Abstract
Background
Racial disparities in African-American (AA) kidney transplant have persisted for nearly 40 years, with limited data available on the scope of this issue in the contemporary era of transplantation.
Methods
Descriptive retrospective cohort study of US registry data including adult solitary kidney transplants between Jan 1, 2005 to Dec 31, 2009.
Results
60,695 recipients were included; 41,426 Caucasians (68%) and 19,269 AAs (32%). At baseline, AAs were younger, had lower college graduation rates, were more likely to be receiving public health insurance and have diabetes. At one-year post-transplant, AAs had 62% higher risk of graft loss (RR 1.62, 95% CI 1.50–1.75) which increased to 93% at five years (RR 1.93, 95% CI 1.85–2.01). Adjusted risk of graft loss, accounting for baseline characteristics, was 60% higher in AAs (HR 1.61 [1.52–1.69]). AAs had significantly higher risk of acute rejection and delayed graft function.
Conclusion
AAs continue to experience disproportionately high rates of graft loss within the contemporary era of transplant, which are related to a convergence of an array of socioeconomic and biologic risk factors.
Keywords: African Americans, Kidney Transplant, Graft Loss, Acute Rejection
Summary for the Table of Contents
This was a cohort study of US registry data in adult kidney transplant recipients from 2005 to 2009 demonstrating that AAs continue to experience disproportionately high rates of graft loss within the contemporary era of transplant, which are likely related to a convergence of an array of socioeconomic and biologic risk factors.
INTRODUCTION
The first kidney transplant was performed in 1954, when Dr. Joseph E. Murray transplanted a living donor kidney from one twin to another at Brigham Hospital in Boston, MA. Since that time, kidney transplantation has grown from an experimental procedure to the treatment option of choice in eligible patients with end-stage renal disease (ESRD). It has been clearly demonstrated that this procedure dramatically extends both the length of quality of a person’s life, as compared to remaining on dialysis.1,2
Persistent racial disparities in kidney transplant graft survival have been well documented over this same period; first reported in 1977 and extended through contemporary eras.3 Although graft survival rates have dramatically improved over the past 40 years, based on the most recent data, racial disparities in graft outcomes have remained.4 There have been numerous studies focused on trying to understand the prevailing risk factors that disproportionately impact African-American (AA) kidney transplant recipients. Previous research has demonstrated that AAs have a number of significant disadvantages that likely contribute to this disparity, including gene variants, socioeconomics, reduced access to pre-emptive transplants and living donors, and a higher burden of comorbidities.5–13
Since the 1990s, there have been substantial changes to how organs are allocated, improvements in HLA antibody measurement and matching techniques, and significant advancements in immunosuppressant medications.14 Since this time, there is paucity in published studies determining if these changes have impacted the magnitude of racial disparities in kidney transplant outcomes.7 Over this same timeframe, many changes have occurred to the transplant registry with regards to the type and completeness of baseline demographics and transplant variables. With a lack of published studies assessing disparities in AA recipients since these changes, it is currently unclear if they have impacted these inequalities. Thus, the objective of this study was to utilize U.S. national registry data from a more contemporary timeframe of 2005 to 2009 and describe racial disparities in AA kidney transplantation, allowing for an updated assessment to guide future interventions.15,16
MATERIAL AND METHODS
Study Design and Patients
This was a retrospective analysis of the UNOS registry database, which was linked to the Social Security Death Master File (SSDMF) to obtain accurate patient death dates. The UNOS registry contains data regarding every organ donation and transplant event occurring in the U.S. since October 1, 1987.17 After local IRB approval and signing a data use agreement (DUA), we obtained Standard Transplant Analysis and Research (STAR) de-identified datasets in SAS format, which were pre-linked to the SSDMF data. The time period for this study focused on transplant events occurring between Jan 1, 2005 and Dec 31, 2009, with follow up through December 31, 2014. Patients were included if they were adult recipients (≥18 years of age at the time of transplant) of kidney transplants which occurred within the U.S. during the pre-specified timeframe. Pediatrics, recipients of non-renal organs and those that were not either AA or Caucasian were excluded.
Outcome Measurements
The primary outcome measure for this study was death-censored graft loss at one, three and five years post-transplant, which is defined as either a return to chronic dialysis or retransplantation. Patients that died with a functioning allograft were not included as graft loss events, but were censored at the date of death. We also analyzed mortality rates at one, three and five years post-transplant. Overall graft loss, a composite of either graft loss or death, was analyzed at the same time periods. Additional outcomes that were assessed included delayed graft function (defined as the need for dialysis within 7 days of transplant), acute rejection (defined as either biopsy proven or empirically treated) at any time after transplant, and graft function (defined as the serum creatinine [mg/dL] at last follow up).
