Abstract
Objective
Although the CDC growth charts are widely used, BMIz is known to be uninformative above the 97th percentile. We compared the relations of BMIz and other BMI metrics (%BMIp95, percent of 95th percentile, and ΔBMIp95, BMI minus 95th percentile) to circumferences, skinfolds and fat mass. We were particularly interested in the differences among children with severe obesity (%BMIp95 ≥ 120).
Methods
We used data from 30,003 2- to 19-year-olds who were examined from 1999-2000 through 2013-14 in NHANES.
Results
The theoretical maximum BMIz based on the growth charts varied by more than 3-fold across ages. The BMI metrics were strongly intercorrelated, but BMIz was less strongly related to the adiposity measures than were ΔBMIp95 and %BMIp95. Among children with severe obesity, circumferences and triceps skinfold showed almost no association with BMIz (r ≤ 0.10), whereas associations with %BMIp95 and ΔBMIp95 ranged from r=0.32 to 0.79. Corresponding associations with fat mass ÷ height2 ranged from r=0.40 (BMIz) to r=0.82 (%BMIp95) among 8- to 19-year-olds.
Conclusions
Among children with severe obesity, BMIz is only weakly associated with other measures of body fatness. Very high BMIs should be expressed relative to the CDC 95th percentile, particularly in studies that evaluate obesity interventions.
Keywords: children, NHANES, growth charts, obesity, z-scores
Introduction
The 2000 Centers for Disease Control and Prevention (CDC) growth charts (1, 2) are widely used to classify obesity (BMI ≥ 95th percentile for a child's sex and age) among 2- to 19-year-olds. In these growth charts, 10 percentiles of BMI between the 3rd and 97th were estimated using various smoothing methods (1, 3). These percentiles were then used to derive L (normality transformation for skewness), M (central tendency) and S (dispersion) parameters that allow for the estimation of z-scores and percentiles for any child (4).
The use of the LMS parameters in the CDC growth charts, however, is known to yield z-scores for very high BMIs that can differ substantially from the estimates based on the data (3, 5). This is, in part, because these parameters in the CDC growth charts were derived from already-smoothed percentiles ≤ 97th (z-score of 1.88), rather than from the underlying data as originally proposed (4). In addition, it is difficult to accurately estimate extreme values. This has led to the use of 120% of the 95th percentile of BMI rather than the LMS-extrapolated 99th percentile, to classify severe obesity (5, 6).
Although the CDC growth charts were constructed as references for clinical care and in the estimation of prevalences, BMI-for-age z-scores (BMIz) have been widely used in all types of analyses, including obesity interventions (7–10). This has occurred despite investigators having emphasized the limitations of very high BMIz values (11–15). A wide range of very high BMIs can map to similar z-scores, and BMIz values in the growth charts have a theoretical maximum (14, 16). Further, when neither the baseline nor follow-up BMIz can be estimated accurately, an examination of ΔBMIz can lead to erroneous conclusions.
The objective of the current study is to describe the relationship of various BMI metrics to other anthropometric indices of obesity (arm and waist circumferences, and triceps skinfold thickness) and to fat mass assessed by dual X-ray absorptiometry (DXA). We were particularly interested in comparing the magnitudes of these associations among children with severe obesity.
Methods
Sample and Measurements
We used data from the National Health and Nutrition Examination Survey (NHANES) from 8 cycles conducted from 1999-2000 through 2013-2014 (17). NHANES employs a multi-stage, stratified, cluster sampling design to select a representative sample of the US civilian, non-institutionalized population. The surveys were approved by the ethics review board, and parental permission was obtained for subjects < 18 y of age.
We focus on 2- to 19-year-olds who had weight and height measurements, and after excluding pregnant girls, the resulting sample size was 30,003. Race and ethnicity were self-reported, and subjects were classified as white non-Hispanic, black non-Hispanic, Mexican-American, or other (which includes other Hispanics and multi-racial persons). Weight, height, waist circumference, and mid upper-arm circumference were measured in a standardized fashion (18). Triceps skinfold thickness was measured through the 2009-10 surveys, but not in more recent cycles.
DXA scans were acquired in NHANES 1999-2006 for boys and non-pregnant girls who were ≥ 8 y using a Hologic QDR 4500A fan-beam densitometer (Hologic Inc., Bedford MA) (19, 20). The current analyses focus on fat mass (kg) and fat mass index (fat mass/ht2). The 1999-2000 DXA data for girls are not available in the publicly released data and are not included in the current analyses.
We used the NHANES DXA Multiple Imputation Data Files (19) in the analyses. These imputations, which were performed by the National Center for Health Statistics, used sequential regression to impute (estimate) missing DXA values for 5 complete datasets from non-missing DXA measurements and characteristics such as sex, race-ethnicity, age, BMI and waist circumference (21).
