Abstract
We evaluated late effects of AdhAQP1 administration in five subjects in a clinical trial for radiation-induced salivary hypofunction (http://www.clinicaltrials.gov/ct/show/NCT00372320?order=). All were identified as initially responding to human aquaporin-1 (hAQP1) gene transfer (Baum et al, 2012). They were followed for 3-4 years after AdhAQP1 delivery to one parotid gland. At intervals we examined salivary flow, xerostomic symptoms, saliva composition, vector presence and efficacy in the targeted gland, clinical laboratory data, and adverse events. All displayed marked increases (71-500% above baseline) in parotid flow 3-4.7 years after treatment, with improved symptoms for ~ 2-3 years. There were some changes in [Na+] and [Cl−] consistent with elevated salivary flow, but no uniform changes in secretion of key parotid proteins. There were no clinically significant adverse events, nor consistent negative changes in laboratory parameters. One subject underwent a core needle biopsy of the targeted parotid gland 3.1 years post treatment and displayed evidence of hAQP1 protein in acinar, but not duct, cell membranes. All subjects responding to hAQP1 gene transfer initially had benefits for much longer times. First generation adenoviral vectors typically yield transit effects, but these data show beneficial effects can continue years after parotid gland delivery.
Keywords: Adenoviral vector, aquaporin-1, clinical, parotid, radiation damage, xerostomia
INTRODUCTION
Head and neck cancers are among the most common malignancies worldwide, with the majority of patients being treated at least in part with radiation. It has long been recognized that during radiation therapy damage can occur to healthy salivary glands 1,2. This is surprising given the generally low rate of turnover of mammalian salivary epithelial cells 3,4. While methods of radiation have greatly improved 2,5, and can significantly limit the damage to normal tissue adjacent to the tumor, radiation-induced salivary hypofunction is still a significant clinical problem because of (i) the large number of patients with already existing radiation-induced gland damage and (ii) the fact that the most technologically advanced instruments to focus radiation and minimize gland damage are primarily found in academic medical centers in relatively wealthy countries. While treatment with sialogogues (e.g., Salagen, Evoxac) can be beneficial for some patients with radiation-damaged salivary glands (Radiation Therapy Oncology Group, RTOG, grade 1 6), there currently is no suitable conventional therapy for most patients (grades 2-4).
More than 20 years ago we began an effort that ultimately led to a Phase I /II clinical gene therapy trial for patients in RTOG grades 2 and 3, i.e., with some glandular epithelial tissue remaining 7-10. The gene delivered encoded the water channel protein human aquaporin-1 (hAQP1 11), and was administered to subjects using a first generation, serotype 5, adenoviral (Ad5), vector termed AdhAQP1 12. Eleven subjects were treated with AdhAQP1 in this clinical trial. All eleven enrolled subjects showed no evidence of disease presence for at least five years (range ~5.5-11.5) following completion of their radiation therapy 12. The early results from that trial, through day 42 post-vector delivery, have been reported 12 and five of eleven treated subjects were identified as responding positively to the gene transfer maneuver. The positive response in these five individuals was defined as an increased salivary flow from the targeted parotid gland, as well as in the improvement of two key symptomatic benefits (amount of saliva, and the level of dryness, in their mouth), during the initial 42-day study period 12. Importantly, the peak increase of parotid salivary flow observed occurred much later (from 7-42 days post-vector administration) than was seen in pre-clinical animal models (rat, miniature pig; ~3 days) 12. For the originally approved clinical protocol, patients were required to be seen through day 360 after vector administration. However, the protocol was amended based on our observations with the first responder-subject (#19, see below), who exhibited a positive response to gene transfer on day 7 12. Although his initial peak increase in salivary flow declined thereafter, we measured the occurrence of a second, later elevation in parotid salivary flow rate on days 180 and 360, both of which were well above his baseline value (see below). Accordingly, the approved protocol was modified to permit all responders to AdhAQP1 administration to be evaluated for two additional time points, at least 1- and 2-years following their completion of the original 360-day protocol. All five responder-subjects consented to this extended evaluation. It is the purpose of the present study to describe results from all five responder-subject evaluations following the initially reported day 42-time period 12.
RESULTS
Supplemental Table 1 provides several general clinical characteristics of the five responder-subjects studied herein; most of these were reported earlier 12.
Evaluation of adverse events and clinical laboratory parameters
All adverse events (AEs) occurring after day 42 that were reported by these five subjects were analyzed. Our previous report 12 described AEs occurring through day 42. The subjects reported a total of 21 AEs during the post-treatment period from day 42 through the final time point (~3-4.7 years). Of these 21 AEs, 18 were regarded as unrelated to either the treatment with AdhAQP1 or to the study procedures used. Of the remaining three AEs, one (soreness in a parotid duct) was considered unrelated to treatment, but definitely related to a study procedure. The second AE (an oral candidal infection) was considered unrelated to treatment and unlikely related to study procedures. The third (an upper respiratory tract infection) was considered unlikely related to treatment and unrelated to study procedures. Of the 21 AEs, 18 were considered mild (grade 1), but three, all unrelated to treatment or study procedures, were serious and required hospitalization (hip fracture from a motorcycle accident; elective surgery for degenerative hip disease, and an episode of severe abdominal pain).
All clinical laboratory parameters measured (clinical chemistries and hematologies), for all five subjects from day 90 through the completion of all follow-up visits, were evaluated. There were no consistent, significant changes related to the study (AdhAQP1 administration or related procedures) for any parameter measured.
Parotid salivary secretion
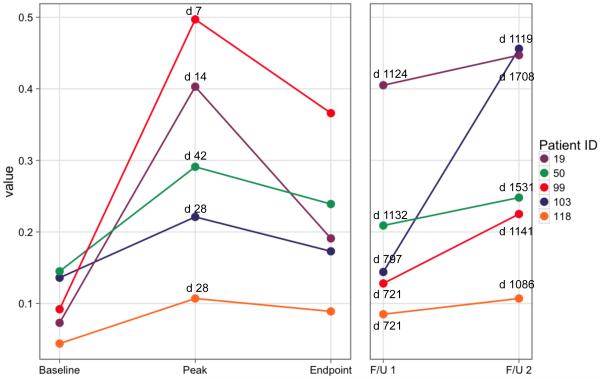
None of the non-responder subjects showed any increase in parotid flow rate after the original day 42 data reported. Figure 1 shows the individual salivary flow rates, at several key time points, obtained from each of the five subjects considered to be positive responders to hAQP1 gene transfer 12 , over their entire course of observation. All subjects were seen for minimally three years after gene transfer, while the first two responders sequentially were seen over a longer interval (# 19 for ~4.7 years; # 50 for ~4.2 years). Each person’s parotid salivary flow results were then compared to their baseline parotid flow rate at the first pre-vector delivery visit (Baseline). It is clear visually in Figure 1 and in tabular form for these key time points (Table 1) that following their initial peak in salivary flow after gene transfer (on days 7-42), there was a decline in salivary flow rate, but each subject’s parotid flow rate was still well above baseline at the end of the original 360-day study. Furthermore, all subjects still exhibited significantly elevated salivary flow rates from the targeted parotid gland at their final study-visit, i.e., from 1086 to 1708 days after the gene transfer procedure (Figure 1, Table 1). An initial examination of salivary flow rates at these key time points from the untreated, contralateral parotid glands indicated there was little change (Table 1). This examination was prompted by a recently reported observation of increased salivary flow in contralateral parotid glands of irradiated miniature pigs following non-viral AQP1 gene transfer 13. Furthermore, the initial impression from these key time points was confirmed when the flow rates from both the treated and untreated glands were assessed with considerable statistical detail; see below, Tables 2 and 3).
Figure 1.
