Abstract
Background
Ofatumumab is a humanized anti-CD20 monoclonal antibody that has recently garnered interest as a potential therapeutic agent for nephrotic syndrome. We report our center's experience in administering ofatumumab to 5 pediatric patients with idiopathic nephrotic syndrome.
Methods
Between March 2015 and November 2016, 5 patients were treated with ofatumumab. One patient had post-transplant recurrent focal segmental glomerulosclerosis (FSGS) which had been resistant to plasmapheresis and numerous immunosuppressive agents. Four patients had nephrotic syndrome in their native kidneys, one with initial steroid-resistant disease, and the others with subsequent development of steroid-resistance. Two of the patients were treated with a desensitization protocol after experiencing hypersensitivity reactions to ofatumumab.
Results
One patient did not complete ofatumumab treatment due to infusion reactions. Of the 4 remaining patients, 3 achieved complete remission after treatment, and 1 partial remission. One of the patients achieving complete remission represents the first reported case of successful treatment of post-transplant recurrent FSGS using ofatumumab. Two patients who received ofatumumab with our desensitization protocol were able to complete their treatments after initially experiencing hypersensitivity reactions.
Conclusions
Ofatumumab may be an effective treatment for refractory childhood nephrotic syndrome and post-transplant recurrent FSGS. A desensitization protocol may be helpful to address hypersensitivity reactions.
Keywords: ofatumumab, nephrotic syndrome, focal segmental, glomerulosclerosis, children
Introduction
Treatment of nephrotic syndrome (NS) in children remains challenging. The current mainstay of therapy is prednisone/prednisolone given for weeks to months at presentation and with each disease relapse, exposing children to adverse steroid effects on metabolism, growth, and behavior [1]. Furthermore, 7.4-19.6% of children are resistant to corticosteroid therapy [2-4]. Second-line immunosuppressive agents used for those intolerant of or resistant to corticosteroids confer additional side-effects and have expected response rates of only 20-50% [5]. Patients who have treatment refractory NS inevitably progress to end-stage renal disease (ESRD) [6]. A further challenge is that focal segmental glomerulosclerosis (FSGS), one of the most common histologic subtypes of childhood NS, has a 15-30% risk of recurrence in transplanted kidneys [7]. Thus, identifying additional therapeutic agents is critical.
Ofatumumab is a human anti-CD20 monoclonal antibody indicated for the treatment of chronic lymphocytic leukemia. Recently, 3 reports on a total of 11 children were published on the use of ofatumumab for the treatment of refractory NS [8-10]. Remission was induced in 9 of the reported 11 children with multi-drug resistant NS [8-10]. This has spurred the off-label use of ofatumumab for childhood NS treatment, despite little information on dosing, efficacy, and side effects in this disease.
We reviewed our center's experience with ofatumumab in childhood NS. Between March 2015 and November 2016, 5 patients were treated with ofatumumab, including 4 patients with NS involving their native kidneys and one patient with recurrence of FSGS in his transplanted kidney. Our approach to administering ofatumumab safely to those who had hypersensitivity reactions is also described.
Materials and Methods
We performed a retrospective review of all pediatric NS patients treated with ofatumumab between March 2015 and November 2016 at the Children's Healthcare of Atlanta. There were 5 patients in total. All patients presented with NS between ages 1 and 18 years and had the clinical diagnosis of idiopathic NS (edema; urine protein/creatinine ratio (UPCR) > 2 mg/mg, or ≥ 300 mg/dL or 3+ protein on urine dipstick; and hypoalbuminemia ≤ 2.5 g/dL [1]), without evidence of secondary causes (e.g. lupus). Clinical course and laboratory results were collected from the electronic medical records. Ofatumumab dosing, administration, and reported side effects were obtained from the electronic medical records and confirmed with the dispensing pharmacy.
Ofatumumab Regimen and Desensitization Protocol
We based our ofatumumab treatment protocol on the report by Basu, with a first dose of 300 mg/1.73m2 followed by 5 weekly doses of 2000 mg/1.73m2 [8]. Our dosing regimen and administration is described in Table 1. We subsequently developed a desensitization protocol (Table 1) for patients who developed hypersensitivity reactions with our standard ofatumumuab regimen. This protocol is adopted from that reported by Galvão and Castells [11] and was utilized for Patients 4 and 5. The protocol was used for those who had mild-to-moderate infusion reactions - i.e. skin and subcutaneous tissue findings only or features suggesting respiratory, cardiovascular, or gastrointestinal involvement without hypoxia, hypotension, or neurologic compromise, as define by Brown [12].
