Abstract
Objective
Examine interactive relations of race and socioeconomic status (SES) to magnetic resonance imaging (MRI) - assessed global brain outcomes with previously demonstrated prognostic significance for stroke, dementia, and mortality
Methods
Participants were 147 African Americans (AAs) and Whites (ages 33 to 71 years; 43% AA; 56% female; 26 % below poverty) in the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) SCAN sub-study. Cranial MRI was conducted using a 3.0 Tesla unit. White matter (WM) lesion volumes and total brain, gray matter (GM) and WM volumes were computed. An SES composite was derived from education and poverty status.
Results
Significant interactions of race and SES were observed for WM lesion volume (b = 1.38; η2 = 0.036; p = .028), total brain (b = 86.72; η2 = 0.042; p = <.001), GM (b = 40.16; η2 = 0.032; p = .003), and WM (b = 46.56; η2 = 0.050; p < .001). AA participants with low SES exhibited significantly greater WM lesion volumes than white participants with low SES. White participants with higher SES had greater brain volumes than all other groups (albeit within normal range).
Conclusions
Low SES was associated with greater WM pathology – a marker for increased stroke risk – in AAs. Higher SES was associated with greater total brain volume – a putative global indicator of brain health and predictor of mortality - in Whites. Findings may reflect environmental and interpersonal stressors encountered by AAs and those of lower SES and could relate to disproportionate rates of stroke, dementia, and mortality.
Keywords: subclinical brain pathology, brain volume, MRI, socioeconomic status, race
INTRODUCTION
Although disparities between African Americans (AAs) and Whites are apparent for all leading causes of death in the United States, the differential mortality associated with stroke is most pronounced (1). Indeed, AAs have twice the risk of incident stroke than Whites. AA-White disparities extend to multiple additional neurological endpoints including post-stroke dementia, vascular dementia, and Alzheimer's disease (2–4). Self-identified AA race further confers greater risk for cognitive decline, decreased physical function, disability, frailty, and magnetic resonance imaging (MRI)-assessed subclinical brain pathology (5–12).
Three of the most commonly derived MRI-based indices of subclinical brain pathology include white matter (WM) lesions, subclinical or “silent” brain infarcts, and brain atrophy. WM lesions reflect cerebral small vessel disease commonly associated with vascular risk factors (and/or cerebral amyloid angiopathy) and include diffuse areas of nonspecific injury (13). Subclinical or “silent” infarcts are characterized by focal areas of infarction (e.g., lacunes) without clinical signs of stroke (14). These types of pathology can be characterized by clinical ratings or reflected in a quantitative WM lesion volume score. Brain (or cerebral) atrophy refers to glial, synaptic, dendritic, and/or neuronal loss with age and/or pathology that is reflected in lesser brain volume assessed quantitatively or by clinical rating. However, it’s important to note that there is much variability in “normal” brain volumes with no clear cut scores as to what constitutes pathology. Nonetheless, each of these MRI-based measures of brain health are of notable public health concern as they have demonstrated prognostic significance for future cognitive decline, progression to dementia, stroke, and mortality, even among initially healthy individuals (14–20). Furthermore, total brain volume has been suggested to be a global indicator of brain health that has predictive utility with respect to all- cause mortality risk (21).
AAs are more likely than Whites to have lower socioeconomic status (SES), another potent risk factor for stroke (22), dementia (23), cognitive and functional decline and disability (6, 24), and subclinical brain pathology (25, 26). Relations of AA race to clinical brain health endpoints (e.g., stroke) remain significant following statistical adjustment for SES (27). However, lower SES may potentiate poor brain health outcomes among AAs. In that regard, a more pronounced relation of SES to stroke has indeed been reported in Black versus White men (23). Furthermore, both AAs and persons of lower SES have a greater prevalence and/or more severe cardiovascular and metabolic risk factors such as smoking, hypertension, and diabetes (28) that are known to confer risk for stroke, dementia, and subclinical brain pathology (13,14). However, to our knowledge, it is unknown whether lower SES is associated with greater subclinical brain pathology among AAs than Whites.
Noble and colleagues have demonstrated elegantly that the relation of lower family SES to lesser MRI-assessed brain volumes in children is independent of genetic markers of African ancestry (29). However, race is a social construct, and it is possible that self-identified AAs are more vulnerable to SES influences on brain health, in part, due to exposure to multiple environmental and interpersonal stressors in the United States (28).
Individual level SES is also a social construct with multiple indicators such as income, education, occupation, and wealth. However, no single SES indicator fully captures all complexities of social disadvantage (30). Further, it’s been argued that examination of single SES indicators oversimplifies the construct and can overestimate the effects attributable to that particular variable. Therefore, the utility of combining SES indicators has been suggested and may better capture dimensions of cumulative risk. Here we created a composite index of SES predicated on both education and poverty status.
