Abstract
Epidemiological studies show that maternal immune activation (MIA) during pregnancy is a risk factor for autism. However, mechanisms for how MIA affects brain development and behaviors in offspring remain poorly described. To determine whether placental interleukin-6 (IL-6) signaling is required for mediating MIA on the offspring, we generated mice with restricted deletion of the receptor for IL-6 (IL-6Rα) in placental trophoblasts (Cyp19-Cre+;Il6rafl/fl), and tested offspring of Cyp19-Cre+;Il6rafl/fl mothers for immunological, pathological and behavioral abnormalities following induction of MIA. We reveal that MIA results in acute inflammatory responses in the fetal brain. Lack of IL-6 signaling in trophoblasts effectively blocks MIA-induced inflammatory responses in the placenta and the fetal brain. Furthermore, behavioral abnormalities and cerebellar neuropathologies observed in MIA control offspring are prevented in Cyp19-Cre+;Il6rafl/fl offspring. Our results demonstrate that IL-6 activation in placenta is required for relaying inflammatory signals to the fetal brain and impacting behaviors and neuropathologies relevant to neurodevelopmental disease.
Keywords: Maternal immune activation (MIA), Maternal-placental-fetal axis, Interleukin-6 (IL-6), Placenta, Hindbrain development, Autism spectrum disorder (ASD)
1. INTRODUCTION
Autism spectrum disorder (ASD) is a range of complex neurodevelopmental disorders, characterized by difficulties in social communication, and repetitive and stereotyped behaviors (Association, 2013). To date, the prevalence for ASD in the United States is one in 68 children (Investigators, 2014) and diagnoses worldwide are 62 per 10,000 people (Elsabbagh et al., 2012). Monozygotic twins studies indicate the concordance rate for ASD ranges between 60 and 91% (Ronald and Hoekstra, 2011), which suggests both genetic and non-genetic factors (e.g. environment) could contribute to the etiology of ASD.
Epidemiological studies suggest that maternal infection is a principal non-genetic risk factor for ASD (Brown, 2012). Analyses of large cohorts of ASD patients found that inpatient diagnosis of severe infection during pregnancy are associated with increased ASD risk (Atladottir et al., 2010; Lee et al., 2014). The diversity of infections and the observation that many are not transmitted to the fetus (Fatemi et al., 2012; Shi et al., 2005) suggest that maternal immune activation (MIA), rather than microbial pathogenesis, is responsible for increasing the risk of ASD in the offspring. This emphasis on MIA rather than a specific pathogen is supported by animal models that involve injection of non-pathogenic antigens, such as polyinosinic-polycytidylic acid (poly(I:C))(Boksa, 2010; Meyer, 2014). Stimulation of the maternal immune system in these cases causes offspring to develop behavioral and neuropathological features of ASD similar to those seen in maternal infection (Boksa, 2010; Knuesel et al., 2014; Meyer, 2014).
The molecular mechanisms underlying abnormal neurodevelopment and behavior in MIA are poorly understood, though cytokines appear to be critical (Boksa, 2010; Knuesel et al., 2014). The immune dysregulation in MIA offspring persists postnatally (Garay et al., 2013; Hsiao et al., 2012). MIA causes long-lasting and region specific changes of brain cytokines in the offspring that vary based on developmental time point (Garay et al., 2013). Furthermore, MIA leads to decreases in splenic and mesenteric regulatory T cells (Tregs) and increases in interleukin (IL)-6 and IL-17 production in splenic CD4+ T cells in adult offspring (Hsiao et al., 2012). IL-6 levels are elevated in fetal brain after MIA induction (Connor et al., 2012; Meyer et al., 2006; Wu et al., 2015). Blocking IL-6, but not IL-1β or interferon gamma (IFNγ), after induction of MIA in pregnant mice prevents behavioral abnormalities in the offspring (Smith et al., 2007). Furthermore, injection of recombinant IL-6 alone into mice is sufficient to promote similar behavioral phenotypes seen in the MIA model (Hsiao and Patterson, 2011), arguing for a causal role for IL-6 signaling in this context. The importance of IL-6 in mediating MIA effects on brain and behavior in rodents is also supported by the studies shown in human ASD subjects, wherein IL-6 is increased in ASD subjects (Ashwood et al., 2011; Li et al., 2009; Masi et al., 2014; Vargas et al., 2005; Wei et al., 2011). IL-6 is both necessary and sufficient for mediating the effects of MIA on the development of ASD-related behavioral abnormalities, suggesting that tracing the pathways of MIA-induced IL-6 signaling may reveal novel mechanisms by which maternal insults disrupt fetal neurodevelopment.
The significance of the placenta in the occurrence of psychiatric disorders has been suggested by several studies. The concordance rate of monochorionic twins for schizophrenia is 60%, while dichorionic twins is only 10.7% (Davis et al., 1995). Examining the histology of the placenta from individuals with ASD reveals that about 3 – 8-fold increased odds of having trophoblast inclusion in the ASD groups compared to controls (Anderson et al., 2007; Walker et al., 2013). These correlations indicate that the uterine environment should also be considered when evaluating potential etiologies for psychiatric disorders.
During infection, increased cytokine levels in the maternal environment might directly transmit signals to the fetus through the placenta (Dahlgren et al., 2006; Zaretsky et al., 2004). Placenta is of fetal origin, juxtaposed against the maternal decidua (D) layer, and represents the primary molecular connection between the mother and its developing fetus. IL-6 production signaling in the placenta, particularly in the spongiotrophoblast (SP) layer, following induction of MIA has been reported (Hsiao and Patterson, 2011). Furthermore, the MIA-induced alterations in IL-6 signaling pathways in the placenta, and placental hormone production following MIA are prevented upon immune-activation of Il6−/− pregnant mice (Hsiao and Patterson, 2011), revealing dependence on placental IL-6. Whether placental IL-6 signaling is involved in relaying the detrimental effects of MIA to the developing embryo is unknown.
To understand whether IL-6 signaling in the placenta plays a role in modulating the MIA response, we crossed placental trophoblast specific Cre mice (Cyp19-Cre)(Wenzel and Leone, 2007) with IL-6Rα loxp-flanked mice (Il6rafl/fl)(McFarland-Mancini et al., 2010) to generate trophoblast-specific IL-6Rα knockout mice (Cyp19-Cre+;Il6rafl/fl). The Cyp19 gene encodes aromatase cytochrome P450 converting androgens to estrogens, which plays an important role in uterine and placental growth and differentiation (Furbass et al., 2008). Different promoter regions of the Cyp19 gene drive its expression into different tissues (Rawn and Cross, 2008). The Cyp19-Cre 5912 founder line specifically expresses Cre recombinase at placental trophoblast precursor cells during the early stage of embryogenesis and shows minimal expression of Cre recombinase in fetal tissues (Wenzel and Leone, 2007), which allows us to examine the functionality of IL-6 in MIA model specifically in placental trophoblast population. Herein, we reveal that immune activation in the placenta perturbs fetal brain development during gestation, resulting in ASD-like behavioral symptoms in offspring. These findings support a growing appreciation of environmental risk factors for mental disorders.
2. MATERIALS AND METHODS
2.1. Mice
Wild-type C57BL/6N mice were obtained through Caltech’s barrier animal facility (originally from Charles River, Wilmington, MA, USA). Il6−/− (002650; B6.129S2-Il6tm1Kopf/J; Bar Harbor, ME, USA) and Il6rafl/fl mouse lines were obtained from Jackson Laboratory (012944; B6;SJL-Il6ratm1.1Drew/J012944; Bar Harbor, ME, USA). Cyp19-Cre mouse (5912 line) was obtained by Dr. Gustavo Leone from Ohio State University (Wenzel and Leone, 2007). ROSA::LSL-lacZ mouse was kindly provided by Dr. David J. Anderson at Caltech. Ate1−/− mice were kindly provided by Dr. Alexander J. Varshavsky at Caltech (Brower and Varshavsky, 2009). Mice were maintained at Caltech’s barrier animal facility and transferred to Caltech’s Broad animal facility for experiments. All mice were group housed (2–5 mice per cage) with a 13 hours light/11 hours dark cycle (lights on at 06:00) at 21–23°C and 45% relative humidity within a range of 30–70% in ventilated cages (Super Mouse 750™, Lab Products Inc, Seaford, DE, USA). Pregnant and lactating mice were fed a mix of half 5053 PicoLab Rodent Diet and half 5058 PicoLab Rodent Diet (5053, Lad Diet, St. Louis, MO, USA). All experiments were performed under the approval of the California Institute of Technology Institutional Animal Care and Use Committee (IACUC).
2.2. Generation and genotyping of placental trophoblast IL-6R α knockout mice
Placental trophoblast IL-6Rα knockout mice, Cyp19-Cre+; Il6rafl/fl, were generated by crossing two mouse lines- Il6rafl/fl and Cyp19-Cre (5912 line). To yield a congenic strain of Cyp19-Cre+; Il6rafl/fl mice, the Cyp19-Cre+ mouse line was backcrossed to C57BL/6 for at least 8 generations. After backcrossing, C57BL/6 Cyp19-Cre+ mice were crossed with Il6rafl/fl mice, which were originally derived and maintained on C57BL/6J background. F1 offspring were Il6rafl/+ and Cyp19-Cre+;Il6rafl/+. These were then crossed to yield F2: Il6ra+/+, Il6rafl/+, Il6rafl/fl, Cyp19-Cre+; Il6ra+/+, Cyp19-Cre+;Il6rafl/+, and Cyp19-Cre+; Il6rafl/fl. Offspring of Il6rafl/fl and Cyp19-Cre+; Il6rafl/fl genotypes were maintained for experiments.
Mice were weaned at the age of 3 weeks. Then the mice were labeled by ear punch and tail snips were collected immediately after weaning. gDNA was extracted using a standard DNA extraction protocol. PCR was performed to amplify a fragment of Il6 flox allele or wild-type allele. Cre was detected in a separate round of PCR. Primer sequences are listed below.
Il6ra flox allele: Forward 5′-GAA GGA GGA GCT TGA CCT TGG-3′; Reverse: 5′-AAC CAT GCC TAT CAT CCT TTG G-3′;
Cre: Forward 5′-GGC GTT TTC TGA GCA TAC CTG-3′; 5′-CAT TCT CCC ACC GTC AGT ACG-3′.
For genotyping placentas and fetuses, a piece of tail from the fetus was processed using the standard gDNA extraction and PCR procedure, as described above. The genotype of the placenta can be determined by the genotype of the corresponding fetus.
2.3. Timed-mating for C57BL/6N wild-type, Il6−/−, and Cyp19-Cre+;Il6afl/fl, mutant mice
We adopted a trio timed-mating strategy (two female and one male) to minimize the number of sires and limit variation. The females were transferred to a clean cage one day before the introduced of the male into the cage. Timed-mating pairs were set up in the late phase of the light period. Vaginal plugs were checked the following morning. The day of vaginal plug presence was considered embryonic day 0.5 (E0.5). Three independent mouse lines were used in this study- C57BL/6N wild-type line, Il6−/− mutant line, and Cyp19-Cre+;Il6afl/fl mutant line. For the timed-mating of Il6−/− mutant line for Luminex cytokine array study, two kinds of breeding pairs were used- wild-type sire x Il6−/− dam and Il6−/− sire x wild-type dam. The genotype of offspring yielded from the two kinds of breeding pairs is Il6+/−. For the timed-mating of Cyp19-Cre+;Il6afl/fl mutant line, the pair we used was Il6rafl/fl sire x Cyp19-Cre+;Il6afl/fl dam. The genotype of offspring we yield from the breeding pair included Il6rafl/fl and Cyp19-Cre+;Il6afl/fl. All female mice were 8–16 weeks of age, with no prior pregnancies.