BMI Transformations
Body mass index (BMI) was calculated as kg/m2. BMIz was calculated by expressing a child's BMI relative to children in the CDC growth charts (1). The L (power transformation for skewness) M (median), and S (dispersion) parameters in Cole's LMS method (4) allows for the estimation of a child's z-score with:
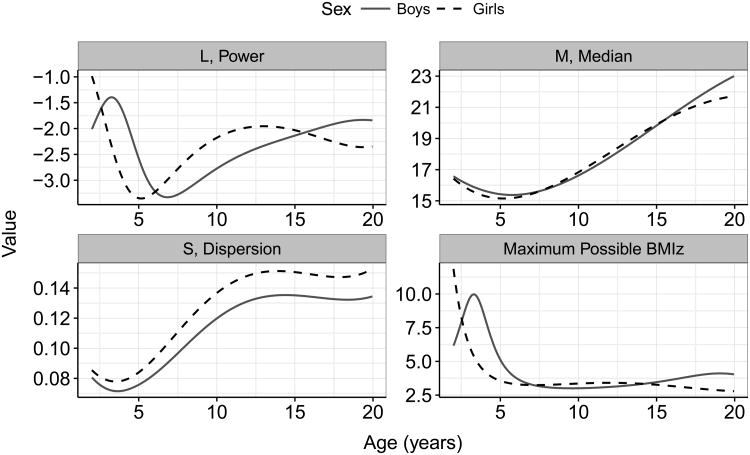
Figure 1 show the L, M, and S estimates in the CDC growth charts (http://www.cdc.gov/growthcharts/percentile_data_files.htm). Because L is large and negative, if a child's BMI is very large relative to the median BMI, (BMI ÷ M)L approaches 0 and the maximum BMIz value that is possible (−1) ÷ (L × S). An 8-year-old boy with a BMI of 80 kg/m2, for example, would have a (BMI ÷ M)L of 0.006 and a BMIz of 3.1.
Figure 1.
Sex- and age-specific values of L, M, and S in the CDC growth charts. The lower, right panel shows the maximum values of BMIz that are theoretically possible based on the CDC growth charts.
Obesity is defined as a BMI ≥ 95th percentile of the CDC growth charts (1, 2). We refer to a BMI that is expressed as a percentage of the 95th percentile as %BMIp95, and severe obesity as a %BMIp95 ≥ 120 (6). Several analyses also focus on children with %BMIp95 levels of 100 to <120; we refer to this category as moderate obesity.
We also examined other BMI metrics that account for sex and age. Because of the upper limit of BMIz, CDC proposed using ‘modified’ z-scores to identify extreme values; these were constructed by extrapolating ½ of the distance between 0 and 2 z-scores to more extreme values (16, 22). In addition to %BMIp95, which expresses a child's BMI as a percentage of the (sex- and age-specific) 95th percentile, we also examine ΔBMIp95 (BMI – 95th percentile) which is the distance (in kg/m2) from the 95th percentile. For example, an 8-year-old boy (95th percentile, 20.5 kg/m2) with a BMI of 25.3 kg/m2, would have a ΔBMIp95 of +4.8 kg/m2 and a BMIp95 of 124%, whereas a similarly aged boy with a BMI of 18 kg/m2 would have a ΔBMIp95 of -2.5 kg/m2 (2.5 units below the 95th percentile).
The CDC SAS program for the Growth Charts (23) calculates the 95th percentile of BMI, %BMIp95 and ΔBMIp95, as well as BMIz and the modified z-score for each child.
Statistical Methods
All analyses used the examination sample weights and accounted for the sample design using the survey package in R (24). About 3% to 4% of the 30,003 2- to 19-year-olds in the analyses were missing information on triceps skinfold thickness and waist circumference, and we imputed these missing values using the ‘aregImpute’ function in the Hmisc package (25). Because children who were missing information on these measures tended to have high BMIs, an analysis of only non-missing data would be biased, and multiple imputation replaces these missing values with estimates based on information from correlated variables (26). Predictors in these imputations included sex, race-ethnicity, age, BMI, DXA-calculated fat mass and fat mass index (kg of fat/m2), leg length, circumferences, and survey cycle. One imputed data set was created for each of the 5 DXA data sets, and the 5 sets of results were combined (27).
Descriptive characteristics of various characteristics are contrasted across three categories (non-obese, moderate obesity and severe obesity) of BMIp95. Before examining the relation of the various BMI metrics to levels of the circumferences, skinfolds and fat mass, we adjusted these other characteristics for sex and age by regressing each characteristic on sex, age (modeled with splines) and the sex × age interaction. The residuals from these models, referred to as ‘adjusted levels’, were then used in the correlational analyses. We also examined the correlations between the various BMI metrics and levels of circumferences, skinfolds and DXA measures within categories of sex, age (<12 y vs. ≥ 12 y) and BMI. We used lowess, a non-parametric smoother, to illustrate the relation of several of the examined characteristics to age.
Results
Table 1 shows levels of various characteristics among non-obese children, children with moderate obesity (BMIp95, 100% to 119%), and children with severe obesity. About 11% of the children had a BMI ≥ 97th percentile, and 5% had a %BMIp95 ≥ 120%. In the current study, 15 children had a %BMIp95 > 200%, with a maximum of 230% (BMI, 63 kg/m2).
