Abstract
As our world’s population ages, cardiovascular diseases (CVD) will become an increasingly urgent public health problem. A key antecedent to clinical CVD and many other chronic disorders of aging is age-related arterial dysfunction, characterized by increased arterial stiffness and impaired arterial endothelial function. Accumulating evidence demonstrates that diet and nutrition may favorably modulate these arterial functions with aging, but many important questions remain. In this review, we will summarize the available information on dietary patterns and nutritional factors that have been studied for their potential to reduce arterial stiffness and improve endothelial function with age, with an emphasis on: 1) underlying physiological mechanisms, and 2) emerging areas of research on nutrition and arterial aging that may hold promise for preventing age-related CVD.
Keywords: endothelial dysfunction, arterial stiffness, diet, nutrition
CARDIOVASCULAR DISEASE, DIET AND ARTERIAL FUNCTION
Our world’s population is aging. In fact, the number of adults over age 65 is projected to more than double by the year 2050 (Harper, 2014). Because aging predisposes us to a variety of chronic degenerative diseases, this shift in population demographics is expected to cause major socioeconomic and health care problems in the near future (Olshansky et al., 2009). A key concern among the many chronic diseases that will contribute to this aging-driven “epidemic” is cardiovascular disease (CVD), which remains the leading cause of death in modern societies. Indeed, it is estimated that >40% of the U.S. population will have one or more form of clinical CVD by the year 2030 (Heidenreich et al., 2011).
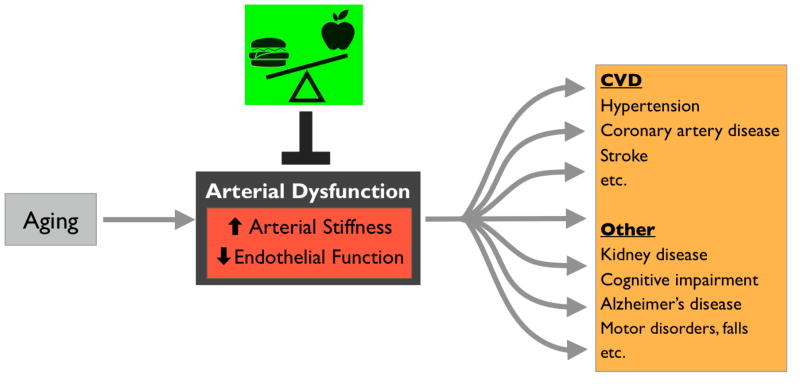
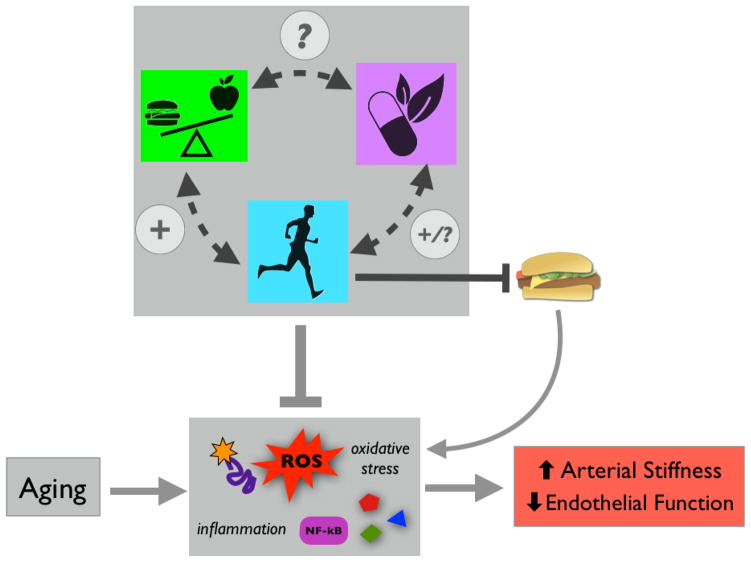
Healthy lifestyle and diet clearly have the potential to reduce CVD risk (Mozaffarian et al., 2011). However, research on dietary interventions typically focuses on reducing one or more traditional CVD risk factors (e.g., systolic blood pressure, LDL cholesterol, etc.). Although these factors certainly play a role in determining CVD risk, the major risk factor for CVD is advancing age itself (Mozaffarian et al., 2016). This is largely a result of age-related declines in arterial function that make us more susceptible to clinical CVD as we grow older (Najjar et al., 2005; Seals et al., 2014). Two particularly important functional changes that occur in arteries as we age are: 1) stiffening of the large elastic arteries (the aorta carotid arteries), and 2) a decline systemic vascular endothelial function (Figure 1) (Lakatta and Levy, 2003). Both of these functional declines predict future CVD risk in older adults, and both are “macro-scale” physiological parameters that integrate the influences of other risk factors (Mitchell et al., 2010; Seals et al., 2011). As such, they may in some ways be more reflective of overall cardiovascular health than traditional risk factors alone—especially in the context of aging (Lakatta and Levy, 2003). Moreover, it is increasingly clear that in addition to CVD, reduced arterial function contributes to a growing list of chronic systemic disorders of aging (e.g., cognitive and motor impairments) (Heffernan et al., 2012; Stanimirovic and Friedman, 2012). This is because appropriate blood flow/pressure and nutrient supply are critical for active muscles and organs, so arterial dysfunction leads to tissue-level stresses that may precipitate neurodegenerative diseases, kidney disease, and likely many other age-related conditions. Thus, there is significant interest in specific healthy lifestyle interventions, including dietary/nutritional strategies, for preventing or reversing age-related arterial stiffening and endothelial dysfunction.
Figure 1.
Aging leads to arterial dysfunction (increased stiffness and reduced endothelial function) that predisposes us to cardiovascular diseases (CVD) and other chronic disorders. Diet/nutrition has the potential to modulate/prevent arterial dysfunction with aging.
In this review, we will briefly summarize the physiology and underlying mechanisms of arterial aging, and then discuss specific lifestyle dietary/nutritional strategies that appear to prevent or reverse it. In some cases, there is limited direct evidence for the effects of nutritional factors on arterial aging per se (i.e., in the absence of pre-existing disease and/or risk factors). In those instances, we will focus on strategies for which there are compelling data from studies in at-risk human populations, with a focus on middle-aged and older adults (over age 40), and/or results of translational investigations using preclinical models. Finally, we will provide insight into the mechanisms underlying the potential beneficial effects of these nutritional strategies whenever possible.
ARTERIAL AGING
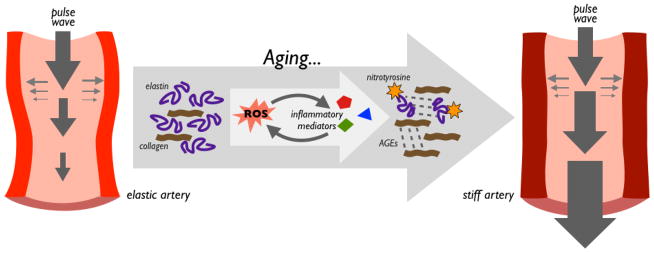
Large elastic arteries (e.g., the aorta and carotid arteries) are designed to expand and recoil elastically as the heart beats in order to buffer the energy of the pulse wave, and to drive blood distally to our tissues and cells (Santos-Parker et al., 2014). However, these arteries stiffen progressively with aging (Avolio et al., 1985). This forces the heart to work harder (pushing against stiff arteries), and leads to higher blood pressure, as well as greater pulsatile flow that damages other tissues, especially high-perfusion organs such as the brain, kidneys and eyes (Seals, 2014) (Figure 2). Age-related declines in arterial elasticity result primarily from structural changes that occur in the arterial wall as we age, including increases in collagen (fibrosis), fragmentation and degradation of elastin, and the formation of advanced glycation end-products—all of which reduce elasticity and confer stiffness (Cavalcante et al., 2011). These cellular changes are driven by the development of oxidative stress (excess production and reduced degradation of reactive oxygen species, ROS), which is marked by age-related increases in nitrotyrosine and other markers of oxidative protein damage (Zieman, 2005). Chronic low-grade inflammation exacerbates these processes and interacts synergistically with oxidative stress (Wang et al., 2014), but the exact order and relationship between these events are uncertain.
Figure 2.
A key function of large elastic arteries is to buffer energy of the pulse wave. As large elastic arteries age, oxidative stress (characterized by excess reactive oxygen species, ROS) and inflammation alter structural protein composition of the arterial wall. Age-related reductions in elastin, increases in collagen, and buildup of oxidatively damaged proteins and advanced glycation end products (AGEs) results in stiffer arterial walls and greater pulse wave velocity/pressure.
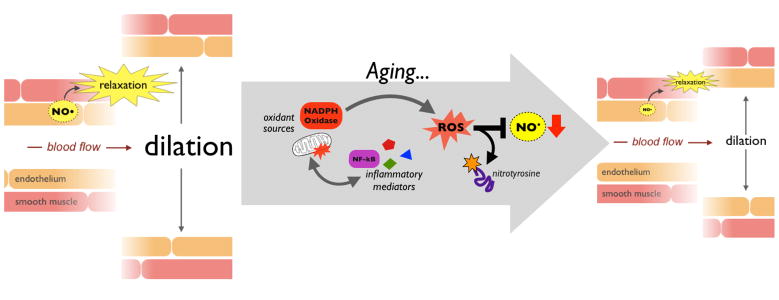
Compared to arterial stiffening, the mechanisms underlying arterial endothelial dysfunction with aging are somewhat clearer. This is because the vascular endothelium, a single-cell layer lining the interior of arteries, is accessible in both preclinical models and healthy human subjects (whereas samples of the arterial wall are not). The endothelium plays a central role in controlling vascular tone, metabolism, immune function, thrombosis, and many other processes (Cines et al., 1998; Seals et al., 2011). It also is responsible for coordinating circulation throughout the body, by dilating as necessary to allow increased blood flow. A central mediator of these processes is the vascular-protective molecule nitric oxide (NO), which is produced by endothelial NO synthase (eNOS) and subsequently diffuses to the smooth muscle layer of the artery causing it to relax (dilation) (Figure 3). This dilation in response to NO (endothelium-dependent dilation) is an excellent biomarker of endothelial function with aging, as it declines starting in early middle age (Celermajer et al., 1994). Indeed, as we age, ROS from various sources (e.g., dysfunctional mitochondria and oxidant enzymes such as NADPH oxidase) scavenge NO, reducing its bioavailability and damaging endothelial cell proteins, as indicated by increases in cellular nitrotyrosine levels (Donato et al., 2015). Chronic inflammation, characterized by activation of central inflammatory mediators like nuclear factor kB (NF-kB) and increases in circulating inflammatory cytokines, also impairs endothelial cell function, and synergizes with oxidative stress (Figure 3). Collectively, these events underlie the progressive decline in endothelial function observed with aging (Seals et al., 2011).
Figure 3.
Endothelium-dependent dilation in response to nitric oxide (NO) is an important indicator of overall arterial endothelial function. Aging is associated with increases in oxidative stress (marked by excess reactive oxygen species, ROS) and inflammation driven by nuclear factor kB (NF-kB) and other cytokine mediators. Together, these processes reduce the bioavailability of NO, resulting in reduced endothelium-dependent dilation with age.
Importantly, both arterial stiffness and reduced endothelial function predict future CVD risk in healthy middle-aged and older adults (Mitchell et al., 2010; Widlansky et al., 2003; Yeboah et al., 2007), and both can be measured in preclinical/animal models, as well as in clinical settings (human subjects) (Seals et al., 2014). This makes it possible to evaluate mechanisms and interventions for improving arterial aging, including nutritional strategies, in time-efficient preclinical/animal experiments, and to translate these findings to clinical studies in middle aged/older adults when appropriate. Stiffness is commonly assessed by determining aortic pulse wave velocity or carotid artery compliance (stiffer arteries result in greater pulse wave velocity and reduced compliance), whereas endothelial function is evaluated by measuring increases in blood flow in response to a pharmacological stimulus, such as acetylcholine, or arterial dilation induced by physiological stimuli (e.g., increased shear stress/flow within the blood vessel). The details of these measurements are reviewed elsewhere (Cavalcante et al., 2011; Santos-Parker et al., 2014; Seals et al., 2014); for the purposes of this review, we will simply focus on the influence of healthy lifestyle and nutrition on age-related arterial function/dysfunction, as measured by arterial stiffness and endothelium-dependent dilation.
NUTRITIONAL FACTORS AND INTERVENTIONS
In many cases, evidence for the effects of diet/nutrition on arterial aging phenotypes and the underlying mechanisms is somewhat limited. In part, this is likely because dietary intervention trials are challenging to perform and involve many factors that might contribute to changes in arterial function and/or underlying mechanisms (e.g., different foods and micronutrients in any particular diet). It also is difficult to conduct preclinical investigations of food-based diets (e.g., to feed mice a Mediterranean diet), thus limiting mechanistic insight into the effects of diet/nutrition on cells and tissues. Likewise, translating observations from animal models to clinical settings is often challenging, as the standardized chow used in preclinical studies is often much lower in fat than the typical westernized human diet. Nevertheless, there are promising data in support of several broad dietary patterns that protect against arterial aging, and clear translational evidence for a number of more targeted nutritional approaches. The following is a summary of current information on these dietary/nutritional strategies reported to promote healthy arterial aging.
Diet Composition
At the broadest level, general dietary patterns have strong modulatory effects on arterial aging (Table 1). Different study designs and differences among baseline/control diets (e.g., between trials performed in different countries) make this a challenging area of research. Nevertheless, based on clinical data from observational studies and controlled trials, certain diets do appear to be particularly protective, especially among middle-aged and older adults who are overweight/obese, or have elevated systolic blood pressure and/or cholesterol. These include the traditional Mediterranean and DASH diets, which emphasize fruits and vegetables, whole grains, nuts, legumes, seeds, low-fat dairy, moderate amounts of lean meat and fish, and limited consumption of refined/sugary foods (Al-Solaiman et al., 2009; Schwingshackl and Hoffmann, 2014; van de Laar et al., 2013). Although the Mediterranean diet is less well defined than the DASH diet (and may differ among countries where it is consumed), both diets improve endothelial function and reduce arterial stiffness in certain populations (Blumenthal et al., 2010; Klonizakis et al., 2013; Lee et al., 2015; Marin et al., 2013; Shenoy et al., 2010). Evidence suggests that key common factors in these diets may be an emphasis on unprocessed, plant-based foods, and sources of fat (e.g., olive oil and nuts vs. meats and dairy) (Mozaffarian et al., 2011). Similarly, although there is less evidence, vegetarian diets appear to be protective against some expressions of arterial aging in middle-aged and older, at-risk adults (Lin et al., 2001). More general dietary patterns, such as higher intake of fruits, vegetables, fiber and fish are also associated with improved arterial aging phenotypes (reduced stiffness in some cases, improved endothelium-dependent dilation in others) in the same groups (Blanch et al., 2015; Klonizakis et al., 2013; McCall et al., 2009; van Bussel et al., 2011), and intervention trials demonstrate that increasing the intake of these foods improves function in middle-aged/older subjects (McCall et al., 2009; van Bussel et al., 2015). The exact mechanisms by which these macro-scale dietary patterns modulate arterial aging are not certain, but evidence suggests that reduced oxidative stress and inflammation play a role in most cases (Chistiakov et al., 2015; Marin et al., 2013).
Table 1.
Broad dietary patterns and specific foods that modulate arterial function with aging.
| Diet/factor | Effects | Evidence* | |
|---|---|---|---|