Table 1. Ofatumumab Regimen and Desensitization Protocol.
| Standard Ofatumumab Regimena | ||||||
| Premedications | ||||||
| Drug | Route | Dose | ||||
| Acetaminophen | Oral | 15 mg/kg (max 650 mg) | ||||
| Diphenhydramine | IV | 0.5-1 mg/kg (max 50 mg) | ||||
| Methylprednisolone | IV | 1 mg/kg (max 60 mg) | ||||
| Dose | Concentration | Rate (mL/hr) | ||||
| Dose 1 | 300 mg/1.73m2 | 300 mg/1.73m2 in 1,000 mL NS | 6 incremental steps, 30 min each: 12, 25, 50, 100, 200, 300 Final step: 400 | |||
| Dose 2 | 2000 mg/1.73m2 (max 2000 mg) | 2000 mg/1.73m2 in 1,000 mL NS | Same as Dose 1 | |||
| Dose 3 | Same as Dose 2 | Same as Dose 2 | 4 incremental steps, 30 min each: 25, 50, 100, 200 Final step: 400 | |||
| Dose 4 | Same as Dose 2 | Same as Dose 2 | Same as Dose 3 | |||
| Dose 5 | Same as Dose 2 | Same as Dose 2 | Same as Dose 3 | |||
| Dose 6 | Same as Dose 2 | Same as Dose 2 | Same as Dose 3 | |||
| Desensitization Protocol for Patients with Allergic Reactions to Ofatumumab | ||||||
| Premedications | ||||||
| Drug | Route | Dose | ||||
| Acetaminophen | Oral | 15 mg/kg (max 650 mg) | ||||
| Diphenhydramine | IV | 0.5-1 mg/kg (max 50 mg) | ||||
| Methylprednisolone | IV | 1 mg/kg (max 60 mg) | ||||
| Cetirizine | Oral | 5-10 mg | ||||
| Montelukast | Oral | 5-10 mg | ||||
| Ranitidine | Oral | 75-125 mg | ||||
| Infusion Protocol for Each Ofatumumab Doseb | ||||||
| Step | Concentration (mg/mL) | Rate (mL/hr) | Time (min) | Dose per Step (mg) | Cumulative Dose (mg) | Cumulative time (min) |
| 1 | 0.04 | 2.5 | 15 | 0.025 | 0.025 | 15 |
| 2 | 0.04 | 5 | 15 | 0.05 | 0.075 | 30 |
| 3 | 0.04 | 10 | 15 | 0.1 | 0.175 | 45 |
| 4 | 0.04 | 20 | 15 | 0.2 | 0.375 | 60 |
| 5 | 0.40 | 5 | 15 | 0.5 | 0.875 | 75 |
| 6 | 0.40 | 10 | 15 | 1 | 1.875 | 90 |
| 7 | 0.40 | 20 | 15 | 2 | 3.875 | 105 |
| 8 | 0.40 | 40 | 15 | 4 | 7.875 | 120 |
| 9 | 1.98 | 10 | 15 | 4.96 | 12.835 | 135 |
| 10 | 1.98 | 20 | 15 | 9.92 | 22.755 | 150 |
| 11 | 1.98 | 40 | 15 | 19.84 | 42.595 | 165 |
| 12 | 1.98 | 80 | 15 | 39.68 | 82.275 | 180 |
| 13 | 1.98 | 120 | 15 | 59.53 | 141.805 | 195 |
| 14 | 1.98 | 160 | 15 | 79.37 | 221.175 | 210 |
| 15 | 1.98 | 200 | 15 | 99.21 | 320.385 | 225 |
| 16 | 1.98 | 240 | 210.6 | 1671.73 | 1992.115 | 435.6 |
Results
Patient 1
The patient is a 16-year-old male with onset of steroid dependent (SD) NS at age 9. Renal biopsy showed minimal change disease (MCD) with mesangial IgA deposition and mild mesangial hypercellularity. Mycophenolate mofetil (MMF), tacrolimus, and IV methylprednisolone failed to improve disease control, though adherence was poor. Two doses of IV rituximab 375 mg/m2 were given in the 4th year after diagnosis (course #1), followed by a remission lasting approximately 8 months. Relapse occurred within 3 months after B cell recovery (defined as CD19 >5% of lymphocytes), whereupon he received another two doses of IV rituximab 375 mg/m2 (course #2). This second course of rituximab did not result in depletion of B cells (defined as CD19 <1% of lymphocytes) and the NS persisted despite ongoing high-dose prednisone. He suffered numerous complications including pulmonary emboli, deep vein thromboses, and arterial thromboses. Ofatumumab treatment was initiated 3 months after the second course of rituximab. He received a first dose of ofatumumab of 300 mg/1.73m2 followed by 4 weekly doses of 2000 mg/1.73m2. B cell depletion was achieved by 1 week and persisted 5 months after the first dose. Remission was achieved within 3 weeks and lasted 13 months, whereupon a third course of IV rituximab, three doses of 375mg/m2, were given over a 6-week period. B cell depletion was again achieved with the third course of rituximab, and NS remitted 2 months later. At his last follow- up, he had been in remission for 3 months.
