Abstract
The molecules and pathways of the gut-brain axis represent new targets for developing methods to diagnose and treat psychiatric disorders. Manipulation of the gut microbiome with probiotics may be a therapeutic strategy with the potential to relieve gastrointestinal (GI) comorbidities and improve psychiatric symptoms. Candida albicans and Saccharomyces cerevisiae, commensal yeast species, can be imbalanced in the unhealthy human microbiome, and these fungal exposures were previously found elevated in schizophrenia. In a longitudinal, double-blind, placebo-controlled, pilot investigation of 56 outpatients with schizophrenia, we examined the impact of probiotic treatment on yeast antibody levels, and the relationship between treatment and antibody levels on bowel discomfort and psychiatric symptoms. We found that probiotic treatment significantly reduced C. albicans antibodies over the 14-week study period in males, but not in females. Antibody levels of S. cerevisiae were not altered in either treatment group. The highest levels of bowel discomfort over time occurred in C. albicans-seropositive males receiving the placebo. We observed trends toward improvement in positive psychiatric symptoms in males treated with probiotics who were seronegative for C. albicans. Results from this pilot study hint at an association of C. albicans seropositivity with worse positive psychiatric symptoms, which was confirmed in a larger cohort of 384 males with schizophrenia. In conclusion, the administration of probiotics may help normalize C. albicans antibody levels and C. albicans-associated gut discomfort in many male individuals. Studies with larger sample sizes are warranted to address the role of probiotics in correcting C. albicans-associated psychiatric symptoms.
ClinicalTrials.gov identifier: NCT01242371
Keywords: Gastroenterology, fungus, infection, mental health, autism, sex differences
1. Introduction
In mental health research, understanding how the gut-brain axis might be altered could aid the development of novel primary and adjunctive therapies for neuropsychiatric disorders such as schizophrenia. Although gastrointestinal (GI) inflammation and dysbioses have been identified as comorbidities in schizophrenia [1–5], treatment trials of gut microbiome modulators such as probiotics are limited. In one of the first published randomized, placebo-controlled trials of probiotic supplementation in outpatients diagnosed with schizophrenia, we previously reported the amelioration of GI functioning in many patients receiving the probiotic treatment [6]. Analysis of blood samples obtained from individuals in this cohort found significant alterations in immune proteins and associated pathways between treatment groups, which were suggestive of a probiotic-mediated improvement of GI epithelial and immune pathologies [7].
Preclinical investigations of the gut-brain axis have largely focused on characterizing how gut bacteria may modulate brain function via immune, neuronal and endocrine pathways [8, 9]. However, other organisms, including fungi, are also components of the human microbiome and these taxa likely contribute to inter- and intra-kingdom dynamics of resident commensals [10, 11]. We have previously demonstrated that exposure to members of the human mycobiome, the yeast species, Candida albicans and Saccharomyces cerevisiae, was elevated in individuals with schizophrenia compared to non-psychiatric controls [2, 12]. Furthermore, antibody levels against one of these yeast species, C. albicans, were associated with GI disturbances and lower scores on cognitive tests in people with schizophrenia [12]. In immune-competent, healthy individuals, commensal yeast interactions with resident bacteria are stable and balanced; however, during bacterial dysbioses or species depletion, such as following the intake of antibiotics, potentially pathogenic yeast infections can occur [13]. The administration of probiotics to restore the gut microbiota and improve GI conditions shows promise in combatting overgrowth of yeast commensals, and of Candida spp. in particular [14–16].
For the current study of the double-blind, placebo-controlled probiotic cohort [6, 7], we tested the hypothesis that the manipulation of the intestinal microbiome by probiotics in schizophrenia will ameliorate yeast imbalances, as evident by a decrease in the levels of antibodies to C. albicans and S. cerevisiae. We also investigated the interactive effects of probiotic treatment and yeast seropositivity on bowel difficulties and severity of psychiatric symptoms over a 14 week course of probiotic or placebo treatment.
