Abstract
Objective
To test the hypothesis that HIV infection impairs the beneficial effects of weight-loss on insulin-sensitivity and adipose-tissue inflammation and endoplasmic reticulum (ER) stress.
Methods
A prospective clinical trial to evaluate the effects of moderate diet-induced weight-loss on body-composition, metabolic function and adipose-tissue biology in women with obesity who were HIV-seronegative (HIV−) or HIV-positive (HIV+). Body-composition, multi-organ insulin-sensitivity (assessed by using a 2-stage hyperinsulinemic-euglycemic clamp procedure with stable isotopically-labeled tracer infusions), and adipose-tissue expression of markers of inflammation, autophagy and ER stress were evaluated in 8 HIV− and 20 HIV+ women with obesity before and after 6%–8% diet-induced weight-loss.
Results
Although weight-loss was not different between groups (~7.5%), the decrease in fat-free-mass was greater in HIV+ than HIV− subjects (−4.4±0.7 % vs −1.7±1.0%, P < 0.05). Weight loss improved insulin-sensitivity in adipose-tissue (suppression of palmitate rate of appearance [Ra]), liver (suppression of glucose Ra) and muscle (glucose disposal) similarly in both groups. Weight-loss did not affect adipose-tissue expression of markers of inflammation or ER stress in either group.
Conclusions
Moderate diet-induced weight-loss improves multi-organ insulin-sensitivity in HIV+ women to the same extent as women who are HIV−. However, weight loss causes a greater decline in FFM in HIV+ than HIV− women.
Keywords: diabetes, energy restriction, HIV
Introduction
Combination antiretroviral therapy (cART) increases survival in HIV infected (HIV+) people. Unfortunately, this therapeutic success is associated with an increased prevalence of obesity, the metabolic syndrome, type 2 diabetes (T2D), and cardiovascular disease (CVD), particularly in women (1, 2, 3). The adverse metabolic effects of obesity are especially pertinent in the setting of HIV, because HIV+ people already have a 2–4 fold increased risk of developing T2D and CVD than BMI-matched, HIV seronegative (HIV−) people (2, 4, 5). Although obesity increases the risk of T2D in people with HIV, increased BMI does not increase the risk of CVD events (6).
The reason for the high prevalence of T2D in HIV+ people is unclear, but several hypotheses have been proposed. The use of cART, itself, can have adverse metabolic effects, including adipose tissue inflammation (7), impaired insulin signaling and β-cell dysfunction (8, 9). Furthermore, several protease-inhibitors (PIs) increase endoplasmic reticulum (ER) stress, which can induce inflammation and apoptosis (9, 10, 11). The induction of ER stress can also induce autophagy, the orderly degradation and recycling of cellular components. PIs can have adverse effects on autophagy, which could contribute to HIV associated insulin resistance and attenuate the metabolic benefits of weight loss (12, 13).
Moderate (5–10%) weight loss in obese people without HIV infection improves all features of the metabolic syndrome (14, 15). Although weight loss is recommended for obese HIV+ people (16), it is not known whether weight loss actually confers metabolic benefits in this group. We are aware of only one prospective study that evaluated the effect of weight loss on metabolic outcomes in obese HIV+ patients (17). In that study, 7% weight loss induced by a low-calorie diet in combination with a supervised exercise program did not result in metabolic benefits. Similarly, Fitch et al were able to reduce waist circumference, but not BMI, using a lifestyle-intervention program however again found no benefit on lipid profile or fasting blood glucose concentration (18). The reason for the absence of a therapeutic metabolic effect is unclear, but might involve adverse effects of cART, direct effects of HIV on metabolic function, HIV-induced inflammation, and inadequate power to detect a therapeutic effect because of the small number of subjects who had metabolic testing.
The purpose of the present study was to perform a prospective trial to evaluate the effect of moderate 6–8% weight loss, induced by a low-calorie diet, on CVD risk factors, liver and skeletal muscle insulin sensitivity and plasma and adipose tissue markers of inflammation in HIV− and HIV+ women with obesity and who had evidence of insulin-resistant glucose metabolism. We hypothesized that the therapeutic effects of weight loss on insulin sensitivity and markers of inflammation, autophagy and ER stress would be blunted in HIV+ women compared with HIV− women.
Methods
Study subjects
Twenty women who were obese and HIV+ (age 37±2 yrs, BMI 43.8±2.9 kg/m2, 17 African American, AA, 3 Caucasian) and 8 women who were obese and HIV− (age 40±1 yrs, BMI 39±2 kg/m2, 6 AA, 2 Caucasian) enrolled in the study (see supplemental Figure 1). All women completed a history and physical examination, standard blood tests, and an oral glucose tolerance test. All participants were “insulin-resistant” based on a homeostasis model assessment of insulin resistance (HOMA-IR) value > 3.0, impaired fasting glucose or impaired glucose tolerance according to American Diabetes Association criteria (19) and had ≥2 additional ATP III criteria for metabolic syndrome (20). Potential participants who consumed an excessive amount of alcohol (> 2 drinks/day), had a history of hepatitis B or C, or took medications that affect glucose metabolism were excluded. All participants were weight stable (≤2% change in body weight) and sedentary (<1 hour of exercise per week) for at least two months before beginning the study. All HIV+ women were receiving stable cART, had a plasma CD4+ T-cell count >200 cells/PL, and an undetectable plasma viral load (< 50 copies HIV RNA/ml) for at least 6 months before enrollment. The study was approved by the Human Studies Committee of Washington University School of Medicine (St Louis, MO). Written informed consent was obtained from all subjects. The study was registered with clinicaltrials.gov, NCT00857298.
