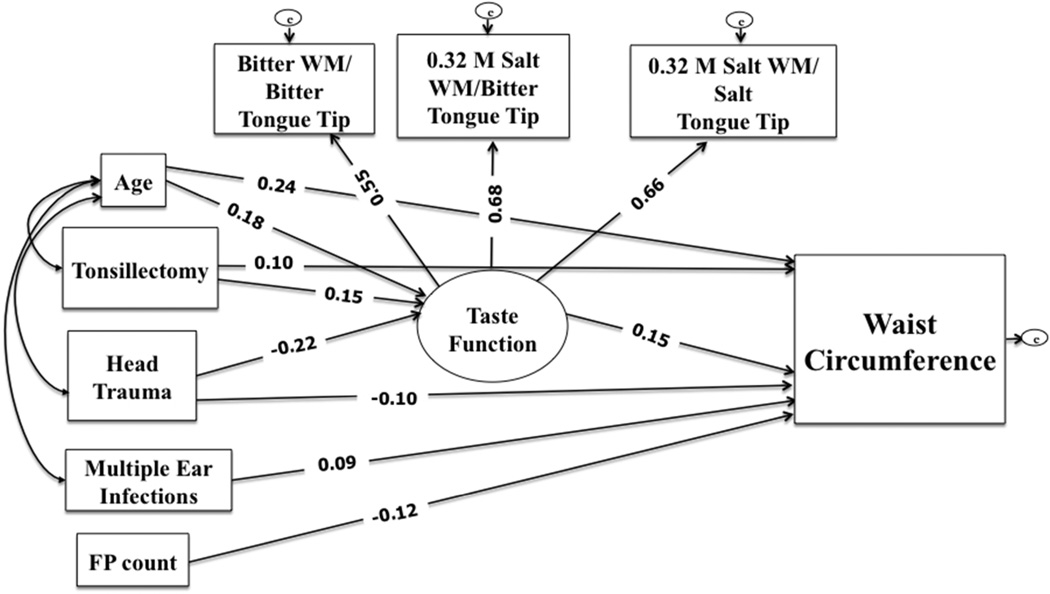
Confirmatory factor analyses identified a latent construct of taste functioning, defined by three indicators of whole-mouth to tongue-tip taste ratios (Fig 1) with good fit indices (CFI=0.99; TLI=0.99; RMSEA=0.03). The three taste ratio indicators were correlated (Table 3) with high degree of internal consistency (Cronbach’s alpha = 0.72), and factor loadings >0.5 (Fig 1). Based on the hypothesized conceptual model and supporting bivariate analyses, an overall SEM examined direct or indirect associations between the three taste-related risk factors, the latent variable taste functioning, and waist circumference, with age, FP count included as covariates; race/ethnicity was removed as a covariate since it did not adequately explain differences in the exposures or outcomes. Non-significant paths (P>0.10) were trimmed and the re-specified model fit was tested before being provisionally accepted. The final model shown had all paths significant and good fit indices [X2 =21.9 (df=13), P=0.06; CFI=0.98; TLI=0.96; RMSEA=0.04].
Figure 1.
Structural equation model testing direct and indirect associations between taste-related risk factors, taste functioning indicated by three taste ratios, and waist circumference. All standardized path coefficients shown were significant (P<0.05). The model showed good fit indices [X2 = 21.9 (df=13), P=0.06; CFI=0.98; TLI=0.96; RMSEA=0.04]
Abbreviations: WM, whole-mouth; FP, fungiform papillae; CFI, Comparative Fit Index; RMSEA, Root Mean Squared Error of Approximation; TLI, Tucker-Lewis Index.
Figure 1 shows the final model with standardized parameter estimates. The latent variable taste functioning was independently predicted by tonsillectomy and head trauma after mutually adjusting for each other, as well as age. Age and tonsillectomy history were associated positively with latent variable taste functioning, indicating that women who were older and had tonsillectomy had diminished sensations on the tongue-tip compared to the whole-mouth; women with head trauma had lower ratios and thus head trauma associated negatively with latent variable taste functioning. FP count was initially included as a covariate in these paths but was removed due to lack of association. The taste functioning latent variable was not associated with history of multiple ear infections after controlling for age and other taste-related risks. Accordingly, this path also was removed.
