Abstract
Objective
To determine if immediate compared to deferred initiation of antiretroviral therapy (ART) in healthy persons living with HIV (PLWH) had a more favorable impact on health-related quality of life (QOL), or self-assessed physical, mental and overall health status.
Design
QOL was measured in START (Strategic Timing of Antiretroviral Therapy), which randomized healthy ART naive PLWH with >500 CD4+ cells/μl from 35 countries to immediate versus deferred ART.
Methods
At baseline, months 4 and 12, then annually, participants completed a visual analogue scale (VAS) for “perceived current health” and the Short-Form 12-Item Health Survey version 2 from which were computed: (1) General health (GH) perception; (2) Physical Component Summary (PCS), and (3) Mental Component Summary (MCS); the VAS and GH were rated from 0=lowest to 100=highest.
Results
QOL at study entry was high (mean scores: VAS=80.9, GH=72.5, PCS=53.7, MCS=48.2). Over a mean follow-up of 3 years, changes in all QOL measures favored the immediate group (p<0.001); estimated differences were: VAS=1.9, GH=3.6, PCS=0.8, MCS=0.9. When QOL changes were assessed across various demographic and clinical subgroups, treatment differences continued to favor the immediate group. QOL was poorer in those experiencing primary outcomes; however, when excluding those with primary events, results remained favorable for immediate ART recipients.
Conclusions
In an international randomized trial in ART-naive participants with >500 CD4+ cells/μl, there were modest but significant improvements in self-assessed QOL among those initiating ART immediately compared to deferring treatment, supporting patient-perceived health benefits of initiating ART as soon as possible after an HIV diagnosis.
Keywords: HIV, antiretroviral therapy, quality of life, clinical trial, mental health
INTRODUCTION
With early initiation of antiretroviral therapy (ART) and greater life expectancy [1-3], quality of life (QOL) is an important holistic measure assessing health of persons living with HIV (PLWH). Given the World Health Organization's definition of health as "a state of complete physical, mental and social wellbeing, and not merely the absence of disease or infirmity" [4], QOL reflects one's subjective assessment of physical, emotional, and functional well-being [5,6]. QOL in PLWH on ART has been assessed in a number of studies [7-20]. Because HIV-related illnesses and the requirement for life-long medication can both affect QOL, it has become an increasingly important outcome in the comparison of HIV treatment strategies.
We measured QOL in the Strategic Timing of Antiretroviral Therapy (START) study, which enrolled adult PLWH from 35 countries; participants were ART naive, had no history of AIDS, were in good health, and had CD4+ counts of >500 cells/μl [21]; participants were randomized to either immediate ART initiation or deferring ART until the CD4+ count decreased to <350 cells/μl, or until development of AIDS or another condition requiring ART use. Through a mean of three years follow-up, the risk of the primary composite endpoint (serious AIDS or non-AIDS events, or death from any cause) was 57% lower in the immediate group.
We previously reported overall high QOL among the primarily healthy population entering this study [19]. In this analysis, we evaluated whether initiation of ART allowed patients to maintain or even improve this QOL, including their self-assessed physical, mental and overall health status, relative to deferring ART.
METHODS
QOL measures
From 2009-2013, 4,684 participants were enrolled and randomized to the immediate or deferred ART groups. The initial ART regimen was to be consistent with U.S. Department of Health and Human Services guidelines [1] and approved in the country of enrollment, but was not further specified. START was approved by institutional review boards or ethics committees at each clinical site; written consent was obtained from all participants. This analysis includes data accrued through May 2015, when START results were unblinded and ART recommended to all participants in the deferred group.
QOL measures were obtained at baseline, months 4 and 12, and annually thereafter. Health-related QOL outcomes were assessed by participant self-administered questionnaires, containing a single item visual analogue scale (VAS), and the Short-Form 12-Item Health Survey version 2 (SF-12v2). The VAS asked participants to rate from 0 to 100 their perceived current state of health [22]. The SF-12v2 asked respondents to choose one of three or five responses that best described their self-perceived current or recent physical and mental health [23]. We analyzed three QOL outcome measures derived from the SF-12v2: general health (GH), the Physical Component Summary score (PCS), and the Mental Component Summary score (MCS). On the SF-12v2 GH question, participants' perceived general health is self-rated on a 5-item Likert scale; we scored these responses as 0 (=“poor”), 20, 50, 80, or 100 (=“excellent”). PCS and MCS summary scores are weighted averages based on responses to all SF-12v2 items, and are standardized to have a mean score of 50 and a standard deviation (SD) of 10 in a U.S. reference population [23]. For all measures, higher values denote better QOL.
Statistical Analyses
All analyses were performed for each of the four QOL outcomes, unless noted otherwise. Our primary analysis compared the immediate and deferred groups by intent-to-treat for changes in QOL from baseline through follow-up, using longitudinal mixed models adjusted for visit and baseline QOL. Changes from baseline to individual follow-up times were also compared between the treatment groups using unadjusted t-tests.
To investigate possible cohort effects, we repeated treatment group comparisons for participants who had 4 or more years of follow-up. Also, to further elucidate the effect of ART, we censored follow-up in the deferred group at ART initiation, and excluded participants from the immediate group who did not start ART within the first year.
