Abstract
INTRODUCTION
Recommendations for colorectal cancer screening encourage patients to choose among various screening methods based on individual preferences for benefits, risks, screening frequency and discomfort. We devised a model to illustrate how individuals with varying tolerance for screening complications risk might decide on their preferred screening strategy.
METHODS
We developed a discrete-time Markov mathematical model that allowed hypothetical individuals to maximize expected lifetime utility by selecting screening method, start age, stop age and frequency. Individuals could choose from stool-based testing every 1-3 years, flexible sigmoidoscopy every 1-20 years with annual stool-based testing, colonoscopy every 1-20 years, or no screening. We compared the life expectancy gained from the chosen strategy with the life expectancy available from a benchmark strategy of decennial colonoscopy.
RESULTS
For an individual at average risk of colorectal cancer who was risk-neutral with respect to screening complications (and therefore was willing to undergo screening if it would actuarially increase life expectancy), the model predicted that they would choose colonoscopy every 10 years, from age 53-73 years, consistent with national guidelines. For a similar individual who was moderately averse to screening complications risk (and therefore required a greater increase in life expectancy to accept potential risks of colonoscopy), the model predicted that they would prefer flexible sigmoidoscopy every 12 years with annual stool-based testing, with 93% of the life expectancy benefit of decennial colonoscopy. For an individual with higher risk aversion, the model predicted that they would prefer 2 lifetime flexible sigmoidoscopies, 20 years apart, with 70% of the life expectancy benefit of decennial colonoscopy.
CONCLUSION
Mathematical models may formalize how individuals with different risk attitudes choose between various guideline-recommended colorectal cancer screening strategies.
In June 2016, the United States Preventive Services Task Force (USPSTF) updated its colorectal cancer screening guidelines, advising patients to choose between various methods (colonoscopy, flexible sigmoidoscopy, CT colonography or stool-based testing) based on their individual preferences for test frequency, preparation, discomfort, benefits and risks.1 From a population health perspective, all of the recommended methods had roughly similar estimates of life-years gained (200-275) per 1,000 persons who adhered to the guidelines.2 On average, each person screened was forecast to gain 2-3 months of life expectancy.
However, gains from screening are not evenly distributed across the population. Nearly all individuals will experience minimal benefits (such as peace of mind) or no benefits with negligible harm (from test preparation discomfort), a few individuals will gain many years and the rare individual will experience serious harm. As highlighted by the USPSTF,1 the optimal screening strategy for an individual must be determined in balance of these important dimensions, and may depend on how an individual values different outcomes based on attitudes toward risk.
In this study, we sought to develop a basic model to formalize how individuals with different risk attitudes might come to different conclusions about their preferred method and frequency of colorectal cancer screening. By “risk,” we mean additional life expectancy gained or lost through screening, rather than screening discomfort, fear of cancer, or similar issues. Our model was not intended for clinical implementation, but simply to quantify one way in which individuals might decide between various possibilities for colorectal cancer screening. In contrast to previous work on patient preferences, which analyzed informed decision making between guideline recommendations (for example, decennial colonoscopy or annual fecal occult blood testing),3-5 we allowed hypothetical patients to choose any method and frequency of screening that they desired, even if it was more or less often than USPSTF recommendations.
METHODS
Our Methods involved 3 steps. First, we modeled a hypothetical individual’s decision to screen for disease. Second, we used national cancer registry data to apply the model to colorectal cancer screening. Third, we rank-ordered the results, to find optimal screening strategies from a hypothetical individual’s perspective.
Model
We built a discrete-time Markov decision model through which a hypothetical individual evaluated benefits and risks of colorectal cancer screening with decision points every year (at the current age and each future age). The individual’s goal was to maximize expected lifetime utility. At every age, the individual had an option to engage in screening to improve the probability of long-term survival, in exchange for a risk of screening complications that would slightly reduce the probability of near-term survival. Therefore, in our first step, we obtained an individual’s baseline survival probability to each future age, using national life expectancy tables.6
Second, we assessed the potential increase in survival probabilities that a hypothetical individual could achieve through colorectal cancer screening, informed by benefits and risks of 3 methods recommended by the US Preventive Services Task Force[USPSTF]: stool-based testing (an average of FIT, FIT-DNA, and HSgFOBT), flexible sigmoidoscopy with annual stool-based testing, and colonoscopy.1,2 Screening benefits were based on the relative risk reduction[RRR] in probability of colorectal cancer diagnosis (through polypectomy) and increase in post-diagnosis relative survival rates (because of early detection). Benefits were estimated from data on the proportion of the general population that obtained vs. did not obtain screening. For example, for a white female aged 60 years, her estimated probability of colorectal cancer diagnosis was 0.020% with colonoscopy and 0.087% absent screening (Figure 17-9). In this case, a 77% relative risk reduction (1 – 0.020% probability of diagnosis with colonoscopy ÷ 0.060% national average) would be allocated to each stage of diagnosis (local, regional, distant/metastatic, unstaged), due to colonoscopy. We excluded CT (virtual) colonography because of minimal historic data available to inform RRR, and lack of Medicare coverage.10
Figure 1.