|
Mediterranean, DASH, vegetarian(?) diets | ↑ endothelial function ↓ arterial stiffness |

|

|
Higher fruit/vegetable intake | ↑ endothelial function ↓ arterial stiffness |

|

|
Higher fish intake | ↑ endothelial function ↓ arterial stiffness |

|

|
High fat diet | ↓ endothelial function ? arterial stiffness |

|

|
Low fat diet | ↑ endothelial function ? arterial stiffness |

|
|
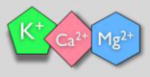
Specific electrolytes | ↑ endothelial function ↓ arterial stiffness (some) |

|

|
Sodium restriction | ↑ endothelial function ↓ arterial stiffness |
|

|
Nuts, tea, coffee, cocoa, whole grains, legumes, olive oil | ↑ endothelial function ↓ arterial stiffness (some) |

|
Human and mouse symbols represent clinical and preclinical evidence, respectively, and number of symbols reflects approximate weight of evidence. For details, see references/discussion in text.
In contrast to healthy diet, less is known about the effects of suboptimal dietary patterns on arterial aging. However, preclinical (mouse model) evidence demonstrates that “Western” diet (high fat/sugar) increases arterial stiffness (DeMarco et al., 2015; Henson et al., 2014) and reduces endothelial function (Lesniewski et al., 2013) with age. Direct evidence for this in humans is limited and less consistent. Most reports do indicate that diets high in saturated fat reduce endothelial function (although primarily in middle-aged/older adults with pre-existing risk factors) (Fuentes et al., 2008; Keogh et al., 2005), but the influence of these diets on arterial stiffness is less clear; some have found that high fat diet increases stiffness, while others have observed no effects (Hall, 2009; Sanders et al., 2013). Thus, there is a clear need for future trials aimed at establishing the effects of suboptimal (e.g., Western) diet on arterial functions in humans with aging—especially because this is the diet consumed by most middle-aged/older adults in developed (and developing) countries.
In addition to broad dietary patterns, evidence suggests that some individual micronutrients common to many foods have powerful effects on arterial function with aging, and may contribute to the more general effects of certain diets (Mozaffarian et al., 2011). For example, specific electrolytes (e.g., potassium, magnesium, and calcium) appear to improve endothelial function in middle-aged/older adults (Aaron and Sanders, 2013; Blanch et al., 2015; Blanch et al., 2014; Joris et al., 2016)—although the effects of these micronutrients on arterial stiffness remain to be determined. Other micronutrients exert adverse effects on arterial aging, as demonstrated by studies in which intake of these micronutrients is reduced. Lower sodium intake, in particular, is associated with greater endothelial function and reduced arterial stiffness in middle-aged and older adults (Jablonski et al., 2009), and dietary sodium restriction improves endothelial function and reduces stiffness in these same groups (Gates et al., 2004; Jablonski et al., 2013). Similarly, although the effects of high fat diet on arterial function are somewhat uncertain, it does seem that dietary fat restriction improves endothelial function in adults with pre-existing CVD risk factors—especially when the overall dietary pattern is also healthy (Fuentes et al., 2001; Mohler et al., 2013). Again, these interventions appear to reduce markers of systemic oxidative stress and inflammation, but the specifics of how this occurs are unclear.
Finally, the effects of certain dietary patterns on arterial aging may also be due to greater intake of specific foods in these diets. The consumption of whey protein and dairy, for instance, is associated with enhanced endothelial function and lower arterial stiffness in middle-aged or older adults (Ballard et al., 2013; Crichton et al., 2012). Similarly, data from controlled trials demonstrate that cocoa products, including dark (not milk) chocolate, improve endothelial function and reduce arterial stiffness (Corti et al., 2009; Heiss et al., 2015), as do fermented dairy, tea and soy (Jauhiainen et al., 2010; Lin et al., 2016; Pase et al., 2011; Schreuder et al., 2014). Besides these well studied foods, accumulating evidence also suggests that consuming olive oil, seeds, whole grains, nuts, legumes and coffee is associated with CVD risk-lowering effects, and this is likely to be mediated in part by preservation of endothelial function and reductions in arterial stiffness (Mozaffarian et al., 2011; Ros and Hu, 2013; Uemura et al., 2013). As is the case with broad dietary patterns, specific foods are thought to improve arterial function primarily by reducing oxidative stress and inflammation (Seals et al., 2014; Wu et al., 2015). However, beyond these macro-mechanistic processes, it is often difficult to isolate specific signaling pathways activated by nutritional factors, perhaps because foods contain numerous bioactive components, and thus activate multiple cellular/physiological signaling cascades.
Energy Intake
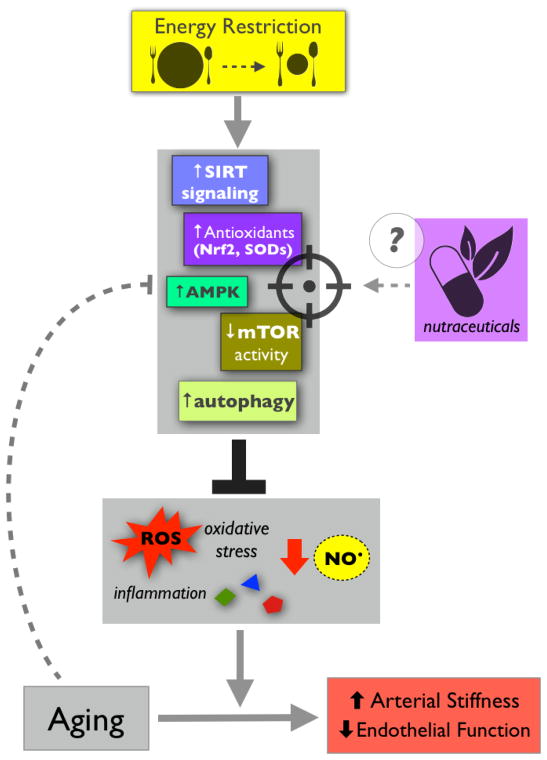
Whereas diet quality (patterns/foods) certainly modulates arterial aging, in many ways the effects and underlying mechanisms of quantity are even clearer. Energy restriction in the absence of malnutrition (caloric restriction, CR) is associated with beneficial effects on numerous physiological parameters with aging (Ingram et al., 2006; Mattson and Wan, 2005). Arterial functions are no exception, and there is strong translational evidence for this (Figure 4). In mice, long term (lifelong) CR protects against arterial stiffening and preserves endothelial function with aging by preventing age-related changes in collagen and elastin, maintaining NO bioavailability, and reducing vascular ROS production and oxidative stress (Donato et al., 2013). Evidence also indicates that briefer periods of energy restriction can recapitulate the effects of long-term CR. In old mice, for example, 8 weeks of CR restores endothelial function to levels observed in young animals, again by reducing oxidative stress and increasing NO bioavailability (Rippe et al., 2010). In humans, there is some evidence that CR improves select cardiovascular parameters (Meyer et al., 2006), but direct translational evidence for its effects on vascular function is limited because clinical trials of CR per se are few. However, the preclinical observations described above are consistent with studies of weight loss in overweight/obese middle-aged and older adults (short-term CR, effectively). In fact, a ~30% reduction in energy intake over 12 weeks, without any increase in physical activity, greatly improves endothelial function (Pierce et al., 2008) and ameliorates arterial stiffness (Dengo et al., 2010) in these subjects. Reductions in fat mass may play a role in these improvements, but nevertheless, this evidence suggests that energy restriction powerfully protects against (and/or reverses) arterial aging phenotypes.
Figure 4.
Energy restriction prevents/reverses age-related arterial dysfunction by stimulating key cellular signaling networks that reduce oxidative stress/inflammation and increase nitric oxide bioavailability. These energy-sensitive networks, which tend to become impaired with aging, may be novel targets for alternative nutritional therapies, such as nutraceuticals.
In spite of the compelling evidence for long and short term energy restriction, these strategies are unlikely to be successful for preventing arterial aging in most modern societies, where high calorie diets are a common lifestyle feature and most adults are unlikely to adhere to CR (Mattson et al., 2014). As a result, there is significant interest in practical alternatives for mimicking the effects of CR. In this context, intermittent fasting (IF) may be particularly promising. IF strategies include simple, alternate day fasting (i.e., eating every other day), as well as interval-based approaches (e.g., the “5:2 diet”—unrestricted food intake 5 days of the week coupled with fasting or significant calorie reduction 2 days a week) (Harvie et al., 2011; Varady et al., 2013). Recent reports suggest that both of these diets improve various CVD risk factors and, in some cases, markers of arterial function in aged mice (Kroeger et al., 2012; Razzak et al., 2011). Direct evidence in humans is limited, but IF paradigms do promote weight loss and improve select CVD risk factors, at least in certain populations (Varady et al., 2013). This suggests that IF in general may mimic CR and could be a viable strategy for preventing/reversing arterial aging. Still, even IF-based approaches require strong adherence. Moreover, the weight loss associated with both CR and IF could have adverse effects on normal weight older adults who are at risk for age-related declines in muscle mass and bone mineral density (Miller and Wolfe, 2008). Thus, there is interest in more feasible approaches, such as periodic fasting (e.g., 4 consecutive days of fasting once per month) (Mattson et al., 2014). However, it may also be possible to mimic CR via even simpler methods, like time-restricted feeding (TRF). TRF involves restricting normal calorie intake to a 6–12 hour window, and fasting for the remainder of the day (Rothschild et al., 2014). This may mimic the effects of CR but reduce the risk of poor adherence and unwanted weight loss in older adults. Like alternate day and interval-based fasting, there is compelling preclinical evidence for TRF (Chaix et al., 2014; Hatori et al., 2012), but it has not been studied in older human subjects and, therefore, is an important area for future investigation.