Stimulated parotid salivary flow rates of the targeted gland at key time points from each subject responding positively to AdhAQP1 treatment. The color-coding for subjects in this figure are the same as in Figure 3, and as those published in Baum et al 12. The Y-axis shows salivary flow in mL/min from the targeted parotid gland. The X-axis shows five key time points in this study, with the specific days for each indicated above data points in the figure. Baseline represents each subject’s initial visit to the NIH Clinical Center prior to any procedures being performed. The initial peak increase in salivary flow represents the time point (varied from day 7-42) following AdhAQP1 administration when the subject’s parotid salivary flow was maximal. Both the Baseline and initial peak data were presented previously in Baum et al 12. The endpoint of the originally approved study was on day 360 visit after vector delivery for each subject. Thereafter, the original clinical protocol was amended and we received permission to evaluate the effects of AdhAQP1 administration to all five responder-subjects for an additional two years of follow-up. The exact days following vector administration for the two follow-up visits are different for each subject (shown also in Table 1). They are shown in the figure and are as follows: #19 (days 1124 and 1708, respectively), #50 (1132 and 1531), #99 (721 and 1141), #103 (797 and 1119), and #118 (721 and 1086).
Table 1.
Parotid salivary flow rates at key time points*
| Subject | Baseline | Peak l flow rate (day) |
Day 360/ Endpoin t flow rate |
Follow- up 1 flow rate (day) |
Follow- up 2 flow rate (day) |
|---|---|---|---|---|---|
| 19 Treated | 0.073 |
0.403
(7) |
0.191 |
0.405
(1124) |
0.447
(1708) |
| Untreated | 0.13 | NR | NR | 0.068 | 0.268 |
| 50 Treated | 0.145 |
0.291
(42) |
0.239 |
0.209
(1132) |
0.248
(1531) |
| Untreated | 0.0 | 0.0 | 0.0 | 0.0 | 0.13 |
| 99 Treated | 0.092 |
0.497
(14) |
0.366 |
0.128
(721) |
0.225
(1141) |
| Untreated | tubing | tubing | tubing | tubing | 0.03 |
| 103 Treated | 0.136 |
0.221
(28) |
0.172 |
0.144
(797) |
0.456
(1119) |
| Untreated | 0.458 | 0.256 | 0.107 | 0.103 | 0.3 |
| 118 Treated | 0.044 | 0.107 (28) | 0.089 | 0.085 (721) | 0.107 (1086) |
| Untreated | 0.173 | NR | 0.0 | 0.145 | 0.238 |
Parotid saliva flow rates are given in ml/min/gland and were obtained as described previously 12. All five subjects positively responding to AdhAQP1 treatment were seen for two additional follow-up visits after the original final visit at day 360. The number of days after AdhAQP1 administration for each of the initial and follow-up visits is shown in parentheses. Baseline represents salivary flow rate in the targeted gland prior to vector delivery. Data are shown for both the treated and the untreated, i.e., contralateral, glands. NR = no data recorded for that time point. Tubing means saliva was in the tubing of the collection device, but was unable to be quantified.
Table 2.
GEE Modeling of Stimulated Parotid Salivary Flow Rate with Time – Treated Gland
| Time | SPFR | 95% CI | Robust SE | p-value |
|---|---|---|---|---|
|
| ||||
| Model 1a | ||||
| Visit 2 | 0.142 | 0.102, 0.182 | 0.020 | <0.001* |
| Visit 3 | −0.008 | −0.043, 0.026 | 0.018 | 0.632 |
| Visit 4 | 0.087 | 0.000, 0.175 | 0.045 | 0.051 |
| Visit 5 | 0.145 | −0.008, 0.298 | 0.078 | 0.063 |
| Visit 6 | 0.177 | 0.062, 0.291 | 0.058 | 0.002* |
| Visit 7 | 0.152 | −0.007, 0.311 | 0.081 | 0.061 |
| Visit 8 | 0.131 | 0.093, 0.169 | 0.019 | <0.001* |
| Visit 9 | 0.114 | 0.039, 0.189 | 0.038 | 0.003* |
| Visit 10 | 0.099 | 0.048, 0.151 | 0.026 | <0.001* |
| Visit 11 | 0.176 | 0.113, 0.239 | 0.032 | <0.001* |
| Visit 12 | 0.146 | 0.038, 0.254 | 0.055 | 0.008* |
| Visit 13 | 0.207 | 0.031, 0.384 | 0.090 | 0.021* |
| Visit 14 | 0.184 | 0.098, 0.270 | 0.044 | <0.001* |
| Visit 15 (follow-up 1) | 0.166 | 0.055, 0.277 | 0.057 | 0.003* |
| Visit 16 (follow-up 2) | 0.269 | 0.140, 0.397 | 0.066 | <0.001* |
|
| ||||
| Model 1b | ||||
|
| ||||
| Time | 0.009 | 0.005, 0.013 | 0.002 | <0.001* |
|
| ||||
| Model 2a | ||||
|
| ||||
| Peak 1 | 0.262 | 0.130, 0.395 | 0.068 | <0.001* |
| Follow-up 1 | 0.153 | 0.041, 0.265 | 0.057 | 0.007* |
| Follow-up 2 | 0.255 | 0.128, 0.382 | 0.065 | <0.001* |
|
| ||||
| Model 2b | ||||
|
| ||||
| Time | 0.049 | 0.006, 0.091 | 0.022 | 0.025* |
SPFR: Stimulated parotid salivary flow rate. Model 1a - all 16 visits in the model with visits treated as a categorical time variable. Visit 1 is the reference (baseline). Model 1b - all 16 visits in the model with visits treated as continuous time variable. Model 2a - 4 visits (stimulated parotid salivary flow rate at peak 1, follow-up 1 and follow-up 2 visits were compared to baseline flow rates) in the model with visits treated as categorical time variable. Visit 1 is the reference (baseline). Model 2b - 4 visits in the model with visits treated as continuous time variable. Estimates are rounded to three decimal places.
Statistically significant.
Table 3.
GEE Modeling of Stimulated Parotid Salivary Flow Rate with Time-Contralateral Gland
| Time | SPFR | 95% CI | Robust SE | p-value |
|---|---|---|---|---|
|
| ||||
| Model 1a | ||||
| Visit 2 | −0.015 | −0.087, 0.057 | −0.015 | 0.677 |
| Visit 3 | −0.135 | −0.235, −0.034 | −0.135 | 0.009* |
| Visit 4 | −0.081 | −0.223, 0.061 | −0.081 | 0.262 |
| Visit 5 | −0.067 | −0.181, 0.047 | −0.067 | 0.251 |
| Visit 6 | −0.124 | −0.209, −0.038 | −0.124 | 0.005* |
| Visit 7 | 0.023 | −0.072, 0.118 | 0.023 | 0.636 |
| Visit 8 | −0.061 | −0.128, 0.007 | −0.061 | 0.079 |
| Visit 9 | −0.099 | −0.252, 0.054 | −0.099 | 0.204 |
| Visit 10 | −0.134 | −0.264, −0.004 | −0.134 | 0.043* |
| Visit 11 | 0.028 | −0.057, 0.113 | 0.028 | 0.520 |
| Visit 12 | −0.121 | −0.272, 0.031 | −0.121 | 0.118 |
| Visit 13 | 0.049 | −0.035, 0.133 | 0.049 | 0.255 |
| Visit 14 | −0.145 | −0.291, 0.001 | −0.145 | 0.051 |
| Visit 15 (follow-up 1) | −0.099 | −0.244, 0.046 | −0.099 | 0.179 |
| Visit 16 (follow-up 2) | 0.034 | −0.086, 0.155 | 0.034 | 0.576 |
|
| ||||
| Model 1b | ||||
|
| ||||
| Time | 0.001 | −0.003, 0.005 | 0.002 | 0.666 |
|
| ||||
| Model 2a | ||||
|
| ||||
| Peak 1 | −0.058 | −0.142, 0.026 | 0.043 | 0.177 |
| Follow-up 1 | −0.110 | −0.264, 0.044 | 0.079 | 0.163 |
| Follow-up 2 | 0.046 | −0.083, 0.175 | 0.066 | 0.489 |
|
| ||||
| Model 2b | ||||
|
| ||||
| Time | 0.002 | −0.047, 0.051 | 0.025 | 0.940 |
SPFR: Stimulated parotid gland salivary flow rate. Estimates rounded to three decimal places.
statistically significant. Model 1a: In the contralateral gland, the stimulated parotid flow was lower at most visits compared to the baseline rate, with a statistically significant decline in flow rate at visits 3, 6, and 10. The slight increase in flow rate at visits 7, 11 and 16 was not of statistical significance; visit 1 is reference (baseline). Model 1b: There was no significant change in stimulated parotid salivary flow rate with each sequential visit in the contralateral gland. Model 2a: In the contralateral gland, stimulated parotid salivary flow rate at peak 1, follow-up 1 and follow-up 2 visits were compared to baseline flow rates and the change in flow rates was not of statistical significance. Model 2b: Comparing only the peak, follow-up 1 and follow-up 2 flow rates to the baseline flow rate, there was no significant change in stimulated parotid salivary flow rate with each sequential visit in the contralateral gland.