2. Methods
2.1 Probiotic study design
Details regarding study design and patient recruitment for this clinical trial were previously described [6]. In brief, inclusion criteria included an age of 18–65, written informed consent, DSM-IV diagnosis of schizophrenia or schizoaffective disorder [17], outpatient status, scores on the Positive and Negative Syndrome Scale (PANSS) indicative of at least moderately severe psychosis [18], and currently on at least 8 weeks of antipsychotic medication. A stable regime of antipsychotics was maintained over the course of the study. Exclusion criteria included mental retardation, clinically significant somatic condition, history of intravenous drug use, DSM-IV diagnosis of substance abuse or dependence within the last 3 months, recent participation in investigational new drug trial, pregnancy, use of antibiotic medication within the last 14 days, and celiac disease. Medical records and self-reports were examined for evidence of GI, genitourinary (G-UR) and respiratory (RESP) disturbances.
A baseline PANSS score and blood sample were taken at the beginning of the study for each participant. Each participant received a 2-week placebo run-in and then were randomized to adjunctive probiotics or placebo, once per day with food, for 14 weeks. The probiotic formulation, “Bifiform Balance” (Ferrosan A/S, Pfizer, Søborg, Denmark) contained approximately 109 colony-forming units (cfus) of Lactobacillus rhamnosus strain GG and 109 cfus of Bifidobacterium animalis subsp. lactis Bb12. Participants met weekly with staff to monitor side effects and GI function and biweekly to score PANSS psychiatric symptoms. GI function was assessed as a “bowel score” on a scale of 1 to 4 regarding self-reported assessment of difficulty moving their bowels over the previous week, with higher numbers indicating more difficulty. During these weekly meetings, information regarding dietary intake of calories, carbohydrates, fats, fiber, protein, sodium and sugar was also obtained. A second blood sample was taken at the end of the 14-week study period.
All participants gave written informed consent and study approval was granted by the institutional review boards of Sheppard Pratt Health System and the Johns Hopkins School of Medicine. A Data Safety Monitoring Board oversaw the study and the study received a ClinicalTrials.gov identifier number: NCT01242371.
2.2 Parent study cohort
The participants in the probiotic study were drawn from an ongoing primary study of individuals with and without psychiatric disorders recruited by the Stanley Research Program at Sheppard Pratt Health System in Baltimore, MD, U.S.A. Study details of participants with schizophrenia and controls of this main cohort have been previously described [19, 20]. Limited clinical and laboratory information from 619 of these samples including the clinical trial cohort were available to investigate our preliminary finding linking C. albicans seropositivity with positive symptoms on PANSS.
2.3 Laboratory procedures
Study start and study end blood samples were obtained by venipuncture, and plasma separated and assessed for antibodies. Anti-C. albicans IgG levels were measured according to the manufacturer’s protocol using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Abcam, Cambridge, MA, U.S.A.). Anti-Saccharomyces cerevisiae IgG antibodies (ASCA) were also measured by ELISA according to the manufacturer’s protocol using a commercially available kit (Orgentec, Mainz, Germany). Each 96-well plate tested contained kit standards as well as study sample replicates for use as internal controls of reproducibility. C. albicans seropositivity designations were assigned according to standards run with the commercial ELISA kit, as previously described [12]. The Limit of Detection (LOD) and Limit of Quantitation (LOQ) were defined as three times and ten times the standard deviation of the blanks, respectively. These values were 0.17 for LOD and 0.55 for LOQ.
2.4 Data analyses
Power analyses were undertaken to determine if sufficient sample sizes were present for this retrospective analysis. Data analyses were performed at Johns Hopkins and investigators were blinded to treatment groups; data was unblinded by study coordinators at Sheppard Pratt Health System. Because we previously detected significantly different case:control patterns of C. albicans and S. cerevisiae IgG in males versus females with schizophrenia [2, 12], we performed the data analyses separately for both sexes for the present study. We first compared starting mean antibody levels between treatment groups with analyses of variance (ANOVA)s. We then used repeated measures ANOVAs to determine if mean antibody levels differed according to treatment group and to the beginning versus end of the study. Repeated measures ANOVAs were also used to determine if bowel and PANSS scores differed between seropositive and seronegative groups over time. For more exploratory analyses of the PANSS data, post-hoc tests and basic comparisons of continuous data, one-tailed t-tests were performed to maximize detection of informative significant differences in mean levels of the selected variable between groups. Chi-square tests were used to examine group differences between categorical data. Multiple linear regressions were performed to detect correlations between quantitative demographic and dietary variables with C. albicans IgG levels.