2.4. Maternal Immune Activation (MIA) by poly(I:C)
The induction of MIA is previously described (Chow et al., 2016). Briefly, Potassium salt poly(I:C) (P9582; Sigma, St. Louis, MO, USA) was used to induce MIA. 20mg/kg poly(I:C) was dissolved in 0.9% sodium chloride (Hospira, Inc, Lake Forest, IL, USA). Either poly(I:C) or saline was intraperitoneally injected into pregnant mice on embryonic day 12.5 (E12.5). Poly(I:C) was prepared at 40 mg/ml of the actual weight of poly(I:C) powder.
2.5. Harvesting Embryo and placenta at E12.5 from pregnant mice after MIA
Three hours (hr), 6 hr or 24 hr after poly(I:C) injection, mice were sacrificed by cervical dislocation without anesthesia. Placenta and fetal brains were harvested from poly(I:C)- and saline-injected pregnant mice, based on prior work in MIA on the placenta (Hsiao and Patterson, 2011). Fetal brains were dissected under a stereomicroscope (M5A, Wild Heerbrugg, Switzerland). For gene expression analysis, tissues were stored in RNAlater (Qiagen, Gaithersburg, MD, USA) at −80° C until later used. For immunohistochemistry, fetal brains and placenta were postfixed in 4% paraformaldehyde at 4° C for 30–60 min and then cryopreserved in 30% sucrose at room temperature overnight. Tissues were then embedded in OCT (Tissue-Tek, Torrance, CA, USA), frozen in 2-methybutane on dry ice and stored at −80 ° C until used. For LCM, each embryo was immediately embedded in OCT and frozen in 2-methybutane on dry ice and stored at −80 degrees until used.
2.6. Harvesting adult brain for histology
Adult mice were euthanized using Euthasol. Mice were perfused via the cardiovascular system with PBS followed by 4% paraformaldehyde (Alfa Aesar, Ward Hill, MA, USA). Brains were removed and postfixed in 4% paraformaldehyde overnight at 4°C. Postfixed brains were cryopreserved in 30% sucrose for 3 days at 4° C and then embedded in OCT (Tissue-Tek, Torrance, CA, USA). The embedded brains were kept at −80° C until sectioning.
2.7. RNA extraction and RT-PCR
RNA extraction of placenta and fetal brain was based on the manufacturer’s protocol (Trizol; Life Technologies, Grand Island, NY, USA). The RNA concentration and quality were measured by NanoDrop (Thermo Scientific, Wilmington, DE, USA). Before reverse transcription, RNA was treated with DNase I (Promega, San Luis Obispo, CA, USA) to eliminate genomic DNA contamination. 1μg RNA from each sample was reverse transcribed by using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA).
2.8. Quantitative PCR (qPCR)
The gene expression of fetal brain subregions analysis was measured using Power SYBR Green PCR master mix (Life Technologies, Carlsbad, CA, USA) on ABI Prism 7900HT system (Life Technologies, Carlsbad, CA, USA). All other mRNA expression was measured using FastStart Universal SYBR Green master mix with ROX (Roche, El Cerrito, CA, USA) on the ABI 7300 real-time PCR system (Life Technologies, Carlsbad, CA, USA). Gene expression was normalized to β-actin mRNA. Data are presented as fold-change in gene expression in each group relative to that in the maternal saline control group. The primer sequences were adapted from the Harvard PrimerBank database (Spandidos et al., 2010).
2.9. Luminex cytokine array
Fetal brains were dissected at 3 hours post-poly(I:C) injection and homogenized in Tissue Extraction Reagent I (Invitrogen, Life Technologies, Carlsbad, CA, USA) containing EDTA-free protease inhibitors (Roche, El Cerrito, CA, USA). For cytokine profiling, mouse 20-plex cytokine arrays (Invitrogen, Life Technologies, Carlsbad, CA, USA) were analyzed on the Luminex FLEXMAP 3D platform by the Clinical Immunobiology Correlative Studies Laboratory at the City of Hope (Duarte, CA). These cytokines, chemokines growth factors were chosen because of the relevance to the level of IL-6. Cytokine levels were normalized to total protein content as detected by BCA assay.
2.10. Cryosectioning
For fetal brain sectioning, whole embryos were sectioned sagittally at a thickness of 30 μm and adhered to a Superfrost Plus microscope slide (Fisher Scientific, Tustin, CA, USA). For placenta sectioning, placentas were cut sagittally at a thickness of 16 μm. Slides were stored at −80° C until staining. For adult brain sectioning, 50 μm sagittal sections were cut at −20° C and stored as free-floating in PBS at 4° C until staining.
2.11. Immunohistochemistry
For colorimetric staining, the sections were postfixed with 4% paraformaldehyde for 10 min at room temperature. To eliminate endogenous peroxidase activity, the sections were incubated with 0.6 % hydrogen peroxide (Sigma, St. Louis, MO, USA) for 30 min at room temperature. For pSTAT3 staining, antigen retrieval was conducted after endogenous peroxidase elimination. The slides were incubated in 10 mM sodium citrate pH 6.0 for 30 min in a 95 °C water bath. After incubation, slides were equilibrated to room temperature. To prevent non-specific binding of antibodies, the sections were incubated with blocking solution (10% goat serum, 0.1% triton X-100, and 0.02% sodium azide in PBS) for 1 hour at room temperature. After blocking, the sections were incubated with primary antibody made in blocking solution overnight at room temperature. On the next day, the sections/slides were incubated with biotinylated secondary antibody for 2 hours at room temperature. Lastly, the sections were incubated in VECTASTAIN ABC kit (Vector Laboratories Inc, Burlingame, CA, USA) for 1 hour at room temperature and then developed using the VECTOR NovaRED peroxidase substrate kit (Vector Laboratories Inc, Burlingame, CA, USA) according to the manufacturer’s instructions. Between each step, slides were thoroughly washed with PBS. The sections were dehydrated by xylene and then mounted with Permount mounting medium (Fisher Scientific, Tustin, CA, USA).
For fluorescence staining, the sections were postfixed with 4% paraformaldehyde for 10 min at room temperature. After fixation, the sections/slides were incubated in primary antibody made in blocking solution (10% horse serum, 0.1% triton X-100, and 0.02% sodium azide in PBS) overnight at room temperature. The next day, sections were incubated wtih fluorescence-conjugated secondary antibody for 2 hours at room temperature. Between each step, sections were thoroughly washed with PBS. ProLong gold, anti-fade mounting medium (Molecular Probe, Life Technologies, Carlsbad, CA, USA) was applied to the slide before coverslip mounting.
Free-floating sections were mounted to Superfrost Plus microscope slides (Fisher Scientific, Tustin, CA, USA) after staining. For slide staining, a barrier was drawn on the edge of microscope slide by using ImmEdge Hydrophobic Barrier Pen (Vector Laboratories Inc, Burlingame, CA, USA).
The primary antibodies and their dilutions were rabbit anti-IL-6Rα (1:500; Santa Cruz, CA, USA), rabbit anti-Phosphorylation of the transcription factor Signal Transducer and Activator of Transcription 3 (pSTAT3; 1:500; Cell Signaling, Danvers, MA, USA), mouse anti-NeuN (1:500; Millipore, Darmstadt, Germany), rabbit anti-GFAP (1:500; DAKO, Glostrup, Denmark), rabbit anti-calbindin (1:1000; Abcam, Cambridge, MA, USA), mouse anti-GAD67 (1:1000; Millipore, Darmstadt, Germany), mouse anti-parvalbumin (1:1000; Sigma, St. Louis, MO, USA). The fluorescence-conjugated secondary antibodies were donkey anti-rabbit (1:1000), and donkey anti-mouse (1:1000) (all from Molecular Probes, Life Technologies, Carlsbad, CA, USA). The biotinylated secondary antibody was biotinylated goat anti-rabbit IgG antibody (1:200; Vector Laboratories Inc, Burlingame, CA, USA). Colorimetric staining images were taken using the Nikon DIAPHOT 300 (Nikon, Tokyo, Japan) with SPOT software (V4.6, Sterling Heights, MI, USA). Confocal imaging was done using the Zeiss LSM 5 Exciter inverted laser scanning microscope (Carl Zeiss MicroImaging Gmbh, Jena, Germany) with Zen 2009 software (Carl Zeiss MicroImaging Gmbh, Jena, Germany). Quantification of pSTAT3, calbinidin, and parvalbumin positive cells was analyzed using ImageJ software (NIH, Bethesda, MD, USA).
For quantification of pSTAT3+ cells in placental spongiotrophoblast, the region of interest (ROI) was first selected by a segmented line. All images were converted to 8 bit, thresholds were set between 2.71 and 0.37 after calibration. The optical density for the ROI was analyzed by measurement of area fraction. The final value of optical density for each sample is and average of 5–6 sections. For the quantification of pSTAT3+ cells in fetal brain, positive cells were manually counted by the cell counter from serial sections of whole fetal brains. The final number of positive cells is an average of multiple sections. For quantification of calbindin+ and parvalbumin+ cells in adult cerebellar lobule VII, confocal images for each sample were collected from different focal planes with 2.5 μm intervals, converted to Z-stacks and merged into a 2D image by maximum pixel intensity using Zen 2009 software. Positively stained cells were quantified using a manual cell counter. Cerebellum sections for each mouse were collected every 0.2 mm. The coordination for lobule VII quantification was from medial to lateral (ML) −0.04 mm to 1.20 mm relative to Bregma (bilateral). The length of the Purkinje cell layer was measured by a segmented line and further calibrated to the actual length. The final number of positive cells reported is averaged from multiple sections.
2.12. X-gal staining
Brain sections were incubated with rinse buffer (pH 7.3 100 mM sodium phosphate, 2 mM MgCl2, 0.01% sodium deoxycholate, 0.02% NP-40) 3 times for 40 min at room temperature. After rinsing, sections were stained in the rinse solution containing 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 1 mg/ml X-gal (from 25 mg/ml stock in dimethylformamide) for 24 hours. After staining, sections were postfixed in 4% paraformaldehyde for 10 min at room temperature.
2.13. Laser capture microdissection (LCM) and transcriptome amplification
Fetal brains were cut into 16 μm sections on PALM Membrane Slides (415190-9081-000, MembraneSlide, Carl Zeiss, North York, ON, Canada). Cryostat blades and workstations were cleaned with RNaseZap (Applied Biosystems, Life Technologies, Carlsbad, CA, USA) to prevent RNA degradation. 10 sagittal sections were used for LCM. Duration was limited to <30 min per slide to prevent RNA degradation. For each fetal brain section, the hindbrain was localized by morphology under the Axio Observer.Z1 confocal microscope (Zeiss; Thornwood, NY). Conservative regions of interest encompassing approximately 100 cell nuclei were microdissected (energy: 50–53; focus: 73) using the PALM Microbeam system and PALMRobo software 4.3 (Zeiss; Thornwood, NY) and immediately catapulted into an AdhesiveCap 200 microcentrifuge tube (415190-9181-000, Carl Zeiss, North York, ON, Canada Zeiss). RNA isolation was performed immediately as described above, with the RNeasy Micro Plus kit (Qiagen, Gaithersburg, MD, USA). Genomic DNA was removed using gDNA eliminator columns (Qiagen, Gaithersburg, MD, USA). Total RNA was amplified and reverse-transcribed using the QuantiTect Whole Transcriptome Amplification kit (Qiagen, Gaithersburg, MD, USA). 100 ng cDNA was used for qPCR, according to the methods described above.