Patient 2
The patient is a 13-year-old male with onset of steroid resistant NS at age 3. Renal biopsy showed MCD. He experienced severe medication side effects while on multiple immunosuppressants – prednisone, cyclosporine, MMF, and tacrolimus. IV rituximab 375mg/m2 was thus given 5 years after diagnosis, followed by some improvement in relapse frequency. Eight years following diagnosis, a second dose of rituximab was complicated by symptoms consistent with serum sickness. Thus, treatment with ofatumumab was tried, but it was aborted when he developed rash and nasal congestion with the first dose. These symptoms resolved with IV methylprednisolone and diphenhydramine. At his last follow-up, he was having infrequent relapses on tacrolimus, MMF, and prednisone.
Patient 3
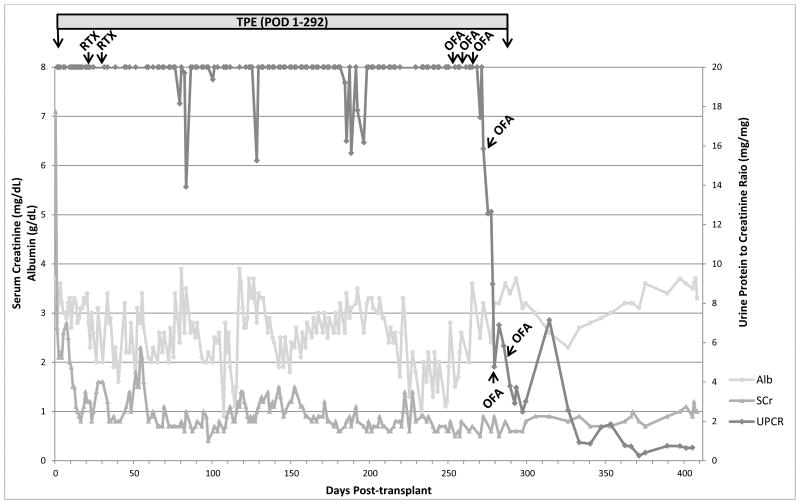
The patient is a 13-year-old male who was diagnosed with SDNS at 3 years of age. Renal biopsy showed diffuse foot process effacement and mesangial hypercellularity, suggestive of early FSGS. Tacrolimus and IV methylprednisolone failed to improve disease control, and he suffered from severe steroid side effects and disease complications including pulmonary emboli. He reached ESRD 6 years after diagnosis and underwent deceased-donor renal transplantation 2 years later with basiliximab and methylprednisolone induction. Maintenance immunosuppression comprised MMF, tacrolimus, and prednisone. He rapidly displayed signs of disease recurrence with a UPCR of 8.04 mg/mg within 24 hours post procedure. He was started on daily plasmapheresis beginning post-operative day (POD) 1. The plasmapheresis frequency was decreased to 4 days a week after nearly 3 weeks of daily treatment. Rituximab 375 mg/m2 was given on POD 20 and 28, with no plasmapheresis for 48 hours following each treatment to prevent drug removal. Additional treatments for the FSGS recurrence included twice weekly IV methylprednisolone 20 mg/kg and conversion of tacrolimus and MMF to cyclosporine and cyclophosphamide. Eight months after transplant, despite ongoing immunosuppressant therapy plus plasmapheresis at 4 sessions per week, severe proteinuria and edema persisted. Ofatumumab was given at 300 mg/1.73m2 for the first dose, followed by five weekly doses of 2000 mg/1.73m2. Plasmapheresis sessions were held for at least 48 hours following each ofatumumab dose to prevent drug removal. The patient developed itching and rash with the first infusion which resolved with a brief pause and IV diphenhydramine. Urine protein dramatically downtrended thereafter (Figure 1), becoming subnephrotic within 12 weeks after the first ofatumumab dose. Plasmapheresis was stopped after completion of ofatumumab, and the patient was able to remain free of edema and maintain nearly normal serum albumin levels despite no longer receiving albumin replacement with plasmapheresis. UPCR reached a nadir of 0.16 to 0.41 mg/mg and remained in this range at last follow-up 9 months after ofatumumab treatment.
