Abstract
Objective
Implant based reconstruction rates have risen among radiation treated breast cancer patients in the United States. The aim of this study is to assess the morbidity associated with various breast reconstruction techniques in radiated patients.
Methods
From the MarketScan Commercial Claims and Encounters database, we selected breast cancer patients who had undergone mastectomy, radiation and breast reconstruction from 2009 to 2012. We obtained demographic and clinical treatment data including data on the timing of radiation relative to breast reconstruction. We recorded complications and failures after implant and autologous reconstruction. We developed a multivariable logistic regression model with postoperative complications as the dependent variable and patient demographic and clinical variables including method and timing of reconstruction as independent variables.
Results
4,781 radiated patients who met the inclusion criteria were selected. A majority of the patients (n=3,846, 80%) were reconstructed with implants. Overall complication rates were 45.3% and 30.8% for patients with implant and autologous reconstruction respectively. Failure of reconstruction occurred in 29.4% of patients with implant reconstruction compared to 4.3% for patients with autologous reconstruction. In multivariable logistic regression, radiated patients with implant reconstruction had 2 times the odds of having any complication and 11 times the odds of failure relative to patients with autologous reconstruction.
Conclusions
Implant based breast reconstruction in the radiated patient, though popular, is associated with significant morbidity. Failures of reconstruction with implants in these patients approach 30% in the short term, suggesting a need for careful shared decision-making with full disclosure of the potential morbidity.
Keywords: Breast Reconstruction, Implant Breast Reconstruction, Autologous Breast Reconstruction, Radiation, Complications, Morbidity
Introduction
Radiation therapy is an increasingly common adjunct in breast cancer therapy by providing loco-regional control and improved survival in selected groups of patients (1). Although radiation has beneficial oncologic effects, the collateral damage to the chest wall and breast soft tissue negatively impacts breast reconstruction (2-7). To minimize complications from breast reconstruction in radiated fields, some recommend delayed autologous breast reconstruction as the ideal approach (8). Delaying reconstruction avoids radiation exposure of the tissue used for reconstruction and autologous flaps provide the benefit of replacing some of the breast and chest wall soft tissue exposed to radiation.
In recent years, implant reconstruction rates in the United States have risen in the radiated patient population. Using the SEER database, Agarwal and colleagues evaluated immediate reconstruction trends among radiated patients and found a steady increase in implant only reconstruction from 27% to 52%, with a concomitant decrease in autologous reconstruction from 56% to 32%over the past decade (9). There are concerns about the outcomes of implant reconstructions in patients who have a history of radiation or require post-mastectomy radiation therapy. A systematic review of the literature on the morbidity associated with implant reconstruction before or after radiation exposure found a pooled reconstruction failure rate of approximately 20%.Additionally, other studies have reported implant removal in as much as 30% of patients who underwent immediate breast reconstruction with subsequent post-mastectomy radiation therapy (10). However, other studies with variations in the sequence of reconstruction stages have reported fewer failures with immediate implant reconstruction in the setting of post mastectomy radiation therapy (11, 12, 13). This lack of agreement in the published literature warrants a longitudinal population level examination of breast reconstruction outcomes for radiated patients. Previous large database studies with a focus on reconstruction of the radiated breast have suffered from an inability to follow patients longitudinally after they have undergone radiation and reconstruction; this was especially true for delayed breast reconstruction (9). Use of a claims database in this current study uniquely allowed for the identification of patients who underwent mastectomy, radiation therapy and reconstruction with detailed longitudinal follow up.
Hence, the aim of this study is to compare the morbidity associated with implant and autologous breast reconstruction techniques in a national sample of radiated patients, with the goal of better defining the optimal approach to reconstruction in these patients.
Methods
Data source and study cohort
We identified patients who underwent breast reconstruction following mastectomy in the 2009-2013 Truven Health MarketScan® Research Databases, including the MarketScan Commercial Claims and Encounters Database and the Medicare Supplemental and Coordination of Benefits Database. These databases capture patient-level utilization of medical services, payments, and enrollment across inpatient and outpatient settings. They represent the healthcare utilization of approximately 50 million active employees, early retirees, Medicare-eligible retirees with employer-provided Medicare Supplemental plans, and their dependents each year.