2.14. Behavior
Experimental procedures
All mice that underwent behavior testing were maintained under the same conditions as described in subsection 2.1. Mice were weaned at the age of 3 weeks. There is no significant difference in litter sizes across treatment groups (Saline: 7.3±1.2 offspring, MIA: 7.3±2.2 offspring) and sex ratios across treatment groups (Saline Cre-: Male 1.8±0.8, Female 1.4±1.1; Saline Cre+: Male 2.8±1.5, Female 1.6±1.1; MIA Cre-: Male 1.9±2.3, Female 1.9±1.9; MIA Cre+: Male 1±1.7, Female 2.6±2.1). Both male and female mice were behaviorally tested (male female ratio is 1:1). The male mice were tested prior to female mice in order to avoid the interference of female scent. All behavior tests were performed at 13:00–19:00 daily. The experimenter was blinded from the genotypes while performing behavioral testing and analyzing behavioral data.
Timeline
Marble burying was performed at 7 weeks of age. The 3-chamber social test was performed at 8 weeks of age. All the apparatuses were cleaned with 70% ethanol and then tap water between subjects. Cage bedding was not changed 3 days prior to behavioral testing. Mice were acclimated to the testing room at least 30 min before each behavior test.
3 chamber social test
The design and procedure for the 3 chamber social test was modified from previous literature (Yang et al., 2011). The social chamber is a 40 (width) × 20 (length) × 22 (height) cm Plexiglas box divided equally into 3 chambers by transparent walls made by Plexiglas with the opening doors (10 cm width × 5 cm height). The procedure consisted of two consecutive phases- habituation and sociability. In habituation phase, mouse was placed in the center of the social chamber for 10 min and allowed to freely explore each compartment. In the sociability phase, the testing mouse was enclosed in the center compartment of social chamber with the doors closed. Two inverted steel wire cups were placed in each of the two side chambers. An unfamiliar, strain-, age- and gender- matched mouse was placed in one of the inverted wire cups. The unfamiliar mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). The other inverted wire cup represented a novel object. After setting up, the doors were opened, and the mouse was allowed to investigate the chamber for 10 min. The behavior was recorded by a video camera mounted above the apparatus. Ethovision (Noldus Information Technology, Leesburg, VA, USA) was used to analyze the duration of the mouse in each chamber. Mice were only excluded from the data if they showed biased preference to a certain side of chamber during habituation phase.
Marble burying test
Marble burying is a test for repetitive behavior. The procedure has been previously described (Malkova et al., 2012) with modification. The mouse was first acclimated to a test cage with compressed, 5-cm deep clean, Aspen pine bedding. After this habituation the mouse was returned to its home cage. 20 navy blue glass marbles (15 mm diameter) were gently placed on the bedding of the test cage (4 × 5 arrangement). The mouse was then returned to this test cage and, after 10 min, the number of buried marbles was counted. The criteria for a buried marble was over 50% of the marble covered by bedding. The marbles were cleaned with 70% alcohol, dried, and submerged in Aspen pine bedding between each test.
2.15. Statistic analysis
All data are represented as mean ± standard error mean (SEM). The fetus/offspring used for qRT-PCR, cytokine array, immunohistochemistry were collected from at least 3 independent litters per group. The offspring used for behavior testing were pooled from at least from 6 independent litters per group. The data were analyzed by using a litter as an n number in order to minimize potential confounding litter effect. All offspring were used for each litter. Specifically, the readouts of the offspring from each litter were averaged and used the averaged number to be the biological replicate (Wu et al., 2015). Analysis with the two-tailed unpaired t test was used to compare data between two groups. Data with two variable factors were analyzed by two-way ANOVA with post-hoc test. The data analyzed using Prism 6.0 (Graphpad, La Jolla, CA, USA) and with SigmaStat 3.5 (Systat, San Jose, CA, USA). p value is used to justify the significance between groups. When p is smaller than 0.05, the groups are considered as different. The number of asterisks indicates the difference in the Figures.
3. RESULTS
3.1. Increased IL-6 expression levels in MIA fetal brain
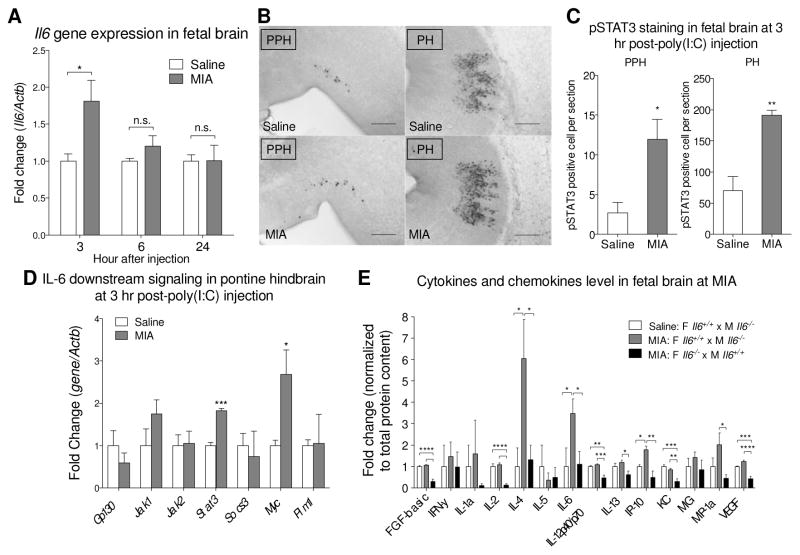
To determine the temporal pro-inflammatory responses of MIA on the developing offspring, we analyzed fetal brain Il6 gene expression at different time point after injection of poly(I:C) to dams at embryonic day 12.5 (E12.5). Brain Il6 levels are higher in fetuses from poly(I:C)-treated dams compared to saline treated dams at 3 hours post-injection (Fig. 1A). Il6 levels return to baseline at 6 and 24 hours post-poly(I:C) injection (Fig. 1A). Thus, the rapid and transient elevation of Il6 gene expression in fetal brain represents an acute inflammatory insult to the developing fetus.
Fig. 1.
MIA induces an acute immune response in the fetal brain. (A) The level of IL-6 in fetal brain was measured by qPCR. Fetal brains from poly(I:C)-injected dam exhibit elevated Il6 gene expression at 3 hours post-injection (t10 = 2.732, p = 0.0211; Student’s two-tailed unpaired t test). No difference in Il6 gene expression is observed between saline and MIA fetal brains at 6 and 24 hours post-injection. (3 hr: Saline n = 6 litters (16 embryos); MIA n = 6 litters (18 embryos); 6 hr and 24 hr: Saline n = 3 litters (9 embryos); MIA n = 3 litters (9 embryos). Il6 gene expression was normalized to β-actin. Gene expression was normalized to β-actin. (B) pSTAT3 is increased in MIA fetal hindbrain at 3 hours post-poly(I:C) injection. Representative images of pSTAT3 staining in sagittal sections of the MIA fetal prepontine hindbrain (PPH) and pontine hindbrain (PH). Scale bar = 200 μm. (C) Quantification of pSTAT3+ cells in the fetal PPH (t7 = 3.038, p = 0.0189) and PH (t6 = 3.888, p = 0.0081) (Student’s two-tailed unpaired t test). Saline n = 4 litters (7 embryos); MIA n = 5 litters (5 embryos). (D) Downstream signaling molecules of the IL-6 pathway in fetal MIA and control LCM samples are analyzed by qPCR. The results are normalized to β-actin using the ddCT method. Stat3 (t4 = 9.483, p = 0.0007) and Myc (t4 = 2.810, p = 0.0483) expression are significantly increased in the PH area after MIA (Student’s two-tailed unpaired t test). Saline n = 3 litters (7–9 embryos); MIA n = 3 litters (9–10 embryos). (E) Luminex cytokine array indicates that MIA-mediated alterations in fetal brain cytokines and chemokines depend on maternal IL-6 genotype. Values were normalized to white bar. Data shows that maternal poly(I:C) injection induces cytokine levels in fetal brain and that maternal IL-6 is required for induction of fetal brain IL-4 (Saline WT dam v.s. MIA WT dam p = 0.0176; MIA WT dam v.s. MIA KO dam p = 0.0238), IL-6 (Saline WT dam v.s. MIA WT dam p = 0.0397; MIA WT dam v.s. MIA KO dam p = 0.0465), IP-10 (Saline WT dam v.s. MIA WT dam p = 0.0201; MIA WT dam v.s. MIA KO dam p = 0.0012) and for maintaining baseline levels of FGF-basic (Saline WT dam v.s. MIA KO dam p < 0.0001; MIA WT dam v.s. MIA KO dam p < 0.0001), IL-2 (Saline WT dam v.s. MIA KO dam p < 0.0001; MIA WT dam v.s. MIA KO dam p < 0.0001), KC (Saline WT dam v.s. MIA KO dam p = 0.0002; MIA WT dam v.s. MIA KO dam p = 0.0012), VEGF (Saline WT dam v.s. MIA KO dam p = 0.0004; MIA WT dam v.s. MIA KO dam p < 0.0001) (One-way ANOVA with Fisher’s LSD post-hoc test). All groups n = 4 litters (4 embryos per group). Cytokine levels were normalized to total protein content. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 v.s. saline control. Data are presented as mean ± SEM. n.s.: not significant.
3.2. The transcription factor STAT3 and downstream signaling are activated in the hindbrain of the MIA fetus
pSTAT3 is a downstream activation marker of cytokine signaling. Immunohistochemistry analysis of pSTAT3 in the brain of embryos at 3 hours post- poly(I:C) injection revealed spatially restricted immune activation following MIA. Several central and peripheral areas in the fetus display positive pSTAT3 staining, including the hindbrain, trigeminal ganglion (Fig. 1B, Supplementary Fig. S1–S3). The most dramatic changes in pSTAT3 levels are in hindbrain areas—specifically the prepontine hindbrain (PPH) and pontine hindbrain (PH) (Fig. 1B, low magnification in Supplementary Fig. S1A and detailed annotation in Fig. S1B). These two hindbrain areas develop into the cerebellum and brainstem (Puelles et al., 2013), sites of dysfunction in some cases of autism (Courchesne, 1997; Rodier et al., 1996; Wang et al., 2014). The distribution of pSTAT3+ cells is significantly higher in the MIA fetal brain compared to saline controls within the PPH and PH (Fig. 1C), and largely localize to the lateral side of the fetal brain.
To further analyze regional-specific pro-inflammatory responses identified by pSTAT3 staining, we collected the PH by LCM (Supplementary Fig. S1C), and examined the expression of genes relevant to the JAK/STAT3 signaling pathway. Following MIA induction, Stat3 and Myc expressions are significantly increased in the PH (Fig. 1D). This is consistent with the finding that Myc is a central “hub” in signaling pathways enriched in the ASD brain (Ziats and Rennert, 2011). Taken together, these data reveal that MIA induces increased cytokines expression and activation of STAT3 signaling pathways within specific brain regions in the developing fetus.