Experimental Design
Body composition assessments
Body fat and fat-free mass (FFM) were determined by using dual-energy-X-ray absorptiometry (21). Visceral and abdominal subcutaneous adipose tissue volumes were quantified by using magnetic resonance imaging (22). Intrahepatic triglyceride content was measured by using magnetic resonance spectroscopy (23). MRI was not able to be performed in 4 HIV+ subjects and 2 HIV− subjects.
Blood pressure
Systolic and diastolic blood pressures were measured by taking the average of 2 measurements made 5 minutes apart during resting conditions, 30 minutes before the clamp procedure was started.
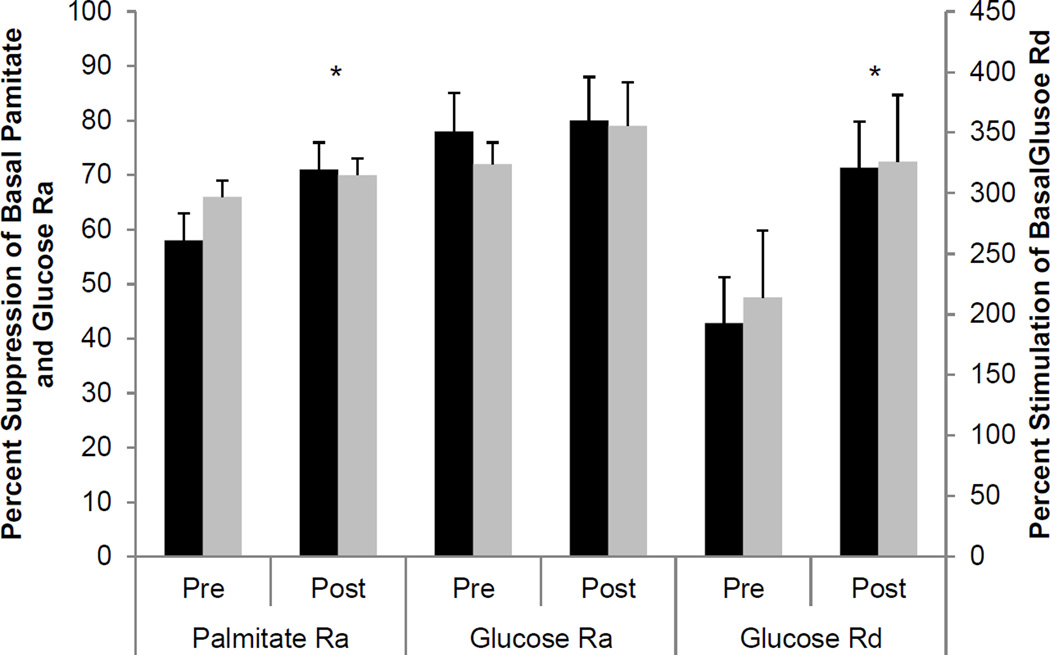
Hyperinsulinemic-euglycemic-clamp procedure (Figure 1
Figure 1.
Percent suppression of palmitate Ra and glucose Ra during stage 1 of the hyperinsulinemic-euglycemic clamp procedure and percent stimulation of glucose Rd during stage 2 of the hyperinsulinemic-euglycemic clamp procedure before and after weight loss in HIV− (black bars) and HIV+ subjects (grey bars). * Main effect of time, P < 0.05
All subjects were studied during the follicular phase of their menstrual cycle. A two-stage hyperinsulinemic-euglycemic clamp procedure was performed in conjunction with infusion of [2,2-2H2]-palmitate to assess adipose tissue lipolytic rate, and [6,6-2H2]-glucose to measure endogenous glucose production and glucose disposal rates.
Subjects were admitted to the Clinical Research Unit the day before the clamp procedure. At 1900h, subjects consumed a standard meal, containing 15 kcal·kg·FFM−1 and then fasted (except for water) and rested in bed until completion of the clamp procedure the next day. The following morning, at 0500h, a catheter was inserted into an antecubital vein of one arm to infuse insulin, dextrose and stable isotopically-labeled tracers, and another catheter was inserted into a contralateral hand vein, which was placed in a thermostatically controlled (55°C) box to obtain arterialized venous blood samples (24). At 0600h, a primed (22.5 µmol·kg−1), continuous infusion (0.25 µmol·kg−1·min−1) of [6,6-2H2]glucose was started. At 120 minutes, a continuous infusion (0.035 µmol·kg−1·min−1) of 2H2-palmitate was started. At 210 minutes (stage 1), the insulin infusion was initiated at 7 mU·m2 body surface area−1·min−1 (initiated with priming doses of 28 mU·m2 body surface area−1·min−1 for 5 minutes followed by 14 mU·m2 body surface area−1·min−1 for 5 minutes) and continued for 3 hours. At 390 minutes (stage 2), the insulin infusion rate was increased to 50 mU·m2 body surface area−1·min−1 (initiated with priming doses of 200 mU·m2 body surface area−1·min−1 for 5 minutes followed by 100 mU·m2 body surface area−1·min−1 for 5 minutes) and continued for 3.5 hours. Plasma glucose concentration was measured every 10 min during insulin infusion and maintained at ~100 mg/dl by infusing 20% dextrose containing 2.5% [6,6-2H2]glucose. The infusion rate of [6,6-2H2]glucose and [2,2-2H2]palmitate was decreased by 50% during stage 1 and by 75% of the basal rate during stage 2 to account for the expected decline in hepatic glucose production. Blood samples were taken every 10 min during the last 30 minutes of the basal period and stage 1 and stage 2 of the clamp procedure to determine plasma glucose concentration, plasma palmitate and glucose tracer-to-tracee ratios (TTRs), and plasma hormone concentrations. Resting energy expenditure was measured by using indirect calorimetry during the basal period of the clamp procedure (Parvo Medics' TrueOne® 2400, Sandy, UT) (15). Adipose tissue biopsies were obtained during the basal stage of the clamp procedure as described previously (25).