Table 1. Descriptive Characteristics among 2- to 19-year-olds in NHANES, 1999-2000 through 2013-14a.
| Non-Obese (%BMIp95 < 100) | Moderate Obesity (%BMIp95 of 100% to 119 | Severe obesity (%BMIp95 ≥ 120) | |
|---|---|---|---|
|
|
|||
| N (unweighted) | 24,564 | 3,600 | 1,839 |
| Prevalence | 84% | 11% | 5% |
| Boys (%) | 51 ± 1%b | 53 ± 1% | 54 ± 2% |
| White non-Hispanics (%) | 59 ± 1% | 53 ± 2% | 44 ± 3% |
| Black non-Hispanics (%) | 14 ± 1% | 16 ± 1% | 23 ± 2% |
| Mexican-Americans (%) | 13 ± 1 % | 17 ±1% | 19 ± 2% |
| Age (y) | 10.9 ± 0.1 | 11.4 ± 0.1 | 12.9 ± 0.1 |
| BMI (kg/m2) | 18.7 ± 0.04 | 26.0 ± 0.1 | 34.1 ± 0.2 |
| BMIz (SD) | 0.13 ± 0.01 | 1.95 ± 0.01 | 2.52 ± 0.01 |
| Modified BMIz (SD) | -0.03 ± 0.01 | 1.92 ± 0.01 | 3.46 ± 0.03 |
| ΔBMIp95 (kg/m2) | -4.9 ± 0.03 | 2.0 ± 0.04 | 8.9 ± 0.1 |
| %BMIp95 (%) | 80 ± 1 | 108 ± 1 | 135 ± 4 |
| Weight-for-age z (SD) | 0.13 ± 0.01 | 1.88 ± 0.01 | 2.69 ± 0.01 |
| Height-for-age z (SD) | 0.07 ± 0.01 | 0.50 ± 0.03 | 0.70 ± 0.04 |
| Waist circumference (cm) | 65.3 ± 0.1 | 84.2 ± 0.5 | 103.4 ± 0.5 |
| Waist/height | 0.47 ± 0.001 | 0.57 ± 0.001 | 0.67 ± 0.001 |
| Triceps skinfold (mm) | 12.1 ± 0.1 | 21.7 ± 0.2 | 28.3 ± 0.2 |
| Subscapular skinfold (mm) | 9.3 ± 0.1 | 19.2 ± 0.2 | 27.0 ± 0.3 |
| % Body fatc | 26.4 ± 0.1 | 37.8 ± 0.2 | 42.5 ± 0.3 |
| Fat Mass Index (kg/m2)c | 5.5 ± 0.04 | 10.8 ± 0.1 | 15.2 ± 0.2 |
| Fat mass (kg)c | 13.8 ± 0.1 | 28.0 ± 0.4 | 40.1 ± 0.6 |
Chi-square tests indicated that the difference in sex across the 3 BMI categories was statistically significant at the 0.05 level and that difference in race-ethnicity were statistically significant at the 0.00001 level. An examination of continuous variables (beginning with age) across the 3 BMI categories indicated that all trends were statistically significant (p < 0.001).
Values are mean ± standard error
DXA measurements were available only for 8 – to 19-year-olds in the 1999-2000 through 2005-2006 cycles. There were 8345, 1340, and 780 children with DXA measurements in the 3 groups based on %BMIp95.
Severe obesity tended to be more prevalent among boys than girls (p = 0.04) and among both black and Mexican-American children than among white children (p < 0.0001). Among children with severe obesity, the mean BMIz was 2.5 SDs and the mean ΔBMIp95 was 8.9 kg/m2. As compared to children without obesity, those with severe obesity had higher mean levels of both weight-for age and height-for-age, as well as higher levels of the of circumferences, triceps skinfolds and DXA-calculated body fat.
The bottom, right panel of Figure 1 shows the maximum values of BMIz that are theoretically possible at each age, based on the LMS values in the CDC growth charts; these values have been previously published (14). Among boys, the theoretical maximum BMIz is greater than 5 among 2- to 4-year-olds, but decreases to about 3 between ages of 6 and 12 y, and then increases to about 4 at age 18 y. Among girls, the maximum possible BMIz value decreases rapidly from > 11 (age 2 y) to about 3.5 (ages 6 to 15 y); this maximum further decreases to 2.8 at age 19 y.
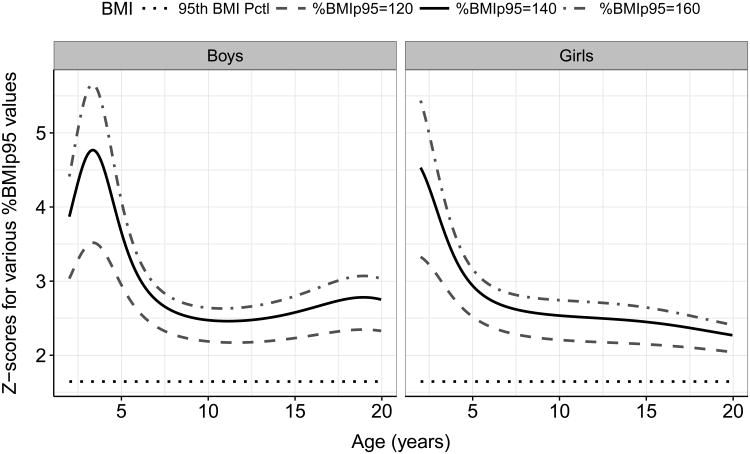
Figure 2 shows, for the CDC growth charts, BMIz values corresponding to levels of %BMIp95 between 100% (the CDC 95th percentile) and 160%. As seen for the maximum possible BMIz (Figure 1), these very high BMIz values also vary by sex and age so that a constant %BMIp95 value is associated with substantially different z-scores. Among girls, z-scores become more compressed at older ages, so that z-score differences (between constant %BMIp95 levels) become smaller. For example, a 2-year-old girl with a %BMIp95 of 140 would have a BMIz of about 4.5, whereas a 17-year-old girl with the same %BMIp95 would have a BMIz of 2.3. Among boys, the BMIz differences across %BMIp95 levels was more complex, and were most compressed at about age 10 y. A very similar pattern (data not shown) was seen if BMIz values were plotted against values of ΔBMIp95 that ranged from +6 to +18 kg/m2.