To evaluate the effect of clinical events on QOL, we performed three analyses. First, we assessed whether QOL measures declined prior to serious non-AIDS events, serious AIDS events, or all-cause deaths. For those who developed one of these events, we evaluated QOL changes from baseline to the visit immediately prior to the event. Results were compared to QOL changes in event-free participants, using t-tests adjusted for baseline QOL. In order to avoid bias due to shorter follow-up for those with an event, we performed this analysis separately for two follow-up times: (1) QOL visits through month 12, and (2) through month 36. For example, we evaluated QOL changes in those developing an event in the first 12 months, compared to changes from baseline to month 12 in those who were event-free during this period. Mean change in QOL was estimated without adjustments.
Second, we assessed whether QOL measures declined after developing serious AIDS or non-AIDS events. For those who developed an event, we evaluated QOL changes from baseline to the first QOL measurement after the event occurred; comparisons to event-free participants followed through 12 and 36 months were as described above. Third, in order to assess whether QOL treatment differences could be explained by different numbers of clinical events developing in immediate and deferred participants, we repeated the primary comparison of change in QOL between these groups after excluding any participant who experienced an event.
We summarized mean changes in CD4+ cell counts from baseline to each visit with 95% confidence intervals, and compared treatment groups for changes in CD4+ count throughout follow-up using a longitudinal mixed model adjusted for baseline CD4+ and visit. In order to assess the effect of changes in CD4+ counts on QOL, we included the time-update change in CD4+ count in longitudinal mixed models for change in QOL, in addition to baseline QOL, visit, and treatment group indicator.
We previously reported that QOL scores among participants entering this study differed by characteristics such as gender, age and geographic region [19]. We assessed the homogeneity of the treatment effect on QOL changes across various baseline characteristics in subgroup analyses, by testing for an interaction between subgroup variables with the treatment group indicator in longitudinal mixed models; for subgroups defined by categorizing a continuous variable, the continuous variable was used in the interaction test. The investigated subgroups were pre-specified in the START study protocol for the primary endpoint analyses. We also evaluated subgroups based upon the initial pre-specified ART regimen including whether or not the regimen included efavirenz, any protease inhibitor, or tenofovir; numbers on other ART drugs were much smaller.
All analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC) and R version 3 [24]. All tests are two-sided, and p-values ≤0.05 denote significance.
RESULTS
Study population
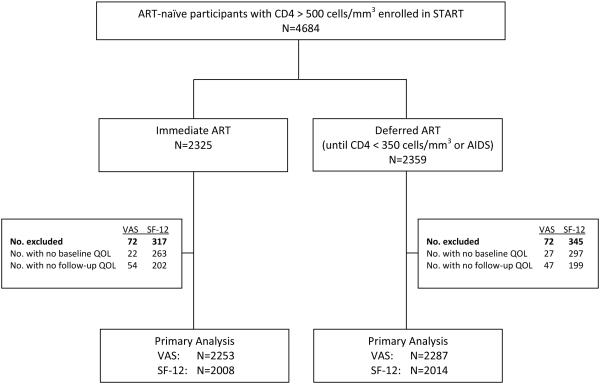
Our analyses included the 4,561 (97%) of the START participants who had a baseline and at least one follow-up QOL measure (2262 in the immediate and 2299 in the deferred group). Participants were enrolled from 35 countries. Because of translations for the SF-12v2 were not available for participants in Uganda and Nigeria who required questionnaires in their native language, only the VAS was collected for these participants. In total, 4,540 participants had a baseline and follow-up VAS, and 4022 answered all twelve items on the SF-12v2 (Figure 1).
Figure 1.
Number of participants enrolled in the START study who were included in the quality of life primary analysis, by ART treatment arm.
Baseline characteristics are summarized in Table 1, and were similar for the immediate and deferred groups. In the immediate group, the pre-specified ART regimen contained a non-nucleoside reverse transcriptase inhibitor (NNRTI) for 79% of participants, of which 95% were pre-specified efavirenz; for 18% the regimen included a protease inhibitor, and for 4% an integrase inhibitor. The nucleoside/nucleotide (NRTI) regimen included tenofovir for 89%, zidovudine for 8% and abacavir for 3%.
Table 1.