The basic idea behind alternative energy restriction strategies like TRF is to stimulate the same signaling pathways as CR and, in doing so, exert many of the same beneficial physiological effects (Ingram et al., 2006; Mattson et al., 2014). Indeed, the efficacy of CR for preventing/reversing arterial aging (and other age-related conditions) is likely due to the activation of important, conserved cellular signaling networks (Figure 4). In general, energy restriction reduces arterial oxidative stress and inflammation, and increases nitric oxide bioavailability (Seals et al., 2014; Ungvari et al., 2008). Upstream of these effects, improvements in arterial function as a result of CR are generally associated with modulation of key energy sensing networks, most of which are impaired with aging (Donato et al., 2015). Important examples include the sirtuin-1 (SIRT-1) and AMP-activated protein kinase (AMPK) signaling pathways (Donato et al., 2011; Lesniewski et al., 2012). At the same time, CR generally represses cellular growth mediators that are associated with aging and disease, such as mammalian target of rapamycin (mTOR) (Mattson and Wan, 2005). All of these proteins/systems are sensitive to decreases in markers of cellular energy levels, such as NAD:NADH ratios (SIRT-1), ATP concentrations (AMPK), and amino acid and glucose availability (mTOR). Under low energy conditions, they activate (SIRT-1 and AMPK) and/or de-repress (mTOR) cellular energy generating systems and stress resistance pathways, such as protein catabolism, fatty acid oxidation, autophagy and mitochondrial homeostasis networks—all of which protect against oxidative stress and inflammation (Donato et al., 2015; Mattson and Wan, 2005; Ungvari et al., 2008). The specifics of how this occurs are not well defined, but it seems likely that because energy-sensing networks generally increase oxidative phosphorylation, this would lead to more mitochondrial ROS production and a subsequent need for cellular responses to suppress oxidative stress. Energy-sensing networks also broadly accelerate the degradation/recycling of damaged cellular components (to generate energy production substrates) that may stimulate inflammatory signaling (Kroemer et al., 2010), thereby decreasing low-grade inflammation. Consistent with this notion, CR is reported to activate endogenous anti-oxidant/inflammatory systems, especially those under control of the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2), and ROS-scavenging superoxide dismutase (SOD) enzymes (Martin-Montalvo et al., 2011; Rippe et al., 2010). Because the coordinated activation of these important, cell-protective signaling pathways is thought to underlie the broad efficacy of energy restriction, they represent novel targets for alternative arterial aging therapies, including nutrition-based treatments, such as nutraceuticals (discussed below) (Figure 4).
Nutraceuticals
Concept and Examples
Healthy diet and energy restriction (when appropriate) are likely to be the most effective nutritional approaches for preventing and treating arterial aging. As such, they should be the “first line” strategies emphasized in public health/policy efforts (Mozaffarian, 2016; Seals et al., 2016). However, for a number of reasons (e.g., poor education, financial constraints, social/lifestyle influences, and even environmental cues), many middle-aged and older adults do not engage in healthy lifestyle behaviors. As a result, there is significant interest in easily deliverable treatments that may induce effects similar to healthy diet and lifestyle. One possibility is that pharmaceutical drugs could be “repurposed” for treating arterial aging; for example, certain antihypertensive drugs appear to also reduce arterial stiffness in middle aged/older adults (Hayashi et al., 2006), and anti-inflammatory agents improve endothelial function in the same groups (Pierce et al., 2009). Other examples are reviewed elsewhere (Alfaras et al., 2016). This is a promising area of research, but many middle-aged and older adults/consumers are also interested in more “natural” treatments, such as nutracueticals—individual food ingredients/components with bioactive properties that may benefit human health (and in this case, arterial function) (Seals et al., 2014; Zuchi et al., 2010).
Nutraceuticals comprise a broad range of dietary supplements, functional foods (any altered food or ingredient that could have a beneficial effect beyond that provided by nutrients it traditionally contains), and medical foods (foods formulated to be consumed or administered under the supervision of a physician for dietary management of a disease or condition) (Ferrari, 2004; Wrick, 2005). Broadly, the concept is based on: 1) the idea that the benefits of certain dietary patterns may be a result of nutrients that are particularly abundant in these diets, and 2) the potential ability of these compounds to suppress established macro-mechanistic processes that contribute to physiological/arterial dysfunction, such as oxidative stress and inflammation. Classic examples include omega-3 polyunsaturated fatty acids (anti-inflammatory compounds abundant in Mediterranean and other diets), and antioxidant vitamins (C, E, etc., in most high-fruit/vegetable diets). However, results from intervention trials with these agents have been largely inconsistent, at least in the context of arterial aging. Omega-3s seem to improve arterial functions in some populations but not others (Pase et al., 2011; Wang et al., 2012), and the reported effects often vary from one study to the next. Similarly, despite the central role of oxidative stress in arterial aging, chronic supplementation with antioxidant vitamins generally has not been successful for reducing CVD risk, and their effects on arterial aging phenotypes are unclear at best (Kris-Etherton et al., 2004). On one hand, acute administration of high dose antioxidants temporarily reverses endothelial dysfunction, and in some cases arterial stiffness, in older humans and mice (Eskurza et al., 2004; Wilkinson et al., 1999). On the other hand, whereas chronic antioxidant supplementation is generally effective for reversing arterial aging phenotypes in old mice and humans with pre-existing disease/risk factors, it seems to have no effect in older, otherwise healthy middle-aged/older adults (Ashor et al., 2015b; Ashor et al., 2014; Eskurza et al., 2004). Other examples, such as folate and vitamin D, do exert protective effects on arterial aging in select populations (Jablonski et al., 2011; Liu et al., 2013; Moat et al., 2004), but trials investigating these compounds in middle-aged/older adults without clinical disease have been generally inconclusive (Massaro et al., 2010).
Arguably, the problem with nutraceuticals like omega-3s and exogenous antioxidants is that they are somewhat non-specific; that is, they may have anti-oxidant/inflammatory effects, but they do not “target” any particular cellular signaling pathways or compartments. Thus, there is growing interest in more targeted compounds for treating precise cellular causes of arterial aging and/or boosting specific, cell-protective pathways (Table 2) (Ingram et al., 2006; Seals et al., 2016; Zuchi et al., 2010). For example, given the role of mitochondria in mediating vascular oxidative stress (Dai et al., 2012), mitochondria-specific antioxidants, such as mitoquinone (MitoQ, a mitochondria-targeted version of the naturally occurring coenzyme Q10 in meats and vegetable oils, among other food sources) (DiNicolantonio et al., 2015), may be good candidates for preventing arterial aging. Indeed, MitoQ supplementation completely restores endothelial function and NO bioavailability in old mice (Gioscia-Ryan et al., 2014). Preliminary data also suggest that MitoQ reduces arterial stiffness in these same animals, and a pilot clinical trial is underway to examine its efficacy in middle-aged and older human subjects (clinicaltrials.gov/ct2/show/NCT02597023).
Table 2.
Nutracueticals studied for their effects on arterial function with aging.
| Nutraceutical | Target(s) | Effects | Evidence | |
|---|---|---|---|---|

|
Omega-3 fish oils |

|
↑/↔ endothelial function ↓/↔ arterial stiffness |
|

|
† Green tea polyphenols † Soy isoflavones † Coffee polyphenols Sulforaphane † Lactotripeptides † Allicin † Spermidine Capsaicin Shogaol Carnosol † Cocoa flavonols Lycopene Quercetin † NAD+ precursors |

