Abstract
Seventy-three women with PTSD resulting from rape or physical assault participated in a loud-tone procedure, while skin conductance (SC), heart rate and electromyogram responses were recorded. Pearson correlations were examined between each psychophysiological response and Clinician-Administered PTSD Scale (CAPS) symptom scores. Significant correlations were adjusted for each remaining individual PTSD symptom score. Heart rate response (HRR) significantly correlated with CAPS total score and with CAPS nightmares. The relationship between HRR and nightmares remained significant after controlling for each of the other 16 individual PTSD symptoms, for the remaining reexperiencing cluster, and for CAPS total score. The zero-order correlations between SC response and nightmares and between EMG response and nightmares were both not significant. The association of nightmares with larger HRR in the absence of an association with larger SC response likely reflects reduced parasympathetic tone. Thus, our findings indirectly support a role for reduced parasympathetic tone in PTSD nightmares.
Keywords: Psychophysiology, heart rate response, skin conductance response, electromyography, posttraumatic stress disorder, traumatic stress, anxiety disorders
1. Introduction
Although the association between psychophysiological responses to startling stimuli and PTSD has been extensively researched, the association between the former and individual PTSD symptoms has received little attention. Psychophysiological measures such as skin conductance response (SCR), heart rate response (HRR), and orbicularis oculi electromyogram (eyeblink) response (EMGR) to loud tones have been shown to be altered in PTSD (Orr, Lasko, Shalev, & Pitman, 1995; Shalev, Orr, Peri, Schreiber, & Pitman, 1992). However, the pattern of psychophysiological reactivity to loud tones has differed across studies. For example, increased SCR has been observed in some studies (e.g., (Shalev et al., 1992), but not others (e.g., (Orr et al., 1995). Similarly, increased startle EMGR in veterans with PTSD has been observed in some studies (Butler et al., 1990; Orr et al., 1995), but not others (e.g., (Shalev et al., 1992). In contrast to these differing findings, increased HRRs to loud tones has been consistently observed in PTSD (Carson et al., 2007; Orr et al., 1995; Orr et al., 2003; Paige, Reid, Allen, & Newton, 1990; Shalev et al., 1992; Shalev et al., 2000) and appears to be an acquired feature of this disorder (Orr et al., 2003; Shalev et al., 2000). Functionally, whereas SCR has been suggested to be a relatively pure measure of sympathetic activation (Lang, Bradley, & Cuthbert, 1990; Orr et al., 1995), it is likely that reduced parasympathetic tone explains the consistent finding of increased HRR to loud tones (Orr et al., 1995).
Polyvagal theory posits that there are three phylogenetically derived structures responsible for autonomic regulation leading to three neurophysiological states and three behavioral survival strategies (Porges, 2001). Relevant to the issue of HRR regulation, when the myelinated vagus fibers originating in the nucleus ambiguus in the medulla become deactivated, the sympathetic nervous system increases cardiac output and HRR unopposed by the vagus’ “vagal brake.”
According to DSM-IV, PTSD is a clinical syndrome that includes 17 symptoms falling into three different domains: re-experiencing, avoidance and numbing of general responsiveness, and increased arousal (American Psychiatric Association, 1994). Each of these domains comprises specific diagnostic criteria (i.e., symptoms). To date, relatively little is known about the autonomic basis of individual clinical symptoms in PTSD. The present study examined relationships between psychophysiological reactivity to loud tones and individual DSM-IV PTSD symptoms.
2. Methods
2.1 Participants
Data were obtained in the context of a cognitive behavioral therapy study of women with PTSD resulting from rape or physical assault (n=150) (Griffin, Resick, & Galovski, 2012; Resick et al., 2008). A subset of these women (n=73) were tested in the psychophysiology laboratory prior to beginning therapy by measuring their HR, SC and EMG responses during exposure to a series of loud tones. Of the 73 women, 54 were victims of sexual assault (74%), and 19 were the victims of first degree physical assault (26%). Their mean age was 35 years (SD=11.5). The mean time since the assault was 166 months (13.75 years, SD=170.7), range 4–639 months. Participants’ racial origins were as follows: 19 (26%) African-American, 50 (68%) Caucasian, and 4 (6%) other. Their ethnic origin was: 51 (70%) non-Hispanic and 1 (1%) Hispanic; data from 21 (29%) were missing.