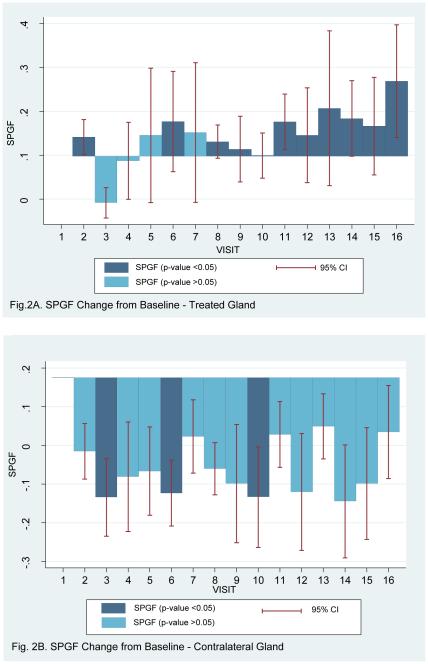
Model 1a, shown in Table 2 (16 visits including baseline visit), reports the GEE modeling with uniform correlation structure with the Huber White estimator of variance 14. A graphic representation of some of these data, the dynamics of salivary flow from the targeted parotid gland, is shown in Figure 2A. Consistent with this visual impression, the GEE model indicated that the stimulated parotid salivary flow from the targeted parotid gland continued to be statistically significantly higher at follow-up visit 1 with a 0.17 ml/min (95%CI: 0.06-0.28, SE: 0.06, p-value=0.003) increase from baseline flow rate, and at follow-up visit 2 with a 0.27 ml/min (95%CI: 0.14-0.40, SE: 0.07, p-value <0.001) increase from baseline flow rate. Furthermore, the stimulated parotid saliva flow rate from the treated gland increased by 0.01 ml/min (95% CI: 0.005-0.013, SE: 0.002: p-value <0.001) with every sequential visit (Model 1b). Models 2a and 2b (4 visits including baseline visit), shown in Table 2, also report stimulated parotid salivary flow rate at three key time points, namely the study-visit with the initial peak salivary flow rate (peak 1), follow-up visit 1 and follow-up visit 2. The GEE model (Model 2a) with uniform correlation structure with the Huber White estimator of variance indicated that the stimulated parotid salivary flow rate from the treated gland continued to be statistically significantly higher on these three visits, with a 0.26 ml/min (95%CI:0.13-0.40 SE:0.07, p-value <0.001) increase in flow rate at peak 1, a 0.15 ml/min (95%CI:0.04-0.26 SE:0.06, p-value =0.007) increase in flow rate at follow-up visit 1, and 0.26 ml/min (95%CI:0.13-0.38 SE:0.06, p-value <0.001) increase in flow rate at follow-up visit 2, compared to baseline flow rate. Furthermore, the stimulated parotid salivary flow from the treated gland increased by 0.05 ml/min (95% CI: 0.006-0.091, SE: 0.02, p-value = 0.025) with every sequential visit, when only the peak 1, follow-up visit 1 and follow-up visit 2 were compared to the baseline visit (Model 2b).
Figure 2.
Depiction of the changes from baseline for stimulated parotid salivary flow rates at each time point in this clinical trial from the AdhAQP1-treated gland (A) and the contralateral untreated gland (B). Statistical significance is indicated using the dark (p<0.05) and light blue (p>0.05) shading of each bar, and the 95% confidence intervals are depicted. Bars above the line represent an increase in salivary flow rate, while those below indicate a decrease in salivary flow rate. Statistical analysis used GEE modeling as presented in Tables 2 and 3. Visit numbers represent the following time points: 1 (baseline), 2 (6 hours), 3 (day 1), 4 (day 2), 5 (day 3), 6 (day 7), 8 (day 28), 9 (day 42), 10 (day 90), 11 (day 120), 12 (day 150), 13 (day 180, 14 (day 360), 15 (follow-up visit 1), 16 (follow-up visit 2). The exact times for the two follow-up visits are presented in Figure 1 and its legend.
As noted above, we also examined stimulated parotid flow rates in the contralateral parotid glands in more detail using GEE modeling. However, again, we could not detect any consistent effects of AdhAQP1 treatment on stimulated saliva in the contralateral parotid glands (Table 3). A graphic representation of the dynamics of salivary flow from the contralateral parotid gland is shown in Figure 2B.
Evaluating xerostomic symptoms
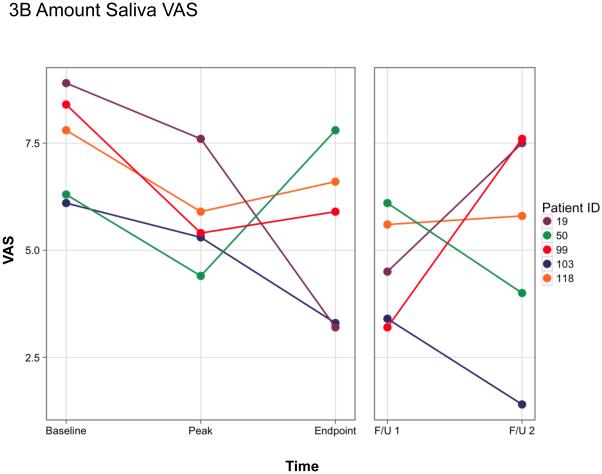
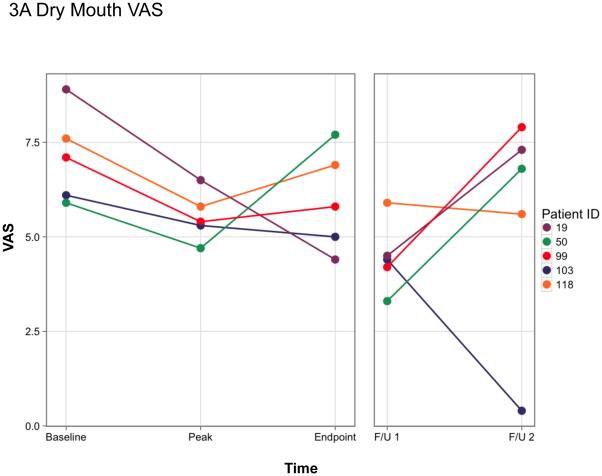
For the present study we evaluated the same two, key xerostomic symptoms previously reported using a validated visual analog scale (VAS, see supplemental Figure 1 15). The subjects were asked to rate (i) the dryness in their mouth (Dry Mouth; Figure 3A) and (ii) how much saliva was in their mouth (Amount Saliva; Figure 3B) at the beginning of each study visit prior to any saliva collections. The time points shown in Figure 3 are the same key time points shown in Figure 1 and Table 1. As reported previously 12, when compared to the baseline visit each of these five subjects showed improvement (i.e., a lower VAS score) in these two subjective assessments at the time of their initial elevation in parotid salivary flow (during days 7-42). Thereafter, the results were more variable, but all five individuals reported some improvement in these two symptoms at either (or both) the 2- or 3-year time point. However, by the second follow-up visit, three subjects (19, 50, 99) felt that their mouths were almost as dry as at the start of the trial, and both subjects 19 and 99 believed the saliva in their mouth was at or near baseline levels (Figures 3A, B).
Figure 3.