Figure 2.
Calculated BMIz values from the CDC growth charts associated with various levels of %BMIp95 and with the CDC 95th percentile of BMI.
Table 2 shows correlations among the BMI metrics, circumferences, skinfolds and fat mass among all children (top rows), those with moderate obesity (middle), and those with severe obesity (bottom). Overall, correlations among the BMI metrics were r ≥ 0.90 except for BMIz. For example, %BMIp95 (3nd column) was very strongly correlated with modified BMIz (r=0.93) and ΔBMIp95 (r=0.98). In contrast, BMIz was less strongly associated with %BMIp95 and ΔBMIp95 (r=0.81 and 0.87). In addition, BMIz consistently showed weaker (about 0.05 to 0.10 lower) correlations with the other adiposity measures than did both %BMIp95 and ΔBMIp95. Correlations with adjusted fat mass, for example, ranged from r=0.81 (BMIz) to 0.93 (%BMIp95 and ΔBMIp95.)
Table 2.
Weighted correlations among the BMI metrics and other adiposity measures, NHANES 1999-2000 through 2013-14.
| BMI Metrics | Circumferences | DXA Measuresa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| BMIz | Modified BMIz | %BMIp95 | ΔBMIp95 | Armb | Waistb | Waist / Height | Triceps Skinfoldb | Fat massb | Fat mass index | |
| All subjects (N=30,003) | ||||||||||
| BMIz | 1.00 | 0.84 | 0.78 | 0.76 | 0.73 | 0.81 | 0.78 | |||
| Modified BMIz | 0.97 | 1.00 | 0.88 | 0.83 | 0.80 | 0.76 | 0.88 | 0.84 | ||
| %BMIp95 | 0.87 | 0.93 | 1.00 | 0.93 | 0.90 | 0.86 | 0.81 | 0.93 | 0.87 | |
| ΔBMIp95 | 0.81 | 0.88 | 0.98 | 1.00 | 0.92 | 0.88 | 0.83 | 0.79 | 0.93 | 0.84 |
| Moderate Obesity (%BMIp95: 100 – 119) | ||||||||||
| BMIz | 1.00 | 0.20 | 0.21 | 0.26 | 0.14 | 0.43 | 0.26 | |||
| Modified BMIz | 0.99 | 1.00 | 0.21 | 0.22 | 0.26 | 0.15 | 0.42 | 0.22 | ||
| %BMIp95 | 0.79 | 0.82 | 1.00 | 0.52 | 0.50 | 0.45 | 0.36 | 0.46 | 0.39 | |
| ΔBMIp95 | 0.66 | 0.69 | 0.96 | 1.00 | 0.60 | 0.58 | 0.52 | 0.41 | 0.54 | 0.48 |
| Severe Obesity (%BMIp95 ≥ 120 | ||||||||||
| BMIz | 1.00 | 0.10 | 0.10 | 0.18 | 0.07 | 0.65 | 0.40 | |||
| Modified BMIz | 0.87 | 1.00 | 0.36 | 0.30 | 0.35 | 0.19 | 0.66 | 0.55 | ||
| %BMIp95 | 0.48 | 0.80 | 1.00 | 0.70 | 0.55 | 0.56 | 0.32 | 0.71 | 0.71 | |
| ΔBMIp95 | 0.30 | 0.61 | 0.93 | 1.00 | 0.79 | 0.63 | 0.62 | 0.34 | 0.83 | 0.82 |
DXA measurements were available only for 8 – to 19-year-olds in the 1999-2000 through 2005-2006 cycle (N=10,465). Of these subjects, 1340 were moderately obese, and 780 were severely obese.
Levels of arm circumference, waist circumference, triceps skinfold, and DXA-calculated fat mass were sex- and age-adjusted.
Of the 30,000 subjects, 3600 had moderate obesity and 1839 had severe obesity. All children with severe obesity had a LMS-extrapolated BMI percentile > 98th.
Although the magnitudes of the observed correlations were weaker among children with moderate obesity (middle) and those with severe obesity (bottom), due at least in part to the restricted ranges of BMI values, the decreases were most evident for BMIz. Among children with severe obesity, for example, correlations between BMIz and levels of both ΔBMIp95 and %BMIp95 were of r < 0.5. Further, among children with severe obesity, BMIz showed almost no association with adjusted levels of the two circumferences and triceps skinfold (r ≤ 0.10), and only a moderate (r=0.40) correlation with fat mass index, which was not available among 2- to 7-year-olds. In general, ΔBMIp95 showed the strongest associations with the other body size measures among children with either moderate or severe obesity. For example, correlations with waist to height among children with severe obesity were r=0.18 (BMIz), r=0.56 (%BMIp95) and r=0.62 (ΔBMIp95), while the comparable associations among children with moderate obesity ranged from r=0.26 (BMIz) to 0.52 (ΔBMIp95).