Baseline characteristics of the 4,561 START participants who had both baseline and follow-up quality of life (QOL)
| Total | Immediate ART Group |
Deferred ART Group |
|
|---|---|---|---|
| Number of participants | 4,561 | 2,262 | 2,299 |
| Demographics | |||
| Age (years), median [IQR] | 36 [29, 44] | 36 | 36 |
| Women (%) | 26.8% | 26.5% | 27.0% |
| Race/Ethnicity (%) | |||
| Asian | 8.4% | 8.5% | 8.2% |
| Black | 30.2% | 30.0% | 30.4% |
| Latino/Hispanic | 13.9% | 14.1% | 13.7% |
| White/Other | 48.5% | 48.2% | 48.8% |
| Geographic region (%) | |||
| High income | 45.8% | 46.0% | 45.5% |
| North America | 10.8% | 10.6% | 11.1% |
| Europe and Israel | 32.7% | 33.1% | 32.3% |
| Oceania (Australia) | 2.3% | 2.3% | 2.2% |
| Low/middle income | 54.2% | 54.0% | 54.5% |
| South American and Mexico | 25.2% | 25.1% | 25.4% |
| Asia | 7.7% | 7.6% | 7.7% |
| Africa | 21.3% | 21.3% | 21.4% |
| Likely mode of infection (%) | |||
| Sexual contact, same sex | 55.3% | 56.4% | 54.2% |
| Sexual contact, opposite sex | 38.1% | 37.0% | 39.2% |
| Injection drug use | 1.4% | 1.5% | 1.2% |
| Other or unknown | 5.2% | 5.0% | 5.4% |
| Clinical Data | |||
| CD4+ (cells/μL), median [IQR] | 651 [583, 763 ] | 651 | 651 |
| Nadir CD4+ (cells/μL), median [IQR] | 552 [487, 653] | 550 | 553 |
| HIV RNA (copies/mL), median [IQR] | 12,800 [3,057, 43,005] | 13,169 | 12,356 |
| Framingham 10-year risk of CHD (%) | 1.9% | 1.9% | 1.9% |
| Pre-specified antiretroviral therapy | |||
| NNRTI | 78.6% | 79.3% | 77.9% |
| Efavirenz (% of total) | 75.1% | 75.5% | 74.7% |
| Protease inhibitor | 17.6% | 16.8% | 18.4% |
| Tenofovir | 88.9% | 89.3% | 88.5%% |
| Quality of Life † | |||
| Current health (VAS), mean±SD | 80.9 ±15.7 | 81.3 ±15.5 | 80.5 ±15 |
| General health, mean±SD | 72.5 ±21.5 | 73.1 ±21.3 | 71.9 ±21.7 |
| PCS, mean±SD | 53.7 ±7.2 | 53.7 ±7.0 | 53.6 ±7.4 |
| MCS, mean±SD | 48.2 ±10.5 | 48.4 ±10.1 | 48.0 ±10.9 |
Participants marked their “current health” on a visual analogue scale (0-100). The general health, PCS and MCS outcomes were calculated from the SF-12v2 questionnaire.
Abbreviations: IQR=Interquartile range, MCS=mental health component score, NNRTI= non-nucleoside reverse transcriptase inhibitor, PCS=physical health component score, SD= standard deviation, SF-12v2= Short-Form 12-Item Health Survey version 2, VAS=Visual Analogue Scale CHD= coronary heart disease.
Median follow-up of participants was 3 years (range: 4 months to 6 years). ART was used for 95% of follow-up time in the immediate group, and 25% in the deferred group (Figure S1A). In both the immediate and deferred groups, almost all participants suppressed HIV viral load (≤200 copies/mL) while using ART (Figure S1A). Averaged throughout follow-up, CD4+ counts in the immediate group were 196 cells/μL higher than the deferred group (Figure S1B).
QOL scores by treatment arm
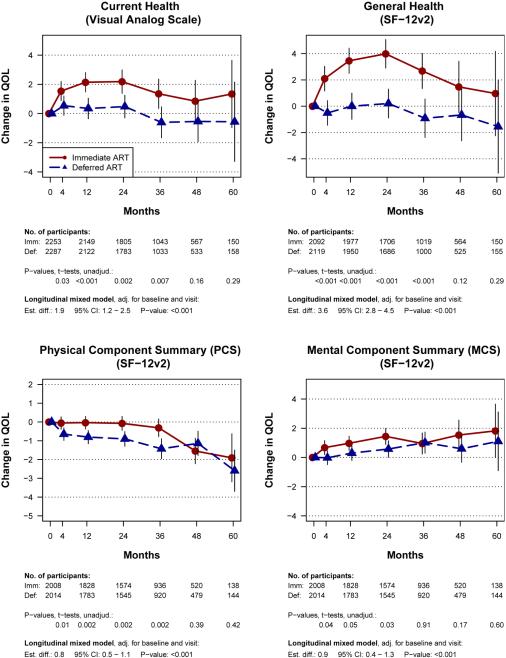
Health related QOL at study entry was generally high (Table 1). Mean baseline VAS and perceived GH scores were 80.9 and 72.5 respectively, and mean PCS and MCS scores were 53.7 and 48.2, respectively. Throughout follow-up, changes in all four QOL measures favored the immediate group (p<0.001) (Figure 2). Estimated differences were: 1.9 (95% CI: 1.2-2.5) for the VAS, 3.6 (95% CI: 2.8-4.5) for GH perception, 0.8 (95% CI: 0.5-1.1) for PCS and 0.9 (95% CI: 0.4-1.3) for MCS. Modest but significant differences were seen as early as 4 months. In the immediate group, VAS (current health) and GH perception improved relative to baseline (especially in the first 24 months), while remaining close to baseline in the deferred group. Visual inspection of the four curves suggests that VAS and GH increases above baseline during the first two years in the immediate group may have primarily reflected increases in MCS, while PCS remained stable (compared to PCS decreases in the deferred group).
Figure 2.
Change from baseline in four quality of life outcomes, for the immediate and deferred ART groups in the Strategic Timing of Antiretroviral Therapy trial. (A) Current health visual analogue scale (VAS), (B) General health perception, (C) Physical component score (PCS), (D) Mental component score (MCS). General Health, PCS and MCS are derived from the SF-12v2 survey.
Footnote: The treatment groups were compared by intent-to-treat, using t-tests for changes from baseline to individual visits, and using longitudinal mixed models for changes from baseline through follow-up. The longitudinal models included baseline QOL, visit and treatment group indicators as independent variables.