2.2 Psychodiagnostics
The standard 1-month version of the Clinician-Administered PTSD Scale (CAPS) for DSM-IV (Blake et al., 1995) was used to assess PTSD symptoms. Clinicians with master’s degree or above served as diagnostic raters, as described in prior publications (Griffin et al., 2012). All individual items are scored as the sum of intensity (0–4) and frequency (0–4) of the symptom, yielding a nine-point (0–8) severity score (Weathers, Keane, & Davidson, 2001).
2.3 Hearing
All participants were screened for ability to hear a pure tone tested at 125, 750, 1000, 3000, and 6000 Hz at a threshold of 35 decibels. No participants showed a hearing loss.
2.4 Laboratory Assessment
Psychophysiological testing took place in a sound-attenuated room adjacent to an observation room containing the physiological equipment. Participants were seated in a comfortable armchair, and the recording electrodes were attached. Participants were instructed that following a 4-minute resting baseline period, they would hear a series of loud tones and should keep their eyes open. The stimuli were 10 consecutive, 95-dB (sound pressure level), 1000 Hz pure tones presented for 500 ms with nearly 0-msec rise and fall times. Tones were presented binaurally over headphones.
2.5 Physiologic Measures
Left orbicularis oculi EMG was recorded using two 4-mm Ag/AgCl surface electrodes positioned according to published recommendations (Fridlund & Cacioppo, 1986). The EMG signals were amplified and filtered to retain the portion from 90–250 Hz. The amplified signal was fed into a contour-following integrator that was set to an integration time constant of 65ms. An EMGR score was calculated by taking the maximum EMG level between 40–200 ms after tone onset minus the average level during the 1 s immediately prior to tone onset (baseline). Skin conductance was recorded using Ag/AgCl 9-mm electrodes filled with isotonic paste and attached to the medial phalanges of the first and third finger of the left hand. An SCR was defined as the maximum conductance level between 0.5–6 seconds after tone onset minus the average level during the 2 s immediately prior to tone onset. Heart rate was recorded using disposable Ag/AgCl electrodes attached to the left wrist and right ankle. Intertrial intervals were varied randomly between 32 and 52 seconds. Peak R-wave signals were detected, and interbeat intervals were converted to HR. Heart rate response was calculated as the maximum HR achieved during the period from 0.5–6 s after tone onset minus the average level during the 5 s immediately prior to tone onset (Griffin et al., 2012).
Data for all physiological signals were continuously sampled at 500 Hz. In order to reduce the variability associated with extreme scores, a square root transformation was performed on the HR, SC, and EMG response scores before statistical analyses. Transformed responses of each psychophysiological measure were averaged across the 10 tone trials.
2.6 Statistical Analysis
As a first step, three sets of parallel Pearson correlations were examined. First, correlations were examined between each of the three psychophysiological responses to the tones (EMGR, SCR, and HRR) and CAPS total score. For this analysis, the threshold for statistical significance was p<0.017 (0.05 ÷ 3 psychophysiological measures). Second, correlations were also examined between each psychophysiological measure and each CAPS cluster score, at a significance threshold of p<0.006 (0.05 ÷ 3 ÷ 3 DSM-IV clusters). Third, correlations were examined between each psychophysiological measure and each of the 17 CAPS individual PTSD symptoms, using a significance threshold of p<0.001 (p<0.05 ÷ 3 ÷ 17 DSM-IV individual symptoms). Partial correlations were also examined to ascertain whether any significant correlation between a given psychophysiological response measure and a given individual CAPS item remained significant (at p<0.05 unadjusted) after adjusting for the effect of the remaining 16 individual PTSD items (separately), CAPS total score (with the individual CAPS item score subtracted), and the score of the specific CAPS cluster to which the individual item belonged (again with the individual CAPS item score subtracted).