Exposure Variables
The primary variable of interest for this analysis was race, which was self-identified as detailed in the UNOS registry. For ease of presentation of the data, we restricted this study to only include non-Hispanic Whites (Caucasians) and non-Hispanic Blacks (AAs). Baseline recipient sociodemographics (age, gender, body mass index [BMI], functional status, education and insurance), comorbidities (reason for ESRD, cardiovascular disease [CVD] comorbid conditions and time on dialysis and waitlist), donor characteristics (age, gender, race, and donor type), transplant characteristics/immunologic risks (HLA mismatches, PRA, cold ischemic time, previous kidney transplant) and immunosuppression (induction and maintenance therapy) were compared between groups. Expanded criteria donor (ECD) was defined as age ≥60 years or age ≥50 years with at least two of the following: history of hypertension, death due to CVA or terminal serum creatinine of ≥1.5 mg/dL. Panel reactive antibody (PRA), which is a measure of recipient sensitization to HLA antigens (pre-existing HLA antibodies) was assessed as 0–100%, reporting both the peak and most current values.
Statistical Analysis
Standard descriptive statistics were used to compare categorical and continuous variables stratified by recipient race. For continuous variables, results are reported as means ± standard deviations (SD); for continuous variables that are not normally distributed, such as time on dialysis, HLA mismatches and PRA, results are reported as medians with interquartile ranges (IQR). Categorical variables are presented as percentages. Statistical comparisons between groups were conducted using the Student’s T-test for two independent samples for continuous variables, the Mann Whitney U test for continuous variables that were not normally distributed and the Chi square test for categorical data. Survival curves were estimated using Cox regression analyses, with both unadjusted (race only) and fully adjusted modeling (all baseline variables listed in the exposure section above). As a sensitivity analysis, to determine the impact of missing data, we conducted multiple imputation and estimated the effect of race on outcomes with this dataset, comparing it with the estimates from the complete case dataset (see supplemental Tables 1 and 2) Statistical significance was based on a two-sided p-value of less than 0.05. Statistical analyses were performed using SPSS (version 23.0, IBM Corp, Armonk, NY) and SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Study population
The complete UNOS STAR file contained 394,359 kidney transplant events that occurred between Oct 1, 1987 and Sept 1, 2014. Of these, 19,313 were excluded for being <18 years of age, 37,810 were excluded for receiving non-renal transplants and 62,813 were excluded for being non-Caucasian or non-AA recipients. Finally, 213,728 recipients were excluded for receiving transplants outside the specified time period (2005–2009), leaving 60,695 transplant recipients in the final study cohort; of which, 41,426 (68%) were Caucasian and 19,269 (32%) were AA (See Supplemental Figure 1). The mean follow up was 5.1±2.4 years.
Baseline recipient sociodemographics
AAs had significantly different baseline characteristics, when compared to Caucasians (Table 1). AAs were, on average, younger (mean age: AA 49.0±12.9 vs. 51.5±13.8 years; p<0.001), more likely to be female (40.5% vs. 37.9%; p<0.001) and had a higher BMI (28.4±5.6 vs. 27.6±5.4 kg/m2; p<0.001). AAs were also more likely to be socioeconomically disadvantaged, including a lower college graduation rate (18.4% vs. 29.0%; p<0.001) and more likely to be receiving public health insurance (72.7% vs. 50.2%; p<0.001). AAs were more likely to have hypertension (92.5% vs. 86.0%; p<0.001) and diabetes (33.3% vs. 29.1%; p<0.001), but less likely to have a history of PVD (3.1% vs. 4.7%; p<0.001) or angina (7.7% vs. 10.3%; p<0.001). Finally, AAs were more likely to be receiving dialysis at the time of transplant (81.3% vs. 56.8%; p<0.001), to be on dialysis for a longer period of time (median years: 4.0 [2.4–6.0] vs. 2.4 [1.3–4.0] years; p<0.001) and to be on the wait list nearly twice as long (median years: 2.1 [0.9–3.8] vs. 1.1 [0.4–2.3]; p<0.001).
Table 1.
