Abstract
Stress and negative affect are known contributors to drug use and relapse, and several known treatments for addictions include strategies for managing them. In the current study, we administered a well-established stress provocation during functional magnetic resonance imaging (fMRI) to 23 participants who completed either mindfulness training (MT; N=11) or the American Lung Association's Freedom From Smoking (FFS; N=12), which is a cognitive-behavioral treatment (CBT) for smoking cessation. Across the entire sample, we found that stress reactivity in several brain regions including the amygdala and anterior/mid insula was related to reductions in smoking after treatment, as well as at 3-month post-treatment follow-up. Moreover, conjunction analysis revealed that these same regions also differentiated between treatment groups such that the MT group showed lower stress-reactivity compared to the FFS/CBT group. This suggests that reduction in stress reactivity may be one of the mechanisms that underlie the efficacy of MT in reducing smoking over time. The findings have important implications for our understanding of stress, the neural and psychological mechanisms that underlie mindfulness-based treatments, and for smoking cessation treatments more broadly.
Keywords: Stress, fMRI, smoking, mindfulness, cognitive-behavioral therapy
Introduction
Cigarette smoking is responsible for 5.4 million deaths per year and is the most preventable cause of morbidity and mortality in developed nations (CDC, 2008a, 2008b; WHO, 2010). Measured in terms of the burden on services such as health care and law enforcement, the loss of productivity in the home or workplace, and premature death and disability, the estimated costs of smoking in the US is 193 billion dollars per year (CDC, 2005). Although the rates of smoking have been declining, 21% of Americans still smoke. And, although over 70% of smokers report wanting to quit, < 5% of unassisted quit attempts are successful (CDC, 2005), and relapse is the most common outcome (Fiore, Bailey, & Cohen, 2000; Fiore, Jaén, & Baker, 2008; Piasecki, 2006). These grim statistics underscore the need to understand the factors that promote relapse, including their underlying neural mechanisms, in order to improve current treatments.
The term “stress” typically refers to processes involving perception, appraisal, and response to potentially harmful, threatening, or challenging events or stimuli (Levine, 2005; Sinha, 2008). Although several types of stress have been defined (e.g., McEwen et al., 2015), here we focus on stress as an acute, negatively-valenced affective state, which is closely related to anxiety (Leuner & Shors, 2013). Several lines of research suggest that such acute stress increases drug use in general and cigarette smoking in particular: (1) Acute stress increases self-administration of drugs (including nicotine) in animal models (Buczek, Le, Wang, Stewart, & Shaham, 1999; Piazza & Moal, 1998; Shaham & Stewart, 1995; Volpicelli, 1987; Zislis, Desai, Prado, Shah, & Bruijnzeel, 2007); (2) Acute stress is associated with drug use and relapse in human prospective studies (Back et al., 2010; Baer & Lichtenstein, 1988; Brewer, Catalano, Haggerty, Gainey, & Fleming, 1998; Brown et al., 1990; Brown, Vik, Patterson, Grant, & Schuckit, 1995; Shiffman & Waters, 2004); (3) Experience-sampling studies (in which drug users provide frequent reports throughout their daily lives) link stress to increased drug use and smoking (Cooney et al., 2007; Epstein, Marrone, Heishman, Schmittner, & Preston, 2010; Preston & Epstein, 2011; Shiffman, 2005; Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996; Shiffman & Waters, 2004); (4) Studies in abstinent smokers link stress and relapse (Doherty, Kinnunen, Militello, & Garvey, 1995; Swan et al., 1988); (5) Laboratory-induced stress increases cigarette craving (Buchmann et al., 2010; Childs & de Wit, 2010) and cigarette smoking (McKee et al., 2011), and magnitude of stress responses and negative affect predict relapse (Back et al., 2010; Sinha, Fox, et al., 2011; Sinha, Garcia, Paliwal, Kreek, & Rounsaville, 2006; Witkiewitz & Villarroel, 2009); (6) In retrospective reports, drug users (including smokers) often cite stressful events and psychological distress as reasons for relapse (Baer & Lichtenstein, 1988; Brandon, 1994; Brownell, Marlatt, Lichtenstein, & Wilson, 1986; Marlatt & Donovan, 2005; Marlatt & Gordon, 1980; O'Connell & Martin, 1987; Swan et al., 1988; Wallace, 1989).
Despite the demonstrated role of stress in smoking, few studies have assessed neural stress responses in smokers (for a review of neural and HPA responses to nicotine, nicotine abstinence, and nicotine cues, see Supplementary Materials). In one study of satiated smokers, stress produced deactivation in limbic (e.g., amygdala, hippocampus, striatum) and prefrontal regions (e.g., ventromedial PFC, anterior cingulate cortex) that predicted increases in subsequent cue-induced craving responses (Dagher, Tannenbaum, Hayashi, Pruessner, & McBride, 2009). On the other hand, Ashare and colleagues (Ashare et al., 2016) reported increased neural stress reactivity in four brain regions, including anterior cingulate, precuneus, and inferior frontal gyrus; further, deprived smokers exhibited significantly greater activation compared to those who were non-deprived. The latter findings are consistent with prior reports in other drug using groups showing stress-induced increases (rather than decreases) in neural activity (e.g., Potenza et al., 2012; Sinha et al., 2005). However, to our knowledge, no previous study assessed whether neural responses to stress may relate to treatment response among smokers undergoing treatment for smoking cessation. Such a study would increase our understanding of the underlying neural mechanisms by which acute stress relates to relapse, which can improve smoking outcomes, and addiction treatment more generally.
Given the role of stress in smoking and relapse, several smoking cessation treatments include components directed at reducing it. For example, cognitive-behavioral treatments (CBTs) may recommend reinterpretation of negative events as more positive, or engaging in a distracting or pleasurable activity to cope with stress (Carroll, 1998; Lando, McGovern, Barrios, & Etringer, 1990). Conversely, mindfulness-based treatments (MBTs) may recommend using mindfulness- and acceptance-based strategies, such as noticing and accepting negative emotions (letting those emotions be exactly as they are, without reacting to them; (Bowen, Chawla, & Marlatt, 2011). The difference between these two orientations in the treatment of substance use and smoking may be important. For example, it is known that using cognitive strategies to regulate negative emotions depends on recruitment of prefrontal cortex (PFC; Buhle et al., 2014), which may be compromised by stress (Arnsten, 2009; Raio, Orederu, Palazzolo, Shurick, & Phelps, 2013). Prefrontal function may also be compromised in some forms of psychopathology, including addictions (e.g., Kober, DeVito, DeLeone, Carroll, & Potenza, 2014; Koenigsberg et al., 2009). In contrast, it has been suggested that mindfully accepting craving and negative emotion may not depend on PFC recruitment, and may therefore be more effective, especially in vulnerable populations, or in vulnerable moments of stress when PFC function may be disrupted (Kober, Buhle, Weber, Ochsner, & Wager, under review; Westbrook et al., 2013). This raises the intriguing possibility that mindfulness-based interventions for substance use and smoking may be particularly effective at reducing stress, which in turn could improve outcomes (Creswell & Lindsay, 2014). Indeed, one of the first mindfulness-based treatments was designed for stress reduction (Kabat-Zinn et al., 1992), and such treatments have been associated with reductions in anxiety and negative affect in anxiety and mood disorders (Goldin & Gross, 2010; Kabat-Zinn et al., 1992; Teasdale, Segal, Ridgeway, & Soulsby, 2000) as well as in healthy adults (e.g., Chambers, Lo, & Allen, 2008). In fact, several recent meta-analyses have established mindfulness' efficacy in reducing negative mood and anxiety symptoms in diverse clinical samples (Goyal et al., 2014; Hofmann, Sawyer, Witt, & Oh, 2010). Thus, examining differences in responses to stress following such treatments for smoking cessation, especially as they relate to smoking outcomes, may provide a route to understanding their mechanisms of action.
Recently, we reported results from a randomized controlled trial (RCT) for smoking cessation, comparing Freedom From Smoking (FFS) – a common cognitive-behavioral treatment (CBT) for smoking cessation issued by the American Lung Association (Lando et al., 1990) – and mindfulness training for smoking (MT; Brewer, Mallik, et al., 2011). Both treatments were effective in reducing smoking, but the MT group demonstrated a greater rate of reduction in cigarette use during treatment, which was maintained during 3 month post-treatment follow-up (RCT N = 87; F(1,1082) = 11.11, p = .001). Furthermore, the MT group showed a trend toward greater 1-week point prevalence abstinence at the end of treatment (36% vs. 15%, χ2(1) = 3.45, p = .06). This difference became statistically significant at the 17-week follow-up endpoint (31% vs. 6%, χ2(1) = 6.32, p = .01; Brewer, Mallik, et al., 2011).
In the current manuscript, we report data from a neuroimaging probe administered to a sub-sample of participants from that clinical trial, immediately following treatment completion. We were especially interested in stress reactivity, and exposed participants to a well-established procedure of individualized script-based stressful scenarios, following our prior work (e.g., Brewer et al., 2009; McKee et al., 2011; Seo et al., 2011; Seo, Tsou, Ansell, Potenza, & Sinha, 2014; Sinha, 2001; Sinha, Catapano, & O'Malley, 1999; Rajita Sinha, Fuse, Aubin, & O'Malley, 2000; Sinha, Lacadie, Skudlarski, & Wexler, 2004; Sinha et al., 2005; Sinha & Tuit, 2012; for review see Sinha, 2009). We then (1) tested whether stress reactivity related to smoking after treatment as well as at the 3-month post-treatment follow-up, and also (2) compared neural activity during stressful scenarios between treatment groups. Given the role of stress in precipitating smoking (McKee et al., 2011), and prior findings that stress reactivity predicts relapse after treatment for other addictions (e.g., Seo et al., 2013; Sinha, Fox, et al., 2011; Sinha et al., 2006), we hypothesized that greater neural stress reactivity will be related to more smoking after treatment. Furthermore, we expected that neural stress-reactivity may be lower in the MT compared to FFS group, given prior work linking mindfulness-based treatments to reductions in stress (Goyal et al., 2014; Hofmann et al., 2010; Kabat-Zinn et al., 1992).