3.3. Maternal IL-6 is required for immune activation in the fetal brain during MIA
Based on the finding that IL-6 is induced directly in the fetal brain following MIA, in addition to previous reports revealing that MIA elevates IL-6 in the maternal blood and placenta, we aimed to determine whether maternal IL-6 is required for the priming of acute inflammatory response in the fetal brain. Wild-type male mice were crossed to Il6−/− females and, in parallel, Il6−/− males were crossed to wild-type females. MIA was induced in pregnant females, generating Il6+/− MIA offspring in both cases. IL-6, IL-4, and IP-10 protein levels are increased in the Il6+/− fetal brain of offspring from wild-type females that have been injected with poly(I:C), compared to saline controls (Fig. 1E). Furthermore, elevation of IL-6, IL-4, and IP10 in the Il6+/− fetal brain were not observed upon injection of poly(I:C) into Il6−/− females, indicating that maternal sources of IL-6 are required for acute induction of cytokines in the fetal brain after MIA. In addition, maternal IL-6 is required for maintaining baseline levels of FGF-basic, IL-2, IL-12p40/p70, KC, and VEGF. These data demonstrate that IL-6 protein expression in the mother exacerbates an inflammatory program in the fetus that may impact brain function and development. Though this finding does not rule out a role for IL-6 signaling outside the fetal brain, it prompted us to examine how IL-6 at the maternal-fetal interface transmits immune activation and behavioral deficits to the fetus.
3.4. Generation of placental trophoblast Il6ra conditional knockout mice
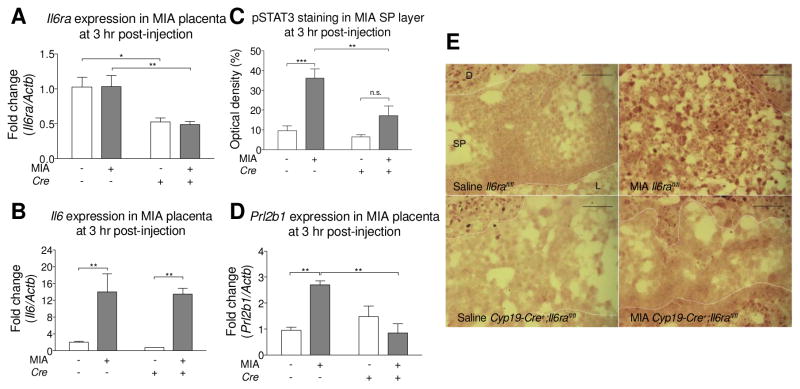
To validate Cyp19-Cre recombinase-mediated Il6ra deletion, we analyzed mRNA and protein levels for the IL-6Rα in the placenta. Analysis of whole placenta RNA extracts from the Cyp19-Cre+;Il6rafl/fl group shows a ~50% reduction in the Il6ra transcript level compared to the Il6rafl/fl group, regardless of MIA induction, which indicates that Cyp19-Cre is specifically localized at SP layer, instead of whole placenta (Fig. 2A). Antibody staining of placental tissue sections for IL-6Rα shows minimal expression in the SP layer of Cyp19-Cre+;Il6rafl/fl mice. In contrast, a few IL-6Rα+ cells can be detected in SP layer of the saline control and placenta from MIA-treated Il6rafl/fl offspring (Supplementary Fig. S4). In addition, knockout of Il6ra does not alter Il6 gene expression in the placenta, as elevation of Il6 is detected in MIA mice regardless of receptor expression (Fig. 2B). To confirm that Cyp19-Cre is not residually expressed directly in the brain, we evaluated β-galactosidase expression in the brains of Cyp19-Cre+;ROSA::LSL-lacZ mice. β-galactosidase staining is absent in the brains of Cyp19-Cre+;ROSA::LSL-lacZ mice, while the positive control shows strong expression across different brain regions (Supplementary Fig. S5). Moreover, IL-6Rα is intact in the calbindin+ cerebellar Purkinje cells and brainstem motor trigeminal nucleus (MO5) of Cyp19-Cre+;Il6rafl/fl brains (Supplementary Fig. S6). No difference is observed in the global distribution of neurons and astrocytes across various adult brain regions in Cyp19-Cre+;Il6rafl/fl offspring compared to Il6rafl/fl offspring (Supplementary Fig. S7). Further, there are no differences in body weights between Cyp19-Cre+;Il6rafl/fl and Il6rafl/fl offspring (Supplementary Fig. S8).
Fig. 2.
Deletion of placental trophoblast Il6rafl/fl decreases STAT3 activation, but not IL-6 production after MIA. (A) The level of IL-6Rα in placenta was measured by qPCR. Il6ra expression is decreased in the Cyp19-Cre+;Il6rafl/fl placenta. There is no effect of MIA on placental Il6ra expression. All groups n = 3 litters (6, 9, 4, 8 placentas were used for Saline Cre−; MIA Cre−; Saline Cre+; MIA Cre+, respectively) (MIA: F(1,8) = 0.08604, p = 0.8774; Cre: F(1,8) = 72.66, p = 0.0017; MIAxCre: F(1,8) = 0.1413, p = 0.8433; Post hoc- Cre− v.s. Cre+: Saline p = 0.0140, MIA p = 0.0091) (Two-way ANOVA with Fisher’s LSD post-hoc test). (B) The level of IL-6 in placenta was measured by qPCR. MIA increases Il6 gene expression in both Il6rafl/fl and Cyp19-Cre+;Il6rafl/fl placentas. There is no effect of Il6ra genotype on placental Il6 gene expression in response to MIA. Il6 gene expression was normalized to β-actin. All groups n = 3 litters (6, 9, 4, 8 placentas were used for Saline Cre−; MIA Cre−; Saline Cre+; MIA Cre+, respectively) (MIA: F(1,8) = 78.65, p = 0.0006; Cre: F(1,8) = 0.4826, p = 0.6780; MIAxCre: F(1,8) = 0.06658, p = 0.8768; Post hoc- Saline v.s. MIA: Cre− p = 0.0054, Cre+ p = 0.0039) (Two-way ANOVA with Fisher’s LSD post-hoc test). (C) Quantification of pSTAT3 optical density in the placental spongiotrophoblast (SP) layer. All groups n = 3 litters (3 placentas per group). (MIA: F(1,8) = 25.34, p = 0.001; Cre: F(1,8) = 8.906, p = 0.0175; MIAxCre: F(1,8) = 4.794, p = 0.06; Post hoc- Saline v.s. MIA: Cre− p = 0.0009, Cre+ p = 0.0791; Cre− v.s. Cre+: Saline p = 0.5895, MIA p = 0.0064) (Two-way ANOVA with Fisher’s LSD post-hoc test) (D) The level of Prolactin family 2 subfamily b member 1 hormone in placenta was measured by qPCR. MIA increases Prl2b1 expression in the Il6rafl/fl placenta. This elevation is prevented in placentas from MIA Cyp19-Cre+;Il6rafl/fl mice. All groups n = 3 litters (5, 6, 5, 6 placentas were used for Saline Cre−; MIA Cre−; Saline Cre+; MIA Cre+, respectively) (MIA: F(1,8) = 3.657, p = 0.0922; Cre: F(1,8) = 5.159, p = 0.0528; MIAxCre: F(1,8) = 16.58, p = 0.0036; Post hoc- Saline v.s. MIA: Cre− p = 0.0029, Cre+ p = 0.1653; Cre− v.s. Cre+: Saline p = 0.2388, MIA p = 0.0020) (Two-way ANOVA with Fisher’s LSD post-hoc test). (E) Representative images of pSTAT3 in the SP layer of saline Il6rafl/fl, MIA Il6rafl/fl, saline Cyp19-Cre+;Il6rafl/fl, and MIA Cyp19-Cre+;Il6rafl/fl placentas. Scale bar = 100 μm. * p < 0.05, ** p < 0.01, *** p < 0.001 between groups. Data are presented as mean ± SEM. D: decidua, L: labyrinth, n.s.: not significant.
3.5. Knockout of placental trophoblast Il6ra blocks MIA induced pSTAT3 activation in spongiotrophoblast layer and placental Prl2b1 gene expression
pSTAT3 is increased in the placenta 3 hours post-poly(I:C) injection, with a maternal IL-6 dependent activation of pSTAT3 in the SP layer (Hsiao and Patterson, 2011). We observed the same effect in MIA-induced Il6rafl/fl mice, as the distribution of pSTAT3+ cells dramatically increase in the SP layer of MIA Il6rafl/fl placentas compared to saline controls, while this increase is not observed in Cyp19-Cre+;Il6rafl/fl mice (Fig. 2C and 2E). Furthermore, knockout of Il6ra in the placental trophoblasts blocks pSTAT3 activation in the SP layer of the placenta following MIA treatment, but not in saline controls (Fig. 2C and 2E). To corroborate the observation that IL-6 signaling is inhibited in the placenta of these mice, levels of Prl2b1 (Prolactin family 2 subfamily b member 1 hormone) were also examined. Prl2b1 is a placenta specific protein expressed in multiple trophoblast lineages (Dai et al., 2000), and we previously demonstrated that Prl2b1 expression is elevated in the placenta of poly(I:C)-treated dams, in a maternal IL-6-dependent manner (Hsiao and Patterson, 2011). An interaction of Cre and MIA is detected in Prl2b1 expression (Fig. 2D). Il6rafl/fl and Cyp19-Cre+;Il6rafl/fl do not differ in placental Prl2b1 expression in saline treated groups; however, for the MIA treatment, Prl2b1 expression is significantly lowered in Cyp19-Cre+;Il6rafl/fl compared to Il6rafl/fl placenta. In addition, in the Il6rafl/fl placenta, Prl2b1 expression is increased in the MIA treated group. Within the Cyp19-Cre+;Il6rafl/fl placenta, there is no difference between saline and MIA groups. Overall, these experiments confirm that knockout of IL-6Rα in the placenta abolishes IL-6-dependent responses in placental pSTAT3 activation and downstream gene expression.
3.6. Inhibition of placental IL-6 signaling prevents immune responses in the fetal brain
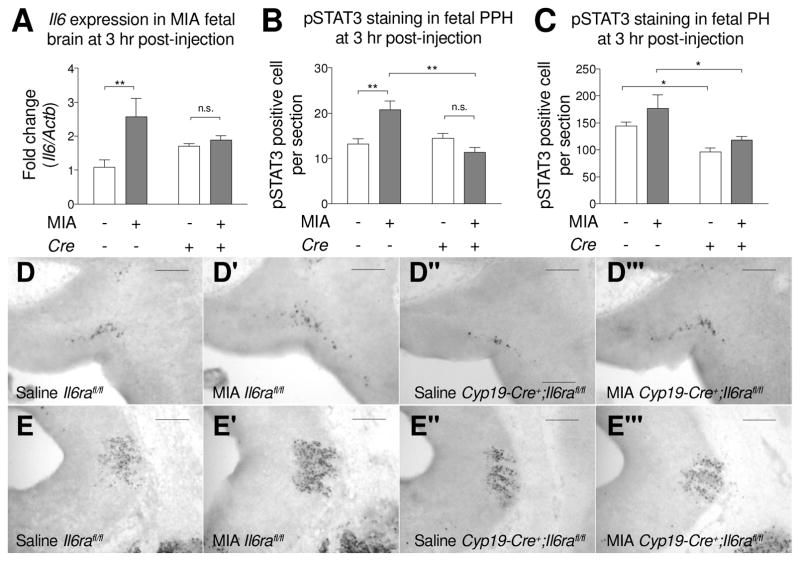
To determine whether placental IL-6 signaling after MIA is required for the elevated Il6 gene expression observed in fetal brain, we analyzed the pro-inflammatory response in fetal brains of Cyp19-Cre+;Il6rafl/fl mice. Gene expression analysis shows that Il6 gene expression is increased in Il6rafl/fl fetal brains after MIA induction, but not in Cyp19-Cre+;Il6rafl/fl fetal brains (Fig. 3A). pSTAT3 activation in the fetal brain is also reduced following inhibition of IL-6 signaling in the placenta (Fig. 3B–3E). Specifically, within the fetal PPH, a main effect for Cre and an interaction for Cre and MIA are detected in fetal brain pSTAT3 activation. Knockout of trophoblast Il6ra results in the reduction of pSTAT3+ cells in the fetal PPH following MIA induction. However, the distribution of pSTAT3+ cells in the fetal PPH is not different between Il6rafl/fl and Cyp19-Cre+;Il6rafl/fl fetuses in saline controls (Fig. 3B and 3D). In addition, for Il6rafl/fl fetal brains, MIA increases the distribution of pSTAT3+ cells in the PPH; however, in Cyp19-Cre+;Il6rafl/fl fetal brains, numbers of pSTAT3+ cells for saline and MIA are similar. Within the fetal PH, knockout of trophoblast Il6ra results in the reduction of pSTAT3+ cells in both saline and MIA groups (Fig. 3C and 3E). There is no difference in pSTAT3+ cells in the PH between saline and MIA in both Il6rafl/fl and Cyp19-Cre+;Il6rafl/fl fetal brains. These data reveal that MIA-induced IL-6 signaling at the maternal-fetal interface of the placenta leads to activation of the immune system within the fetal brain.