When analyses were limited to participants who had ≥4 years of follow-up, the pattern was similar, with all QOL changes favoring the immediate group (Figure S2). After censoring follow-up in the deferred group at ART initiation, and excluding 40 participants in the immediate group who did not start ART within the first year, between-group differences were slightly larger but also followed the same general pattern (Figure S3).
Effect of clinical events on QOL
For participants who developed a primary clinical event within 12 months, we first evaluated QOL changes from baseline to the visit immediately before the event (Table 2A); results were compared to QOL changes in those who did not develop a primary event within 12 months. This analysis was repeated for clinical events within 36 months. For example, those with primary events through Month 36 had mean declines of −2.6 in VAS and −8.2 in GH scores before developing these events, compared to QOL changes of +1.5 and +2.2 among those who did not experience an event through Month 36 (each p<0.001).
Table 2A.
Mean change in quality of life (QOL) from baseline to the last QOL visit prior to primary event (serious AIDS, serious non-AIDS illness, or death [21]), compared to the mean change in QOL among participants who did not experience a primary event. *
| Participants With an Event |
Participants Without Events |
|||||
|---|---|---|---|---|---|---|
| QOL Outcome** | No. | Mean† | No. | Mean‡ | Est Diff (95%CI)§ | p-value§ |
| Current health (VAS) | ||||||
| Through Month 12 | 62 | −1.77 | 4343 | 1.28 | −3.72 (−7.13, −0.30) | 0.033 |
| Through Month 36 | 109 | −2.64 | 4345 | 1.52 | −5.01 (−7.72, −2.30) | <0.001 |
| General health perception | ||||||
| Through Month 12 | 54 | −6.39 | 4006 | 1.69 | −6.99 (−12.0, −1.94) | 0.007 |
| Through Month 36 | 98 | −8.21 | 4019 | 2.19 | −9.47 (−13.3, −5.62) | <0.001 |
| PCS | ||||||
| Through Month 12 | 50 | −2.60 | 3690 | −0.40 | −2.20 (−3.98, −0.43) | 0.015 |
| Through Month 36 | 91 | −2.64 | 3686 | −0.48 | −2.38 (−3.74, −1.03) | <0.001 |
| MCS | ||||||
| Through Month 12 | 50 | −0.15 | 3690 | 0.65 | 0.15 (−2.38, 2.68) | 0.909 |
| Through Month 36 | 91 | −0.79 | 3686 | 1.04 | −1.17 (−3.10, 0.76) | 0.235 |
To minimize bias due to shorter follow-up time among those who experienced events, follow-up was censored at months 12 and 36.
Participants marked their “current health” on a visual analogue scale (0-100). The general health, PCS and MCS outcomes were calculated from the SF-12v2 questionnaire.
Mean change in QoL from baseline to the last visit prior to the event, if this visit was at month 12 or earlier (month 36 or earlier).
Mean change in QoL from baseline to the last visit at or prior to month 12 (month 36).
Estimated in a linear regression model adjusted for baseline QOL..
Abbreviations: Est Dif= Estimated Difference, CI=confidence interval, MCS=mental health component score, PCS=physical health component score, SF-12v2= Short-Form 12-Item Health Survey version 2, VAS=visual analogue scale.
Differences were even greater when we evaluated QOL changes from baseline to the visit immediately after a non-fatal event, again compared to those who were event-free during a given time period (Table 2B). For example, those with an event through Month 36 had mean declines of −7.9 in VAS and −14.4 in GH scores after developing these events, compared to increases of +1.5 and + 2.2 in those without an event (p<0.001).
Table 2B.
Mean change in quality of life (QOL) from baseline to the first QOL visit immediately after a nonfatal primary event (serious AIDS, serious non-AIDS illness [21]), compared to the mean change in QOL among participants who did not experience a primary event.*
| Participants With an Event |
Participants Without Events |
|||||
|---|---|---|---|---|---|---|
| QOL Outcome** | No. | Mean† | No. | Mean‡ | Est Diff (95%CI)§ | p-value§ |
| Current health (VAS) | ||||||
| Through Month 12 | 26 | −8.38 | 4343 | 1.28 | −10.2 (−15.5, −4.98) | <0.001 |
| Through Month 36 | 71 | −7.94 | 4345 | 1.52 | −8.83 (−12.2, −5.48) | <0.001 |
| General health perception | ||||||
| Through Month 12 | 22 | −7.95 | 4006 | 1.69 | −9.41 (−17.3, −1.52) | 0.019 |
| Through Month 36 | 64 | −14.4 | 4019 | 2.19 | −14.3 (−19.1, −9.59) | <0.001 |
| PCS | ||||||
| Through Month 12 | 19 | −4.47 | 3690 | −0.40 | −4.54 (−7.41, −1.67) | 0.002 |
| Through Month 36 | 58 | −5.42 | 3686 | −0.48 | −5.08 (−6.78, −3.38) | <0.001 |
| MCS | ||||||
| Through Month 12 | 19 | −2.83 | 3690 | 0.65 | −2.00 (−6.09, 2.08) | 0.337 |
| Through Month 36 | 58 | −3.36 | 3686 | 1.04 | −3.22 (−5.64, −0.81) | 0.009 |
To minimize bias due to shorter follow-up time among those who experienced events, follow-up was censored at months 12 and 36.