3. Results
3.1 Demographic, psychometric, and psychophysiological measures
Table 1 shows the group mean demographic, psychometric, and psychophysiological scores.
Table 1.
Demographic, Psychometric and Psychophysiological Variables
| Variable | N | Mean | Standard Deviation | Minimum | Maximum |
|---|---|---|---|---|---|
| Age | 73 | 34.8 | 11.5 | 18.0 | 69.0 |
| Years of Education | 73 | 14.2 | 2.4 | 9.0 | 21.0 |
| Months since assault | 73 | 165.5 | 170.7 | 3.9 | 638.7 |
| CAPS Total Score | 73 | 69.1 | 17.8 | 31.0 | 109.0 |
| CAPS B symptoms (Reexperiencing) − Frequency+Intensity | 73 | 17.5 | 6.8 | 5.0 | 35.0 |
| CAPS C symptoms (Avoidance/Numbing) − Frequency+Intensity | 73 | 29.5 | 9.3 | 11.0 | 49.0 |
| CAPS D symptoms (Increased Arousal) − Frequency+Intensity | 73 | 22.1 | 7.1 | 5.0 | 38.0 |
| BDI-2 Total Score | 71 | 26.3 | 10.5 | 7.0 | 51.0 |
| SCR Mean | 58 | 0.8 | 0.5 | 0.0 | 2.1 |
| HRR Mean | 69 | 2.6 | 0.8 | 0.8 | 4.6 |
| EMGR Mean | 72 | 3.7 | 1.9 | 0.5 | 7.9 |
| Resting Baseline EMG | 72 | 6.2 | 5.7 | 0.4 | 25.2 |
| Resting Baseline HR | 73 | 75.1 | 9.2 | 54.9 | 100.2 |
CAPS: Clinician Administered PTSD Scale; BDI: Beck Depression Inventory; SCR: Skin conductance response; HRR: heart rate response: EMGR: electromyographic response; EMG: electromyography.
3.2 Zero-order correlations
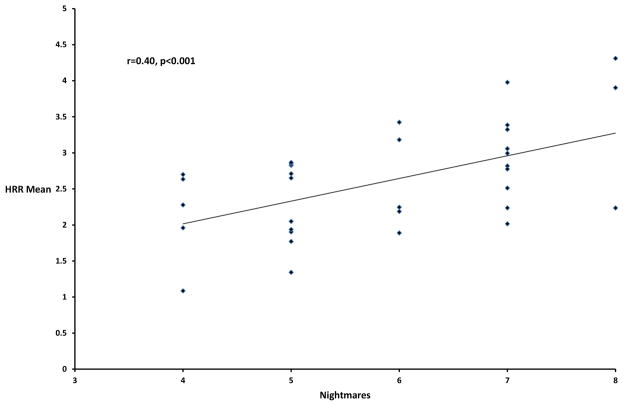
Pearson correlations between the 17 CAPS items and the three psychophysiological response measures are presented in Table 2. For CAPS total score, only the correlation with heart rate response (HRR) was significant (p<0.017). None of the CAPS cluster scores significantly correlated with any of the psychophysiological variables (at p<0.006). For the individual PTSD items, the only significant correlation was between CAPS nightmares (B2) and HRR (at p<0.001). This association is portrayed in the Figure.
Table 2.