|
↑ endothelial function (most cases) ↓ arterial stiffness († some cases) |

|
|
Nitrite/nitrate |
|
↑ endothelial function ↓ arterial stiffness (mice) |

|
|
|
Resveratrol |

|
↑ endothelial function ↓ arterial stiffness (mice) |

|
|
Trehalose |

|
↑ endothelial function ↓ arterial stiffness (mice) |

|
|
|
Curcumin |

|
↑ endothelial function ↓ arterial stiffness (mice) |

|
Human and mouse symbols represent clinical and preclinical evidence, respectively, and the number of symbols reflects approximate weight of evidence. For details, see references/discussion in text.
Nutraceuticals reported to modulate arterial stiffness.
Aside from specific cellular sources of ROS, the most promising targets for candidate nutraceuticals are likely those activated by energy restriction, as this approach appears to exert consistently beneficial effects on function (Alfaras et al., 2016). Indeed, there is strong interest in the concept of “CR mimetics” (Ingram et al., 2006). As discussed above, CR/energy restriction increases NO bioavailability and favorably modulates arterial wall structure by: 1) activating endogenous cellular stress resistance networks (SIRT-1, AMPK, mTOR and autophagy) that have powerful, cell-protective effects, and 2) upregulating endogenous antioxidant systems, such as those controlled by Nrf2. As such, targeting these key players would seem to be a promising approach for nutraceutical interventions, and evidence in support of this concept is growing.
Nutraceuticals studied for their effects on arterial aging-related outcomes via key cellular health pathways (Table 2) include compounds present in coffee (terpenes and polyphenols) and green tea (EGCG and epicatechin), as well as soy isoflavones and cocoa flavonols—all of which seem particularly promising based on findings in both mice and human subjects (Corti et al., 2009; Jimenez et al., 2012; Mubarak et al., 2012; Pase et al., 2011). Bioactive constituents of fruits and vegetables are also of interest, including: quercetin (apples), allicin (garlic), sulforaphane (broccoli), lycopene (tomatoes) and capsaicin (peppers) (Alfaras et al., 2016; Burton-Freeman and Sesso, 2014; Dower et al., 2015; Evans, 2011; Landberg et al., 2012; McCarty et al., 2015). Herbal compounds, such as shogaol (ginger) and carnosol (rosemary) are promising, as well (Kelsey et al., 2010). Many of these nutraceuticals have been shown to reduce oxidative stress/inflammation, activate certain CR-related signaling systems, and improve one or more expressions of arterial aging in preclinical (mouse) models and in some cases cell culture. However, only a few have been studied for their effects in human subjects. Additional clinical trials are underway in some cases (clinicaltrials.gov), but in general, more uniform evidence for the efficacy of these compounds, based on well-controlled trials in middle-aged/older adults, is needed. Other concerns include the inconsistent composition of nutraceutical formulations from one study to the next, and generally less stringent regulations for their use.
Examples with Translational Evidence
In contrast to the many nutraceutical examples based on limited experimental data, there are several for which compelling translational evidence does exist (Table 2). Most of these nutraceuticals trigger a multitude of cellular signaling pathways (much like CR), which may in part explain their beneficial effects on arterial/physiological function. However, it is difficult to know exactly which cellular targets are the most important mediators of any one nutraceutical, as related data from studies of older transgenic animals is generally lacking. Thus, in order to simplify the discussion, we will review these in the context of their most frequently reported cellular targets.
Perhaps the best characterized nutracuetical in the context of arterial aging is nitrite/nitrate—found in high concentrations in green leafy vegetables and beets, among other foods. Diets enriched in these foods improve arterial aging phenotypes (Hobbs et al., 2013), and recent findings demonstrate that this is likely because increased levels of circulating (plasma) and tissue (e.g., skin) nitrite, nitrate and related compounds serve as precursor molecules for increasing NO bioavailability and preventing/treating oxidative stress and inflammation (Oplander et al., 2009; Rocha et al., 2011). As such, these molecules appear to be good nutraceutical candidates for preventing arterial aging. Indeed, short-term supplementation with nitrite restores endothelial function and reverses arterial stiffening in old mice by increasing NO bioavailability, reducing oxidative stress and normalizing structural proteins (Sindler et al., 2011). Moreover, recent reports show that supplementation with nitrite (DeVan et al., 2016) and nitrate (Rammos et al., 2014) improves endothelial function in middle-aged/older adults without clinical CVD. Nitrite seems particularly promising, as it also is reported to improve motor (neuromuscular) and cognitive functions (Justice et al., 2015), and larger trials to establish efficacy and broader mechanisms are underway (clinicaltrials.gov/ct2/show/NCT02393742).
Upstream of NO, other nutraceutical compounds may protect against arterial aging by activating the CR mediator SIRT-1. The polyphenol resveratrol, which is reported to potently activate SIRT-1 (in addition to other cell-protective pathways), improves endothelial function with aging in rodents fed normal chow or high-fat diets (da Luz et al., 2012; Pearson et al., 2008), an effect associated with reduced vascular oxidative stress and inflammation. Resveratrol also improves endothelial function in surgically removed arteries of middle-aged and older patients with hypertension and dyslipidemia (Carrizzo et al., 2013). Clinically, acute resveratrol administration is reported to improve endothelial function in middle-aged and older overweight and obese adults with elevated blood pressure (Wong et al., 2011), and chronic resveratrol supplementation enhances endothelial function in older obese adults (Wong et al., 2013). These observations suggest that SIRT-1 activation with resveratrol may indeed improve endothelial function with aging, but its effects on arterial stiffness are uncertain. There is, however, some evidence in support of other activators of the SIRT-1 network, such as nutraceuticals that boost NAD+ (the cellular trigger for SIRT-1 activity). For example, supplementation with nicotinamide mononucleotide (NMN), a metabolic precursor to NAD+, restores endothelial function and decreases arterial stiffness (de Picciotto et al., 2016), and clinical trials investigating the effects of nicotinamide riboside (NR, a similar compound found in milk and yeast-derived foods) on various physiological parameters, including arterial function, are currently underway (clinicaltrials.gov).
Translational evidence also supports nutraceutical compounds that activate endogenous antioxidants, such as Nrf2. Curcumin, a polyphenol found in the curry spice turmeric (Carmona-Ramirez et al., 2013), is often characterized as a potent Nrf2 activator—although similar to resveratrol, it has various other targets, as well. Curcumin restores endothelial function in old mice to levels of young controls by increasing NO bioavailability and normalizing oxidative stress, and reduces arterial stiffness in these same animals by normalizing arterial structural proteins (Fleenor et al., 2013). Consistent with these preclinical findings, oral supplementation with curcumin improves endothelial function and lowers circulating C-reactive protein (an indicator of inflammation) in healthy postmenopausal women (Akazawa et al., 2012). Conclusive evidence for the effects of curcumin on arterial stiffness and endothelial function in healthy, older adults is lacking, but again, clinical trials are underway (clinicaltrials.gov/ct2/show/NCT01968564).
Downstream of SIRT-1, AMPK and Nrf2, autophagy (the cellular process of recycling damaged macromolecules and organelles) is a central mediator of CR’s beneficial effects on physiological function (Alfaras et al., 2016; Marzetti et al., 2013). Several naturally occurring nutraceuticals may have the potential to boost autophagy. For example, oral supplementation with trehalose, a disaccharide found in mushrooms and honey, stimulates autophagy and reverses endothelial dysfunction and arterial stiffening in old mice by normalizing oxidative stress and structural proteins, while also ameliorating vascular inflammation (LaRocca et al., 2014; LaRocca et al., 2012). The autophagy enhancer spermidine (a polyamine found in grapefruits and fermented soy products) also enhances arterial autophagy and reverses age-associated endothelial dysfunction and arterial stiffness in mice (LaRocca et al., 2013). The exact mechanisms by which trehalose and spermidine induce autophagy are poorly characterized, and like resveratrol and curcumin, they may have multiple targets. Still, this evidence suggests that translational studies of autophagy-enhancing nutraceuticals are warranted. Thus far, clinical trials are limited, but recent evidence demonstrates that trehalose supplementation in middle-aged/older adults does improve endothelial function, although the role of autophagy is not entirely clear (Kaplon et al., 2016). Nevertheless, these observations provide an experimental basis for future studies aimed at determining the effects autophagy-boosting compounds on arterial aging in humans.
OTHER CONSIDERATIONS
Interactions between Lifestyle and Nutritional Factors
An important concern with any approach for improving arterial function with aging is the potential for redundant or adverse effects due to interactions between cellular/physiological signaling pathways. Indeed, many of the mechanisms by which diets and bioactive food ingredients improve function can be characterized as hormetic (i.e., low-level stressors that stimulate protective cellular processes and beneficial physiological outcomes) (Mattson, 2008). Other healthy lifestyle behaviors, such as fasting and physical activity/exercise, are also hormetic stressors (Radak et al., 2008). Thus, for example, it is possible that targeting one cause of arterial aging by supplementing with certain nutraceuticals might negate the hormetic effects of other healthy lifestyle behaviors or diets. Alternatively, some of these factors/interventions might have additive or even synergistically positive effects on arterial function.
One key lifestyle factor that interacts with diet and nutrition is exercise. Aerobic exercise, specifically, is perhaps the most powerful and well-established lifestyle intervention for preventing/reversing arterial aging (Santos-Parker et al., 2014). Cross-sectional studies show that middle-aged and older adults who habitually perform moderate aerobic exercise (e.g., walking, running, swimming) have more elastic arteries and greater endothelial function (Seals, 2014). Moreover, meta-analyses demonstrate that even moderate aerobic exercise interventions (e.g., walking for 12–16 weeks) improve endothelial function and reduce arterial stiffness in middle-aged/older subjects with or without existing CVD or risk factors (Ashor et al., 2015a). In general, these functional improvements associated with exercise are a result of its powerful inhibitory effects on oxidative stress and inflammation (Seals, 2014). This is particularly clear in preclinical studies; for example, voluntary wheel running restores endothelial function and decreases arterial stiffness in old mice by boosting NO bioavailability and reversing age-associated increases in collagen and advanced glycation end-products (Durrant et al., 2009; Fleenor et al., 2010). These preclinical studies also show that in old mice with access to running wheels, vascular expression of key oxidative stress sources (e.g., NADPH oxidase) and inflammatory mediators (e.g., NF-kB) are normalized to levels of young mice, and this is associated with reductions in oxidative protein damage (nitrotyrosine) and pro-inflammatory cytokines.