Baseline sociodemographics for adult kidney transplant recipients transplanted between 2005 and 2009, stratified by race
| Variable | Caucasian (n=41,426) | African American (n=19,269) | p-Value |
|---|---|---|---|
| Mean Age (yrs±SD) | 51.5±13.8 | 49.0±12.9 | <0.001 |
|
| |||
| Female Gender | 37.9% | 40.5% | <0.001 |
|
| |||
| Mean Body Mass Index (kg/m2±SD) | 27.6±5.4 | 28.4±5.6 | <0.001 |
|
| |||
| Median Functional Status (IQR) | 90% (80–90%) | 80% (80–90%) | 0.002 |
|
| |||
| Education | |||
| Below High School | 2.6% | 3.3% | <0.001 |
| High School Education | 43.0% | 50.8% | |
| Some College | 25.3% | 27.5% | |
| College Graduate | 29.0% | 18.4% | |
| Primary Insurance | |||
| Private | 48.9% | 27.3% | <0.001 |
| Medicare | 46.9% | 65.7% | |
| Medicaid | 4.3% | 7.0% | |
|
| |||
| Primary Diagnosis for ESRD | |||
| Hypertension | 16.0% | 41.4% | <0.001 |
| Diabetes | 21.8% | 22.8% | |
| Other | 62.2% | 35.8% | |
|
| |||
| Comorbidities | |||
| Angina | 10.3% | 7.7% | <0.001 |
| Diabetes | 29.1% | 33.3% | <0.001 |
| Cerebrovascular Accident | 3.0% | 2.9% | 0.383 |
| Hypertension | 86.0% | 92.5% | <0.001 |
| Peripheral Vascular Disease | 4.7% | 3.1% | <0.001 |
|
| |||
| Receiving Dialysis at Time of Transplant | 56.8% | 81.3% | <0.001 |
|
| |||
| Median Time on Dialysis (IQR) | 2.4 (1.3–4.0) | 4.0 (2.4–6.0) | <0.001 |
|
| |||
| Median Time on Wait List (IQR) | 1.1 (0.4–2.3) | 2.1 (0.9–3.8) | <0.001 |
AA recipients received organs from younger donors (mean donor age: 38.9±15.4 vs. 40.9±14.5; p<0.001) that were less likely to be female (44.1% vs. 49.8%; p<0.001), more likely to be AA (34.5% vs. 5.2%; p<0.001) and less likely to be living donors (22.0% vs. 47.3%; p<0.001). AAs were also more likely to receive organs from expanded criteria donors (14.8% vs. 12.7%; p<0.001) and cardiac death donors (9.2% vs. 6.0%; p<0.001). AA recipients had greater numbers of HLA mismatches (median: 5 [3–5] vs. 4 [2–5]; p<0.001), a higher peak PRA (median: 2% [0–27] vs. 0% [0–13]; p<0.001) and longer cold ischemic times (15.4 [8.0–22.4] vs. 10.0 [1.3–19.2]; p<0.001).
In terms of immunosuppression, AA were more likely to receive cytolytic induction therapy (60.2% vs. 55.1%; p<0.001) and be discharged on maintenance regimens consisting of tacrolimus (85.1% vs. 82.0%; p<0.001), mycophenolate (89.4% vs. 88.4%; p<0.001), and corticosteroids (70.2% vs. 63.0%; p<0.001).
Outcomes
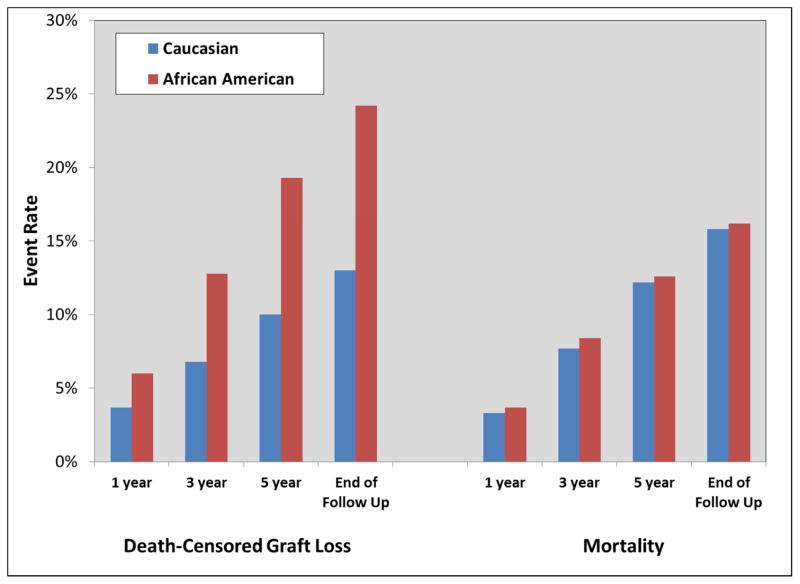
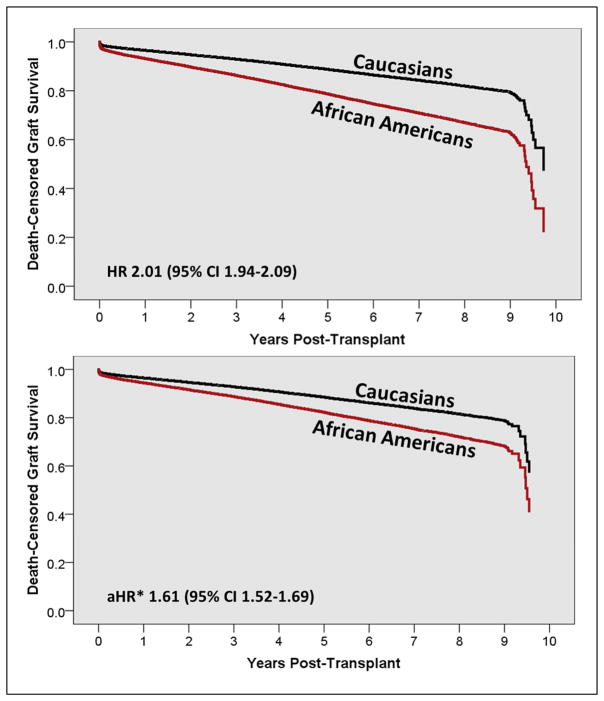