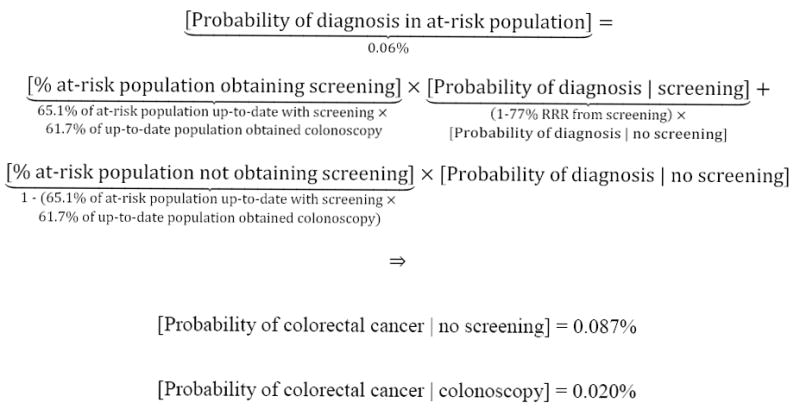
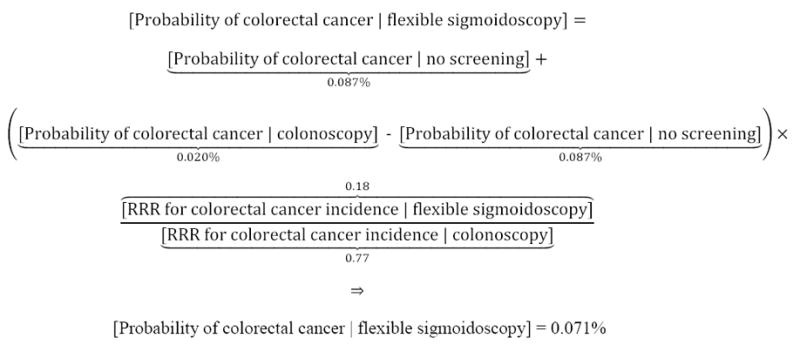
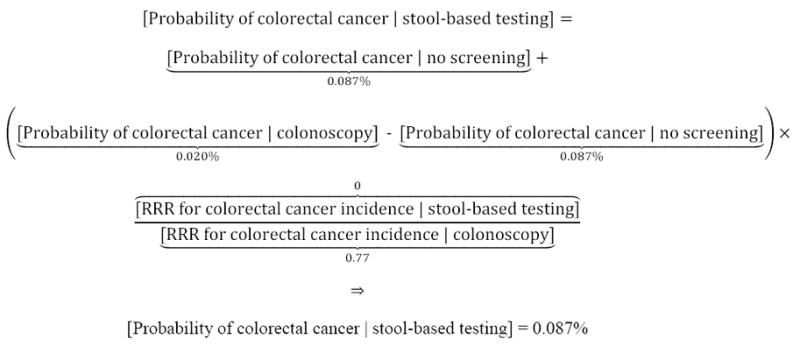
Example of the estimated probability of colorectal cancer, with vs. without various screening methods
For a hypothetical white female aged 60 years, her estimated probability of colorectal cancer was modeled as 0.020% with colonoscopy, 0.071% with flexible sigmoidoscopy and 0.087% with stool-based testing or no screening. The stage-specific probability of diagnosis was estimated by allocating the relative risk reduction for each method to the national probability of diagnosis for each stage. In this example, the national probability of local, regional, distant/metastatic and unstaged diagnoses was 0.0262%, 0.0209%, 0.0109%, and 0.0018%, respectively, for a total probability of 0.0598%.9 Allocating the relative risk reduction for colonoscopy screening (1 – 0.020% probability of diagnosis with colonoscopy ÷ 0.0598% national average = 77%) to each stage, the estimated stage-specific probabilities of diagnosis were 0.0087%, 0.0070%, 0.0036%, and 0.0006%, respectively, with colonoscopy. Similar methods were employed for flexible sigmoidoscopy, stool-based testing and no screening.
A. With colonoscopy
B. With flexible sigmoidoscopy
C. With stool-based testing
Screening risks were based on the probability of fatal complications for each screening method. We also recognized that some individuals may find the risk of serious complications (perforation, hemorrhage, or other events requiring hospital admission) as extremely undesirable, and therefore included a worst-case scenario in which a patient who suffered serious complications would stop screening for the remainder of their life. These risks were modeled to increase with age based on previously-published work.11 While this method overestimated actual harm, we reasoned that it resulted in minimum screening preferences. For example, if we estimated that an individual would choose to screen once every 12 years, then when modeling complications as less impactful on future screening, an individual should choose to screen at least once every 12 years. Supplement 1 provides further details on methodology.
Third, the hypothetical individual considered how much longer they were likely to live from screening, based on 3 possible outcomes (Figure 2). Most likely (often >90% probability), they would have no disease detected, with very small complications risk, resulting in a slight decrease in life expectancy (Figure 2, solid green line). Alternatively, if screening detected mild-moderate disease, then they likely would benefit from early detection. That benefit generally rose with tumor aggressiveness; hence, an upward sloping dotted red line in Figure 2. However, for more advanced tumors, early detection might eventually provide less longevity benefit, and at that point, the dotted red line in Figure 2 began a negative slope. In the extreme, incurable cancer might be diagnosed, in which case early detection had minimal benefit, or for particularly advanced disease, no benefit (Figure 2, dashed blue line). For example, for a 50-year-old average risk white female who chose to screen, the probability of a decrease in life expectancy (no disease with slight complications risk) was 99.4%. The probability of an increase in life expectancy ≤5 additional years was 0.5%, and the probability of an increase in life expectancy >5 additional years was 0.1%. An individual who chose not to screen realized no change in survival probabilities.
Figure 2.
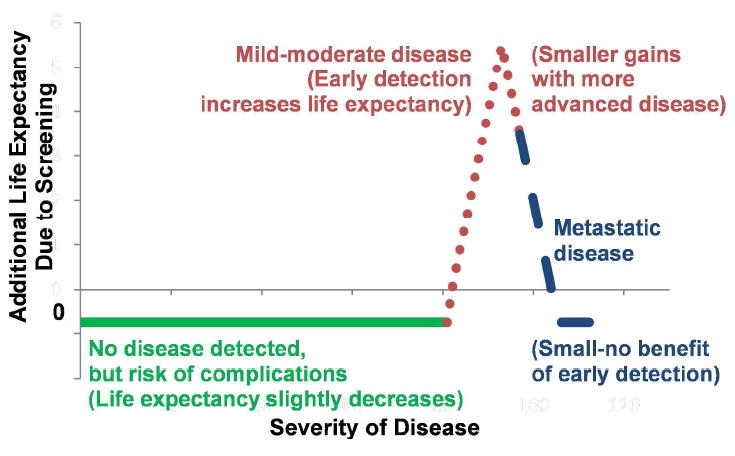
An Individual’s Perspective on Disease Screening
An individual considering whether to screen faces 3 possibilities. Sold green line: no disease detected, with a very a small risk of complications (resulting in a slight decrease in life expectancy). Dotted red line: mild-moderate disease, resulting in benefits from early detection (generally rising with tumor aggressiveness [upward-sloping red line], but with less benefit for more advanced tumors [downward-sloping red line]). Dashed blue line: incurable, metastatic cancer, for which early detection has minimal or no benefit, but still carries complications risk.