It has more generally been recommended that research in health disparities give dual consideration to race and SES (28). Indeed, these variables are often conflated, and it may not be possible to fully disentangle their respective influences on health outcomes. Williams and colleagues emphasize the need to explicitly assess potential interactions of race and SES as sole statistical adjustment of one for the other can yield highly misleading findings (28).
Here, we examined potential interactions of self-identified race and SES to global measures of MRI-assessed WM lesion volumes, and total brain, gray matter (GM), and WM volumes. We posited disparities in these early indicators of brain health such that synergistic associations of race and SES would reveal the greatest vulnerability for lower SES AAs with respect to subclinical brain pathology.
METHODS
Participants
Participants were 147 AA and White adults (aged 33 – 71 years; 43% AA; 56% female; 26 % living in poverty) who had recently participated in Wave 3 of the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) parent study between the years 2009 and 2013. HANDLS is an ongoing longitudinal investigation of race- and SES-related health disparities among AA and White adults residing in 13 Baltimore neighborhoods pre-selected based on their likelihood to yield representative distributions of race and sex across a range of socioeconomic circumstances (31). The HANDLS study was approved by the Institutional Review Board of the National Institute of Environmental Health Sciences, NIH. All participants provided written informed consent. HANDLS study exclusions were 1) outside of the age range 30– 64 years; 2) currently pregnant; 3) within six months of receiving chemotherapy, radiation, or biological treatments for cancer; 4) AIDS diagnosis; 5) unable to provide informed consent due to mental incapacity resulting from drug or alcohol intoxication, severe developmental disability, or dementia; 6) unable to provide at least five data measures on the mobile medical research vehicle; 7) without a verifiable address or valid government issued identification at time of consent.
HANDLS SCAN participants were recruited from the HANDLS parent study with the following additional exclusions: history of dementia, stroke, transient ischemic attack, carotid endarterectomy; MRI contraindications (e.g., indwelling ferromagnetic material); terminal illness (e.g., metastatic cancer, end-stage liver or pulmonary diseases); HIV positive status; or other neurological disorder (e.g., multiple sclerosis). Two hundred twenty-five participants were recruited for the present protocol. Of those, 41 did not complete neuroimaging for the following reasons (n = 12 deemed medically ineligible; n = 19 claustrophia; n= 4 excessive girth; n= 6 equipment or scheduling issues). Of the 184 participants with neuroimaging, 9 were excluded for partial scans (due to motion artifact); 15 for incidental clinical findings, 8 because of a history of cocaine or opiate use, and 5 had missing data for key study variables. Complete data were available for 147 participants in this ongoing investigation.
All participants provided written informed consent and HIPAA consent approved by the University of Maryland, Baltimore's Institutional Review Board (and also approved by the University of Maryland, Baltimore County) for the HANDLS SCAN sub-study. They were then seen by a physician at the University of Maryland General Clinical Research Center for a brief medical evaluation to identify acute medical problems since their last HANDLS visit, review current medications, administer the MRI eligibility checklist, assess potential contraindications to the performance of HANDLS SCAN testing, and complete brief physical function assessment. They next underwent MRI acquisition in the Department of Diagnostic Radiology at the University of Maryland School of Medicine. Individuals received $50 and coverage of transportation costs for their participation.
Measures
Demographic variables
Degree of SES risk was indexed as below median education (0 = 12 years or above; 1 = < 12 years) or income below 125% of the 2004 poverty line ($18,850 per year for a family of 4) relative to family size and household income (0 = above poverty; 1 = below poverty). A composite index of SES was computed as a dichotomous variable, with low SES defined as below median education (< 12 years), and/or income below 125% of the federal poverty line. Participants were classified as high SES if they met neither of these criteria. Also considered were continuous and trichotomous education (0 = > 12 year; 1 = 12 years; 2 = < 12 years); and a trichotomy derived from poverty status and self-reported categories of annual income level (1=below poverty line; 2= above poverty and < $50,000 income; 3 = above poverty and >/= $50,000 income). Self-identified race (0 = White; 1 =AA), sex (0 = female; 1 = male) and age (in years) were also assessed.
Clinical variables
Systolic and diastolic blood pressure were obtained by brachial artery auscultation following a 5 min rest in a seated position. One measure was obtained in each arm; those measures were averaged. Hypertension was defined by self-reported history, use of antihypertensives, and/or resting systolic pressure of >/= 140 mm Hg or diastolic pressure >/= 90 mm Hg.
Blood samples were obtained from an antecubital vein following an overnight fast. Levels of total serum cholesterol, and fasting glucose were assessed by standard laboratory methods at Quest Diagnostics (Chantilly, VA; www.questdiagnostics.com). Diabetes was defined as fasting blood glucose >/= 126 mg/dl, self-reported history, and/or use of relevant medications.