Patients selected had at least one medical claim for breast reconstruction (immediate or delayed) procedures under the diagnosis breast cancer/high risk of breast cancer/family history of breast cancer between January 1st, 2009 and September 30th, 2012. (See Supplemental Digital Content 1, Appendix 1, which shows Diagnosis codes for breast cancer and procedure codes, INSERT LINK) This study period allowed a 15-month follow-up for each patient to enable capture of complications and reconstruction failures following the index reconstruction procedure. In order to directly compare outcomes between prosthesis only and autologous tissue only based procedures, we excluded patients who had a combinations of the two techniques in the same reconstruction episode e.g. implants with a latissimus dorsi flap. Other inclusion criteria were female sex, age 18 years or older, and continuous enrolment for at least 15 months following the index procedure. Finally, to examine the influence of timing of radiation therapy on outcomes following breast reconstruction, we included only patients who received radiation therapy for the management of breast cancer or patients who had claims indicating a history of radiation therapy during the study period. (See Supplemental Digital Content 1, Appendix 2, which shows Diagnosis and procedural codes for radiation therapy, INSERT LINK) We used International Classification of Diseases, 9th version (ICD-9 codes) and the Current Procedural Terminology codes (CPT codes) to identify specific diagnosis and procedures, respectively.
Dependent variables
One of the dependent variables was overall complications following the initial breast reconstruction procedure including infection, wound complications, hematoma, mechanical implant complications (rupture), capsular contractures of implant, fat necrosis and flap venous congestion (14). The second dependent variable was reconstruction failure. (See Supplemental Digital Content 1, Appendix 3, which shows Diagnosis and procedural codes for complications and reconstruction failure, INSERT LINK) All complications captured had to be under a diagnosis of breast cancer to link them to the reconstruction procedure. To capture unsuccessful attempts at reconstruction that occurred during any stage of the reconstructive process, failures were defined as operations resulting in the termination of reconstruction, operations resulting in a repetition of an already performed stage of reconstruction or a change in the reconstructive type at any point during the stipulated observation period. Failures were specifically defined as patients who underwent reoperation in one of the following scenarios: 1) patient had prosthesis procedure as the index procedure and then underwent a removal of the tissue expander or implant without any further reconstruction procedures during the follow-up; 2) patient had prosthesis procedure as the index procedure and then had another tissue expander inserted during the follow-up; 3) patient had tissue expander inserted during the initial reconstruction and underwent exchange during follow-up, but received another or more than one exchange procedure following the first exchange; 4) patient had implant as the index procedure and received a replacement of the initial implant during the follow-up; 5) had prosthesis procedure as the index procedure and then received implant+flap or a flap alone; 6) had autologous tissue placed as the index procedure and then received another flap. Lastly, to count as reconstruction failures, the above 6 scenarios had to occur without a new procedure code for mastectomy, thus eliminating involvement of the contralateral breast as the reason for additional operations.
Independent variables
Key independent variables included the index reconstruction type (prosthesis vs. autologous tissue), timing of radiation therapy (before breast reconstruction vs. after breast reconstruction) and timing of reconstruction (immediate vs. delayed). Immediate reconstruction was defined as breast reconstruction procedures performed on the same day of mastectomy and delayed reconstruction was defined as any reconstruction procedures performed after the mastectomy. Other variables included: age, geographic region, median household income for Metropolitan Statistical Area (MSA), comorbidities and type of insurance plan. We linked the MSA identifier for each patient to the 2010 census data and obtained median household income for their residence. Finally, we identified comorbid conditions using appropriate ICD-9-CM codes, and these were classified using the Elixhauser method. (15, 16) We then included the Elixhauser comorbidity score as an independent variable.
Data Analysis
We described the distribution of the socio-economic, demographic and clinical variables in the study sample according to reconstruction type. Secondly, we described the distribution of complications of each reconstruction technique by timing of reconstruction and timing of radiation therapy. We used the Chi-square test to examine the unadjusted association between each complication and timing of reconstruction and radiation therapy. Lastly, we used a multivariable logistic regression model to examine associations between our previously outlined key independent variables and both dependent variables: 1) occurrence of any complications and 2) reconstruction failure.