Age, the latent variable taste functioning, FP count, and all three taste-related risk factors were independent predictors of waist circumference, after controlling for other variables in the model. Women with histories of tonsillectomy and multiple ear infections had higher waist circumferences than those without these respective histories. Women with head trauma, in contrast, had significantly lower waist circumferences than those without. Greater FP count also was associated with lower waist circumference. The latent variable of taste functioning was associated positively with waist circumference. Additionally, taste functioning partially mediated (indirect effect, β=-0.03, P<0.05) the negative association between head trauma and waist circumference. Overall, the model explained 17.7% of the variance in waist circumference.
Additional models were tested, first without FP count and second, with BMI as the primary outcome variable (data not shown). The model excluding FP count, and with waist circumference as the adiposity measure, showed similar paths of associations with good model fit [X2=16.2 (df=10), P=0.09; CFI=0.98; TLI=0.97; RMSEA=0.04], but explained slightly less variance in waist circumference (16%). The model with BMI as the primary outcome did not show associations between any of the taste-related risk factors and BMI after controlling for age, taste functioning and FP count, yet all other associations were retained and the model had an acceptable fit [X2 = 30.5 (df=13), P<0.01; CFI=0.96; TLI=0.94; RMSEA=0.06], explaining 13.5% variance in BMI.
Discussion
The present study examined an existing laboratory database to identify measures of taste functioning that were significantly associated with taste-related risk factors as well as adiposity among adult females. These taste measures, which have been incorporated into the NIH Toolbox 26 and the NHANES 2013–201411, included tongue-tip and whole-mouth intensities of concentrated quinine and NaCl solutions. Diminished taste intensity on the tongue-tip, measured directly or as a ratio to whole-mouth intensity, was associated with greater adiposity and was explained by reported exposures to taste-related risk factors. Through structural equation modeling, we identified direct associations between risk factors of taste alteration and adiposity, as well as some indirect associations mediated by taste functioning. These associations were significant after excluding or controlling for FP density, an anatomical marker of taste functioning not included in the NIH Toolbox or NHANES protocol.
Direct associations between taste-related risk factors and adiposity have been previously reported. In our study, women with histories of tonsillectomy and multiple ear infections had higher adiposity than women without such histories. These findings are consistent with a recent study3, which reported that histories of tonsillectomy and otitis media were independently associated with higher BMIs. Data7 from the National Health Examination Survey (1963–1970) showed that young children and adolescent girls who had undergone tonsillectomy were more likely to be overweight independent of demographic factors; reported otitis media symptoms also was associated with increased BMI in adolescent girls. Several other studies have linked chronic otitis media with excess adiposity in children.2,8–10 Associations between tonsillectomy and weight also have been mostly studied in children, who show significant weight gain following the surgery.28–30 Case studies of weight loss after tonsillectomy have been reported as well, but only with the presence of dysgeusia.24
Although there are some studies to the contrary31,32, the literature suggests that tonsillectomy and chronic otitis media are associated with diminished or altered taste. For tonsillectomy, most studies on post-operative taste changes have been focused on qualitative symptoms like dysgeusia, which are found to be common but transient.33 Data on measured regional taste loss from tonsillectomy are scarce, presumably because such loss is likely to be unnoticed by patients due to disinhibition from undamaged taste nerves.3,33 In our study, women with tonsillectomy and multiple ear infections had diminished taste sensations on the tongue-tip for both NaCl and quinine. In contrast, Bartoshuk and colleagues3 observed significant differences between individuals with and without tonsillectomy for taste intensities on the posterior but not the anterior tongue; for individuals with otitis media, NaCl and sucrose distributions, but not quinine, were significantly displaced toward lower intensities on the tongue-tip. Similar to our study, Shin and colleagues8 reported that children with chronic otitis media had higher adiposity and lower taste perception on the tongue-tip.