Figure 1. Post-transplant Recurrence Treatment and Laboratory Results for Patient 3.
Abbreviations: Alb, albumin; CsA, cyclosporine; MP, methylprednisolone; OFA, ofatumumab; POD, post-operative day; RTX, rituximab; SCr, serum creatinine; TPE, plasmapheresis; UPCR, urine protein/creatinine ratio
Patient 4
The patient is a 16-year-old male who presented with SDNS at age 11. Renal biopsy was consistent with MCD. By the second year after diagnosis, he was on a multi-drug regimen of MMF, tacrolimus, prednisone, and lisinopril to control relapses. Three years after diagnosis, he displayed persistent disease, for which 2 doses of IV rituximab 375 mg/m2 were given. Post-rituximab, the UPCR transiently improved to a subnephrotic range, but did not normalize. We therefore attempted to give ofatumumab. The patient developed an urticarial rash and lip swelling with the first dose, which resolved with IV diphenhydramine and stopping the infusion. Therapy was switched to IV methylprednisolone and IV cyclophosphamide, and he was able to achieve remission. However, he quickly relapsed when IV methylprednisolone doses were spaced. We developed a desensitization protocol based on published experience [11] (Table 1) and gave the patient ofatumumab at an initial dose of 300 mg/1.73m2, followed by 5 weekly infusions of 2000 mg/1.73m2. He experienced transient nausea and emesis with the first dose but was able to complete the remainder of the treatments uneventfully. The UPCR prior to ofatumumab was 4.32 mg/mg and improved to 1.88 mg/mg after the first dose. Subsequent UPCR ranged from 0.64 to 2.24 mg/mg over the ensuing 6 months of follow-up. The serum albumin normalized 2 months following ofatumumab.
Patient 5
The patient is a 13-year-old male who presented with SDNS at age 6. Renal biopsy performed 5 months after diagnosis showed MCD. Tacrolimus, IV cyclophosphamide, and cyclosporine failed to reduce the frequency of relapses. Two doses of IV rituximab 375 mg/m2 were given 5 years after diagnosis (course #1). Thereafter, he enjoyed a sustained remission of 8 months. He was given a second course of IV rituximab, 2 doses of 375 mg/m2 after recovery of B cells and the development of subnephrotic range proteinuria. Full relapse occurred 6 months later, whereupon a third course of IV rituximab was given, with depletion of B cells with one dose of IV rituximab 375 mg/m2. However, steroid-resistant relapse persisted, with numerous complications. Ofatumumab was first given 5 months later, but the patient developed diffuse hives with the first dose. The rash resolved with stopping infusion and giving IV diphenhydramine. We subsequently readministered IV ofatumumab with our desensitization protocol in an initial dose of 300 mg/1.73m2, followed by 5 weekly doses of 2000 mg/1.73m2. Remission was achieved 4 weeks after the first dose of ofatumumab and has been sustained for 3 months at the time of this report.
Discussion
In this report, we present our experience using ofatumumab to treat 5 pediatric patients with idiopathic NS, including the first report of a patient successfully treated with ofatumumab for FSGS recurrence post-transplant (Patient 3). This patient's dramatic response to ofatumumab even as aggressive and prolonged plasmapheresis and numerous immunosuppressants including rituximab had failed is particularly encouraging for the management of this devastating disease. Similar to prior published reports [8-10], the response in our pre-transplant patients is promising. The 3 patients who were able to complete at least 5 doses of ofatumumab (Patients 1, 4, and 5) each achieved partial or complete remission. These patients all had developed steroid resistance after initial steroid sensitivity and had poor responses to rituximab and numerous other immunosuppressants. Patient 1, with the longest duration of follow-up, enjoyed a relatively long period of remission of 13 months after ofatumumab therapy, including 8 months after recovery of B cells. Follow-up for Patients 4 and 5 was quite short.