Fourth, the hypothetical individual estimated the expected gain in lifetime utility from each screening method as the probability distribution for life-years survived in each scenario (stage-specific disease detected, no disease detected) multiplied by individual utility as a function of remaining life expectancy and a time-discount factor. Recognizing that individuals would vary in their willingness to accept potential screening complications, we personalized utility for risk aversion, defined as the additional life expectancy that an individual would require to accept complications risk and undergo colorectal cancer screening. Specifically, we employed constant relative risk aversion, in which an individual’s risk aversion was expressed as a proportion of their life expectancy and remained constant with age. For example, for a 50-year-old black female who required 5% of her 31.3-year life expectancy to undergo a risky procedure, she would, at age 60 years, require 5% of her then 23.2-year life expectancy.6 This form of risk aversion has been widely investigated12-18 and observed in practice; a recent study found that up to 30% of US-based employees of a large multinational corporation exhibited constant relative risk aversion in selections of health insurance, disability insurance, and 401(k) allocations.18 In this setting, an individual was risk-neutral if they were willing to accept any method that offered at least actuarial gains in life expectancy (life-years gained in the at-risk population divided by size of that population). By contrast, an individual was risk-averse—or hesitant—to accept potential complications if they required a greater than actuarial increase in life expectancy to undergo screening. The concept was distinct from discounting, which would simply consider future life-years to be less valuable than the current life-year, without regard to risk. Figure 3 presents a decision tree, with further details in Supplement 1.
Figure 3.
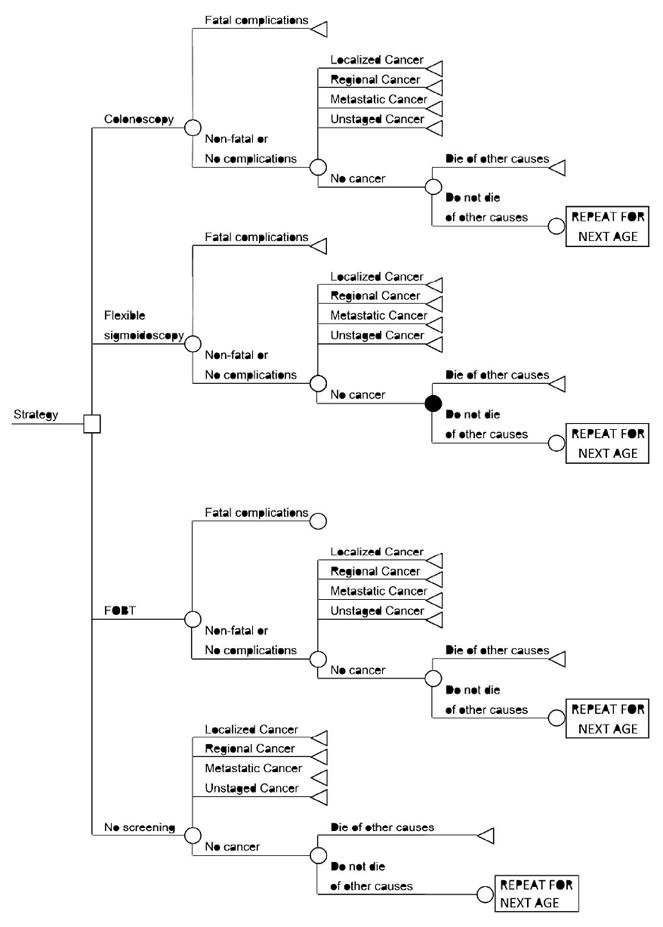
Decision Tree
At every age, an individual faced a decision to screen by colonoscopy, screen by flexible sigmoidoscopy with annual stool-based testing, screen by stool-based testing alone, or not screen.
Finally, the hypothetical individual rank-ordered the results, to determine their preferred age of colorectal cancer screening initiation and cessation, frequency, and method. The model was implemented in STATA/MP 13.1 (College Station, TX).
Inputs
Table 1 shows model inputs and sources.19-27
Table 1.
Model Inputs
| Parameter | Stool-based testing | Flexible Sigmoidosocpy | Colonoscopy | Source |
|---|---|---|---|---|
| Relative risk reduction for colorectal cancer incidence | 0% | 18% | 77% | 7,20 |
| Relative risk reduction for colorectal cancer-specific mortality | 25%* | 28% | 65% | 20,21,23-26 |
| Serious complications, per 100,000 | 1† | 34‡ | 277‡ | 11,19 |
| Screening rate in at-risk population, among up-to-date population | 10.4% | 0.7%§ | 61.7% | 8 |
| Specificity | 92.9%║ | 87.0% | N/A¶ | 2 |
| Parameters independent of screening method | ||||
| Life expectancy, age-specific | 6 | |||
| Probability of colorectal cancer diagnosis, age- and stage-specific | 9 | |||
| Relative survival rate, by stage and years post-diagnosis | 9 | |||
| Proportion of general population up-to-date with screening – 65.1% | 8 | |||
| Delay after first cancer screening, for screening benefits to commence – 5 years | 22,27 | |||
| Discount rate – 3% | Assumption | |||
| Individual risk aversion (Supplement 1) | Assumption | |||
Average for guaiac and immunochemical tests among individuals attending ≥1 round of screening, following meta-analysis.26
To proxy for anxiety, we assumed a minimal risk of complications.
The rate of complications increased with age, based on previous literature.11
Assumption.
Average of FIT, FIT-DNA, and HSgFOBT.
Specificity was employed to transfer individuals with false positive results to future colonoscopy screening.
Assumptions
To improve model feasibility, scalability, and generalizability, we incorporated numerous simplifications. First, we assumed that an individual’s life expectancy could be represented by national data from Vital Statistics. A more realistic model would include comorbidity, ethnicity, socioeconomic status, and geography.