Height and weight were obtained using calibrated equipment, and body mass index was computed as weight divided by height-squared (kg/m2). Waist circumference was measured to the nearest 0.1 cm with a flexible tape measure placed at the midpoint between the lower rib margin and the iliac crest at the end of exhalation during normal breathing.
Cardiovascular disease co-morbidity was coded as present = 1 or absent = 0 based on the presence of any of the following conditions: coronary artery disease, myocardial infarction, peripheral artery disease, or coronary artery bypass surgery. Conditions were documented by a HANDLS physician or nurse practitioner following a comprehensive physical examination and medical history.
Smoking status and alcohol use were dichotomized as 0 = never used and 1 = ever used (i.e., former and current users).
Magnetic Resonance Imaging
Cranial magnetic resonance images were obtained using a Siemens Tim-Trio 3.0 Tesla unit. Volumetric T1-weighted magnetization prepared rapid gradient echo imaging (MP-RAGE) images were obtained covering the entire brain in the sagittal plane at 1.2 mm thickness for a total of 160 slices (TR/TE/TI=2300/2.9/900 ms; FOV 25.6cm). These images were reformatted into axial sections to match the orientation of other anatomical images. Axial FLAIR images were obtained at a slice thickness of 3.0 mm with no gap in two concatenated groups of 24 slices each for a total of 48 slices (TR/TE/TI = 8000/71/2500ms, FOV=23cm). T2-weighted axial images were also obtained at 3mm thickness with no gap using turbo spin-echo acquisition from the same location as the FLAIR images (TR/TE = 6600/93ms, FOV=23cm; turbo factor 7).
Structural MRI scans were preprocessed by applying newly developed in-house techniques in the Section of Biomedical Image Analysis, Department of Radiology at the University of Pennsylvania. The processing steps first include removal of extra-cranial material on T1-weighted image (skull-stripping) using a multi-atlas registration based method that requires minimal manual correction (32), followed by joint bias correction and tissue segmentation into GM, WM, and cerebrospinal fluid using multiplicative intrinsic component optimization (33).
Preprocessed images were co-registered to a common brain atlas (template) using deformable registration via attribute matching and mutual-saliency weighting (34). Regional analysis of volumes examined in normalized space (RAVENS) maps (35) were generated to enable comparative analysis of tissue volumes on the common template space. The RAVENS approach has been extensively validated and applied in various studies (34–36). In this investigation, GM, WM and ventricular RAVENS maps were generated, each quantifying the amount of respective tissue present in each brain region. The RAVENS maps were normalized by individual intracranial volume to adjust for global differences in intracranial size, down-sampled to 2×2×2 mm, and smoothed for incorporation of neighborhood information using an 8mm-diameter Gaussian filter.
A supervised learning based multimodal lesion segmentation technique, which uses a model trained on manually segmented lesions, was applied to segment ischemic lesions (37). The method involved co-registration of T1, T2, FLAIR and PD scans, histogram normalization to a template image, feature extraction, voxel wise label assignment and false-positive elimination. A new multi-atlas label fusion method was applied for segmenting the brain into a set of anatomical regions of interest (ROI; 38). Volumetric measurements for normal and abnormal (with lesion) tissue were calculated within each ROI, as well as in larger anatomical regions obtained by grouping single ROIs within a hierarchical representation.
Statistical Methods
A series of multiple regression analyses (SAS 9.4 PROC GLM) were conducted to assess potential interactive relations of race and the SES composite score to WM lesion volumes, total GM and WM volumes, and total brain volumes. Models included the interactions of race × SES in addition to the first order terms of race and SES. All analyses were adjusted for age and sex. Significant interactions were decomposed by contrasting all groups by t-test using the least squares means procedure in PROC GLM.
Subsequent sensitivity analyses were computed to assess respective influences of hypertension, diabetes, smoking, alcohol, total cholesterol, body mass index, and waist circumference as covariates. Analyses were also repeated with those having CVD diagnoses excluded. Lastly, a series of exploratory analyses examined interactive relations of race with several individual SES indicators including continuous education, dichotomous education, trichotomous education, poverty dichotomy, income trichotomy for each of the outcome measures.
RESULTS
Table 1 shows demographic data for the overall sample, and by SES. Study non-completers had significantly lower levels of education than study completers (p =.006), but were otherwise similar sociodemographically (see Table S1, Supplemental Digital Content 1). Scatterplots of age associations with each MRI outcome are displayed in Supplemental Digital ContentFigures S1–S4.
Table 1.