Of note, 4 out of 5 patients had hypersensitivity reactions to ofatumumab infusion despite pre- medication with diphenhydramine, methylprednisolone, and acetaminophen. Previous studies have reported an incidence of urticaria and rash of >5% [11]. Because we felt that virtually no other treatment options were available for these most difficult NS patients, we developed a desensitization protocol based on that published by Galvão and Castells [11]. This protocol was used for Patients 4 and 5 with successful completion of the 6 doses of ofatumumab. To our knowledge, this is the first published experience using a desensitization protocol for ofatumumab in pediatric patients.
Ofatumumab, like rituximab, is a B cell depleting monoclonal antibody that targets the CD20 antigen expressed on all B cells, excluding plasma cells. Two recent randomized clinical trials found rituximab to be effective in maintaining remission and allowing steroid withdrawal in children with steroid-dependent NS [13, 14]. Presently, there are no published randomized trials of ofatumumab for the treatment of NS. The first case of ofatumumab use was reported by Basu [8], involving a 19-year-old female with multidrug resistant idiopathic NS was who treated with ofatumumab for chronic lymphocytic leukemia and serendipitously achieved NS remission. Subsequent reports of ofatumumab in NS also showed efficacy in inducing remission in multidrug and rituximab resistant NS [8-10]. The clinical features, ofatumumab treatment details, and patient responses of these early reports and the current cases are summarized in Table 2.
Table 2. Review of Reported Cases of Ofatumumab Treatment in Nephrotic Syndrome.
| Author, year | Cases | Age | Previous Therapy | Ofatumumab Regimen | Response | Duration | Adverse Reaction |
|---|---|---|---|---|---|---|---|
| Basu, 2014[8] | 1 | 19 | CTX, Tac, RTX | 300 mg/1.73m2, then 5 weekly doses of 2000 mg/1.73m2 | Remission | 50 weeks (at time of report) | Nonea |
| 2 | 8 | CTX, CsA, Tac, RTX | Same as above | Remission | 42 weeks (at time of report) | None | |
| 3 | 7 | CTX, Tac, RTX, galactose | Same as above | Remission | 8 weeks | None | |
| 4 | 5 | CTX, CsA, Tac, RTX, galactose | Same as above | Remission | 38 weeks (at time of report) | None | |
| 5 | 6 | Tac, MMF, RTX | Same as above | Remission | 25 weeks (at time of report) | None | |
| Bonanni et al., 2015[9] | 6 | 7 | CsA, Tac, RTX | 300 mg/1.73m2, then 700 mg/1.73m2 2 weeks apart | Partial remission | <2 months | Not reported |
| 7 | 16 | CTX, CsA, Tac, interleukin, RTX | Same as above | No response | -- | Not reported | |
| 8 | 14 | CsA, plasma exchange, Tac, RTX | Same as above | Remission | 12 months (at time of report) | Not reported | |
| 9 | 14 | CsA, Tac, RTX, interleukin | Same as above | No response | -- | Not reported | |
| Vivarelli et al., 2016 | 10 | 14 | CsA, MMF, RTX, | 750 mg/1.73m2 | Remission | 15 months (at time of report) | Mild allergic reaction |
| 11 | 3 | CsA, Tac, RTX | 750 mg/1.73m2 | Remission | 19 months (at time of report) | None | |
| Current report | Patient 1 | 16 | MMF, Tac, IV MP, RTX | 300 mg/1.73m2, then 4 weekly doses of 2000 mg/1.73m2 | Remission | 13 months | None |
| Patient 2b | 13 | CsA, MMF, Tac, RTX | N/A | N/A | N/A | Rash and nasal congestion | |
| Patient 3c | 13 | Tac, IV MP, CsA, CTX, RTX, plasma exchange | 300 mg/1.73m2, then 5 weekly doses of 2000 mg/1.73m2 | Remission | 9 months to date | Itching and rash | |
| Patient 4 | 16 | MMF, Tac, RTX, CTX | Same as above | Partial remission | 6 months to date | Rash, angioedema, nausea, emesis | |
| Patient 5 | 13 | Tac, IV MP, CTX, CsA, RTX | Same as above | Remission | 3 months to date | Rash |
One case of “transient infusion reaction” was reported, but the author did not state which patient had the reaction.