Translational studies in human subjects are consistent with these preclinical observations. In older sedentary men, for example, reducing vascular oxidative stress via acute infusion of the antioxidant vitamin C restores endothelium-dependent dilation to levels observed in young controls, but does not further improve function in old endurance exercise-trained subjects (Eskurza et al., 2004), thus demonstrating that reduced oxidative stress underlies exercise-associated improvements in arterial function. Consistent with these observations, the expression of nitrotyrosine and NADPH oxidase is higher in endothelial cells biopsied from arteries of older adults than in cells from young sedentary and old endurance exercise-trained subjects (Pierce et al., 2011). Moreover, the expression of inflammatory cytokines (e.g., IL-6) and NF-kB is lower in older endurance exercise-trained subjects compared with their sedentary counterparts, and this is associated with preserved endothelium-dependent dilation (Walker et al., 2014). Collectively, these translational studies indicate that the suppression of ROS-mediated oxidative stress and vascular inflammation plays a central role in the beneficial effects of aerobic exercise on arterial aging (Figure 5). And, although little information is available on vascular tissue per se, many of the beneficial effects of exercise on arterial function with aging are likely also mediated by activation of the key cellular signaling pathways related to CR (discussed above) (Cacicedo et al., 2011; Ferrara et al., 2008; Lira et al., 2013; Seals et al., 2016).
Figure 5.
Exercise protects against the effects of poor diet and synergizes with healthy diet, but its interactions with nutraceuticals are less certain. The interactive effects of healthy diet and nutraceuticals on oxidative stress/inflammation-mediated arterial aging have generally not been studied.
In general, adverse interactions do not seem to be a problem for the combination of exercise and healthy diet. In fact, some reports demonstrate that healthy (Mediterranean) diet combined with exercise leads to long-lasting improvements in endothelial function in middle-aged and older adults that are even greater than the effects of either intervention alone (Klonizakis et al., 2013). Additional trials investigating the effects of this combination on arterial stiffness, as well as the influence of the DASH diet, are currently underway (Blumenthal et al., 2010; Blumenthal et al., 2013). Less evidence exists for the interactions between energy restriction and exercise (in part because it is difficult to separate the effects of these interventions), but studies comparing the two suggest that both improve arterial aging phenotypes to the same degree (Maeda et al., 2015). Recent studies in middle-aged/older human subjects also suggest that aerobic exercise directly protects arterial function against adverse CVD risk factors related to diet, such as elevated circulating LDL cholesterol and fasting glucose (DeVan et al., 2013; Walker et al., 2009). Evidence for protection from poor diet per se in human subjects is lacking, but: 1) aerobic exercise does protect against Western diet-induced impairments in endothelial function in old mice by increasing resistance to oxidative stress and preserving NO (Lesniewski et al., 2013), and 2) aerobic exercise also prevents acute, postprandial impairments in endothelial function as a result of high fat meals in middle aged adults (Tyldum et al., 2009). Taken together, these observations suggest that aerobic exercise likely enhances the effects of healthy diet/nutrition and reduces the effects of poor diet on arterial function with aging (Figure 5).
In contrast to diet and exercise, the data on interactions between nutraceuticals and healthy lifestyle/diet are less clear. Some examples are encouraging; lactotripeptides (isolated from dairy products), for example, additively enhance the effects of exercise on endothelial function in postmenopausal women (Yoshizawa et al., 2009, 2010). Similarly, supplementation with curcumin is as effective as exercise for improving endothelial function (Akazawa et al., 2012), and may synergistically improve vascular parameters related to arterial stiffness in this same group (Sugawara et al., 2012). The effects of other nutraceuticals, however, are uncertain. Recent reports suggest that resveratrol supplementation may inhibit some of exercise’s physiological benefits (although not in arteries per se) (Gliemann et al., 2013), and studies of omega-3 fish oil supplements combined with exercise have found mixed, but generally benign results (Hill et al., 2007). For the great many nutraceuticals that have not been studied in this way, determining the nature of these potential interactions will be an important line of research in the future.
Remaining Questions
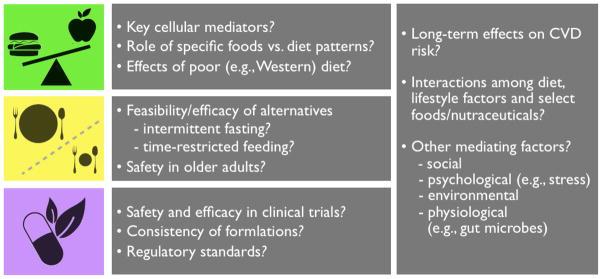
Significant evidence supports certain dietary patterns and nutritional strategies for healthy arterial aging, but many important questions remain for future investigation (Figure 6).
Figure 6.
Important remaining questions and areas of research related to healthy diet, energy restriction and nutraceutical treatments for preventing/reversing age-related arterial dysfunction.
How effective will dietary/nutritional interventions be for reducing long-term CVD risk?
Research into nutritional influences on arterial aging and their role in the development of CVD is relatively new. Thus, although evidence demonstrates that improvements in endothelial function and arterial stiffness are associated with a reduced risk of CVD-related outcomes (Kitta et al., 2009; Modena et al., 2002; Orlova et al., 2010) and it is clear that dietary/nutritional interventions may improve arterial functions with aging, whether or not these improvements will translate to reduced long-term CVD risk remains to be determined. Improvements in arterial function are linked with favorable changes in biological processes that lead to CVD (e.g., reduced oxidative stress/inflammation in the arterial wall), but there is an important need for intervention trials that include follow-up assessments of CVD-related outcomes years, and perhaps decades later.
How do healthy dietary patterns exert their effects on arteries?
Broadly, it is still unclear whether specific components of healthy dietary patterns (e.g., select foods in the Mediterranean diet) are the most important features of these diets. If so, is it possible to supplement other diets with these foods to achieve the same effects, or is overall diet the most influential factor? Or, could particular ingredients within those foods be responsible for the observed benefits, and therefore serve as therapeutic targets of natural or synthetic supplements? And, what is the role of key energy-sensing and anti-oxidant/inflammatory cellular signaling networks in mediating the effects of these diets or foods? Addressing these questions will require more carefully controlled clinical trials to establish efficacy in human subjects, as well as an understanding of the underlying mechanisms (likely via preclinical experiments).
Are alternative energy restriction strategies feasible/effective?
Aside from diet content, quantity (caloric load) is clearly a central mediator of healthy arterial aging. Energy restriction (i.e., CR) appears to be a particularly promising strategy for healthy arterial aging, but it is generally recognized as unfeasible. Other diet-based alternatives, such as protein restriction (Fontana et al., 2008), may be worth investigating for their potential to mimic CR—but concerns about these approaches leading to inappropriate weight/muscle loss in older adults will need to be addressed. Potentially more practical CR alternatives, such as IF and/or TRF, may be effective for mimicking the effects of CR on arterial function, but the efficacy of these strategies remains to be determined. If any are successful in pilot clinical trials, it will be important to establish long-term feasibility and safety in older adults, and to determine whether chronic energy restriction is necessary, or if, for example, several weeks of an IF or TRF regime has sufficiently long-lasting effects on arterial health.
Can nutraceuticals actually mimic the beneficial effects of CR?
Nutraceuticals are a popular and developing area of research; these compounds will probably not be as effective as overall healthy diet for reducing CVD risk, but they may represent promising alternatives and/or complementary approaches for maximizing arterial health. They also are appealing to many consumers and less costly than traditional pharmaceuticals. Unfortunately, the safety and consistency of nutraceutical formulations are not well defined and often vary from one study to the next (Seals et al., 2014), making it difficult to recommend any of these compounds based on existing evidence. Thus, additional well designed clinical trials aimed at translating observations from preclinical studies are needed to determine if nutraceuticals can actually “mimic” the beneficial effects of healthy diet and/or energy restriction on arterial function. More stringent and consistent standards for the composition of nutraceutical formulations (and perhaps additional regulatory oversight) will also be important in this context.
What other dietary/lifestyle factors and mechanisms modulate arterial aging?
Finally, as previously discussed, the adverse effects of poor diet on arterial function with age remain poorly defined. This is a particularly important area of research, as unhealthy (Western) diet may be the norm for most adults in modern countries. Aging adults in these societies are also exposed to numerous environmental factors (e.g., stress, physical inactivity) that may interact with dietary habits to influence arterial function, but many of these are only just being studied. One highly novel and emerging modulator of physiological (and arterial) function that may be affected by these various stresses is the gut microbiome. Dysbiosis of the microbiome has been implicated in various diseases, and appears to be influenced by age, diet and physical activity (exercise) (Conlon and Bird, 2015). However, there is no information about the role of the gut microbiome in mediating changes in arterial function with aging, or the possible influence of consuming a Western diet on this process. Therefore, an important goal for future research will be to determine how age- and diet-related changes to the gut microbiome influence arterial function (i.e., endothelial function and arterial stiffness), as this may be a modifiable target in the quest to prevent age-related CVD.
CONCLUSIONS
With our population rapidly aging, nutritional strategies for preventing or reversing arterial aging and reducing CVD risk will continue to be an important area of research. There is already strong clinical/translational evidence in support of several broad dietary patterns, energy restriction approaches, and nutraceutical compounds for healthy arterial aging. However, many important questions remain. It will be particularly important to further establish which specific diets and foods improve vs. impair function, and which alternative strategies (e.g., intermittent fasting paradigms, select nutracueticals) have the potential to enhance arterial health in older adults for whom healthy diet alone is not enough. Fortunately, there is a growing body of translational evidence that can serve as an experimental platform for designing and conducting future studies to address these questions.
Acknowledgments
