Abstract
Despite improved survival due to combination antiretroviral therapy (cART), youth with perinatally-acquired HIV (PHIV) show cognitive deficits and developmental delay at increased rates. HIV affects the brain during critical periods of development, and the brain may be a persistent reservoir for HIV due to suboptimal blood brain barrier penetration of cART. We conducted structural magnetic resonance imaging (sMRI) and cognitive testing in 40 PHIV youth (mean age=16.7 years) recruited from the NIH Pediatric HIV/AIDS Cohort Study (PHACS) who are part of the first generation of PHIV youth surviving into adulthood. Historical and current HIV disease severity and substance use measures were also collected. Total and regional cortical grey matter brain volumes were compared to a group of 334 typically-developing, HIV-unexposed and uninfected youth (frequency-matched for age and sex) from the Pediatric Imaging, Neurocognition, and Genetics (PING) study (mean age=16.1 years). PHIV youth had smaller (2.8 – 5.1%) total and regional grey matter volumes than HIV-unexposed and uninfected youth, with smallest volumes seen among PHIV youth with higher past peak viral load (VL) and recent unsuppressed VL. In PHIV youth, worse cognitive performance correlated with smaller volumes. This pattern of smaller grey matter volumes suggests that PHIV infection may influence brain development and underlie cognitive dysfunction seen in this population. Among PHIV youth, smaller volumes were also linked to substance use (alcohol use: 9.0 – 13.4%; marijuana use: 10.1 – 16.0%). In this study, collection of substance use information was limited to the PHIV cohort; future studies should also collect substance use information in controls to further address interactions between HIV and substance use on brain volume.
1. Introduction
Worldwide, it is estimated that there are over three million youth living with HIV globally, with the majority of youth acquiring HIV perinatally (Sohn and Hazra, 2013; UNAIDS, 2014). Youth with perinatally-acquired HIV (PHIV) may show cognitive deficits as well as developmental delay even among those with reconstituted immunologic and virologic status, (Cohen et al., 2014; Crowell et al., 2014; Ene et al., 2014; Koekkoek et al., 2008; Linn et al., 2015; Malee et al., 2016; Martin et al., 2006; Nichols et al., 2016; Nozyce et al., 2006; Redmond et al., 2016; Sherr et al., 2014; Sirois et al., 2016; Smith et al., 2012; Smith and Wilkins, 2015) making PHIV a common infectious cause of perinatally-acquired developmental disability globally (Armstrong et al., 1993; Institute of Medicine, 2001; UNAIDS, 2015). Combination antiretroviral therapy (cART) for children with PHIV has resulted in substantial improvements in health with survival beyond childhood and reductions in morbidity and mortality (Brady et al., 2010; Gona et al., 2006; Hazra et al., 2010).
Early HIV infection, immune activation, and viral persistence (due to suboptimal blood brain barrier penetrance of cART regimen) during a critical period of development may be especially detrimental to developing brains in youth with PHIV (Annunziata, 2003; Churchill et al., 2015; Cohen et al., 2015; Ene et al., 2011; González-Scarano and Martín-García, 2005; Kramer-Hammerle et al., 2005; Linn et al., 2015; Martin et al., 2006; Nesbit and Schwartz, 2002; Sarma et al., 2014; Thompson et al., 2011). Brain development is an extended process that begins prenatally and continues throughout the first two decades of life, with increased sensitivity to experience during the first year of life in pathways responsible for sensory, language and higher order cognitive development (Fox et al., 2010; Tierney and Nelson, 2009). Adolescence is also a crucial developmental window marked by a period of rapid brain maturation via synaptic pruning and myelination. White matter volume increases while grey matter volume decreases (Ernst and Mueller, 2008), with parietal grey matter reduction prominent before adolescence, followed by dorsal, mesial, and orbital frontal grey matter reduction during and after adolescence (Sowell et al., 2004).
Studies including neuroimaging combined with cognitive evaluation allow for an in vivo characterization of how HIV and cART may mediate brain development (Thompson and Jahanshad, 2015). In adults, studies of post-mortem tissue and in vivo neuroimaging combined with cognitive testing have revealed atrophy in cortical and subcortical structures that is related to HIV severity and cognitive performance (Ances et al., 2012; Archibald et al., 2004; Becker et al., 2012, 2011; Cohen et al., 2010a; Heindel et al., 1994; Jernigan et al., 1993; Kallianpur et al., 2013; Stout, 1998; Thompson et al., 2005; Thompson and Jahanshad, 2015). Still, the effects of early HIV infection on the underlying brain in adolescents with PHIV have not been well-characterized (Cohen et al., 2015; Hoare et al., 2014; Musielak and Fine, 2015).
Adolescent brains are also subject to environmental influences, including substance use. Neuroimaging and neuropsychological studies in youth who use substances have found structural brain abnormalities, including grey matter volume reductions, as well as cognitive dysfunction (Battistella et al., 2014; Churchwell et al., 2010; Jacobus and Tapert, 2014; Peng et al., 2015; Squeglia et al., 2009; Substance Abuse and Mental Health Services Administration, 2011; Williams et al., 2010; Yakolev and Lecours, 1967). Youth with worse HIV disease severity are more likely to engage in substance use (Williams et al., 2010). Thus, to carefully study effects of PHIV on youth treated with cART, it is important to account for substance use.
We present one of the first studies to investigate the impact of HIV severity and coincident substance use on regional and total brain volumes and their association with cognition in PHIV youth. Other studies on grey matter volumes in PHIV do not focus on regional grey matter or substance use or are in PHIV populations with varying clinical characteristics from our cohort (Cohen et al., 2015; Sarma et al., 2014). Since PHIV youth often exhibit global cognitive functioning, working memory, and processing speed deficits (Crowell et al., 2014; Hazra et al., 2010; Koekkoek et al., 2008; Linn et al., 2015; Raskino et al., 1999), we hypothesized that frontal and parietal regions, regions important for higher-order cognitive functioning, would show volume reduction as compared to typically-developing, HIV-unexposed and uninfected youth and smaller volumes would be associated with worse cognitive performance. We also hypothesized that HIV disease severity and substance use would be associated with reduced cortical grey matter volumes among adolescents with PHIV.
2. Materials and methods
2.1 Study Population
40 PHIV youth from a single site (Ann & Robert H. Lurie Children’s Hospital of Chicago) participating in the Adolescent Master Protocol (AMP) study of the NIH Pediatric HIV/AIDS Cohort Study (PHACS) network were recruited. Institutional review board approvals were obtained. Parents, legal guardians, or youth aged 18 years or older provided written informed consent; minors provided written assent. A control group of 334 typically developing, HIV-unexposed and uninfected youth was generated using frequency-matching for sex and age from the Pediatric Imaging, Neurocognition, and Genetics (PING) study (http://pingstudy.ucsd.edu/welcome.html) Magnetic Resonance Imaging (MRI) database from five sites (Massachusetts General Hospital; Sackler Institute; University of Hawaii; Yale; University of California-Los Angeles). Of note, information regarding alcohol and drug use was not collected in the PING cohort.
2.2 HIV Disease Markers, Substance Use, and Cognitive Functioning in PHIV youth
Visits occurred semi-annually (2007 through 2010), then annually, as described previously (Lewis-de los Angeles et al., 2016; Smith et al., 2012). Lifetime laboratory results including CD4 T-lymphocyte percentages (CD4%), plasma HIV RNA concentration (viral load (VL)), and CDC HIV classification (World Health Organization, 2007) were obtained from medical charts. Past HIV disease severity measures were determined by collecting the lowest known lifetime CD4% (“nadir CD4%”) and highest known lifetime HIV VL (“peak VL”) prior to entry. A measure of ongoing viremia was calculated as percentage of measures exceeding 1,000 copies/mL in the five years prior to neuroimaging. Recent VL was defined as VL closest to neuroimaging.
Substance use (alcohol, tobacco, marijuana, and other illicit drugs) was collected starting at 10 years of age by Audio Computer-Assisted Self-Interview (ACASI), which was used to decrease under-reporting as a result of social stigma (Alperen et al., 2014; Mellins et al., 2011). A binary variable for lifetime prevalence of alcohol, marijuana and tobacco use was created for any self-reported use on ACASI performed within one year preceding neuroimaging.
Standardized neuropsychological examinations (Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV) (6–16 years), and Wechsler Adult Intelligence Scale, Fourth Edition (WAIS-IV) (17 years and older)) were performed. Working memory and processing speed indices, standardized to have mean=100 and standard deviation (SD)=15 in the general population, were used to calculate cognitive proficiency index (CPI), an estimate of the information processing efficiency for learning, problem solving, and higher-order reasoning (Weiss et al., 2006).