3. Results
Of the original 65 patients who were enrolled, 58 (89%) completed the study, and C. albicans and S. cerevisiae data were available for 56 individuals (86%). The basic demographic, lifestyle and clinical data (age, gender, race, total PANSS, smoking status, body mass index (BMI), maternal education as a surrogate for socioeconomic status, and GI, G-UR, RESP disturbances) for these 56 individuals were not statistically different between treatment groups and are listed in Supplemental Table 1. Also in this table are corresponding data for individuals with schizophrenia from the main study cohort, which we will use later to verify a preliminary finding. In Supplemental Table 2 is a power analysis demonstrating that the sample size available for this pilot study was sufficient to detect a difference in mean antibody levels as well as to test for associations with the longitudinal data of bowel difficulty and psychiatric symptom scores. Antipsychotic regimes were not different between probiotic and placebo groups with 26.79% of the individuals receiving clozapine, 21.43% olanzapine, 17.86% risperidone, 10.71% aripiprazole, 19.64% quetiapine, 16.07% haloperidol, and 8.93% ziprasidone. Other medications were also not significantly different between probiotic and placebo groups: 12.50% valproate, 44.64% antidepressants, 35.71% anticonvulsants.
In the clinical trial, starting C. albicans IgG levels were similar for males, but not females, with females receiving placebo having significantly higher antibody levels compared to those who received probiotics (ANOVA, F=4.02, post-hoc t=2.01, p≤0.03). A significant treatment effect of probiotics in females was not detectable, and the remaining analyses were focused on the male subset of participants (n=37). Antibody levels for S. cerevisiae were not significantly different between study starting and ending timepoints for either treatment group and either sex; thus, the remaining analyses were focused on C. albicans. The variables of age, race, smoking, BMI, maternal education were not significantly associated with C. albicans seropositivity.
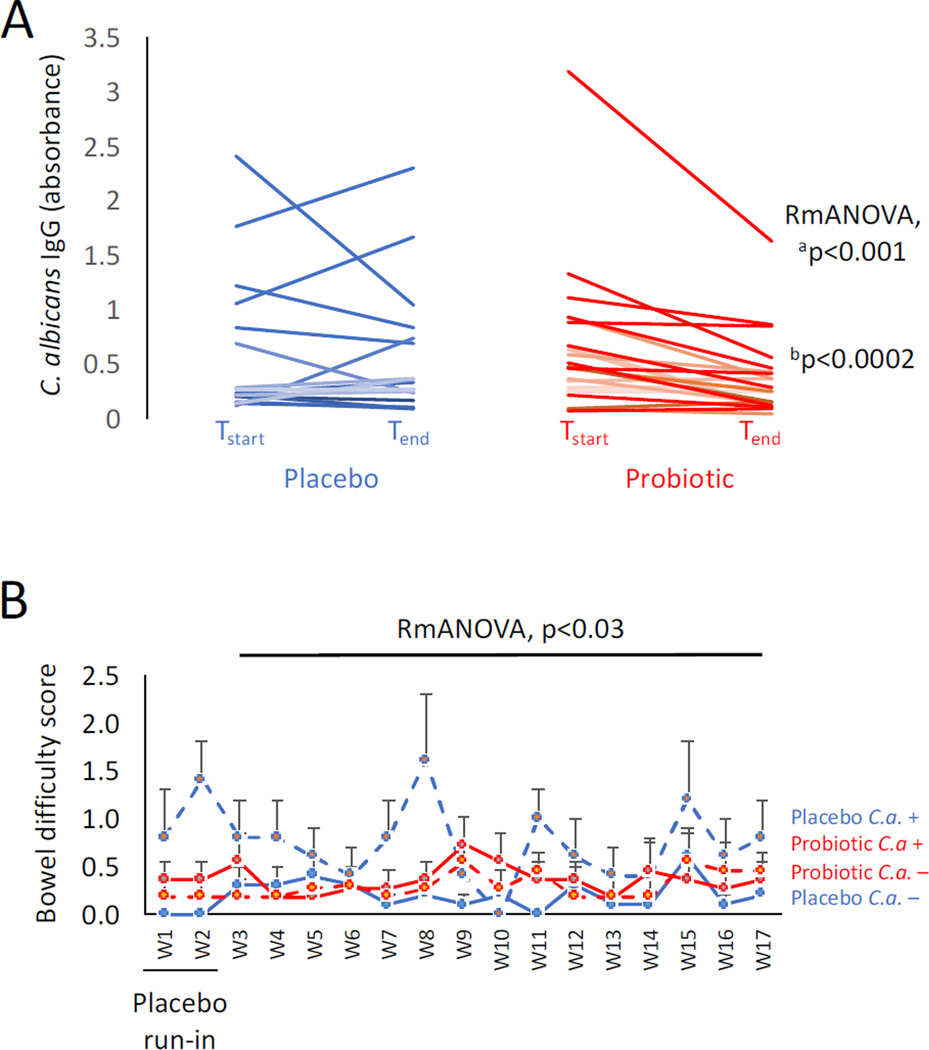
In males, mean C. albicans IgG levels were significantly reduced over time in those receiving probiotics (n=22), but not placebo (n=15) (Figure 1A: Repeated measures ANOVA, F=14.6, p≤0.001). As depicted in Figure 1A, there is one individual in the probiotic group with quite high C. albicans IgG levels at the beginning of the study, and which substantially decreased by the end of the study. To verify that the significant effect of treatment on the group was not being driven by this one individual, the analyses were repeated with this sample removed. A highly statistically significant reduction of antibody levels by probiotics over time remained (Repeated measures ANOVA, F=20.66, p≤0.0002), with overall, only 18.2% of the males (4 of 22) having IgG levels that were not responsive to treatment (vs 44% in the placebo group).