Method
Participants
Twenty-six participants underwent fMRI scanning in this protocol; three participants received only one (of two) negative/stress story or only one neutral/relaxing story and were therefore excluded from analyses. This was due to technical or other difficulties (e.g., scanner error; bathroom break) that limited the length of the scanning session and precluded presentation of all four stories. Therefore, data from 23 participants were considered usable and included in analyses in this paper. All participants were recruited from a smoking-cessation RCT (Brewer, Mallik, et al., 2011). RCT participants were English-speaking adults between 18-60 years of age, smoked ≥10 cigarettes per day, had fewer than 3 months of abstinence in the prior year, and reported interest in quitting smoking. Over 90% of them completed at least high school level education (see Table 1). Participants were excluded from the RCT if they could not read and understand the entire consent form, used psychoactive medications, had a serious or unstable medical condition in the prior 6 months, or met DSM-IV criteria for other substance dependence in the past year. RCT participants were offered participation in the fMRI component if they reported no claustrophobia, colorblindness, history of severe head trauma with loss of consciousness, neurological disorders, or any MRI-contraindicated conditions (e.g., metallic implants). fMRI scanning was conducted within 8 days of the last session of treatment. All participants provided written informed consent in accordance with Yale's Institutional Review Board.
Table 1. Clinical and Demographic Characteristics.
| Overall | MT | FFS | Test Statistics | Significance | |
|---|---|---|---|---|---|
|
|
|||||
| N | 23 | 11 | 12 | ||
| N Females | 7 | 4 | 3 | X2 = .35 | NS p>.5 |
| Age: Years (SD) | 48.3 (6.98) | 48.0 (7.18) | 48.5 (7.10) | t(21) = .43 | NS p>.6 |
| Race | X2 = 4.43 | NS p>.1 | |||
| White | 14 | 8 | 6 | ||
| Black | 8 | 2 | 6 | ||
| Asian | 1 | 1 | 0 | ||
| Hispanic | 1 | 1 | 0 | ||
| Education | X2 =3.63 | NS p>.3 | |||
| College Grad or More | 6 | 3 | 3 | ||
| Partial College | 3 | 1 | 2 | ||
| High School | 12 | 6 | 6 | ||
| Less Than High School | 2 | 1 | 1 | ||
| Body Mass index (SD) | 28.9 (4.5) | 28.5 (4.34) | 29.2 (4.70) | t(21) = .40 | NS p>.6 |
| Pre-Treatment Stress (PSS) | 25.41 (6.84) | 27.56 (4.85) | 23.92 (7.77) | t(20) = 1.24 | NS p>.2 |
| Pre-Treatment Alcohol Per Day | 1.14 (1.54) | 1.3(1.8) | 1.02(1.39) | t(21) = .41 | NS p>.6 |
| Pre-Treatment CPD | 17.97 (9.65) | 20.67 (10.92) | 14.71 (7.45) | t(21) = 1.36 | NS p>.1 |
| Average % Reduction in CPD Post Treatment | 79% | 88% | 71% | Group × Time reported ln text | |
| Average % Reduction in CPD At Follow Up | 60% | 71% | 50% | Group × Time reported in text | |
Note. The fMRI sub-sample was similar to the main RCT sample. Treatment groups did not differ significantly on any dimension pre-treatment. One individual identified as both Black and Hispanic (MT=Mindfulness Training; FFS=Freedom from Smoking; N=number of participants; SD=standard deviation; NS=not significance; PSS=Perceived Stress Scale; CPD=Cigarettes Per Day).
Clinical Assessments
During treatment and at each of the follow-up sessions, self-reported smoking was assessed using the timeline follow-back method (Robinson, Sobell, Sobell, & Leo, 2014; Sobell & Sobell, 1992). Self-reported abstinence was then verified using exhaled carbon monoxide (CO) at CO ≤10 parts per million. The primary outcome measure was average number of cigarettes per day (CPD) across the 4 treatment weeks and through week 17 follow-up (3 month post treatment). Reduction in CPD from pre- to post-treatment and through follow-up was significant for both groups (effect of time: F(1,1115) = 480.79, p < .0001; Brewer, Mallik, et al., 2011). For consistency, we used reduction in CPD from pre- to post-treatment and from pre-treatment to 3-month post-treatment follow-up as the clinical outcome variables in the current manuscript. Pre-treatment stress reactivity was assessed using the Perceived Stress Scale (PSS; Cohen, Kamarck, & Mermelstein, 1983).
Interventions
In the RCT, 84 participants were urn-randomized (Lachin, Matts, & Wei, 1988; Stout, Wirtz, Carbonari, & Del Boca, 1994; Wei & Lachin, 1988) to receive one of two active treatments based on gender (male vs. female), age (>40 years vs. ≤40 years old), race (white vs. non-white), and CPD (>20 vs. ≤20). Both treatments consisted of two weekly group sessions for 4 weeks (8 total sessions) that were manualized and delivered by trained instructors (for a detailed description, see (J. A. Brewer, Mallik, et al., 2011).
Freedom From Smoking (FFS; Lando et al., 1990), is a cognitive-behavioral treatment issued by the American Lung Association, that includes cognitive strategies for coping with cravings and stress/negative emotions, behavior modification, and relapse prevention. It is divided into three stages: preparation, action, and maintenance. In the preparation stage (sessions 1–3), participants examine smoking patterns through self-monitoring, identify triggers, and develop a personalized quit plan. On quit day (session 4), participants affirm their decision to be smoke-free and practice personalized coping strategies for stress and craving (e.g., avoiding high-risk situations). In the maintenance stage (sessions 5-8), participants identify ways to remain smoke-free by maintaining a healthy lifestyle (e.g., exercise, weight management), and discuss relapse prevention and the importance of social support and cognitive and behavioral coping strategies. Homework is recommended after each session, including formal practices (e.g., guided relaxation) and informal techniques (e.g., smoking diaries).
Mindfulness Training (MT) was developed for active smoking cessation based on mindfulness-based relapse prevention (Bowen et al., 2011) and has been described in detail previously (Brewer, Mallik, et al., 2011). Briefly, it includes training in mindfulness as a two component process: (1) attention to present moment experience, even if it includes craving or negative emotion; and (2) an accepting attitude towards this experience (letting it be exactly as it is, without judging it or reacting to it; Bishop et al., 2004; Ludwig & Kabat-Zinn, 2008). Early sessions (1-2) include an introduction to the concept of cue-induced craving, as well as strategies for mindfully working with craving and practicing mindfulness meditation. Session 3 discusses mindfully working with stress and negative emotion, and introduces loving-kindness meditation as a way to work with them through direct well-wishing (e.g., “may I be happy”; Gunaratana, 1991). On quit day (session 4) participants practice mindfulness techniques to cope with craving, and commit to an aspiration to remain smoke free. In subsequent sessions 5-7, participants learn about possible triggers for habitual behavior and additional mindfulness practices (e.g., walking meditation, noting/labeling thoughts and feelings), while acceptance is reinforced as a tool for working with negative emotions and changing habits. The last session summarizes the course and offers ways of maintaining change. Homework is recommended after each session throughout the treatment period, including formal practices (e.g., body scan, loving-kindness meditation) and informal techniques (e.g., mindfulness of craving, smoking, stress, and daily activities).
fMRI Stress Task
During the scanning session, participants listened to two individualized stressful/negative scripts and two individualized neutral/relaxing scripts, based on our prior work (e.g., Brewer et al., 2009; McKee et al., 2011; Seo et al., 2011; Seo et al., 2014; Sinha, 2001; Sinha et al., 1999; Sinha et al., 2000; Sinha et al., 2004; Sinha et al., 2005; for review, see Sinha, 2009; for the published manual, see Sinha & Tuit, 2012). This method was initially adapted from Peter Lang's emotional imagery work and emotional network theory of threat, fear and anxiety (e.g., Lang, 1979; Lang, Levin, Miller, & Kozak, 1983; Sinha, 2009). Such individually-calibrated stressful scenarios were previously shown to elicit neurobiological stress responses in healthy adults as well as individuals with substance use and addiction disorders. Such stress responses include HPA activity, and neural activity in regions associated with negative affect, salience, and arousal, such as amygdala, hippocampus/parahippocampus, insula, thalamus, and striatum (e.g., Seo et al., 2011; Seo et al., 2014; for additional discussion, see supplementary materials).