Second, we assumed that life expectancy was the only factor influencing an individual’s decision to obtain screening. For example, they did not care about quality of life28 or monetary costs. While this assumption is inaccurate, an individual who considered quality of life components should elect to screen at least as often as predicted in our framework, because the individual would recognize that early detection carries a higher probability of less invasive treatments (local excision or partial colectomy), and therefore higher quality of life, than advanced stage detection.
Third, to maintain consistency with assumptions employed in guideline recommendations, we assumed perfect adherence to screening strategies.2,29
Fourth, we assumed that parameters of an individual’s risk aversion, measured from the economics literature,12-18 would apply to colorectal cancer screening. This assumption was based on prior work suggesting that economic risk aversion predicts healthy behaviors such as responsible alcohol use, tobacco abstention, and seat belt use.30 To the extent that individuals might be more risk-averse with regard to survival than wealth, our assumption would be too conservative, because the model would underweight the importance of complications risk. In this situation, individuals would be expected to choose less intensive screening than found in our framework and further expand discrepancies between national guidelines and patient-selected screening strategies.
Fifth, we assumed perfect rationality, perfect information, and perfect ability to incorporate information. These assumptions were employed as a first step in better understanding the implications of patient-centered disease screening as compared with guideline recommendations.
Screening strategies
We considered an age of initiation and cessation between 50-75 years, with stool-based testing every 1-3 years, flexible sigmoidoscopy every 1-20 years with annual stool-based testing, or colonoscopy every 1-20 years. To maintain consistency with clinical guidelines, a positive result on stool-based testing or flexible sigmoidoscopy prompted an individual to transfer to colonoscopy,1,2,29,31 at which time they could select a new screening frequency and age of cessation (Supplement 1). As a result, over time, most individuals who chose stool-based testing eventually transferred to colonoscopy; an individual undergoing annual stool-based testing had a 33%, 54% and 79% probability of undergoing colonoscopy over 5, 10, and 20 years, respectively.
Scenarios
To illustrate the model, we simulated patient characteristics for 2 types of hypothetical individuals. First, we considered individuals of average risk, who were either risk-neutral (requiring only actuarial gains in life expectancy to undergo screening—about 2 months for colonoscopy) or had varying degrees of risk aversion (moderate or high, requiring 5- or 8-month increases in life expectancy to undergo colonoscopy, respectively) (Supplement 1). Parameters were based on prior estimates of risk aversion (Supplement 1, section “Utility and risk aversion”).12-18 Although we employed discrete levels of risk aversion to illustrate the model, we would expect a continuum of parameters in practice. Second, we considered individuals with special considerations for screening (≥2 first-degree relatives with history of colorectal cancer, relative risk[RR] of colorectal cancer-specific mortality=4.25;32,33 high risk of adverse event owing to severe systemic disease [American Society of Anesthesiologists class III], RR of severe complications=1.66 (colonoscopy) or 2.10 (flexible sigmoidoscopy);34 both factors; or Crohn’s colitis diagnosed at age 25, absolute risk of colorectal cancer=0.50%/year beginning 10 years after diagnosis32). Individuals with above-average benefits (family history or Crohn’s colitis) were permitted to start screening at age 40 years.
Sensitivity Analyses
We conducted a one-way sensitivity analysis of input parameters across plausible ranges, with particular attention to allowing screening to continue through age 85 years. We also considered model predictions for individuals who poorly understood the benefits of early detection. These individuals mistakenly assumed that screening benefits would occur with certainty; for example, a 100% probability of living 0.3 years longer, rather than a 5% probability of 1-year longer survival, 3% probability of 5-year longer survival and 1% probability of 10-year longer survival. With less potential for early detection to allow survival to later ages, we expected risk-averse individuals to choose less intensive screening than in the main analysis.
Validation
The model was considered externally valid if (1) model predictions for risk-neutral individuals—who, like national guidelines, weighed benefits and risks equally—were similar to USPSTF recommendations1 and (2) the magnitude of model-predicted increases in life expectancy were similar to predictions for life-years gained per 1000 individuals in the decision analysis accompanying USPSTF recommendations.2,29 The model was considered internally valid if it predicted less intensive screening when risk aversion, the discount rate, or the risk of complications increased; and more intensive screening when the risk of developing colorectal cancer increased.
Role of the Funding Source
This study was funded by the National Institutes of Health, National Center for Advancing Translational Sciences (grant KL2TR000440, administered by the Clinical and Translational Science Collaborative of Cleveland). The funding source had no role in the design, conduct, and analysis of this study or in the decision to submit the manuscript for publication.
RESULTS
Table 2 shows the model’s predictions for when a hypothetical individual with average risks and benefits would choose to screen for colorectal cancer, solely based on their individual attitudes toward risk, rather than population health goals. A hypothetical risk-neutral individual was predicted to choose colonoscopy every 10 years from ages 53-73 years. They had an associated gain in life expectancy of 0.27 years overall or 5.02 years conditional on diagnosis, which may be thought of as expected benefits of early detection. The lifetime risk of developing colorectal cancer was 3.31%. In exchange, the individual anticipated an 872 in 100,000 (0.872%) lifetime probability of serious complications from screening. Owing to lower benefits of early detection from alternate screening methods, which were not fully offset by reduced complications risk, the optimal flexible sigmoidoscopy and stool-based testing strategies afforded ≤87% the expected utility of colonoscopy.
Table 2.