Results
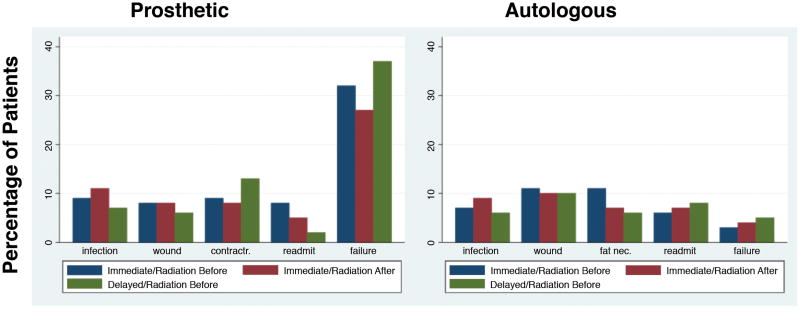
We identified 4,781 patients who met the inclusion criteria of breast reconstruction and radiation therapy for a breast cancer diagnosis. Eighty percent (3,846) underwent implant-based reconstruction and 20% (935) underwent autologous reconstruction. Table 1 shows the social, demographic and clinical characteristics of patients by reconstruction type (implant vs. autologous tissue). Overall complication rates were 45.3% and 30.8% for patients with implant-based reconstruction and autologous reconstruction respectively (Tables 2 and 3). Additionally, failure of reconstruction occurred in 29.4% of patients with implant-based reconstruction compared to 4.3% for patients with autologous reconstruction. Figure 1 shows the comparison of individual complication and failure rates of patients who underwent implant-based and autologous reconstruction by the timing of reconstruction (immediate vs. delayed) and timing of radiation (pre- vs. post-reconstruction). The figure shows no consistent pattern of complication or failure rates by timing of reconstruction and radiation in either group of patients; however, it shows a considerable difference in failure rates between radiated patients who underwent implant-based compared to autologous reconstruction.
Table 1. Patient demographic and clinical characteristics (n=4781).
| Implant Reconstruction n=3846 | Autologous Reconstruction n=935 | P values | |
|---|---|---|---|
| Age | <0.001 | ||
| 18 -34 | 225 (6%) | 44 (5%) | |
| 35-44 | 1019 (26%) | 219 (23%) | |
| 45-54 | 1524 (40%) | 395 (42%) | |
| 55-64 | 865 (22%) | 222 (24%) | |
| 65 and older | 213 (6%) | 55 (6%) | |
| Quartile of median house income | <0.001 | ||
| Quartile1 (<=$46,910) | 575 (15%) | 134 (14%) | |
| Quarile2 ($46,910 to $51,920) | 672 (17%) | 167 (18%) | |
| Quartile3 ($51,920 to $58,900) | 1074 (28%) | 251 (27%) | |
| Quartile4 (> $58,900) | 1073 (28%) | 254 (27%) | |
| Missing | 452 (12%) | 129 (14%) | |
| Region | <0.001 | ||
| Northeast | 835 (22%) | 206 (22%) | |
| North Central | 921 (24%) | 195 (21%) | |
| South | 1236 (32%) | 340 (36%) | |
| West | 779 (20%) | 176 (19%) | |
| Missing | 75 (2%) | 18 (2%) | |
| Comorbidity score | <0.001 | ||
| Quartile1 (<=12) | 939 (24%) | 313 (33%) | |
| Quartile2 (13-20) | 986 (26%) | 240 (26%) | |
| Quartile3 (21-27) | 1090 (28%) | 209 (22%) | |
| Quartile4 (>=27) | 831 (22%) | 173 (19%) | |
| Timing of radiation | 0.202 | ||
| Before reconstruction | 971 (25%) | 597 (64%) | |
| After reconstruction | 2875 (75%) | 338 (36%) | |
| Timing of reconstruction | <0.001 | ||
| Immediate | 3205 (83%) | 487 (52%) | |
| Delayed | 641 (17%) | 448 (48%) |
Table 2. Postoperative complications in patients who underwent implant reconstruction with variations in the timing of reconstruction (n=3846).
| Total | Immediate implant recon + radiation before recon (n=330) | Immediate implant recon + radiation after recon (n=2875) | Delayed implant recon + radiation before recon (n=641) | P values* | |
|---|---|---|---|---|---|
| Any complications | 1744 (45 %) | 156 (47 %) | 1263 (44 %) | 325 (51 %) | 0.505 |
| Prosthesis failure | 1133 (29 %) | 105 (32 %) | 790 (27 %) | 238 (37 %) | 0.117 |
| Infection | 404 (11 %) | 29 (9 %) | 330 (11 %) | 45 (7 %) | <0.001 |
| Wound complications | 287 (7 %) | 27 (8 %) | 221 (8 %) | 39 (6 %) | 0.658 |
| Hematoma | 75 (2 %) | 9 (3 %) | 63 (2 %) | 3 (0 %) | 0.005 |
| Mechanical complications | 417 (11 %) | 28 (8 %) | 284 (10 %) | 105 (16 %) | 0.045 |
| Capsular Contracture of Implant | 355 (9 %) | 31 (9 %) | 238 (8 %) | 86 (13 %) | 0.61 |
| Readmission | 190 (5 %) | 25 (8 %) | 155 (5 %) | 10 (2 %) | 0.033 |
: P values calculated from Chi-square test.