Pearson Correlation Coefficients Between Psychophysiological Measures and CAPS Scores
| CAPS Scores (freq + int) | Description | CAPS Means | HRR | SCR | EMGR |
|---|---|---|---|---|---|
| CAPS TOTAL | CAPS Total Score | 69.1 | 0.30* | −0.14 | −0.05 |
| CAPS B1 | Distressing recollections | 3.6 | 0.27 | 0.10 | 0.20 |
| CAPS B2 | Distressing dreams | 5.4 | 0.40*** | 0.03 | −0.07 |
| CAPS B3 | Reliving the trauma | 1.1 | −0.01 | −0.15 | −0.02 |
| CAPS B4 | Psychological distress at cue exposure | 3.6 | 0.04 | −0.25 | −0.09 |
| CAPS B5 | Physiological reactivity to cue exposure | 3.6 | 0.26 | 0.15 | 0.10 |
| CAPS B | Reexperiencing the trauma | 17.5 | 0.31 | −0.02 | 0.07 |
| CAPS C1 | Avoiding thoughts, feelings, or conversations | 5.6 | 0.11 | −0.17 | 0.13 |
| CAPS C2 | Avoiding activities, places, or people | 4.4 | 0.19 | 0.16 | −0.09 |
| CAPS C3 | Inability to recall aspects of traumatic event | 2.4 | 0.01 | −0.06 | 0.06 |
| CAPS C4 | Diminished interest in significant activities | 3.9 | 0.05 | 0.04 | −0.06 |
| CAPS C5 | Feeling detached or estranged from others | 5.4 | 0.32 | 0.03 | 0.19 |
| CAPS C6 | Restricted range of affect | 4.1 | −0.07 | −0.09 | −0.14 |
| CAPS C7 | Sense of foreshortened future | 3.3 | 0.15 | −0.07 | −0.07 |
| CAPS C | Avoiding stimuli/numbing of responsiveness | 29.5 | 0.21 | −0.02 | −0.03 |
| CAPS D1 | Difficulty falling/staying asleep | 5.4 | −0.09 | −0.39 | 0.08 |
| CAPS D2 | Irritability/outbursts of anger | 4.0 | 0.20 | −0.07 | 0.04 |
| CAPS D3 | Difficulty concentrating | 4.8 | 0.11 | −0.10 | −0.08 |
| CAPS D4 | Hypervigilance | 4.8 | 0.15 | −0.05 | −0.30 |
| CAPS D5 | Exaggerated startle response | 3.4 | 0.13 | −0.11 | −0.08 |
| CAPS D | Increased arousal | 22.1 | 0.19 | −0.30 | −0.15 |
For CAPS Total Score, threshold p<0.017*
For CAPS Cluster Score, threshold p<0.006**
For CAPS Item Score, threshold p<0.001***
CAPS: Clinician Administered PTSD Scale.
Figure.
Correlation between Clinician-Administered PTSD Scale Nightmares item scores and mean heart rate response averaged across the 10 tone trials (HRR Mean)
3.3 Partial correlations
The relationship between HRR and nightmares remained significant after controlling for each of the other 16 individual PTSD symptoms (all partial r’s ≥ 0.33; p’s ≤ 0.007). Additionally, the correlation between HRR and nightmares remained significant after controlling for the remaining cluster B score (reexperiencing cluster, with the nightmares item score subtracted; r=0.35, p=0.004), and after controlling for CAPS total score (with the nightmares item score subtracted; r=0.33, p=0.007).
4. Discussion
The major finding of the present study is that in a group of 73 women who had experienced rape or physical assault, self-reported nightmare severity (CAPS item B2, which is part of the re-experiencing cluster of PTSD symptoms) was significantly associated with greater HRR, but not with greater SCR, to a series of sudden, loud tones. Whereas HR is modulated by both the sympathetic (positively) and parasympathetic (negatively) nervous systems, SC is only modulated by the former (Orr et al., 2012). Thus, the observed absence of a significant statistical sympathetic contribution to nightmare severity, in the presence of a significant (negative) statistical parasympathetic contribution, suggests that posttraumatic nightmares may be associated with reduced parasympathetic activity. Consistent with polyvagal theory (Porges, 2001), our findings suggest that PTSD nightmares are associated with relative suppression of the “vagal brake” on the heart, which leads to relative disinhibition of the sympathetic nervous system influence on the heart.