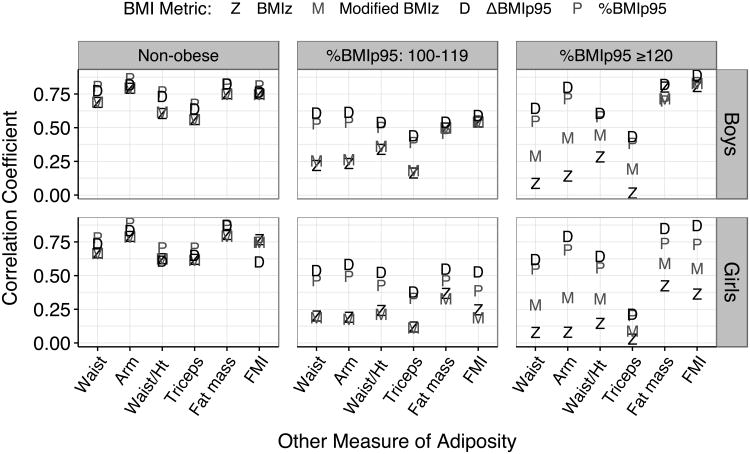
We then examined the relation of the various BMI metrics to the other adiposity measures within categories of BMI status and sex (Figure 3). For both boys (upper panels) and girls, associations with the other body size measures were generally stronger for %BMIp95 and ΔBMIp95 than for BMIz, and the magnitudes of the differences among the correlations were largest for children with severe obesity (right panels). Of the 36 sets of correlations (2 sexes × 3 obesity groups × 6 body size measures), 24 were strongest for ΔBMIp95 and 11 were strongest for %BMIp95. Only for levels of fat mass index among non-obese girls, was the correlation with BMIz slightly higher than that with %BMIp95 (r = 0.77 vs 0.75). Among children with severe obesity, several of the associations between BMIz and adjusted levels of circumferences and skinfolds were < 0.30. The largest difference in the magnitudes of the 4 BMI metrics among children with severe obesity was seen for adjusted levels of arm circumference among girls, with correlations ranging from r=0.08 (BMIz) to r=0.79 (ΔBMIp95).
Figure 3.
Relation of BMIz, modified BMIz, %BMIp95 and ΔBMIp95 to other body size measures, by sex and BMI status. Values of arm circumference, waist circumference, triceps skinfold and fat mass were adjusted for sex and age.
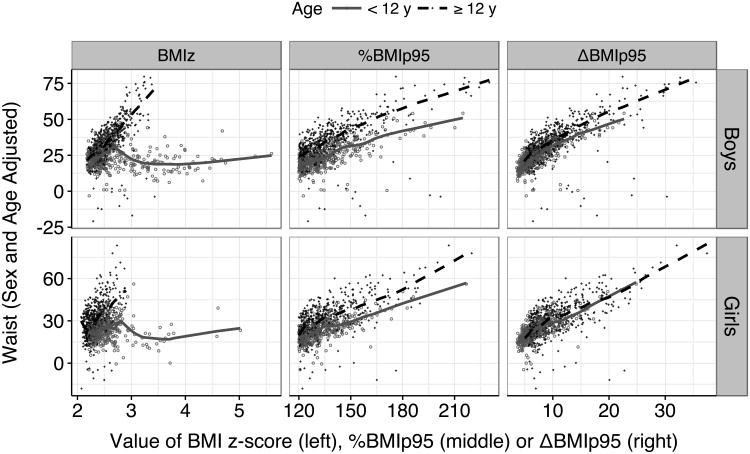
The relations of BMIz, %BMIp95 and ΔBMIp95 to adjusted levels of waist circumference are shown in Figure 4 among children with severe obesity. BMIz (left panels) showed a strong association with adjusted waist circumference among 12- to 19-year-olds, but almost no association with BMIz values above 2.5 among younger children (solid line). In contrast, both %BMIp95 and ΔBMIp95 showed strong associations with waist circumference over their entire ranges in both age groups.
Figure 4.
Relation of various BMI metrics to the sex- and age-adjusted levels of waist circumference among children with severe obesity
Discussion
About 6% of 2- to 19-year-olds currently have a BMI that is greater than or equal to 120% of the CDC 95th percentile (i.e., %BMIp95 ≥ 120) (28), and there is much interest in the evaluation and treatment of these children (6, 29). Although the report accompanying the CDC growth charts (1) noted that extrapolation outside the 3rd through 97th percentiles (z-scores of ± 1.88) should be interpreted cautiously, BMIz values remain widely used in cross-sectional and longitudinal analyses of children with severe obesity (7, 8, 10, 30, 31). Our results emphasize that BMIz functions poorly as an indicator of adiposity among children with obesity, particularly among those with severe obesity. Among children with severe obesity, BMIz is, in general, much less strongly associated with circumferences, skinfolds and fat mass than are ΔBMIp95 and %BMIp95. Because a wide range of very high BMI values can map to essentially the same z-score, which varies by sex and age, differences between children who have different levels of adiposity, as well as longitudinal BMIz changes (32), can be obscured. Many of the limitations of BMIz, particularly when based on the CDC growth charts, have been emphasized in previous studies (11–14), and our results highlight the weak relationship of BMIz to levels of other measures of fatness among children who are severely obese.