Figure 1. Reduction of C. albicans IgG antibodies and improvement of bowel difficulty in males receiving probiotics.
Panel A: Males with schizophrenia had significantly lower C. albicans antibody levels following a 14-week regimen of probiotics (n=22) compared to those receiving placebo (n=15). Tstart refers to study start and Tend refers to study end. Repeated measures ANOVA (RmANOVA) showed significantly reduced antibody levels associated with the probiotic treatment group over time. ap≤0.001 refers to the p-value including all individuals; bp≤0.0002 refers to the p-value with the elevated outlier removed. Panel B: Males receiving the placebo who were C. albicans seropositive reported the most bowel difficulties over the 14-week study. C.a.+ refers to C. albicans seropositive; C.a.− refers to C. albicans seronegative. W in the x axis refers to study week. Error bars designate standard errors of the mean for each group at each time point.
To identify a possible source location of exposure to C. albicans, we compared IgG levels between those who were positive or negative for disturbances to the GI, G-UR and RESP systems, all of which are typical resident sites of C. albicans. We found that C. albicans IgG levels were consistently elevated in those patients with GI conditions compared to those without and this finding was significant in both males and females (T-tests, both t=1.72, p≤0.05). G-UR conditions were only associated with elevated C. albicans in females (t=1.79, p≤0.05) and RESP conditions were not significantly associated with C. albicans in either sex.
To determine if the reduction in C. albicans IgG levels by probiotics was associated with amelioration of gut dysfunction, we compared a score of bowel movement difficulty during the course of the study for C. albicans seropositive males versus seronegative males for each treatment group. Males in the placebo group who were seropositive for C. albicans IgG (n=5), had significantly higher bowel scores indicative of increased difficulty with bowel movements over time than those who were C. albicans IgG seronegative (n=10) (Figure 1B, repeated measures ANOVA, F=1.79, p≤0.03). Bowel scores between seropositive (n=11) and seronegative (n=11) individuals were not significantly different in the probiotic group, and these differences were not significantly different from the seronegative individuals in the placebo group. C. albicans levels were not statistically associated with dietary intake of calories, carbohydrates, fats, fiber, protein, sodium and sugar.