Although much of the research into autonomic mechanisms in PTSD implicates sympathetic nervous system dysfunction (Busso, McLaughlin, & Sheridan, 2014; Mellman, Knorr, Pigeon, Leiter, & Akay, 2004; Morris & Rao, 2013), there is an increasing literature supporting parasympathetic dysfunction in PTSD as well. Sahar and colleagues investigated HR response in 29 trauma-exposed subjects, 14 with PTSD and 15 without, while at rest and during an arithmetic challenge (Sahar, Shalev, & Porges, 2001). The investigators found that the non-PTSD trauma group showed increased respiratory sinus arrhythmia during the arithmetic challenge, suggesting myelinated vagal (i.e., parasympathetic) modulation of their HR response, whereas the PTSD group did not. PTSD subjects have been found to have significantly lower HR variability and respiratory sinus arrhythmia at rest compared with both trauma-exposed (Cohen et al., 1997; Jovanovic, Norrholm, Sakoman, Esterajher, & Kozaric-Kovacic, 2009; Norte et al., 2013) and trauma-unexposed healthy subjects (Cohen et al., 1998; Jovanovic et al., 2009), suggesting an impairment in the myelinated vagus inhibition of the sympathetic nervous system influence on the heart (Porges, 2001). Although the literature has produced inconsistent results on resting baseline HR in PTSD, a recent meta-analysis of psychophysiological data showed that PTSD subjects had higher resting HR, higher diastolic blood pressure, but similar systolic blood pressure compared with subjects without PTSD. The meta-analysis concluded that PTSD exerts stronger effects on the resting phase (on diastolic blood pressure) than on the activation phase (on systolic blood pressure) of the cardiac cycle, which is consistent with the hypothesis that the relationship between PTSD and elevated resting HR is mediated primarily by reduced parasympathetic activity rather than increased sympathetic activity (Pole, 2007).
Results from two studies suggest a relationship between reduced parasympathetic tone and nightmares, albeit not in PTSD populations. Simor et al. used electroencephalography, electrocardiography, and electromyography to compare individuals suffering from nightmares and healthy controls. The investigators found that individuals with nightmares had lower HR variability during pre-rapid eye movement (REM) and non-REM sleep periods, suggesting attenuated parasympathetic regulation (Simor et al., 2014). Nielsen and colleagues found that during a REM-recovery sleep night, individuals suffering from nightmares had higher low-frequency spectrum power (reflecting greater sympathetic influence), lower high-frequency spectrum power (reflecting lower parasympathetic influence), and higher low-frequency/high-frequency ratio (reflecting higher sympathovagal ratio) for HR during REM and stage 2 sleep, compared to normal controls (Nielsen et al., 2010). The authors concluded that the individuals with nightmares showed evidence of increased sympathetic activity during REM sleep, compared with healthy controls, and this sympathetic activity was related to nightmares. However, the lower high-frequency spectrum results also suggest decreased parasympathetic activity in individuals with nightmares. In addition, whereas healthy controls shifted from sympathetic to parasympathetic activity during recovery from REM sleep deprivation, individuals with nightmares remained in a mode of elevated sympathetic activity. Thus, although there is some evidence of parasympathetic dysfunction in nightmares in general, and there is evidence of impaired balance between the sympathetic and parasympathetic systems in PTSD, our study is the first to suggest an association between parasympathetic dysfunction and nightmares in PTSD subjects.
Alpha-1-adrenergic receptors participate in the regulation of nightmares in PTSD (Raskind et al., 2003; Raskind et al., 2013; Thompson, Taylor, McFall, Barnes, & Raskind, 2008), and more generally in sleep (Berridge, Schmeichel, & Espana, 2012). Most medications used to treat PTSD nightmares, as well as exaggerated startle, reduce adrenergic activity in the CNS (Lipov & Kelzenberg, 2012; Ostrowski & Delahanty, 2014). Prazosin, which is an α1-adrenergic receptor antagonist, reduces CNS sympathetic outflow throughout the brain and is used to treat PTSD nightmares (Raskind et al., 2003; Raskind et al., 2013; Thompson et al., 2008), and more generally disturbed sleep (Berridge et al., 2012). In a placebo-controlled study, prazosin was associated with improved total sleep time, increased REM sleep time, and increased mean REM period duration without alteration of sleep-onset latency (Taylor et al., 2008). Clonidine, an α2-adrenergic receptor agonist that suppresses sympathetic nervous system outflow, is similarly used to treat PTSD, although the research evidence is less robust and based primarily on case reports (Aurora et al., 2010). Cyproheptadine, a potent antagonist of serotonin, histamine, and acetylcholine, has been used in the treatment of PTSD-related nightmares, although the data for its use are of low quality and conflicting (Aurora et al., 2010).