Table 3. Postoperative complications in patients who underwent autologous reconstruction with variations in the timing of reconstruction (n=935).
| Total | Immediate autologous recon + radiation before recon (n=149) | Immediate autologous recon + radiation after recon (n=338) | Delayed autologous recon + radiation before recon (n=448) | P values* | |
|---|---|---|---|---|---|
| Any complications | 288 (31 %) | 55 (37 %) | 110 (33 %) | 123 (27 %) | 0.505 |
| Autologous failure | 40 (4 %) | 4 (3 %) | 14 (4 %) | 22 (5 %) | <0.001 |
| Infection | 69 (7 %) | 11 (7 %) | 31 (9 %) | 27 (6 %) | <0.001 |
| Wound complications | 95 (10 %) | 16 (11 %) | 35 (10 %) | 44 (10 %) | 0.658 |
| Hematoma | 27 (3 %) | 8 (5 %) | 10 (3 %) | 9 (2 %) | 0.005 |
| Fat necrosis | 70 (7 %) | 17 (11 %) | 25 (7 %) | 28 (6 %) | <0.001 |
| Congestion | 61 (7 %) | 15 (10 %) | 23 (7 %) | 23 (5 %) | <0.001 |
| Readmission | 67 (7 %) | 9 (6 %) | 24 (7 %) | 34 (8 %) | 0.033 |
: P values calculated from Chi-square test.
Figure 1.
Comparison of the rates of individual complications and failure of reconstruction between patients who underwent prosthesis versus autologous breast reconstruction based on the timing of reconstruction.
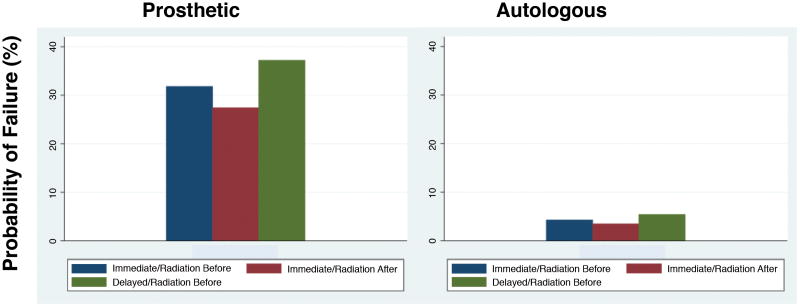
Adjusted estimates from logistic regression models (Table 4 and 5) show that patients who undergo implant-based reconstruction have 2 times the odds of having any of the complications examined compared to their counterparts who underwent autologous reconstruction. Additionally, patients with implant-based reconstruction had 11 times the odds of reconstruction failure compared to their counterparts with autologous reconstruction. Lastly, the highest probability of reconstruction failure was among patients with implant-based delayed reconstruction and pre-reconstruction radiation. The probability of failure for the average patient who had pre-reconstruction radiation and delayed implant-based reconstruction was 37.2%. The lowest probability of failure was amongst patients with immediate autologous reconstruction with post-reconstruction radiation who had a failure probability of 3.5%. Figure 2 demonstrates the probability of reconstruction failure for all 6 categories of patients.
Table 4. stimating the odds that patients developed any complication/had a failure of reconstruction within 15 months of reconstruction (n=4781)*.