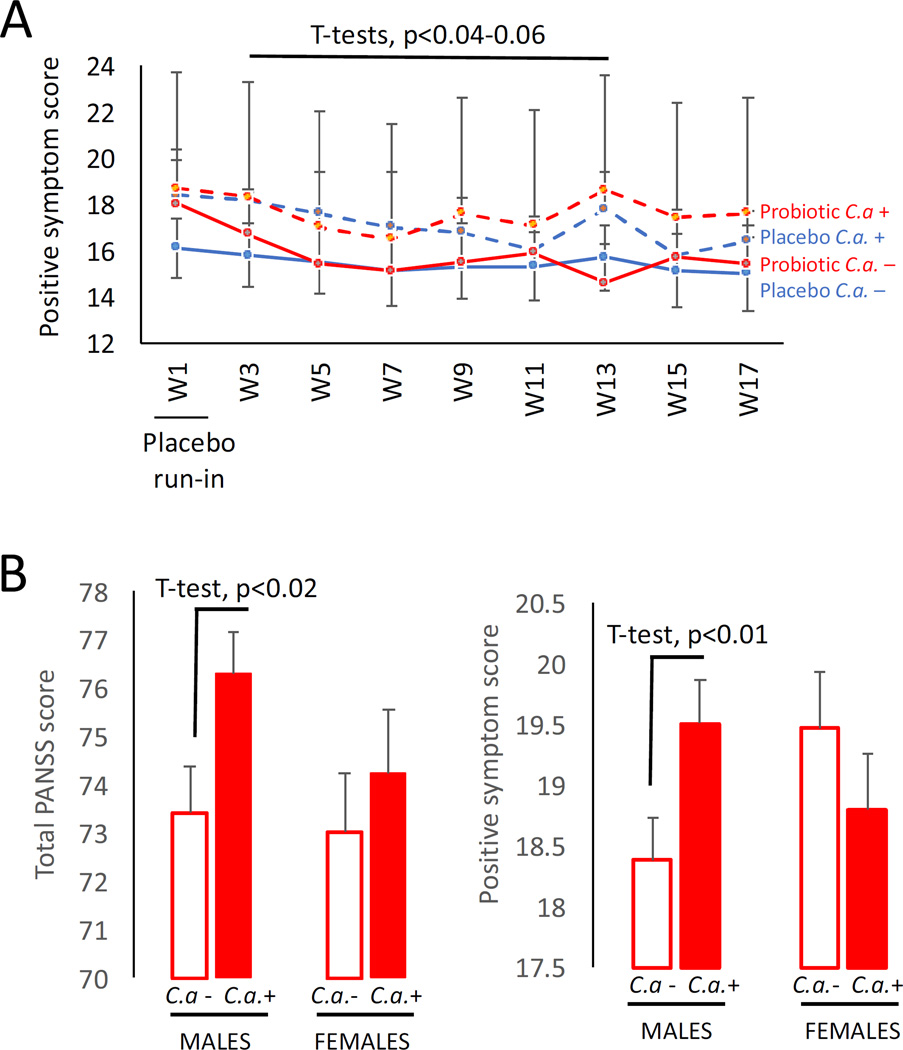
We then performed a longitudinal comparison of PANSS psychiatric symptom scores for C. albicans seropositive vs. seronegative males. Although PANSS scores were not statistically altered in the longitudinal analyses, we detected several trends for association of C. albicans with positive symptom scores (Figure 2A). For example, C. albicans seropositive males generally had elevated positive symptom scores at each week compared to those who were seronegative, irrespective of treatment group. For certain weeks such as study week 13, these differences in positive symptom scores approached significance in the probiotic group (T-test, t=1.62, p≤0.06). Furthermore, the change in score between the study start and the 13-week time point was significantly greater in C. albicans-seronegative males receiving probiotics compared to those who were seropositive, indicating a more accelerated improvement of positive symptoms in the C. albicans-seronegative subset (T-test, t=1.8, p≤0.04).