At one-year post-transplant, AAs had 62% higher risk of death-censored graft loss (RR 1.62, 95% CI 1.50–1.75); at three years, this increased to 88% higher risk (RR 1.88, 95% CI 1.79–1.98); and 93% higher risk of death-censored graft loss at five years (RR 1.93, 95% CI 1.85–2.01, Table 3 and Figure 1). Unadjusted and fully adjusted death-censored graft survival estimates are displayed in Figure 2. In the unadjusted model, AAs had twice the risk of graft loss, as compared to Caucasians (HR 2.01, top of Figure 2). After adjusting for baseline sociodemographics, donor information and transplant characteristics, the independent influence of AA race on graft loss was still significant, but reduced by 20%, to a HR of 1.61 (bottom of Figure 2). Mortality rates (see Table 2 and Figure 1), were similar between AAs and Caucasians.
Table 3.
Graft and patient outcomes for adult kidney transplant recipients transplanted between 2005 and 2009, stratified by race
| Outcome | Caucasian (n=41,426) | African American (n=19,269) | p-Value |
|---|---|---|---|
| Delayed Graft Function | |||
| Overall | 12.9% | 25.8% | <0.001 |
| Deceased Donor | 21.1% | 31.4% | |
| Living Donor | 3.7% | 5.8% | |
|
| |||
| Acute Rejection | |||
| 6 month | 6.7% | 8.7% | <0.001 |
| 1 year | 7.6% | 9.7% | |
| Overall | 8.4% | 11.1% | |
|
| |||
| Death Censored Graft Loss | |||
| 1 year | 3.7% | 6.0% | <0.001 |
| 3 year | 6.8% | 12.8% | |
| 5 year | 10.0% | 19.3% | |
|
| |||
| Death | |||
| 1 year | 3.3% | 3.7% | 0.010 |
| 3 year | 7.7% | 8.4% | 0.002 |
| 5 year | 12.2% | 12.6% | 0.261 |
|
| |||
| Overall Graft Loss | |||
| 1 year | 6.4% | 9.0% | <0.001 |
| 3 year | 12.8% | 18.9% | |
| 5 year | 19.5% | 28.0% | |
|
| |||
| Median SrCr at Last Follow Up (mg/dL±SD) | 1.6 (1.5–4.5) | 2.2 (1.5–4.5) | <0.001 |
Figure 1.
Clinical outcomes for adult kidney transplant recipients transplanted between 2005 and 2009, stratified by race, which include death-censored graft loss (left side of Figure) and mortality (right side of Figure), at 1, 3, 5 years and end of follow-up
Figure 2.
Death-censored graft survival curve estimates using Cox regression, stratified by race. The top figure is unadjusted and the bottom figure is adjusted for baseline recipient, donor and transplant characteristics
*adjusted for age, sex, functional status, insurance, education. BMI, comorbidities, transplant characteristics, donor characteristics and baseline immunosuppression.
Table 2.