Scripts were developed for each participant in a prior session, using a scene development interview, as previously described (Sinha, 2009; Sinha & Tuit, 2012). Briefly, each stressful script was based on a recent personal event that was experienced as very stressful, as indicated by a rating of 8 or greater on a 10-point likert scale ranging from 1 (“not at all stressful”) to 10 (“the most stressful event in my entire life”). Such stressful scenarios included breaking with a significant other, hearing about the loss of a family member, legal problems, and marital conflict situations (see Supplementary Materials for sample scripts). The neutral scripts were developed from the participants' description of a personal neutral or relaxing situation. Participants related the details of each scenario to an interviewer and reported physiologic, emotional, and cognitive responses during the event on a response checklist (e.g., “your heart skipped a beat,” “this can't be happening, you think,” “you can't take it anymore”). The interviewer integrated all the data and developed the personalized scripts using standard techniques (Sinha, 2009; Sinha & Tuit, 2012). All scripts were then recorded by one of the researchers for use during the fMRI scanning. During each of 4 functional runs, participants first provided a resting baseline for 30 seconds, and then heard the instruction “close your eyes and imagine the following situation as if it were happening right now.” Then, one of the individualized scenarios was played via headphones (the order of scenarios was randomized). Each scenario lasted about 3 minutes, and was followed by the instruction “please stop imagining and lay still,” followed by a cooldown period (See Figure 1 for schematic representation). Before and after each run, participants rated the vividness of the imagined scenario, as well as their stress and craving on the same 10-point scale as before.
Figure 1. Schematic Representation of Each Run.
During each of 4 functional runs, participants first experienced a resting baseline for 30 seconds, and then heard the instruction “close your eyes and imagine the following situation as if it were happening right now.” Then, one of four individualized scenarios was played via headphones (two stressful/negative and two neutral/peaceful scenarios, presented in random order). Each scenario lasted 3 minutes, and was followed by the instruction “please stop imagining and lay still,” followed by a cooldown period. Before and after each run, participants rated their stress and craving on a 10-point scale; and after each scenario they rated the vividness of the imagined scenario.
fMRI Data Acquisition, Preprocessing, and Analysis
Data Acquisition
Images were obtained using a 1.5 Tesla Sonata MRI scanner with standard eight-channel head coil (Siemens AG, Erlangen, Germany). Functional images were collected via T2*-weighted gradient-recalled single-shot echo-planar pulse sequence (TR/TE = 2000/35ms; flip angle = 85°; field of view = 220×220mm; 28 × 4mm slices). High-resolution 3D Magnetization Prepared Rapid Gradient Echo (MPRAGE) structural images were also collected (TR/TE = 2400/3.54ms; flip angle = 8°, FOV = 192 × 192; 160 × 1.2mm slices).
Preprocessing
All functional images were inspected for signal-to-noise ratio and motion in excess of one voxel; no participants were excluded from analyses for poor quality or excessive motion. Three initial volumes from each run were removed prior to preprocessing to allow for signal stabilization. Functional images were preprocessed using SPM8 (Wellcome Functional Imaging Laboratory, London, UK), following our prior work (e.g., Kober et al., 2010). This included slice-time correction to the first slice of each volume; motion correction; normalization of the mean functional image to the SPM functional template in Montreal Neurological Institute (MNI) space; warping of functional images to template space; reslicing into isometric 3×3×3 mm3 voxels; and smoothing of functional images using a 6mm Gaussian kernel.
Analysis
First-level robust regression was implemented in MATLAB 7.3 (Mathworks, Natick, MA), via the NeuroElf platform (NeuroElf.net). This procedure uses the standard general linear model but with iteratively reweighted least squares using the bisquare weighting function to reduce the effects of outliers (Wager, Keller, Lacey, & Jonides, 2005), following our prior work (Brewer, Worhunsky, et al., 2011; Buhle et al., 2013; Kober et al., under review; Kober et al., 2014). Neutral and stressful scenarios were modeled as blocks, as were the instruction periods. Motion parameters were modeled as regressors of no interest. Subsequently, we performed a second-level, random-effects analysis to compare activity during stress and neutral scenarios between groups, using NeuroElf (e.g., Brewer, Worhunsky, et al., 2011; Buhle et al., 2013; Kober et al., under review; Kober et al., 2014). Results were familywise-error (FWE) corrected at p < .05 using the procedure first established, tested, and popularized by AFNI (“AlphaSim”; Cox, 1996). This process currently entails two steps. First, smoothness is estimated directly from the residual maps. Then, Monte Carlo simulation is used to estimate cluster size for the intensity threshold (Xiong, Gao, Lancaster, & Fox, 1995) to reach a combined familywise-error threshold.
To assess the relationship between stress-related brain activity and smoking, we computed whole-brain correlations between neural activity during stress scenarios and % reduction in CPD from pre- to post-treatment, as well as from pre-treatment to the 3 month post-treatment follow up, as reported in the original clinical trial (Brewer, Mallik, et al., 2011). Results were similarly FWE corrected at p < .05. To assess whether any stress-responsive regions were associated with smoking at both timepoints, we conducted a formal conjunction analysis between the two correlation maps. To assess whether any stress-responsive regions both differentiated between treatment groups and were associated with smoking at both timepoints, we performed another conjunction between the contrast map [stress (FFS>MT)] and the two correlations.
Results
Participants
Eleven participants from the mindfulness and 12 participants from the CBT/FFS treatment groups participated in the fMRI scan. Demographic and participant characteristics are summarized in Table 1. Participants in the two treatment groups did not differ in age, education, race, BMI, alcohol use, or stress reactivity. Importantly, although the MT group smoked more pre-treatment, this difference was not statistically significant (similar to the main RCT; Brewer, Mallik, et al., 2011).
Smoking Outcomes
Although the fMRI subsample is smaller than that of the full RCT (Brewer, Mallik, et al., 2011), we replicated the analyses from the primary paper, for consistency, and found that smoking outcomes mirrored those in the full RCT. Specifically, both treatments reduced smoking, but the MT group demonstrated a greater rate of reduction in cigarette use during treatment, which was maintained during 3 month post-treatment follow-up (group * time F(1,372) = 21.00, p < .001). Furthermore, the MT group showed a trend toward greater 1-week point prevalence abstinence at the end of treatment (55% vs. 23%, χ2(1) = 2.42, p = .11, d = .70). This difference became statistically significant at the 17-week follow-up (44% vs. 7%, χ2(1) = 4.09, p = .04, d = .95).
Behavioral Results
During scanning, participants reported being able to vividly imagine all scenarios (MVIVIDNESS = 8.58, SD = 1.16; ratings were only available for 17 participants due to technical errors). First, as a manipulation check, we assessed the changes in ratings of stress and craving from pre- to post- stress and neutral scenarios. As expected, stress/negative scenarios increased ratings of stress (t(16) = 3.78, p = .002, d = .6), whereas neutral scenarios did not (t(16) = 1.33, p > .2). Similarly, stress scenarios increased ratings of craving (t(16)=2.58, p =.02, d = .36) whereas neutral scenarios did not (t(16) = -1.69, p > .1). Then, we compared post-scenario ratings between stressful and neutral scenarios. As expected, stress and craving ratings following stress stories were significantly higher than ratings following neutral stories (anxiety: t(16) = 2.42, p = .028, d = .3; craving: t(16) = 2.72, p =.015, d = .36). The MT and FFS groups did not differ on any of these self-report measures (all ps > .2).
fMRI Results
Correlations with Smoking
Across all participants, neural activity during the stressful scenarios was negatively correlated with post-treatment CPD reduction in a large cluster that included peaks in bilateral amygdala, anterior insula, mid insula, hippocampus, parahippocampal gyrus, thalamus, middle occipital gyrus, midbrain, cerebellum, and right posterior insula, as well as a second region spanning the midline across cuneus/precuneus and posterior cingulate cortex (Table 2A; see Supplementary Figures S1-S2 for full results). The negative correlation indicates that those individuals with the greatest stress reactivity in those regions showed the lowest reduction in smoking from pre- to post- treatment.
Table 2.
Neuroimaging Results: Stress-reactive regions that differ between treatment groups and relate to smoking outcomes.