Fig. 3.
Deletion of placental trophoblast Il6ra prevents MIA-induced acute responses in the fetal brain. (A) Fetal brain IL-6 gene expression was measured by qPCR. MIA increases Il6 gene expression in the Il6rafl/fl fetal brain, but not in Cyp19-Cre+;Il6rafl/fl fetal brain. Il6 gene expression was normalized to β-actin. All groups n = 3 litters (8, 7, 8, 12 fetal brains were used for Saline Cre−; MIA Cre−; Saline Cre+; MIA Cre+, respectively). (MIA: F(1,8) = 8.043, p = 0.0219; Cre: F(1,8) = 0.01323, p = 0.9113; MIAxCre: F(1,8) = 4.883, p = 0.0581; Post hoc- Saline v.s. MIA: Cre− p = 0.0073, Cre+ p = 0.6696) (Two-way ANOVA with Fisher’s LSD post-hoc test) (B–C) Quantification of pSTAT3+ cells in the fetal (B) prepontine hindbrain (PPH) (MIA: F(1,8) = 2.956, p = 0.1239; Cre: F(1,8) = 9.251, p = 0.0160; MIAxCre: F(1,8) = 15.96, p = 0.0040; Post hoc- Saline v.s. MIA: Cre− p = 0.0037, Cre+ p = 0.1463; Cre− v.s. Cre+: Saline p = 0.5192, MIA p = 0.0011) and (C) pontine hindbrain (PH) (MIA: F(1,8) = 3.757, p = 0.0886; Cre: F(1,8) = 14.50, p = 0.0052; MIAxCre: F(1,8) = 0.1662, p = 0.6942; Post hoc- Cre− v.s. Cre+: Saline p = 0.0429, MIA p = 0.0176). All groups n = 3 litters (6, 7, 3, 6 fetal brains were used in Saline Cre−; MIA Cre−; Saline Cre+; MIA Cre+, respectively) (Two-way ANOVA with Fisher’s LSD post-hoc test). pSTAT3+ cells are more abundant in MIA-induced in Il6rafl/fl fetal PPH compared to saline controls. Reduction of pSTAT3+ cells in the fetal PPH is observed in Cyp19-Cre+;Il6rafl/fl compared to Il6rafl/fl fetus following MIA. (D–E) Elevation of pSTAT3 in MIA fetal hindbrain is prevented when knockout of Il6ra in plcantal trophoblast. Representative images of pSTAT3 staining in sagittal sections of saline Il6rafl/fl, MIA Il6rafl/fl, saline Cyp19-Cre+;Il6rafl/fl and MIA Cyp19-Cre+;Il6rafl/fl fetal PPH (D) and PH (E). Scale bar = 200 μm. * p < 0.05, ** p < 0.01 between groups. Data are presented as mean ± SEM. n.s.: not significant.
3.7. Knockout of placental Il6ra prevents cerebellum neuropathology in MIA offspring
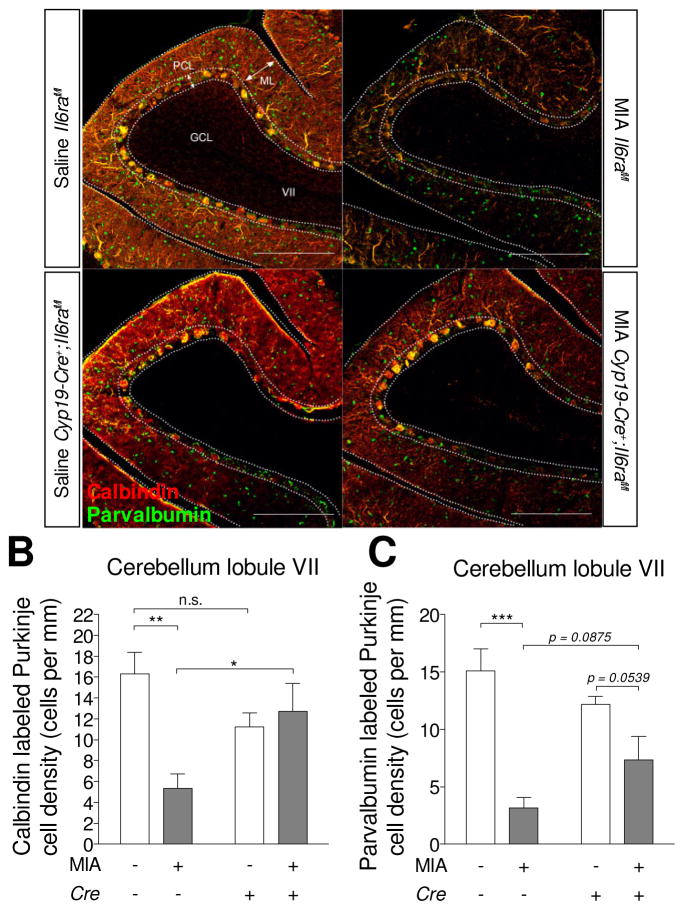
Loss of Purkinje cells in lobule VII of the cerebellum is one of the neuropathological phenotypes seen in the MIA model as well as in ASD (Naviaux et al., 2013; Shi et al., 2009; Skefos et al., 2014). To determine whether inhibition of placental trophoblast IL-6 signaling can prevent the loss of Purkinje cell in adulthood, we visualized calbindin+ Purkinje cells in adult brain sections from control and trophoblast IL-6Rα deficient offspring born to immune-activated mothers. A reduction in calbindin+ Purkinje cells was observed in the cerebellar lobule VII of Il6rafl/fl offspring following MIA compared to saline Il6rafl/fl controls (Fig. 4A and 4A′). The reduction of calbindin+ Purkinje cells in the cerebellar lobule VII by MIA is not observed in Cyp19-Cre+;Il6rafl/fl offspring (Fig. 4A‴ and B). Due to an interaction of Cre and MIA, we further analyzed calbindin+ Purkinje cells between Il6rafl/fl and Cyp19-Cre+;Il6rafl/fl offspring. Remarkably, for the MIA group, Cyp19-Cre+;Il6rafl/fl offspring display significantly greater densities of calbindin+ Purkinje cells in the cerebellar lobule VII compared to Il6rafl/fl offspring (Fig. 4A′ and 4A‴). However, in saline control animals, calbindin+ Purkinje cells do not differ between Il6rafl/fl and Cyp19-Cre+;Il6rafl/fl offspring (Fig. 4A and 4A″). These results suggest that elevated placental IL-6 signaling in response to MIA is required to perturb the neurodevelopment of Purkinje cells in lobule VII.
Fig. 4.
Deletion of placental trophoblast Il6ra prevents cerebellar neuropathology and behavioral abnormalities in MIA offspring. (A) Representative images of calbindin and parvalbumin immunofluorescence staining in the offspring adult brain. Cerebellar Purkinje cells are labeled by calbindin (red) and co-stained for parvalbumin (green). Scale bar = 200 μm. Quantification of (B) calbindin+ and (C) parvalbumin+ Purkinje cell density at cerebellar lobule VII (Calbindin- MIA: F(1,8) = 24.14, p = 0.0410; Cre: F(1,8) = 1.392, p = 0.5752; MIAxCre: F(1,8) = 41.85, p = 0.0126; Post hoc- Saline v.s. MIA: Cre− p =0.0040, Cre+ p = 0.6008; Cre− v.s. Cre+: Saline p = 0.1012, MIA p = 0.0280)(Parvalbumin- MIA: F(1,8) = 30.45, p = 0.0006; Cre: F(1,8) = 0.1830, p = 0.6801; MIAxCre: F(1,8) = 5.403, p = 0.0486; Post hoc- Saline v.s. MIA: Cre− p = 0.0005, Cre+ p = 0.0539; Cre− v.s. Cre+: Saline p = 0.2167, MIA p = 0.0875). All groups n = 3 litters (1–2 offspring per group) (Two-way ANOVA with Fisher’s LSD post-hoc test). * p < 0.05, ** p < 0.01, *** p < 0.001 between groups (Data are presented as mean ± SEM. ML: molecular layer, GCL: granule cell layer, PCL: Purkinje cell layer. n.s.: not significant.
Dysfunction of inhibitory neurotransmission has been described in ASD patients and mouse models (Coghlan et al., 2012). To understand whether the inhibitory neurons of Purkinje cell populations are affected by MIA, we co-stained calbindin with the inhibitory neuronal markers – parvalbumin and glutamate decarboxylase 67 (GAD67). Co-staining indicates that Purkinje cells that express calbindin also express parvalbumin and GAD67 in lobule VII (Fig. 4A and Supplementary Fig. S9). Offspring whose mothers were treated with MIA not only contain fewer calbindin+ cells, but also display lower proportions of parvalbumin+ cells in the Purkinje cell layer (PCL) of lobule VII. This reduction is more pronounced in Il6rafl/fl offspring (Fig. 4A and 4C). In addition, although an interaction of Cre and MIA is detected, there is no detectable difference between Il6rafl/fl and Cyp19-Cre+;Il6rafl/fl offspring in both saline and MIA groups (Fig. 4A and 4C). The reduction of parvalbumin+ cells by MIA is restricted to the PCL, whereas parvalbumin+ cells are intact in molecular layer (ML) of the cerebellum (Fig. 4A). There is no detectable difference in GAD67 staining in the PCL between offspring of saline and MIA Il6rafl/fl mice (Supplementary Fig. S9). Collectively, these data show that activation of the IL-6 pathway in the placenta leads to MIA-induced Purkinje cell defects in offspring, a neuropathology also found in ASD.