Because of the LMS transformation, BMIz values can differ substantially among children who have similar levels of %BMIp95, ΔBMIp95 and other measures of adiposity. For example, there were 2 boys (ages 3 and 9 y) in the current study who had a %BMIp95 of about 140, but the older boy had a markedly lower BMIz (2.5 vs 4.7). This BMIz difference resulted from the sex and age changes in the L and S parameters in the CDC growth charts which affect z-scores > 1.88 (97th percentile). Previous studies (11–15) have concluded that LMS-based z-scores should not be used to assess BMI changes. Our results indicate that among children with severe obesity, even very large (e.g., >1 SD) BMIz differences may simply reflect differences in sex or age rather than body size.
The attenuation and confounding of very high BMIz values could influence the results of longitudinal studies that include a large proportion of children who have severe obesity. For example, a 17-year-old girl with a BMI of 50 kg/m2 would have a BMIz of 2.6 based on the CDC LMS parameters (33). (The theoretical maximum BMIz for this sex/age is 3.1.) If this girl were to gain an additional 29 kg over 2 years, her BMI would increase by about 10 kg/m2, but her BMIz would remain constant. In contrast, there would be large increases in %BMIp95 (from 169% to 193%) and ΔBMIp95 (from +29 to +38 kg/m2) reflecting her large weight increase.
The influence of the sex/age differences in the L and S parameters on very high BMIz levels may account for some of the conflicting results in the literature. For example, an intervention study (34) of children with severe obesity (mean BMI, 37 kg/m2) reported a small, statistically significant decrease in BMIz (-0.03 SD) along with a statistically significant 1 kg/m2 increase in BMI. These problems would be particularly relevant for intervention studies that contain a large proportion of children with severe obesity, and if analyses focused only on ΔBMIz (7, 30, 31, 35), it is possible that the conclusions could be incorrect. Both cross-sectional and longitudinal studies that include a large proportion of children with severe obesity should express BMI levels relative to the CDC 95th percentile.
In the current study, BMIz showed a moderate to strong association with both ΔBMIp95 and %BMIp95 (r=0.81 to 0.87) among all children, but much weaker associations (r < 0.50) among children with severe obesity. Although the relationships of %BMIp95 and ΔBMIp95 to the other measures of adiposity were generally similar, there are situations when 1 might be preferred. A 1-unit increase in ΔBMIp95 for example, would indicate that a child's BMI increased by 1 kg/m2 more than expected based on sex and change in age, and this might be easier to interpret than a change in %BMIp95. However, for comparisons of children across a wide range of ages over which BMI levels vary substantially, %BMIp95 might be preferred as a 1 unit BMI difference should likely be interpreted differently among 2-year-olds than among 18-year-olds. It is possible that neither ΔBMIp95 nor %BMIp95 would be best in all situations, and that both metrics could be investigated. It should also be noted that %BMIp95 and ΔBMIp95 resemble other BMI metrics that have been proposed, such as sympercents (11, 12, 36) and percent over BMI (12). Additional analyses (data not shown) of sympercents, defined in the current study as the difference, on the natural log scale, between a child's BMI and the 95th percentile of BMI, indicated that this metric was strongly associated (r=0.99) with %BMIp95.
There are several limitations of the current study that should be considered when interpreting our results. Our results concerning the extrapolation of the LMS calculations apply to children with obesity, and particularly those with severe obesity. However, even in the entire sample of children, BMIz was less strongly correlated with the other adiposity measures than were ΔBMIp95 and %BMIp95 (Table 2). It should also be realized that attenuation of the LMS-estimated z-scores in the CDC growth charts was recognized by CDC, and a set of modified z-score were developed to address this limitation (16). These modified z-scores are based on extrapolating the distance between 0 and 2 SDs to more extreme BMI values and therefore do not have an upper limit. However, the appropriateness of applying this fixed distance to very high BMI values is uncertain, and a somewhat different approach was used in the WHO growth standards (37). It should also be realized that all of the adiposity measures in the current study, including circumferences, triceps skinfold and DXA measurements, become increasingly difficult to accurately assess among children and adults with severe obesity (38, 39). For example, about 27% of all children with severe obesity in the current study had a DXA measurement that was imputed from other characteristics (21). It should be also realized it would have been optimal to have examined differences between the BMI metrics using a gold-standard measure of adiposity rather than with indirect measures (e.g., circumferences) or with DXA measurements that were available for only a subset of the children.