Participants marked their “current health” on a visual analogue scale (0-100). The general health, PCS and MCS outcomes were calculated from the SF-12v2 questionnaire.
Mean change in QOL from baseline to the first visit after the event, if this visit was at month 12 or earlier (month 36 or earlier).
Mean change in QOL from baseline to the last visit at or prior to month 12 (month 36).
Estimated in a linear regression model adjusted for baseline QOL.
Abbreviations: Est Dif= Estimated Difference, CI=confidence interval, MCS=mental health component score, NNRTI= non-nucleoside reverse transcriptase inhibitor, PCS=physical health component score, SF-12v2= Short-Form 12-Item Health Survey version 2, VAS=visual analogue scale.
Since primary events were associated with a lower QOL, we evaluated whether QOL differences between immediate and deferred groups could be explained by more participants experiencing events in the deferred group. We repeated the analyses shown in Figure 2, excluding those who developed a primary event. Differences between immediate and deferred groups for all four QOL measures were virtually unchanged, with estimated differences of 1.8, 3.6, 0.8 and 0.8 for the VAS, GH, PCS and MCS, respectively (each p<0.001) (Figure S4).
Effect of CD4+ count change on QOL
To determine if immediate-deferred differences could be explained by differences in CD4+ count, we estimated associations of time-updated change in CD4+ cell counts with change in QOL using longitudinal mixed models. We found that for every 100 cell CD4+ increase, VAS and GH scores increased by only 0.2 (p<0.001) and 0.4 points (p<0.001), respectively. Change in CD4+ count, even if statistically significant, therefore had a quantitatively minimal impact on QOL changes.
Subgroup analysis
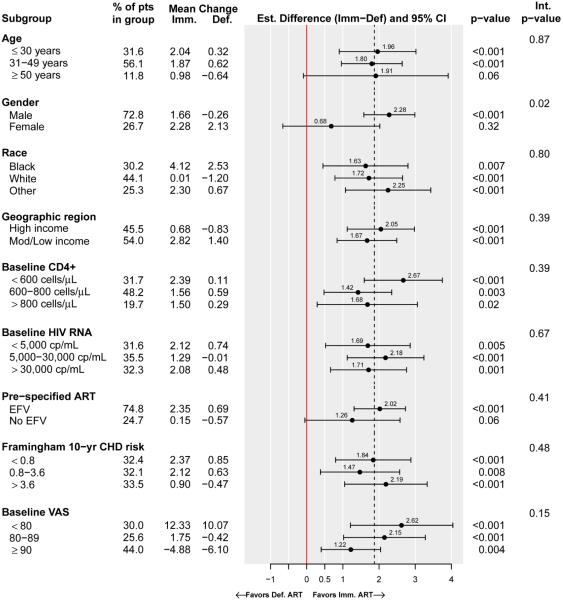
QOL changes were assessed within and across subgroups formed by different baseline demographic and clinical characteristics; Figure 3 illustrates the subgroup analyses for the VAS. Across all subgroups, treatment differences favored the immediate group for all QOL outcomes. The estimated immediate-deferred group differences in VAS were similar across all subgroup categories except for gender, where men had a greater difference compared to women (2.3 in men compared to 0.7 in women, p=0.02 for interaction). VAS differences favoring the immediate group tended to be greater for those with the lower baseline VAS scores, although the differences in treatment effect were not significant (p=0.15 for interaction between baseline VAS and the subgroup indicator).
Figure 3.
Subgroup analyses for changes in current health (Visual Analogue Scale), Strategic Timing of Antiretroviral Therapy trial
Footnotes: 1) Estimates within each subgroup were calculated using longitudinal mixed models that contained visit, baseline QOL and treatment group indicator. An interaction p-value ≤ 0.05 is evidence for heterogeneity in the treatment effect across the subgroups; the estimates and p-values for the interaction between the treatment group indicator and the participant characteristic defining the subgroups were calculated using longitudinal mixed models that contained baseline QOL, visit, and subgroup and treatment group indicators. For subgroups that were formed by discretizing continuous variables (e.g., age), the interaction effect was estimated using the continuous variable.
2) Additionally, subgroups by ART type were analyzed for participants whose pre-specified ART regimen contained (or did not contain) protease inhibitors (17.6% of participants), or tenofovir (88.4%). Treatment effects were homogeneous across these subgroups.
The treatment effect on QOL was homogeneous across subgroups formed by different types of ART regimens, including subgroups by whether or not the pre-specified regimen contained efavirenz (Figure 3 for VAS, p=0.41 for homogeneity of the treatment effect), protease inhibitors or tenofovir.
Subgroup analyses for the GH, PCS and MCS are presented in Figure S5A-C. Since we investigated many subgroups with many comparisons, subgroup analyses should be interpreted with caution due to a possible inflation of Type I error. However, changes typically favored the immediate group, and in no case did QOL changes significantly favor the deferred group. In general, differences favoring the immediate group were greatest for those with the lowest baseline QOL scores. For both GH and PCS estimated immediate-deferred group differences in QOL were homogeneous across all subgroup categories except for gender, where men again had a significantly greater difference compared to women; for MCS group differences were similar across all subgroups including gender.