| Peak-Coordinates | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Regions of Activation | R/L/Bi | x | y | z | k | Vol(mm3) | Peak Statistic | Mean Statistic | Cohen d |
| A. Stress Reactivity Correlates with % Smoking Reductiion at End of Treatment (Week 4) | |||||||||
| Bilateral Amygdala, Anterior/Mid Insula, Hippocampus, Parahippocampal Gyrus, Thalamus, Middle Occipital Gyrus, Cerebellum, Midbrain, and Right Posterior insula, Superior/Middle Temporal Gyrus | Bi | -15 | -51 | -45 | 3250 | 87750 | -0.77 | -0.51 | 1.18 |
| Cuneus, Precuneus, Posterior Cingulate | Bi | -3 | -69 | 21 | 164 | 4428 | -0.69 | -0.52 | 1.22 |
| B. Stress Reactivity Correlates with % Smoking Reduction at FollowUp (Week17) | |||||||||
| Hipoocampus, Parahippocampal Gyrus, Posterior inferior Temporal Gyrus, Middle Occipital Gyrus | R | 30 | -30 | -6 | 180 | 4860 | -0.77 | -0.52 | 1.22 |
| Cerebellum | L | -39 | -90 | -24 | 143 | 3861 | -0.70 | -0.50 | 1.15 |
| Caudate, Superior/Middle Temporal Gyri | R | 24 | -39 | 12 | 165 | 4455 | -0.69 | -0.51 | 1.18 |
| Middle Occipital, Parahippocampal Gyrus, Caudate | L | -27 | -72 | -3 | 152 | 4104 | -0.67 | -0.51 | 1.18 |
| Amygdala, Anterior/Mid insula, Posterior insula, Superior Temporal Gyrus, Parahippocampal Gyrus | L | -39 | -6 | -3 | 208 | 5616 | -0.66 | -0.49 | 1.12 |
| Thalamus. Cerebellum | Bi | -6 | -42 | -9 | 276 | 7452 | -0.63 | -0.49 | 1.12 |
| C. Group Differences: Stress Scenarios (FFS>MT) | |||||||||
| Amygdala, Anterior/Mid insula, Posterior insula, Putamen, Thalamus, Parahippocampal Gyrus | L | -24 | -6 | 15 | 344 | 9288 | 5.72 | 2.64 | 1.10 |
| Right Thalamus, Putamen, Bilateral Midbrain, Cerebellum | Bi | 9 | -3 | -6 | 371 | 10017 | 4.00 | 2.48 | 1.03 |
| Posterior Parahippocampal/Hippocampal Gyri, Cerebellum | Bi | 6 | -66 | -42 | 380 | 10260 | 3.97 | 2.48 | 1.03 |
| D. Conjunction of A & B: Stress Reactivity Correlates with % Smoking at Weeks 4 & 17 | |||||||||
| Hippocampus, Parahippocampal Gyrus, Posterior Insula | R | 30 | -27 | -9 | 95 | 2565 | 0.0008 | 0.010 | - |
| Amygdala, Anterior/Mid Insula, Parahippocampal Gyrus | L | -30 | -9 | -18 | 75 | 2025 | 0.003 | 0.020 | - |
| E. Conjunction of A & B & C: Stress Reactivity Correlates with Smoking Outcomes and Differentiates Between Groups | |||||||||
| Posterior Cingulate/Posterior Caudate | R | 21 | -48 | 12 | 22 | 594 | 0.007 | 0.027 | - |
| Posterior Hippocampus/Parahippocampal Gyrus | R | 24 | -45 | -15 | 22 | 594 | 0.008 | 0.026 | - |
| Hippocampus/Parahippocampal Gyrus | R | 30 | -24 | -9 | 15 | 405 | 0.010 | 0.024 | - |
| Amygdala | L | -30 | -9 | -18 | 12 | 324 | 0.016 | 0.030 | - |
| Anterior/Mid insula | L | -42 | -3 | -12 | 11 | 297 | 0.026 | 0.036 | - |
Note. Peak activations xyz are in MNI coordinates. R/L/Bi refer to lateralization of activation as Right, Left, or Bilateral. K refers to number of 3×3×3 voxels in each cluster. Volume is expressed in mm3. (a-b) Peak/mean statistics are correlation coefficient r. Results are whole-brain familywise error-corrected at p < .05. (c) Peak/mean statistics are t values. Results are whole-brain familywise error-corrected at p < .05. (d-e) Peak statistics for conjunctions represent the maximum (i.e. least significant) p statistic following conjunction conventions (Nichols, Brett, Andersson, Wager, & Poline, 2005). Mean statistics represent the average maximum (i.e. least significant) p statistic following conjunction convention. Cohen d effects sizes are provided for illustration, calculated from the mean (rather than peak) statistic in each cluster. These should be interpreted with caution as effect sizes estimated from imaging data may be inflated (e.g., Vul, Harris, Winkielman, & Pashler, 2009; Yarkoni, 2009).
Further, neural activity during the stressful scenarios was negatively correlated with CPD reduction at the 3-month follow-up in several regions including left amygdala, anterior/mid insula, posterior insula, parahippocampal gyrus, caudate and middle occipital gyrus, right hippocampus, hippocampal gyrus, inferior temporal gyrus and middle occipital gyrus, and bilateral portions of thalamus and cerebellum (Table 2B; Supplementary Figures S3-S4 for full results). Again, the negative correlation indicates that those individuals with the greatest stress reactivity in those regions showed the lowest reduction in smoking from pre-treatment to 3-month follow-up.
Differences between treatment groups
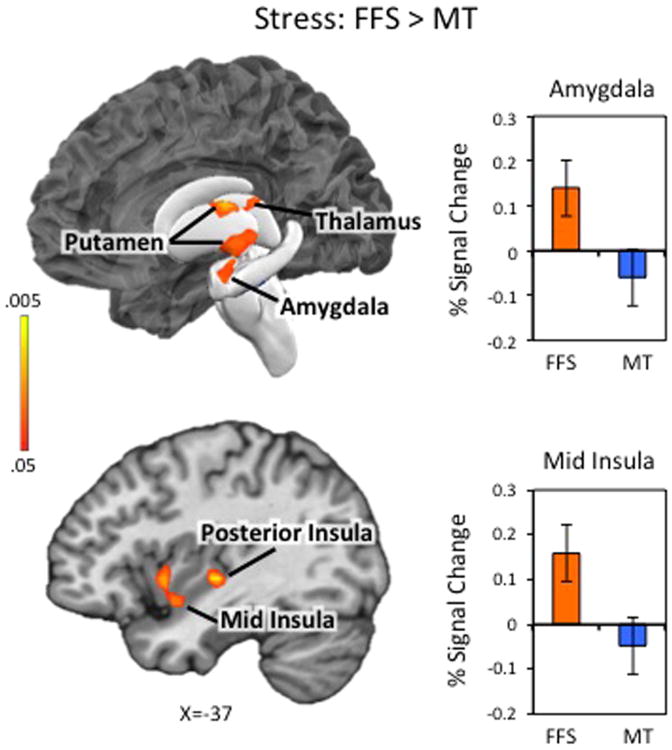
Next, we compared neural activity during neutral and stressful scenarios between groups. There were no significant group differences in brain activity during neutral scenarios. During stressful scenarios, participants in the FFS group (vs. MT) exhibited increased neural reactivity in several brain regions including left amygdala, anterior, middle, and posterior insula, and bilateral portions of parahippocampal gyrus and hippocampus, putamen, thalamus, midbrain and cerebellum (See Table 2C; Figure 2; Supplementary Figures S5-6 for full results). The MT group did not show greater neural reactivity in any region during stressful stories.
Figure 2. Stress Reactivity: Comparison Between Treatment Groups.
Neural activity during stressful scenarios was contrasted between the Freedom from Smoking (FFS) group and the Mindfulness Training (MT) group [FFSSTRESS>MTSTRESS]. The FFS group exhibited greater stress-related neural activity in left amygdala, anterior, middle, and posterior insula, and bilateral portions of parahippocampal gyrus and hippocampus, putamen, thalamus, midbrain and cerebellum (See Table 2A; See Supplementary Figures S5-S6 for full results). The MT group did not show greater neural reactivity in any region during stressful stories. Bar graphs represent the extracted cluster-averaged percent signal change in amygdala (top) and insula (bottom). Error bars represent standard errors. Results are familywise-error corrected (FWE) at p < .05. Left side of the brain is displayed on the left.
Identifying commonalities
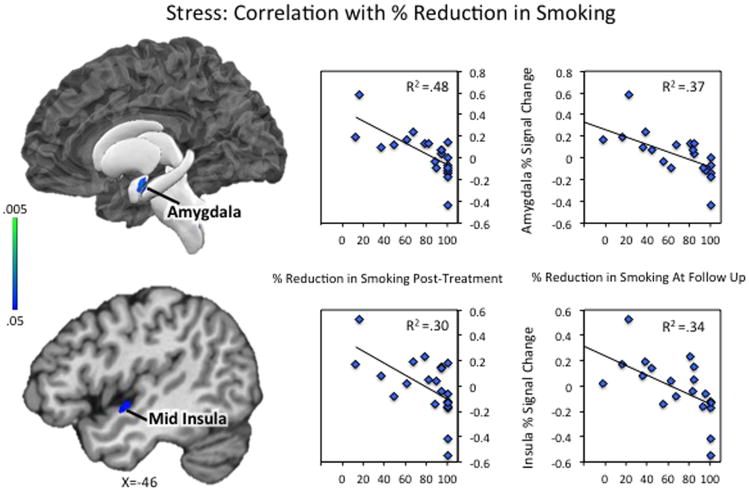
A formal conjunction analysis between the two correlation maps revealed a set of regions that were responsive to stressful scenarios, and correlated with CPD reduction at both timepoints. Those included the left amygdala, extending into the anterior/mid insula and parahippocampal gyrus, as well as right hippocampus, parahippocampal gyrus, and posterior insula (Table 2D; Figure 3; Supplementary Figures S7-8 for full results). A second conjunction between the two correlation maps and the between-group contrast [stress (FFS>MT)] identified a few small regions that were related to smoking outcome and differed significantly between the two groups. Those included left amygdala and anterior/mid insula and right posterior parahippocampal gyrus (Table 2E; Supplementary Figures S9-10 for full results). Notably, in these regions, the MT group showed lower stress reactivity, and lower activity was related to better outcomes (greater reduction in smoking) after treatment and at 3-month follow-up.
Figure 3. Stress Reactivity: Correlations with % Reduction in Smoking.
Neural activity during stressful scenarios was correlated with % reduction in cigarettes per day from pre- to post-treatment (left scatter plots) and % reduction in cigarettes per day from pre-treatment to 3-month follow-up (right scatter plots). Full correlation results are displayed in Supplementary Figures S1–S4. A formal conjunction analysis between the two correlation maps revealed a set of regions that were responsive to stressful scenarios, and correlated with CPD reduction at both timepoints. Those included the left amygdala, extending into the anterior/mid insula (as shown here) and parahippocampal gyrus, as well as right hippocampus, parahippocampal gyrus, and posterior insula (shown in Supplementary Figure S7-S8). Scatter plots represent the extracted cluster-averaged percent signal change during stress scenarios in regions identified in the conjunction analysis.