Although LMS-extrapolated z-scores for children who have very high BMI levels are widely used (7, 30, 31, 35), with some investigators specifically recommending the use of ΔBMIz among children with severe obesity (http://stokes.chop.edu/web/zscore), this approach is not optimal. BMIz levels among children with severe obesity (1) can differ substantially from the empirical estimates, (2) have an effective upper limit resulting in the mapping of very high BMIs to similar z-scores, and (3) can differ solely due to differences in sex and age. Investigators using the CDC growth charts to calculate BMIz values for analyses should be aware of these limitations. Although the drawbacks of BMIz would be unlikely to markedly influence the analysis of population-based studies, the results of studies that include a large proportion of children who have severe obesity should be verified using %BMIp95 or ΔBMIp95. For studies that include many children with extreme BMI values, including those focused on obesity interventions, the analyses should likely emphasize %BMIp95, ΔBMIp95 or another metric based on the 95th percentile rather than BMIz.
What is already known on this subject.
BMI-for-age z-scores based on the CDC growth charts are widely used
BMI percentiles and z-scores for very high BMIs based on the LMS parameters in these growth charts can differ substantially from values that are directly estimated from the data. Therefore, 120% of the 95th percentile, rather than the LMS 99th percentile, is used to classify severe obesity
Previous investigators have emphasized the difficulties in interpreting z-scores and changes in these z-scores for children with very high BMIs
What does this study add?
BMI-for-age z-scores among children who have obesity, and particularly those who have severe obesity, show weaker correlations with other measures of adiposity, such as triceps skinfold, circumference and DXA-calculated fat mass than do other BMI metrics that adjust for sex and age.
Rather than using BMI-for-age z-scores for very high BMIs, these BMIs should be expressed relative to the 95th percentile either as the distance (kg/m2) from the sex- and age-specific 95th percentile or as a percentage of the 95th percentile.
Acknowledgments
NB and ET were supported by funds from the Centers for Disease Control and Prevention (RFA-DP-11-007). ET was supported by funds from the Centers for Disease Control and Prevention (U18DP003370) and by grant K24 DK10589 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviations
- BMI
body mass index
- CDC
Centers for Disease Control and Prevention
- NHANES
National Health and Nutrition Examination Survey
- DXA
dual-energy x-ray absorptiometry
Footnotes
Disclosure: The authors declare no conflict of interest
The findings and conclusions in this report do not represent the official position of the Centers for Disease Control.
References
- 1.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002;11:1–190. [PubMed] [Google Scholar]
- 2.Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. Natl Health Stat Report. 2010:1–5. [PubMed] [Google Scholar]
- 3.Flegal KM, Cole TJ. Construction of LMS parameters for the Centers for Disease Control and Prevention 2000 growth charts. Natl Health Stat Report. 2013;9:1–3. [PubMed] [Google Scholar]
- 4.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11:1305–19. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 5.Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, Curtin LR. Characterizing extreme values of body mass index-for-age by using the 2000 Centers for Disease Control and Prevention growth charts. Am J Clin Nutr. 2009;90:1314–1320. doi: 10.3945/ajcn.2009.28335. [DOI] [PubMed] [Google Scholar]
- 6.Kelly AS, Barlow SE, Rao G, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: A Scientific Statement from the American Heart Association. Circulation. 2013;128:1689–712. doi: 10.1161/CIR.0b013e3182a5cfb3. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Cai L, Wu Y, et al. What childhood obesity prevention programmes work? A systematic review and meta-analysis. Obes Rev. 2015;16:547–565. doi: 10.1111/obr.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siwik V, Kutob R, Ritenbaugh C, et al. Intervention in overweight children improves body mass index (BMI) and physical activity. J Am Board Fam Med. 2013;26:126–37. doi: 10.3122/jabfm.2013.02.120118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelley GA, Kelley KS, Pate RR. Effects of exercise on BMI z-score in overweight and obese children and adolescents: a systematic review with meta-analysis. BMC Pediatr. 2014;14:225. doi: 10.1186/1471-2431-14-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCormick EV, Dickinson LM, Haemer MA, Knierim SD, Hambidge SJ, Davidson AJ. What can providers learn from childhood body mass index trajectories: a study of a large, safety-net clinical population. Acad Pediatr. 2014;14:639–45. doi: 10.1016/j.acap.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Cole TJ, Faith MS, Pietrobelli A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI%, BMI z-score or BMI centile? Eur J Clin Nutr. 2005;59:419–425. doi: 10.1038/sj.ejcn.1602090. [DOI] [PubMed] [Google Scholar]
- 12.Paluch RA, Epstein LH, Roemmich JN. Comparison of methods to evaluate changes in relative body mass index in pediatric weight control. Am J Hum Biol. 2007;19:487–94. doi: 10.1002/ajhb.20608. [DOI] [PubMed] [Google Scholar]
- 13.Berkey CS, Colditz GA. Adiposity in adolescents: change in actual BMI works better than change in BMI z score for longitudinal studies. Ann Epidemiol. 2007;17:44–50. doi: 10.1016/j.annepidem.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Woo JG. Using body mass index Z-score among severely obese adolescents: a cautionary note. Int J Pediatr Obes. 2009;4:405–410. doi: 10.3109/17477160902957133. [DOI] [PubMed] [Google Scholar]
- 15.Kakinami L, Henderson M, Chiolero A, Cole TJ, Paradis G. Identifying the best body mass index metric to assess adiposity change in children. Arch Dis Child. 2014;99:1020–4. doi: 10.1136/archdischild-2013-305163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Modified z-scores in the CDC growth charts. [WWW document]. URL http://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/BIV-cutoffs.pdf.