Model-Predicted Colorectal Cancer Screening Preferences
| Risk Aversion | Method* | Ages (y) | Frequency (y) | Gain in Life Expectancy | Expected Lifetime | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Overall (y) | Overall Relative to Decennial Colonoscopy | Conditional on Diagnosis (y) | Risk of Diagnosis | Risk of Serious Complications (in 100,000) | Utility, Relative to Optimal Strategy | ||||
| None (Risk-Neutral) | Colonoscopy | 53-73 | 10 | 0.27 | 100% | 5.02 | 3.31% | 872 | 100% |
| Flexible sigmoidoscopy | 51-75 | 12 | 0.25 | 93% | 5.29 | 4.92% | 421 | 87% | |
| Stool-based testing† | 50-75 | 1 | 0.23 | 85% | 4.56 | 3.97% | 697 | 62% | |
| Moderate | Flexible sigmoidoscopy | 51-75 | 12 | 0.25 | 93% | 5.29 | 4.92% | 421 | 100% |
| Colonoscopy | 51-75 | 12 | 0.26 | 96% | 4.89 | 3.36% | 882 | 93% | |
| Stool-based testing† | 50-75 | 1 | 0.23 | 85% | 4.56 | 3.97% | 697 | 59% | |
| High | Flexible sigmoidoscopy | 55-75 | 20 | 0.19 | 70% | 3.66 | 5.58% | 274 | 100% |
| Colonoscopy | 50-70 | 20 | 0.13 | 48% | 3.43 | 5.18% | 586 | 82% | |
| Stool-based testing† | 50-75 | 1 | 0.23 | 85% | 4.56 | 3.97% | 697 | 55% | |
Hypothetical individuals chose colorectal cancer screening strategies based on their individual attitudes toward risk, rather than population health goals.
Flexible sigmoidoscopy with annual stool-based testing.
For stool-based testing, high expected lifetime benefits and risks occurred because of the potential for a false-positive result to transfer an individual to colonoscopy screening. An individual undergoing annual stool-based testing had a 33%, 54% and 79% probability of being transferred to colonoscopy over 5, 10, and 20 years, respectively.
By contrast, a moderately risk-averse individual overweighed complications risk relative to early detection benefits. They were predicted to prefer flexible sigmoidoscopy with annual stool-based testing every 12 years from ages 51-75 years, with a slightly lower life expectancy gain of 0.25 years, or 93% of the life expectancy gain for a risk-neutral individual (0.25/0.27 years). A more highly risk-averse individual was predicted to prefer flexible sigmoidoscopy with annual stool-based testing every 20 years, at ages 55 and 75 years, with a lower life expectancy gain of 0.19 years, or 70% of the life expectancy gain for a risk-neutral individual (0.19/0.27 years). In the context of guideline-recommended screening, one might view a moderately risk-averse individual as choosing flexible sigmoidoscopy but consistently being 2 years late for follow-up screenings; whereas, a more highly risk-averse individual initially chose flexible sigmoidoscopy but then switched to stool-based testing before deciding to obtain another flexible sigmoidoscopy at age 75 years.
Table 3 illustrates the model’s simulations for individuals with special considerations for colorectal cancer screening. For risk-averse individuals with above-average benefits from screening, model-predicted screening was generally less frequent than national guidelines but more frequent than for average-risk individuals. For example, risk-averse individuals with Crohn’s colitis were predicted to most prefer colonoscopy every 2-4 years, compared with every 1-2 years in national guidelines.35 This result confirmed the model’s expectation that individuals with above-average benefits would choose both more frequent screening than average-risk individuals (because of amplified benefits with similar risks) and less frequent screening than national guidelines (because of risk aversion). Elevated risk of bleeding did not meaningfully change optimal screening strategies.
Table 3.
Preferences for Individuals with Special Considerations for Colorectal Cancer Screening
| Risk Aversion | Method* | Ages (y) | Frequency (y) |
|---|---|---|---|
| Family history of colorectal cancer | |||
| None (Risk-Neutral) | Colonoscopy | 45-75 | 10 |
| Moderate | Colonoscopy | 45-75 | 10 |
| High | Colonoscopy | 45-75 | 10 |
| High risk of bleeding from potential polypectomy | |||
| None (Risk-Neutral) | Colonoscopy | 53-75 | 11 |
| Moderate | Flexible sigmoidoscopy | 55-75 | 10 |
| High | Flexible sigmoidoscopy | 50-64 | 14 |
| Family history of colorectal cancer and high risk of bleeding | |||
| None (Risk-Neutral) | Colonoscopy | 45-75 | 10 |
| Moderate | Colonoscopy | 53-75 | 11 |
| High | Colonoscopy | 51-75 | 12 |
| Crohn’s colitis | |||
| None (Risk-Neutral) | Colonoscopy | 36-75 | 1 |
| Moderate | Colonoscopy | 41-75 | 2 |
| High | Colonoscopy | 43-75 | 4 |
Risk-averse, hypothetical individuals with special considerations for colorectal cancer screening chose colorectal cancer screening less often than guideline recommendations, but more often than average-risk individuals.
Flexible sigmoidoscopy with annual stool-based testing.
Sensitivity analyses
When screening was allowed to continue to age 85 years, individuals generally chose later ages of initiation and cessation (Table 4). Alternatively, when the benefits of early detection were poorly understood (because hypothetical individuals only understood mean benefits, rather than the distribution of possible outcomes), moderately risk-averse individuals chose biennial stool-based testing (ages 61-75 years), while more highly risk-averse individuals chose no screening (Table 4).
Table 4.
Sensitivity Analyses
| Predicted Screening
|
|||
|---|---|---|---|
| Risk Aversion | Method | Ages | Frequency |
| Sensitivity Analysis #1: Screening allowed to continue through age 85 y | |||
| None (Risk-Neutral) | Colonoscopy | 51-84 y | Every 11 years |
| Moderate | Colonoscopy | 59-85 y | Every 13 years |
| High | Colonoscopy | 55-85 y | Every 15 years |
| Sensitivity Analysis #2: Individuals who poorly understood the benefits of early detection | |||
| None (Risk-Neutral) | Colonoscopy | 53-75 y | Every 11 years |
| Moderate | Stool-based testing | 61-75 y | Every 2 years |
| High | No screening | - | - |
We considered 2 sensitivity analyses for hypothetical individuals: (1) screening allowed to continue through age 85 y and (2) individuals who poorly understood the benefits of early detection, by only understanding the mean benefits of screening rather than the distribution of possible outcomes.
Validation
The model satisfied external and internal validity criteria (Supplement 2).