Visual analog scale (VAS) measurements of two key xerostomic symptoms at key time points in responder-subjects. A. Rate the dryness in your mouth; B. Rate how much saliva is in your mouth. A lower score indicates an improvement in the symptom. The color-coding for subjects in this figure are the same as in Figure 1, and as those published in Baum et al 12. Baseline and initial peak data were presented previously in Baum et al 12. As noted in the legend to Figure 1, the endpoint of the originally approved study was on day 360 visit after vector delivery for each subject. The two follow-up visits are different for each subject (presented in Figure 1). They are as follows: #19 (days 1124 and 1708, respectively), #50 (1132 and 1531), #99 (721 and 1141), #103 (797 and 1119), and #118 (721 and 1086). The Y-axis represents the visual analogue scale numerical value [10 cm scale, with 10 being the driest mouth (A) or the lowest amount of saliva (B) imaginable]. The form used for the visual analogue scale measurements can be found in Supplemental Figure 1 and was derived from Pai et al 15.
Serum neutralizing antibodies
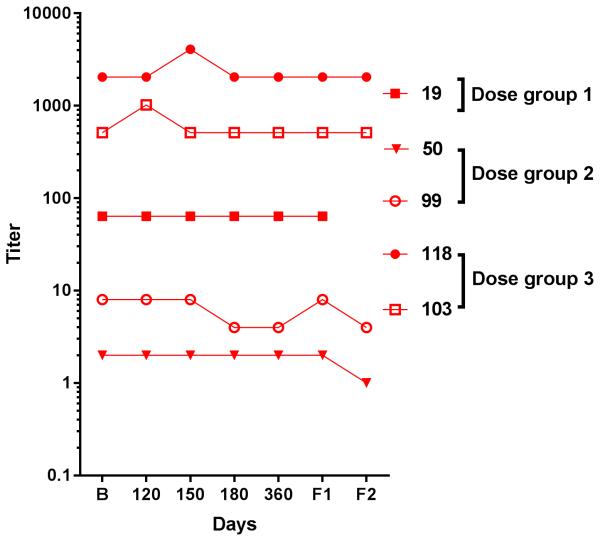
Previously, we have reported serum neutralizing antibody (NAb) levels at baseline 12 and over the initial 42 days post-AdhAQP1 treatment 16. As shown in Figure 4, compared to baseline serum NAb levels measured in the five responder-subjects, little change occurred throughout the study, extending to 3+ years post-treatment, with serum NAb titers at or below those measured at baseline.
Figure 4.
Anti-Ad5 serum neutralizing antibody titers in responder-subjects. Anti-Ad5 neutralizing serum antibodies employed a previously described assay 36,37. The assay tests the ability of serum dilutions to block the transduction of 293 cells by an Ad5 vector, AdCMVLuc encoding luciferase. The titers (Y-axis) represent serum dilutions resulting in a 50% inhibition of transduction. The X-axis represents time points in the study. B = baseline, while all other time points shown are days following AdhAQP1 administration. Individual subject symbols are indicated. No sample was available to perform this assay at the second follow-up visit of subject 19. The exact days of each follow-up visit are listed in the legends of Figures 1 and 3, and Table 1.Note that serum neutralizing antibody titers found in all time points through day 42 have recently been reported and were generally similar to those shown in the figure 16.
Analysis of saliva composition
Table 4 shows the concentrations of Na+, K+ and Cl− in parotid saliva at baseline and at or near the initially observed increase in salivary flow. For both subjects with saliva samples available for analysis at the first salivary peak (#s 19 and 50), there was a dramatic increase in the concentrations of all three electrolytes. Subject # 99 showed a similar increase, albeit not as marked, with a saliva sample obtained at day 7. His initial peak of salivary flow was at day 14, but the day 7 flow rate was still >2-fold that at baseline. Subjects 103 and 118 had their initial peak salivary flow rates on day 28, however, samples were only available for analysis on day 7. Flow rates for both subjects were ~30% above their baseline levels, and electrolyte levels were only modestly elevated, if at all (Table 4).
Table 4.
Parotid saliva electrolyte composition *
| Subject (electrolyte) | Baseline | Day 7 | day 42 | |
|---|---|---|---|---|
| 19 | Na+ | 18.8 | 110.6 | 55 |
| K+ | 15.8 | 27.3 | 16.3 | |
| Cl− | 22.6 | 109.9 | 57 | |
| 50 | Na+ | 12.8 | 35.2 | 83 |
| K+ | 12.9 | 10.6 | 16.7 | |
| Cl− | 17.1 | 36.2 | 81.3 | |
| 99 a | Na+ | 4.1 | 37.9 | 0.0 |
| K+ | 18.5 | 22 | 25.6 | |
| Cl− | ND | 40.1 | 22.2 | |
| 103b | Na+ | 0.0 | 7.7 | 0.0 |
| K+ | 22 | 24.5 | 20.6 | |
| Cl− | 10.2 | 19 | 12.1 | |
| 118 c | Na+ | 0.9 | 4.3 | 2.7 |
| K+ | 21.9 | 25.8 | 29.9 | |
| Cl− | 14.6 | ND | 19.5 | |
The gray shaded areas represent electrolyte concentrations (mM) in samples from the subject’s initial peak flow, i.e., day 7 for # 19 and day 42 for # 50. ND = not determined.
The initial peak flow rate for this subject was on day 14. The day 7 flow was 0.24 ml/min/gland, and was substantially above baseline (0.092).
The initial peak flow rate for this subject was on day 28. The day 7 flow was 0.184 ml/min/gland, 36% above baseline (0.135).
The initial peak flow for this subject was on day 28. The day 7 flow was 0.058 ml/min/gland, 31% above baseline (0.044).
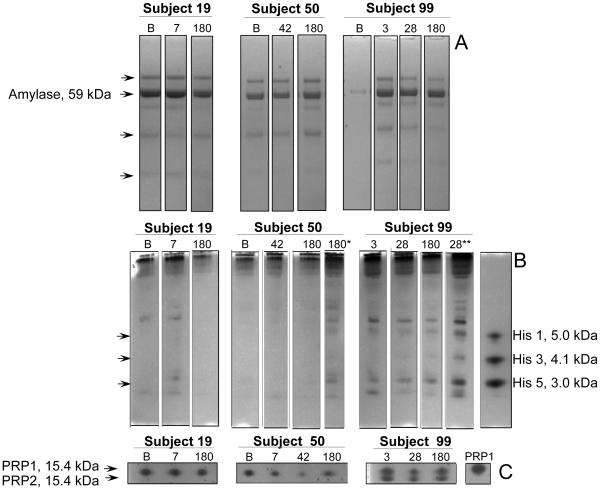
There were sufficient quantities of parotid saliva available to evaluate key parotid acinar cell secretory proteins in subjects 19, 50 and 99 (Figure 5). When comparing levels of amylase, histatins 1, 3 and 5, and acidic proline-rich proteins (PRPs) 1 and 2 at baseline (B) with the initial peak salivary flow increase for # 19 (day 7) and # 50 (day 42) no consistent pattern of change was observed. For subject # 99 there was only a minimal amount of baseline saliva available, and none of the peak saliva, so useful comparisons were made of day 28 with day 3 saliva samples, which were of a similar level to the peak (day 14) and baseline flow rates, respectively. Again, the general impression is one of no consistent change in the output of the examined key parotid secretory proteins (Figure 5).
Figure 5.
Assessment of protein composition in patient parotid saliva samples by BisTris PAGE (A), cationic PAGE (B) and anionic PAGE (C). Left panels: samples from subject 19; middle panels: samples from subject 50; right panel, samples from subject 99. Volumes loaded in A, B and C were 10 ul, 25 ul and 50 ul, respectively. In B, samples indicated with * and ** indicate 50 ul and 75 ul volumes were loaded, respectively. Numbers above the lanes refer to the day post treatment, with B in that position indicating baseline. The migration positions and molecular weight (MW) of the major proteins in each of the three PAGE types are indicated: Amylase in A; histatins (His) 1, 3 and 5 in B; and proline-rich proteins 1 and 2 (PRP1, PRP2) in C. The MW standards used in A, B and C were 10 ul BioRad MW standard, 8 ug histatin 1, 3, and 5 each, and 20 ug PRP1, respectively. Note low histatin levels in the subject samples, but increasing the saliva amounts analyzed revealed their presence. Note furthermore that all subjects expressed acidic PRPs and that different isoforms were expressed by the three subjects.