Weight-loss intervention and repeat studies
The energy content of the diet was designed to cause a 1000 kcal/day energy deficit based on each subject’s estimated energy requirement (calculated as 1.5 × measured resting energy expenditure). Participants received weekly dietary counseling from research dietitians. Meal replacements (Optifast™, Nestle HealthCare Nutrition, Geneva, Switzerland) were provided to replace two meals/day, and subjects were instructed to eat a third meal each day containing regular food. When the targeted 6%–8% weight-loss was achieved, dietary intake was adjusted to maintain a stable body weight for 2 weeks before repeating the body composition assessments, the hyperinsulinemic-euglycemic clamp procedure and abdominal subcutaneous adipose biopsies.
Sample Analyses
Plasma substrate and hormone concentrations
Plasma glucose concentration was determined by using an automated glucose analyzer (YSI 2300 STAT Plus; Yellow Springs Instrument Co, Yellow Springs, OH). Plasma insulin and leptin concentrations were measured by using a chemiluminescent immunometric method (Immulite; Siemens, Los Angeles, CA). Plasma C-reactive protein (CRP) concentration was measured by using a particle-enhanced immunoturbidometric assay (Roche Diagnostics, IN, USA). HbA1C was measured by using a commercial assay (Roche Diagnostics, IN, USA). Plasma FFA concentrations were quantified by gas chromatography (Hewlett-Packard 5890-II, Palo Alto, CA) (26).
Plasma glucose and palmitate isotopic enrichment
Plasma palmitate and glucose TTRs were measured by using selected ion-monitoring electron impact ionization GC-MS (Hewlett-Packard MSD 5973 system) as previously described (27, 28).
mRNA isolation and quantitative PCR
Frozen adipose tissue samples were homogenized in TRIzol Reagent (Life Technologies) for total RNA isolation according to the manufacturer's protocol. cDNA synthesis was performed (Life Technologies). Quantitative real-time PCR (qRT-PCR) was performed using Power Sybr Green in the ABI 7500 Fast real-time PCR system (Applied Biosystems) to determine mRNA expression levels of the salient genes in adipose tissue samples before and after weight-loss. Primer sequences are provided in Supplementary Table 1. qRT-PCR results were analyzed by comparing the sample threshold crossing (Ct) after normalization to the housekeeping gene acidic ribosomal phosphoprotein P0 (36B4) (ΔCt). Changes in the threshold crossing (ΔCt) were used to calculate relative levels of each mRNA using the formula 2−ΔCt.
Calculations
Glucose and palmitate rates of appearance (Ra) into plasma and glucose rate of disappearance (Rd) from plasma were calculated as previously described (29). Insulin sensitivity in: 1) the liver was assessed as the suppression of glucose Ra during low-dose insulin infusion; 2) skeletal muscle was assessed as the increase in glucose Rd during high-dose insulin infusion ; and 3) adipose tissue was assessed as the suppression of palmitate Ra during low-dose insulin infusion (30).
Statistical Analysis
Baseline subject characteristics were compared by using the two-tailed Student’s t-test. A two-way analysis of variance with repeated measures was used to compare between and within-group differences in the outcome measures induced by weight loss. Tukey’s post-hoc procedure was used to locate differences if a significant group × time interaction was found. All P-values ≤ 0.05 were considered statistically significant. Data are expressed as means ± SEM. All data were analyzed by using SPSS (version 18; IBM, New York, USA).
Results
Only 19 of the 28 subjects enrolled in the study achieved the targeted 6–8% weight loss and completed the study; 13 HIV+ (10 African-Americans, 3 Caucasians), and 6 HIV− women (4 African-American, 2 Caucasians). Among the HIV+ women initially enrolled, one dropped out due to intolerance of intravenous catheters, one was lost to follow-up and 5 did not meet weight loss goals. Among the HIV− women, 2 were withdrawn because they did not meet weight loss goals. All HIV+ women were treated with emtricitabine and tenofovir and either non-nucleoside reverse-transcriptase inhibitor based therapy (nevirapine (N=3) or efavirenz, (N=4)),or ritonavir-boosted PIs (darunavir (N=2) or atazanavir (N=4)), or raltegravir. The mean duration of known HIV serostatus was 12±2 years (range 4–21 yrs) and the mean duration of cART was 7±1 yrs (range 4–13 yrs). The time to achieve goal weight loss was greater in the HIV+ than the HIV seronegative group (18±2 weeks vs 12±1 weeks, P=0.006). The flow of study subjects is summarized in supplemental Figure 1.