Figure 2. C. albicans seropositivity is associated with higher positive psychiatric symptoms on PANSS.
Panel A: Trends toward elevated positive PANSS symptom scores over time were observed in C. albicans seropositive (C.a. +) individuals in the pilot probiotic study. C.a.− refers to C. albicans seronegative. W in the x axis refers to study week. Error bars designate standard errors of the mean for each group at each time point. Panel B: Significantly worse psychiatric symptoms in males with C. albicans seropositivity compared to seronegative males were confirmed in an analysis of PANSS scores in the main Sheppard Pratt schizophrenia cohort. Shown are differences in total PANSS scores and in positive symptom subset scores; similarly significant differences related to seropositivity in males were observed for general PANSS scores (not shown). No differences between groups were observed for negative PANSS scores or for females for any of the PANSS comparisons.
To better address whether or not actual quantitative differences in these psychiatric scores exist and can be attributed to C. albicans independently of a probiotic treatment response, we examined C. albicans data from the main schizophrenia cohort and found that males who were C. albicans seropositive (n=165) had significantly elevated total PANSS scores than males who were C. albicans seronegative (n=219) (Figure 2B, T-tests, t=2.15, p≤0.02). The significant differences in total PANSS scores were found to be a function of elevated positive (Figure 2B, t=2.2, p≤0.01) and general (t=1.7, p≤0.04) symptoms, but not negative psychiatric symptoms. Total and subset PANSS scores were not significantly different between seropositive and seronegative females in this main study cohort.
4. Discussion
In our longitudinal, double-blind, placebo-controlled pilot study, a probiotic formulation containing the bacterial species, Lactobacillus rhamnosus and Bifidobacterium animalis, was associated with the significant reduction over time of C. albicans IgG in males with schizophrenia compared to males who received the placebo. Furthermore, our data suggest that the improvement of bowel function by probiotics may in part be due to correction of yeast overgrowth, as C. albicans-seropositive males receiving placebo reported the most bowel difficulty. Because the highest C. albicans levels were consistently in those individuals with GI ailments and not genitourinary or respiratory disturbances, it is likely that microbial dysbioses in the gut are the predominant source of C. albicans exposures in these males. In spite of sample size limitations, we detected trends towards increased positive psychiatric symptom scores associated with C. albicans seropositivity, which were then verified in the larger main cohort from which the probiotic study was derived.
Results from this study highlight the physiological heterogeneity of schizophrenia and the difficulties inherent to identifying the most applicable participants for clinical trials. We were not able to include the female study arm in the data analyses due to significant, presumably random, differences in starting antibody levels of C. albicans for the two treatment groups; however, we have also previously found that analyses of C. albicans exposures in women with psychiatric disorders were hampered by the greater susceptibility of the female reproductive tract to yeast infections, irrespective of mental health status. Indeed, for the female individuals evaluated in the current study, C. albicans IgG was consistently elevated in those who had genitourinary and GI conditions, while in males corresponding elevations were only found with GI disturbances. Previously, we found that C. albicans-seropositivity conferred to females with schizophrenia and bipolar disorder a greater risk for memory deficits on cognitive tests than females with these disorders who were seronegative [12]. Cognitive function was not assessed in the present study, but analyses of the PANSS psychiatric symptom data gave a preliminary indication that probiotics might also improve positive symptom scores in males, and particularly in those who were not C. albicans positive. While the effect of probiotic treatment combined with C. albicans seropositivity on psychiatric symptoms was not straightforward, the hints at an interactive effect of these variables here and the C. albicans-associated cognitive deficits detected in our previous study, together suggest that these interactive effects should be addressed and disentangled in larger-scale, carefully designed clinical trials.