| ANY COMPLICATIONS | FAILURE | |||
|---|---|---|---|---|
| Patient characteristics | Odds ratios and 95%CI | P values | Odds ratios and 95%CI | P values |
| Type of reconstruction | ||||
| Implant | Ref | Ref | ||
| Autologous | 0.48 (0.40-0.57) | <0.001 | 0.09 (0.07-0.13) | <0.001 |
| Timing of reconstruction and radiation | ||||
| Immediate reconstruction and radiation before reconstruction | Ref | Ref | ||
| Immediate reconstruction and radiation after reconstruction | 0.73 (0.59-0.91) | 0.005 | 0.81 (0.62-1.05) | 0.107 |
| Delayed reconstruction and radiation before reconstruction | 0.96 (0.75-1.22) | 0.718 | 1.27 (0.95-1.70) | 0.103 |
| Age | ||||
| 18 -34 | Ref | Ref | ||
| 35-44 | 0.97 (0.72-1.29) | 0.818 | 0.92 (0.66-1.29) | 0.644 |
| 45-54 | 0.93 (0.70-1.23) | 0.608 | 0.89 (0.64-1.23) | 0.474 |
| 55-64 | 1.06 (0.79-1.43) | 0.684 | 1.06 (0.75-1.49) | 0.742 |
| 65 and older | 0.80 (0.55-1.16) | 0.24 | 0.71 (0.45-1.11) | 0.131 |
| Quartile of median house income | ||||
| Quartile1 (<=$46,910) | Ref | Ref | ||
| Quarile2 ($46,910 to $51,920) | 0.91 (0.74-1.13) | 0.411 | 0.83 (0.65-1.06) | 0.128 |
| Quartile3 ($51,920 to $58,900) | 1.08 (0.89-1.31) | 0.434 | 0.92 (0.74-1.14) | 0.449 |
| Quartile4 (> $58,900) | 1.08 (0.88-1.33) | 0.456 | 0.83 (0.65-1.05) | 0.125 |
| Region | ||||
| Northeast | Ref | Ref | ||
| North Central | 1.04 (0.86-1.27) | 0.671 | 1.07 (0.85-1.36) | 0.551 |
| South | 1.14 (0.94-1.38) | 0.189 | 1.35 (1.07-1.70) | 0.010 |
| West | 1.26 (1.03-1.53) | 0.025 | 1.20 (0.94-1.52) | 0.139 |
| Comorbidity score | ||||
| Quartile1 (<=12) | Ref | Ref | ||
| Quartile2 (13-20) | 1.28 (1.07-1.53) | 0.006 | 1.01 (0.82-1.25) | 0.895 |
| Quartile3 (21-27) | 1.30 (1.09-1.55) | 0.004 | 0.91 (0.74-1.13) | 0.397 |
| Quartile4 (>=27) | 1.71 (1.41-2.07) | <0.001 | 1.31 (1.06-1.64) | 0.015 |
Adjusted odds ratios calculated from multivariable logistic regression models, controlling for all the variables in this table.
Table 5. Estimating the odds that patients developed any complication/had a failure of reconstruction within 15 months of reconstruction by the timing of reconstruction(n=4781)*.
| ANY COMPLICATIONS | FAILURE | |||
|---|---|---|---|---|
| Patient characteristics | Odds ratios and 95%CI | P values | Odds ratios and 95%CI | P values |
| Among patients who received immediate reconstruction | ||||
| Implant | Ref | Ref | ||
| Autologous | 0.59 (0.47-0.73) | <0.001 | 0.10 (0.06-0.16) | <0.001 |
| Among patients who received delayed reconstruction | ||||
| Implant | Ref | Ref | ||
| Autologous | 0.34 (0.26-0.46) | <0.001 | 0.09 (0.06-0.15) | <0.001 |
Adjusted odds ratios calculated from multivariable logistic regression models, controlling for all patient characteristics.
Figure 2.
Comparison of the probability of reconstruction failure between patients who underwent prosthesis versus autologous based breast reconstruction based on the timing of reconstruction.
Discussion
In this large national claims-based database study, we found, as has been shown in other studies, (9, 17,18) that radiated breast cancer patients predominantly underwent implant breast reconstruction. Additionally, a majority (77%) of all radiated patients underwent immediate reconstruction with subsequent radiation. Over the 15 month follow-up period, overall postoperative complications were higher in patients with implant reconstruction compared to autologous reconstruction (45.3% vs 30.8%, p<0.001). Complications were highest (51%) in patients who underwent post-radiation delayed implant breast reconstruction. Patients who underwent delayed autologous breast reconstruction had the lowest overall complication rate at 27%. Delayed implant breast reconstruction also had the highest rate of reconstruction failure at 37%. Reconstruction failure in the setting of immediate implant reconstruction was significantly higher than failure observed in patients undergoing immediate autologous reconstruction (27.9% vs 3.7%, p<0.001).
The fact that breast or chest wall radiation is associated with greater morbidity with post mastectomy breast reconstruction is generally accepted. However, there is far less agreement in the plastic surgery community on how best to reconstruct patients who require radiation therapy. Deciding on options for breast reconstruction should require consideration of patient preferences, anatomic constraints and safety in terms of reasonably successful outcomes of reconstruction with avoidance of adverse effects to the patient. This study particularly addresses the issue of safety.