Otitis media and tonsillectomy are hypothesized to contribute to excess adiposity through changes in taste perception. In this study, tonsillectomy and otitis media were associated with both diminished taste and excess adiposity, but taste failed to show a significant mediating effect. One explanation may be that these chronic infections can alter oral sensations beyond taste, which were not measured in this study. Disruptions in the central inhibitory taste circuits from taste nerve damage, for example, can alter other oral sensations including retronasal olfaction and tactile sensations from fatty foods and are thought to explain the enhanced palatability of energy-dense fat/sweet foods in adults with histories of tonsillectomy and otitis media.3 Alternatively, otitis media and tonsillectomy may contribute to excess adiposity through non-dietary mechanisms, such as inflammatory mediators and decreased systemic catecholamines.8,30
In contrast to tonsillectomy and ear infections, history of head trauma was significantly associated with decreased central adiposity in our study. Head trauma in our sample was defined by either loss of consciousness or concussion due to head injury, and hence is likely to capture the milder spectrum of traumatic brain injury (TBI). In the literature, associations between TBI and weight changes have been mixed, with reports of both weight gain and loss following the injury.34–36 The negative association between head trauma and central adiposity in our study was statistically mediated, in part, by taste functioning. Specifically, women with head trauma reported significantly greater intensities for NaCl on the tongue-tip and had lower whole-mouth to tongue-tip ratios. One possible explanation is that mild chorda tympani damage from head trauma may have released inhibition of cranial nerve V, resulting in heightened somatosensory sensations from taste-irritant stimuli like concentrated NaCl.3 Conversely, more severe damage, or damage to more than one nerve such as by tonsillectomy, may result in reductions in overall taste sensations.3 It is unclear why we observed the negative associations between head trauma and adiposity in our sample. However, clinical reports of eating disorders, including anorexia following TBI have been reported previously.37,38 Head trauma also may cause olfactory dysfunction and subsequently decrease appetite or desire to eat.11
Our study had several limitations. Causal relationships between taste-related risk factors, taste functioning and adiposity cannot be established due to our cross-sectional data. We were unable to verify the presence of the self-reported risk factors by physical exams or medical records. Self-reported childhood otitis media by adults, in particular, may be susceptible to recall bias, however its validity and reliability have been demonstrated previously.39,40 Our sample had lower rates of obesity than is typically seen among adult females in the U.S. Additionally, other confounding factors such as physical activity, dietary intake and early-life risk factors of otitis media (e.g., breastfeeding status/duration, exposure to air pollutants) were not evaluated. The observed taste-adiposity associations may have been mediated by differences in food preference and intake, and should be examined in future studies. Since taste perception is modulated by the endocrine system, it is possible that metabolic disorders such as obesity may influence taste perception rather than vice versa.4 Longitudinal studies are needed to evaluate this possibility of reverse causation. Our database consisted of a relatively homogenous sample, which increases the ability to examine the taste-adiposity relationship, but decreases the generalizability of the findings.
Despite the limitations, our study supports the utility of regional and whole-mouth taste assessments included in the NIH Toolbox and NHANES 2013–2014 taste protocols, and their relevance to nutrition-related health outcomes. In our study, diminished taste function was associated with increased adiposity, and was partly explained by common risk factors for taste alterations. The present study provides a framework for analyzing population-based studies employing the NIH Toolbox or the related NHANES 2013–2014 taste protocol. Examination of the NHANES 2013–2014 taste data will provide an opportunity to confirm the present study findings in a national study and also elucidate if dietary factors mediate the observed taste-adiposity associations.
Supplementary Material
What is already known about this subject?
Variation in taste functioning, associated with taste gene polymorphisms, taste papilla density and environmental exposures, has been shown to explain differences in preference for and/or consumption of foods and beverages (e.g., sweets, fatty foods, vegetables, alcoholic-beverages) that are linked with the risk for obesity. Studies investigating associations between taste functioning and weight are few and inconsistent in their findings, with some reporting that individuals with obesity have lower taste functioning and others reporting no such differences.