Analysis of parotid gland biopsy samples
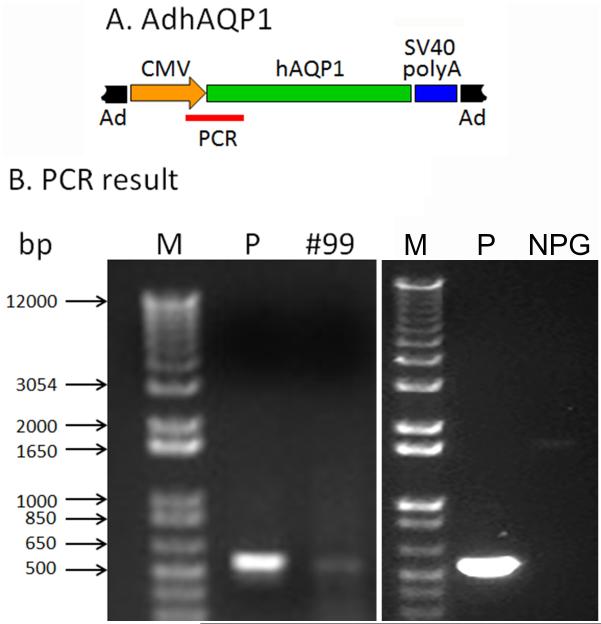
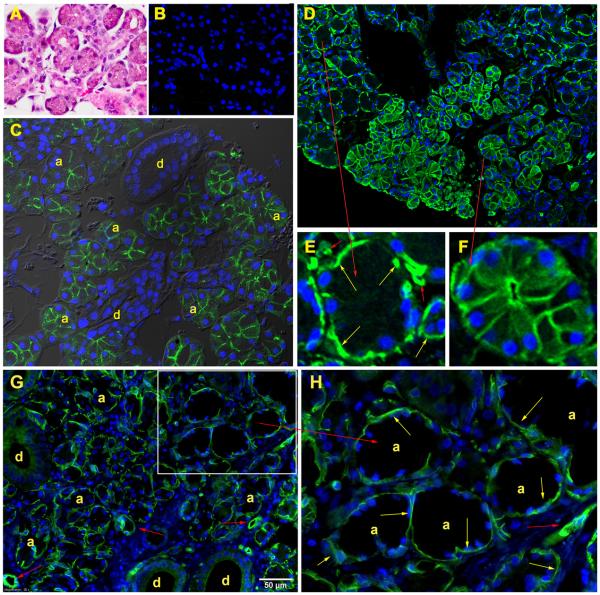
Two subjects underwent a modified sialoendoscopic biopsy of the targeted parotid gland at the time of their first long-term follow-up visit. DNA isolated from both samples was examined for the presence of AdhAQP1 using a conventional PCR assay. The appropriate PCR amplicon was detected in the sample from only one subject, # 99, (Figure 6 left panel; # 103 was negative). As can also be seen in Figure 6 (right panel), when this PCR reaction was performed on a sample of normal human parotid gland (NPG) no amplicon was detected. An ultrasound guided core needle biopsy was performed on subject # 19, and yielded considerably more tissue. That tissue was examined by light and immunofluoresence microscopy (Figure 7). Panel A depicts the general histological appearance of the biopsied tissue, showing the presence of acinar and duct cells with H & E staining. Panel B shows results of immunostaining with a control antibody, and only the appearance of DAPI-stained nuclei is visible. Panel C shows the tissue sample stained with an antibody to aquaporin-5, which is a normal component of the luminal membranes of human parotid acinar cells (a) but not present in duct cells (d) except those of the intercalated duct region. Panel D shows the tissue sample stained with an antibody to human aquaporin-1, which normally is only present in myoepithelial and vascular endothelial cells in the human parotid gland 17. Panel E is an enlarged region of a hAQP1-negative acinus, with only immunostained myoepithelial and endothelial cells observed, while panel F shows an enlarged region of an acinus that was transduced by AdhAQP1. This acinus and many adjacent acini are still expressing hAQP1 in their luminal and basolateral membranes 3.1 years after AdhAQP1 treatment. Panel G shows the results obtained when a biopsy specimen from a normal human parotid gland was stained with anti-human AQP1 antibody. The immunofluorescence staining is found only in two cell types: myoepithelial and vascular endothelial cells (Panel H shows an enlarged view), and is comparable to the results shown in Panel E.
Figure 6.
Results of conventional PCR assay with DNA extracted from tissue obtained with a modified sialoendoscopic biopsy of subject # 99. See SUBJECTS AND METHODS for details of the PCR reaction conditions and primers. A. Schematic diagram of AdhAQP1 with the region of the PCR amplicon shown as a red line. The Ad5 genome is shown in black. The human cytomegalovirus promoter (CMV) is shown in orange. The human aquaporin-1 transgene is shown in green, followed by the SV40 polyadenylation signal shown in blue. B. PCR Result. Lane M contains DNA standard markers (sizes shown to left). In the left panel, lane P is the positive control sample and shows the 565 bp amplicon as obtained from an extract of AdhAQP1. Lane #99 shows the same 565 bp amplicon, which was obtained from DNA extracted from the targeted parotid gland tissue of subject 99 at follow-up visit 1 (day 721 after AdhAQP1 administration). In the right panel, lanes M and P are the same, but the sample in the lane labeled NPG was obtained from the normal parotid gland of a male volunteer who had not been administered AdhAQP1.
Figure 7.
Images from core needle biopsy specimen obtained from subject #19 at follow-up visit 1 (day 1124 after AdhAQP1 administration). A. H&E staining of the parotid gland tissue sample, showing the presence of acini and ducts. B. Control for immunofluorescence staining using normal rabbit IgG as the primary antibody. The nuclei are stained using DAPI and have a blue color. C. Tissue stained with an antibody to human AQP5. The immunofluorescence staining observed is localized only to the luminal membrane of acinar cells (a) and the closely adjacent intercalated duct region. Larger ducts (d) do not express AQP5 and are unstained. D. Tissue stained with an antibody to human AQP1. The immunofluorescence staining is found in three cell types: myoepithelial, vascular endothelial and acinar. Normally, AQP1 is only present in myoepithelial and vascular endothelial cells (Gresz et al 17). Acinar cells that can be seen expressing AQP1 (right central and bottom portion of panel) were transduced with AdhAQP1 administered to subject # 19 1124 days previously. E. An enlarged region of Panel D showing the presence of AQP1 in myoepithelial (yellow arrows) and vascular endothelial cells (red, smaller arrows) and the negative staining of a non-transduced acinus. F. An enlarged region of Panel D showing the abundant presence of AQP1 in the basolateral and luminal membranes of a transduced acinus. G. AQP1 localization in a biopsy specimen from a normal, male human volunteer’s parotid gland, i.e., without AdhAQP1 transduction. There is no immunofluorescence staining in acinar cells. H. An enlarged region of Panel G clearly showing the absence of AQP1 staining in acinar (a) and duct (d) cells, but its presence in myoepithelial cells (yellow arrows) and vascular endothelial cells (smaller red arrows). See SUBJECTS AND METHODS for details on the staining procedures and antibodies used.
DISCUSSION
First generation Ad5 vectors, such as AdhAQP1, have been used frequently in pre-clinical and clinical gene therapy studies 18-21. While these vectors lead to very efficient gene transfer and high levels of transgenic protein production, they are also considered problematic because they elicit potent innate, cellular and humoral immune responses. In great part, because of this immunoreactivity, first generation Ad5 vectors are considered only to yield transient expression of the delivered transgene 21-23, typically for no more than a week or two, with a peak at ~days 2 or 3. Compared to Ad5 vector use systemically (intravascular delivery) or to a variety of organs, relatively few studies have involved delivery of a first generation Ad5 vector to salivary glands. All but one of these studies were performed in preclinical, animal models (mouse, rat, miniature pig and macaque), and all studies in these animal models displayed the typical pattern of transgene expression described above 24-27 s.