With the growing popularity of implant based breast reconstruction in radiated patients, the literature is replete with multiple single center retrospective studies conducted to assess postoperative outcomes with this reconstructive approach. For example, Brooks and colleagues evaluated 560 women who underwent tissue expander/implant reconstruction at the Cleveland Clinic between 2000 and 2006 (19). Major complications requiring additional operative interventions occurred in 45.4% of radiated breasts in comparison to 21.2% in non-radiated breasts. Among radiated patients, 19.6% required implant removal and 10.3% were ultimately reconstructed with flaps. Similar findings were reported by Ascherman and colleagues, in their single surgeon experience with tissue expander/implant reconstruction in radiated patients (3). In that series, overall complications in radiated breasts was 40.7% and specific complications necessitating implant removal or replacement occurred in 18.5% of the radiated breasts. Lastly, radiated patients reconstructed with implants at the Memorial Sloan Kettering Cancer Center, when compared to similar non-radiated patients reported significantly lower quality of life and satisfaction with their reconstructed breasts (20). With some variability of reported complications in the existing literature (10), findings on complications in this current study with a 45.3% implant complication rate and 29.4% failure, agree with the published literature. These complication rates in our study translated to 2 and 11 times greater odds of complications and failures of reconstruction respectively, with implant based reconstruction in radiated patients compared to autologous reconstruction (Tables 4 and 5).
Existing literature also provides robust evidence that autologous flap alternatives are associated with better success rates and high levels of patient satisfaction (21, 22). A systematic review of the published literature on autologous reconstruction outcomes in the face of radiation revealed relatively low pooled flap failure rates with reconstruction performed before (4%) or after delivery of radiation therapy (1%) (23). Given that the results of our study are based on a broader nationwide sample of patients and institutions and are in general agreement with existing studies, we suggest the following implications: implant-only reconstruction in the radiated patient should be an exception and not the rule. From the standpoint of safety i.e. reasonably successful outcomes of reconstruction with avoidance of adverse effects to the patient, our study findings with flap failures ranging from 3% to 5%, support the notion that autologous tissue reconstruction has a lower risk for complications and failure for radiated patients.
The primary concerns with autologous reconstruction in radiated patients have to do with the timing of reconstruction relative to radiation exposure. Specifically, these concerns center on exposure of flaps to radiation i.e. immediate reconstruction with subsequent post-mastectomy radiation therapy. Multiple studies have reported fat necrosis, flap volume loss and contraction as reasons to avoid flap exposure to radiation therapy, (4, 6, 24) favoring delaying reconstruction for a period after radiation. However, the trade-offs of delaying reconstruction include patients having to live without a breast for extended periods, intraoperative challenges working with radiated recipient vessels and more prominent scars with the delayed flap inset. Kronowitz et al. championed the delayed-immediate method to mitigate the soft tissue deficit issues of delayed reconstructions (24). Tissue expanders are placed at the time of mastectomy, allowing for expansion and preservation of the soft tissue envelope even after exposure to radiation. This modification also has its problems with complications requiring expander removal in about a third of cases (25). Finally, existing data on the clinically significant effects of radiation on autologous flaps is mixed. Mirzabeigi et al' s recent evaluation of 127 abdominal based free flap reconstructions exposed to radiation did find a higher incidence of volume loss and fat necrosis when compared to similar non-radiated reconstructions (26). However, the radiated patients did not experience a higher rate of complications and required an equivalent number of revision procedures as non-radiated patients to achieve a complete reconstruction (24). In contrast, volumetric assessments performed by Chatterjee and colleagues on flaps exposed to radiation therapy demonstrated a volume decrease in the radiated flaps that was not significantly different from similar non-radiated flaps (27). The variation in reports on the effect of radiation on flaps likely has to do with the unavoidable differences in radiation protocols from institution to institution. Additionally, most studies on this topic provide relatively short term follow up data with radiation changes expected to occur over many years. Nevertheless, it appears that the incidences of clinically significant volumetric loss and fat necrosis are somewhat ambiguous and not clearly defined. Furthermore, these changes may not affect the ultimate outcome of an autologous breast reconstruction exposed to radiation, especially when anticipated and adjusted for with initial flap oversizing.
This study has limitations. Although the MarketScan database represents a large population, it lacks information on race/ethnicity and only includes commercially insured patients, typically of working age limiting its generalizability. Reconstruction type may be influenced by non-clinical factors including surgeon preference, patient preference or financial implications for reimbursement that cannot be accounted for in the database. Lastly, other pertinent clinical factors such as body mass index, race, smoking history, prior surgeries that may preclude autologous reconstruction, or preference of reconstruction method by the patient or the surgeon are not provided in the database. Despite these limitations, we present data that provides some insight into the morbidity associated with varying approaches to breast reconstruction in patients undergoing radiation therapy.
Conclusion
Implant breast reconstruction in patients exposed to radiation therapy, though popular, is associated with significant morbidity. Failures of reconstruction with this method approach 30% in the short term. Appreciating the importance of patient preferences, there is still a need for careful shared decision making with full disclosure of the potential morbidity associated with implant-only reconstruction in radiated patients. Also, given that autologous reconstruction of the radiated breast results in significantly fewer complications and failures, it is critical that reconstructive surgeons reassess their approach to surgical management in this growing patient population.