Discussion
We found that lower neural reactivity to stressful scenarios in amygdala, mid-insula, and hippocampal regions related to greater reduction in smoking after treatment and at 3-month follow-up. Moreover, we found that reactivity in the same regions was significantly lower in individuals who underwent MT compared to FFS. In addition, we reported that the MT group showed a greater reduction in smoking in both timepoints following treatment, and a significantly higher rate of point-prevalence abstinence at follow-up. Taken together, these results suggest that MT reduces stress reactivity in these brain regions, and that this reduction is one of the clinically-relevant mechanisms that may underlie its efficacy as a smoking cessation treatment. This is the first demonstration of this kind, and has important implications for our understanding of stress, the neural and psychological mechanisms that underlie mindfulness-based treatments, and for smoking cessation treatments more broadly.
Stress Reactivity and Smoking
First, these results join prior reports linking stress reactivity to drug use in general (Back et al., 2010; Brewer et al., 1998; Brown et al., 1990; Brown et al., 1995; Preston & Epstein, 2011; Sinha, 2001; Witkiewitz & Villarroel, 2009) and smoking in particular (Baer & Lichtenstein, 1988; Cooney et al., 2007; Epstein et al., 2010; McKee et al., 2011; Shiffman, 2005; Shiffman et al., 1996; Shiffman & Waters, 2004). Some of these studies have specifically shown that physiological and neural responses to stress relate to or predict drug use and relapse (Back et al., 2010; Seo et al., 2013; Sinha, Fox, et al., 2011; Sinha et al., 2006). Importantly, to our knowledge, this is the first demonstration of this relationship in smokers, whereby neural stress reactivity is negatively correlated with smoking outcomes, suggesting a broad role for stress reactivity across various substances, including nicotine cigarettes.
More specifically, we found that stress reactivity related to outcome in the amygdala and insula. The amygdala is an almond-shaped structure comprised of several subnuclei, which have distinct anatomical projections and serve different functions (Amaral, Price, Pitkanen, & Carmichael, 1992; Freese & Amaral, 2009). Nevertheless, the responsivity of amygdala to stress provocation in this study is not surprising given its role in detecting motivationally-salient stimuli (Kim et al., 2011), and in implementing core affect and emotion (for meta-analytic reviews, see Kober et al., 2008; Lindquist, Wager, Kober, Bliss-Moreau, & Barrett, 2012). Similarly, the insula may be conceptualized as a core affect region involved in awareness of affective feelings and bodily sensations (Craig, 2002, 2009; Lindquist et al., 2012). Consistently, both amygdala and insula have been implicated in the pathophysiology of anxiety and anxiety disorders (Damsa, Kosel, & Moussally, 2009; Etkin & Wager, 2007). In addition, functional and structural neuroplastic changes have previously been shown in both insula and amygdala following mindfulness meditation training (e.g., Farb et al., 2007; Goldin & Gross, 2010; Hölzel et al., 2011; Lazar et al., 2005; Lutz et al., 2014), with one study specifically linking reduction in amygdala density with stress reduction (Holzel et al., 2010).
Stress Induced Craving
Interestingly, the amygdala and insula have also both been implicated in drug craving (Chase, Eickhoff, Laird, & Hogarth, 2011; Garavan, 2010; Jasinska, Stein, Kaiser, Naumer, & Yalachkov, 2014; Mihov & Hurlemann, 2012), including cigarette craving (Engelmann et al., 2012; Kober et al., 2010; Kuhn & Gallinat, 2011; Naqvi, Rudrauf, Damasio, & Bechara, 2007). This is relevant to the present study, as it has been suggested that stress increases drug use specifically via stress-induced increases in craving (Li & Sinha, 2008; Potenza et al., 2012; Sinha, 2007, 2008; Sinha, Shaham, & Heilig, 2011). This link was demonstrated in laboratory studies in which stress and negative affect cues were found to increase negative affect, cortisol, heart rate, self-reported craving and cue reactivity (Childress et al., 1994; Coffey et al., 2002; Cooney, Litt, Morse, Bauer, & Gaupp, 1997; Fox, Hong, Siedlarz, & Sinha, 2008; Hyman, Fox, Hong, Doebrick, & Sinha, 2007; Sinha, 2001; Sinha et al., 1999; Sinha et al., 2006; Sinha et al., 2005; Sinha & Li, 2007; for review, see Sinha, Shaham, et al., 2011). Prospective clinical studies have also related acute stress to craving for cigarettes (Doherty et al., 1995; McKee et al., 2011) and other drugs (Sinha, Fox, et al., 2011; Sinha et al., 2006) and further linked such stress-induced craving to drug use and relapse (McKee et al., 2011; Sinha et al., 2006). In the present study, participants across groups also reported increased craving following stressful scenarios, along with increased stress-related neural activity. However, compared to FFS, the MT group showed lower stress-related neural activity, and it is possible that the reduction in smoking seen with MT may be attributable to relative reduction in such stress-induced reactivity (Witkiewitz et al., 2014). Alternatively, reductions in these regions may reflect the decoupling of craving and smoking behavior; in the larger clinical trial, we found strong correlations between craving and smoking at baseline that were attenuated at the end of treatment in the MT group (Brewer, Mallik, et al., 2011). Further, this decoupling of craving and smoking was moderated by informal mindfulness practice (Elwafi, Witkiewitz, Mallik, & Brewer, 2013). While both interpretations are plausible, future studies are needed to specifically compare between them.
Implications for our Understanding of Mindfulness
It has long been known that mindfulness-based treatments reduce stress and anxiety, including in anxiety and mood disorders (as exemplified by Mindfulness-Based Stress Reduction; Goldin & Gross, 2010; Kabat-Zinn et al., 1992; Teasdale et al., 2000) and healthy adults (e.g., Chambers et al., 2008). In terms of neural activity, several studies have linked mindfulness to reduced neural reactivity to affective stimuli. In mindfulness-based emotion regulation studies comparing mindfulness (as an instructed transient mindful state) to non-mindfulness trials, mindfulness was associated with reduced amygdala and parahippocampal reactivity during perception of negative images (Lutz et al., 2014) and reduced reactivity to cigarette cues in subgenual anterior cingulate (in cigarette smokers Westbrook et al., 2013). Following 8 weeks of mindfulness training, Goldin and Gross (2010) reported faster decrease in amygdala activity to negative self-beliefs in socially anxious patients. Similarly, reductions in amygdala activity to negative emotional images was reported in healthy adults following training (Desbordes et al., 2012). Studies with trait mindfulness are also consistent with reduced reactivity to affective stimuli: higher trait mindfulness is associated with lower amygdala reactivity to negative faces (Creswell, Way, Eisenberger, & Lieberman, 2007), lower resting-state amygdala activity (Way, Creswell, Eisenberger, & Lieberman, 2010) and smaller amygdala volume (Taren, Creswell, & Gianaros, 2013). A recent EEG study also found lowered late positive potential to negative and erotic images in individuals with higher trait mindfulness (Brown, Goodman, & Inzlicht, 2013); for extended discussion, see Supplementary Materials).
In substance users, however, only a few small studies have been published on this topic. Those reported reductions in depression, anxiety, and stress (Zgierska et al., 2008), and in physiological markers of stress (Brewer et al., 2009) following mindfulness-based treatments. Here we find reduced neural stress reactivity following MT in cigarette smokers, compared to FFS, and no differences in recruitment of typical “cognitive control” regions (e.g., dorsolateral prefrontal cortex; Buhle et al., 2014). This pattern of results is consistent with the view that mindfulness may lower emotional reactivity via “bottom up” mechanisms (rather than by increasing cognitive regulation of emotion; (Chiesa, Serretti, & Jakobsen, 2013; Kober et al., under review; Westbrook et al., 2013). This is further consistent with the Buddhist view that mindfulness prevents emotional reactivity (“the second arrow”; Teasdale & Chaskalson, 2011a).
The findings are further consistent with the “stress buffering account” of mindfulness (Creswell & Lindsay, 2014), which makes the specific prediction that mindfulness effects should be most potent in populations, such as smokers, in which stress is known to exacerbate the condition – and that this reduction would directly relate to reduced severity of the condition (i.e. smoking), as we report herein. As such, the present results may be the first direct evidence of this model, in showing that MT was associated with reduced neural reactivity to stress, which was further related to reduced smoking post treatment and at follow-up. Future studies should investigate the effects of MT on stress reactivity both pre- and post-treatment, as well as their effect on smoking and drug use outcomes.
Broad Implications for Treatment
Following previous reports implicating stress reactivity as a contributory factor in smoking relapse, this work suggests that, by reducing stress reactivity, MT may lead to improved smoking outcomes. One obvious implication would be that smoking cessation treatments should include strategies for stress-reduction. However, this is already the case: both treatments investigated in this RCT already included techniques for stress reduction. What is important, then, is that each treatment did so using different psychological orientation and strategies. Indeed, while CBT-type treatments focus on changing the content of thought and emotions (e.g., reappraising negative events, “finding the silver lining,” reducing negative affect), mindfulness-based treatments change one's relationship to thoughts and emotions (e.g., acceptance of negative events, letting thoughts be as they are, tolerating emotions; Gilpin, 2008; Shapiro, Carlson, Astin, & Freedman, 2006). This difference may be implicated in the differences in neural stress reactivity observed herein, as it has been previously proposed that awareness and acceptance (rather than avoidance and reduction) of emotional states is a mechanism of behavioral change across various disorders (e.g., Baer, 2003; Fjorback, Arendt, Ørnbøl, Fink, & Walach, 2011; Greenberg, 2002; Hayes & Feldman, 2004; Roemer & Orsillo, 2003; Teasdale & Chaskalson, 2011b).