Demographic and health variables for the overall sample, and by SES
| Overall N=147 |
Low SES N = 63 |
High SES N = 84 |
||
|---|---|---|---|---|
| Variable | M (SD) or N (%) |
M (SD) or N (%) |
M (SD) or N (%) |
p |
| Age (years) | 52.1 (9.5) | 49.9 (9.3) | 53.7 (9.4) | 0.016 |
| Education (years) | 12.9 (3.2) | 11.0 (2.7) | 14.2 (2.8) | <.001 |
| Low Education (%) | 37 (25.1) | 37 (58.7) | 0.0 | |
| Below Poverty (%) | 43 (29.3) | 43 (68.3) | 0.0 | |
| Female (%) | 82 (55.8) | 42 (66.7) | 41 (48.8) | 0.042 |
| African American (%) | 85 (57.8) | 32 (50.8) | 30 (35.7) | 0.13 |
| Ever Smoker (%) | 104 (70.7) | 48 (76.2) | 56 (66.7) | 0.34 |
| Ever Alcohol Use (%) | 132 (89.8) | 55 (87.3) | 76 (90.5) | 0.57 |
| Hypertension (%) | 67 (45.6) | 32 (49.2) | 31 (36.9) | 0.12 |
| Diabetes (%) | 24 (16.3) | 10 (15.9) | 14 (16.7) | >.99 |
| CVD comorbidities (%) | 3 (2.0) | 3 (4.8) | 0.0 | 0.17 |
| Body mass index (kg/m2) | 30.2 (6.7) | 29.9 (7.2) | 30.5 (36.4) | 0.60 |
| Waist circumference (cm) | 103.5 (16.2) | 101.8 (17.5) | 104.7 (15.2) | 0.30 |
| Total cholesterol (mg/dl) | 188.2 (43.4) | 182.8 (38.5) | 191.7 (46.1) | 0.25 |
| Total brain volume (cc) | 975.6 (102.4) |
948.1 (87.9) | 996.1 (108.0) |
0.005 |
| Gray matter volume (cc) | 517.6 (54.7) | 504.3 (47.8) | 527.5 (57.6) | 0.011 |
| White matter volume (cc) | 458.0 (50.6) | 443.8 (43.6) | 468.6 (53.1) | 0.003 |
| White matter lesion volume (cc) | 2.1 (1.8) | 2.0 (2.0) | 2.2 (1.6) | 0.67 |
CVD comorbidities variable includes participants with any of the following: coronary artery disease, myocardial infarction, peripheral artery disease, and coronary artery bypass surgery.
t-tests were used to compare continuous variables; Fisher’s exact test was used to compare categorical variables.
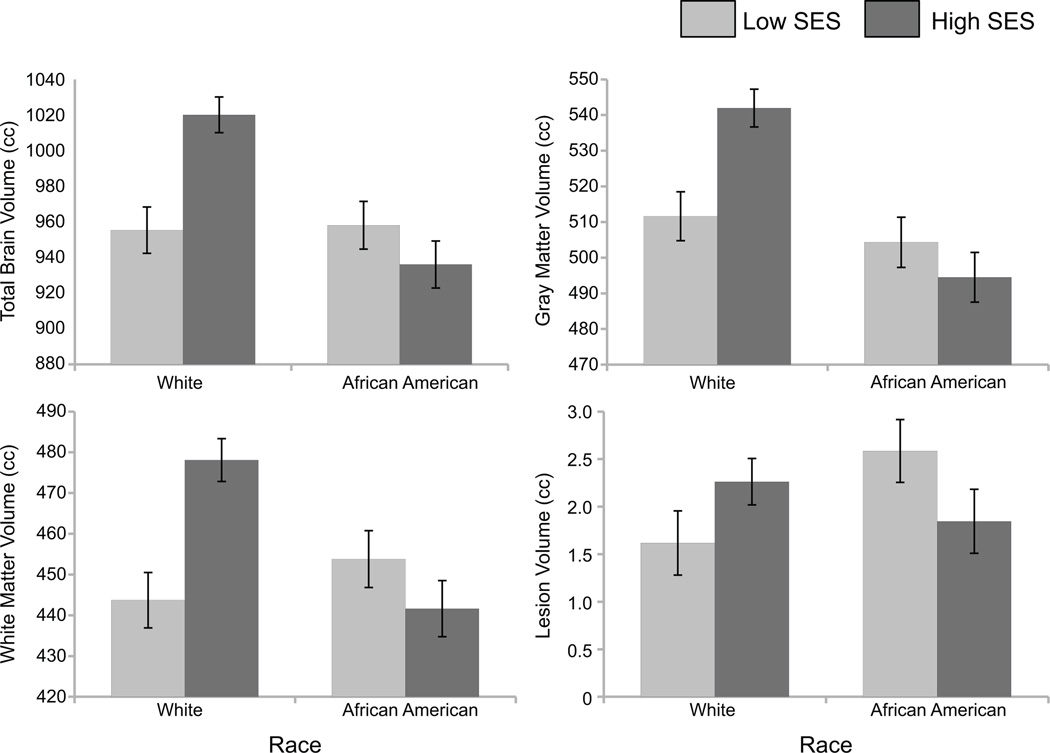
As described in Table 2 and illustrated in Figure 1, significant interactions between race and the SES composite were observed for WM lesion volume (p = .028)1, in addition to total brain volume (p <.001), GM volume (p = .003), and WM volume (p< .001). Significant group contrasts showed that AA participants with low SES exhibited significantly greater WM lesion volumes compared with white participants with low SES (p<.05). In addition, higher SES Whites had greater total brain, WM, and GM volumes than low SES Whites, low SES AAs, and higher SES AAs (p’s < .05).