The authors are supported by U.S. National Institutes of Health awards AG013038, AG006537, AG000279, TR001082, NS063964, and the Glenn Foundation and the American Federation for Aging Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aaron KJ, Sanders PW. Role of dietary salt and potassium intake in cardiovascular health and disease: a review of the evidence. Mayo Clin Proc. 2013;88:987–995. doi: 10.1016/j.mayocp.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akazawa N, Choi Y, Miyaki A, Tanabe Y, Sugawara J, Ajisaka R, Maeda S. Curcumin ingestion and exercise training improve vascular endothelial function in postmenopausal women. Nutr Res. 2012;32:795–799. doi: 10.1016/j.nutres.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Al-Solaiman Y, Jesri A, Zhao Y, Morrow JD, Egan BM. Low-Sodium DASH reduces oxidative stress and improves vascular function in salt-sensitive humans. J of Hum Hypertens. 2009;23:826–835. doi: 10.1038/jhh.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaras I, Di Germanio C, Bernier M, Csiszar A, Ungvari Z, Lakatta EG, de Cabo R. Pharmacological strategies to retard cardiovascular aging. Circ Res. 2016;118:1626–1642. doi: 10.1161/CIRCRESAHA.116.307475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashor AW, Lara J, Siervo M, Celis-Morales C, Oggioni C, Jakovljevic DG, Mathers JC. Exercise modalities and endothelial function: a systematic review and dose-response meta-analysis of randomized controlled trials. Sports Med. 2015a;45:279–296. doi: 10.1007/s40279-014-0272-9. [DOI] [PubMed] [Google Scholar]
- Ashor AW, Siervo M, Lara J, Oggioni C, Afshar S, Mathers JC. Effect of vitamin C and vitamin E supplementation on endothelial function: a systematic review and meta-analysis of randomised controlled trials. Br J Nutr. 2015b;113:1182–1194. doi: 10.1017/S0007114515000227. [DOI] [PubMed] [Google Scholar]
- Ashor AW, Siervo M, Lara J, Oggioni C, Mathers JC. Antioxidant vitamin supplementation reduces arterial stiffness in adults: a systematic review and meta-analysis of randomized controlled trials. J Nutr. 2014;144:1594–1602. doi: 10.3945/jn.114.195826. [DOI] [PubMed] [Google Scholar]
- Avolio AP, Deng FQ, Li WQ, Luo YF, Huang ZD, Xing LF, O’Rourke MF. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation. 1985;71:202–210. doi: 10.1161/01.cir.71.2.202. [DOI] [PubMed] [Google Scholar]
- Ballard KD, Kupchak BR, Volk BM, Mah E, Shkreta A, Liptak C, Ptolemy AS, Kellogg MS, Bruno RS, Seip RL, Maresh CM, Kraemer WJ, Volek JS. Acute effects of ingestion of a novel whey-derived extract on vascular endothelial function in overweight, middle-aged men and women. Br J Nutr. 2013;109:882–893. doi: 10.1017/S0007114512002061. [DOI] [PubMed] [Google Scholar]
- Blanch N, Clifton PM, Keogh JB. A systematic review of vascular and endothelial function: effects of fruit, vegetable and potassium intake. Nutr Metab Cardiovasc Dis. 2015;25:253–266. doi: 10.1016/j.numecd.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Blanch N, Clifton PM, Petersen KS, Willoughby SR, Keogh JB. Effect of high potassium diet on endothelial function. Nutr Metab Cardiovasc Dis. 2014;24:983–989. doi: 10.1016/j.numecd.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin PH, Caccia C, Johnson J, Waugh R, Sherwood A. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch Intern Med. 2010;170:126–135. doi: 10.1001/archinternmed.2009.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal JA, Smith PJ, Welsh-Bohmer K, Babyak MA, Browndyke J, Lin PH, Doraiswamy PM, Burke J, Kraus W, Hinderliter A, Sherwood A. Can lifestyle modification improve neurocognition? Rationale and design of the ENLIGHTEN clinical trial. Contemp Clin Trials. 2013;34:60–69. doi: 10.1016/j.cct.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton-Freeman B, Sesso HD. Whole food versus supplement: comparing the clinical evidence of tomato intake and lycopene supplementation on cardiovascular risk factors. Adv Nutr. 2014;5:457–485. doi: 10.3945/an.114.005231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacicedo JM, Gauthier MS, Lebrasseur NK, Jasuja R, Ruderman NB, Ido Y. Acute exercise activates AMPK and eNOS in the mouse aorta. Am J Physiol Heart Circ Physiol. 2011;301:H1255–1265. doi: 10.1152/ajpheart.01279.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Ramirez I, Santamaria A, Tobon-Velasco JC, Orozco-Ibarra M, Gonzalez-Herrera IG, Pedraza-Chaverri J, Maldonado PD. Curcumin restores Nrf2 levels and prevents quinolinic acid-induced neurotoxicity. J Nutr Biochem. 2013;24:14–24. doi: 10.1016/j.jnutbio.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Carrizzo A, Puca A, Damato A, Marino M, Franco E, Pompeo F, Traficante A, Civitillo F, Santini L, Trimarco V, Vecchione C. Resveratrol improves vascular function in patients with hypertension and dyslipidemia by modulating NO metabolism. Hypertension. 2013;62:359–366. doi: 10.1161/HYPERTENSIONAHA.111.01009. [DOI] [PubMed] [Google Scholar]
- Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011;57:1511–1522. doi: 10.1016/j.jacc.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20:991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistiakov DA, Revin VV, Sobenin IA, Orekhov AN, Bobryshev YV. Vascular endothelium: functioning in norm, changes in atherosclerosis and current dietary approaches to improve endothelial function. Mini Rev Med Chem. 2015;15:338–350. doi: 10.2174/1389557515666150226114031. [DOI] [PubMed] [Google Scholar]
- Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2015;7:17–44. doi: 10.3390/nu7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti R, Flammer AJ, Hollenberg NK, Luscher TF. Cocoa and cardiovascular health. Circulation. 2009;119:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.827022. [DOI] [PubMed] [Google Scholar]
- Crichton GE, Elias MF, Dore GA, Abhayaratna WP, Robbins MA. Relations between dairy food intake and arterial stiffness: pulse wave velocity and pulse pressure. Hypertension. 2012;59:1044–1051. doi: 10.1161/HYPERTENSIONAHA.111.190017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Luz PL, Tanaka L, Brum PC, Dourado PM, Favarato D, Krieger JE, Laurindo FR. Red wine and equivalent oral pharmacological doses of resveratrol delay vascular aging but do not extend life span in rats. Atherosclerosis. 2012;224:136–142. doi: 10.1016/j.atherosclerosis.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Dai DF, Chen T, Johnson SC, Szeto H, Rabinovitch PS. Cardiac aging: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal. 2012;16:1492–1526. doi: 10.1089/ars.2011.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Picciotto NE, Gano LB, Johnson LC, Martens CR, Sindler AL, Mills KF, Imai S, Seals DR. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15:522–530. doi: 10.1111/acel.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarco VG, Habibi J, Jia G, Aroor AR, Ramirez-Perez FI, Martinez-Lemus LA, Bender SB, Garro M, Hayden MR, Sun Z, Meininger GA, Manrique C, Whaley-Connell A, Sowers JR. Low-dose mineralocorticoid receptor blockade prevents western diet-induced arterial stiffening in female mice. Hypertension. 2015;66:99–107. doi: 10.1161/HYPERTENSIONAHA.115.05674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengo AL, Dennis EA, Orr JS, Marinik EL, Ehrlich E, Davy BM, Davy KP. Arterial destiffening with weight loss in overweight and obese middle-aged and older adults. Hypertension. 2010;55:855–861. doi: 10.1161/HYPERTENSIONAHA.109.147850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVan AE, Eskurza I, Pierce GL, Walker AE, Jablonski KL, Kaplon RE, Seals DR. Regular aerobic exercise protects against impaired fasting plasma glucose-associated vascular endothelial dysfunction with aging. Clin Sci (Lond) 2013;124:325–331. doi: 10.1042/CS20120291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVan AE, Johnson LC, Brooks FA, Evans TD, Justice JN, Cruickshank-Quinn C, Reisdorph N, Bryan NS, McQueen MB, Santos-Parker JR, Chonchol MB, Bassett CJ, Sindler AL, Giordano T, Seals DR. Effects of sodium nitrite supplementation on vascular function and related small metabolite signatures in middle-aged and older adults. J Appl Physiol (1985) 2016;120:416–425. doi: 10.1152/japplphysiol.00879.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNicolantonio JJ, Bhutani J, McCarty MF, O’Keefe JH. Coenzyme Q10 for the treatment of heart failure: a review of the literature. Open Heart. 2015;2:e000326. doi: 10.1136/openhrt-2015-000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Magerko KA, Lawson BR, Durrant JR, Lesniewski LA, Seals DR. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol. 2011;589:4545–4554. doi: 10.1113/jphysiol.2011.211219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Morgan RG, Walker AE, Lesniewski LA. Cellular and molecular biology of aging endothelial cells. J Mol Cell Cardiol. 2015;89:122–135. doi: 10.1016/j.yjmcc.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Walker AE, Magerko KA, Bramwell RC, Black AD, Henson GD, Lawson BR, Lesniewski LA, Seals DR. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell. 2013;12:772–783. doi: 10.1111/acel.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower JI, Geleijnse JM, Gijsbers L, Schalkwijk C, Kromhout D, Hollman PC. Supplementation of the pure flavonoids epicatechin and quercetin affects some biomarkers of endothelial dysfunction and inflammation in (pre)hypertensive adults: a randomized double-blind, placebo-controlled, crossover trial. J Nutr. 2015;145:1459–1463. doi: 10.3945/jn.115.211888. [DOI] [PubMed] [Google Scholar]
- Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans PC. The influence of sulforaphane on vascular health and its relevance to nutritional approaches to prevent cardiovascular disease. EPMA J. 2011;2:9–14. doi: 10.1007/s13167-011-0064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, Rengo G, Rossi F, Filippelli A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008;11:139–150. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- Ferrari CK. Functional foods, herbs and nutraceuticals: towards biochemical mechanisms of healthy aging. Biogerontology. 2004;5:275–289. doi: 10.1007/s10522-004-2566-z. [DOI] [PubMed] [Google Scholar]
- Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-beta1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol. 2010;588:3971–3982. doi: 10.1113/jphysiol.2010.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS, Sindler AL, Marvi NK, Howell KL, Zigler ML, Yoshizawa M, Seals DR. Curcumin ameliorates arterial dysfunction and oxidative stress with aging. Exp Gerontol. 2013;48:269–276. doi: 10.1016/j.exger.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7:681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes F, Lopez-Miranda J, Perez-Martinez P, Jimenez Y, Marin C, Gomez P, Fernandez JM, Caballero J, Delgado-Lista J, Perez-Jimenez F. Chronic effects of a high-fat diet enriched with virgin olive oil and a low-fat diet enriched with alpha-linolenic acid on postprandial endothelial function in healthy men. Br J Nutr. 2008;100:159–165. doi: 10.1017/S0007114508888708. [DOI] [PubMed] [Google Scholar]
- Fuentes F, Lopez-Miranda J, Sanchez E, Sanchez F, Paez J, Paz-Rojas E, Marin C, Gomez P, Jimenez-Pereperez J, Ordovas JM, Perez-Jimenez F. Mediterranean and low-fat diets improve endothelial function in hypercholesterolemic men. Ann Intern Med. 2001;134:1115–1119. doi: 10.7326/0003-4819-134-12-200106190-00011. [DOI] [PubMed] [Google Scholar]
- Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension. 2004;44:35–41. doi: 10.1161/01.HYP.0000132767.74476.64. [DOI] [PubMed] [Google Scholar]
- Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol. 2014;592:2549–2561. doi: 10.1113/jphysiol.2013.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliemann L, Schmidt JF, Olesen J, Bienso RS, Peronard SL, Grandjean SU, Mortensen SP, Nyberg M, Bangsbo J, Pilegaard H, Hellsten Y. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J Physiol. 2013;591:5047–5059. doi: 10.1113/jphysiol.2013.258061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall WL. Dietary saturated and unsaturated fats as determinants of blood pressure and vascular function. Nutr Res Rev. 2009;22:18–38. doi: 10.1017/S095442240925846X. [DOI] [PubMed] [Google Scholar]
- Harper S. Economic and social implications of aging societies. Science. 2014;346:587–591. doi: 10.1126/science.1254405. [DOI] [PubMed] [Google Scholar]
- Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, Son TG, Maudsley S, Carlson OD, Egan JM, Flyvbjerg A, Howell A. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond) 2011;35:714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Miyagawa K, Sato K, Ueda R, Dohi Y. Temocapril, an Angiotensin converting enzyme inhibitor, ameliorates age-related increase in carotid arterial stiffness in normotensive subjects. Cardiology. 2006;106:190–194. doi: 10.1159/000093024. [DOI] [PubMed] [Google Scholar]