Body composition
Baseline body composition and fat distribution were not different between the HIV− and HIV+ groups (Table 1). Mean weight loss was not different between the HIV− and HIV+ subjects (−7.3±0.1% vs −7.7±0.1%, respectively), and caused a similar reduction in visceral adipose tissue volume and intrahepatic triglyceride (IHTG) content in both groups (Table 1). In contrast, weight loss caused a greater decrease in both whole body and appendicular (limb) fat-free mass in HIV+ than HIV− subjects (Table 1).
Table 1.
Body composition and cardiometabolic variables before and after weight loss.
| HIV− before | HIV− after | Percent difference |
HIV+ before |
HIV+ after | Percent difference |
|
|---|---|---|---|---|---|---|
| Body weight (kg) | 113±17 | 105±6 | −7.3±0.1* | 108±5 | 100±4 | −7.7±0.1* |
| BMI (kg/m2) | 39.9±2.2 | 36.9±1.9 | −7.1±1.0* | 39.1±1.6 | 36.3±1.5 | −7.2±0.8* |
| Fat-free mass (kg) | 55.7±3.0B | 54.8±2.9A, B | −1.7±1.0B | 51.1±2.0B | 48.9±1.9A, B | −4.4±0.7B |
| Appendicular fat-free mass (kg) |
25.5±.9 | 25.4±1.1* | −0.6±2.4 | 25.2±1.3 | 23.7±1.1*, B | −5.8±0.7B |
| Fat mass (% body weight) | 45.9±2.4 | 44.0±2.3 | −4.4±1.6* | 49.7±1.6 | 48.0±1.4 | −3.2±0.8* |
| Total abdominal adipose tissue (cm3) |
5052±574 | 4815±618 | −6.4±4.9* | 5227±428 | 4757±461* | −7.4±3.1* |
| Visceral adipose tissue (cm3) |
1139±187 | 1034±183 | −12±4* | 1164±140 | 997±137* | −14±4* |
| VAT:TAT ratio | 0.22±0.03 | 0.22±0.03 | −5.1±4.7* | 0.24±0.03 | 0.22±0.03* | −7.1±3.0* |
| IHTG content (%) | 7.5±2.4 | 5.4±1.5 | −16.8±43.8* | 4.6±1.7 | 2.2±1.0* | −26.3±18.6* |
| Systolic BP (mmHg) | 126±5 | 117±6 | −7±4* | 122±3 | 118±4 | −4±2* |
| Diastolic BP (mmHg) | 76±5 | 69±5 | −9±3* | 72±3 | 69±3 | −4±3* |
| Glucose (mg/dl) | 91.5±3.0 | 87.6±2.5 | −4.0±3.3 | 94.1±2.0 | 91.7±1.6 | −2.2±2.0 |
| Insulin (µU/L) | 18.3±3.3 | 8.8±2.6 A, B | −54.0±7.3B | 16.6±2.5 | 14.2±1.9B | −11.1±10.5B |
| C-peptide (ng/ml) | 3.2±0.5 | 2.2±0.4A, B | −28.2±9.4 | 2.8±0.3 | 2.7±0.2B | −3.9±6.6B |
| Leptin (µg/L) | 39.0±6.6 | 36.2±6.3 | −8.6±11.8* | 53.1±4.5 | 39.8±4.3 | −20.4±6.4* |
| Hemoglobin A1C (%) | 5.8±0.2 | 5.3±0.2 | −7.9±2.9 | 5.7±0.1 | 5.6±0.1 | −3.2±2.0 |
| FFA (µmol/ml) | 550±66 | 527±85 | −3.1±7.9 | 661±45 | 662±58 | −1.1±8.4 |
| LDL-C (mg/dl) | 111±14 | 100±7 | −9±5 | 103±10 | 101±12 | −1±4 |
| HDL-C (mg/dl) | 43±3 | 39±3A | −9±3* | 42±2 | 39±2 A | −7±3* |
| Triglyceride (mg/dl) | 128±28 | 92±28 | −17±15 | 112±18 | 119±22 | 12±12 |
Values are means±SE.
VAT:TAT, visceral adipose tissue (VAT) area as percent total abdominal adipose tissue (TAT) area; IHTG, intrahepatic triglyceride content; FFA, free-fatty acid; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
P<0.05 for main effect of time.
Value is different from corresponding value at baseline, P<0.05.
Value is different from corresponding value in other group, P<.0.05.
Metabolic variables
Weight loss decreased systolic and diastolic blood pressures (P < 0.05) and tended to decrease fasting blood glucose concentration (P=0.08) in both groups, without any differences between groups (Table 1). Fasting plasma insulin and C-peptide concentrations were not different between groups at baseline, and were lower after weight loss in the HIV− (P < 0.01), but not the HIV+ group. Accordingly, the percent change in fasting plasma insulin concentration was greater in the HIV− than the HIV+ group (P < 0.03).
By design, plasma glucose concentrations during the high-dose insulin infusion of the euglycemic-hyperinsulinemic clamp procedure were the same before and after weight loss in the HIV− (101.1±0.7 and 101.7±1.7 mg/dl) and HIV+ (101.0±1.0 and 101.9±1.1 mg/dl) groups, and were not different between groups. Plasma insulin concentrations during the clamp procedure were also the same before and after weight loss in both HIV− (81.0±4.3 and 86.1±9.3 µU/ml) and HIV+ (74.6±4.6 and 74.6±5.6 µU/ml) groups.