Environmental exposures, including tonsillectomy and otitis media, have been associated with depressions in regional taste functioning, altered taste sensations, and elevated adiposity. No study to date has examined whether taste functioning mediates the relationship between taste-related risk factors and adiposity.
What does your study add?
This study shows direct and indirect associations between reported risk factors of taste alteration, measured taste functioning, and adiposity among over 400 women. Depressed taste intensity on the tongue-tip was associated with greater adiposity, with the former being partially explained by reported exposures to common risk factors for taste alterations. Additionally, direct associations were identified between taste-related risk factors and adiposity, as well as some indirect associations mediated by taste functioning.
Lastly, we provide a framework for analyzing population-based studies that incorporate key taste measures identified in our study, including the NHANES 2013–2014 taste data and studies employing the NIH Toolbox Taste Assessment.
Acknowledgments
Funding agencies: This research was supported by the United States Department of Agriculture Hatch Project as well as an Interagency Agreement (Y1-DC-0013) between the National Institute on Deafness and Other Communication Disorders (NIDCD), National Institutes of Health (NIH) and the National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention (CDC). Also, Dr. Shristi Rawal was partially supported by the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Author contributions: SR: conception of analysis design, data analysis, interpretation of data, and initial drafting of manuscript. THM: statistical assistance and interpretation of data, drafting and revision of manuscript. HJH: interpretation of data, drafting and revision of manuscript. HS: interpretation of data, drafting and revision of manuscript. VBD: conception of study, interpretation of data, drafting and revision of manuscript.
Disclosure: The authors declared no conflicts of interest.
References
- 1.Tepper BJ, Banni S, Melis M, Crnjar R, Tomassini Barbarossa I. Genetic sensitivity to the bitter taste of 6-n-propylthiouracil (PROP) and its association with physiological mechanisms controlling body mass index (BMI) Nutrients. 2014;6:3363–3381. doi: 10.3390/nu6093363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snyder DJ, Bartoshuk LM. Oral sensory nerve damage: Causes and consequences. Rev Endocr Metab Disord. 2016;17:149–158. doi: 10.1007/s11154-016-9377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartoshuk LM, Catalanotto F, Hoffman H, Logan H, Snyder DJ. Taste damage (otitis media, tonsillectomy and head and neck cancer), oral sensations and BMI. Physiol Behav. 2012;107:516–526. doi: 10.1016/j.physbeh.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Depoortere I. Taste receptors of the gut: emerging roles in health and disease. Gut. 2014;63:179–190. doi: 10.1136/gutjnl-2013-305112. [DOI] [PubMed] [Google Scholar]
- 5.Kim UK, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299:1221–1225. doi: 10.1126/science.1080190. [DOI] [PubMed] [Google Scholar]
- 6.Rawal S, Hayes JE, Wallace MR, Bartoshuk LM, Duffy VB. Do polymorphisms in the TAS1R1 gene contribute to broader differences in human taste intensity? Chem Senses. 2013;38:719–728. doi: 10.1093/chemse/bjt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffman HJ, Losonczy KG, Bartoshuk LM, Himes JH, Snyder DJ, Duffy VB. Taste damage from tonsillectomy or otitis media may lead to overweight children: The U.S. National Health Examination Surveys (NHES), 1963–1970. St. Pete Beach, FL: 9th Int Symposium on Recent Advances in Otitis Media; 2007. Jun, June 2007. [Google Scholar]
- 8.Shin IH, Park DC, Kwon C, Yeo SG. Changes in taste function related to obesity and chronic otitis media with effusion. Arch Otolaryngol Head Neck Surg. 2011;137:242–246. doi: 10.1001/archoto.2011.23. [DOI] [PubMed] [Google Scholar]