Table 2.
Sociodemographic variable and brain volume measures (N = 147)*
| Outcome | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Brain Volume | Gray Matter Volume | White Matter Volume | White Matter Lesion Volume |
|||||||||
| Predictor | b | part η2 | p | b | part η2 | P | b | part η2 | p | b | part η2 | p |
| Age | −1.81 | 0.026 | 0.007 | −1.34 | 0.050 | <.001 | −0.47 | 0.007 | 0.17 | 0.031 | 0.023 | 0.075 |
| Sex | 122.64 | 0.340 | <.001 | 63.91 | 0.324 | <.001 | 58.72 | 0.319 | <.001 | −0.052 | 0.0002 | 0.87 |
| Race | −83.95 | 0.089 | <.001 | −47.47 | 0.100 | <.001 | −36.48 | 0.069 | <.001 | −0.417 | 0.007 | 0.31 |
| SES | −64.73 | 0.053 | <.001 | −30.35 | 0.041 | <.001 | −34.38 | 0.061 | <.001 | −0.645 | 0.017 | 0.13 |
| SES × Race | 86.72 | 0.042 | <.001 | 40.16 | 0.032 | 0.003 | 46.56 | 0.050 | <.001 | 1.384 | 0.036 | 0.028 |
Data reflect unstandardized regression coefficients (b), semipartial η2, and p-values
Sex coded as: 0=female, 1=male
Race coded as: 0=White, 1=African American
Figure 1.
Least-squares (adjusted) brain volume means according to race and SES. Error bars represent standard error of the mean. Means adjusted for age and sex.
Results of sensitivity analyses (see Table 3) indicated that the interactions of race and SES remained significant following adjustment for concurrent hypertension, diabetes, body mass index, waist circumference, total cholesterol, smoking, and alcohol use. Findings also remained significant following exclusion of those with CVD comorbidity.
Table 3.
The associations of the interaction between SES and race with brain volume measures adjusted for additional covariates; base model includes age, sex, race, SES, and race*SES interaction (N = 147)*
| Outcome | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Brain Volume | Gray Matter Volume | White Matter Volume |
White Matter Lesion Volume | |||||||||
| Covariate | b | part η2 | p | b | part η2 | p | b | part η2 | p | b | part η2 | p |
| Base Model | 86.72 | 0.042 | <.001 | 40.16 | 0.032 | 0.003 | 46.56 | 0.050 | <.001 | 1.38 | 0.036 | 0.028 |
| Alcohol Use | 81.29 | 0.036 | 0.003 | 36.84 | 0.026 | 0.009 | 44.46 | 0.044 | 0.002 | 1.49 | 0.039 | 0.027 |
| Cigarette Use | 78.70 | 0.035 | 0.003 | 34.92 | 0.024 | 0.010 | 43.78 | 0.044 | 0.002 | 1.53 | 0.042 | 0.020 |
| Hypertension | 81.05 | 0.037 | 0.002 | 36.19 | 0.026 | 0.008 | 44.86 | 0.046 | 0.001 | 1.44 | 0.037 | 0.027 |
| Diabetes | 81.20 | 0.037 | 0.002 | 36.26 | 0.026 | 0.008 | 44.94 | 0.046 | 0.001 | 1.52 | 0.041 | 0.021 |
| Waist circumference | 81.36 | 0.036 | 0.002 | 35.84 | 0.025 | 0.010 | 45.52 | 0.046 | 0.001 | 1.42 | 0.036 | 0.030 |
| Body Mass Index | 80.09 | 0.036 | 0.002 | 35.84 | 0.026 | 0.009 | 44.25 | 0.045 | 0.001 | 1.48 | 0.040 | 0.023 |
| Total cholesterol | 80.20 | 0.034 | 0.003 | 35.63 | 0.024 | 0.012 | 44.57 | 0.043 | 0.002 | 1.59 | 0.044 | 0.018 |
| CVD excluded | 89.33 | 0.044 | <.001 | 41.46 | 0.034 | 0.002 | 47.88 | 0.052 | <.001 | 1.27 | 0.030 | 0.045 |
Data reflect unstandardized regression coefficients (b), semipartial η2, and p-values illustrating associations of the SES × Race interaction with brain volume measures with adjustment for additional individual covariates, each in separate analyses.