2.3 Image Acquisition and Processing
MRI scans were performed using standardized PING acquisition protocols (Jernigan et al., 2015; Lewis-de los Angeles et al., 2016; Uban et al., 2015) on a single 3.0 Tesla Siemens Magnetom Tim Trio scanner (Siemens Medical Solutions, Erlangen, Germany) with a 12-channel head coil. Structural MRI used a T1-weighted, MP-RAGE sequence (sagittal, TR/TE/TI=2,170/4.37/1,100ms, FOV=256x256 mm, flip angle=7o, voxel resolution=1x1x1.2 mm3, scan time=8:08). The same imaging protocol was used for PING 3T Siemens imaging sites with exception of an 8-channel head coil. Structural MRIs were analyzed with FreeSurfer (http://surfer.nmr.mgh.harvard.edu), producing 85 cortical and subcortical grey matter regions of interests (ROIs) along with ROI and total grey matter volumes (Desikan et al., 2006). Total grey matter volume was calculated by summing all cortical, subcortical and cerebellum grey matter volumes. A priori cortical ROIs (left and right hemisphere postcentral gyrus, precentral gyrus, rostral middle frontal gyrus, superior frontal cortex, and superior parietal cortex) were selected due to their roles in higher-order cognitive functioning, which may be impaired in PHIV youth (Crowell et al., 2014; Hazra et al., 2010; Koekkoek et al., 2008; Linn et al., 2015; Raskino et al., 1999). Quality control and processing of PHIV and uninfected youth were executed with the PING processing portal (Jernigan et al., 2015).
2.4 Statistical Analyses
2.4.1 Between-group analyses
Total grey matter and 10 a priori cortical ROIs volumes were identified as primary outcomes. The remaining 74 ROIs were analyzed as secondary outcomes. Descriptive statistics and graphical methods were used to confirm the normality assumption for volume measures. Volumes were compared between PHIV youth and HIV-unexposed and uninfected youth using linear regression models. Results were reported for models with and without adjusting for age at scan, sex, race, caregiver education attainment, annual household income (Noble et al., 2015), and intracranial volume. Caregiver education was classified as high school education and below vs. greater than high school. Annual household income was classified as $30,000 and below vs. greater than $30,000. Percent change in volume as compared to HIV-unexposed and uninfected youth was calculated based on adjusted means.
2.4.2 Within-group analyses in PHIV youth
Among PHIV youth, associations of ROI volumes with HIV severity and substance use were first evaluated using Spearman correlations for continuous HIV disease measures (i.e., log-transformed peak RNA VL, nadir CD4%) or two-sample t-tests for dichotomized measures (i.e., HIV CDC classification, HIV VL>400 copies/mL, current CD4% < 15%, and history of substance use). General linear modeling was performed to adjust for age at scan and sex. Age at nadir CD4% and age at peak VL were also included in adjusted models with nadir CD4% and peak VL as HIV markers, respectively. Statistical significance for the 10 primary ROIs as well as total grey matter was set at 0.05 while the Benjamini-Liu approach to control the false discovery rate (FDR) with a threshold of 0.10 was used to control for multiple comparisons among the 74 secondary ROIs (Benjamini et al., 2001).
For evaluating associations of volume with cognitive functioning, we considered the 10 primary ROIs as well as total grey matter. Linear regression was used to evaluate associations between ROI volume as well as total grey matter and working memory, processing speed, and cognitive proficiency indices, adjusting for sex and age at scan.
Three sets of sensitivity analyses were conducted: 1) including substance use in models of associations of brain volumes with HIV measures for grey matter volumes; 2) adjusting for total grey matter volume in models evaluating associations between brain volumes and substance use for secondary ROIs; 3) including substance use, sex, and age at scan in analyses evaluating associations of brain volumes with cognition.
3. Results
3.1 Study Population
The 40 PHIV youth had a mean age at scan of 16.7 years; 53% were female, 73% were black (one did not report race), and 13% Hispanic (Table 1). The 334 PING typically-developing controls had a mean age of 16.1 years; 48% were female, 19% black, and 24% Hispanic (Table 1). PING youth more often than PHIV youth had caregivers who completed more than high school (72% vs 53%) and had annual household income > $30,000 (73% vs 55%) (Table 1). Forty-three percent of youth with PHIV had a CPI < 85 (more than 1 SD below norm) and average working memory scores were significantly lower than general population means of 100 (mean=87.6, p<0.001), as reported previously (Lewis-de los Angeles et al., 2016; Uban et al., 2015). At MRI scan, 85% of PHIV youth had suppressed VL (<400 copies/mL) and 92% were receiving cART (Table 1).
Table 1.
Characteristics of Study Population by HIV Infection Status
| HIV Infection Status | ||||
|---|---|---|---|---|
| Characteristic | PHIV (N=40) | PING Control (N=334) | P- Value* | |
| Age at the time of neuroimaging scan (years) | Mean (SD) | 16.7 (2.4) | 16.1 (2.7) | 0.14 |
| Median (Q1, Q3) | 17.1 (14.9, 18.2) | 15.8 (13.7, 18.5) | ||
| Min, Max | 11.6, 20.7 | 11.6, 20.7 | ||
| Sex | Female | 21 (53%) | 159 (48%) | 0.56 |
| Male | 19 (48%) | 175 (52%) | ||
| Black Race | Yes | 29 (73%) | 65 (19%) | <0.001 |
| No | 10 (25%) | 269 (81%) | ||
| Unknown | 1 (3%) | 0 (0%) | ||
| Ethnicity | Hispanic or Latino | 5 (13%) | 79 (24%) | 0.11 |
| Not Hispanic or Latino | 35 (88%) | 255 (76%) | ||
| Caregiver education attainment | ≤ High school | 19 (48%) | 76 (23%) | 0.001 |
| > High school | 21 (53%) | 242 (72%) | ||
| Unknown | 0 (0%) | 16 (5%) | ||
| Annual household income | ≤ $30,000 | 18 (45%) | 71 (21%) | 0.002 |
| > $30,000 | 22 (55%) | 244 (73%) | ||
| Unknown | 0 (0%) | 19 (6%) | ||
| HIV Disease Severity Measures | Nadir CD4% | 16.5 (8.0, 23.8) | ||
| Nadir CD4% < 15% | 17 (43%) | |||
| Age at nadir CD4% (years) | 5.7 (2.2, 10.9) | |||
| Most recent CD4% | 35.9 (27.6, 42.9) | |||
| Age at most recent CD4% (years) | 17.0 (14.9, 18.2) | |||
| Log peak HIV RNA VL (copies/mL) | 5.7 (5.2, 5.9) | |||
| Age at peak RNA (years) | 2.5 (0.6, 5.3) | |||
| Most recent RNA count > 400 copies/mL | 6 (15%) | |||
| Age at most recent RNA (years) | 17.0 (14.9, 18.2) | |||
| % of VL > 1000 copies/mL in past 5 years | 6.5 (0.0, 29.0) | |||
| Substance use by ACASI | Alcohol | 14 (35%) | ||
| Tobacco use | 10 (25%) | |||
| Marijuana use | 14 (35%) | |||
| Illicit drug use excluding marijuana a | 3 (8%) | |||
| ARV regimen at the time of scanning | Combination ARV regimenb | 37(92%) | ||
| Not on combination ARV, but on ARVs | 1 (3%) | |||
| Not on any ARVs | 2 (5%) | |||
| WISC-IV/WAIS-IV indices (mean, SD) | Cognitive Proficiency Index | 90.3 (16.0) | ||
| Working Memory Index | 87.6 (16.8) | |||
| Processing Speed Index | 95.4 (14.2) | |||
Values presented are median and interquartile range (IQR), or N (%), unless otherwise specified. SD = standard deviaton.
ACASI=audio computer-assisted self-interview; ARV=antiretroviral; PING=Pediatric Imaging, Neurocognition, and Genetics; PHIV=perinatally-acquired HIV; VL=viral load; cART = combination anti-retroviral therapy; WISC-IV=Wechsler Intelligence Scale for Children, Fourth Edition; WAIS-IV=Wechsler Adult Intelligence Scale-Fourth Edition.
P-value by Wilcoxon rank sum test for continuous measures and by Chi-Square test for categorical measures
Illicit drugs include: inhalants, amphetamine, cocaine, methamphetamine, crack, sedatives/barbiturates, ecstasy, hallucinogens, heroin.
Combination ARV regimen defined as regimen including at least 3 drugs from at least 2 drug classes.
As described previously for other neuroimaging studies in this cohort (Herting et al., 2015; Lewis-de los Angeles et al., 2016; Uban et al., 2015), for PHIV youth, the mean interval between scanning and assessment of recent disease markers was 1.8 months (SD=3.5 months) with 83% of recent VL measures within three months prior to scanning. Mean interval between scanning and cognitive testing was 3.8 months (SD=5.8 months). All but 2 participants completed cognitive assessments within 1 year of neuroimaging, with 31 (77%) within 3 months. One fourth of PHIV youth reported tobacco use, 35% alcohol, and 35% marijuana use. 13 youth (33%) reported both alcohol and marijuana use, and 8 youth (20%) reported use of tobacco, alcohol, and marijuana. Due to small numbers reporting illicit drug use beyond marijuana (3/40, 8%), this measure was not considered further in statistical analyses (Table 1). There was no difference in substance use by race.