- 9.Peracchio HL, Henebery KE, Sharafi M, Hayes JE, Duffy VB. Otitis media exposure associates with dietary preference and adiposity: a community-based observational study of at-risk preschoolers. Physiol Behav. 2012;106:264–271. doi: 10.1016/j.physbeh.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Nelson HM, Daly KA, Davey CS, Himes JH, Synder DJ, Bartoshuk LM. Otitis media and associations with overweight status in toddlers. Physiol Behav. 2011;102:511–517. doi: 10.1016/j.physbeh.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman HJ, Rawal S, Li CM, Duffy VB. New chemosensory component in the U.S. National Health and Nutrition Examination Survey (NHANES): first-year results for measured olfactory dysfunction. Rev Endocr Metab Disord. 2016;17:221–240. doi: 10.1007/s11154-016-9364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kveton JF, Bartoshuk LM. The effect of unilateral chorda tympani damage on taste. Laryngoscope. 1994;104:25–29. doi: 10.1288/00005537-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Dinehart ME, Hayes JE, Bartoshuk LM, Lanier SL, Duffy VB. Bitter taste markers explain variability in vegetable sweetness, bitterness, and intake. Physiol Behav. 2006;87:304–313. doi: 10.1016/j.physbeh.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Duffy VB, Peterson J, Bartoshuk LM. Associations between taste genetics, oral sensations and alcohol intake. Physiol Behav. 2004;82:435–445. doi: 10.1016/j.physbeh.2004.04.060. [DOI] [PubMed] [Google Scholar]
- 15.Bartoshuk LM, Duffy VB, Hayes JE, Moskowitz HR, Snyder DJ. Psychophysics of sweet and fat perception in obesity: problems, solutions and new perspectives. Philos Trans R Soc Lond B Biol Sci. 2006;361:1137–1148. doi: 10.1098/rstb.2006.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sartor F, Donaldson LF, Markland DA, Loveday H, Jackson MJ, Kubis HP. Taste perception and implicit attitude toward sweet related to body mass index and soft drink supplementation. Appetite. 2011;57:237–246. doi: 10.1016/j.appet.2011.05.107. [DOI] [PubMed] [Google Scholar]
- 17.Overberg J, Hummel T, Krude H, Wiegand S. Differences in taste sensitivity between obese and non-obese children and adolescents. Arch Dis Child. 2012;97:1048–1052. doi: 10.1136/archdischild-2011-301189. [DOI] [PubMed] [Google Scholar]
- 18.Simchen U, Koebnick C, Hoyer S, Issanchou S, Zunft HJ. Odour and taste sensitivity is associated with body weight and extent of misreporting of body weight. Eur J Clin Nutr. 2006;60:698–705. doi: 10.1038/sj.ejcn.1602371. [DOI] [PubMed] [Google Scholar]
- 19.Low YQ, Lacy K, Keast R. The role of sweet taste in satiation and satiety. Nutrients. 2014;6:3431–3450. doi: 10.3390/nu6093431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Cordero E, Malacara-Hernandez JM, Martinez-Cordero C. Taste perception in normal and overweight Mexican adults. Appetite. 2015;89:192–195. doi: 10.1016/j.appet.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Duffy VBHJ, Davidson AC, Kidd JR, Kidd KK, Bartoshuk LM. Vegetable intake in college-aged adults is explained by oral sensory phenotypes and TAS2R38 genotype. Chemosens Percept. 2010;3:137–148. doi: 10.1007/s12078-010-9079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes JE, Duffy VB. Oral sensory phenotype identifies level of sugar and fat required for maximal liking. Physiol Behav. 2008;95:77–87. doi: 10.1016/j.physbeh.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattes RD, Cowart BJ, Schiavo MA, Arnold C, Garrison B, Kare MR, et al. Dietary evaluation of patients with smell and/or taste disorders. Am J Clin Nutr. 1990;51:233–240. doi: 10.1093/ajcn/51.2.233. [DOI] [PubMed] [Google Scholar]
- 24.Scinska A, Jodkowska A, Korkosz A, Kukwa W, Sienkiewicz-Jarosz H. Post-tonsillectomy dysgeusia with weight loss: possible involvement of soft palate. J Laryngol Otol. 2008;122:e5. doi: 10.1017/S002221510700117X. [DOI] [PubMed] [Google Scholar]
- 25.Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, et al. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav. 2004;82:109–114. doi: 10.1016/j.physbeh.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 26.Coldwell SE, Mennella JA, Duffy VB, Pelchat ML, Griffith JW, Smutzer G, et al. Gustation assessment using the NIH Toolbox. Neurology. 2013;80:S20–S24. doi: 10.1212/WNL.0b013e3182872e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guidelines on Overweight and Obesity: Electronic Textbook. National Heart, Lung and Blood Institute. Available from: http://www.nhlbi.nih.gov/healthpro/guidelines/current/obesity-guidelines/e_textbook/txgd/4142.htm.