Thus, in the literature, drugs that treat PTSD nightmares primarily target putatively increased sympathetic activity. However, although the sympatholytic prazosin has been found effective in reducing PTSD nightmares, the percent reduction is only approximately 50% (Raskind et al., 2003; Raskind et al., 2007; Raskind et al., 2013). This leaves substantial room for a parasympathetic contribution to PTSD nightmares. Our results potentially suggest a role for decreased parasympathetic activity in posttraumatic nightmares and a new direction for pharmacologic interventions that increase parasympathetic activity at bedtime.
The present study has several limitations. Although we correlated CAPS symptoms with the psychophysiological measurements, these two sets of data were not obtained simultaneously but on consecutive days. Also, the psychophysiological responses were recorded over a period of time that lasted several minutes, whereas the CAPS symptoms were assessed for the past month. These considerations could dilute associations between subjective (symptom self-report) and objective (psychophysiology) domains.
The correlation results preclude making causal inferences, e.g., that decreased parasympathetic tone underlies PTSD nightmares. Such inferences derive from our reasoning about the data, rather than from the data themselves, and our reasoning is indirect because we did not assess psychophysiological reactivity to the loud tones in the sleep laboratory, e.g., while the subjects were experiencing nightmares. Another limitation of our archival dataset, collected between October 2000 and August 2005, is that it did not contain the 4-minute resting baseline heart rate recordings, from which heart rate variability (HRV), a measure of parasympathetic tone, could be derived. Future research should consider obtaining HRV prior to presenting the loud tone stimuli, so as to allow for an examination of the relationship between HRV and heart rate reactivity to the loud tones. From prior research, it is known that decreased parasympathetic tone is associated with PTSD and that this changes with treatment (Griffin et al., 2012). Experiments in which parasympathetic tone were to be modified, e.g., by drugs, and the effect on nightmares measured, could help clarify causation.
Acknowledgments
This work was partly supported by an National Institute of Mental Health career development award grant 1K23MH097844-01A1 awarded to Kaloyan S. Tanev, and by National Institute of Mental Health Grant 2-R01-MH51509 awarded to Patricia A. Resick.
Footnotes
Conflict of interest statement:
Dr. Tanev does not have any conflict of interest impacting his authorship in this article. Dr. Orr does not have any conflict of interest impacting his authorship in this article. Dr. Pace-Schott does not have any conflict of interest impacting his authorship in this article. Dr. Griffin does not have any conflict of interest impacting his authorship in this article. Dr. Pitman does not have any conflict of interest impacting his authorship in this article. Dr. Resick does not have any conflict of interest impacting her authorship in this article.
Informed consent was obtained from subjects prior to their participation in this trial.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, D.C: American Psychiatric Association; 1994. [Google Scholar]
- Aurora RN, Zak RS, Auerbach SH, Casey KR, Chowdhuri S, Karippot A … American Academy of Sleep Medicine. Best practice guide for the treatment of nightmare disorder in adults. Journal of Clinical Sleep Medicine : JCSM : Official Publication of the American Academy of Sleep Medicine. 2010;6(4):389–401. [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Schmeichel BE, Espana RA. Noradrenergic modulation of wakefulness/arousal. Sleep Medicine Reviews. 2012;16(2):187–197. doi: 10.1016/j.smrv.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Busso DS, McLaughlin KA, Sheridan MA. Media exposure and sympathetic nervous system reactivity predict PTSD symptoms after the boston marathon bombings. Depression and Anxiety. 2014;31(7):551–558. doi: 10.1002/da.22282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler RW, Braff DL, Rausch JL, Jenkins MA, Sprock J, Geyer MA. Physiological evidence of exaggerated startle response in a subgroup of vietnam veterans with combat-related PTSD. The American Journal of Psychiatry. 1990;147(10):1308–1312. doi: 10.1176/ajp.147.10.1308. [DOI] [PubMed] [Google Scholar]