DISCUSSION
In this population of ART-naïve PLWH with CD4+ counts >500 cells/μL and in good health, we found modest but significant changes in QOL measures favoring the immediate compared to the deferred ART groups for all four QOL measures. This finding was consistent across a variety of subgroups and in multiple sensitivity analyses.
For PLWH using ART, QOL may reflect perceived or real harms from treatment as well as underlying HIV disease. Patients who enrolled in START were generally healthy with relatively high baseline QOL scores [19], and avoiding decreases in QOL after initiating ART would be a desired outcome. Initiation of ART in those with minimal symptoms has been described as a "balancing act" between on the one hand preventing HIV disease and improving survival, and on the other hand not exposing patients to ART side effects [25,26]. Such adverse events include gastrointestinal symptoms, neuropsychiatric problems, or metabolic abnormalities, although newer ART drugs are associated with fewer side effects than older regimens [1,16]. For patients who started ART immediately, the goal of avoiding decreases in QOL after drug initiation was met. During the first 24 months after beginning ART, QOL for all four measures increased or remained stable, and was consistently above QOL in the deferred group.
Why participants in the immediate arm benefited to a greater degree in QOL measures is not entirely clear. Increases in CD4+ count were associated only with very small changes in QOL scores within treatment groups. Given that almost all participants suppressed HIV viral load while using ART, one cannot separate change in viral load from other ART effects. Although QOL was poorer in those who developed a primary clinical event, our analysis also indicated that lower QOL in the deferred group could not be explained solely by their developing a higher number of such events; when excluding those with primary events, our results for treatment group differences were virtually unchanged.
One possible explanation for our results is that deferred participants had a greater number of clinical symptoms and minor illnesses not meeting the criteria for clinical events, but reflected in poorer self-assessed physical QOL; the decline in PCS scores in this group over the first three years would be consistent with this hypothesis. A second possibility is that initiating ART may have led to improvements in self-perceived emotional status in the immediate group. The early increase in MCS scores in this group would be consistent with this hypothesis. Participants were not blinded to treatment arm, and some patients may have been encouraged by the belief that initiating ART represented a positive intervention in the management of their HIV infection. Other participants who feared serious ART side effects may have felt relief if they did not occur. Further studies investigating these theories are important areas for investigation.
Although the treatment effect on QOL was statistically significant, group differences and changes from baseline were relatively modest. On a scale of 0-100, estimated differences between the immediate and deferred ART groups were 1.9 for VAS and 3.6 for perceived GH. Several factors may have limited the potential for larger QOL differences. Because study enrollees were healthy with higher CD4+ counts, we would not expect dramatic decreases in QOL after entering the study, even in the absence of ART. Confirming this expectation, in deferred group participants not on ART, VAS and GH scores did not decrease for the first 24 months. In addition, because many participants entered the study with already high scores, there was limited room for further large increases. Supporting this argument, QOL score differences favoring the immediate ART initiation group were greatest for persons with the lowest baseline QOL scores. Nevertheless, despite all these factors, immediate ART resulted in higher QOL than deferred ART over the first few years.
Our conclusions of a positive impact of ART on QOL are consistent with other studies. In a study comparing continuous to CD4+ guided episodic ART use, group differences through 36 months favoring continuous use were +1.20 for VAS and +2.07 for GH [8]. In another study of patients starting ART primarily with advanced HIV disease (median CD4+ counts of 162.5 cells/μL and 38% with AIDS illnesses), PCS and MCS scores (45.3 and 42.9 at baseline) improved through 12 months by +2.6 and +1.8, respectively [27].
In our analysis of demographic and clinical subgroups, QOL changes consistently favored the immediate group over a wide variety of participant characteristics. Our finding of greater immediate-deferred differences among men compared to women should be interpreted with caution, since we evaluated many subgroups, and since our study was not designed to specifically evaluate gender differences in self assessed QOL, which may be influenced by many factors [25,28]. Our finding that treatment arm differences were significantly greater for men than women in PCS but not MCS scores suggests gender differences on overall QOL life measures (VAS and GH) primarily reflect greater improvement among men in self-assessed physical symptoms; reasons for such differences require additional evaluation.
More than three quarters of ART regimens used contained an NNRTI, almost always efavirenz, and two NRTIs that most often included tenofovir. Our subgroup analysis found that QOL changes favored the immediate group irrespective of the pre-specified ART regimen, and the size of the treatment effect was homogeneous across the subgroups by pre-specified efavirenz, protease inhibitors, or tenofovir. These results need to be interpreted with caution, however, since the START study was not designed to determine whether specific ART drugs or regimens differentially affect QOL. Pre-specified regimens were investigator-determined, decisions concerning which drug to use may have been based on various clinical considerations. Nevertheless, our analysis does suggest that those on different regimens all benefitted from earlier ART initiation.
Our QOL measures were based upon the SF-12v2 questionnaire and the VAS. Although containing only twelve items, the SF-12 was derived from the larger SF-36 and has been demonstrated to be a reliable and valid measure of this larger scale [29-31]. The VAS has also been shown to be a valid and reliable measure when evaluated against longer multi-item scales for overall QOL [22]. Although longer item QOL surveys may give more precise data on specific sub-domains, the VAS and SF-12v2 provide valid summary information, and are practical and efficient in settings such as large trials where multiple other data collection forms are also required.