- 28.Bonuck KA, Freeman K, Henderson J. Growth and growth biomarker changes after adenotonsillectomy: systematic review and meta-analysis. Arch Dis Child. 2009;94:83–91. doi: 10.1136/adc.2008.141192. [DOI] [PubMed] [Google Scholar]
- 29.Conlon BJ, Donnelly MJ, McShane DP. Tonsillitis, tonsillectomy and weight disturbance. Int J Pediatr Otorhinolaryngol. 1997;42:17–23. doi: 10.1016/s0165-5876(97)00105-5. [DOI] [PubMed] [Google Scholar]
- 30.Jeyakumar A, Fettman N, Armbrecht ES, Mitchell R. A systematic review of adenotonsillectomy as a risk factor for childhood obesity. Otolaryngol Head Neck Surg. 2011;144:154–158. doi: 10.1177/0194599810392328. [DOI] [PubMed] [Google Scholar]
- 31.Mueller CA, Khatib S, Landis BN, Temmel AF, Hummel T. Gustatory function after tonsillectomy. Arch Otolaryngol Head Neck Surg. 2007;133:668–671. doi: 10.1001/archotol.133.7.668. [DOI] [PubMed] [Google Scholar]
- 32.Seaberg RM, Chadha NK, Hubbard BJ, Gordon KA, Allemang BA, Harrison BJ, et al. Chorda tympani nerve function in children: relationship to otitis media and body mass index. Int J Pediatr Otorhinolaryngol. 2010;74:1393–1396. doi: 10.1016/j.ijporl.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Landis BN, Lacroix JS. Postoperative/posttraumatic gustatory dysfunction. Adv Otorhinolaryngol. 2006;63:242–254. doi: 10.1159/000093763. [DOI] [PubMed] [Google Scholar]
- 34.Jourdan C, Brugel D, Hubeaux K, Toure H, Laurent-Vannier A, Chevignard M. Weight gain after childhood traumatic brain injury: a matter of concern. Dev Med Child Neurol. 2012;54:624–628. doi: 10.1111/j.1469-8749.2012.04291.x. [DOI] [PubMed] [Google Scholar]
- 35.Crenn P, Hamchaoui S, Bourget-Massari A, Hanachi M, Melchior JC, Azouvi P. Changes in weight after traumatic brain injury in adult patients: A longitudinal study. Clin Nutr. 2014;33:348–353. doi: 10.1016/j.clnu.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Schechter PJ, Henkin RI. Abnormalities of taste and smell after head trauma. J Neurol Neurosurg Psychiatry. 1974;37:802–810. doi: 10.1136/jnnp.37.7.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castano B, Capdevila E. Eating disorders in patients with traumatic brain injury: a report of four cases. NeuroRehabilitation. 2010;27:113–116. doi: 10.3233/NRE-2010-0586. [DOI] [PubMed] [Google Scholar]
- 38.Lewin J, Sumners D. Anorexia due to brain injury. Brain Inj. 1992;6:199–201. doi: 10.3109/02699059209029660. [DOI] [PubMed] [Google Scholar]
- 39.Kvestad E, Kvaerner KJ, Roysamb E, Tambs K, Harris JR, Magnus P. The reliability of self-reported childhood otitis media by adults. Int J Pediatr Otorhinolaryngol. 2006;70:597–602. doi: 10.1016/j.ijporl.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Stephenson H. Evaluation of self-report by adults of childhood otitis media histories. Audiology. 1995;34:124–134. doi: 10.3109/00206099509071906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.