Baseline donor characteristics, immunologic risk factors and immunosuppression for adult kidney transplant recipients transplanted between 2005 and 2009, stratified by race
| Variable | Caucasian (n=41,426) | African American (n=19,269) | p-Value |
|---|---|---|---|
| Mean Donor Age (yrs±SD) | 40.9±14.5 | 38.9±15.4 | <0.001 |
|
| |||
| Donor Female Gender | 49.8% | 44.1% | <0.001 |
| Donor Race | |||
| Caucasian | 87.0% | 54.3% | <0.001 |
| African-American | 5.2% | 34.5% | |
| Other | 7.7% | 11.2% | |
|
| |||
| Living Donor | 47.3% | 22.0% | <0.001 |
|
| |||
| Expanded Criteria Donor | 12.7% | 14.8% | <0.001 |
|
| |||
| Donor after Cardiac Death | 6.0% | 9.2% | <0.001 |
|
| |||
| Median HLA Mismatches (IQR) | 4 (2–5) | 5 (3–5) | <0.001 |
| A Mismatches (IQR) | 1 (1–2) | 2 (1–2) | <0.001 |
| B Mismatches (IQR) | 1 (1–2) | 2 (1–2) | <0.001 |
| DR Mismatches (IQR) | 1 (0–2) | 1 (1–2) | <0.001 |
|
| |||
| Median Peak PRA (IQR) | 0% (0–13%) | 2% (0–27%) | <0.001 |
|
| |||
| Median Current PRA (IQR) | 0% (0–7%) | 0% (0–12%) | <0.001 |
|
| |||
| Current PRA >20% | 17.7% | 21.0% | <0.001 |
|
| |||
| Current PRA >80% | 6.1% | 7.4% | <0.001 |
|
| |||
| Mean Cold Ischemic Time | 10.0 (1.3–19.2) | 15.4 (8.0–22.4) | <0.001 |
|
| |||
| Previous Kidney Transplant | 12.7% | 9.4% | <0.001 |
|
| |||
| Induction Therapy | |||
| IL-2 Receptor Antagonist | 27.2% | 20.6% | <0.001 |
| Cytolytic Therapy | 55.1% | 60.2% | |
|
| |||
| Immunosuppression at Discharge | |||
| Tacrolimus | 82.0% | 85.1% | <0.001 |
| Cyclosporine | 10.8% | 7.8% | <0.001 |
| Mycophenolate | 88.4% | 89.4% | <0.001 |
| Azathioprine | 0.8% | 0.4% | <0.001 |
| mTOR Inhibitor | 6.5% | 5.3% | <0.001 |
| Corticosteroids | 63.0% | 70.2% | <0.001 |
AAs also experienced significant disparities for other outcomes; most notably delayed graft function, acute rejection and renal function at last follow up. In deceased donor transplant recipients, AAs had 48% higher risk of developing DGF as compared to Caucasians (RR 1.48, 95% CI 1.45–1.53) and had higher risk of having acute rejection. The median serum creatinine at last follow up was 2.2 mg/dL in AAs, as compared to 1.6 mg/dL in Caucasians; this translates into an estimated GFR that is approximately 20 mL/min lower in AAs.
The impact of missingness was assessed using multiple imputation and comparing estimates from the complete case dataset to those from the imputed datasets. The level of missingness is displayed in supplemental Table 1. Most variables had very low frequencies of missing data, with the exception of history of angina, history of CVA, history of hypertension and cold ischemic time. However, the impact of missingness on estimates was negligible, as demonstrated in supplemental Table 2. In adjusted models, the estimates from the complete case dataset mirrored those from the imputed dataset for all outcomes, including DGF, acute rejection, graft loss and death.
DISCUSSION
The results of this study demonstrate that contemporary adult AA kidney transplant recipients continue to experience disproportionately higher rates of death-censored graft loss; even within adjusted modeling accounting for baseline variables and within the setting of potent immunosuppression regimens,18,19 changes to the organ allocation system7,20 and modern HLA antibody surveillance techniques.14,21,22 The higher rates of graft loss in AAs are likely to be multifactorial, as these patients have meaningful differences in a vast array of baseline factors known to significantly influence graft outcomes. These include sociodemographics, immunologic risks and donor characteristics. As compared to previous studies, and despite a significant focus on racial disparities research in transplantation, these baseline risk factors have significantly differed between AAs and Caucasians since national tracking began in the late 1980s. However, even within fully adjusted models, there appears to be significant risk for AAs, suggesting there are additional explanatory factors that are not adequately captured in these registry datasets.7
For sociodemographics, AAs kidney transplant recipients are, on average, younger, have a higher BMI, are less likely to be college graduates and are more likely to be on publically funded health insurance. AAs also are more likely to have hypertension or diabetes, spend longer on dialysis and once listed, await transplantation. Previous studies have demonstrated that these factors may be risks for graft loss, and thus, are likely important mediators of racial disparities.5–7,23 A number of these factors are largely immutable, but studies have attempted to mitigate their influence on graft outcomes. Yet, there is limited empirical evidence to suggest that these efforts have made any impact on this disparity at a national level. AAs also have substantial immunologic and donor derived