3.8. Knockout of placental Il6ra prevents behavioral abnormalities in the MIA model
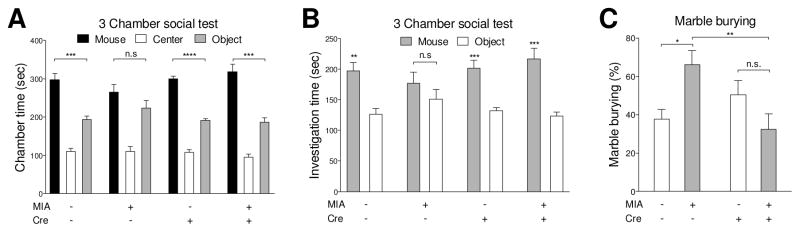
MIA offspring exhibit behavioral abnormalities relevant to ASD (Hsiao et al., 2013). Here, we reveal that placental IL-6 signaling during gestation is a key mediator of MIA-induced impairments in social and marble burying behaviors (Fig. 5A–C). In the 3-chamber social test, the time spent in mouse chamber (social) and object chamber (asocial) is not different in MIA Il6rafl/fl mice (Fig. 5A), whereas offspring in other group spend more time in the mouse chamber compared to the object chamber (Fig. 5A). Similarly, when we further analyzed the investigation time toward stranger mouse and object, MIA Il6rafl/fl offspring have no preference in investigating a stranger mouse and an object (Fig. 5B), whereas other groups investigate the stranger mouse more than object (Fig. 5B). Thus, placental IL-6 signaling impacts social interaction in offspring. Finally, in the marble burying paradigm for repetitive/stereotyped behavior, an interaction of Cre and MIA is detected in marble burying behavior. Following MIA treatment, knockout of placental Il6ra results in the reduction of offspring marble burying behavior when compared to Il6rafl/fl offspring. However, for the saline treatment groups, marble burying behavior does not differ between Cyp19-Cre+;Il6rafl/fl and Il6rafl/fl offspring (Fig. 5C). Furthermore, MIA results in the increase of marble burying behavior in Il6rafl/fl offspring, whereas MIA has no effect on marble burying behavior in Cyp19-Cre+;Il6rafl/fl offspring (Fig. 5C). In addition, no sexual dimorphism in MIA-related behavioral abnormalities was observed in Cyp19-Cre+;Il6rafl/fl offspring (Supplementary Fig. S10). These results indicate that placental IL-6 signaling is required for MIA-induced behavioral abnormalities in social and marble burying behaviors.
Fig. 5.
Deletion of placental trophoblast Il6ra prevents behavioral abnormalities in MIA offspring. (A–B) Offspring social behavior was assessed by 3 chamber social test. Maternal saline treated offspring display social preference, whereas they spend more time in the mouse chamber over than object chamber. On the contrary, MIA Il6rafl/fl offspring do not have social preference. Deletion of placental IL-6Rα in MIA offspring display social preference (A) Mouse v.s. Object: Student’s two-tailed unpaired t test; Chamber duration: Saline Cre− t10 = 5.505, p = 0.0003, MIA Cre− t10 = 1.503, p = 0.1637, Saline Cre+ t10 = 12.71, p < 0.0001, MIA Cre+ t10 = 6.032, p = 0.0001. (B) Mouse v.s. Object: Student’s two-tailed unpaired t test Cup investigation time: Saline Cre− t10 = 4.195, p = 0.0018, MIA Cre− t10 = 1.081, p = 0.3053, Saline Cre+ t10 = 5.1777, p = 0.0004, MIA Cre+ t10 = 4.957, p = 0.0006. All groups n = 6 litters (22, 22, 19, 25 offspring were used for Saline Cre−; MIA Cre−; Saline Cre+; MIA Cre+, respectively). (C) Stereotypic/repetitive behavior was measured by marble burying test. Increased repetitive marble burying behavior is seen in MIA Il6rafl/fl offspring, but abrogated in MIA Cyp19-Cre+;Il6rafl/fl offspring (MIA: F(1,20) = 0.5537, p = 0.4655; Cre: F(1,8) = 2.156, p = 0.1576; MIAxCre: F(1,8) = 10.55, p = 0.0040; Post hoc- Saline v.s. MIA: Cre− p = 0.0105, Cre+ p = 0.0919; Cre− v.s. Cre+: Saline p = 0.2226, MIA p = 0.0033)(Two-way ANOVA with Fisher’s LSD post-hoc test). All groups n = 6 litters (22, 22, 26, 25 offspring were used for Saline Cre−; MIA Cre−; Saline Cre+; MIA Cre+, respectively). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 between groups. Data are presented as mean ± SEM. n.s.: not significant.
4. DISCUSSION
The ASD risk factor, MIA, initiates an immunologic program that leads to offspring with abnormalities in fetal brain development and behavioral deficits. Our study demonstrates that placental IL-6 signaling, specifically in the trophoblast, is required for MIA-induced acute immune activation in the fetal brain, as well as downstream neuropathologies and behavioral impairments. These findings contribute to the increasing appreciation that interactions at the maternal-placental-fetal interface play an important role in relaying the effects of maternal gestational insults to the developing embryo.
However, the events linking transiently elevated fetal brain cytokines to neuropathologies and behavioral deficits remain to be defined. Previous studies of the MIA model reveal that although MIA itself is transient, it results in dynamic and long-lasting changes to cytokine profiles across gross regions of the early postnatal and adult brain (Garay et al., 2013). In addition, MIA in mothers leads to durable programming of persistent immune dysregulation in adult offspring (Hsiao et al., 2012). Epigenetic alterations have been reported in MIA, and may mediate the long-term effects of transient immune activation (Connor et al., 2012). Therefore, transient induction of IL-6 in the fetal brain, during critical period of neurodevelopment, appear to set in motion events that lead to adverse neuropathological and behavioral outcomes in adulthood.
Herein, we demonstrate that initiating the MIA cascade through placental IL-6 signaling leads to adverse effects in the offspring. Three different pathways may contribute to the link between placental IL-6 expression and downstream responses in the fetal brain. First, the placenta may initiate a feed-forward cycle of IL-6 induction in the embryo. Maternal IL-6 can directly cross the placenta and reach the fetus during mid-gestation, but not late gestation in rats, which aligns well with the finding that the placenta exhibits higher permeability during mid-gestation compared to late gestation (Dahlgren et al., 2006). Interestingly, expression of suppressor of cytokine signaling 3 (SOCS3), a key factor involved in the negative inhibition of IL-6 activity, is absent in the SP layer of placenta between E7–E14, while both STAT3 and pSTAT3 are present (San Martin et al., 2013), which may support the notion that IL-6 triggers a feed-forward cycle of cytokine signaling to propagate the MIA inflammatory signal in the absence of its native suppressor.
A second potential mechanism underlying the link between placental IL-6 signaling and fetal brain status is modulation of placental hormones. Hormone imbalance could profoundly impact placental and embryonic development. We find herein that placental Prl2b1 gene expression is upregulated after MIA, but absent in placenta lacking trophoblast-specific IL-6 signaling. Prl2b1 is speculated to play a role in the regulation of placental angiogenesis and uterine growth (Dai et al., 2000). Further, placental corticotrophin-releasing factor (CRF) signaling is another pathway that may affect MIA response. Placenta derived CRF secretion has been shown in response to different conditions during pregnancy, including infectious stress (Florio et al., 2002). In vitro studies reveal that CRF can inhibit IL-6 production induced by endotoxin in human mononuclear cells (Hagan et al., 1992), which suggest other possible mechanisms for CRF activity in contributing to MIA dependent outcomes. Future studies warrant examination of additional placental hormones and functional evaluation of how changes in their expression impact embryonic development.
A third pathway by which placental IL-6Rα signaling may lead to altered neurodevelopment involves an effect of MIA directly on placental permeability. In the placenta, tight junction proteins provide a barrier between the mother and fetus in regulating the exchange of materials, and the permeability of the placenta can be altered during inflammation. Chorioamnionitis-induced upregulation of proinflammatory cytokines decreases expression of tight junction proteins in trophoblastic and endothelial cells of the placenta, facilitating infection (Tossetta et al., 2014). Furthermore, co-culture of human umbilical vein endothelial cells with the placental trophoblast cells from women with preeclampsia results in decreased barrier function of endothelial cells. The endothelial tight junctions become more vulnerable, which indicates that placenta trophoblast-derived factors are able to change the vascular permeability in the placenta (Wang et al., 2004). How such changes in the placenta alter the metabolic and nutrient milieu in the developing embryo is unclear. However, we recently discovered that MIA offspring display increased gut permeability, which allows potentially neurotoxic molecules to leak into the circulation and impact behavior (Hsiao et al., 2013). Tracking metabolites from mother to fetus following placental IL-6 signaling may reveal insights into biological links between placental status and neurodevelopment.
Notably, the role of soluble IL-6Rα (sIL-6Rα) in the MIA model is still not clear. In mouse, 93.4% of circulating sIL-6Rα is contributed by granulocytes, macrophages, and hepatocytes as demonstrated by a conditional IL-6Rα knockout approach (McFarland-Mancini et al., 2010). Cyp19-Cre is expressed in trophoblast precursor cells and predominately distributed within the spongiotrophoblasts, labyrinth trophoblasts, and giant cells of the placenta (Wenzel and Leone, 2007). While the Cyp19-Cre+;Il6rafl/fl mice used in this study exhibited intended deficiencies in placental IL-6R gene expression, whether signaling through sIL-6Rα occurs on IL-6R-deficient trophoblasts is unclear. sIL-6Rα is thought to be derived by either proteolytic cleavage of the membrane moiety to liberate the extracellular portion of the receptor or by alternative splicing of the IL-6R transcript. It is possible that sIL-6Rα derived from non-trophoblastic sources in the placenta or from the maternal bloodstream could bind to gp130 on IL-6R-deficient trophoblasts; however, our data showing that MIA-associated STAT3 activation is abrogated in trophoblast cells from Cyp19-Cre+;Il6rafl/fl mice suggests that signaling through any available sIL-6Rα is not a major confounding factor. Additional studies are warranted to examine the role of sIL-6Rα in the MIA model.
Although we observe that blocking IL-6 signaling in MIA placenta successfully prevents detrimental effects to the offspring, whether direct infiltration of inflammatory cells into the placenta is involved in this MIA response remains unclear. We previously demonstrated MIA increased CD69 expression in decidual natural killer cells (uterine NK, uNK), macrophages and granulocytes (Hsiao and Patterson, 2011). The elevation of CD69 expression in leukocyte populations was unchanged when induced MIA in IL-6 deficient dam, indicating that placental inflammatory cell activation is independent of IL-6 action (Hsiao and Patterson, 2011). In preliminary studies, we observed no overt infiltration of uNK cells or myeloid cells from the decidua or maternal blood spaces into the placenta at 3, 6 or 24 hours post MIA induction, but whether other subtypes of immune cells or other time points may be affected requires further study.
Our study demonstrates that blocking IL-6 signaling in the placenta prevents the development of a robust neuropathology in ASD, a spatially-restricted deficit in Purkinje cells in lobule VII of the cerebellum. Cerebellar injury at birth is a strong risk factor for ASD (Wang et al., 2014). Postmortem studies reveal Purkinje cell abnormalities in ASD patients (Skefos et al., 2014; Whitney et al., 2008). Most pSTAT3+ cells in the fetal brain of MIA offspring are located at cerebellum primordium of PPH. The cerebellum arises from the PPH, specifically rhombomere 1 at E9 of gestation, and develops into the wing-like cerebellar primordium by E12.5 in mice. Purkinje cells arise around E10–E13 (Hashimoto and Mikoshiba, 2003; Miale and Sidman, 1961). Postmitotic Purkinje cells undergo a migration process at E12.5–E15 and start to express calbindin at E14 (Sillitoe and Joyner, 2007; Wang and Zoghbi, 2001). MIA is induced during this critical window of Purkinje cell development at E12.5. In addition, the other region with elevated pSTAT3 is located at the trigeminal ganglion afferents area of PH. It has been demonstrated that by altering hindbrain transcription factors during embryonic stage, the nerve ascending from the trigeminal ganglion ectopically projects into the cerebellum instead of hindbrain (Oury et al., 2006). Based on these finding, hypothetically, induction of MIA in the fetus at E12.5 might directly disrupt cerebellum development, Purkinje cells generation and hindbrain neural circuitry formation. Blocking IL-6 signaling in the placenta during the acute phase of infection is capable of reducing STAT3 activation in the cerebellar primordium of the fetus, and also prevents the deficiency in Purkinje cells seen in the cerebellum of MIA offspring.