At baseline, basal glucose Ra, glucose Rd and palmitate Ra and the effect of insulin infusion on glucose and palmitate kinetics were not different between HIV− and HIV+ subjects (Figure 1). Both the absolute and relative decreases in palmitate Ra during stage 1 of the clamp procedure were greater after weight loss than before weight loss in both groups, without a difference between groups (Figure 1). Weight loss did not affect basal glucose Ra, but tended to cause a greater decline in glucose Ra during low-dose insulin infusion in both groups, but the decreases were not statistically significant (Figure 1). Both the absolute and relative increase in glucose Rd during insulin infusion were greater after weight loss than before weight loss in both groups, without a difference between groups (Figure 1).
Plasma immune and inflammatory markers
At baseline, mean plasma CRP concentration was not different between groups, and tended to decrease after weight loss in both HIV− (from 5.9±1.9 to 4.4±1.5 mg/L) and HIV+ (from 5.2±1.3 to 4.2±1.0 mg/L) subjects, but the changes were not statistically significant (P=0.14). Plasma HIV viremia was undetectable in HIV+ subjects at baseline and remained undetectable after weight loss. In addition, weight loss did not affect CD4+ T-cell counts (641±79 cells/µL before and 701±81 cells/µL after weight loss, P=0.29).
Adipose tissue biology
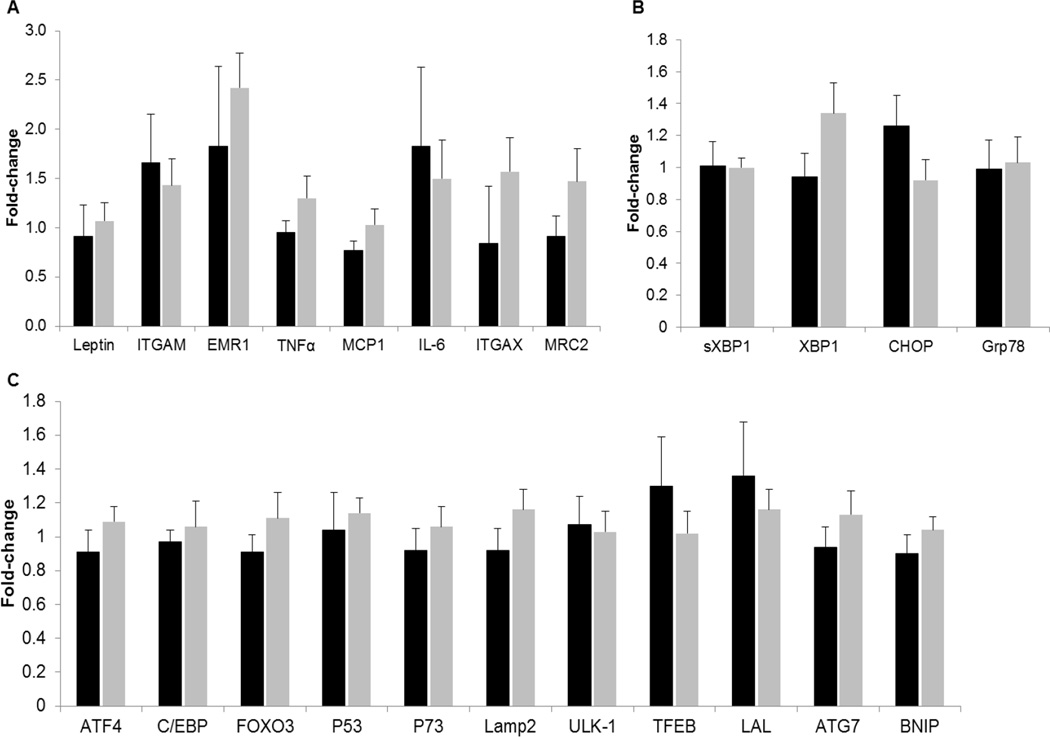
Gene expression of markers of inflammation (ITGAM, EMR1, TNFα, MCP1, IL-6, ITGAX, MRC2) (Figure 2A), ER stress (sXBP1, XPB1, CHOP and Grp78) (Figure 2B) or autophagy (ATF4, C/EBP, FOXO3, P53, P73, Lamp2, ULK1, TFEB, LAL, ATG7, BNIP) (Figure 2C) were not different between groups at baseline (data not shown) and did not change with weight loss in either HIV− or HIV+ groups..
Figure 2.
Fold-changes in adipose tissue gene expression of markers of inflammation (A), autophagy (B), and endoplasmic reticulum stress (C) after weight loss in HIV – (black bars) and HIV+ subjects (grey bars)
ITGAM, integrin α-M (CD11b); EMR1, EGR-like module-containing mucin-like hormone receptor-1 like 1; TNFα, tumor necrosis factor α; MCP1, monocyte chemoattractant protein 1, IL-6, interleukin 6; MRC2, mannose-receptor 2; ITGAX, integrin alpha X (CD11c). mRNA expression normalized to the housekeeping control gene acidic ribosomal phosphoprotein P0 (36B4); ATF4, activating transcription factor 4; CEBP, CCAAT enhancer-binding protein; FOXO3, forkhead box O3; lysosomal-associated membrane protein 2; ULK-1, unc-51 like autophagy activating kinase; TFEB, transcription factor EB; LAL, lysosomal acid phosphatase; ATG7, autophagy-related protein 7; BNIP BCL2/adenovirus E1B protein-interacting protein. mRNA expression normalized to the housekeeping control gene acidic ribosomal phosphoprotein P0 (36B4); sXBP1, spliced x-box binding protein 1; XBP1, x-box binding protein 1; CHOP, C/EBP homologous protein; Grp78, glucose-regulated protein 78.