There is only a single human study reported involving gene transfer to a salivary gland, the same as the one described herein 12. This study employed the first generation Ad5 vector, AdhAQP1. Our previous report described initial results from this study, through day 42 following delivery of AdhAQP1 to a single, irradiation-damaged salivary gland 12. The present study reports results from the long-term follow-up of the five subjects described previously to have responded positively to AdhAQP1 delivery. The initial report of this clinical trial 12 was remarkable in showing that the peak of transgene expression, inferred to be the peak of increased parotid saliva flow rate, occurred on days 7-42, i.e., over a much later time frame than anticipated. Studies with AdhAQP1 and other Ad5 vectors in salivary glands in all animal models were quite similar to each other and, as indicated above, showed peak transgene expression on days 2 or 3, which then returned to background levels by two weeks. The present report is even more remarkable in that all five subjects who responded positively to hAQP1 gene transfer initially, still displayed substantially elevated levels of parotid saliva flow 3-4.7 years after AdhAQP1 administration. In addition, we showed that most subjects also experienced relief from two key xerostomic symptoms for at least two years after treatment. Because of the unique nature of our results it is important to try to understand how these findings might have occurred.
Globally, there are likely two key reasons. First, it is generally considered that the significant immune response to a first generation Ad5 vector delivery results in the complete removal of the vector from the targeted tissue. However, as we have shown previously in rat salivary glands, that is not the case 30. Following administration of a dose of 109 vector particles/ rat submandibular gland, we found that 0.1% of the delivered vector dose, i.e., 106 vector particles, was still present in gland tissue 6-12 months later 28. In that study, one of the vectors used (AdCMV-hEpo) was identical to AdhAQP1, including the same promoter (hCMV), differing only in the transgene used, i.e., the cDNA encoding human erythropoietin (hEpo) versus hAQP1.
Also as shown in that study, serum hEpo levels in response to the AdCMV-hEpo vector were at background levels between days 14-19 after administration, despite the continued presence of the vector in rat submandibular glands 28. The explanation for the absence of hEpo expression, and the second reason, came from a very recent study by our group 29. In that study, it was shown that the hCMV promoter is substantially methylated in rodent salivary glands, a chemical modification that effectively silences its ability to function as a promoter and, thus, drive transgene expression. For example, after administration of 109 particles of AdhAQP1 in rat submandibular glands, the hCMV promoter was ~30%, 65% and 90% methylated on days 2, 7 and 14, respectively. Similarly, in rat submandibular epithelial (A5) cells in vitro, 7 days after AdhAQP1 exposure >90% of the hCMV promoter was methylated. Conversely, the hCMV promoter was completely non-methylated 7 days after AdhAQP1 was added to cultures of primary cells from human parotid and minor salivary glands, as well as to cultures of two human salivary gland cell lines (HSG, HSY 29). Indeed, comparing hAQP1 functional expression (cell volume regulation in response to an osmotic challenge) in AdhAQP1-transduced A5 cells and HSG cells 7 days after vector treatment, we observed the absence of volume regulation in A5 (rat) cells, but normal volume regulation in HSG (human) cells, with the latter indicating effective transgenic hAQP1 function. Thus, the aggregate results of both Zheng et al studies 28,29 predict that the AdhAQP1 vector will still be (i) present in human parotid glands long after its administration, and (ii) capable of directing the expression of functional hAQP1. The PCR result shown in Figure 6 confirms the first prediction, while the immunofluorescence results shown in panels D and F of Figure 7 confirm the second prediction.
When we originally began working to develop a gene therapy treatment for radiation-induced xerostomia employing the hAQP1 cDNA, we anticipated that most of a targeted parotid gland’s acinar cells would be destroyed or severely damaged, and that the majority of the administered AdhAQP1 vector would likely target, and lead to fluid secretion from, the normally water impermeable duct cells 8-10. However, that clearly is not the case in humans, given several results presented herein. Firstly, Figure 7 shows that the major cell type expressing hAQP1 protein following AdhAQP1 administration is the acinar cell. Normally, only myoepithelial and vascular endothelial cells express hAQP1 in human parotid glands (Gresz et al 17; Figure 7D, E herein), but Figure 7F shows abundant transduction of acinar cells. Secondly, the conclusion that remaining acinar cells are possible targets of administered AdhAQP1 is supported by the results of secretory protein electrophoresis (Figure 5). With the two subjects for whom sufficient baseline parotid saliva samples were available (#s 19 and 50), polyacrylamide gel electrophoresis shows the clear presence of two major types of acinar cell secretory proteins, amylase and PRP1 (Figure 5), with low levels of a third acinar protein group (histatins) also visible. Comparable results were seen with samples from subject 99, albeit not with the baseline and peak saliva samples, but from samples with comparable parotid flow rates. Finally, the conclusion is also supported by the salivary electrolyte composition results (Table 4). These results are most striking for subject #s 19 and 50, for whom true baseline and peak saliva samples were available for analysis. With both subjects, the concentrations of Na+ and Cl− at peak initial parotid salivary flow rates (days 7 and 42, respectively) were very high, consistent with the acinar secretion of a true isotonic primary fluid that experienced little NaCl reabsorption as it rapidly passed through the ductal tree 30,31. Clearly acinar cells can be targets for AdhAQP1 transduction in irradiated human parotid glands, a conclusion that is consistent with recent experiments after AdhAQP1 transduction of irradiated mouse submandibular glands 32.
The mechanism by which transgenic hAQP1 expression facilitates increased salivary flow in the radiation damaged acinar cells is not yet fully understood and requires additional study. However, we speculate that since hAQP1 is non-polarized in its membrane distribution, i.e., present in luminal and basolateral membranes, the mechanism may involve radiation damage to, and defective function of, the aquaporin isoforms normally found in human acinar cells: AQP5 in the luminal membrane and AQP3 in the basolateral membrane 17. If that occurred, the transgenic hAQP1 could replace the function of these AQP isoforms in acinar cells and permit salivary flow. It is also not clear why there were no duct cells expressing hAQP1 in the core needle biopsy sample from subject # 19. A possible consideration is that while the extended expression of transgenic hAQP1 in acinar cells in situ results from the hCMV promoter likely not being methylated in transduced acinar cells, the promoter may have been methylated in duct cells targeted by the vector. There was no way for us to examine this possibility under the protocol approved for the present study, but it should be testable in the future.
A final major finding in the present study supports a key conclusion of the initial 42-day report 12, i.e., that AdhAQP1 delivery to a single human parotid gland is safe. Herein we found no significant vector or procedure-related AEs following day 42 of the study until the final follow-up subject visits, 3-4 years later. Additionally, there were no consistent, significant changes related to the study in any clinical chemistry or hematology parameter measured.
The original purpose of the AdhAQP1 clinical trial (http://www.clinicaltrials.gov/ct/show/NCT00372320?order=) was considered essentially to be a proof of concept, i.e., that hAQP1 gene transfer to an irradiation damaged salivary gland would lead to increased fluid secretion from the targeted gland. It was expected that a positive result would unlikely lead to a long term benefit and that the AdhAQP1 vector would be rapidly cleared from the targeted gland (http://osp.od.nih.gov/sites/default/files/RAC_minutes_12-05.pdf). First generation Ad5 vectors may not be ideal gene therapy vectors. However, when used at modest doses and delivered locally to a parotid gland, conditions that do not elicit a marked immune response 12, Ad5 vectors can be useful for salivary gland gene transfer in humans. As shown herein, all five responder-subjects in the AdhAQP1 clinical trial experienced significant objective and subjective benefits over a considerable time period following vector administration.
SUBJECTS AND METHODS
General methods
As previously described 12, the Phase I/II clinical trial (NIH protocol 06-D-0206) to test the safety and efficacy of AdhAQP1-mediated gene transfer to a single, previously irradiated parotid gland was approved by the NIDCR Institutional Review Board, the NIH Biosafety Committee, the Recombinant DNA Advisory Committee, the FDA (IND BB-13,102), as well as an independent Data and Safety Monitoring Board. Five enrolled subjects (all males; #s 19, 50, 99, 103, 118) were considered to be positive responders based on increased parotid salivary flow rates, following 2% citric acid stimulation (dorsal surface swabbing of the tongue), as well as a measured improvement in two key xerostomic symptoms using a visual analogue scale developed by Pai et al 15. Parotid salivary flow rates measured by the methods used herein are widely employed, but can show from15-45% variability according to several studies 33-35. The two xerostomic symptoms reported earlier 12 and herein exhibit moderate (rate the dryness of your mouth) and marginal (rate how much saliva is in your mouth) reproducibility 15. While these two questions could reflect interdependent symptoms, they were used to indicate self-perceived oral dryness and oral moisture, respectively. Both are reported herein because of their use in our earlier publication in defining positive responder-subjects 12. The responder-subjects were in the first three AdhAQP1 vector dosage groups: 4.8×107 (# 19), 2.9×108 (#s 50, 99), and 1.3×109 (#s 103, 118) vector particles/gland. As noted earlier, general clinical characteristics of these subjects were previously reported in Baum et al 12 and a summary also can be found in supplemental Table 1. All methods were as previously reported 12, except for the following used only for the present study.