3.2 Comparison of Brain Volume Measures in PHIV youth compared to HIV-unexposed and uninfected youth
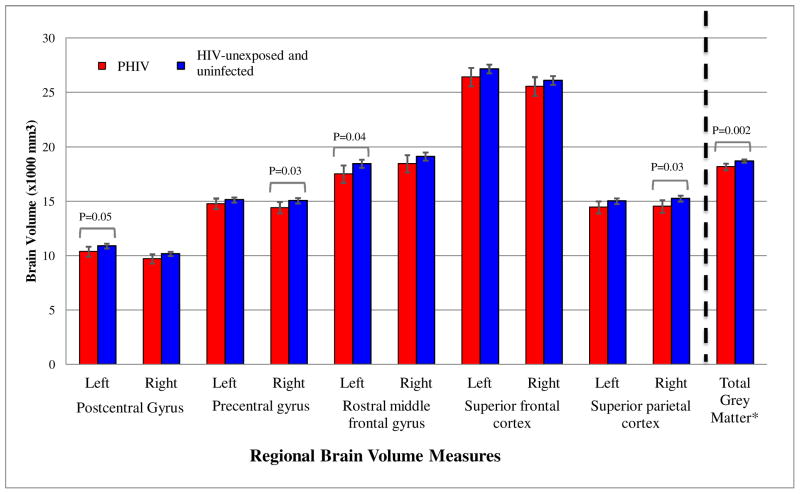
In unadjusted models, compared to typically-developing, HIV-unexposed and uninfected youth, PHIV youth had significantly smaller volumes for all 10 primary ROIs as well as total grey matter, with 7.5% to 15.0% reductions (p < 0.001) (Fig. 1). In adjusted models, volume differences were attenuated but remained significant for left hemisphere (LH) and right hemisphere (RH) postcentral gyrus, right hemisphere (RH) precentral gyrus, LH rostral middle frontal gyrus, RH superior parietal, and for total grey matter volumes, with 2.0 to 5.0% volume reductions (p < .05) (Fig. 1; Fig. 2; Supplemental Table 1). RH postcentral volume and LH superior parietal cortex trended toward significantly smaller volumes (p = 0.06 and p = 0.07, respectively).
Figure 1.
Adjusted Mean Regional Brain Volumes (95% CI) by HIV Infection Status
Adjusted models include sex, race, age at scan, caregiver education, annual household income, and intracranial volume.
* The unit of brain volume for total grey matter is (× 105 mm3).
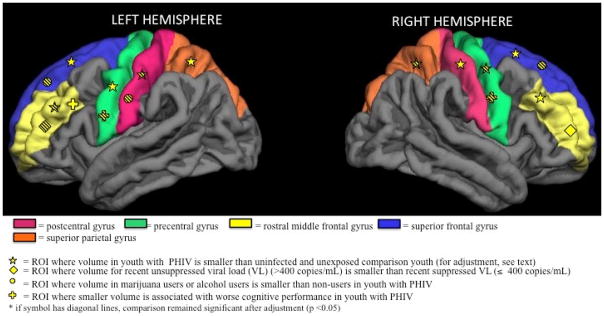
Figure 2.
Schematic of 10 primary regions of interest (ROI) and volume associations visualized using a Freesurfer template brain
3.3 Association of Brain Volume Measures with HIV Disease Severity
Among PHIV youth, log peak VL negatively correlated with volumes of bilateral rostral middle frontal gyrus (Spearman r=−0.39 and −0.43, p=0.01) and superior frontal cortex (r=−0.35 and −0.35, p=0.03), and with total grey matter (r=−0.35, p=0.03) (Fig. 2). Youth with recent unsuppressed VL (>400 copies/mL) had 11% to 14% smaller volumes for these same brain regions (Table 2). Associations attenuated after adjustment for sex and age at neuroimaging (p=0.06, 0.31, 0.11, 0.07, 0.12 respectively). Mean total grey matter volume was 10% smaller for those with recent unsuppressed VL (1575.6 vs. 1751.1 x103 mm3, p=0.06); after adjustment this decrease attenuated to 7.7% (p=0.12). For the other six primary ROIs, all correlations with peak VL were negative (range −0.08 to −0.22), and all mean volumes for those with recent unsuppressed VL were consistently smaller than for those with VL<400 copies/mL, but did not attain statistical significance. In contrast, there were no associations of any primary ROI volume with nadir or recent CD4%, CDC HIV classification, or ongoing viremia (data not shown).
Table 2.
Whole and Regional Brain Volumetric Parameters by Recent Unsuppressed VL in PHIV youth
| Unadjusted Comparison | Adjusted Comparison | |||||||
|---|---|---|---|---|---|---|---|---|
| Most Recent Viral Load (copies/mL) | Most Recent Viral Load (copies/mL) | |||||||
|
|
|
|||||||
| Brain Volume Parameters (x103 mm3) | >400 (N=6) Mean (SD) |
≤400 (N=34) Mean (SD) |
Percent Decrease for>400 to ≤400 | p-value | >400 (N=6) Adjusted Mean | ≤400 (N=34) Adjusted Mean | Percent Decrease for >400 to ≤400 | p-value |
| Postcentral gyrus | ||||||||
| Left hemisphere | 9.67 (0.97) | 9.69 (1.53) | 0.2% | 0.98 | 9.99 | 9.67 | 3.2% | 0.60 |
| Right hemisphere | 8.69 (0.82) | 9.17 (1.52) | 5.2% | 0.45 | 9.00 | 9.16 | 1.6% | 0.80 |
| Precentral gyrus | ||||||||
| Left hemisphere | 13.22 (2.31) | 14.13 (2.25) | 6.4% | 0.37 | 13.49 | 14.14 | 4.6% | 0.45 |
| Right hemisphere | 12.79 (2.24) | 13.84 (2.11) | 7.6% | 0.27 | 13.19 | 13.84 | 4.6% | 0.45 |
| Rostral middle frontal gyrus | ||||||||
| Left hemisphere | 14.69 (2.32) | 17.14 (2.73) | 14.3% | 0.05 | 14.84 | 17.15 | 13.5% | 0.05 |
| Right hemisphere | 15.83 (3.73) | 17.80 (3.05) | 11.1% | 0.17 | 16.43 | 17.78 | 7.6% | 0.31 |
| Superior frontal gyrus | ||||||||
| Left hemisphere | 22.47 (2.53) | 25.64 (3.89) | 12.4% | 0.06 | 22.86 | 25.63 | 10.8% | 0.11 |
| Right hemisphere | 21.55 (2.99) | 24.79 (3.63) | 13.1% | 0.05 | 21.89 | 24.80 | 11.7% | 0.07 |
| Superior parietal cortex | ||||||||
| Left hemisphere | 13.61 (1.80) | 13.53 (2.14) | 0.6% | 0.94 | 13.98 | 13.52 | 3.3% | 0.61 |
| Right hemisphere | 12.28 (2.48) | 13.66 (2.42) | 10.1% | 0.21 | 12.77 | 13.65 | 6.5% | 0.39 |
| Total grey matter volume | 1575.6 (147.9) | 1751.1 (216.2) | 10.0% | 0.06 | 1615.42 | 1750.29 | 7.7% | 0.12 |
Significant differences are bolded.
In evaluating associations of HIV severity with the 74 secondary ROI volumes, no significant findings were identified after applying the Benjamini-Liu approach to control the FDR at a level of 0.10 (Supplemental Table 2) (Benjamini et al., 2001). Even at an uncorrected significance level of p<0.05, only isolated findings were observed for nadir and current CD4% (3–4 ROIs each), while slightly more associations were observed for peak and current VL (7 ROIs each).
3.4 Association of Brain Volume Measures with Substance Use
PHIV youth who reported alcohol or marijuana use were older than those who did not (mean = 18.4 vs. 16.1 and 18.7 vs. 15.9 years, respectively). After adjusting for age at scan and sex, PHIV youth reporting alcohol or marijuana use had significantly smaller LH postcentral gyrus, bilateral superior frontal gyri, and total grey matter volumes, ranging from 9–16% smaller (Table 3; Fig. 2). For other primary ROI volumes, adjusted means were consistently smaller for those who reported alcohol or marijuana use as compared to those who did not (except for LH precentral gyrus volume), but none attained statistical significance (Table 3).
Table 3.