We hypothesize that the loss of Purkinje cells in lobule VII of the cerebellum in MIA adult offspring is due to an alterations in developmental trajectory. Previous studies revealed that deficiencies in lobule VII Purkinje cells could be observed as early as postnatal day 11 in offspring of mothers infected with influenza. Moreover, abnormal migration of Purkinje cells was also observed at this stage, with ectopic placement of some Purkinje cells within the cerebellar lobules (Shi et al., 2009). Based on these findings, we speculate that the loss of Purkinje cells observed in adult MIA offspring may result from a common mechanism as seen in the infection model, where the generalized maternal inflammatory response causes the loss of Purkinje cells in lobule VII in offspring during early development.
Traditionally, the cerebellum is regarded as the brain region responsible for controlling motor-related tasks. More recent evidence suggests that the cerebellum may be an essential component in processing external sensory inputs to govern movement and higher cognitive function (Wang et al., 2014). The association between ASD-like behaviors and the loss of cerebellar Purkinje cells has been reported in several mouse models with features of ASD-like behavior (Fujita et al., 2012; Reith et al., 2013; Tsai et al., 2012). Functional MRI studies demonstrate the connectivity of cerebellum lobule VII and the pre-prefrontal and posterior-parietal cortices, which implies lobule VII might be involved in higher cognitive function (Habas et al., 2009; O’Reilly et al., 2010). Disruption of cerebellar lobule VII development at the embryonic stage may produce cognitive impairment in offspring, which again points to the importance of intervention of immune activation in the maternal environment during the acute phase of infection.
The role of trigeminal afferent neurons and associated brain nuclei is relatively not well understood in ASD. Whether MIA disrupts hindbrain pattern formation and development of trigeminal system via activation of inflammatory signaling in the PH, a brain region that receives inputs from the trigeminal ganglion, will need further investigation. Prenatal exposure of valproic acid (VPA) is another model of an environmental risk factor for autism. Intriguingly, injection of VPA between E11.5 and 12.5 leads to reduction of motor neuron number in the trigeminal motor nucleus in rat pups (Rodier et al., 1996). Furthermore, VPA injection decreases immunoreactivity of neurofilaments in the trigeminal nerves of the embryo (Tashiro et al., 2011). Collectively, these studies suggest a possible mechanism by which MIA and other maternal insults could perturb development of trigeminal innervations.
As IL-6 is a proinflammatory cytokine in response to bacterial and viral infections, the prophylactic effect of blocking IL-6 signaling in placenta could widely applicable across different modes of maternal infection. We hypothesize that this preventive effect is not only limited to maternal toll-like receptor 3 (TLR3) activation, as we demonstrate by using poly(I:C), but can also be effective for other types of maternal immune activation, e.g. prenatal activation of TLR4 by administration of the bacterial endotoxin lipopolysaccharide (LPS). Prenatal challenge with LPS similarly increases placental IL-6 during the acute phase of infection in both in vivo and in vitro models (Anton et al., 2012; Bell et al., 2004; Bloise et al., 2013; Jin et al., 2015; Urakubo et al., 2001). Furthermore, histopathology approaches indicate that prenatal administration of LPS causes mesenchymal hyperplasia, infiltration of neutrophils in the placental labyrinth and increase of fibrin deposition at both the spongiotrophoblast and labyrinth zone (Jin et al., 2015). Moreover, maternal LPS challenge has also been shown to activate pSTAT3 in the fetal brain. This pSTAT3 activation in the fetal brain can be neutralized by IL-6 antibody (Mouihate and Mehdawi, 2016), indicating IL-6/STAT3 signaling is the key pathway for prenatal LPS administration. These results lead to a converging mechanism by which maternal infection impacts placental and fetal development via placental IL-6 signaling. Our findings support a causal role for placental IL-6/IL-6Rα/STAT3 in MIA models.
In conclusion, our study reveals that MIA induces an IL-6 surge in the maternal-placental-fetal axis, resulting in acute fetal brain responses and long-term changes in brain development and behavior. We identify signaling of placentally-derived IL-6 through IL-6Rα on placenta trophoblasts as a key step for relaying the MIA response from mother to fetus. Blocking IL-6 signaling in the placenta by trophoblast-specific deletion of IL-6Rα effectively prevents downstream immune responses in the fetal brain and precludes the development of Purkinje cell deficits and ASD-related behaviors in adult offspring. These data contribute to the increasing appreciation for the important role of maternal-fetal interactions at the placenta in guiding embryonic neurodevelopment. Moreover, our findings suggest that blocking placental IL-6 signaling during the acute phase of infection can serve as a preventative therapeutic for the development of neuropathological and behavioral symptoms of ASD in At-risk populations.
Supplementary Material
Highlights.
IL-6 downstream signaling is activated in specific regions of fetal hindbrain after MIA.
Placental IL-6Rα knockout prevents MIA induced inflammatory responses in placental-fetal axis
MIA-induced behavioral abnormalities are prevented in placental IL-6Rα knockout mice
MIA-induced cerebellar neuropathologies are prevented in placental IL-6Rα knockout mice
Acknowledgments
We acknowledge H. Chu, A. Khoshnan, B.D. Needham, T.R. Sampson, C.E. Schretter, and G. Sharon for critically reviewing the manuscript; Professor Gustavo Leone for providing the Cyp19-Cre mouse line; Professor D.J. Anderson for providing ROSA::LSL-lacZ mice; Professor A.J. Varshavsky for providing Ate1−/− mouse; Professor J.M. Allman for use of the LCM equipment; Professor B.J. Wold for use of a cryostat; Dr. B. Williams for training and use of LCM and cryostat equipment; Dr. A. Collazo and Caltech Biological Imaging Center for the training and use of confocal microscope; L. Rodriguez for administrative assistance; J. Gutierrez, K.F. Lee, J. Rodriguez, L.C. Sandoval, N.A. Verduzco for caring of animals. This work was supported by NIH Conte Center Award (NIH 5P50MH086383-04, to P.H.P.); Autism Speaks (#7670, to P.H.P); postdoctoral fellowship from National Science Council, Taiwan (NSC 101-2917-I-564-039, to W.-L.W.); Autism Speaks Weatherstone Predoctoral Fellowship (to E.Y.H.) and NIH/NRSA Predoctoral Fellowship (to E.Y.H.); Caltech Undergraduate Research Fellowship (SURF) (to Z.Y.) and Amgen Scholars Program at Caltech (to Z.Y.); the Heritage Medical Research Institute (to S.K.M.), Simons Foundation (to S.K.M.), and NIH (MH100556 to S.K.M.).
Footnotes
FINANCIAL DISCLOSURES
All authors declare no conflicts of interest related to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson GM, Jacobs-Stannard A, Chawarska K, Volkmar FR, Kliman HJ. Placental trophoblast inclusions in autism spectrum disorder. Biological psychiatry. 2007;61:487–491. doi: 10.1016/j.biopsych.2006.03.068. [DOI] [PubMed] [Google Scholar]
- Anton L, Brown AG, Parry S, Elovitz MA. Lipopolysaccharide induces cytokine production and decreases extravillous trophoblast invasion through a mitogen-activated protein kinase-mediated pathway: possible mechanisms of first trimester placental dysfunction. Human reproduction. 2012;27:61–72. doi: 10.1093/humrep/der362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain, behavior, and immunity. 2011;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association, A.P. Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Association; Washington, D.C: 2013. [Google Scholar]
- Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. Journal of autism and developmental disorders. 2010;40:1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- Bell MJ, Hallenbeck JM, Gallo V. Determining the fetal inflammatory response in an experimental model of intrauterine inflammation in rats. Pediatric research. 2004;56:541–546. doi: 10.1203/01.PDR.0000139407.89883.6B. [DOI] [PubMed] [Google Scholar]
- Bloise E, Bhuiyan M, Audette MC, Petropoulos S, Javam M, Gibb W, Matthews SG. Prenatal endotoxemia and placental drug transport in the mouse: placental size-specific effects. PloS one. 2013;8:e65728. doi: 10.1371/journal.pone.0065728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain, behavior, and immunity. 2010;24:881–897. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Brower CS, Varshavsky A. Ablation of arginylation in the mouse N-end rule pathway: loss of fat, higher metabolic rate, damaged spermatogenesis, and neurological perturbations. PloS one. 2009;4:e7757. doi: 10.1371/journal.pone.0007757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Developmental neurobiology. 2012;72:1272–1276. doi: 10.1002/dneu.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow KH, Yan Z, Wu WL. Induction of Maternal Immune Activation in Mice at Mid-gestation Stage with Viral Mimic Poly(I:C) Journal of visualized experiments: JoVE. 2016 doi: 10.3791/53643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan S, Horder J, Inkster B, Mendez MA, Murphy DG, Nutt DJ. GABA system dysfunction in autism and related disorders: from synapse to symptoms. Neuroscience and biobehavioral reviews. 2012;36:2044–2055. doi: 10.1016/j.neubiorev.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor CM, Dincer A, Straubhaar J, Galler JR, Houston IB, Akbarian S. Maternal immune activation alters behavior in adult offspring, with subtle changes in the cortical transcriptome and epigenome. Schizophrenia research. 2012;140:175–184. doi: 10.1016/j.schres.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E. Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Current opinion in neurobiology. 1997;7:269–278. doi: 10.1016/s0959-4388(97)80016-5. [DOI] [PubMed] [Google Scholar]
- Dahlgren J, Samuelsson AM, Jansson T, Holmang A. Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatric research. 2006;60:147–151. doi: 10.1203/01.pdr.0000230026.74139.18. [DOI] [PubMed] [Google Scholar]
- Dai G, Wang D, Liu B, Kasik JW, Muller H, White RA, Hummel GS, Soares MJ. Three novel paralogs of the rodent prolactin gene family. The Journal of endocrinology. 2000;166:63–75. doi: 10.1677/joe.0.1660063. [DOI] [PubMed] [Google Scholar]
- Davis JO, Phelps JA, Bracha HS. Prenatal development of monozygotic twins and concordance for schizophrenia. Schizophrenia bulletin. 1995;21:357–366. doi: 10.1093/schbul/21.3.357. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcin C, Montiel-Nava C, Patel V, Paula CS, Wang C, Yasamy MT, Fombonne E. Global prevalence of autism and other pervasive developmental disorders. Autism research: official journal of the International Society for Autism Research. 2012;5:160–179. doi: 10.1002/aur.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Rooney RJ, Mori S, Kornfield TE, Reutiman TJ, Kneeland RE, Liesch SB, Hua K, Hsu J, Patel DH. The viral theory of schizophrenia revisited: abnormal placental gene expression and structural changes with lack of evidence for H1N1 viral presence in placentae of infected mice or brains of exposed offspring. Neuropharmacology. 2012;62:1290–1298. doi: 10.1016/j.neuropharm.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florio P, Severi FM, Ciarmela P, Fiore G, Calonaci G, Merola A, De Felice C, Palumbo M, Petraglia F. Placental stress factors and maternal-fetal adaptive response: the corticotropin-releasing factor family. Endocrine. 2002;19:91–102. doi: 10.1385/endo:19:1:91. [DOI] [PubMed] [Google Scholar]