Discussion
The results from this study provide the first evidence that moderate diet-induced weight loss improves metabolic function in insulin-resistant, HIV+ women who are obese. Matched weight loss caused a similar improvement in multi-organ insulin sensitivity, decline in intra-abdominal adipose tissue volume and IHTG content, and decreased systolic and diastolic blood pressure in both groups. These cardiometabolic improvements were not associated with alterations in adipose tissue gene expression of markers of inflammation, autophagy or ER stress. In contrast, weight loss caused a greater decrease in FFM in HIV+ than in HIV− women. These results demonstrate that moderate diet-induced weight loss has important therapeutic cardiometabolic effects in HIV+ women with obesity.
Our finding that moderate (~7%) weight loss improves skeletal muscle insulin sensitivity, assessed as insulin-mediated glucose disposal rate during insulin infusion, differ from the results of a previous study that found a 7% weight loss achieved by dietary modification and physical activity failed to improve insulin sensitivity, assessed by using the frequently-sampled intravenous glucose tolerance test (17). The results from two other studies found that lifestyle therapy in patients with HIV infection resulted in a decrease in waist circumference without a change in body weight, but failed to show any beneficial metabolic benefits (18, 31). The reason for the different findings between our study and the previous study that found weight loss in HIV+ patients did not improve insulin sensitivity could be related to differences in cART regimens and in the methods used to assess insulin action. Our patients received ritonavirboosted darunavir or atazanavir and emtricitabine with tenofovir, which could have less adverse metabolic effects than older (unspecified) PIs used in the previous study (32, 33, 34). In addition, we assessed insulin sensitivity by using a two-stage hyperinsulinemic-euglycemic clamp procedure in conjunction with a stable isotopically labeled glucose tracer infusion, which is a more sensitive measure of insulin action than the frequently-sampled intravenous glucose tolerance test (35). Nonetheless, despite the improvement we observed in insulin sensitivity, the decline in basal plasma insulin concentration was blunted in HIV+ compared with HIV− subjects. The absence of a decline in plasma insulin concentrations after weight loss in obese HIV-infected people has been reported previously (17). The reason for persistent basal hyperinsulinemia is not known.
Data from studies conducted in rodent models and in human subjects suggest that ER stress and unfolded protein response-pathways are increased in the setting of obesity and hyperinsulinemia and could be involved in the pathogenesis of insulin resistance (36, 37). However our data do not support the notion that decreased adipose tissue inflammation, autophagy or ER stress are responsible for the weight loss-induced improvement in cardiometabolic outcomes. Adipose tissue markers of these biological processes did not change after weight loss in either HIV− or HIV+ subjects. Our results are consistent with data from previous clinical studies demonstrating that moderate weight loss does not affect markers of inflammation in adipose tissue (38), and we are not aware of any studies that evaluated the effect of moderate weight loss on autophagy or ER stress. However, marked (~30%) weight loss reduces markers of ER stress (39),
Diet induced weight loss caused a greater decrease in whole-body and appendicular FFM in HIV+ women than HIV− women. The mechanism responsible for the difference in FFM loss between groups is not known, but could be due to insulin-resistant protein metabolism in skeletal muscle, in conjunction with the catabolic effects of a hypocaloric diet. We previously found that whole-body proteolytic rates are increased and insulin-mediated suppression of muscle protein breakdown is impaired in insulin resistant HIV+ people (40). It is unlikely that differences in protein intake between groups confounded our findings because subjects received at least 42 grams (~0.75g/kgFFM/day) of protein from liquid shakes (Optifast™, Nestle Health Care Nutrition, Geneva, Switzerland) in addition to the dietary protein in their main meals each day. Nonetheless, it is unlikely the small (~1.3 kg) greater decline in FFM in HIV+ subjects had adverse effects on physical function.
Our study has several limitations. First, we only included women, so our findings might not apply to men. However, obesity is approximately three times more common in HIV+ women than HIV+ men, so our results are relevant to a large proportion of people with HIV infection. Nonetheless, future studies conducted in men will be needed to confirm whether the results we found in women are also found in men. Second, our subjects were being treated with several different antiretroviral regimens; therefore we cannot determine whether specific cART regimens influence the metabolic benefits of weight loss. Third, the number of subjects in each group was small, which decreased our ability to detect differences between the groups. However, we used precise and reproducible measures of metabolic function (35), and found the differences in key outcome measures between groups were very small making it unlikely we missed physiologically-important differences. Finally, although we did not find differences between groups in either adipose tissue or systemic markers of inflammation, our assessment of systemic inflammation (plasma CRP concentration) was not robust, so it is possible that additional and more sensitive markers (e.g. soluble CD14, CD163, MIP-1a) could have detected an effect of HIV infection and weight loss.
In summary, moderate weight loss improved multi-organ insulin sensitivity and several other key risk factors for cardiovascular disease in women with obesity and HIV-infection to the same degree that weight loss improved these outcome measures in women with obesity but without HIV-infection. However, weight loss in HIV+ women caused a greater decline in FFM than the same amount of weight loss in HIV− women.
Supplementary Material
What is known
Obesity is extremely common in women with HIV infection, and is associated with insulin resistance, dyslipidemia and cardiovascular disease.
HIV infection and its treatment are associated with a markedly increased risk of diabetes and cardiovascular disease, possibly due to adipose-tissue inflammation and/or ER stress.