However, thus far, only a few studies directly compared mindfulness-based to cognitive-behavioral treatments (e.g., Smith et al., 2008), and even fewer did so for substance use disorders (Bowen et al., 2014) including our prior RCT in smoking cessation (Brewer, Mallik, et al., 2011). This highlights the need for additional studies that examine the relationship between treatment type, mastery of particular strategies, neural activity, and drug use or smoking outcomes. The current results are at the very least consistent with the idea that the techniques taught in MT – including noticing and accepting negative affect and craving – are important treatment targets in smoking cessation and may be more potent than cognitive-behavioral strategies taught in FFS. This, in turn, suggests that adding mindfulness-based strategies might enhance the efficacy of active cessation treatments, as has been shown recently in comparing standard relapse prevention and mindfulness-based relapse prevention (Bowen et al., 2014).
Strengths and limitations
One limitation of this study was the group sample sizes: there were only 12 participants in the FFS group and 11 in MT. Nevertheless, 23 participants were included in the main correlational analysis – that directly relates neural stress reactivity to treatment outcome – and this sample size exceeds the minimum standards for a study of this type (e.g., Carter, Heckers, Nichols, Pine, & Strother, 2008). The data were also collected on 1.5T scanner, which typically has lower signal-to-noise ratio (SNR) than 3T scanners. However, data were carefully quality-checked by the imaging center staff and the authors, and found to have normal SNR, and sufficient contrast-to-noise ratio (which is most important in this context). Another limitation is that self-report ratings were not available for the full sample due to technical difficulties during data acquisition. In addition, the smokers in our study participated in the fMRI session after completing smoking cessation treatment; therefore, changes from pre- to post-treatment were impossible to assess. Furthermore, because we excluded individuals taking psychoactive medications or who met DSM-IV criteria for any substance dependence, we did not collect information on substance use except cigarettes and alcohol. Nonetheless, random assignment from a community-based sample is a strength, and the groups did not differ in any pre-treatment clinical characteristics, including smoking, alcohol use, and stress reactivity (measured via the PSS). Indeed, participants were randomized into groups; thus, the post-treatment data allows for cautious consideration of treatment effects.
Conclusions
We presented results from an fMRI stress probe administered following MT or FFS treatment for smoking cessation. We found that neural reactivity in regions including amygdala and insula related to smoking outcomes after treatment and at 3-month post-treatment follow-up. Activity in these regions also differentiated between treatment groups such that those who underwent MT showed lower stress reactivity in these regions. The results implicate reduction in stress reactivity as a mechanism of MT treatment-related change, and suggest that treatments that target stress reactivity hold particular promise for smoking cessation.
Supplementary Material
Acknowledgments
The authors wish to sincerely thank Kathleen Carroll and Bruce Rounsaville for their help with design of the clinical trial; Candace Minnix-Cotton for help in data collection; Cameron DeLeone for help with initial data organization; Charla Niche for help with analysis of the clinical trial and smoking outcome data; Jochen Weber for NeuroElf support; Paul Whalen for helpful comments on the data; Bethany Goodhue, Rebecca Boswell, Matthew Schafer, and Shosuke Suzuki for helpful comments on the manuscript; Theresa Babucio and the staff of the Psychotherapy Development Center for help with management and analysis of clinical variables.
This work was funded by K12 DA00167 to HK and JAB; P50 DA09241 pilot funds to HK and JAB; UL1-DE019586 and PL1-DA024859 to RS; and the U.S. Veterans Affairs New England Mental Illness Research, Education, and Clinical Center (MIRECC)
HK, JAB, KLH, and RS designed the research, JAB collected the data, HK analyzed the data, HK wrote the paper, all authors provided revisions, and approved of the final manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. Wiley; 1992. [Google Scholar]
- Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;10(6):410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Lerman C, Cao W, Falcone M, Bernardo L, Ruparel K, et al. Loughead J. Nicotine withdrawal alters neural responses to psychosocial stress. Psychopharmacology. 2016 doi: 10.1007/s00213-016-4299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Hartwell K, DeSantis SM, Saladin M, McRae-Clark AL, Price KL, et al. Kreek MJ. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug and Alcohol Dependence. 2010;106(1):21–27. doi: 10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer JS, Lichtenstein E. Classification and prediction of smoking relapse episodes: an exploration of individual differences. Journal of consulting and clinical psychology. 1988;56:104–110. doi: 10.1037//0022-006x.56.1.104. [DOI] [PubMed] [Google Scholar]
- Baer RA. Mindfulness training as a clinical intervention: A conceptual and empirical review. Clinical Psychology: Science and Practice. 2003;10(2):125–143. [Google Scholar]
- Bishop SR, Lau M, Shapiro S, Carlson L, Anderson ND, Carmody J, et al. Devins G. Mindfulness: A Proposed Operational Definition. Clinical Psychology: Science and Practice. 2004;11(3):230–241. [Google Scholar]
- Bowen S, Chawla N, Marlatt GA. Mindfulness-based relapse prevention for addictive behaviors: A clinician's guide. New York, NY: The Guilford Press; 2011. [Google Scholar]
- Bowen S, Witkiewitz K, Clifasefi SL, Grow J, Chawla N, Hsu SH, et al. Larimer ME. Relative efficacy of mindfulness-based relapse prevention, standard relapse prevention, and treatment as usual for substance use disorders: a randomized clinical trial. JAMA psychiatry. 2014;71(5):547–556. doi: 10.1001/jamapsychiatry.2013.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH. Negative affect as motivation to smoke. Current Directions in Psychological Science. 1994;3(2):33–37. [Google Scholar]
- Brewer DD, Catalano RF, Haggerty K, Gainey RR, Fleming CB. A meta-analysis of predictors of continued drug use during and after treatment for opiate addiction. Addiction. 1998;93(1):73–92. [PubMed] [Google Scholar]
- Brewer JA, Mallik S, Babuscio TA, Nich C, Johnson HE, Deleone CM, et al. Rounsaville BJ. Mindfulness training for smoking cessation: Results from a randomized controlled trial. Drug and Alcohol Dependence. 2011;119(1-2):72–80. doi: 10.1016/j.drugalcdep.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Sinha R, Chen JA, Michalsen RN, Babuscio TA, Nich C, et al. Rounsaville BJ. Mindfulness Training and Stress Reactivity in Substance Abuse: Results from a Randomized, Controlled Stage I Pilot Study. Substance Abuse. 2009;30(4):306–317. doi: 10.1080/08897070903250241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Gray JR, Tang YY, Weber J, Kober H. Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of the National Academy of Sciences. 2011;108(50):20254–20259. doi: 10.1073/pnas.1112029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, Goodman RJ, Inzlicht M. Dispositional mindfulness and the attenuation of neural responses to emotional stimuli. Social Cognitive and Affective Neuroscience. 2013;8:93–99. doi: 10.1093/scan/nss004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Vik PW, McQuaid JR, Patterson TL, Irwin MR, Grant I. Severity of psychosocial stress and outcome of alcoholism treatment. Journal of abnormal psychology. 1990;99(4):344–348. doi: 10.1037//0021-843x.99.4.344. [DOI] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. Journal of Studies on Alcohol and Drugs. 1995;56(5):538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- Brownell KD, Marlatt GA, Lichtenstein E, Wilson GT. Understanding and preventing relapse. American Psychologist. 1986;41(7):765–782. doi: 10.1037//0003-066x.41.7.765. [DOI] [PubMed] [Google Scholar]
- Buchmann AF, Laucht M, Schmid B, Wiedemann K, Mann K, Zimmermann US. Cigarette craving increases after a psychosocial stress test and is related to cortisol stress response but not to dependence scores in daily smokers. Journal of Psychopharmacology. 2010;24(2):247–255. doi: 10.1177/0269881108095716. [DOI] [PubMed] [Google Scholar]
- Buczek Y, Le AD, Wang A, Stewart J, Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology. 1999;144(2):183–188. doi: 10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Kober H, Ochsner KN, Mende-Siedlecki P, Weber J, Hughes BL, et al. Wager TD. Common representation of pain and negative emotion in the midbrain periaqueductal gray. Social Cognitive and Affective Neuroscience. 2013;8(6):609–616. doi: 10.1093/scan/nss038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle JT, Silvers J, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. Ochsner KN. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral cortex. 2014;24(11):2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM. A Cognitive-Behavioral Approach: Treating Cocaine Addiction. Rockville, MD: National Institute of Drug Abuse; 1998. NIH Publication No. 98-4308. [Google Scholar]
- Carter CS, Heckers S, Nichols T, Pine DS, Strother S. Optimizing the Design and Analysis of Clinical Functional Magnetic Resonance Imaging Research Studies. Biological psychiatry. 2008;64:842–849. doi: 10.1016/j.biopsych.2008.06.014. [DOI] [PubMed] [Google Scholar]
- CDC - Center for Disease Control and Prevention. Annual Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity Losses - United States, 1997-2001. Morbidity and Mortality Weekly Report. 2005;54:625–628. [PubMed] [Google Scholar]
- CDC - Center for Disease Control and Prevention. Annual Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity Losses --- United States, 2000-2004. Office on Smoking and Health, editor. Morbidity and Mortality Weekly Report. 2008a;57:1226–1228. [PubMed] [Google Scholar]
- CDC - Center for Disease Control and Prevention. Cigarette Smoking Among Adults - United States, 2007. Office on Smoking and Health, editor. Morbidity and Mortality Weekly Report. 2008b;56:1157–1161. [PubMed] [Google Scholar]