DISCUSSION
The most recent USPSTF colorectal cancer screening guidelines illustrate a transition to screening recommendations based on patient preferences. Rather than recommending a single screening practice for all at-risk individuals, the guidelines present numerous screening possibilities, and suggest that individuals and their healthcare providers choose a method based on the patient’s opinion surrounding benefits, risks, screening frequency, and other issues of importance to each individual patient.
Prior work has considered personalization in the context of risk factors (such as age,36 sex,36 family history37 and comorbidity38,39—each used to inform the optimal stop age and frequency for colonoscopy), competing needs for other preventive care services40 and genetics.41 Each of these studies determined an optimal screening strategy based on disease risk factors, with the goal of improving longevity. By contrast, in our setting, individuals had a more complex set of objectives, seeking to maximize longevity subject to their risk attitudes. Such complexity is emblematic of the multiple dimensions patients consider when making preventive health decisions. Therefore, we sought to quantify one possible decision process through which a risk-averse individual might decide between colorectal cancer screening options. Our approach differed from quality-adjusted life-years (QALYs) in that the potential to lose life-years was weighted more heavily than the potential to gain life-years, with exact weightings determined by each individual’s risk aversion. (In principle, a weighted analysis could be performed with QALYs but rarely is.) Thus, two individuals with identical disease risk factors and treatment burdens (e.g., disutility from taking a medicine)42,43 but different risk attitudes could have different optimal screening strategies. We found that more risk-averse individuals (in the context of the potential to lose life expectancy through screening complications) preferred to screen less intensively than individuals who were more focused on the potential to gain additional life expectancy from early detection. For example, while we predicted that a risk-neutral individual would choose decennial colonoscopy (consistent with national guidelines), we predicted that a moderately risk-averse individual would choose flexible sigmoidoscopy every 12 years (with annual stool-based testing) and that a highly risk-averse individual would choose flexible sigmoidoscopy every 20 years. These decision processes are pertinent, for in contrast to public health issues such as whether to fluoridate drinking water (an outcome that must be provided for all or no residents of a geographic area), it is possible to make cancer screening decisions one individual at a time, resulting in different outcomes for different individuals.44 In an era of patient-centered care, this question needs to be considered carefully.
Our findings have two additional, exploratory implications. First, because our findings suggest that rational individuals will choose to obtain screening at or around the guideline-recommended age of initiation (50 years), but less frequently than recommended thereafter, future research might consider whether public service campaigns should devote more attention to screening frequency. Little is known about individuals’ chosen frequency for obtaining colorectal cancer screening;45,46 surveys led by the CDC allow estimation of the percentage of individuals who are up-to-date with guideline recommendations, but not respondents’ age of initiation or time between screenings. Second, as illustrated by our sensitivity analysis, individuals who choose not to screen around age 50 years may not fully understand the benefits of early detection, a finding similar to prior qualitative evidence.48 Future work should consider whether a general discussion of the benefits of early detection would improve screening compliance. For example, a provider might tell a patient, “The most likely outcome of a colonoscopy is that you will be uncomfortable for 2 days and then move on with your life. However, in the unlikely event that you do have cancer, we can treat it early, and you’ll probably live an extra 4-5 years.” In our model, understanding such information (a distribution of outcomes in the main analysis) was enough to drive a patient to obtain voluntary, regular screening. On the other hand, although we found that patients would generally choose a later age of cessation if screening were permitted to age 85, we did not model aspects such as quality of life, which may decrease with age.
Naturally, it is neither practical to convey nor absorb all information presented in our model.47 Before providers could consider implementing our framework to help patients decide on their preferred colorectal cancer screening strategy, numerous limitations would need to be addressed. First, we created a basic model that focused on life expectancy gains from screening. We did not account for discomfort from screening, which is a far more common complaint than serious complications,48 or for the disutility associated with cancer-specific mortality. Second, while our framework serves as a reasonable first proxy to model individual utility, it does not fully account for processes such as adenoma size.2,28,29 Third, we did not incorporate comorbidity, although prior work suggests that this is possible.38,40,49 Fourth, we ignored monetary costs, which may encourage individuals to obtain lower-priced stool-based testing. Fifth, consistent with the methods employed in USPSTF recommendations, we did not risk stratify decisions based on prior colorectal cancer screening results.2,29 Sixth, we did not consider individuals who may be “risk-seeking” (willing to face more than actuarially fair risk in exchange for an increase in life expectancy), or patients whose primary risk aversion was to cancer morbidity or treatment (which might result in patients preferring more frequent colonoscopy). Seventh, a more realistic model would consider adherence rates. We followed the USPSTF in limiting our analysis to full adherence,2,29 because little is known about screening method-specific adherence, or the change in adherence rates with age,2 but these remain important developments for future work. Eighth, model input parameters had varying levels of evidence, with some parameters from cohort or case-control studies and others from randomized controlled trials. In studies that used an intention-to-treat analysis, the mortality reduction from screening was diluted with non-attenders to screening.
Conclusion
Quantitative models can improve understanding of how individuals may choose among various guideline-recommended colorectal cancer screening strategies. Future research might consider how to improve this framework into a shared decision-making process for clinical care.
Supplementary Material
Acknowledgments
Funding: Financial support for this study was provided in part by the National Institutes of Health, National Center for Advancing Translational Sciences (grant KL2TR000440, administered by the Clinical and Translational Science Collaborative of Cleveland). The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Footnotes
Meetings: This work was presented at the 36th Annual Meeting of the Society for Medical Decision Making, October, 2014 and the 38th Annual Meeting of the Society of General Internal Medicine, April 2015.