Besides absence of blinding to treatment arm, our study has several other limitations. Those from different regions and cultures may attach different interpretations and significance to specific symptoms or functional status, affecting how they respond to specific QOL items [19,32,33]. PCS and MCS scores were standardized based upon U.S. populations [23], and the significance of a specific numerical QOL score may vary across countries. However, because of randomization, and because we investigated within-subject changes from baseline, such differences should minimally affect our treatment comparisons and our overall conclusion that immediate ART initiation contributes to a more favorable subjective sense of well-being. In addition, our results are consistent with the superiority of early ART as reported for major clinical outcomes in START, where events were adjudicated by a blinded endpoint review committee [21].
In summary, in this large randomized clinical trial of ART-naïve PLWH from multiple countries who were in generally good health and who had >500 CD4+ cells/μL at enrollment, there were modest but significant QOL improvements during follow-up in those immediately initiating ART compared to those who deferred treatment. Our conclusions held for measures of overall general health, for summary scales of physical and mental health and well-being, for different sensitivity analyses, and were consistent across various participant characteristics. The improvements in patient-perceived health with immediate ART adds to the growing body of evidence that supports recommendations to start ART as soon as possible after HIV diagnosis, irrespective of CD4+ cell count [1,2].
Supplementary Material
ACKNOWLEDGEMENTS
We thank all participants, investigators, and funders who made the START study possible. The START trial was designed and conducted by the International Network for Strategic Initiatives in Global HIV Trials (INSIGHT), funded by NIH Grants UM1-AI068641 and UM1-AI120197. See N Engl J Med 2015; 373:795-807 for the complete list of START investigators. Antiretroviral drugs were donated to the central drug repository by AbbVie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmith-Kline/ViiV Healthcare, Janssen Scientific Affairs, and Merck. The funders did not contribute to the study design, data analysis, or content of the manuscript. Preliminary data from this analysis was presented at the 2016 Conference on Retroviruses and Opportunistic Infections, Boston, MA. Poster #475. The START ClinicalTrials.gov identifier is NCT00867048, and START EudraCT number is 2008-006439-12.
Footnotes
The number of co-authors reflects the organization and coordination of the primary study, as well as multiple roles involved in preparation of this manuscript.
AUTHOR CONTRIBUTIONS:
AL and BG were lead on planning and overseeing this analysis and writing of the manuscript, with assistance from NE, EG and ED. All co-authors were involved in helping to develop the study design and interpret study results. BG and NE performed the statistical analysis and prepared figures and tables. AL, BG, NE and ED represented the START Statistical and Data Management Center. EG, RK, CC, FC, SD, EF, JS and SE represented the International Coordinating Centers and local sites where data collection and follow-up of study participants occurred. All co-authors reviewed interim drafts of this manuscript, and approved the final version.
REFERENCES
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed September 15, 2016.
- 2.World Health Organization . Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. WHO; Geneva: 2015. www.who.int/hiv/pub/guidelines/earlyrelease-arv/en. Accessed September 15, 2016. [PubMed] [Google Scholar]
- 3.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8(12):e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Preamble to the constitution of the World Health Organization as adopted by the International Health Conference; New York. 19 June – 22 July 1946; signed on 22 July 1946 by the representatives of 61 States (Official Records of the World Health Organization, no. 2, p. 100) and entered into force on 7 April 1948. [Google Scholar]
- 5.Cella D. Quality of life: Concepts and definition. J Pain Symptom Management. 1994;9(3):186–92. doi: 10.1016/0885-3924(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention . Measuring health days: population assessment of health-related quality of life. CDC; Atlanta: 2000. www.cdc.gov/hrqol/pdfs/mhd.pdf. Accessed September 15, 2016. [Google Scholar]
- 7.Burgoyne RW, Tan DH. Prolongation and quality of life for HIV-infected adults treated with highly active antiretroviral therapy (HAART): a balancing act. J Antimicrob Chemother. 2008;61(3):469–73. doi: 10.1093/jac/dkm499. [DOI] [PubMed] [Google Scholar]
- 8.Burman WJ, Grund B, Roediger MP, et al. The impact of episodic CD4 cell count-guided antiretroviral therapy on quality of life. J Acquir Immune Defic Syndr. 2008;47(2):185–93. doi: 10.1097/QAI.0b013e31815acaa4. [DOI] [PubMed] [Google Scholar]
- 9.Beard J, Feeley F, Rosen S. Economic and quality of life outcomes of antiretroviral therapy for HIV/AIDS in developing countries: a systematic literature review. AIDS Care. 2009;21(11):1343–56. doi: 10.1080/09540120902889926. [DOI] [PubMed] [Google Scholar]