Adjusted Mean Primary Whole and Regional Brain Volume Measures by Self-reported Substance Use among 40 PHIV youth
| Alcohol Use | Marijuana Use | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Adjusted Means | Adjusted Means | |||||||||
| Brain Volumes (x 103 mm3) | Yes (n=14) | No (n= 24) | Adjusted Mean Difference | Percent Decrease | P-value* | Yes (n=14) | No (n=24) | Adjusted Mean Difference | Percent Decrease | P- value* |
| Postcentral gyrus | ||||||||||
| Left hemisphere | 8.77 | 10.02 | −1.25 (−2.11, −0.40) | 12.5% | 0.01 | 8.69 | 10.07 | −1.39 (−2.37, −0.41) | 13.7% | 0.01 |
| Right hemisphere | 8.54 | 9.26 | −0.72 (−1.68, 0.24) | 7.8% | 0.14 | 8.42 | 9.34 | −0.92 (−2.00, 0.16) | 9.9% | 0.09 |
| Precentral gyrus | ||||||||||
| Left hemisphere | 13.98 | 14.15 | −0.17 (−1.59, 1.24) | 1.2% | 0.80 | 14.10 | 14.08 | 0.02(−1.59, 1.64) | −0.1% | 0.98 |
| Right hemisphere | 13.53 | 13.68 | −0.15 (−1.45, 1.15) | 1.1% | 0.82 | 13.48 | 13.72 | −0.24 (−1.72, 1.24) | 1.8% | 0.74 |
| Rostral middle frontal gyrus | ||||||||||
| Left hemisphere | 16.06 | 17.37 | −1.31 (−3.43, 0.80) | 7.5% | 0.22 | 16.26 | 17.26 | −1.00(− −3.45, 1.44) | 5.8% | 0.41 |
| Right hemisphere | 16.75 | 18.13 | −1.39 (−3.72, 0.95) | 7.6% | 0.24 | 17.06 | 17.95 | −0.88 (−3.58, 1.82) | 5.0% | 0.51 |
| Superior frontal gyrus | ||||||||||
| Left hemisphere | 22.93 | 26.31 | −3.39 (−6.11, −0.66) | 12.9% | 0.02 | 22.79 | 26.40 | −3.61 (−6.75, −0.46) | 13.7% | 0.03 |
| Right hemisphere | 22.18 | 25.62 | −3.43 (−5.95, −0.92) | 13.4% | 0.01 | 21.75 | 25.88 | −4.13 (−6.96, −1.30) | 16.0% | 0.01 |
| Superior parietal cortex | ||||||||||
| Left hemisphere | 13.06 | 13.84 | −0.78 (−2.31, 0.75) | 5.6% | 0.31 | 13.16 | 13.79 | −0.63 (−2.38, 1.13) | 4.6% | 0.47 |
| Right hemisphere | 12.75 | 13.86 | −1.11 (−2.76, 0.54) | 8.0% | 0.18 | 12.75 | 13.87 | −1.11(−3.00, 0.78) | 8.1% | 0.24 |
| Total grey matter volume | 1626.3 | 1786.9 | −160.6 (−296.1, −25.1) | 9.0% | 0.02 | 1613.7 | 1794.9 | −181.3 (−335.9, −26.6) | 10.1% | 0.02 |
PHIV = perinatally-acquired HIV
p-values from linear regression models for each volume measure controlling for age at scan and sex. Significant models are bolded.
There was no association of tobacco use with total grey matter or 10 primary ROI volumes in either unadjusted or adjusted models (data not shown). There were no significant findings of associations between substance use and secondary ROI volumes, after controlling for multiple comparisons with FDR of 0.1. A large percentage of these volumes demonstrated negative associations based on an uncorrected p<0.05 (19/74 for alcohol, 17/74 for marijuana) (see Supplemental Table 2).
3.5 Associations of Brain Volumes with Cognitive Functioning
Among the 10 primary ROIs, bilateral precentral gyrus, LH rostral middle frontal gyrus, and total grey matter volumes positively correlated with cognitive proficiency (CPI) (p=0.01 to 0.05), working memory (WMI) (p=0.004 to 0.05), and processing speed (PSI) (p=0.02 to 0.03) indices after adjusting for sex and age at scan (see Table 4 and Fig. 2). Specifically, the strongest associations with CPI, WMI, and PSI were with bilateral precentral gyri volumes (b = 2.68 to 4.18, p=0.01 to 0.03). Associations with secondary ROI volumes were consistently positive, although none attained statistical significance.
Table 4.
Adjusted associations of primary regional brain volume and total grey matter volume with cognitive functioning among 40 PHIV youth
| Cognitive Proficiency Index | Working Memory Index | Processing Speed Index | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Brain Volumes (mm3) | Estimated Coefficient (95% CI) | P-value | Estimated Coefficient (95% CI) | P-value | Estimated Coefficient (95% CI) | P-value |
| Postcentral gyrus | ||||||
| Left hemisphere | 0.29 (−3.77, 4.35) | 0.89 | 0.52 (−3.81, 4.85) | 0.81 | 0.28 (−3.25, 3.82) | 0.87 |
| Right hemisphere | 1.37 (−2.77, 5.52) | 0.51 | 1.08 (−3.35, 5.52) | 0.62 | 1.74 (−1.85, 5.32) | 0.33 |
| Precentral gyrus | ||||||
| Left hemisphere | 3.47 (0.87, 6.06) | 0.01 | 3.53 (0.75, 6.32) | 0.01 | 2.86 (0.58, 5.13) | 0.02 |
| Right hemisphere | 3.71 (1.09, 6.33) | 0.01 | 4.18 (1.42, 6.94) | 0.004 | 2.68 (0.32, 5.04) | 0.03 |
| Rostral middle frontal gyrus | ||||||
| Left hemisphere | 1.90 (0.01, 3.79) | 0.05 | 2.02 (−0.00, 4.03) | 0.05 | 1.46 (−0.21, 3.12) | 0.08 |
| Right hemisphere | 1.44 (−0.35, 3.23) | 0.11 | 1.52 (−0.39, 3.43) | 0.12 | 1.23 (−0.33, 2.79) | 0.12 |
| Superior frontal gyrus | ||||||
| Left hemisphere | 1.03 (−0.36, 2.42) | 0.14 | 1.09 (−0.40, 2.57) | 0.15 | 0.81 (−0.40, 2.03) | 0.18 |
| Right hemisphere | 1.13 (−0.36, 2.61) | 0.13 | 1.25 (−0.33, 2.83) | 0.12 | 0.91 (−0.39, 2.21) | 0.16 |
| Superior parietal cortex | ||||||
| Left hemisphere | 0.57 (−2.16, 3.30) | 0.67 | 1.14 (−1.75, 4.03) | 0.43 | 0.13 (−2.25, 2.51) | 0.91 |
| Right hemisphere | 1.34 (−1.03, 3.72) | 0.26 | 1.42 (−1.11, 3.96) | 0.26 | 1.18 (−0.89, 3.25) | 0.25 |
| Total grey matter volume | 0.03 (0.01, 0.06) | 0.01 | 0.04 (0.01, 0.06) | 0.01 | 0.03 (0.00, 0.05) | 0.02 |
PHIV = youth with perinatally-acquired HIV; LH=left hemisphere; RH=right hemisphere. Each row represents separate model results for each brain volume measure as a predictor of each cognitive outcome, adjusting for sex and age at scan. Significant models are bolded.
3.6 Sensitivity analyses
Adjustment for substance use in models evaluating associations between primary ROI volumes and HIV disease severity as well as cognitive function did not alter findings. Similarly, adjustment for total grey matter volume in models evaluating associations of HIV severity and substance use measures with secondary ROI volumes did not alter findings, with no associations identified based on a FDR level of 0.10. Positive associations observed between primary brain volumes and cognitive function persisted after adjustment for substance use. Bilateral superior frontal volumes positively correlated with CPI, WMI, and PSI after adjustment for substance use.
4. Discussion
The current study is one of the first to examine relationships among PHIV infection, disease severity, and substance use on the brain grey matter and cognitive outcomes in PHIV youth. We found that PHIV youth had reduced total grey matter volume as well as reduced volumes in the rostral middle frontal, postcentral, precentral, and superior parietal gyri, compared to similarly-aged HIV-unexposed and uninfected youth. These patterns persisted after adjusting for sex, age at scan, race, caregiver education attainment, annual household income, and intracranial volume. We found that increased peak VL correlated with reduced total grey matter, bilateral rostral middle and superior frontal volumes. Youth with recent unsuppressed VL had smaller volumes for these regions, although associations attenuated after adjustment for sex, age at neuroimaging, and age at peak VL. Moreover, PHIV youth who reported alcohol and marijuana use showed further reduction in grey matter volumes compared with PHIV youth who reported no use. Finally, smaller volumes of primary ROIs and total grey matter correlated with lower performance on standardized measures of working memory, processing speed, and cognitive proficiency. Patterns of smaller volumes appeared to be symmetric bilaterally for these tested associations: 1) PHIV youth vs. typically-developing, HIV-unexposed and uninfected youth 2) higher peak VL and 3) recent unsuppressed VL for regions that were significantly different or trended towards significance. In addition to these selective losses associated with PHIV, higher VL, substance use, and poorer cognitive performance were also associated with smaller total grey matter volumes. Total decreased grey matter volume may be due to abnormal development in primary ROIs as well as subtler abnormalities in the rest of the brain that did not reach statistical significance after multiple comparisons with our sample size.