risk factors for graft loss. For donor characteristics, AAs are more than half as likely to receive a transplant from a living donor, which is an important factor driving long-term graft survival.24–26 AAs are significantly more likely to receive an expanded criteria or deceased after cardiac death. AAs are younger at the time of transplant and have longer estimated post-transplant survival (EPTS) and thus would greatly benefit from higher quality donor organs. It is yet to be determined if the new organ allocation system, implemented in December of 2014, which removed expanded criteria donor and attempts to match EPTS with donor organ quality (KDPI) will improve upon this issue.27 Immunologically, it is well established that AAs are more likely to be sensitized to HLA antigens and to have more mismatches.28 This analysis demonstrates that these immunologic risk factors continue to exist and likely impact immunosuppression choice and acute rejection risk. Despite the increased use of potent immunosuppression in AAs, including cytolytic induction therapy, tacrolimus and mycophenolate, this analysis demonstrated that AAs had 32% higher risk of acute rejection. However, as compared to previous analyses, acute rejection rates have been substantially decreased in AAs.8
Likely due to reduced donor quality and immunologic risk, AAs had twice the overall risk of developing DGF after transplant, which is a major risk factor for the development of acute rejection, diminished renal function and graft loss.29,30 Ischemia reperfusion injury is the predominant cause of DGF, and numerous studies of novel pharmaceutical agents aimed at reducing the incidence and/or severity of DGF are currently ongoing.31–33 It will be interesting to see if these studies enroll enough AA recipients to determine if these agents can influence racial disparities in any appreciable manner.
It is clear that interventions aimed at improving outcomes in AAs need to be multidimensional, if they hope to significantly reduce disparities in kidney transplantation. Previous studies have predominantly focused on reducing disparities through manipulation of immunosuppression regimens and improving access to living donation. Although the former has demonstrated some success in reducing acute rejection rates, no interventions to date have significantly increased living donation rates in AA patients. Multimodal interventions that focus on prevailing explanatory factors for these disparities are needed. Endeavors that limit the impact of socioeconomic and health care access disadvantages while also delivering a care model that is culturally acceptable and patient-centric are needed if researchers hope to substantially improve equity in transplant access on outcomes.8,34–37
The strengths of this analysis are that it includes a detailed assessment of all contemporary adult patients undergoing solitary renal transplantation within the United States; thus, the study population is a full representation of the target population. The analysis provides easily interpretable and clinically relevant comparisons that detail the substantial number of disadvantages AA recipients encounter. However, there are a number of important limitations with the study that are worthy of discussion. First, the data utilized in this analysis was acquired through a national registry, relying on each U.S. transplant center to submit accurate data. Although death events were validated through the SSMDF, the vast majority of data was not validated. Previous studies demonstrate the UNOS registry to be highly accurate.15,17 Another limitation related to the use of this national registry is that a number of important baseline and follow up variables were not captured. These include risk factors for graft loss, such as medication nonadherence, changes in socioeconomics and/or health insurance and clinical events (cardiovascular disease and cancer).12,13,23,38 It is likely that these are strong mediating factors that influence AA disparities. Thus, this analysis does not provide a fully comprehensive assessment of the predominant causes of racial disparities in transplant. Finally, it should be noted that this analysis solely focused on AA disparities and only compared outcomes to Caucasians.
CONCLUSION
In summary, the results of this analysis of adult contemporary kidney transplant recipients demonstrate that AAs continue to experience considerable disparities in graft outcomes. These disparities occur within the context of the modern era of transplantation, including the use of potent immunosuppression, an updated organ allocation schema and modern HLA matching and antibody measurement techniques. AAs have a substantial number of sociodemographic and immunologic disadvantages and risk factors, which are likely to be strong contributors to disparities.