- 17.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics: National Health and Nutrition Examination Survey Data. Questionnaires, Datasets, and Related Documentation. 2016 [WWW document]. URL http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
- 18.National Center for Health Statistics (NCHS), Center for Health Statistics N, National Center for Health Statistics (NCHS) Anthropometry Procedures Manual. National Health and Nutrition Examination Survey (NHANES) 2014 [WWW document]. URL http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/Anthropometry_Procedures_Manual.pdf.
- 19.National Center for Health Statistics. The 1999-2006 Dual Energy X-ray Absorptiometry (DXA) Multiple Imputation Data Files and Technical Documentation. 2016 [WWW document]. URL https://wwwn.cdc.gov/Nchs/Nhanes/Dxx/dxa.aspx.
- 20.National Center for Health Statistics. National Health and Nutrition Examination Survey: Technical Documentation for the 1999-2004 Dual Energy X-Ray Absorptiometry (DXA) Multiple Imputation Data files. [WWW document]. URL http://www.cdc.gov/nchs/data/nhanes/dxa/dxa_techdoc.pdf.
- 21.Schenker N, Borrud LG, Burt VL, et al. Multiple imputation of missing dual-energy X-ray absorptiometry data in the National Health and Nutrition Examination Survey. Stat Med. 2010;30:260–276. doi: 10.1002/sim.4080. [DOI] [PubMed] [Google Scholar]
- 22.Freedman DS, Lawman HG, Skinner AC, McGuire LC, Allison DB, Ogden CL. Validity of the WHO cutoffs for biologically implausible values of weight, height, and BMI in children and adolescents in NHANES from 1999 through 2012. Am J Clin Nutr. 2015;102:1000–6. doi: 10.3945/ajcn.115.115576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC) A SAS Program for the 2000 CDC Growth Charts. [WWW document]. URL https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm.
- 24.Lumley T. Survey: Analysis of complex survey samples. R package version 3.30-3. [WWW document]. URL http://cran.r-project.org/web/packages/survey/index.html.
- 25.Harrell FE. Hmisc: Harrell Miscellaneous. 2016 [WWW document]. URL http://cran.r-project.org/web/packages/Hmisc/index.html.
- 26.Donders ART, van der Heijden GJMG, Stijnen T, Moons KGM, Donders ART. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59:1087–1091. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Lumley T. mitools: Tools for multiple imputation of missing data. [WWW document]. URL https://cran.r-project.org/web/packages/mitools/
- 28.Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA. 2016;315:2292–9. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gulati AK, Kaplan DW, Daniels SR. Clinical tracking of severely obese children: a new growth chart. Pediatrics. 2012;130:1136–40. doi: 10.1542/peds.2012-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kreier F, Genco ŞM, Boreel M, et al. An individual, community-based treatment for obese children and their families: the solution-focused approach. Obes Facts. 2013;6:424–32. doi: 10.1159/000355909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hampl S, Odar Stough C, Poppert Cordts K, Best C, Blackburn K, Dreyer Gillette ML. Effectiveness of a hospital-based multidisciplinary pediatric weight management program: Two-year outcomes of PHIT Kids. Child Obes. 2016;12:20–5. doi: 10.1089/chi.2014.0119. [DOI] [PubMed] [Google Scholar]
- 32.Butte NF, Cai G, Cole SA, et al. Metabolic and behavioral predictors of weight gain in Hispanic children: the Viva la Familia Study. Am J Clin Nutr. 2007;85:1478–85. doi: 10.1093/ajcn/85.6.1478. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention (CDC) Percentile data files with LMS values. [WWW document]. URL http://www.cdc.gov/growthcharts/percentile_data_files.htm.
- 34.Skelton JA, DeMattia LG, Flores G. A pediatric weight management program for high-risk populations: a preliminary analysis. Obesity (Silver Spring) 2008;16:1698–701. doi: 10.1038/oby.2008.243. [DOI] [PubMed] [Google Scholar]
- 35.Baughcum AE, Gramling K, Eneli I. Severely obese preschoolers in a tertiary care obesity program: characteristics and management. Clin Pediatr (Phila) 2015;54:346–52. doi: 10.1177/0009922814555975. [DOI] [PubMed] [Google Scholar]
- 36.Cole TJ, Kryakin YV. Sympercents: symmetric percentage differences on the 100 log(e) scale simplify the presentation of log transformed data. Stat Med. 2002;21:2287–90. doi: 10.1002/sim.1008. [DOI] [PubMed] [Google Scholar]
- 37.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weight-for- age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization; 2006. [Google Scholar]
- 38.Haroun D, Wells JC, Williams JE, Fuller NJ, Fewtrell MS, Lawson MS. Composition of the fat-free mass in obese and nonobese children: matched case-control analyses. Int J Obes. 2005;29:29–36. doi: 10.1038/sj.ijo.0802834. [DOI] [PubMed] [Google Scholar]
- 39.Bray GA, Greenway FL, Molitch ME, Dahms WT, Atkinson RL, Hamilton K. Use of anthropometric measures to assess weight loss. Am J Clin Nutr. 1978;31:769–73. doi: 10.1093/ajcn/31.5.769. [DOI] [PubMed] [Google Scholar]