Prior neuroimaging studies in adults with HIV have reported that HIV severity was related to reduced global and regional brain volumes in both untreated patients and those treated with cART (Ances et al., 2012; Archibald et al., 2004; Becker et al., 2012, 2011; Cohen et al., 2010b; Heindel et al., 1994; Jernigan et al., 1993; Kallianpur et al., 2013; Stout, 1998; Thompson et al., 2005; Thompson and Jahanshad, 2015). Findings on cortical changes varied but overlapped between studies. Generalized cerebral grey matter atrophy has been shown to correlate with worse disease (Cohen et al., 2010b; Kallianpur et al., 2013). Some studies have identified frontal and parietal lobe grey matter volume loss (Cohen et al., 2010b; Thompson et al., 2005); others have shown volume loss in temporal and limbic regions (Jernigan et al., 1993). Cortical thinning has been reported in postcentral and precentral gyri with degree of atrophy correlating with cognitive impairment in HIV+ adults (Thompson et al., 2005). Our study of PHIV youth identifies reduced volume in the prefrontal cortex, a brain region not specifically reported in studies of adults with horizontally-transmitted HIV. Importantly, prefrontal regions undergo tremendous development during adolescence (Giedd, 2004; Giedd et al., 1999; Paus, 2005; Sowell et al., 2004, 1999). Differences in findings between our study and prior studies could be due in part to differing patient populations (adolescents with PHIV vs. adults with horizontally-transmitted HIV) and different environmental exposures (Thompson et al., 2005).
Although brain volumes in adults with horizontally-transmitted HIV have been studied, the long-term effects of HIV infection treated with cART on developing brains of adolescents have not been extensively examined (Cohen et al., 2015; Jernigan et al., 2011; Sarma et al., 2014; Thompson et al., 2005). Importantly, PHIV youth are exposed to HIV and cART during critical developmental periods of the brain. Other studies of our PHIV cohort have found overall and regional white matter microstructure and functional connectivity as well as subcortical deformation differences in those with worse HIV disease severity and/or compared to controls (Herting et al., 2015; Lewis-de los Angeles et al., 2016; Uban et al., 2015). In a recent study on another cohort of PHIV youth, Sarma et al. (Sarma et al., 2014) found that compared to age-matched controls, adolescents with PHIV had reduced white matter volume, but increased grey matter volumes in superior frontal and temporal gyri. Differences in findings between our studies may be due to the smaller sample size, more severe HIV disease (recent HIV viral load) and later onset of cART in the PHIV youth in the Sarma et al study (Sarma et al., 2014). The authors suggest that larger grey matter volume may be due to inflammation. Another recent study on a slightly younger cohort of PHIV youth by Cohen et al. (Cohen et al., 2015) found that youth had lower overall grey matter volumes, but did not study regional differences compared to controls. We also found lower total grey matter volume in our cohort of PHIV, a geographically-different and older clinical population than in the Cohen et al. study. Importantly, our study also considered additional effects of substance use on brain structure as well as an examination of relationships between regional brain differences and cognition.
Substance use in adolescence may lead to aberrant development during a vulnerable period of significant brain maturation and/or or pre-existing structural brain differences may lead to increased substance use (Jacobus et al., 2015; Squeglia and Gray, 2016). Previous studies have demonstrated that adolescents who are more likely to drink alcohol have thinner frontal cortices (Brumback et al., 2016; Silveri et al., 2016) and distributed regions in the frontal, parietal, temporal, and occipital lobes (in prediction models that including neuroimaging data (Silveri et al., 2016; Squeglia et al., 2016). A larger study of PHIV youth in the PHACS AMP study found that substance use among PHIV youth may lead to greater risky behavior (Alperen et al., 2014). In our neuroimaging cohort, we found that substance use among PHIV youth may exacerbate total and regional grey matter reductions. Notably, PHIV youth who reported substance use had reductions in bilateral frontal gyri, regions involved in executive functioning, logical thinking, goal setting, planning, and self-control, which may be associated with decision-making and risky behaviors (Fowler et al., 2007; Steinberg, 2007). While these findings are consistent with some studies on brain volume reductions among HIV-uninfected adolescents who use alcohol or marijuana, they also differ from others identifying increased volumes with marijuana use (Jacobus et al., 2015; Lopez-Larson et al., 2011). Differing patterns found in our study may be due to dose-related effects; we examined ever-use, not frequency or duration of use. Moreover, concurrent marijuana and alcohol could have complex and perhaps interacting effects on brain structure (Price et al., 2015; Squeglia et al., 2014, 2009; Subramaniam et al., 2016).
An additional consideration for interpreting our findings is that socioeconomic status may affect brain development. Previous studies have demonstrated that altered grey matter measures such as volume, cortical thickness, and surface area in distributed cortical and subcortical regions are associated with socioeconomic status (Hair et al., 2015; Jednoróg et al., 2012; Noble et al., 2015; Piccolo et al., 2016). Despite differences in socioeconomic status between our HIV-unexposed and uninfected youth and PHIV youth cohorts, regional and total grey matter volume differences persisted after controlling for socioeconomic status. Unlike some previous studies, socioeconomic status alone was not associated with regional brain volume differences.
There are some potential limitations to consider when interpreting our results. One limitation is the difficulty of teasing apart individual effects of PHIV, cART, and substance use on brain volumes. Although we attempted to address this limitation by including a measure of persistence of unsuppressed VL, we observed no associations with this measure; other cumulative measures of HIV severity may add additional insight on HIV effects on the brain. Another limitation is the availability of only a single neuroimaging study during adolescence, making it more difficult to isolate effects of HIV, treatment, and preexisting pathology. A further limitation is that we only report cognitive and substance use findings in the PHIV youth and did not have comparable measures for the typically-developing control cohort. However, it should be noted that the relationship between brain volume changes in the general population is relatively well-researched and our volume findings in many regions in PHIV youth overlap with previous findings on cognition and substance use-brain volume findings in typically-developing youth (Crowell et al., 2014; Hazra et al., 2010; Koekkoek et al., 2008; Linn et al., 2015; Raskino et al., 1999).
Finally, while our study only evaluated brain volumes with respect to HIV infection status and past HIV disease severity measures such as peak HIV RNA load and nadir CD4%, examining measures of inflammation (e.g. C-reactive protein (CRP) and interleukins) with respect to brain volumes may deepen our understanding of structural brain changes in youth with PHIV. Other studies have found that cognitive performance is related to a variety of cytokines (Correia et al., 2013) and that certain cytokine markers, especially IL-6 and IL-16, significantly relate to brain volumes (Gongvatana et al., 2014) in adult HIV patients. Proton magnetic resonance spectroscopy studies (MRS) have found that in adult HIV patients, elevated neurofilament light chain in the cerebrospinal fluid correlated with MRS abnormalities in the anterior cingulate, frontal white matter, and parietal grey matter (Peluso et al., 2013). In youth with PHIV, the relationship of brain volumes to inflammatory markers has not yet been studied, though studies in youth with PHIV have found that neopterin and sCD14 levels are elevated (Blokhuis et al., 2016; Sainz et al., 2014; Syed et al., 2013) and that aggregate measures of fibrinogen, CRP, and IL-6 are associated with decreased processing speed in youth with PHIV and in uninfected youth with perinatal HIV exposure (Kapetanovic et al., 2014). Future studies should aim to assess effects of PHIV, cART, and substance use with longitudinal neuroimaging and include diverse measures of HIV disease severity and inflammation, which may provide more information on when the brain is most vulnerable to HIV and substance use, as well as when treatment is most effective.
With increased longevity due to cART, preservation of neurological function becomes important for quality of life and adequate functional outcomes. Our study provides preliminary insight into the effects of HIV and substance use on the brain in PHIV youth as well as potential clinical biomarkers for evaluating HIV-related brain atrophy and cART efficacy in the brain. PHIV youth may demonstrate distinct and particular vulnerability to HIV compared to adults due to exposure to HIV and antiretroviral treatment during critical developmental periods.
Supplementary Material
Supplemental Table 1: Brain Volumetric Parameters for PHIV+ vs. PING Controls
Supplemental Table 2. Uncorrected Significant Associations of HIV Disease Severity Measures and Self-reported Substance Use with 74 Secondary Brain Volume Measures*
Highlights.
Youth with PHIV have smaller grey matter volumes compared to HIV-unexposed youth.
In PHIV youth, worse HIV disease severity correlated with smaller brain volumes.
In PHIV youth, alcohol and marijuana use were associated with smaller brain volumes.
In PHIV youth, reduction in brain volumes was associated with poorer cognitive performance.