Supplementary Material
Supplemental Table 1 – Missing data assessment for all baseline and outcome variables
Supplemental Table 2 – Comparison of adjusted hazard ratios between the complete case and multiple imputation data sets
Legend – Study cohort flowchart displaying the method in which the study population was created, with specifics regarding the number and reasons for exclusions
Acknowledgments
Funding Source: This work was supported by the National Institutes of Health NIDDK under Award Number K23DK099440.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morris P, Knechtle SJ. Kidney transplantation-principles and practice. Elsevier Health Sciences; 2013. [Google Scholar]
- 2.Rana A, Gruessner A, Agopian VG, et al. Survival benefit of solid-organ transplant in the united states. JAMA Surgery. 2015;150(3):252–259. doi: 10.1001/jamasurg.2014.2038. [DOI] [PubMed] [Google Scholar]
- 3.Opelz G, Mickey MR, Terasaki PI. Influence of race on kidney transplant survival. Transplant Proc. 1977;9(1):137–142. [PubMed] [Google Scholar]
- 4.Matas A, Smith J, Skeans M, et al. OPTN/SRTR 2012 annual data report: Kidney. Am J Transplant. 2014;14(S1):11–44. doi: 10.1111/ajt.12579. [DOI] [PubMed] [Google Scholar]
- 5.Young CJ, Gaston RS. Renal transplantation in black Americans. N Engl J Med. 2000;343(21):1545–1552. doi: 10.1056/NEJM200011233432107. [DOI] [PubMed] [Google Scholar]
- 6.Young CJ, Gaston RS. African Americans and renal transplantation: Disproportionate need, limited access, and impaired outcomes. Am J Med Sci. 2002;323(2):94–99. doi: 10.1097/00000441-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Young CJ, Kew C. Health disparities in transplantation: Focus on the complexity and challenge of renal transplantation in African Americans. Med Clin N Am. 2005;89:1003–1031. doi: 10.1016/j.mcna.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Malat GE, Culkin C, Palya A, Ranganna K, Kumar MSA. African American kidney transplantation survival. Drugs. 2009;69(15):2045–2062. doi: 10.2165/11318570-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Taber DJ, Meadows HB, Pilch NA, Egede LE. The impact of diabetes on ethnic disparities in kidney transplantation. Ethnicity and Disease. 2013;23:238–44. [PubMed] [Google Scholar]
- 10.Taber DJ, Gebregziabher MG, Srinivas TR, Chavin KD, Baliga PK, Egede LE. African-American race modifies the influence of tacrolimus concentrations on acute rejection and toxicity in kidney transplant recipients. Pharmacotherapy. 2015 doi: 10.1002/phar.1591. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taber DJ, Pilch NA, Meadows HB, et al. The impact of cardiovascular disease and risk factor treatment on ethnic disparities in kidney transplant. J Cardiovasc Pharmacol Ther. 2013;18:243–50. doi: 10.1177/1074248412469298. [DOI] [PubMed] [Google Scholar]
- 12.Taber DJ, Hamedi M, Rodrigue JR, et al. Quantifying the race stratified impact of socioeconomics on graft outcomes in kidney transplant recipients. Transplantation. 2015 doi: 10.1097/TP.0000000000000931. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taber DJ, Douglass K, Srinivas T, et al. Significant racial differences in the key factors associated with early graft loss in kidney transplant recipients. Am J Nephrol. 2014;40(1):19–28. doi: 10.1159/000363393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirk AD, Knechtle SJ, Larsen CP, Madsen JC, Pearson TC, Webber SA. Textbook of Organ Transplantation. John Wiley & Sons; 2014. [Google Scholar]
- 15.Hanto DW. Quality control of the OPTN/UNOS transplant registry. Transplantation. 2004;77(8):1309–1310. doi: 10.1097/01.tp.0000120943.94789.e4. [DOI] [PubMed] [Google Scholar]
- 16.Leppke S, Leighton T, Zaun D, et al. Scientific registry of transplant recipients: collecting, analyzing, and reporting data on transplantation in the united states. Transplant Rev. 2013;27(2):50–56. doi: 10.1016/j.trre.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Andre M, Huang E, Everly M, Bunnapradist S. Clin Transpl. 2014. The UNOS renal transplant registry: review of the last decade; pp. 1–12. [PubMed] [Google Scholar]
- 18.Meier-Kriesche H, Li S, Gruessner R, et al. Immunosuppression: Evolution in practice and trends, 1994–2004. Am J Transplant. 2006;6:1111–31. doi: 10.1111/j.1600-6143.2006.01270.x. [DOI] [PubMed] [Google Scholar]
- 19.Matas A, Smith J, Skeans M, et al. OPTN/SRTR 2013 annual data report: Kidney. Am J Transplant. 2015;15(S2):1–34. doi: 10.1111/ajt.13195. [DOI] [PubMed] [Google Scholar]
- 20.Gaston RS, Ayres I, Dooley LG, Diethelm AG. Racial equity in renal transplantation: The disparate impact of HLA-based allocation. J Am Med Assoc. 1993;270(11):1352–6. [PubMed] [Google Scholar]