Prior studies have found that women with HIV infection and obesity have blunted improvements in insulin-sensitivity and other cardiovascular risk factors when they undergo moderate weight-loss compared to HIV-seronegative women.
What this study adds
Moderate weight-loss caused similar improvements in insulin sensitivity, between the HIV seropositive and seronegative groups.
Despite similar amounts of weight loss, women with HIV infection lost significantly more fat-free mass and appendicular fat-free mass than HIV seronegative women.
Weight-loss did not reduce markers of inflammation or ER stress in adipose tissue in either seropositive and seronegative groups.
Acknowledgments
The authors’ responsibilities were as follows—DNR, KEY, and SK: designed the research; DNR and WTC: conducted the research; DNR: analyzed the data and had primary responsibility for the final content; NAA and TP designed, conducted and analyzed the adipocyte mRNA expression, and DNR, NAA, KEY and SK: wrote the manuscript. All authors read and approved the final manuscript.
This work was supported by the Doris Duke Foundation Clinical Scientist Development Award, National Institutes of Health Grants DK-56341 (Nutrition and Obesity Research Center), DK096982, DK20579 (Diabetes Research Center), P41-GM-103422 (Biomedical Mass Spectrometry Resource), UL1-RR-024992 (Clinical and Translational Science Award).
The authors thank Freida Custodio and Jennifer Shew (Washington University School of Medicine, St. Louis, MO) for their technical assistance, the staff of the Clinical Research Unit (Washington University School of Medicine, St. Louis, MO) and the study participants.
Footnotes
Clinical trials.gov identifier: NCT00857298
None of the authors had any potential conflicts of interest to disclose.
References
- 1.Mondy K, Overton ET, Grubb J, Tong S, Seyfried W, Powderly W, et al. Metabolic syndrome in HIV-infected patients from an urban, midwestern US outpatient population. Clin Infect Dis. 2007;44:726–734. doi: 10.1086/511679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan RC, Kingsley LA, Sharrett AR, Li X, Lazar J, Tien PC, et al. Ten-year predicted coronary heart disease risk in HIV-infected men and women. Clin Infect Dis. 2007;45:1074–1081. doi: 10.1086/521935. [DOI] [PubMed] [Google Scholar]
- 3.Koethe JR, Jenkins CA, Lau B, Shepherd BE, Justice AC, Tate JP, et al. Rising Obesity Prevalence and Weight Gain Among Adults Starting Antiretroviral Therapy in the United States and Canada. AIDS Res Hum Retroviruses. 2016;32:50–58. doi: 10.1089/aid.2015.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179–1184. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 5.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. The Journal of clinical endocrinology and metabolism. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Womack JA, Chang CC, So-Armah KA, Alcorn C, Baker JV, Brown ST, et al. HIV infection and cardiovascular disease in women. Journal of the American Heart Association. 2014;3:e001035. doi: 10.1161/JAHA.114.001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes care. 33:2244–2249. doi: 10.2337/dc10-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murata H, Hruz PW, Mueckler M. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. J Biol Chem. 2000;275:20251–20254. doi: 10.1074/jbc.C000228200. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S, Carper MJ, Lei X, Cade WT, Yarasheski KE, Ramanadham S. Protease inhibitors used in the treatment of HIV+ induce beta-cell apoptosis via the mitochondrial pathway and compromise insulin secretion. American journal of physiology. 2009;296:E925–E935. doi: 10.1152/ajpendo.90445.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Djedaini M, Peraldi P, Drici MD, Darini C, Saint-Marc P, Dani C, et al. Lopinavir co-induces insulin resistance and ER stress in human adipocytes. Biochem Biophys Res Commun. 2009;386:96–100. doi: 10.1016/j.bbrc.2009.05.148. [DOI] [PubMed] [Google Scholar]
- 11.Kraus M, Malenke E, Gogel J, Muller H, Ruckrich T, Overkleeft H, et al. Ritonavir induces endoplasmic reticulum stress and sensitizes sarcoma cells toward bortezomib-induced apoptosis. Mol Cancer Ther. 2008;7:1940–1948. doi: 10.1158/1535-7163.MCT-07-2375. [DOI] [PubMed] [Google Scholar]
- 12.Nunez CE, Rodrigues VS, Gomes FS, Moura RF, Victorio SC, Bombassaro B, et al. Defective regulation of adipose tissue autophagy in obesity. Int J Obes (Lond) 2013;37:1473–1480. doi: 10.1038/ijo.2013.27. [DOI] [PubMed] [Google Scholar]
- 13.Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. The American journal of clinical nutrition. 1992;56:320–328. doi: 10.1093/ajcn/56.2.320. [DOI] [PubMed] [Google Scholar]
- 15.Kirk E, Reeds DN, Finck BN, Mayurranjan SM, Patterson BW, Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology. 2009;136:1552–1560. doi: 10.1053/j.gastro.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aberg JA, Kaplan JE, Libman H, Emmanuel P, Anderson JR, Stone VE, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:651–681. doi: 10.1086/605292. [DOI] [PubMed] [Google Scholar]