Acknowledgments
Funding sources: National Institute of Health (NIH)
We thank the children and families for their participation in the Pediatric HIV/AIDS Cohort Study (PHACS), and the individuals and institutions involved in the conduct of PHACS. PHACS was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development. PHACS was co-funded by NIDA, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard T. H. Chan School of Public Health (HD052102) (Principal Investigator: George Seage; Project Director: Julie Alperen) and the Tulane University School of Medicine (HD052104) (Principal Investigator: Russell Van Dyke; Co-Principal Investigator: Kenneth Rich; Project Director: Patrick Davis). C.P.L.D.L.A was additionally supported by the Northwestern University Training Program in the Neuroscience of Human Cognition and the Northwestern University Medical Scientist Training Program. Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (PI: Julie Davidson).
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) with co-funding by the National Institute on Drug Abuse (NIDA), the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart, Lung, and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism through cooperative agreements with the Harvard T. H. Chan School of Public Health (HD052102) and the Tulane University School of Medicine (HD052104). This work was also supported by NIDA (RC2 DA029475) through the Pediatrics Imaging-Genomics Data Resource (PING) study. C.P.L-dlA. was additionally supported by the Northwestern University Training Program in the Neuroscience of Human Cognition (NIH T32 NS047987), the Northwestern University Medical Scientist Training Program (NIH T32 GM008152), and the Dr. John N. Nicholson Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alperen J, Brummel S, Tassiopoulos K, Mellins CA, Kacanek D, Smith R, Seage GR, Moscicki A-B. Prevalence of and risk factors for substance use among perinatally human immunodeficiency virus–infected and perinatally exposed but uninfected youth. J Adolesc Heal. 2014;54:341–349. doi: 10.1016/j.jadohealth.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Ortega M, Vaida F, Heaps J, Paul R. Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr. 2012;59:469–477. doi: 10.1097/QAI.0b013e318249db17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziata P. Blood-brain barrier changes during invasion of the central nervous system by HIV-1: Old and new insights into the mechanism. J Neurol. 2003;250:901–906. doi: 10.1007/s00415-003-1159-0. [DOI] [PubMed] [Google Scholar]
- Archibald SL, Masliah E, Fennema-Notestine C, Marcotte TD, Ellis RJ, McCutchan JA, Heaton RK, Grant I, Mallory M, Miller A, Jernigan TL. Correlation of in vivo neuroimaging abnormalities with postmortem human immunodeficiency virus encephalitis and dendritic loss. Arch Neurol. 2004;61:369–376. doi: 10.1001/archneur.61.3.369. [DOI] [PubMed] [Google Scholar]
- Armstrong FD, Seidel JF, Swales TP. Pediatric HIV infection: a neuropsychological and educational challenge. J Learn Disabil. 1993;26:92–103. doi: 10.1177/002221949302600202. [DOI] [PubMed] [Google Scholar]
- Battistella G, Fornari E, Annoni J-M, Chtioui H, Dao K, Fabritius M, Favrat B, Mall J-F, Maeder P, Giroud C. Long-Term Effects of Cannabis on Brain Structure. Neuropsychopharmacology. 2014:2041–2048. doi: 10.1038/npp.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Maruca V, Kingsley La, Sanders JM, Alger JR, Barker PB, Goodkin K, Martin E, Miller EN, Ragin A, Sacktor N, Selnes O. Factors affecting brain structure in men with HIV disease in the post-HAART era. Neuroradiology. 2012;54:113–121. doi: 10.1007/s00234-011-0854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Sanders J, Madsen SK, Ragin A, Kingsley L, Maruca V, Cohen B, Goodkin K, Martin E, Miller EN, Sacktor N, Alger JR, Barker PB, Saharan P, Carmichael OT, Thompson PM. Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav. 2011;5:77–85. doi: 10.1007/s11682-011-9113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–284. doi: 10.1016/S0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- Blokhuis C, Kootstra NA, Caan MWA, Pajkrt D. Neurodevelopmental delay in pediatric HIV/AIDS: Current perspectives. Neurobehav HIV Med. 2016;7:1–13. doi: 10.2147/NBHIV.S68954. [DOI] [Google Scholar]
- Brady MT, Oleske JM, Williams PL, Elgie C, Mofenson LM, Dankner WM, Van Dyke RB. Declines in Mortality Rates and Changes in Causes of Death in HIV-1-Infected Children During the HAART Era. JAIDS J Acquir Immune Defic Syndr. 2010;53:86–94. doi: 10.1097/QAI.0b013e3181b9869f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumback T, Worley M, Nguyen-Louie TT, Squeglia LM, Jacobus J, Tapert SF. Neural predictors of alcohol use and psychopathology symptoms in adolescents. Dev Psychopathol. 2016;28:1209–1216. doi: 10.1017/S0954579416000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill MJ, Deeks SG, Margolis DM, Siliciano RF, Swanstrom R. HIV reservoirs: what, where and how to target them. Nat Rev Microbiol. 2015:1–6. doi: 10.1038/nrmicro.2015.5. [DOI] [PubMed] [Google Scholar]
- Churchwell JC, Lopez-Larson M, Yurgelun-Todd DA. Altered Frontal Cortical Volume and Decision Making in Adolescent Cannabis Users. Front Psychol. 2010;1:1–8. doi: 10.3389/fpsyg.2010.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R, Harezlak J, Gongvatana A, Buchthal S, Schifitto G, Clark U, Paul R, Taylor M, Thompson P, Tate D, Alger J, Brown M, Zhong J, Campbell T, Singer E, Daar E, McMahon D, Tso Y, Yiannoutsos CT, Navia B. Cerebral metabolite abnormalities in human immunodeficiency virus are associated with cortical and subcortical volumes. J Neurovirol. 2010a;16:435–444. doi: 10.3109/13550284.2010.520817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R, Harezlak J, Schifitto G, Hana G, Clark U, Gongvatana A, Paul R, Taylor M, Thompson P, Alger J, Brown M, Zhong J, Campbell T, Singer E, Daar E, McMahon D, Tso Y, Yiannoutsos CT, Navia B. Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J Neurovirol. 2010b;16:25–32. doi: 10.3109/13550280903552420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Caan M, Mutsaerts H, Scherpbier H, Kuijpers T, Reiss P, Majoie C, Pajkrt D. Cerebral injury in perinatally HIV-infected children compared to matched healthy controls. Neurology. 2015 Nov;:1–10. doi: 10.1212/WNL.0000000000002209. [DOI] [PubMed] [Google Scholar]
- Cohen S, ter Stege JA, Geurtsen GJ, Scherpbier HJ, Kuijpers TW, Reiss P, Schmand B, Pajkrt D. Poorer Cognitive Performance in Perinatally HIV-Infected Children Versus Healthy Socioeconomically Matched Controls. Clin Infect Dis. 2014:1–20. doi: 10.1093/cid/ciu1144. [DOI] [PubMed] [Google Scholar]
- Correia S, Cohen R, Gongvatana A, Ross S, Olchowski J, Devlin K, Tashima K, Navia B, Delamonte S. Relationship of plasma cytokines and clinical biomarkers to memory performance in HIV. J Neuroimmunol. 2013;265:117–123. doi: 10.1016/j.jneuroim.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell CS, Malee KM, Yogev R, Muller WJ. Neurologic disease in HIV-infected children and the impact of combination antiretroviral therapy. Rev Med Virol. 2014;24:57-316–331. doi: 10.1002/rmv. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Ene L, Duiculescu D, Ruta S. How much do antiretroviral drugs penetrate into the central nervous system? J Med Life. 2011;4:432–439. [PMC free article] [PubMed] [Google Scholar]
- Ene L, Franklin DR, Burlacu R, Luca AE, Blaglosov AG, Ellis RJ, Alexander TJ, Umlauf A, Grant I, Duiculescu DC, Achim CL, Marcotte TD. Neurocognitive functioning in a Romanian cohort of young adults with parenterally-acquired HIV-infection during childhood. J Neurovirol. 2014;20:496–504. doi: 10.1007/s13365-014-0275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Mueller SC. The adolescent brain: Insights from functional neuroimaging research. Dev Neurobiol. 2008;68:729–743. doi: 10.1002/dneu.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Kassed CA, Chang L. Imaging the addicted human brain. Sci Pract Perspect. 2007;3:4–16. doi: 10.1151/spp07324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S, Levitt P, Nelson CA. How the Timing and Quality of Early Experiences Influence the Development of Brain Architecture. NIH Public Access. 2010;81:28–40. doi: 10.1111/j.1467-8624.2009.01380.x.How. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural Magnetic Resonance Imaging of the Adolescent Brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. \r1021/1/77 [pii] [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gona P, Van Dyke RB, Williams PL, Dankner WM, Chernoff MC, Nachman Sa, Seage GR. Incidence of opportunistic and other infections in HIV-infected children in the HAART era. JAMA. 2006;296:292–300. doi: 10.1001/jama.296.3.292. [DOI] [PubMed] [Google Scholar]