CVD comorbidities variable includes participants with any of the following: coronary artery disease, myocardial infarction, peripheral artery disease, and coronary artery bypass surgery.
As displayed in Table 4, findings from the exploratory multiple regression analyses in which several individual SES indicators were substituted for the SES composite score yielded significant race by SES indicator interactions for total brain volume, GM, and WM volumes across most measures. However, findings for WM lesion volume were not significant for any individual SES indicator.
Table 4.
Multivariate analyses of the interactions between Education and Income with Race as related to brain volume measures (N = 147)
| Outcome | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Brain Volume | Gray Matter Volume | White Matter Volume | White Matter Lesion Volume |
|||||||||
| Interaction Term | b | part η2 | P | b | part η2 | p | b | part η2 | p | b | part η2 | p |
| Continuous Education × Race | −9.42 | 0.016 | 0.041 | −5.26 | 0.017 | 0.028 | −4.16 | 0.013 | 0.085 | −0.065 | 0.003 | 0.56 |
| Trichotomous Education × Race | ||||||||||||
| Education 2nd Tertile × Race |
−62.56 | 0.014 | 0.051 | −31.82 | 0.013 | 0.058 | −30.74 | 0.014 | 0.068 | −0.834 | 0.009 | 0.28 |
| Education 3rd Tertile × Race |
−87.01 | 0.025 | 0.010 | −43.30 | 0.022 | 0.014 | −43.71 | 0.026 | 0.013 | −0.884 | 0.009 | 0.28 |
| Dichotomous Education × Race | 73.54 | 0.024 | 0.012 | 37.00 | 0.021 | 0.015 | 35.53 | 0.024 | 0.017 | 0.949 | 0.013 | 0.18 |
| Poverty Status × Race | 58.33 | 0.016 | 0.038 | 29.00 | 0.014 | 0.049 | 29.33 | 0.017 | 0.046 | 0.689 | 0.007 | 0.32 |
| Trichotomous Income × Race | ||||||||||||
| Income 2nd Tertile × Race |
−49.74 | 0.011 | 0.090 | −23.64 | 0.009 | 0.12 | −26.10 | 0.012 | 0.090 | −0.497 | 0.004 | 0.49 |
| Income 3rd Tertile × Race |
−94.71 | 0.017 | 0.034 | −47.88 | 0.015 | 0.040 | −46.84 | 0.017 | 0.045 | 0.758 | 0.018 | 0.12 |
Data reflect unstandardized regression coefficients (b), semipartial η2, and p-values illustrating associations between Education × Race and Income × Race interactions with brain volume measures for various education and income variables
DISCUSSION
Both self-identified AA race and lower SES have known associations with a plethora of poor clinical brain health outcomes such as stroke and dementia. However, race and SES are commonly conflated due to the substantially greater prevalence of lower SES among racial/ethnic minorities. To our knowledge, this is the first investigation to explicitly address potential differences in several global indices of MRI-assessed brain health outcomes according to race 1Similar findings were noted for the ratio of WM lesion volume to total white matter volume. and SES. Partially consistent with our hypothesis, low SES AAs displayed greater WM pathology than low SES Whites, although no other groups differed significantly. Contrary to expectations, our findings also showed that higher SES Whites had greater mean GM, WM, and total brain volumes than low SES Whites, low SES AAs, and higher SES AAs. The above findings are notable in that greater WM lesions and lesser global brain volumes have demonstrated prognostic significance for poor clinical outcomes such as stroke, dementia, cognitive decline, disability, and/or mortality (14–21).
The present results suggest that the combination of low SES (indexed by either low education, living in poverty, or both) and self-identified AA race may indeed confer the greatest vulnerability for WM lesion burden. These findings remained significant following a series of adjustments for concurrent vascular risk factors commonly associated with WM pathology. WM lesions are typically acquired across the middle to later adult lifespan and are a form of subclinical brain pathology that may have particularly important predictive utility for future stroke (15, 19). WM lesions have also shown robust associations with dementia, cognitive performance and decline, and physical function (15–19), and thus may be a key risk marker for poor, clinically significant brain health outcomes.
Interpretation of findings related to global brain volumes is more complicated. From a clinical perspective, it has been suggested that brain volume may be a global indicator of brain health that is pertinent to risk for mortality and dementia (21). Thus, a combination of higher SES and self-identified White race may confer the greatest advantage for brain health. That higher SES did not similarly advantage AAs may, in part, reflect unique stressors encountered by such persons in their work and neighborhood contexts. For instance, higher SES AAs report greater levels of perceived discrimination than lower SES AAs (39). It has further been suggested that AAs disproportionately experience multiple additional dimensions of social disadvantage compared to Whites (30).