Supplementary Material
Supplemental Digital Content 1. Appendix 1: Diagnosis codes for breast cancer and procedure codes for breast reconstruction, Appendix 2: Diagnosis and procedural codes for radiation therapy, Appendix 3: Diagnosis and procedural codes for complications and reconstruction failure, INSERT LINK.
Acknowledgments
Support for this study was provided in part by grants from the Michigan Institute for Clinical and Health Research (to Adeyiza O Momoh and Erika D Sears), a Mentored Clinical Scientist Award for Patient Centered Outcomes Research (1K08-HS023313-01) (to J.F.W.) and a Mid-career Investigator Award in Patient-Oriented Research (2K24 AR053120-06) (to Kevin C Chung),
Footnotes
Authorship Roles: Matthew D. Chetta, MD: Study design, data analysis, manuscript writing, final approval of manuscript.
Oluseyi Aliu, MD MS: Study design, data analysis, manuscript writing, final approval of manuscript.
Lin Zhong, MD MPH: Data collection, data analysis, manuscript writing, final approval of manuscript.
Erika D. Sears, MD, MS: Study design, manuscript writing, final approval of manuscript.
Jennifer F. Waljee, MD MPH: Study design, manuscript writing, final approval of manuscript.
Kevin C. Chung, MD, MS: Study design, data analysis, manuscript writing, final approval of manuscript.
Adeyiza O. Momoh, MD: Study design, data analysis, manuscript writing, final approval of manuscript.
Presented at the American Society of Reconstructive Microsurgery meeting, January 2016, in Scottsdale, Arizona.
Disclosure Statement: None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript
References
- 1.Early Breast Cancer Trialists' Collaborative Group. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spear SL, Onyewu C. Staged breast reconstruction with saline-filled implants in the irradiated breast: recent trends and therapeutic implications. Plast Reconstr Surg. 2000;105:930. doi: 10.1097/00006534-200003000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Ascherman JA, Hanasono MM, Newman MI, et al. Implant reconstruction in breast cancer patients treated with radiation therapy. Plast Reconstr Surg. 2006;117:359. doi: 10.1097/01.prs.0000201478.64877.87. [DOI] [PubMed] [Google Scholar]
- 4.Tran NV, Chang DW, Gupta A, et al. Comparison of immediate and delayed free TRAM flap breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2001;108:78. doi: 10.1097/00006534-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Williams JK, Carlson GW, Bostwick J, III, et al. The effects of radiation treatment after TRAM flap breast reconstruction. Plast Reconstr Surg. 1997;100:1153. doi: 10.1097/00006534-199710000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Rogers NE, Allen RJ. Radiation effects on breast reconstruction with the deep inferior epigastric perforator flap. Plast Reconstr Surg. 2002;109:1919. doi: 10.1097/00006534-200205000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Spear SL, Ducic I, Low M, et al. The effect of radiation therapy on pedicled TRAM flap breast reconstruction: outcomes and implications. Plast Reconstr Surg. 2005;115:84–95. [PubMed] [Google Scholar]
- 8.Kronowitz SJ, Robb GL. Radiation therapy and breast reconstruction: a critical review of the literature. Plast Reconstr Surg. 2009;124:395–408. doi: 10.1097/PRS.0b013e3181aee987. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal S, Kidwell KM, Farberg A, Kozlow JH, Chung KC, Momoh AO. Immediate reconstruction of the radiated breast: recent trends contrary to traditional standards. Ann Surg Oncol. 2015;22(8):2551–9. doi: 10.1245/s10434-014-4326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Momoh AO, Ahmed R, Kelley BP, et al. A systematic review of complications of implant-based breast reconstruction with prereconstruction and postreconstruction radiotherapy. Ann Surg Oncol. 2014;21:118–124. doi: 10.1245/s10434-013-3284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarthy CM, Pusic AL, Disa JJ, McCormick BL, Montgomery LL, Cordeiro PG. Unilateral postoperative chest wall radiotherapy in bilateral tissue expander/implant reconstruction patients: a prospective outcomes analysis. Plast Reconstr Surg. 2005;116:1642–7. doi: 10.1097/01.prs.0000187794.79464.23. [DOI] [PubMed] [Google Scholar]