Patient did not complete ofatumumab therapy.
Patient with post-transplant focal segmental glomerulosclerosis recurrence who had also received post-transplant immunosuppression therapy – basiliximab, solumedrol, mycophenolate mofetil, tacrolimus
Abbreviations: CsA, cyclosporine; CTX, cyclophosphamide; FR, frequency relapsing; IV, intravenous; MMF, mycophenolate mofetil; MP, methylprednisolone; N/A, not applicable; RTX, rituximab; SD, steroid dependent; SR, steroid resistant; Tac, tacrolimus; Tx, treatment
It is unclear how B cell depleting agents like rituximab and ofatumumab affect the pathogenesis of NS. B cells have been shown to be increased in the active phase of NS and are found in significantly higher numbers in FSGS glomeruli versus controls [15, 16], though whether this represents a direct role of B cells in the disease is not established. Immunoglobulins have also been implicated in the pathogenesis of NS. Dantal et al. showed in 4 patients with post-transplant recurrent FSGS that anti- immunoglobulin immunoadsorption resulted in the removal of factor(s) involved in proteinuria, suggesting that the putative factor(s) is bound to immunoglobulin. Rituximab may act mechanistically by B cell depletion, though persistent remission after B cell recovery suggests a disease-modifying effect beyond total B cell depletion. Colucci et al. reported that delayed reconstitution of switched memory B cells after rituximab is predictive of sustained disease remission and suggests that this subpopulation may play a role in NS pathogenesis [17], though the exact mechanism is unclear. In Patients 1 and 3 of our study, we observed that the disease remission induced by ofatumumab persisted for 8 months and 3 months, respectively, after recovery of B cells. Interestingly, Patient 3 in our series, as well as Patient 5 with his later courses of rituximab, did not achieve remission with rituximab despite B cell depletion but did respond to ofatumumab. Our observations therefore suggest that the effects of ofatumumab and rituximab involve more than B cell depletion. How ofatumumab is able to induce a more favorable response in some patients compared to rituximab is unclear. While ofatumumab is known to bind CD20 via a different epitope and with greater avidity than rituximab and promotes a more potent complement- dependent cytotoxicity [18], the relevance of these differences to the treatment of NS is unclear at this time.
Our experience with ofatumumab for the treatment of refractory NS and recurrent post-transplant FSGS is encouraging, and the desensitization protocol we describe may be helpful to address hypersensitivity reactions that appear to be common with this medication. Prospective studies with larger sample sizes will be required to determine the safety and efficacy of this novel therapeutic agent.
Acknowledgments
Chia-shi Wang is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454. The content is solely the responsibility of the authors and does not necessary represent the official views of the National Institutes of Health.
Footnotes
Compliance with Ethical Standards: Ethical approval. The study was approved by the Children's Healthcare of Atlanta Institutional Review Board. For this type of study, formal consent is not required.
Disclosure. The authors declare that they have no potential conflicts of interest to disclose.