Our findings were specific to the yeast C. albicans, as we did not detect altered antibodies for either treatment group over time for a second species of yeast, S. cerevisiae. In a study of patients with the inflammatory bowel disorder, Crohn’s disease, McKenzie et al found elevation of S. cerevisiae antibodies but not C. albicans antibodies and concluded that Crohn’s disease likely was characterized not simply by generalized permeability of the gut, but of a specific hypersensitivity associated with dietary antigens [21]. We have previously found elevated antibodies to S. cerevisiae in individuals with schizophrenia compared to controls, and this immune response was in fact significantly associated with food antigen hypersensitivity [2]. Thus, the heightened exposure to C. albicans documented in schizophrenia likely reflects a slightly different immunogenic process, in part perhaps generated by lifestyle variables, but also reflective of certain gut dysbioses that are correctable with probiotic treatment.
The interpretation of our study results is limited by the exploratory nature of our investigation and small sample sizes, particularly with respect to the subgroup analyses. As such, there may be unmeasured demographic, lifestyle and medication factors contributing to the effects that we found. In spite of these limitations, this pilot study enabled detection of a biological improvement attributable to probiotic treatment, which was measurable with a blood biomarker and which was relevant to the brain. This achievement was possible because the study was composed of a well-characterized sample set and incorporated a randomized, double blind trial design, which was less subject to bias than a typical observational study. Furthermore, by identifying C. albicans as a potential confounder of probiotic studies, we can design more powerful investigations, and our power analyses suggest sample sizes that future studies should consider in order to rigorously understand the probiotic/C. albicans interface with psychiatric symptoms. These future studies would entail a comprehensive assessment of serum biomarkers of gut dysbioses with actual microbiome biospecimens.
Finally, the development of new treatment options of serious psychiatric disorders has been staggeringly static. The wave of genetic research and widely applied GWAS data has not yet met with the identification of a multitude of new treatment targets as originally anticipated. A growing acceptance of a role for environmental factors contributing to psychiatric disease etiology has resulted in a currently promising new array of treatment modalities residing within the framework of the gut-brain axis. As the mechanisms guiding gut-brain activities become better understood, development and refinement of the most appropriate biomarkers to use to design and monitor individualized treatments of psychiatric disorders will most certainly follow. Our study helps to identify an important determinant, C. albicans exposure status, for understanding specific variables that influence the microbiome and contribute to a microbial dysbiosis that is relevant to schizophrenia. Future studies should be directed at translating these findings into practical methods for the diagnosis and treatment of schizophrenia and related disorders.
Supplementary Material
Highlights.
In schizophrenia, elevated Candida yeast antibody levels were lowered by probiotics.
Probiotics compared to placebo also relieved yeast-related bowel discomfort over time.
Candida albicans seropositivity was associated with worse psychiatric symptoms.
Acknowledgments
Acknowledgements/Funding
This work was supported by a NIMH P50 Silvio O. Conte Center at Johns Hopkins (grant# MH-94268) and by the Stanley Medical Research Institute. RHY is a member of the Stanley Medical Research Institute Board of Directors and Scientific Advisory Board.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies. None of the other authors report any potential conflicts of interest.
References