- Gongvatana A, Correia S, Dunsiger S, Gauthier L, Devlin KN, Ross S, Navia B, Tashima KT, DeLaMonte S, Cohen RA. Plasma Cytokine Levels are Related to Brain Volumes in HIV-infected Individuals. J Neuroimmune Pharmacol. 2014;9:740–750. doi: 10.1007/s11481-014-9567-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Scarano F, Martín-García J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Hair NL, Hanson JL, Wolfe BL, Pollak SD. Association of Child Poverty, Brain Development, and Academic Achievement. JAMA Pediatr. 2015;53706:1–8. doi: 10.1001/jamapediatrics.2015.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazra R, Siberry GK, Mofenson LM. Growing up with HIV: children, adolescents, and young adults with perinatally acquired HIV infection. Annu Rev Med. 2010;61:169–85. doi: 10.1146/annurev.med.050108.151127. [DOI] [PubMed] [Google Scholar]
- Heindel WC, Jernigan TL, Archibald SL, Achim CL, Masliah E, Wiley CA. The Relationship of Quantitative Brain Magnetic Resonance Imaging Measures to Neuropathologic Indexes of Human Immunodeficiency Virus Infection. Arch Neurol. 1994;51:1129–1135. doi: 10.1001/archneur.1994.00540230067015. [DOI] [PubMed] [Google Scholar]
- Herting MM, Uban KA, Williams PL, Gautam P, Huo Y, Malee K, Yogev R, Csernansky J, Wang L, Nichols S, Van Dyke R, Sowell ER. Default mode connectivity in youth with perinatally acquired HIV. Medicine (Baltimore) 2015;94:e1417. doi: 10.1097/MD.0000000000001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare J, Ransford GL, Phillips N, Amos T, Donald K, Stein DJ. Systematic review of neuroimaging studies in vertically transmitted HIV positive children and adolescents. Metab Brain Dis. 2014;29:221–229. doi: 10.1007/s11011-013-9456-5. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Neurological , Psychiatric , and Developmental Disorders: Meeting the Challenge in the Developing Workd. Program. 2001 doi: 10.1016/S0035-9203(02)90116-1. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Meruelo AD, Castro N, Brumback T, Giedd JN, Tapert SF. Cortical thickness in adolescent marijuana and alcohol users: A three-year prospective study from adolescence to young adulthood. Dev Cogn Neurosci. 2015 doi: 10.1016/j.dcn.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Tapert SF. Effects of cannabis on the adolescent brain. Curr Pharm Des. 2014;20:2186–93. doi: 10.2174/13816128113199990426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jednoróg K, Altarelli I, Monzalvo K, Fluss J, Dubois J, Billard C, Dehaene-Lambertz G, Ramus F. The influence of socioeconomic status on children’s brain structure. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald S, Hesselink JR, Atkinson JH, Velin Ra, McCutchan Ja, Chandler J, Grant I. Magnetic resonance imaging morphometric analysis of cerebral volume loss in human immunodeficiency virus infection. The HNRC Group. Arch Neurol. 1993;50:250–255. doi: 10.1001/archneur.1993.00540030016007. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Taylor MJ, Theilmann RJ, Julaton MD, Notestine RJ, Wolfson T, Letendre SL, Ellis RJ, Heaton RK, Gamst AC, Franklin DR, Clifford DB, Collier AC, Gelman BB, Marra C, McArthur JC, McCutchan JA, Morgello S, Simpson DM, Grant I. Clinical factors related to brain structure in HIV: the CHARTER study. J Neurovirol. 2011;17:248–257. doi: 10.1007/s13365-011-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Brown TT, Hagler DJ, Akshoomoff N, Bartsch H, Newman E, Thompson WK, Bloss CS, Murray SS, Schork N, Kennedy DN, Kuperman JM, McCabe C, Chung Y, Libiger O, Maddox M, Casey BJ, Chang L, Ernst TM, Frazier Ja, Gruen JR, Sowell ER, Kenet T, Kaufmann WE, Mostofsky S, Amaral DG, Dale AM. The Pediatric Imaging, Neurocognition, and Genetics (PING) Data Repository. Neuroimage. 2015;124:1149–1154. doi: 10.1016/j.neuroimage.2015.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallianpur KJ, Shikuma C, Kirk GR, Shiramizu B, Valcour V, Chow D, Souza S, Nakamoto B, Sailasuta N. Peripheral blood HIV DNA is associated with atrophy of cerebellar and subcortical gray matter. Neurology. 2013;80:1792–9. doi: 10.1212/WNL.0b013e318291903f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapetanovic S, Griner R, Zeldow B, Nichols S, Leister E, Gelbard HA, Miller TL, Hazra R, Mendez AJ, Malee K, Kammerer B, Williams PL. Biomarkers and neurodevelopment in perinatally HIV-infected or exposed youth. AIDS. 2014;28:355–364. doi: 10.1097/QAD.0000000000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koekkoek S, de Sonneville LMJ, Wolfs TFW, Licht R, Geelen SPM. Neurocognitive function profile in HIV-infected school-age children. Eur J Paediatr Neurol. 2008;12:290–297. doi: 10.1016/j.ejpn.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Lewis-de los Angeles CP, Alpert KI, Williams PL, Malee K, Huo Y, Csernansky JG, Yogev R, Van Dyke RB, Sowell ER, Wang L. Deformed subcortical structures are related to past HIV disease severity in youth with perinatally acquired HIV infection. J Pediatric Infect Dis Soc. 2016;5:S6–S14. doi: 10.1093/jpids/piw051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn K, Fay A, Meddles K, Isbell S, Lin PN, Thair C, Heaps J, Paul R, Mar SS. HIV-Related Cognitive Impairment of Orphans in Myanmar With Vertically Transmitted HIV Taking Antiretroviral Therapy. Pediatr Neurol. 2015 doi: 10.1016/j.pediatrneurol.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Lopez-Larson MP, Bogorodzki P, Rogowska J, McGlade E, King JB, Terry J, Yurgelun-Todd D. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behav Brain Res. 2011;220:164–172. doi: 10.1016/j.bbr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malee KM, Smith RA, Mellins CA. Brain and Cognitive Development Among U.S. Youth With Perinatally Acquired Human Immunodeficiency Virus Infection. J Pediatr Infect Dis Soc. 2016;5:S1–S5. doi: 10.1093/jpids/piw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SC, Wolters PL, Toledo-Tamula MA, Zeichner SL, Hazra R, Civitello L. Cognitive Functioning in School-Aged Children With Vertically Acquired HIV Infection Being Treated With Highly Active Antiretroviral Therapy (HAART) Dev Neuropsychol. 2006;30:633–657. doi: 10.1207/s15326942dn3002_1. [DOI] [PubMed] [Google Scholar]
- Mellins Ca, Tassiopoulos K, Malee K, Moscicki AB, Patton D, Smith R, Usitalo A, Allison SM, Van Dyke R, Seage GR. Behavioral health risks in perinatally HIV-exposed youth: co-occurrence of sexual and drug use behavior, mental health problems, and nonadherence to antiretroviral treatment. AIDS Patient Care STDS. 2011;25:413–22. doi: 10.1089/apc.2011.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musielak KA, Fine JG. An updated systematic review of neuroimaging studies of children and adolescents with perinatally acquired HIV. J Pediatr Neuropsychol. 2015 doi: 10.1007/s40817-015-0009-1. [DOI] [Google Scholar]
- Nesbit CE, Schwartz SA. In Vitro and Animal Models of Human Immunode ciency Virus Infection of the Central Nervous System. Society. 2002;9:515–524. doi: 10.1128/CDLI.9.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols SL, Chernoff MC, Malee KM, Sirois PA, Woods SP, Williams PL, Yildirim C, Delis D, Kammerer B. Executive Functioning in Children and Adolescents With Perinatal HIV Infection and Perinatal HIV Exposure. J Pediatr Infect Dis Soc. 2016;5:S15–S23. doi: 10.1093/jpids/piw049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, Akshoomoff N, Amaral DG, Bloss CS, Libiger O, Schork NJ, Murray SS, Casey BJ, Chang L, Ernst TM, Frazier JA, Gruen JR, Kennedy DN, Van Zijl P, Mostofsky S, Kaufmann WE, Kenet T, Dale AM, Jernigan TL, Sowell ER. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18:773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozyce ML, Lee SS, Wiznia A, Nachman S, Mofenson LM, Smith ME, Yogev R, McIntosh K, Stanley K, Pelton S. A behavioral and cognitive profile of clinically stable HIV-infected children. Pediatrics. 2006;117:763–770. doi: 10.1542/peds.2005-0451. [DOI] [PubMed] [Google Scholar]