- 12.Cordeiro PG, Albornoz CR, McCormick B, Hu Q, Van Zee K. The impact of postmastectomy radiotherapy on two-stage implant breast reconstruction: an analysis of long-term surgical outcomes, aesthetic results, and satisfaction over 13 years. Plast Reconstr Surg. 2014;134(4):588–95. doi: 10.1097/PRS.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 13.Ho A, Cordeiro P, Disa J, et al. Long-term outcomes in breast cancer patients undergoing immediate 2-stage expander/implant reconstruction and postmastectomy radiation. Cancer. 2011;118:2552–2559. doi: 10.1002/cncr.26521. [DOI] [PubMed] [Google Scholar]
- 14.Jagsi R, Jiang J, Momoh AO, et al. Complications after mastectomy and immediate breast reconstruction for breast cancer: a claims-based analysis. Ann Surg. 2016;263(2):219–27. doi: 10.1097/SLA.0000000000001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Medical care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 16.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Medical care. 2009;47(6):626–33. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 17.Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in U.S. breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131(1):15–23. doi: 10.1097/PRS.0b013e3182729cde. [DOI] [PubMed] [Google Scholar]
- 18.Jagsi R, Jing J, Momoh AO, et al. Trends and variations in use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. J Clin Oncol. 2014;32:919–926. doi: 10.1200/JCO.2013.52.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brooks S, Djohan R, Tendulkar R, Nutter B, Lyons J, Dietz J. Risk factors for complications of radiation therapy on tissue expander breast reconstructions. Breast J. 2012;18(1):28–34. doi: 10.1111/j.1524-4741.2011.01182.x. [DOI] [PubMed] [Google Scholar]
- 20.Albornoz CR, Matros E, McCarthy CM, et al. Implant breast reconstruction and radiation: a multicenter analysis of long-term health-related quality of life and satisfaction. Ann Surg Oncol. 2014;21(7):2159–64. doi: 10.1245/s10434-014-3483-2. [DOI] [PubMed] [Google Scholar]
- 21.Lee BT, Adesiyun TA, Colakoglu S, et al. Postmastectomy radiation therapy and breast reconstruction: an analysis of complications and patient satisfaction. Ann Plast Surg. 2010;64(5):679–83. doi: 10.1097/SAP.0b013e3181db7585. [DOI] [PubMed] [Google Scholar]
- 22.Atisha D, Alderman AK, Lowery JC, Kuhn LE, Davis J, Wilkins EG. Prospective analysis of long-term psychosocial outcomes in breast reconstruction: two-year postoperative results from the Michigan Breast Reconstruction Outcomes Study. Ann Surg. 2008;247(6):1019–28. doi: 10.1097/SLA.0b013e3181728a5c. [DOI] [PubMed] [Google Scholar]
- 23.Kelley BP, Ahmed R, Kidwell KM, Kozlow JH, Chung KC, Momoh AO. A systematic review of morbidity associated with autologous breast reconstruction before and after exposure to radiotherapy: are current practices ideal? Ann Surg Oncol. 2014;21(5):1732–8. doi: 10.1245/s10434-014-3494-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kronowitz SJ, Hunt KK, Kuerer HM, et al. Delayed-immediate breast reconstruction. Plast Reconstr Surg. 2004;113:1617–1628. doi: 10.1097/01.prs.0000117192.54945.88. [DOI] [PubMed] [Google Scholar]
- 25.Kronowitz SJ, Lam C, Terefe W, et al. A multidisciplinary protocol for planned skin-preserving delayed breast reconstruction for patients with locally advanced breast cancer requiring postmastectomy radiation therapy: 3-year follow-up. Plast Reconstr Surg. 2011;127:2154–66. doi: 10.1097/PRS.0b013e3182131b8e. [DOI] [PubMed] [Google Scholar]
- 26.Mirzabeigi MN, Smartt JM, Nelson JA, Fosnot J, Serletti JM, Wu LC. An assessment of the risks and benefits of immediate autologous breast reconstruction in patients undergoing postmastectomy radiation therapy. Ann Plast Surg. 2013;71(2):149–55. doi: 10.1097/SAP.0b013e31824b3dcc. [DOI] [PubMed] [Google Scholar]
- 27.Chatterjee JS, Lee A, Anderson W, et al. Effect of postoperative radiotherapy on autologous deep inferior epigastric perforator flap volume after immediate breast reconstruction. Br J Surg. 2009;96:1135–1140. doi: 10.1002/bjs.6693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Appendix 1: Diagnosis codes for breast cancer and procedure codes for breast reconstruction, Appendix 2: Diagnosis and procedural codes for radiation therapy, Appendix 3: Diagnosis and procedural codes for complications and reconstruction failure, INSERT LINK.