Additional methods
Anti-Ad5 neutralizing serum antibodies employed a previously described assay 36,37. The assay tests the ability of serum dilutions to block the transduction of 293 cells by an Ad5 vector, AdCMVLuc encoding luciferase. All assays reported herein were performed at one time and the titers indicated represent serum dilutions resulting in a 50% inhibition of transduction. Some samples of all five subjects’ parotid saliva, at or near the initially observed increase in salivary flow, were available for ionic composition analysis (Na+, K+, Cl−). This was performed using previously reported methods 38. Additionally, parotid saliva samples from three subjects (#s 19, 50, 99) were available for secretory protein analysis using well-described polyacrylamide gel electrophoresis methods 39. Additionally, two subjects were approved for and agreed to a modified sialoendoscopic biopsy of the targeted parotid gland, which was obtained at their first long-term follow-up visit (# 99, day 721; # 103, day 797). However, since little gland tissue was obtained, we also received approval to perform an ultrasound-guided core needle biopsy in the targeted gland of one subject (#19; day 1124), who consented to the procedure. Tissue samples from subject #s 99 and 103 were analyzed for the presence of vector DNA using the polymerase chain reaction (PCR; see below), while the tissue obtained from the ultrasound-guided core needle biopsy was examined by immunofluorescence microscopy (also see below).
PCR assay
The tissue samples from subject #s 99 and 103 were placed in 100 µl saline solution and genomic DNA directly extracted using the QIAamp®DNA blood mini kit (QIAGEN, Gaithersburg, MD). One-half of the obtained DNA (from 20 ng to 146 ng) was used in a conventional PCR assay. Primer 1 (5’-CGTGTACGCTGGGAGGTCTATATAA-3’) is a forward primer from the human cytomegalovirus (hCMV) promoter, which was used to drive hAQP1 expression in AdhAQP1. Primer 2 (5’-TACAGAGAGGCCGATGGCAA-3’) is a reverse primer from sequences in hAQP1. PCR was performed at 50°C for 1 min, 72°C for 1 min and 94°C for 1 min, for 40 cycles. Ten µl of the PCR reaction mixture was electrophoresed on a 1% agarose gel. If the expected band was not present (it was not for both subjects’ samples), then, 5 µl from that first PCR reaction was used to perform a second round of PCR under the same conditions. Again, the expected band was not present on the resulting agarose gel, so 5 µl from second reaction mixture was used to perform a third and final round of PCR. The positive control was DNA extracted from the pure AdhAQP1 viral vector, and that PCR was performed separately from both subjects’ samples. The resulting 565 bp amplicon from this PCR assay could only be derived from tissue samples in which the AdhAQP1 vector genome was present.
Immunofluorescence staining
The ultrasound-guided core needle biopsy sample obtained from # 19’s targeted parotid gland was fixed in 10% formalin and embedded in paraffin. Sections (5 µm) were treated exactly as previously described 29. The primary antibodies used herein were rabbit monoclonal anti-aquaporin-1, rabbit monoclonal anti-aquaporin-5 and normal rabbit IgG as an antibody control (all from Abcam, Inc., Cambridge, MA, USA). The secondary antibody was Alexa Fluor 488 donkey anti rabbit IgG (H+L) (Invitrogen) and used as described 29. Additionally, sections of this sample were stained conventionally with hematoxylin and eosin (H&E).
Statistical analyses
To determine if the change in salivary flow rates in the AdhAQP1-targeted parotid gland of all previously designated responder-subjects was statistically significantly different over time, longitudinal analyses were undertaken. First, exploratory data analysis was performed. Next, correlation structure between multiple salivary flow measurements was analyzed. Models with independent, exchangeable/uniform, autoregressive (AR1), and unstructured correlation structures were compared. Autocorrelation function (ACF) and quasi-Akaike Information Criterion (QIC) were computed for model selection. Generalized Estimating Equation (GEE) models with uniform correlation structure with Huber White estimator of variance were then constructed 14. These models were constructed to: 1) compare stimulated parotid salivary flow rate at all post-treatment visits to baseline values; 2) compare baseline flow rates to those at the initial peak (during the first 42 study days; reported 12) and both long term follow-up visits (days 721-1708 following AdhAQP1-mediated gene transfer); and 3) determine the change in flow rate with time, i.e., over a period of 16 visits and over a period of 4 keys visits (baseline, initial peak, follow-up 1 and 2). Similarly, GEE models were constructed to assess changes in stimulated parotid salivary flow rates in the contralateral, non-targeted parotid gland.
Supplementary Material
ACKNOWLEDGMENT
We are most grateful to the five responder-subjects (#s 19, 50, 99, 103, 118), who gave so generously of their time and themselves for from 3-4.7 years. They enabled this long-term follow-up study to be conducted. Additionally, we are sincerely appreciative of the Intramural Research Program of the National Institute of Dental and Craniofacial Research, which supported this study from its initial conception through its completion.
Footnotes
DISCLOSURES OF POSSIBLE CONFLICTS OF INTEREST
B. J. Baum currently serves as a consultant to GSK, but was not so engaged during this study. None of the other authors has any relationship to disclose.