References
- 1.Lombel RM, Gipson DS, Hodson EM, Kidney Disease: Improving Global Outcomes Treatment of steroid-sensitive nephrotic syndrome: new guidelines from KDIGO. Pediatr Nephrol. 2013;28:415–426. doi: 10.1007/s00467-012-2310-x. [DOI] [PubMed] [Google Scholar]
- 2.McKinney PA, Feltbower RG, Brocklebank JT, Fitzpatrick MM. Time trends and ethnic patterns of childhood nephrotic syndrome in Yorkshire, UK. Pediatr Nephrol. 2001;16:1040–1044. doi: 10.1007/s004670100021. [DOI] [PubMed] [Google Scholar]
- 3.Primary nephrotic syndrome in children: clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A Report of the International Study of Kidney Disease in Children. Kidney Int. 1981;20:765–771. doi: 10.1038/ki.1981.209. [DOI] [PubMed] [Google Scholar]
- 4.Wong W. Idiopathic nephrotic syndrome in New Zealand children, demographic, clinical features, initial management and outcome after twelve-month follow-up: results of a three-year national surveillance study. J Paediatr Child Health. 2007;43:337–341. doi: 10.1111/j.1440-1754.2007.01077.x. [DOI] [PubMed] [Google Scholar]
- 5.Lombel RM, Hodson EM, Gipson DS, Kidney Disease: Improving Global Outcomes Treatment of steroid-resistant nephrotic syndrome in children: new guidelines from KDIGO. Pediatr Nephrol. 2013;28:409–414. doi: 10.1007/s00467-012-2304-8. [DOI] [PubMed] [Google Scholar]
- 6.Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet. 2003;362:629–639. doi: 10.1016/S0140-6736(03)14184-0. [DOI] [PubMed] [Google Scholar]
- 7.Amaral S, Neu A. Recurrent FSGS Postkidney Transplant: Moving the Needle Forward. Clin J Am Soc Nephrol. 2016;11:1932–1934. doi: 10.2215/CJN.09520916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basu B. Ofatumumab for rituximab-resistant nephrotic syndrome. N Engl J Med. 2014;370:1268–1270. doi: 10.1056/NEJMc1308488. [DOI] [PubMed] [Google Scholar]
- 9.Bonanni A, Rossi R, Murtas C, Ghiggeri GM. Low-dose ofatumumab for rituximab-resistant nephrotic syndrome. BMJ Case Rep. 20152015 doi: 10.1136/bcr-2015-210208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vivarelli M, Colucci M, Bonanni A, Verzani M, Serafinelli J, Emma F, Ghiggeri G. Ofatumumab in two pediatric nephrotic syndrome patients allergic to rituximab. Pediatr Nephrol. 2017;32:181–184. doi: 10.1007/s00467-016-3498-y. [DOI] [PubMed] [Google Scholar]
- 11.Galvao VR, Castells MC. Hypersensitivity to biological agents-updated diagnosis, management, and treatment. J Allergy Clin Immunol Pract. 2015;3:175–185. doi: 10.1016/j.jaip.2014.12.006. quiz 186. [DOI] [PubMed] [Google Scholar]
- 12.Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004;114:371–376. doi: 10.1016/j.jaci.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 13.Ravani P, Rossi R, Bonanni A, Quinn RR, Sica F, Bodria M, Pasini A, Montini G, Edefonti A, Belingheri M, De Giovanni D, Barbano G, Degl'Innocenti L, Scolari F, Murer L, Reiser J, Fornoni A, Ghiggeri GM. Rituximab in Children with Steroid-Dependent Nephrotic Syndrome: A Multicenter, Open-Label, Noninferiority, Randomized Controlled Trial. J Am Soc Nephrol. 2015;26:2259–2266. doi: 10.1681/ASN.2014080799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iijima K, Sako M, Nozu K, Mori R, Tuchida N, Kamei K, Miura K, Aya K, Nakanishi K, Ohtomo Y, Takahashi S, Tanaka R, Kaito H, Nakamura H, Ishikura K, Ito S, Ohashi Y, Rituximab for Childhood- onset Refractory Nephrotic Syndrome Study Group Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2014;384:1273–1281. doi: 10.1016/S0140-6736(14)60541-9. [DOI] [PubMed] [Google Scholar]
- 15.Pereira WD, Brito-Melo GEA, Guimaraes FTL, Carvalho TGR, Mateo EC, Silva ACSE. The role of the immune system in idiopathic nephrotic syndrome: a review of clinical and experimental studies. Inflamm Res. 2014;63:1–12. doi: 10.1007/s00011-013-0672-6. [DOI] [PubMed] [Google Scholar]
- 16.Benz K, Buttner M, Dittrich K, Campean V, Dotsch J, Amann K. Characterisation of renal immune cell infiltrates in children with nephrotic syndrome. Pediatr Nephrol. 2010;25:1291–1298. doi: 10.1007/s00467-010-1507-0. [DOI] [PubMed] [Google Scholar]
- 17.Colucci M, Carsetti R, Cascioli S, Casiraghi F, Perna A, Rava L, Ruggiero B, Emma F, Vivarelli M. B Cell Reconstitution after Rituximab Treatment in Idiopathic Nephrotic Syndrome. J Am Soc Nephrol. 2016;27:1811–1822. doi: 10.1681/ASN.2015050523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farhadfar N, Litzow MR. New monoclonal antibodies for the treatment of acute lymphoblastic leukemia. Leuk Res. 2016;49:13–21. doi: 10.1016/j.leukres.2016.07.009. [DOI] [PubMed] [Google Scholar]