It is critical to note that there are no clear cut-offs regarding ranges of brain volume that are considered “normal’ versus pathological. The largely overlapping distributions of brain volumes among all of our subgroups of participants suggest that our findings reflect variability within a normal range. Furthermore, MRI-assessed brain size is only one of multiple morphological and functional characteristics that are associated with functional status. Such global macrostructural measures do not capture key microstructural variables (e.g., synaptic density), and anatomical or functional connectivity (40). Thus, one must refrain from interpretation of these findings with respect to individual level functioning. Rather, just as incrementally higher levels of blood pressure, even within the normal range, confer greater relative risk for stroke, dementia, and other poor brain health outcomes, it is possible that a similar association applies to the normal spectrum of brain volume. If brain volume is indeed a robust indicator of overall brain health, this may translate into population level mortality significance.
Importantly, it is impossible to tease apart the degree to which the present findings regarding brain volumes are attributable to neurodevelopmental factors versus atrophic changes acquired over the life span. In that regard, a growing body of literature reveals that (a) AAs are more likely than Whites to have been exposed to early life disadvantage including low SES (41); (b) that children of lower SES show lesser brain volumes in multiple regions (e.g., prefrontal, hippocampal, amygdala) (42, 43); and (c) that lower childhood SES predicts lesser adult brain volumes (44 45). Thus, the lesser brain volumes in AAs and lower SES Whites may, in part, have roots in lower childhood SES. However, the presence of greater WM lesion volumes in low SES AA than low SES Whites, which were unlikely to be present in childhood, suggests possible cumulative impact of various life-long exposures that are more pronounced for AAs and those of lower SES. These may include multi-level variables such as income inequality, neighborhood stress, toxic environmental exposures, racial/ethnic discrimination, lesser access to healthy food and health care, poorer health habits, greater cardiovascular and metabolic risk factors, and greater overall burden of systemic disease. The “brain battering hypothesis” has previously proposed that these factors may explain race-related disparities in cognitive aging (5).
More generally, the present findings related to brain GM, WM, and total brain volumes are consistent with results of prior investigations showing that higher levels of education or income confer greater health benefits for Whites than AAs with respect to various systemic health outcomes such as coronary heart disease, subclinical atherosclerosis, and inflammatory markers (46, 47). However, an alternative explanation is that SES indicators differ systematically for AAs and Whites (28). For example, the average quality of education is better for Whites than for AAs. Furthermore, for each level of income, Whites have more wealth than AAs.
The present investigation has several notable strengths. First, the HANDLS parent study, from which the present participants were recruited, was explicitly designed to disentangle respective influences of self-identified AA and White race from poverty status. Thus, there was a diverse spectrum of SES within our AA and White samples. Second, our SES indicator combined two variables - poverty status and education - that have potent influences on multiple health endpoints. The current work also has several limitations. Specifically, the findings do not generalize to ethnic minority populations other than AAs. In addition, results may be unique to the urban environment of Baltimore city. We did not have a full spectrum of SES indicators such as occupational status or wealth, nor did we have detailed characterization of annual income. It will be important to address both singular and cumulative influences of multiple SES indicators and determine whether they are linked to different mechanistic pathways to brain health. Future studies should also consider whether relations of race and SES to brain health outcomes are further moderated by age or sex, issues that we were underpowered to address herein. Relatedly, our sample size conferred limited statistical power to detect group differences that are small in magnitude, or to explore multi-level predictors of brain volumes within our study subgroups. We expect to examine the latter issue as our sample size increases. Lastly, we did not correct our group contrasts for multiple comparisons due to concerns about potential Type II error in this novel and largely exploratory study. Thus, the chance of Type I error remains a concern.
CONCLUSIONS
In sum, associations of self-identified race and SES with MRI-assessed outcomes were not uniform across all measures but rather subject to effect modification. Among persons of lower SES, AAs displayed a greater burden of WM lesions than Whites. It is possible that these findings may translate into a more pronounced risk for stroke and other poor clinical brain health outcomes in lower SES AAs, at least in part, reflecting the pernicious impact of poverty for AAs. With respect to global brain volumes, higher SES was associated with greater total brain, GM, and WM volumes in Whites but not AAs. These findings may, in part, reflect contextual stressors encountered by higher SES AAs, and may have important prognostic significance for future clinical brain health outcomes and mortality. Although MRI-based indices of brain health are only one in a complex and interrelated set of risk indicators relevant to clinical outcomes, further identification and reduction of such risk factors may assist in the elimination of race and SES related brain health disparities. It will be critical understand the multi-level mechanisms underlying these disparities, and to determine whether early interventions can alter trajectories toward poorer clinical brain health outcomes.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This research was support by NIH grant RO1 AG034161, the National Institute on Aging’s Intramural Research Program ZO1–AG000194, and P30 AG028747.