- Fujita E, Tanabe Y, Momoi MY, Momoi T. Cntnap2 expression in the cerebellum of Foxp2(R552H) mice, with a mutation related to speech-language disorder. Neuroscience letters. 2012;506:277–280. doi: 10.1016/j.neulet.2011.11.022. [DOI] [PubMed] [Google Scholar]
- Furbass R, Selimyan R, Vanselow J. DNA methylation and chromatin accessibility of the proximal Cyp 19 promoter region 1.5/2 correlate with expression levels in sheep placentomes. Molecular reproduction and development. 2008;75:1–7. doi: 10.1002/mrd.20756. [DOI] [PubMed] [Google Scholar]
- Garay PA, Hsiao EY, Patterson PH, McAllister AK. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain, behavior, and immunity. 2013;31:54–68. doi: 10.1016/j.bbi.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan P, Poole S, Bristow AF. Immunosuppressive activity of corticotrophin-releasing factor. Inhibition of interleukin-1 and interleukin-6 production by human mononuclear cells. The Biochemical journal. 1992;281( Pt 1):251–254. doi: 10.1042/bj2810251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Mikoshiba K. Mediolateral compartmentalization of the cerebellum is determined on the “birth date” of Purkinje cells. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:11342–11351. doi: 10.1523/JNEUROSCI.23-36-11342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:12776–12781. doi: 10.1073/pnas.1202556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain, behavior, and immunity. 2011;25:604–615. doi: 10.1016/j.bbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Investigators, A.a.D.D.M.N.S.Y.P; Prevention, C.f.D.C.a, editor. Surveillance Summaries. 2014. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2010; pp. 1–21. [PubMed] [Google Scholar]
- Jin SJ, Liu Y, Deng SH, Liao LH, Lin TL, Ning Q, Luo XP. Neuroprotective effects of activated protein C on intrauterine inflammation-induced neonatal white matter injury are associated with the downregulation of fibrinogen-like protein 2/fibroleukin prothrombinase and the inhibition of pro-inflammatory cytokine expression. International journal of molecular medicine. 2015;35:1199–1212. doi: 10.3892/ijmm.2015.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, Toovey S, Prinssen EP. Maternal immune activation and abnormal brain development across CNS disorders. Nature reviews Neurology. 2014;10:643–660. doi: 10.1038/nrneurol.2014.187. [DOI] [PubMed] [Google Scholar]
- Lee BK, Magnusson C, Gardner RM, Blomstrom S, Newschaffer CJ, Burstyn I, Karlsson H, Dalman C. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain, behavior, and immunity. 2014 doi: 10.1016/j.bbi.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, Ji L, Brown T, Malik M. Elevated immune response in the brain of autistic patients. Journal of neuroimmunology. 2009;207:111–116. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain, behavior, and immunity. 2012;26:607–616. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi A, Quintana DS, Glozier N, Lloyd AR, Hickie IB, Guastella AJ. Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Molecular psychiatry. 2014 doi: 10.1038/mp.2014.59. [DOI] [PubMed] [Google Scholar]
- McFarland-Mancini MM, Funk HM, Paluch AM, Zhou M, Giridhar PV, Mercer CA, Kozma SC, Drew AF. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. Journal of immunology. 2010;184:7219–7228. doi: 10.4049/jimmunol.0901929. [DOI] [PubMed] [Google Scholar]
- Meyer U. Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biological psychiatry. 2014;75:307–315. doi: 10.1016/j.biopsych.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miale IL, Sidman RL. An autoradiographic analysis of histogenesis in the mouse cerebellum. Experimental neurology. 1961;4:277–296. doi: 10.1016/0014-4886(61)90055-3. [DOI] [PubMed] [Google Scholar]
- Mouihate A, Mehdawi H. Toll-like receptor 4-mediated immune stress in pregnant rats activates STAT3 in the fetal brain: role of interleukin-6. Pediatric research. 2016;79:781–787. doi: 10.1038/pr.2015.86. [DOI] [PubMed] [Google Scholar]
- Naviaux RK, Zolkipli Z, Wang L, Nakayama T, Naviaux JC, Le TP, Schuchbauer MA, Rogac M, Tang Q, Dugan LL, Powell SB. Antipurinergic therapy corrects the autism-like features in the poly(IC) mouse model. PloS one. 2013;8:e57380. doi: 10.1371/journal.pone.0057380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cerebral cortex. 2010;20:953–965. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oury F, Murakami Y, Renaud JS, Pasqualetti M, Charnay P, Ren SY, Rijli FM. Hoxa2- and rhombomere-dependent development of the mouse facial somatosensory map. Science. 2006;313:1408–1413. doi: 10.1126/science.1130042. [DOI] [PubMed] [Google Scholar]
- Puelles L, Harrison M, Paxinos G, Watson C. A developmental ontology for the mammalian brain based on the prosomeric model. Trends in neurosciences. 2013;36:570–578. doi: 10.1016/j.tins.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Rawn SM, Cross JC. The evolution, regulation, and function of placenta-specific genes. Annual review of cell and developmental biology. 2008;24:159–181. doi: 10.1146/annurev.cellbio.24.110707.175418. [DOI] [PubMed] [Google Scholar]
- Reith RM, McKenna J, Wu H, Hashmi SS, Cho SH, Dash PK, Gambello MJ. Loss of Tsc2 in Purkinje cells is associated with autistic-like behavior in a mouse model of tuberous sclerosis complex. Neurobiology of disease. 2013;51:93–103. doi: 10.1016/j.nbd.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J. Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. The Journal of comparative neurology. 1996;370:247–261. doi: 10.1002/(SICI)1096-9861(19960624)370:2<247::AID-CNE8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Ronald A, Hoekstra RA. Autism spectrum disorders and autistic traits: a decade of new twin studies. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2011;156B:255–274. doi: 10.1002/ajmg.b.31159. [DOI] [PubMed] [Google Scholar]
- San Martin S, Fitzgerald JS, Weber M, Parraga M, Saez T, Zorn TM, Markert UR. Stat3 and Socs3 expression patterns during murine placenta development. European journal of histochemistry: EJH. 2013;57:e19. doi: 10.4081/ejh.2013.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Smith SE, Malkova N, Tse D, Su Y, Patterson PH. Activation of the maternal immune system alters cerebellar development in the offspring. Brain, behavior, and immunity. 2009;23:116–123. doi: 10.1016/j.bbi.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Tu N, Patterson PH. Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. International journal of developmental neuroscience: the official journal of the International Society for Developmental Neuroscience. 2005;23:299–305. doi: 10.1016/j.ijdevneu.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Sillitoe RV, Joyner AL. Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annual review of cell and developmental biology. 2007;23:549–577. doi: 10.1146/annurev.cellbio.23.090506.123237. [DOI] [PubMed] [Google Scholar]
- Skefos J, Cummings C, Enzer K, Holiday J, Weed K, Levy E, Yuce T, Kemper T, Bauman M. Regional alterations in purkinje cell density in patients with autism. PloS one. 2014;9:e81255. doi: 10.1371/journal.pone.0081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandidos A, Wang X, Wang H, Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic acids research. 2010;38:D792–799. doi: 10.1093/nar/gkp1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro Y, Oyabu A, Imura Y, Uchida A, Narita N, Narita M. Morphological abnormalities of embryonic cranial nerves after in utero exposure to valproic acid: implications for the pathogenesis of autism with multiple developmental anomalies. International journal of developmental neuroscience: the official journal of the International Society for Developmental Neuroscience. 2011;29:359–364. doi: 10.1016/j.ijdevneu.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Tossetta G, Paolinelli F, Avellini C, Salvolini E, Ciarmela P, Lorenzi T, Emanuelli M, Toti P, Giuliante R, Gesuita R, Crescimanno C, Voltolini C, Di Primio R, Petraglia F, Castellucci M, Marzioni D. IL-1beta and TGF-beta weaken the placental barrier through destruction of tight junctions: an in vivo and in vitro study. Placenta. 2014;35:509–516. doi: 10.1016/j.placenta.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, Steinberg J, Crawley JN, Regehr WG, Sahin M. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488:647–651. doi: 10.1038/nature11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakubo A, Jarskog LF, Lieberman JA, Gilmore JH. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophrenia research. 2001;47:27–36. doi: 10.1016/s0920-9964(00)00032-3. [DOI] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Annals of neurology. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Walker CK, Anderson KW, Milano KM, Ye S, Tancredi DJ, Pessah IN, Hertz-Picciotto I, Kliman HJ. Trophoblast inclusions are significantly increased in the placentas of children in families at risk for autism. Biological psychiatry. 2013;74:204–211. doi: 10.1016/j.biopsych.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SS, Kloth AD, Badura A. The cerebellum, sensitive periods, and autism. Neuron. 2014;83:518–532. doi: 10.1016/j.neuron.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VY, Zoghbi HY. Genetic regulation of cerebellar development. Nature reviews Neuroscience. 2001;2:484–491. doi: 10.1038/35081558. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lewis DF, Gu Y, Zhang Y, Alexander JS, Granger DN. Placental trophoblast-derived factors diminish endothelial barrier function. The Journal of clinical endocrinology and metabolism. 2004;89:2421–2428. doi: 10.1210/jc.2003-031707. [DOI] [PubMed] [Google Scholar]
- Wei H, Zou H, Sheikh AM, Malik M, Dobkin C, Brown WT, Li X. IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. Journal of neuroinflammation. 2011;8:52. doi: 10.1186/1742-2094-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel PL, Leone G. Expression of Cre recombinase in early diploid trophoblast cells of the mouse placenta. Genesis. 2007;45:129–134. doi: 10.1002/dvg.20276. [DOI] [PubMed] [Google Scholar]
- Whitney ER, Kemper TL, Bauman ML, Rosene DL, Blatt GJ. Cerebellar Purkinje cells are reduced in a subpopulation of autistic brains: a stereological experiment using calbindin-D28k. Cerebellum. 2008;7:406–416. doi: 10.1007/s12311-008-0043-y. [DOI] [PubMed] [Google Scholar]
- Wu WL, Adams CE, Stevens KE, chow KH, Freedman R, Patterson PH. The interaction between maternal immune activation and alpha 7 nicotinic acetylcholine receptor in regulating behaviors in the offspring. Brain, behavior, and immunity. 2015 doi: 10.1016/j.bbi.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Silverman JL, Crawley JN. Automated three-chambered social approach task for mice. Current protocols in neuroscience/editorial board, Jacqueline N. Crawley … [et al.] 2011;Chapter 8(Unit 8):26. doi: 10.1002/0471142301.ns0826s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretsky MV, Alexander JM, Byrd W, Bawdon RE. Transfer of inflammatory cytokines across the placenta. Obstetrics and gynecology. 2004;103:546–550. doi: 10.1097/01.AOG.0000114980.40445.83. [DOI] [PubMed] [Google Scholar]
- Ziats MN, Rennert OM. Expression profiling of autism candidate genes during human brain development implicates central immune signaling pathways. PloS one. 2011;6:e24691. doi: 10.1371/journal.pone.0024691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.