- 1.Castro-Nallar E, Bendall ML, Perez-Losada M, Sabuncyan S, Severance EG, Dickerson FB, Schroeder JR, Yolken RH, Crandall KA. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ. 2015;3:e1140. doi: 10.7717/peerj.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Severance EG, Alaedini A, Yang S, Halling M, Gressitt KL, Stallings CR, Origoni AE, Vaughan C, Khushalani S, Leweke FM, et al. Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophrenia Research. 2012;138:48–53. doi: 10.1016/j.schres.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Severance EG, Gressitt KL, Stallings CR, Origoni AE, Khushalani S, Leweke FM, Dickerson FB, Yolken RH. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophrenia Research. 2013;148:130–137. doi: 10.1016/j.schres.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yolken RH, Severance EG, Sabunciyan S, Gressitt KL, Chen O, Stallings C, Origoni A, Katsafanas E, Schweinfurth LAB, Savage CLG, et al. Metagenomic Sequencing Indicates That the Oropharyngeal Phageome of Individuals With Schizophrenia Differs From That of Controls. Schizophrenia Bulletin. 2015;41:1153–1161. doi: 10.1093/schbul/sbu197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fond G, Boukouaci W, Chevalier G, Regnault A, Eberl G, Hamdani N, Dickerson F, Macgregor A, Boyer L, Dargel A, et al. The "psychomicrobiotic": Targeting microbiota in major psychiatric disorders: A systematic review. Pathol Biol (Paris) 2015;63:35–42. doi: 10.1016/j.patbio.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Dickerson FB, Stallings C, Origoni A, Katsafanas E, Savage CL, Schweinfurth LA, Goga J, Khushalani S, Yolken RH. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord. 2014;16 doi: 10.4088/PCC.13m01579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomasik J, Yolken RH, Bahn S, Dickerson FB. Immunomodulatory Effects of Probiotic Supplementation in Schizophrenia Patients: A Randomized, Placebo-Controlled Trial. Biomark Insights. 2015;10:47–54. doi: 10.4137/BMI.S22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 9.Yarandi SS, Peterson DA, Treisman GJ, Moran TH, Pasricha PJ. Modulatory Effects of Gut Microbiota on the Central Nervous System: How Gut Could Play a Role in Neuropsychiatric Health and Diseases. J Neurogastroenterol Motil. 2016;22:201–212. doi: 10.5056/jnm15146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghannoum MA, Jurevic RJ, Mukherjee PK, Cui F, Sikaroodi M, Naqvi A, Gillevet PM. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010;6:e1000713. doi: 10.1371/journal.ppat.1000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suhr MJ, Hallen-Adams HE. The human gut mycobiome: pitfalls and potentials-a mycologist's perspective. Mycologia. 2015 doi: 10.3852/15-147. [DOI] [PubMed] [Google Scholar]
- 12.Severance EG, Gressitt KL, Stallings CR, Katsafanas E, Schweinfurth LA, Savage CL, Adamos MB, Sweeney KM, Origoni AE, Khushalani S, et al. Candida albicans exposures, sex specificity and cognitive deficits in schizophrenia and bipolar disorder. NPJ Schizophr. 2016;2:16018. doi: 10.1038/npjschz.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J, Sudbery P. Candida albicans, a major human fungal pathogen. J Microbiol. 2011;49:171–177. doi: 10.1007/s12275-011-1064-7. [DOI] [PubMed] [Google Scholar]
- 14.Mailander-Sanchez D, Wagener J, Schaller M. Potential role of probiotic bacteria in the treatment and prevention of localised candidosis. Mycoses. 2012;55:17–26. doi: 10.1111/j.1439-0507.2010.01967.x. [DOI] [PubMed] [Google Scholar]
- 15.Matsubara VH, Wang Y, Bandara HM, Mayer MP, Samaranayake LP. Probiotic lactobacilli inhibit early stages of Candida albicans biofilm development by reducing their growth, cell adhesion, and filamentation. Appl Microbiol Biotechnol. 2016;100:6415–6426. doi: 10.1007/s00253-016-7527-3. [DOI] [PubMed] [Google Scholar]
- 16.Davar R, Nokhostin F, Eftekhar M, Sekhavat L, Bashiri Zadeh M, Shamsi F. Comparing the Recurrence of Vulvovaginal Candidiasis in Patients Undergoing Prophylactic Treatment with Probiotic and Placebo During the 6 Months. Probiotics Antimicrob Proteins. 2016 doi: 10.1007/s12602-016-9218-x. [DOI] [PubMed] [Google Scholar]
- 17.APA. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. 4th. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 18.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 19.Dickerson F, Stallings C, Origoni A, Katsafanas E, Schweinfurth LA, Savage CL, Khushalani S, Yolken R. Pentraxin 3 is reduced in bipolar disorder. Bipolar disorders. 2015;17:409–414. doi: 10.1111/bdi.12281. [DOI] [PubMed] [Google Scholar]
- 20.Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Yang S, Yolken R. C-reactive protein is elevated in schizophrenia. Schizophrenia Research. 2013;143:198–202. doi: 10.1016/j.schres.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 21.McKenzie H, Main J, Pennington CR, Parratt D. Antibody to selected strains of Saccharomyces cerevisiae (baker's and brewer's yeast) and Candida albicans in Crohn's disease. Gut. 1990;31:536–538. doi: 10.1136/gut.31.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.