- Carson MA, Metzger LJ, Lasko NB, Paulus LA, Morse AE, Pitman RK, Orr SP. Physiologic reactivity to startling tones in female vietnam nurse veterans with PTSD. Journal of Traumatic Stress. 2007;20(5):657–666. doi: 10.1002/jts.20218. [DOI] [PubMed] [Google Scholar]
- Cohen H, Kotler M, Matar MA, Kaplan Z, Loewenthal U, Miodownik H, Cassuto Y. Analysis of heart rate variability in posttraumatic stress disorder patients in response to a trauma-related reminder. Biological Psychiatry. 1998;44(10):1054–1059. doi: 10.1016/s0006-3223(97)00475-7. S0006-3223(97)00475-7 [pii] [DOI] [PubMed] [Google Scholar]
- Cohen H, Kotler M, Matar MA, Kaplan Z, Miodownik H, Cassuto Y. Power spectral analysis of heart rate variability in posttraumatic stress disorder patients. Biological Psychiatry. 1997;41(5):627–629. doi: 10.1016/s0006-3223(96)00525-2. S0006322396005252 [pii] [DOI] [PubMed] [Google Scholar]
- Griffin MG, Resick PA, Galovski TE. Does physiologic response to loud tones change following cognitive-behavioral treatment for posttraumatic stress disorder? Journal of Traumatic Stress. 2012;25(1):25–32. doi: 10.1002/jts.21667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Sakoman AJ, Esterajher S, Kozaric-Kovacic D. Altered resting psychophysiology and startle response in croatian combat veterans with PTSD. International Journal of Psychophysiology : Official Journal of the International Organization of Psychophysiology. 2009;71(3):264–268. doi: 10.1016/j.ijpsycho.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97(3):377–395. [PubMed] [Google Scholar]
- Lipov E, Kelzenberg B. Sympathetic system modulation to treat post-traumatic stress disorder (PTSD): A review of clinical evidence and neurobiology. Journal of Affective Disorders. 2012;142(1–3):1–5. doi: 10.1016/j.jad.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Knorr BR, Pigeon WR, Leiter JC, Akay M. Heart rate variability during sleep and the early development of posttraumatic stress disorder. Biological Psychiatry. 2004;55(9):953–956. doi: 10.1016/j.biopsych.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Morris MC, Rao U. Psychobiology of PTSD in the acute aftermath of trauma: Integrating research on coping, HPA function and sympathetic nervous system activity. Asian Journal of Psychiatry. 2013;6(1):3–21. doi: 10.1016/j.ajp.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen T, Paquette T, Solomonova E, Lara-Carrasco J, Colombo R, Lanfranchi P. Changes in cardiac variability after REM sleep deprivation in recurrent nightmares. Sleep. 2010;33(1):113–122. doi: 10.1093/sleep/33.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norte CE, Souza GG, Vilete L, Marques-Portella C, Coutinho ES, Figueira I, Volchan E. They know their trauma by heart: An assessment of psychophysiological failure to recover in PTSD. Journal of Affective Disorders. 2013;150(1):136–141. doi: 10.1016/j.jad.2012.11.039. [DOI] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Macklin ML, Pineles SL, Chang Y, Pitman RK. Predicting post-trauma stress symptoms from pre-trauma psychophysiologic reactivity, personality traits and measures of psychopathology. Biology of Mood & Anxiety Disorders. 2012;2(1):8-5380-2-8. doi: 10.1186/2045-5380-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Shalev AY, Pitman RK. Physiologic responses to loud tones in vietnam veterans with posttraumatic stress disorder. Journal of Abnormal Psychology. 1995;104(1):75–82. doi: 10.1037//0021-843x.104.1.75. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Hu FB, Shalev AY … Harvard/Veterans Affairs Post-traumatic Stress Disorder Twin Study Investigators. Physiologic responses to sudden, loud tones in monozygotic twins discordant for combat exposure: Association with posttraumatic stress disorder. Archives of General Psychiatry. 2003;60(3):283–288. doi: 10.1001/archpsyc.60.3.283. yoa10353 [pii] [DOI] [PubMed] [Google Scholar]