- Heffernan KS, Chale A, Hau C, Cloutier GJ, Phillips EM, Warner P, Nickerson H, Reid KF, Kuvin JT, Fielding RA. Systemic vascular function is associated with muscular power in older adults. J Aging Res. 2012;2012:386387. doi: 10.1155/2012/386387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ American Heart Association Advocacy Coordinating, C., Stroke, C., Council on Cardiovascular, R., Intervention, Council on Clinical, C., Council on, E., Prevention, Council on, A., Thrombosis, Vascular, B., Council on, C., Critical, C., Perioperative, Resuscitation, Council on Cardiovascular, N., Council on the Kidney in Cardiovascular, D., Council on Cardiovascular, S., Anesthesia, Interdisciplinary Council on Quality of, C., Outcomes, R. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- Heiss C, Sansone R, Karimi H, Krabbe M, Schuler D, Rodriguez-Mateos A, Kraemer T, Cortese-Krott MM, Kuhnle GG, Spencer JP, Schroeter H, Merx MW, Kelm M. Impact of cocoa flavanol intake on age-dependent vascular stiffness in healthy men: a randomized, controlled, double-masked trial. Age (Dordrecht, Netherlands) 2015;37:9794. doi: 10.1007/s11357-015-9794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson GD, Walker AE, Reihl KD, Donato AJ, Lesniewski LA. Dichotomous mechanisms of aortic stiffening in high-fat diet fed young and old B6D2F1 mice. Physiol Rep. 2014;2:e00268. doi: 10.1002/phy2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AM, Buckley JD, Murphy KJ, Howe PR. Combining fish-oil supplements with regular aerobic exercise improves body composition and cardiovascular disease risk factors. Am J Clin Nutr. 2007;85:1267–1274. doi: 10.1093/ajcn/85.5.1267. [DOI] [PubMed] [Google Scholar]
- Hobbs DA, George TW, Lovegrove JA. The effects of dietary nitrate on blood pressure and endothelial function: a review of human intervention studies. Nutr Res Rev. 2013;26:210–222. doi: 10.1017/S0954422413000188. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Zhu M, Mamczarz J, Zou S, Lane MA, Roth GS, deCabo R. Calorie restriction mimetics: an emerging research field. Aging Cell. 2006;5:97–108. doi: 10.1111/j.1474-9726.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57:63–69. doi: 10.1161/HYPERTENSIONAHA.110.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski KL, Gates PE, Pierce GL, Seals DR. Low dietary sodium intake is associated with enhanced vascular endothelial function in middle-aged and older adults with elevated systolic blood pressure. Ther Adv Cardiovasc Dis. 2009;3:347–356. doi: 10.1177/1753944709345790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, Seals DR. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol. 2013;61:335–343. doi: 10.1016/j.jacc.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauhiainen T, Ronnback M, Vapaatalo H, Wuolle K, Kautiainen H, Groop PH, Korpela R. Long-term intervention with Lactobacillus helveticus fermented milk reduces augmentation index in hypertensive subjects. Eur J Clin Nutr. 2010;64:424–431. doi: 10.1038/ejcn.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez R, Duarte J, Perez-Vizcaino F. Epicatechin: endothelial function and blood pressure. J Agric Food Chem. 2012;60:8823–8830. doi: 10.1021/jf205370q. [DOI] [PubMed] [Google Scholar]
- Joris PJ, Plat J, Bakker SJ, Mensink RP. Long-term magnesium supplementation improves arterial stiffness in overweight and obese adults: results of a randomized, double-blind, placebo-controlled intervention trial. Am J Clin Nutr. 2016;103:1260–1266. doi: 10.3945/ajcn.116.131466. [DOI] [PubMed] [Google Scholar]
- Justice JN, Johnson LC, DeVan AE, Cruickshank-Quinn C, Reisdorph N, Bassett CJ, Evans TD, Brooks FA, Bryan NS, Chonchol MB, Giordano T, McQueen MB, Seals DR. Improved motor and cognitive performance with sodium nitrite supplementation is related to small metabolite signatures: a pilot trial in middle-aged and older adults. Aging (Albany NY) 2015;7:1004–1021. doi: 10.18632/aging.100842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplon RE, Hill SD, Bispham NZ, Santos-Parker JR, Nowlan MJ, Snyder LL, Chonchol M, LaRocca TJ, McQueen MB, Seals DR. Oral trehalose supplementation improves resistance artery endothelial function in healthy middle-aged and older adults. Aging (Albany NY) 2016 doi: 10.18632/aging.100962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey NA, Wilkins HM, Linseman DA. Nutraceutical antioxidants as novel neuroprotective agents. Molecules. 2010;15:7792–7814. doi: 10.3390/molecules15117792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh JB, Grieger JA, Noakes M, Clifton PM. Flow-mediated dilatation is impaired by a high-saturated fat diet but not by a high-carbohydrate diet. Arterioscler Thromb Vasc Biol. 2005;25:1274–1279. doi: 10.1161/01.ATV.0000163185.28245.a1. [DOI] [PubMed] [Google Scholar]
- Kitta Y, Obata JE, Nakamura T, Hirano M, Kodama Y, Fujioka D, Saito Y, Kawabata K, Sano K, Kobayashi T, Yano T, Nakamura K, Kugiyama K. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol. 2009;53:323–330. doi: 10.1016/j.jacc.2008.08.074. [DOI] [PubMed] [Google Scholar]
- Klonizakis M, Alkhatib A, Middleton G, Smith MF. Mediterranean diet- and exercise-induced improvement in age-dependent vascular activity. Clin Sci (Lond) 2013;124:579–587. doi: 10.1042/CS20120412. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Lichtenstein AH, Howard BV, Steinberg D, Witztum JL Nutrition Committee of the American Heart Association Council on Nutrition, P.A., Metabolism. Antioxidant vitamin supplements and cardiovascular disease. Circulation. 2004;110:637–641. doi: 10.1161/01.CIR.0000137822.39831.F1. [DOI] [PubMed] [Google Scholar]
- Kroeger CM, Klempel MC, Bhutani S, Trepanowski JF, Tangney CC, Varady KA. Improvement in coronary heart disease risk factors during an intermittent fasting/calorie restriction regimen: Relationship to adipokine modulations. Nutr Metab (Lond) 2012;9:98. doi: 10.1186/1743-7075-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Landberg R, Naidoo N, van Dam RM. Diet and endothelial function: from individual components to dietary patterns. Curr Opin Lipidol. 2012;23:147–155. doi: 10.1097/MOL.0b013e328351123a. [DOI] [PubMed] [Google Scholar]
- LaRocca TJ, Gioscia-Ryan RA, Hearon CM, Jr, Seals DR. The autophagy enhancer spermidine reverses arterial aging. Mech Ageing Dev. 2013;134:314–320. doi: 10.1016/j.mad.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca TJ, Hearon CM, Jr, Henson GD, Seals DR. Mitochondrial quality control and age-associated arterial stiffening. Exp Gerontol. 2014;58:78–82. doi: 10.1016/j.exger.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce GL, Seals DR. Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol. 2012;590:3305–3316. doi: 10.1113/jphysiol.2012.229690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Pase M, Pipingas A, Raubenheimer J, Thurgood M, Villalon L, Macpherson H, Gibbs A, Scholey A. Switching to a 10-day Mediterranean-style diet improves mood and cardiovascular function in a controlled crossover study. Nutrition (Burbank, Los Angeles County, Calif) 2015;31:647–652. doi: 10.1016/j.nut.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Lesniewski LA, Zigler MC, Durrant JR, Donato AJ, Seals DR. Sustained activation of AMPK ameliorates age-associated vascular endothelial dysfunction via a nitric oxide-independent mechanism. Mech Ageing Dev. 2012;133:368–371. doi: 10.1016/j.mad.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski LA, Zigler ML, Durrant JR, Nowlan MJ, Folian BJ, Donato AJ, Seals DR. Aging compounds western diet-associated large artery endothelial dysfunction in mice: prevention by voluntary aerobic exercise. Exp Gerontol. 2013;48:1218–1225. doi: 10.1016/j.exger.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CL, Fang TC, Gueng MK. Vascular dilatory functions of ovo-lactovegetarians compared with omnivores. Atherosclerosis. 2001;158:247–251. doi: 10.1016/s0021-9150(01)00429-4. [DOI] [PubMed] [Google Scholar]
- Lin QF, Qiu CS, Wang SL, Huang LF, Chen ZY, Chen Y, Chen G. A cross-sectional study of the relationship between habitual tea consumption and arterial stiffness. J Am Coll Nutr. 2016;35:354–361. doi: 10.1080/07315724.2015.1058197. [DOI] [PubMed] [Google Scholar]
- Lira VA, Okutsu M, Zhang M, Greene NP, Laker RC, Breen DS, Hoehn KL, Yan Z. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J. 2013;27:4184–4193. doi: 10.1096/fj.13-228486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZM, Woo J, Wu SH, Ho SC. The role of vitamin D in blood pressure, endothelial and renal function in postmenopausal women. Nutrients. 2013;5:2590–2610. doi: 10.3390/nu5072590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Zempo-Miyaki A, Sasai H, Tsujimoto T, So R, Tanaka K. Lifestyle modification decreases arterial stiffness in overweight and obese men: dietary modification vs. exercise training. Int J Sport Nutr Exerc Metab. 2015;25:69–77. doi: 10.1123/ijsnem.2013-0107. [DOI] [PubMed] [Google Scholar]
- Marin C, Yubero-Serrano EM, Lopez-Miranda J, Perez-Jimenez F. Endothelial aging associated with oxidative stress can be modulated by a healthy mediterranean diet. International journal of molecular sciences. 2013;14:8869–8889. doi: 10.3390/ijms14058869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Montalvo A, Villalba JM, Navas P, de Cabo R. NRF2, cancer and calorie restriction. Oncogene. 2011;30:505–520. doi: 10.1038/onc.2010.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Csiszar A, Dutta D, Balagopal G, Calvani R, Leeuwenburgh C. Role of mitochondrial dysfunction and altered autophagy in cardiovascular aging and disease: from mechanisms to therapeutics. Am J Physiol Heart Circ Physiol. 2013;305:H459–476. doi: 10.1152/ajpheart.00936.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro M, Scoditti E, Carluccio MA, De Caterina R. Nutraceuticals and prevention of atherosclerosis: focus on omega-3 polyunsaturated fatty acids and Mediterranean diet polyphenols. Cardiovasc Ther. 2010;28:e13–19. doi: 10.1111/j.1755-5922.2010.00211.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Hormesis defined. Ageing Res Rev. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Allison DB, Fontana L, Harvie M, Longo VD, Malaisse WJ, Mosley M, Notterpek L, Ravussin E, Scheer FA, Seyfried TN, Varady KA, Panda S. Meal frequency and timing in health and disease. Proc Natl Acad Sci U S A. 2014;111:16647–16653. doi: 10.1073/pnas.1413965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem. 2005;16:129–137. doi: 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- McCall DO, McGartland CP, McKinley MC, Patterson CC, Sharpe P, McCance DR, Young IS, Woodside JV. Dietary intake of fruits and vegetables improves microvascular function in hypertensive subjects in a dose-dependent manner. Circulation. 2009;119:2153–2160. doi: 10.1161/CIRCULATIONAHA.108.831297. [DOI] [PubMed] [Google Scholar]
- McCarty MF, DiNicolantonio JJ, O’Keefe JH. Capsaicin may have important potential for promoting vascular and metabolic health. Open Heart. 2015;2:e000262. doi: 10.1136/openhrt-2015-000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TE, Kovacs SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol. 2006;47:398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- Miller SL, Wolfe RR. The danger of weight loss in the elderly. J Nutr Health Aging. 2008;12:487–491. doi: 10.1007/BF02982710. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moat SJ, Lang D, McDowell IF, Clarke ZL, Madhavan AK, Lewis MJ, Goodfellow J. Folate, homocysteine, endothelial function and cardiovascular disease. J Nutr Biochem. 2004;15:64–79. doi: 10.1016/j.jnutbio.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40:505–510. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- Mohler ER, 3rd, Sibley AA, Stein R, Davila-Roman V, Wyatt H, Badellino K, Rader DJ, Klein S, Foster GD. Endothelial function and weight loss: comparison of low-carbohydrate and low-fat diets. Obesity (Silver Spring) 2013;21:504–509. doi: 10.1002/oby.20055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133:187–225. doi: 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Appel LJ, Van Horn L. Components of a cardioprotective diet: new insights. Circulation. 2011;123:2870–2891. doi: 10.1161/CIRCULATIONAHA.110.968735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart Disease and Stroke Statistics-2016 Update: a report from the American Heart Association. Circulation. 2016;133:e38–60. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Mubarak A, Bondonno CP, Liu AH, Considine MJ, Rich L, Mas E, Croft KD, Hodgson JM. Acute effects of chlorogenic acid on nitric oxide status, endothelial function, and blood pressure in healthy volunteers: a randomized trial. J Agric Food Chem. 2012;60:9130–9136. doi: 10.1021/jf303440j. [DOI] [PubMed] [Google Scholar]
- Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Goldman DP, Zheng Y, Rowe JW. Aging in America in the twenty-first century: demographic forecasts from the MacArthur Foundation Research Network on an Aging Society. Milbank Q. 2009;87:842–862. doi: 10.1111/j.1468-0009.2009.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oplander C, Volkmar CM, Paunel-Gorgulu A, van Faassen EE, Heiss C, Kelm M, Halmer D, Murtz M, Pallua N, Suschek CV. Whole body UVA irradiation lowers systemic blood pressure by release of nitric oxide from intracutaneous photolabile nitric oxide derivates. Circ Res. 2009;105:1031–1040. doi: 10.1161/CIRCRESAHA.109.207019. [DOI] [PubMed] [Google Scholar]
- Orlova IA, Nuraliev EY, Yarovaya EB, Ageev FT. Prognostic value of changes in arterial stiffness in men with coronary artery disease. Vasc Health Risk Manag. 2010;6:1015–1021. doi: 10.2147/VHRM.S13591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pase MP, Grima NA, Sarris J. The effects of dietary and nutrient interventions on arterial stiffness: a systematic review. Am J Clin Nutr. 2011;93:446–454. doi: 10.3945/ajcn.110.002725. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ, Seals DR. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension. 2008;52:72–79. doi: 10.1161/HYPERTENSIONAHA.108.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, Donato AJ, LaRocca TJ, Eskurza I, Silver AE, Seals DR. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell. 2011;10:1032–1037. doi: 10.1111/j.1474-9726.2011.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-{kappa}B activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation. 2009;119:1284–1292. doi: 10.1161/CIRCULATIONAHA.108.804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Koltai E, Taylor AW, Goto S. Exercise, oxidative stress and hormesis. Ageing Res Rev. 2008;7:34–42. doi: 10.1016/j.arr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Rammos C, Hendgen-Cotta UB, Sobierajski J, Bernard A, Kelm M, Rassaf T. Dietary nitrate reverses vascular dysfunction in older adults with moderately increased cardiovascular risk. J Am Coll Cardiol. 2014;63:1584–1585. doi: 10.1016/j.jacc.2013.08.691. [DOI] [PubMed] [Google Scholar]
- Razzak RL, Abu-Hozaifa BM, Bamosa AO, Ali NM. Assessment of enhanced endothelium-dependent vasodilation by intermittent fasting in Wistar albino rats. Indian J Physiol Pharmacol. 2011;55:336–342. [PubMed] [Google Scholar]
- Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010;9:304–312. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha BS, Gago B, Pereira C, Barbosa RM, Bartesaghi S, Lundberg JO, Radi R, Laranjinha J. Dietary nitrite in nitric oxide biology: a redox interplay with implications for pathophysiology and therapeutics. Curr Drug Targets. 2011;12:1351–1363. doi: 10.2174/138945011796150334. [DOI] [PubMed] [Google Scholar]
- Ros E, Hu FB. Consumption of plant seeds and cardiovascular health: epidemiological and clinical trial evidence. Circulation. 2013;128:553–565. doi: 10.1161/CIRCULATIONAHA.112.001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild J, Hoddy KK, Jambazian P, Varady KA. Time-restricted feeding and risk of metabolic disease: a review of human and animal studies. Nutr Rev. 2014;72:308–318. doi: 10.1111/nure.12104. [DOI] [PubMed] [Google Scholar]
- Sanders TA, Lewis FJ, Goff LM, Chowienczyk PJ. SFAs do not impair endothelial function and arterial stiffness. Am J Clin Nutr. 2013;98:677–683. doi: 10.3945/ajcn.113.063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Parker JR, LaRocca TJ, Seals DR. Aerobic exercise and other healthy lifestyle factors that influence vascular aging. Adv Physiol Educ. 2014;38:296–307. doi: 10.1152/advan.00088.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreuder TH, Eijsvogels TM, Greyling A, Draijer R, Hopman MT, Thijssen DH. Effect of black tea consumption on brachial artery flow-mediated dilation and ischaemia-reperfusion in humans. Appl Physiol Nutr Metab. 2014;39:145–151. doi: 10.1139/apnm-2012-0450. [DOI] [PubMed] [Google Scholar]
- Schwingshackl L, Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta-analysis of intervention trials. Nutr Metab Cardiovasc Dis. 2014;24:929–939. doi: 10.1016/j.numecd.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Seals DR. Edward F. Adolph Distinguished Lecture: The remarkable anti-aging effects of aerobic exercise on systemic arteries. J Appl Physiol (1985) 2014;117:425–439. doi: 10.1152/japplphysiol.00362.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 2011;120:357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Justice JN, LaRocca TJ. Physiological geroscience: targeting function to increase healthspan and achieve optimal longevity. J Physiol. 2016;594:2001–2024. doi: 10.1113/jphysiol.2014.282665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Kaplon RE, Gioscia-Ryan RA, LaRocca TJ. You’re only as old as your arteries: translational strategies for preserving vascular endothelial function with aging. Physiology (Bethesda) 2014;29:250–264. doi: 10.1152/physiol.00059.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SF, Poston WS, Reeves RS, Kazaks AG, Holt RR, Keen CL, Chen HJ, Haddock CK, Winters BL, Khoo CS, Foreyt JP. Weight loss in individuals with metabolic syndrome given DASH diet counseling when provided a low sodium vegetable juice: a randomized controlled trial. Nutr J. 2010;9:8. doi: 10.1186/1475-2891-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell. 2011;10:429–437. doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanimirovic DB, Friedman A. Pathophysiology of the neurovascular unit: disease cause or consequence? J Cereb Blood Flow Metab. 2012;32:1207–1221. doi: 10.1038/jcbfm.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara J, Akazawa N, Miyaki A, Choi Y, Tanabe Y, Imai T, Maeda S. Effect of endurance exercise training and curcumin intake on central arterial hemodynamics in postmenopausal women: pilot study. Am J Hypertens. 2012;25:651–656. doi: 10.1038/ajh.2012.24. [DOI] [PubMed] [Google Scholar]
- Tyldum GA, Schjerve IE, Tjonna AE, Kirkeby-Garstad I, Stolen TO, Richardson RS, Wisloff U. Endothelial dysfunction induced by post-prandial lipemia: complete protection afforded by high-intensity aerobic interval exercise. J Am Coll Cardiol. 2009;53:200–206. doi: 10.1016/j.jacc.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura H, Katsuura-Kamano S, Yamaguchi M, Nakamoto M, Hiyoshi M, Arisawa K. Consumption of coffee, not green tea, is inversely associated with arterial stiffness in Japanese men. Eur J Clin Nutr. 2013;67:1109–1114. doi: 10.1038/ejcn.2013.132. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res. 2008;102:519–528. doi: 10.1161/CIRCRESAHA.107.168369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bussel BC, Henry RM, Ferreira I, van Greevenbroek MM, van der Kallen CJ, Twisk JW, Feskens EJ, Schalkwijk CG, Stehouwer CD. A healthy diet is associated with less endothelial dysfunction and less low-grade inflammation over a 7-year period in adults at risk of cardiovascular disease. J Nutr. 2015;145:532–540. doi: 10.3945/jn.114.201236. [DOI] [PubMed] [Google Scholar]
- van Bussel BC, Henry RM, Schalkwijk CG, Ferreira I, Feskens EJ, Streppel MT, Smulders YM, Twisk JW, Stehouwer CD. Fish consumption in healthy adults is associated with decreased circulating biomarkers of endothelial dysfunction and inflammation during a 6-year follow-up. J Nutr. 2011;141:1719–1725. doi: 10.3945/jn.111.139733. [DOI] [PubMed] [Google Scholar]
- van de Laar RJ, Stehouwer CD, van Bussel BC, Prins MH, Twisk JW, Ferreira I. Adherence to a Mediterranean dietary pattern in early life is associated with lower arterial stiffness in adulthood: the Amsterdam Growth and Health Longitudinal Study. J Intern Med. 2013;273:79–93. doi: 10.1111/j.1365-2796.2012.02577.x. [DOI] [PubMed] [Google Scholar]
- Varady KA, Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Haus JM, Hoddy KK, Calvo Y. Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr J. 2013;12:146. doi: 10.1186/1475-2891-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AE, Eskurza I, Pierce GL, Gates PE, Seals DR. Modulation of vascular endothelial function by low-density lipoprotein cholesterol with aging: influence of habitual exercise. Am J Hypertens. 2009;22:250–256. doi: 10.1038/ajh.2008.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AE, Kaplon RE, Pierce GL, Nowlan MJ, Seals DR. Prevention of age-related endothelial dysfunction by habitual aerobic exercise in healthy humans: possible role of nuclear factor kappaB. Clin Sci (Lond) 2014;127:645–654. doi: 10.1042/CS20140030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Jiang L, Monticone RE, Lakatta EG. Proinflammation: the key to arterial aging. Trends Endocrinol Metab. 2014;25:72–79. doi: 10.1016/j.tem.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Liang X, Wang L, Lu X, Huang J, Cao J, Li H, Gu D. Effect of omega-3 fatty acids supplementation on endothelial function: a meta-analysis of randomized controlled trials. Atherosclerosis. 2012;221:536–543. doi: 10.1016/j.atherosclerosis.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- Wilkinson IB, Megson IL, MacCallum H, Sogo N, Cockcroft JR, Webb DJ. Oral vitamin C reduces arterial stiffness and platelet aggregation in humans. J Cardiovasc Pharmacol. 1999;34:690–693. doi: 10.1097/00005344-199911000-00010. [DOI] [PubMed] [Google Scholar]
- Wong RH, Berry NM, Coates AM, Buckley JD, Bryan J, Kunz I, Howe PR. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J Hypertens. 2013;31:1819–1827. doi: 10.1097/HJH.0b013e328362b9d6. [DOI] [PubMed] [Google Scholar]
- Wong RH, Howe PR, Buckley JD, Coates AM, Kunz I, Berry NM. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr Metab Cardiovasc Dis. 2011;21:851–856. doi: 10.1016/j.numecd.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Wrick KL. The impact of regulations on the business of nutraceuticals in the united states: yesterday, today, and tomorrow. In: Hasler CM, editor. Regulation of Functional Foods and Nutraceuticals. Blackwell; Oxford, UK: 2005. [Google Scholar]
- Wu CF, Liu PY, Wu TJ, Hung Y, Yang SP, Lin GM. Therapeutic modification of arterial stiffness: an update and comprehensive review. World J Cardiol. 2015;7:742–753. doi: 10.4330/wjc.v7.i11.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M, Maeda S, Miyaki A, Misono M, Choi Y, Shimojo N, Ajisaka R, Tanaka H. Additive beneficial effects of lactotripeptides and aerobic exercise on arterial compliance in postmenopausal women. Am J Physiol Heart Circ Physiol. 2009;297:H1899–1903. doi: 10.1152/ajpheart.00433.2009. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M, Maeda S, Miyaki A, Misono M, Choi Y, Shimojo N, Ajisaka R, Tanaka H. Additive beneficial effects of lactotripeptides intake with regular exercise on endothelium-dependent dilatation in postmenopausal women. Am J Hypertens. 2010;23:368–372. doi: 10.1038/ajh.2009.270. [DOI] [PubMed] [Google Scholar]
- Zieman SJ. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- Zuchi C, Ambrosio G, Luscher TF, Landmesser U. Nutraceuticals in cardiovascular prevention: lessons from studies on endothelial function. Cardiovasc Ther. 2010;28:187–201. doi: 10.1111/j.1755-5922.2010.00165.x. [DOI] [PubMed] [Google Scholar]