- 21.Alheim M, Paul PK, Hauzenberger D, Wikström A. Improved flow cytometry based cytotoxicity and binding assay for clinical antibody HLA crossmatching. Hum Immunol. 2015 doi: 10.1016/j.humimm.2015.09.047. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Orandi B, Garonzik-Wang J, Massie A, et al. Quantifying the risk of incompatible kidney transplantation: A multicenter study. Am J Transplant. 2014;14(7):1573–80. doi: 10.1111/ajt.12786. [DOI] [PubMed] [Google Scholar]
- 23.Butkus DE, Dottes AL, Meydrech EF, Barber WH. Effect of poverty and other socioeconomic variables on renal allograft survival. Transplantation. 2001;72(2):261–6. doi: 10.1097/00007890-200107270-00017. [DOI] [PubMed] [Google Scholar]
- 24.Ojo AO, Hanson JA, Wolfe RA, Leichtman AB, Agodoa LY, Port FK. Long-term survival in renal transplant recipients with graft function. Kidney Int. 2000;57(1):307–13. doi: 10.1046/j.1523-1755.2000.00816.x. [DOI] [PubMed] [Google Scholar]
- 25.Pessione F, Cohen S, Durand D, et al. Multivariate analysis of donor risk factors for graft survival in kidney transplantation. Transplantation. 2003;75(3):361–7. doi: 10.1097/01.TP.0000044171.97375.61. [DOI] [PubMed] [Google Scholar]
- 26.Terasaki PI, Cecka JM, Gjertson DW, Takemoto S. High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med. 1995;333(6):333–6. doi: 10.1056/NEJM199508103330601. [DOI] [PubMed] [Google Scholar]
- 27.Israni AK, Salkowski N, Gustafson S, et al. New national allocation policy for deceased donor kidneys in the united states and possible effect on patient outcomes. J Am Soc Nephrol. 2014;25(8):1842–8. doi: 10.1681/ASN.2013070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padiyar A, Hricik DE. Immune factors influencing ethnic disparities in kidney transplantation outcomes. Expert Rev Clin Immunol. 2011;7:769–78. doi: 10.1586/eci.11.32. [DOI] [PubMed] [Google Scholar]
- 29.Singh SK, Cole EH, Kim SJ. Kidney Transplantation. Springer; 2014. Delayed graft function and kidney transplantation; pp. 143–51. [Google Scholar]
- 30.Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. Am J Transplant. 2011;11(11):2279–96. doi: 10.1111/j.1600-6143.2011.03754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glebova K, Reznik ON, Reznik AO, et al. siRNA technology in kidney transplantation: current status and future potential. BioDrugs. 2014;28(4):345–61. doi: 10.1007/s40259-014-0087-0. [DOI] [PubMed] [Google Scholar]
- 32.O'Neill S, Gallagher K, Hughes J, Wigmore SJ, Ross JA, Harrison EM. Challenges in early clinical drug development for ischemia-reperfusion injury in kidney transplantation. Expert Opinion Drug Discovery. 2015:1–10. doi: 10.1517/17460441.2015.1044967. [DOI] [PubMed] [Google Scholar]
- 33.Ponticelli C. Ischaemia-reperfusion injury: A major protagonist in kidney transplantation. Nephrol Dial Transplant. 2014;29(6):1134–40. doi: 10.1093/ndt/gft488. [DOI] [PubMed] [Google Scholar]
- 34.Garg PP, Diener-West M, Powe NR. Reducing racial disparities in transplant activation: Whom should we target? Am J Kid Dis. 2001;37(5):921–31. doi: 10.1016/s0272-6386(05)80007-1. [DOI] [PubMed] [Google Scholar]
- 35.Gordon EJ, Ladner DP, Caicedo JC, Franklin J. Disparities in kidney transplant outcomes: A review. Sem Nephrol. 2010;30(1):81–9. doi: 10.1016/j.semnephrol.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gordon EJ, Prohaska T, Siminoff LA, Minich PJ, Sehgal AR. Can focusing on self-care reduce disparities in kidney transplantation outcomes? Am J Kidney Dis. 2005;45(5):935–40. doi: 10.1053/j.ajkd.2005.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Padiyar A, Augustine JJ, Bodziak KA, Aeder M, Schulak JA, Hricik DF. Influence of African-American ethnicity on acute rejection after early steroid withdrawal in primary kidney transplant recipients. Transplant Proc. 2010;42(5):1643–47. doi: 10.1016/j.transproceed.2010.02.081. [DOI] [PubMed] [Google Scholar]
- 38.Butler JA, Roderick P, Mullee M, Mason JC, Peveler RC. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: A systematic review. Transplantation. 2004;77(5):769–76. doi: 10.1097/01.tp.0000110408.83054.88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1 – Missing data assessment for all baseline and outcome variables
Supplemental Table 2 – Comparison of adjusted hazard ratios between the complete case and multiple imputation data sets
Legend – Study cohort flowchart displaying the method in which the study population was created, with specifics regarding the number and reasons for exclusions