- 17.Engelson ES, Agin D, Kenya S, Werber-Zion G, Luty B, Albu JB, et al. Body composition and metabolic effects of a diet and exercise weight loss regimen on obese, HIV-infected women. Metabolism: clinical and experimental. 2006;55:1327–1336. doi: 10.1016/j.metabol.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Fitch KV, Anderson EJ, Hubbard JL, Carpenter SJ, Waddell WR, Caliendo AM, et al. Effects of a lifestyle modification program in HIV-infected patients with the metabolic syndrome. AIDS (London, England) 2006;20:1843–1850. doi: 10.1097/01.aids.0000244203.95758.db. [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes A. 2. Classification and Diagnosis of Diabetes. Diabetes care. 2016;39(Suppl 1):S13–S22. doi: 10.2337/dc16-er09. [DOI] [PubMed] [Google Scholar]
- 20.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 21.Genton L, Hans D, Kyle UG, Pichard C. Dual-energy X-ray absorptiometry and body composition: differences between devices and comparison with reference methods. Nutrition. 2002;18:66–70. doi: 10.1016/s0899-9007(01)00700-6. [DOI] [PubMed] [Google Scholar]
- 22.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frimel TN, Deivanayagam S, Bashir A, O'Connor R, Klein S. Assessment of intrahepatic triglyceride content using magnetic resonance spectroscopy. J Cardiometab Syndr. 2007;2:136–138. doi: 10.1111/j.1559-4564.2007.07168.x. [DOI] [PubMed] [Google Scholar]
- 24.Jensen MD, Heiling VJ. Heated hand vein blood is satisfactory for measurements during free fatty acid kinetic studies. Metabolism: clinical and experimental. 1991;40:406–409. doi: 10.1016/0026-0495(91)90152-m. [DOI] [PubMed] [Google Scholar]
- 25.Reeds DN, Mohammed BS, Klein S, Boswell CB, Young VL. Metabolic and structural effects of phosphatidylcholine and deoxycholate injections on subcutaneous fat: a randomized, controlled trial. Aesthet Surg J. 2013;33:400–408. doi: 10.1177/1090820X13478630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reeds DN, Mittendorfer B, Patterson BW, Powderly WG, Yarasheski KE, Klein S. Alterations in lipid kinetics in men with HIV-dyslipidemia. American journal of physiology. 2003;285:E490–E497. doi: 10.1152/ajpendo.00118.2003. [DOI] [PubMed] [Google Scholar]
- 27.Patterson BW, Zhao G, Elias N, Hachey DL, Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res. 1999;40:2118–2124. [PubMed] [Google Scholar]
- 28.Horowitz JF, Coppack SW, Klein S. Whole-body and adipose tissue glucose metabolism in response to short-term fasting in lean and obese women. American Journal of Clinical Nutrition. 2001;73:517–522. doi: 10.1093/ajcn/73.3.517. [DOI] [PubMed] [Google Scholar]
- 29.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 30.Conte C, Fabbrini E, Kars M, Mittendorfer B, Patterson BW, Klein S. Multiorgan insulin sensitivity in lean and obese subjects. Diabetes care. 35:1316–1321. doi: 10.2337/dc11-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cutrono SE, Lewis JE, Perry A, Signorile J, Tiozzo E, Jacobs KA. The Effect of a Community-Based Exercise Program on Inflammation, Metabolic Risk, and Fitness Levels Among Persons Living with HIV/AIDS. AIDS Behav. 2016;20:1123–1131. doi: 10.1007/s10461-015-1245-1. [DOI] [PubMed] [Google Scholar]
- 32.Tomaka F, Lefebvre E, Sekar V, Van Baelen B, Vangeneugden T, Vandevoorde A, et al. Effects of ritonavir-boosted darunavir vs. ritonavir-boosted atazanavir on lipid and glucose parameters in HIV-negative, healthy volunteers. HIV medicine. 2009;10:318–327. doi: 10.1111/j.1468-1293.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- 33.Venhoff N, Setzer B, Melkaoui K, Walker UA. Mitochondrial toxicity of tenofovir, emtricitabine and abacavir alone and in combination with additional nucleoside reverse transcriptase inhibitors. Antiviral therapy. 2007;12:1075–1085. [PubMed] [Google Scholar]
- 34.Guffanti M, Caumo A, Galli L, Bigoloni A, Galli A, Dagba G, et al. Switching to unboosted atazanavir improves glucose tolerance in highly pretreated HIV-1 infected subjects. Eur J Endocrinol. 2007;156:503–509. doi: 10.1530/EJE-06-0648. [DOI] [PubMed] [Google Scholar]
- 35.Magkos F, Fabbrini E, Korenblat K, Okunade AL, Patterson BW, Klein S. Reproducibility of glucose, fatty acid and VLDL kinetics and multi-organ insulin sensitivity in obese subjects with non-alcoholic fatty liver disease. Int J Obes (Lond) 35:1233–1240. doi: 10.1038/ijo.2010.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregor MF, Hotamisligil GS. Thematic review series: Adipocyte Biology. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res. 2007;48:1905–1914. doi: 10.1194/jlr.R700007-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Boden G, Duan X, Homko C, Molina EJ, Song W, Perez O, et al. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 2008;57:2438–2444. doi: 10.2337/db08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, et al. Effects of Moderate and Subsequent Progressive Weight Loss on Metabolic Function and Adipose Tissue Biology in Humans with Obesity. Cell Metab. 2016;23:591–601. doi: 10.1016/j.cmet.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reeds DN, Cade WT, Patterson BW, Powderly WG, Klein S, Yarasheski KE. Whole-body proteolysis rate is elevated in HIV-associated insulin resistance. Diabetes. 2006;55:2849–2855. doi: 10.2337/db06-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.