- Chambers R, Lo BCY, Allen NB. The impact of intensive mindfulness training on attentional control, cognitive style, and affect. Cognitive therapy and research. 2008;32(3):303–322. [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, Hogarth L. The Neural Basis of Drug Stimulus Processing and Craving: An Activation Likelihood Estimation Meta-Analysis. Biological psychiatry. 2011;70(8):785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa A, Serretti A, Jakobsen JC. Mindfulness: Top–down or bottom–up emotion regulation strategy? Clinical psychology review. 2013;33(1):82–96. doi: 10.1016/j.cpr.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Childress AR, Ehrman R, McLellan AT, MacRae J, Natale M, O'Brien CP. Can induced moods trigger drug-related responses in opiate abuse patients? Journal of substance abuse treatment. 1994;11(1):17–23. doi: 10.1016/0740-5472(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Childs E, de Wit H. Effects of acute psychosocial stress on cigarette craving and smoking. Nicotine & Tobacco Research. 2010;12(4):449–453. doi: 10.1093/ntr/ntp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Saladin M, Drobes DJ, Brady KT, Dansky BS, Kilpatrick DG. Trauma and substance cue reactivity in individuals with comorbid posttraumatic stress disorder and cocaine or alcohol dependence. Drug and Alcohol Dependence. 2002;65:115–127. doi: 10.1016/s0376-8716(01)00157-0. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Cooney JL, Pilkey DT, Steinburg HR, Oncken CA. Alcohol and tobacco cessation in alcohol-dependent smokers: Analysis of real-time reports. Psychology of Addictive Behaviors. 2007;21(3):277–286. doi: 10.1037/0893-164X.21.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. Journal of abnormal psychology. 1997;106(2):243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Lindsay EK. How does mindfulness training affect health? A mindfulness stress buffering account. Current Directions in Psychological Science. 2014;23(6):401–407. [Google Scholar]
- Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosomatic Medicine. 2007;69(6):560–565. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- Dagher A, Tannenbaum B, Hayashi T, Pruessner JC, McBride D. An acute psychosocial stress enhances the neural response to smoking cues. Brain research. 2009;1293:40–48. doi: 10.1016/j.brainres.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsa C, Kosel M, Moussally J. Current status of brain imaging in anxiety disorders. Current Opinion in Psychiatry. 2009;22(1):96–110. doi: 10.1097/YCO.0b013e328319bd10. [DOI] [PubMed] [Google Scholar]
- Desbordes G, Negi LT, Pace TWW, Wallace BA, Raison CL, Schwartz EL. Effects of mindful-attention and compassion meditation training on amygdala response to emotional stimuli in an ordinary, non-meditative state. Frontiers in human neuroscience. 2012;6 doi: 10.3389/fnhum.2012.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty K, Kinnunen T, Militello FS, Garvey AJ. Urges to smoke during the first month of abstinence: relationship to relapse and predictors. Psychopharmacology. 1995;119(2):171–178. doi: 10.1007/BF02246158. [DOI] [PubMed] [Google Scholar]
- Elwafi HM, Witkiewitz K, Mallik S, Brewer JA. Mindfulness training for smoking cessation: Moderation of the relationship between craving and cigarette use. Drug and Alcohol Dependence. 2013;130(1-3):222–229. doi: 10.1016/j.drugalcdep.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, et al. Cinciripini PM. Neural substrates of smoking cue reactivity: A meta-analysis of fMRI studies. NeuroImage. 2012;60(1):252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Marrone GF, Heishman SJ, Schmittner J, Preston KL. Tobacco, cocaine, and heroin: Craving and use during daily life. Addictive behaviors. 2010;35(4):318–324. doi: 10.1016/j.addbeh.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. American journal of Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, Anderson AK. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007;2(4):313–322. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ. Treating tobacco use and dependence: Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service; 2000. [Google Scholar]
- Fiore MC, Jaén CR, Baker TB. Treating Tobacco Use and Dependence: 2008 Update. In: Clinical Practice Guideline, editor. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Service, Public Health Service; 2008. [Google Scholar]
- Fjorback L, Arendt M, Ørnbøl E, Fink P, Walach H. Mindfulness- Based Stress Reduction and Mindfulness-Based Cognitive Therapy–a systematic review of randomized controlled trials. Acta Psychiatrica Scandinavica. 2011;124(2):102–119. doi: 10.1111/j.1600-0447.2011.01704.x. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong K, Siedlarz K, Sinha R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology. 2008;33(4):796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese JL, Amaral DG. Neuroanatomy of the primate amygdala. In: Whalen P, Phelps E, editors. The human amygdala. New York, NY: The Guilford Press; 2009. [Google Scholar]
- Garavan H. Insula and drug cravings. Brain Structure and Function. 2010;214(5-6):593–601. doi: 10.1007/s00429-010-0259-8. [DOI] [PubMed] [Google Scholar]
- Gilpin R. The use of Theravāda Buddhist practices and perspectives in mindfulness-based cognitive therapy. Contemporary Buddhism. 2008;9(2):227–251. [Google Scholar]
- Goldin PR, Gross JJ. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion. 2010;10(1):83–91. doi: 10.1037/a0018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal M, Singh S, Sibinga EMS, Gould NF, Rowland-Seymour A, Sharma R, et al. Shihab HM. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA internal medicine. 2014;174(3):357–368. doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg LS. Integrating an emotion-focused approach to treatment into psychotherapy integration. Journal of Psychotherapy Integration. 2002;12(2):154–189. [Google Scholar]
- Gunaratana H. Mindfulness in Plain English. Taipei, Taiwan: The Corporate Body of the Buddha Educational Foundation; 1991. [Google Scholar]
- Hayes AM, Feldman G. Clarifying the construct of mindfulness in the context of emotion regulation and the process of change in therapy. Clinical Psychology: Science and Practice. 2004;11(3):255–262. [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. Journal of consulting and clinical psychology. 2010;78(2):169–183. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Evans KC, Hoge EA, Dusek JA, Morgan L, et al. Lazar SW. Stress reduction correlates with structural changes in the amygdala. Social Cognitive and Affective Neuroscience. 2010;5(1):11–17. doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, Ott U. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspectives on Psychological Science. 2011;6(6):537–559. doi: 10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Fox H, Hong KIA, Doebrick C, Sinha R. Stress and drug-cue-induced craving in opioid-dependent individuals in naltrexone treatment. Experimental and Clinical Psychopharmacology. 2007;15(2):134–143. doi: 10.1037/1064-1297.15.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat-Zinn J, Massion AO, Kristeller J, Peterson LG, Fletcher KE, Pbert L, et al. Santorelli SF. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry. 1992;149:936–943. doi: 10.1176/ajp.149.7.936. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behavioural brain research. 2011;223(2):403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: A meta-analysis of neuroimaging studies. NeuroImage. 2008;42(2):998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Buhle J, Weber J, Ochsner KN, Wager TD. Let it be: Mindful-acceptance down-regulates pain and negative emotion. doi: 10.1093/scan/nsz104. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, DeVito EE, DeLeone CM, Carroll KM, Potenza MN. Cannabis Abstinence During Treatment and One-Year Follow-Up: Relationship to Neural Activity in Men. Neuropsychopharmacology. 2014;39(10):2288–2298. doi: 10.1038/npp.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences. 2010;107(33):14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg HW, Fan J, Ochsner KN, Liu X, Guise KG, Pizzarello S, et al. Goodman M. Neural Correlates of the Use of Psychological Distancing to Regulate Responses to Negative Social Cues: A Study of Patients with Borderline Personality Disorder. Biological psychiatry. 2009;66(9):854–863. doi: 10.1016/j.biopsych.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. European Journal of Neuroscience. 2011;33(7):1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Lachin JM, Matts JP, Wei LJ. Randomization in clinical trials: conclusions and recommendations. Controlled clinical trials. 1988;9(4):365–374. doi: 10.1016/0197-2456(88)90049-9. [DOI] [PubMed] [Google Scholar]
- Lando HA, McGovern PG, Barrios FX, Etringer BD. Comparative evaluation of American Cancer Society and American Lung Association smoking cessation clinics. American Journal of Public Health. 1990;80(5):554–559. doi: 10.2105/ajph.80.5.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ. A bio-informational theory of emotional imagery. Psychophysiology. 1979;16(6):495–512. doi: 10.1111/j.1469-8986.1979.tb01511.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Levin DN, Miller GA, Kozak MJ. Fear behavior, fear imagery, and the psychophysiology of emotion: the problem of affective response integration. Journal of abnormal psychology. 1983;92(3):276–306. doi: 10.1037//0021-843x.92.3.276. [DOI] [PubMed] [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, et al. Benson H. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16(17):1893–1897. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Shors TJ. Stress, anxiety, and dendritic spines: what are the connections? Neuroscience. 2013;251:108–119. doi: 10.1016/j.neuroscience.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30(10):939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Li CSR, Sinha R. Inhibitory control and emotional stress regulation: Neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neuroscience & Biobehavioral Reviews. 2008;32(3):581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist K, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The Brain Basis of Emotion: A meta-analytic review. Behavioral and Brain Sciences. 2012;35(3):121–143. doi: 10.1017/S0140525X11000446. target article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig DS, Kabat-Zinn J. Mindfulness in medicine. Journal of the American Medical Association. 2008;300(11):1350–1352. doi: 10.1001/jama.300.11.1350. [DOI] [PubMed] [Google Scholar]
- Lutz J, Herwig U, Opialla S, Hittmeyer A, Jäncke L, Rufer M, et al. Brühl AB. Mindfulness and emotion regulation—an fMRI study. Social Cognitive and Affective Neuroscience. 2014;9(6):776–785. doi: 10.1093/scan/nst043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, Donovan DM. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. New York: Guilford Press; 2005. [Google Scholar]