REFERENCES
- 1.Jensen SB, Pedersen AM, Vissink A, Andersen E, Brown CG, Davies AN, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: prevalence, severity and impact on quality of life. Support Care Cancer. 2010;18:1039–1060. doi: 10.1007/s00520-010-0827-8. [DOI] [PubMed] [Google Scholar]
- 2.Vissink A, van Luijk P, Langendijk JA, Coppes RP. Current ideas to reduce or salvage radiation damage to salivary glands. Oral Dis. 2015;21:e1–e10. doi: 10.1111/odi.12222. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz-Arad D, Arber L, Arber N, Zajicek G, Michaeli Y. The rat parotid gland – a renewing cell population. J Anat. 1988;161:143–151. [PMC free article] [PubMed] [Google Scholar]
- 4.Redman RS. Proliferative activity by cell type in the developing rat parotid gland. Anat Rec. 1995;241:529–540. doi: 10.1002/ar.1092410411. [DOI] [PubMed] [Google Scholar]
- 5.Van Luijk P, Pringle S, Deasy JO, Moiseenko VV, Faber H, Hovan A, et al. Sparing the region of the salivary gland containing stem cells preserves saliva production after radiotherapy for head and neck cancer. Sci Transl Med. 2015;7:305ra147. doi: 10.1126/scitranslmed.aac4441. doi:1126scitranslmed.aac4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 7.Mastrangeli A, O’Connell B, Aladib W, Fox PC, Baum BJ, Crystal RG. Direct in vivo adenovirus-mediated gene transfer to salivary glands. Am J Physiol. 1994;266:G1146–G1155. doi: 10.1152/ajpgi.1994.266.6.G1146. [DOI] [PubMed] [Google Scholar]
- 8.Delporte C, O’Connell BC, He X, Lancaster HE, O’Connell AC, Agre P, et al. Increased fluid secretion after adenoviral-medicated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc Natl Acad Sci (USA) 1997;94:3268–3273. doi: 10.1073/pnas.94.7.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitolo JM, Baum BJ. The use of gene transfer for the protection and repair of salivary glands. Oral Dis. 2002;8:183–191. doi: 10.1034/j.1601-0825.2002.02865.x. [DOI] [PubMed] [Google Scholar]
- 10.Shan Z, Li J, Zheng C, Liu X, Fan Z, Zhang C, et al. Increased fluid secretion after adenoviral-mediated transfer of the human aquaporin-1 cDNA to irradiated miniature pig parotid glands. Mol Ther. 2005;11:444–45. doi: 10.1016/j.ymthe.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Preston GM, Agre P. Isolation of the cDNA for the erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci (USA) 1991;88:11110–11114. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baum BJ, Alevizos I, Zheng C, Cotrim AP, Liu S, McCullagh L, et al. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc Natl Acad Sci (USA) 2012;109:19403–19407. doi: 10.1073/pnas.1210662109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Zourelias L, Wu C, Edwards PC, Trombetta M, Passineau MJ. Ultrasound-assisted nonviral gene transfer of AQP1 to the irradiated minipig parotid gland restores fluid secretion. Gene Ther. 2015;22:739–749. doi: 10.1038/gt.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diggle PJ, Heagerty P, Liang K-Y, Zeger SL. Oxford Statistical Science Series. Oxford University Press; Oxford, UK: 2002. Analysis of Longitudinal Data. ISBN978-0-19-852484-7. [Google Scholar]
- 15.Pai S, Ghezzi EM, Ship JA. Development of a Visual Analogue Scale questionnaire of subjective assessment of salivary dysfunction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:311–316. doi: 10.1067/moe.2001.111551. [DOI] [PubMed] [Google Scholar]
- 16.Alevizos I, Zheng C, Cotrim AP, Goldsmith CM, McCullagh L, Berkowitz T, et al. Immune reactivity after adenoviral-mediated cDNA transfer to human parotid glands. Oral Dis. doi: 10.1111/odi.12614. doi: 10.1111/odi.12614, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gresz V, Kwon TH, Hurley PT, Varga G, Zelles T, Nielsen S, et al. Identification and localization of aquaporin water channels in human salivary glands. Am J Physiol. 2001;281:G247–G254. doi: 10.1152/ajpgi.2001.281.1.G247. [DOI] [PubMed] [Google Scholar]
- 18.Harvey BG, Hackett NR, El-Sawy T, Rosengart TK, Hirschowitz EA, Lieberman MD, et al. Variability of human systemic humoral immune responses to adenovirus gene transfer vectors administered to different organs. J Virol. 1999;73:6729–6742. doi: 10.1128/jvi.73.8.6729-6742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harvey BG, Hackett NR, Ely S, Crystal RG. Host responses and persistence of vector genome following intrabronchial administration of an E1 (-) E3(-) adenovirus gene transfer vector to normal individuals. Mol Ther. 2001;3:206–215. doi: 10.1006/mthe.2000.0244. [DOI] [PubMed] [Google Scholar]
- 20.Muona K, Makinen K, Hedman M, Manninen H, Yia-Herttuala S. 10-year safety follow-up in patients with local VEGF gene transfer to ischemic lower limb. Gene Ther. 2012;19:392–395. doi: 10.1038/gt.2011.109. [DOI] [PubMed] [Google Scholar]
- 21.Hendrikx R, Stichling N, Koelen J, Kuryk L, Lipiec A, Greber UF. Innate immunity to adenovirus. Hum Gene Ther. 2014;25:265–284. doi: 10.1089/hum.2014.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seregen SS, Appledorn DM, McBride AJ, Schuldt NJ, Aldhamen YA, Voss T, et al. Transient pretreatment with glucocorticoid ablates inate toxicity of systemically delivered adenoviral vectors without reducing efficacy. Mol Ther. 2009;17:685–696. doi: 10.1038/mt.2008.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laakkonen JP, Yia-Herttuala S. Recent advances in cardiovascular gene therapy and vascular biology. Hum Gene Ther. 2015;26:518–524. doi: 10.1089/hum.2015.095. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Baum BJ, Yamano S, Mankani MH, Sun D, Jonsson M, et al. Adenoviral-mediated gene transfer to mouse salivary glands. J Dent Res. 2000;79:701–708. doi: 10.1177/00220345000790020201. [DOI] [PubMed] [Google Scholar]
- 25.Kagami H, Atkinson JC, Michalek SM, Handelman B, Yu S, Baum BJ, et al. Repetitive adenovirus administration to the parotid gland: role of immunological barriers and induction of oral tolerance. Hum Gene Ther. 1998;10:305–313. doi: 10.1089/hum.1998.9.3-305. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Zheng C, Zhang X, Liu X, Zhang C, Goldsmith CM, et al. Development of a large animal model for gene transfer to salivary glands in vivo. J Gene Med. 2004;6:55–63. doi: 10.1002/jgm.476. [DOI] [PubMed] [Google Scholar]
- 27.Voutetakis A, Zheng C, Metzger M, Cotrim AP, Donahue RE, Dunbar CE, et al. Sorting of transgenic secretory proteins in rhesus macaque parotid glands after adenovirus-mediated gene transfer. Hum Gene Ther. 2008;19:1401–1405. doi: 10.1089/hum.2008.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng C, Vitolo JM, Zhang W, Mineshiba F, Cjiorini JA, Baum BJ. Extended transgene expression from a nonintegrating adenoviral vector containing retroviral elements. Mol Ther. 2008;16:1089–1097. doi: 10.1038/mt.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng C, Baum BJ, Liu X, Goldsmith CM, Perez P, Jang SI, et al. Persistence of hAQP1 expression in human salivary gland cells following AdhAQP1 transduction is associated with a lack of methylation of the hCMV promoter. Gene Ther. 2015;22:758–766. doi: 10.1038/gt.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thaysen JH, Thorn NA, Schwartz IL. Excretion of sodium, potassium, chloride and carbon dioxide in human parotid saliva. Am J Physiol. 1954;178:155–159. doi: 10.1152/ajplegacy.1954.178.1.155. [DOI] [PubMed] [Google Scholar]
- 31.Baum BJ. Principles of saliva secretion. Ann NY Acad Sci. 1993;694:17–23. doi: 10.1111/j.1749-6632.1993.tb18338.x. [DOI] [PubMed] [Google Scholar]
- 32.Teos LY, Zheng C-Y, Liu X, Swaim WD, Goldsmith CM, Cotrim AP, et al. Adenovirus-mediated hAQP1 expression in irradiated mouse salivary glands causes recovery of saliva secretion by enhancing acinar cell volume decrease. Gene Ther. 2016;23:572–579. doi: 10.1038/gt.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atkinson JC, Yeh C-K, Bermudez D, Fox PC, Baum BJ. Longitudinal evaluation of major salivary gland function in HIV-1 infected patients. J Oral Pathol Med. 1989;18:469–470. doi: 10.1111/j.1600-0714.1989.tb01344.x. [DOI] [PubMed] [Google Scholar]
- 34.Percival RS, Challacombe SJ, Marsh PD. Flow rates of resting whole and stimulated parotid saliva in relation to age and gender. J Dent Res. 1994;73:1416–1420. doi: 10.1177/00220345940730080401. [DOI] [PubMed] [Google Scholar]
- 35.Ghezzi EM, Lange LA, Ship JA. Determination of variation of stimulated salivary flow rates. J Dent Res. 2000;79:1874–1878. doi: 10.1177/00220345000790111001. [DOI] [PubMed] [Google Scholar]
- 36.Sprangers MC, Lakhai W, Koudstaal W, Verhoeven M, Koel BF, Vogels R, et al. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J Clin Microbiol. 2003;41:5046–52. doi: 10.1128/JCM.41.11.5046-5052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng C, Nikolov NP, Alevizos I, Cotrim AP, Liu S, McCullagh L, et al. Transient detection of E1-containing adenovirus in saliva after the delivery of a first-generation adenoviral vector to human parotid gland. J Gene Med. 2010;12:3–10. doi: 10.1002/jgm.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamoto T, Brown DA, Catalán M, Gonzalez-Begne M, Romanenko VG, Melvin JE. J Biol Chem. 2009;284:4815–4822. doi: 10.1074/jbc.M808597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campese M, Sun X, Bosch JA, Oppenheim FG, Helmerhorst EJ. Concentration and fate of histatins and acidic proline-rich proteins in the oral environment. Arch Oral Biol. 2009;54:345–353. doi: 10.1016/j.archoralbio.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.