References
- 1.U. S. Preventive Services Task Force. Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, Jr, Garcia FA, Gillman MW, Harper DM, Kemper AR, Krist AH, Kurth AE, Landefeld CS, Mangione CM, Owens DK, Phillips WR, Phipps MG, Pignone MP, Siu AL. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016 Jun 21;315(23):2564–75. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- 2.Knudsen AB, Zauber AG, Rutter CM, Naber SK, Doria-Rose VP, Pabiniak C, Johanson C, Fischer SE, Lansdorp-Vogelaar I, Kuntz KM. Estimation of Benefits, Burden, and Harms of Colorectal Cancer Screening Strategies: Modeling Study for the US Preventive Services Task Force. JAMA. 2016 Jun 21;315(23):2595–609. doi: 10.1001/jama.2016.6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akerkar GA, Yee J, Hung R, McQuaid K. Patient experience and preferences toward colon cancer screening: a comparison of virtual colonoscopy and conventional colonoscopy. Gastrointest Endosc. 2001 Sep;54(3):310–5. doi: 10.1067/mge.2001.117595. [DOI] [PubMed] [Google Scholar]
- 4.Hawley ST, Volk RJ, Krishnamurthy P, Jibaja-Weiss M, Vernon SW, Kneuper S. Preferences for colorectal cancer screening among racially/ethnically diverse primary care patients. Med Care. 2008 Sep;46(9 Suppl 1):S10–6. doi: 10.1097/MLR.0b013e31817d932e. [DOI] [PubMed] [Google Scholar]
- 5.Katz ML, James AS, Pignone MP, Hudson MA, Jackson E, Oates V, Campbell MK. Colorectal cancer screening among African American church members: a qualitative and quantitative study of patient-provider communication. BMC Public Health. 2004 Dec 15;462 doi: 10.1186/1471-2458-4-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arias E. United States Life Tables, 2011. Natl Vital Stat Rep. 2015 Sep 22;64(11):1–63. [PubMed] [Google Scholar]
- 7.Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011 Jan 4;154(1):22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro JA, Klabunde CN, Thompson TD, Nadel MR, Seeff LC, White A. Patterns of colorectal cancer test use, including CT colonography, in the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012 Jun;21(6):895–904. doi: 10.1158/1055-9965.EPI-12-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Surveillance E, and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases Nov 2013 Sub (1973-2011 varying) - Linked To County Attributes - Total US, 1969-2012 Counties. National Cancer Institute, DCCPS, Surveillance Research Program Surveillance Systems Branch; ( www.seer.cancer.gov) released April 2014 (updated 5/7/2014), based on the November 2013 submission. [Google Scholar]
- 10.Centers for Medicare & Medicaid Services. Medicare & You: 2016. CMS Product 10050. US Department of Health and Human Services; [July 6, 2016]. Accessed at http://www.medicare.gov/Pubs/pdf/10050.pdf. [Google Scholar]
- 11.Warren JL, Klabunde CN, Mariotto AB, Meekins A, Topor M, Brown ML, Ransohoff DF. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009 Jun 16;150(12):849–57. W152. doi: 10.7326/0003-4819-150-12-200906160-00008. [DOI] [PubMed] [Google Scholar]
- 12.Laibson D, Repetto A, Tobacmanm J. Estimating discount functions with consumption choices over the lifecycle. NBER Working Paper 13314. 2007 Aug; [Google Scholar]
- 13.Gourinchas P, Parker J. Consumption over the life cycle. Econometrica. 2002 Jan;70(1):47–89. [Google Scholar]
- 14.Mankiw N, Zeldes S. The consumption of stockholders and non-stockholders. Journal of Financial Economics. 1991;29(1):97–112. [Google Scholar]
- 15.Parrino R, Poteshman A, Weisbach M. Measuring investment distortions when risk-averse managers decide whether to undertake risky projects. NBER Working Paper 8763. 2002 Feb; [Google Scholar]
- 16.Pindyck R. Risk aversion and determinants of stock market behavior. The Review of Economics and Statistics. 1998 May;52(2):183–90. [Google Scholar]
- 17.Guiso L, Sapienza P, Zingales L. Time varying risk aversion. NBER Working Paper 19284. 2013 Aug; [Google Scholar]
- 18.Einav L, Finkelstein A, Pascu I, Cullen M. How General are Risk Preferences? Choices under Uncertainty in Different Domains. Am Econ Rev. 2012 Oct;102(6):2606–38. doi: 10.1257/aer.102.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin JS, Piper MA, Perdue LA, Rutter CM, Webber EM, O’Connor E, Smith N, Whitlock EP. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2016 Jun 21;315(23):2576–94. doi: 10.1001/jama.2016.3332. [DOI] [PubMed] [Google Scholar]
- 20.Elmunzer BJ, Hayward RA, Schoenfeld PS, Saini SD, Deshpande A, Waljee AK. Effect of flexible sigmoidoscopy-based screening on incidence and mortality of colorectal cancer: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2012;9(12):e1001352. doi: 10.1371/journal.pmed.1001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Littlejohn C, Hilton S, Macfarlane GJ, Phull P. Systematic review and meta-analysis of the evidence for flexible sigmoidoscopy as a screening method for the prevention of colorectal cancer. Br J Surg. 2012 Nov;99(11):1488–500. doi: 10.1002/bjs.8882. [DOI] [PubMed] [Google Scholar]
- 22.Braithwaite RS. Can life expectancy and QALYs be improved by a framework for deciding whether to apply clinical guidelines to patients with severe comorbid disease? Med Decis Making. 2011 Jul-Aug;31(4):582–95. doi: 10.1177/0272989X10386117. [DOI] [PubMed] [Google Scholar]
- 23.Ransohoff DF. How much does colonoscopy reduce colon cancer mortality? Ann Intern Med. 2009 Jan 6;150(1):50–2. doi: 10.7326/0003-4819-150-1-200901060-00308. [DOI] [PubMed] [Google Scholar]
- 24.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009 Jan 6;150(1):1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 25.Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008 Jun;103(6):1541–9. doi: 10.1111/j.1572-0241.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 26.Hewitson P, Glasziou P, Irwig L, Towler B, Watson E. Screening for colorectal cancer using the faecal occult blood test, Hemoccult. Cochrane Database Syst Rev. 2007;1 doi: 10.1002/14651858.CD001216.pub2. CD001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ko CW, Sonnenberg A. Comparing risks and benefits of colorectal cancer screening in elderly patients. Gastroenterology. 2005 Oct;129(4):1163–70. doi: 10.1053/j.gastro.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 28.Ness RM, Holmes AM, Klein R, Dittus R. Utility valuations for outcome states of colorectal cancer. Am J Gastroenterol. 1999 Jun;94(6):1650–7. doi: 10.1111/j.1572-0241.1999.01157.x. [DOI] [PubMed] [Google Scholar]