- Marlatt GA, Gordon JR. Determinants of Relapse: Implications for the Maintenance of Behavior. In: Davidson PO, Davidson SM, editors. Behavioral Medicine: Changing health lifestyles. New York: Brunner/Mazel; 1980. pp. 410–452. [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C. Mechanisms of stress in the brain. Nature Neuroscience. 2015;18(10):1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Sinha R, Weinberger AH, Sofuoglu M, Harrison EL, Lavery M, Wanzer J. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. Journal of Psychopharmacology. 2011;25(4):490–502. doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihov Y, Hurlemann R. Altered amygdala function in nicotine addiction: insights from human neuroimaging studies. Neuropsychologia. 2012;50(8):1719–1729. doi: 10.1016/j.neuropsychologia.2012.04.028. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315(5811):531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- O'Connell KA, Martin EJ. Highly tempting situations associated with abstinence, temporary lapse, and relapse among participants in smoking cessation programs. Journal of consulting and clinical psychology. 1987;55(3):367–371. doi: 10.1037//0022-006x.55.3.367. [DOI] [PubMed] [Google Scholar]
- Piasecki TM. Relapse to smoking. Clinical psychology review. 2006;26(2):196–215. doi: 10.1016/j.cpr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Moal ML. The role of stress in drug self-administration. Trends in Pharmacological Sciences. 1998;19(2):67–73. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Hong KA, Lacadie CM, Fulbright RK, Bergquist KL, Sinha R. Neural Correlates of Stress-Induced and Cue-Induced Craving: Influences of Gender and Cocaine Dependence. American journal of Psychiatry. 2012;169(4):406–414. doi: 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Epstein DH. Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology. 2011;218(1):29–37. doi: 10.1007/s00213-011-2183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raio CM, Orederu TA, Palazzolo L, Shurick AA, Phelps EA. Cognitive emotion regulation fails the stress test. Proceedings of the National Academy of Sciences. 2013;110(37):15139–15144. doi: 10.1073/pnas.1305706110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the Timeline Followback for Cocaine, Cannabis, and Cigarette Use. Psychology of Addictive Behaviors. 2014;28(1):154–162. doi: 10.1037/a0030992. [DOI] [PubMed] [Google Scholar]
- Roemer L, Orsillo SM. Mindfulness: A promising intervention strategy in need of further study. Clinical Psychology: Science and Practice. 2003;10(2):172–178. [Google Scholar]
- Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, Sinha R. Sex differences in neural responses to stress and alcohol context cues. Human brain mapping. 2011;32(11):1998–2013. doi: 10.1002/hbm.21165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA psychiatry. 2013;70(7):727–739. doi: 10.1001/jamapsychiatry.2013.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Tsou KA, Ansell EB, Potenza MN, Sinha R. Cumulative adversity sensitizes neural response to acute stress: association with health symptoms. Neuropsychopharmacology. 2014;39(3):670–680. doi: 10.1038/npp.2013.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology. 1995;119(3):334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shapiro SL, Carlson LE, Astin JA, Freedman B. Mechanisms of mindfulness. Journal of clinical psychology. 2006;62(3):373–386. doi: 10.1002/jclp.20237. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Dynamic influences on smoking relapse process. Journal of Personality. 2005;73(6):1715–1748. doi: 10.1111/j.0022-3506.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. Journal of consulting and clinical psychology. 1996;64(2):366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ. Negative affect and smoking lapses: a prospective analysis. Journal of consulting and clinical psychology. 2004;72(2):192–201. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158(4):343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. The Role of Stress in Addiction Relapse. Current Psychiatry Reports. 2007;9(5):388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences. 2008;1141(1):105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addiction biology. 2009;14(1):84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O'Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology. 1999;142(4):343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress-and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Archives of General Psychiatry. 2011;68(9):942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O'Malley SS. Psychological stress, drug- related cues and cocaine craving. Psychopharmacology. 2000;152(2):140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress- Induced Cocaine Craving and Hypothalamic-Pituitary-Adrenal Responses Are Predictive of Cocaine Relapse Outcomes. Archives of General Psychiatry. 2006;63(3):324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Wexler BE. Neural Circuits Underlying Emotional Distress in Humans. Annals of the New York Academy of Sciences. 2004;1032(1):254–257. doi: 10.1196/annals.1314.032. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lacadie CM, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, Wexler BE. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology. 2005;183(2):171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CSR. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug and alcohol review. 2007;26(1):25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Sinha R, Shaham Y, Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology. 2011;218(1):69–82. doi: 10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Tuit K. Imagery Script Development Procedures Manual. CreateSpace Independent Publishing Platform; North Charleston: 2012. [Google Scholar]
- Smith BW, Shelley BM, Dalen J, Wiggins K, Tooley E, Bernard J. A pilot study comparing the effects of mindfulness-based and cognitive-behavioral stress reduction. The Journal of Alternative and Complementary Medicine. 2008;14(3):251–258. doi: 10.1089/acm.2007.0641. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back: A Technique for Assessing Self-Reported Alcohol Consumption. In: Litten RZ, Allen J, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. Journal of Studies on Alcohol. 1994;(12):70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Swan GE, Denk CE, Parker SD, Carmelli D, Furze CT, Rosenman RH. Risk factors for late relapse in male and female ex-smokers. Addictive behaviors. 1988;13(3):253–266. doi: 10.1016/0306-4603(88)90052-4. [DOI] [PubMed] [Google Scholar]
- Taren AA, Creswell JD, Gianaros PJ. Dispositional mindfulness co- varies with smaller amygdala and caudate volumes in community adults. PloS one. 2013;8(5):e64574. doi: 10.1371/journal.pone.0064574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale JD, Chaskalson M. How does mindfulness transform suffering? I: The nature and origins of dukkha. Contemporary Buddhism. 2011a;12(1):89–102. [Google Scholar]
- Teasdale JD, Chaskalson M. How does mindfulness transform suffering? II: The transformation of dukkha. Contemporary Buddhism. 2011b;12(1):103–124. [Google Scholar]
- Teasdale JD, Segal ZV, Ridgeway VA, Soulsby JM. Prevention of Relapse/Recurrence in Major Depression by Mindfulness-Based Cognitive Therapy. Journal of consulting and clinical psychology. 2000;68(4):615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR. Uncontrollable events and alcohol drinking. British journal of addiction. 1987;82(4):381–392. doi: 10.1111/j.1360-0443.1987.tb01494.x. [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspectives on Psychological Science. 2009;4(3):274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Tobacco Free Initiative. WHO - World Health Organization. 2010 Retrieved January 29, 2013 from http://www.who.int/tobacco/mpower/tobacco_facts/en/index.html.
- Wager TD, Keller MC, Lacey SC, Jonides J. Increased sensitivity in neuroimaging analyses using robust regression. NeuroImage. 2005;26(1):99–113. doi: 10.1016/j.neuroimage.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Wallace BC. Psychological and environmental determinants of relapse in crack cocaine smokers. Journal of substance abuse treatment. 1989;6(2):95–106. doi: 10.1016/0740-5472(89)90036-6. [DOI] [PubMed] [Google Scholar]
- Way BM, Creswell JD, Eisenberger NI, Lieberman MD. Dispositional mindfulness and depressive symptomatology: correlations with limbic and self-referential neural activity during rest. Emotion. 2010;10(1):12–24. doi: 10.1037/a0018312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei LJ, Lachin JM. Properties of the urn randomization in clinical trials. Controlled clinical trials. 1988;9(4):345–364. doi: 10.1016/0197-2456(88)90048-7. [DOI] [PubMed] [Google Scholar]
- Westbrook C, Creswell JD, Tabibnia G, Julson E, Kober H, Tindle HA. Mindful attention reduces neural and self-reported cue-induced craving in smokers. Social, Cognitive, and Affective Neuroscience. 2013;8(1):73–84. doi: 10.1093/scan/nsr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Bowen S, Harrop EN, Douglas H, Enkema M, Sedgwick C. Mindfulness-based treatment to prevent addictive behavior relapse: Theoretical models and hypothesized mechanisms of change. Substance use & misuse. 2014;49(5):513–524. doi: 10.3109/10826084.2014.891845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Villarroel NA. Dynamic association between negative affect and alcohol lapses following alcohol treatment. Journal of consulting and clinical psychology. 2009;77(4):633–644. doi: 10.1037/a0015647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Gao JH, Lancaster JL, Fox PT. Clustered pixels analysis for functional MRI activation studies of the human brain. Human brain mapping. 1995;3(4):287–301. [Google Scholar]
- Yarkoni T. Big Correlations in Little Studies: Inflated fMRI Correlations Reflect Low Statistical Power-Commentary on Vul et al. (2009) Perspect Psychol Sci. 2009;4(3):294–298. doi: 10.1111/j.1745-6924.2009.01127.x. [DOI] [PubMed] [Google Scholar]
- Zgierska A, Rabago D, Zuelsdorff M, Coe C, Miller M, Fleming M. Mindfulness Meditation for Alcohol Relapse Prevention: A Feasibility Pilot Study. Journal of addiction medicine. 2008;2(3):165–173. doi: 10.1097/ADM.0b013e31816f8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW. Effects of the CRF receptor antagonist d-Phe CRF(12–41) and the α2-adrenergic receptor agonist on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007;53(8):958–966. doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.