- 10.Robberstad B, Olsen JA. The health related quality of life of people living with HIV/AIDS in sub-Saharan Africa - a literature review and focus group study. Cost Eff Resour Alloc. 2010;8:5. doi: 10.1186/1478-7547-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Boer-van der Kolk IM, Sprangers MAG, Prins JM, Smit C, de Wolf F, Nieuwkerk PT. Health-related quality of life and survival among HIV-infected patients receiving highly active antiretroviral therapy: a study of patients in the AIDS Therapy Evaluation in the Netherlands (ATHENA) Cohort. Clin Infect Dis. 2010;50(2):255–63. doi: 10.1086/649216. [DOI] [PubMed] [Google Scholar]
- 12.Marcellin F, Bonono CR, Blanche J, et al. Higher risk of unsafe sex and impaired quality of life among patients not receiving antiretroviral therapy in Cameroon: results from the EVAL survey (ANRS 12-116) AIDS. 2010;24(Suppl 1):S17–25. doi: 10.1097/01.aids.0000366079.83568.a2. [DOI] [PubMed] [Google Scholar]
- 13.Grijsen M, Koster G, van Vonderen M, et al. Temporary antiretroviral treatment during primary HIV-1 infection has a positive impact on health-related quality of life: data from the Primo-SHM cohort study. HIV Med. 2012;13(10):630–5. doi: 10.1111/j.1468-1293.2012.01020.x. [DOI] [PubMed] [Google Scholar]
- 14.Oguntibeju OO. Quality of life of people living with HIV and AIDS and antiretroviral therapy. HIV AIDS. (Auckl) 2012;4:117–24. doi: 10.2147/HIV.S32321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drewes J, Gusy B, Rüden Uv. More than 20 years of research into the quality of life of people with HIV and AIDS--a descriptive review of study characteristics and methodological approaches of published empirical studies. J Int Assoc Provid AIDS Care. 2013;12(1):18–22. doi: 10.1177/1545109712456429. [DOI] [PubMed] [Google Scholar]
- 16.Gakhar H, Kamali A, Holodniy M. Health-related quality of life assessment after antiretroviral therapy: a review of the literature. Drugs. 2013;73(7):651–72. doi: 10.1007/s40265-013-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bunupuradah T, Kosalaraksa P, Vibol U, et al. Impact of antiretroviral therapy on quality of life in HIV-infected Southeast Asian children in the PREDICT study. AIDS Patient Care STDs. 2013;27(11):596–603. doi: 10.1089/apc.2013.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langebeek N, Sprenger HG, Gisolf EH, et al. A simplified combination antiretroviral therapy regimen enhances adherence, treatment satisfaction and quality of life: results of a randomized clinical trial. HIV Med. 2014;15(5):286–90. doi: 10.1111/hiv.12112. [DOI] [PubMed] [Google Scholar]
- 19.Lifson AR, Grandits GA, Gardner EM, et al. Quality of life assessment among HIV-positive persons entering the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med. 2015;16(Suppl 1):88–96. doi: 10.1111/hiv.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mekuria LA, Sprangers MA, Prins JM, Yalew AW, Nieuwkerk PT. Health-related quality of life of HIV-infected adults receiving combination antiretroviral therapy in Addis Ababa. AIDS Care. 2015;27(8):934–45. doi: 10.1080/09540121.2015.1020748. [DOI] [PubMed] [Google Scholar]
- 21.INSIGHT START Study Group Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Boer AGEM, van Lanschot JJB, Stalmeier PFM, et al. Is a single-item visual analogue scale as valid, reliable and responsive as multi-item scales in measuring quality of life? Qual Life Res. 2004;13(2):311–20. doi: 10.1023/B:QURE.0000018499.64574.1f. [DOI] [PubMed] [Google Scholar]
- 23.Ware JE, Jr, Kosinski M, Turner-Bowker DM, Gandek B. How to Score Version 2 of the SF-12 Health Survey (With a Supplement Documenting Version1) QualityMetric Incorporated; Lincoln, RI: 2002. [Google Scholar]
- 24.R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2016. www.R-project.org. Accessed September 15, 2016. [Google Scholar]
- 25.Degroote S, Vogelaers D, Vandijck DM. What determines health-related quality of life among people living with HIV: an updated review of the literature. Arch Public Health. 2014;72(1):40. doi: 10.1186/2049-3258-72-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgoyne RW, Tan DH. Prolongation and quality of life for HIV-infected adults treated with highly active antiretroviral therapy (HAART): a balancing act. J Antimicrob Chemother. 2008;61(3):469–73. doi: 10.1093/jac/dkm499. [DOI] [PubMed] [Google Scholar]
- 27.Mannheimer SB, Wold N, Gardner EM, et al. Mild-to-moderate symptoms during the first year of antiretroviral therapy worsen quality of life in HIV-infected individuals. Clin Infect Dis. 2008;46(6):941–5. doi: 10.1086/528859. [DOI] [PubMed] [Google Scholar]
- 28.Sordo del Castillo L, Ruiz-Perez I, Olry de Labry Lima A. Biological, psychosocial, therapeutic and quality of life inequalities between HIV-positive men and women - a review from a gender perspective. AIDS Rev. 2010;12(2):113–20. [PubMed] [Google Scholar]
- 29.Gandek B, Ware JE, Aaronson NK, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51(11):1171–8. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 30.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Jenkinson C, Layte R, Jenkinson D, et al. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med. 1997;19(2):179–86. doi: 10.1093/oxfordjournals.pubmed.a024606. [DOI] [PubMed] [Google Scholar]
- 32.Scott NW, Fayers PM, Aaronson NK, et al. The relationship between overall quality of life and its subdimensions was influenced by culture: analysis of an international database. J Clin Epidemiol. 2008;61(8):788–95. doi: 10.1016/j.jclinepi.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 33.Lee JW, Jones PS, Mineyama Y, Zhang XE. Cultural differences in responses to a Likert scale. Res Nurs Health. 2002;25(4):295–306. doi: 10.1002/nur.10041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.