- 29.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2008 Nov 4;149(9):659–69. doi: 10.7326/0003-4819-149-9-200811040-00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson LR, Mellor JM. Predicting health behaviors with an experimental measure of risk preference. J Health Econ. 2008 Sep;27(5):1260–74. doi: 10.1016/j.jhealeco.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, Dash C, Giardiello FM, Glick S, Johnson D, Johnson CD, Levin TR, Pickhardt PJ, Rex DK, Smith RA, Thorson A, Winawer SJ American Cancer Society Colorectal Cancer Advisory G, Force USM-ST, American College of Radiology Colon Cancer C. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008 May;134(5):1570–95. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Weinberg DS, Schoen RE. In the clinic Screening for colorectal cancer. Ann Intern Med. 2014 May 6;160(9) doi: 10.7326/0003-4819-160-9-201405060-01005. [DOI] [PubMed] [Google Scholar]
- 33.Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001 Oct;96(10):2992–3003. doi: 10.1111/j.1572-0241.2001.04677.x. [DOI] [PubMed] [Google Scholar]
- 34.Enestvedt BK, Eisen GM, Holub J, Lieberman DA. Is the American Society of Anesthesiologists classification useful in risk stratification for endoscopic procedures? Gastrointest Endosc. 2013 Mar;77(3):464–71. doi: 10.1016/j.gie.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, Dash C, Giardiello FM, Glick S, Johnson D, Johnson CD, Levin TR, Pickhardt PJ, Rex DK, Smith RA, Thorson A, Winawer SJ American Cancer Society Colorectal Cancer Advisory Group, U. S. Multi-Society Task Force, American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008 May;134(5):1570–95. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG, Boer R, Wilschut J, Winawer SJ, Habbema JD. Individualizing colonoscopy screening by sex and race. Gastrointest Endosc. 2009 Jul;70(1):96–108. e1–24. doi: 10.1016/j.gie.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilschut JA, Steyerberg EW, van Leerdam ME, Lansdorp-Vogelaar I, Habbema JD, van Ballegooijen M. How much colonoscopy screening should be recommended to individuals with various degrees of family history of colorectal cancer? Cancer. 2011 Sep 15;117(18):4166–74. doi: 10.1002/cncr.26009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Hees F, Saini SD, Lansdorp-Vogelaar I, Vijan S, Meester RG, de Koning HJ, Zauber AG, van Ballegooijen M. Personalizing colonoscopy screening for elderly individuals based on screening history, cancer risk, and comorbidity status could increase cost effectiveness. Gastroenterology. 2015 Nov;149(6):1425–37. doi: 10.1053/j.gastro.2015.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saini SD, van Hees F, Vijan S. Smarter screening for cancer: possibilities and challenges of personalization. JAMA. 2014 Dec 3;312(21):2211–2. doi: 10.1001/jama.2014.13933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taksler GB, Keshner M, Fagerlin A, Hajizadeh N, Braithwaite RS. Personalized estimates of benefit from preventive care guidelines: a proof of concept. Ann Intern Med. 2013 Aug 6;159(3):161–8. doi: 10.7326/0003-4819-159-3-201308060-00005. [DOI] [PubMed] [Google Scholar]
- 41.Jeon J, Berndt SI, Brenner H, Campbell PT, Chan AT, Chang-Claude J, Du M, Giles G, Gong J, Gruber SB, Harrison TA, Hoffmeister M, LeMarchand L, Li L, P JD, Rennert G, Schoen RE, Slattery ML, White E, Woods MO, Peters U, Hsu L. Comprehensive colorectal cancer risk prediction to inform personalized screening and intervention (Presentation Abstract #2587) American Association for Cancer Research; New Orleans, LA: 2016. Apr 18, [Google Scholar]
- 42.Sussman JB, Vijan S, Choi H, Hayward RA. Individual and population benefits of daily aspirin therapy: a proposal for personalizing national guidelines. Circ Cardiovasc Qual Outcomes. 2011 May;4(3):268–75. doi: 10.1161/CIRCOUTCOMES.110.959239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vijan S, Sussman JB, Yudkin JS, Hayward RA. Effect of patients’ risks and preferences on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med. 2014 Aug;174(8):1227–34. doi: 10.1001/jamainternmed.2014.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zulman DM, Vijan S, Omenn GS, Hayward RA. The relative merits of population-based and targeted prevention strategies. Milbank Q. 2008 Dec;86(4):557–80. doi: 10.1111/j.1468-0009.2008.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Health Interview Survey: Questionnaires, Datasets, and Related Documentation. Centers for Disease Control and Prevention; [January 9, 2015]. Accessed at http://www.cdc.gov/nchs/nhis.htm. [Google Scholar]
- 46.Behavioral Risk Factor Surveillance System: Survey Data and Documentation. Centers for Disease Control and Prevention; [January 9, 2015]. Accessed at http://www.cdc.gov/brfss/data_documentation/index.htm. [Google Scholar]
- 47.Brett AS. Flexible sigmoidoscopy for colorectal cancer screening: more evidence, persistent ironies. JAMA. 2014 Aug 13;312(6):601–2. doi: 10.1001/jama.2014.8613. [DOI] [PubMed] [Google Scholar]
- 48.Beeker C, Kraft JM, Southwell BG, Jorgensen CM. Colorectal cancer screening in older men and women: qualitative research findings and implications for intervention. J Community Health. 2000 Jun;25(3):263–78. doi: 10.1023/a:1005104406934. [DOI] [PubMed] [Google Scholar]
- 49.Cho H, Klabunde CN, Yabroff KR, Wang Z, Meekins A, Lansdorp-Vogelaar I, Mariotto AB. Comorbidity-adjusted life expectancy: a new tool to inform recommendations for optimal screening strategies. Ann Intern Med. 2013 Nov 19;159(10):667–76. doi: 10.7326/0003-4819-159-10-201311190-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