- Organization WH. WHO Case Defenition of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV-Related Disease in Adults and Children 2007 [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Peluso MJ, Meyerhoff DJ, Price RW, Peterson J, Lee E, Young AC, Walter R, Fuchs D, Brew BJ, Cinque P, Robertson K, Hagberg L, Zetterberg H, Gisslén M, Spudich S. Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. J Infect Dis. 2013;207:1703–1712. doi: 10.1093/infdis/jit088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng P, Wang Z, Jiang T, Chu S, Wang S, Xiao D. Brain-volume changes in young and middle-aged smokers: a DARTEL-based voxel-based morphometry study. Clin Respir J. 2015 doi: 10.1111/crj.12393. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- Piccolo LR, Merz EC, He X, Sowell ER, Noble KG. Age-Related Differences in Cortical Thickness Vary by Socioeconomic Status. PLoS One. 2016;11:e0162511. doi: 10.1371/journal.pone.0162511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JS, McQueeny T, Shollenbarger S, Browning EL, Wieser J, Lisdahl KM. Effects of marijuana use on prefrontal and parietal volumes and cognition in emerging adults. Psychopharmacology (Berl) 2015;232:2939–2950. doi: 10.1007/s00213-015-3931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskino C, Pearson Da, Baker CJ, Lifschitz MH, O’Donnell K, Mintz M, Nozyce M, Brouwers P, McKinney RE, Jimenez E, Englund Ja. Neurologic, Neurocognitive, and Brain Growth Outcomes in Human Immunodeficiency Virus-Infected Children Receiving Different Nucleoside Antiretroviral Regimens. Pediatrics. 1999;104:e32–e32. doi: 10.1542/peds.104.3.e32. [DOI] [PubMed] [Google Scholar]
- Redmond SM, Yao T-J, Russell JS, Rice ML, Hoffman HJ, Siberry GK, Frederick T, Purswani M, Williams PL. Longitudinal Evaluation of Language Impairment in Youth With Perinatally Acquired Human Immunodeficiency Virus (HIV) and Youth With Perinatal HIV Exposure. J Pediatr Infect Dis Soc. 2016;5:S33–S40. doi: 10.1093/jpids/piw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainz T, Diaz L, Navarro ML, Rojo P, Blázquez D, Ramos JT, de José MI, Álvarez-Fuente M, Serrano-Villar S, Mellado MJ, Muñoz-Fernández MA. Cardiovascular biomarkers in vertically HIV-infected children without metabolic abnormalities. Atherosclerosis. 2014;233:410–414. doi: 10.1016/j.atherosclerosis.2014.01.025. [DOI] [PubMed] [Google Scholar]
- Sarma MK, Nagarajan R, Keller Ma, Kumar R, Nielsen-Saines K, Michalik DE, Deville J, Church Ja, Thomas MA. Regional brain gray and white matter changes in perinatally HIV-infected adolescents. NeuroImage Clin. 2014;4:29–34. doi: 10.1016/j.nicl.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr L, Croome N, Parra Castaneda K, Bradshaw K, Herrero Romero R. Developmental challenges in HIV infected children—An updated systematic review. Child Youth Serv Rev. 2014;45:74–89. doi: 10.1016/j.childyouth.2014.03.040. [DOI] [Google Scholar]
- Silveri MM, Dager AD, Cohen-Gilbert JE, Sneider JT. Neurobiological signatures associated with alcohol and drug use in the human adolescent brain. Neurosci Biobehav Rev. 2016;70:244–259. doi: 10.1016/j.neubiorev.2016.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois PA, Chernoff MC, Malee KM, Garvie PA, Harris LL, Williams PL, Woods SP, Nozyce ML, Kammerer BL, Yildirim C, Nichols SL. Associations of Memory and Executive Functioning With Academic and Adaptive Functioning Among Youth With Perinatal HIV Exposure and/or Infection. J Pediatr Infect Dis Soc. 2016;5:S24–S32. doi: 10.1093/jpids/piw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Chernoff M, Williams PL, Malee KM, Sirois Pa, Kammerer B, Wilkins M, Nichols S, Mellins C, Usitalo A, Garvie P, Rutstein R. Impact of HIV severity on cognitive and adaptive functioning during childhood and adolescence. Pediatr Infect Dis J. 2012;31:592–8. doi: 10.1097/INF.0b013e318253844b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Wilkins M. Perinatally acquired HIV infection: Long-term neuropsychological consequences and challenges ahead. Child Neuropsychol. 2015;21:234–268. doi: 10.1080/09297049.2014.898744. [DOI] [PubMed] [Google Scholar]
- Sohn AH, Hazra R. The changing epidemiology of the global paediatric HIV epidemic: keeping track of perinatally HIV-infected adolescents. J Int AIDS Soc. 2013;16:18555. doi: 10.7448/ias.16.1.18555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga aW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping Changes in the Human Cortex throughout the Span of Life. Neurosci. 2004;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Squeglia L, Ball T, Jacobus J, Brumback T, McKenna B, Nguyen-Louie T, Sorg S, Paulus M, Tapert S. Neural Predictors of Initiating Alcohol Use during Adolescence. Am J Psychiatry. 2016 doi: 10.1176/appi.ajp.2016.15121587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Gray KM. Alcohol and Drug Use and the Developing Brain. Curr Psychiatry Rep. 2016:18. doi: 10.1007/s11920-016-0689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The Influence of Substance Use on Adolescent Brain Development. Clin EEG Neurosci. 2009;40:31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Rinker Da, Bartsch H, Castro N, Chung Y, Dale AM, Jernigan TL, Tapert SF. Brain volume reductions in adolescent heavy drinkers. Dev Cogn Neurosci. 2014;9:117–125. doi: 10.1016/j.dcn.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Risk Taking in Adolescence. Curr Dir Psychol Sci. 2007;16:55–59. doi: 10.1111/j.1467-8721.2007.00475.x. [DOI] [Google Scholar]
- Stout JC. Progressive Cerebral Volume Loss in Human Immunodeficiency Virus Infection: A Longitudinal Volumetric Magnetic Resonance Imaging Study. Arch Neurol. 1998;55:161–168. doi: 10.1001/archneur.55.2.161. [DOI] [PubMed] [Google Scholar]
- Subramaniam P, McGlade E, Yurgelun-Todd D. Comorbid Cannabis and Tobacco Use in Adolescents and Adults. Curr Addict Reports. 2016:182–188. doi: 10.1007/s40429-016-0101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. State Estimates of Substance Use and Mental Disorders from the 2008–2009. 2011. National Surveys on Drug Use and Health NSDUH Series H-40,HHS Publication No. (SMA) 11–464. [Google Scholar]
- Syed S, Balluz R, Kabagambe E, Meyer Wa, Lukas S, Wilson C, Kapogiannis BG, Nachman S, Sleasman J. Assessment of biomarkers of cardiovascular risk among HIV-1 infected adolescents: role of soluble Vascular Cell Adhesion Molecule (sVCAM) as an early indicator of endothelial inflammation. AIDS Res Hum Retroviruses. 2013;29:493–500. doi: 10.1089/AID.2012.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KA, Cherry CL, Bell JE, McLean CA. Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals. Am J Pathol. 2011;179:1623–1629. doi: 10.1016/j.ajpath.2011.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Dutton Ra, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci U S A. 2005;102:15647–15652. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Jahanshad N. Novel Neuroimaging Methods to Understand How HIV Affects the Brain. Curr HIV/AIDS Rep. 2015;12:289–298. doi: 10.1007/s11904-015-0268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney AL, Nelson Ca. Brain Development and the Role of Experience in the Early Years. Zero Three. 2009;30:9–13. [PMC free article] [PubMed] [Google Scholar]
- Uban KA, Herting MM, Williams PL, Ajmera T, Gautam P, Huo Y, Malee KM, Yogev R, Csernansky JG, Wang L, Nichols SL, Sowell ER. White matter microstructure among youth with perinatally acquired HIV is associated with disease severity. AIDS. 2015;29:1035–1044. doi: 10.1097/QAD.0000000000000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. AIDS by the numbers. 2015:1–11. Jc2571/1/E. doi:JC2571/1/E. [Google Scholar]
- UNAIDS. UNAIDS: The gap report. 2014. [Google Scholar]
- Weiss LG, Saklofski DH, Schwartz DM, Prifitera A, Courville T. Advanced clinical interpretation of the WISC-IV index scores. In: Weiss LG, Saklofske A, Prifitera A, Holdnac J, editors. WISC-IV Advanced Clinical Interpretation. Academic Press; New York: 2006. pp. 140–176. [Google Scholar]
- Williams PL, Leister E, Chernoff M, Nachman S, Morse E, Di Poalo V, Gadow KD. Substance use and its association with psychiatric symptoms in perinatally HIV-infected and HIV-affected adolescents. AIDS Behav. 2010;14:1072–82. doi: 10.1007/s10461-010-9782-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakolev P, Lecours A. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life. Blackwell Scientific; Boston: 1967. pp. 3–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Brain Volumetric Parameters for PHIV+ vs. PING Controls
Supplemental Table 2. Uncorrected Significant Associations of HIV Disease Severity Measures and Self-reported Substance Use with 74 Secondary Brain Volume Measures*