- Ostrowski SA, Delahanty DL. Prospects for the pharmacological prevention of post-traumatic stress in vulnerable individuals. CNS Drugs. 2014;28(3):195–203. doi: 10.1007/s40263-014-0145-7. [DOI] [PubMed] [Google Scholar]
- Paige SR, Reid GM, Allen MG, Newton JE. Psychophysiological correlates of posttraumatic stress disorder in vietnam<br/>veterans. Biol Psychiatry. 1990 Feb 15;27(4):419–30. doi: 10.1016/0006-3223(90)90552-d. [DOI] [PubMed] [Google Scholar]
- Pole N. The psychophysiology of posttraumatic stress disorder: A meta-analysis. Psychological Bulletin. 2007;133(5):725–746. doi: 10.1037/0033-2909.133.5.725. 2007-12463-001 [pii] [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: Phylogenetic substrates of a social nervous system. International Journal of Psychophysiology : Official Journal of the International Organization of Psychophysiology. 2001;42(2):123–146. doi: 10.1016/s0167-8760(01)00162-3. S0167-8760(01)00162-3 [pii] [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, Warren D, … McFall ME. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biological Psychiatry. 2007;61(8):928–934. doi: 10.1016/j.biopsych.2006.06.032. S0006-3223(06)00862-6 [pii] [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, … McFall MM. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: A placebo-controlled study. The American Journal of Psychiatry. 2003;160(2):371–373. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peterson K, Williams T, Hoff DJ, Hart K, Holmes H, … Peskind ER. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from iraq and afghanistan. The American Journal of Psychiatry. 2013;170(9):1003–1010. doi: 10.1176/appi.ajp.2013.12081133. [DOI] [PubMed] [Google Scholar]
- Resick PA, Galovski TE, O’Brien Uhlmansiek M, Scher CD, Clum GA, Young-Xu Y. A randomized clinical trial to dismantle components of cognitive processing therapy for posttraumatic stress disorder in female victims of interpersonal violence. Journal of Consulting and Clinical Psychology. 2008;76(2):243–258. doi: 10.1037/0022-006X.76.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahar T, Shalev AY, Porges SW. Vagal modulation of responses to mental challenge in posttraumatic stress disorder. Biological Psychiatry. 2001;49(7):637–643. doi: 10.1016/s0006-3223(00)01045-3. S0006322300010453 [pii] [DOI] [PubMed] [Google Scholar]
- Shalev AY, Orr SP, Peri T, Schreiber S, Pitman RK. Physiologic responses to loud tones in israeli patients with posttraumatic stress disorder. Archives of General Psychiatry. 1992;49(11):870–875. doi: 10.1001/archpsyc.1992.01820110034005. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Peri T, Brandes D, Freedman S, Orr SP, Pitman RK. Auditory startle response in trauma survivors with posttraumatic stress disorder: A prospective study. The American Journal of Psychiatry. 2000;157(2):255–261. doi: 10.1176/appi.ajp.157.2.255. [DOI] [PubMed] [Google Scholar]
- Simor P, Kormendi J, Horvath K, Gombos F, Ujma PP, Bodizs R. Electroencephalographic and autonomic alterations in subjects with frequent nightmares during pre- and post-REM periods. Brain and Cognition. 2014;91:62–70. doi: 10.1016/j.bandc.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Taylor FB, Martin P, Thompson C, Williams J, Mellman TA, Gross C, … Raskind MA. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: A placebo-controlled study. Biological Psychiatry. 2008;63(6):629–632. doi: 10.1016/j.biopsych.2007.07.001. S0006-3223(07)00639-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CE, Taylor FB, McFall ME, Barnes RF, Raskind MA. Nonnightmare distressed awakenings in veterans with posttraumatic stress disorder: Response to prazosin. Journal of Traumatic Stress. 2008;21(4):417–420. doi: 10.1002/jts.20351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: A review of the first ten years of research. Depression & Anxiety. 2001;13(3):132–156. doi: 10.1002/da.1029. Retrieved from https://phstwlp1.partners.org:2443/login?url=http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=med4&AN=11387733. [DOI] [PubMed] [Google Scholar]