Acronyms
- AA
African Americans
- AIDS
acquired immunodeficiency syndrome
- FLAIR
fluid attenuated inversion recovery
- FOV
field of view
- GM
gray matter
- HANDLS
Healthy Aging in Neighborhoods of Diversity across the Lifespan
- HIV
human immunodeficiency virus
- MP-RAGE
magnetization prepared rapid gradient echo imaging
- MRI
magnetic resonance imaging
- NIA
National Institute on Aging
- NIH
National Institutes of Health
- PD
proton density
- RAVENS
regional analysis of volumes examined in normalized space
- ROI
region of interest
- TE
echo time
- TI
inversion time
- TR
repetition time
- WM
white matter
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to report.
DISCLOSURES
Disclosures: None
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive Summary: Heart Disease and Stroke Statistics-2016 Update: A report from the American Heart Association. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2.Harwood DG, Ownby RL. Ethnicity and dementia. Curr Psychiatry Rep. 2000;2:40–45. doi: 10.1007/s11920-000-0040-4. [DOI] [PubMed] [Google Scholar]
- 3.Tatemichi TK, Desmond DW, Mayeux R, Paik M, Stern Y, Sano M, Remien RH, Williams JB, Mohr JP, Hauser WA. Dementia after stroke: baseline frequency, risks, and clinical features in a hospitalized cohort. Neurology. 1992;42:1185–1193. doi: 10.1212/wnl.42.6.1185. [DOI] [PubMed] [Google Scholar]
- 4.Gorelick PB, Griffith P. Late sequelae of cerebrovascular disease in African Americans: vascular dementia. In: Gillum RF, Gorelick PB, Cooper E, editors. Stroke in Blacks: A Guide to Management and Prevention. Basel, Switzerland: Karger AG; 1999. [Google Scholar]
- 5.Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev. 2008;18:223–254. doi: 10.1007/s11065-008-9064-z. [DOI] [PubMed] [Google Scholar]
- 6.Ostchega Y, Harris T, Hirsch R, Parsons VL, Kington R. Prevalence of functional limitations and disability in older person in the US: data from the National health and Nutrition Examination Survey. J Am Geriatr Soc. 2000;48:1132–1135. doi: 10.1111/j.1532-5415.2000.tb04791.x. [DOI] [PubMed] [Google Scholar]
- 7.Dunlop DD, Song J, Manheim LM, Daviglus ML, Chang RW. Racial/ethnic differences in the development of disability among older adults. Am J Public Health. 2007;97:2209–2215. doi: 10.2105/AJPH.2006.106047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch C, Anderson ML, Newman A, Kop W, Jackson S, Gottdiener J, Tracy R, Fried LP. Cardiovascular Health Study Research Group. The association of race with frailty: the Cardiovascular Health Study. Ann Epidemiol. 2006;16:545–553. doi: 10.1016/j.annepidem.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Bryan RN, Cai J, Burke G, Hutchinson RG, Liao D, Toole JF, Dagher AP, Cooper L. Prevalence and anatomic characteristic of infarct-like lesions on MR images of middle-aged adults: the Atherosclerosis Risk in Communities Study. Am J Neuroradiol. 1999;20:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- 10.Prabhakaran S, Wright CB, Yoshita M, Delapaz R, Brown T, DeCarli C, Sacco RL. Prevalence and determinants of subclinical brain infarction. The Northern Manhattan Study. Neurology. 2008;70:425–430. doi: 10.1212/01.wnl.0000277521.66947.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao D, Cooper L, Cai J, Toole J, Bryan N, Burke G, Shahar E, Nieto J, Mosley T, Heiss G. The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: The ARIC Study. Neuroepidemiology. 1997;16:149–162. doi: 10.1159/000368814. [DOI] [PubMed] [Google Scholar]
- 12.Nyquist PA, Bilgel MS, Gottesman R, Yanek LR, Moy TF, Becker LC, Cuzzocreo J, Prince J, Yousem DM, Becker DM, Kral BG, Vaidya D. Extreme deep white matter hyperintensity volumes are associated with African American Race. Cerebrovasc Dis. 2014;37:244–250. doi: 10.1159/000358117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmahmann JD, Smith EE, Eichler FS, Filley CM. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci. 2008;1142:266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vermeer SE, Longstreth WT, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6:611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 15.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 16.Vermeer SR, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 17.Smith EE, Egorova S, Blacker D, Killiany RJ, Muzikansky A, Dickerson BC, Tanzi RE, Albert MS, Greenberg SM, Guttmann CR. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch Neurol. 2008;65:94–100. doi: 10.1001/archneurol.2007.23. [DOI] [PubMed] [Google Scholar]
- 18.de Groot JC, de Leeuw FE, Oudkerk M, Van Gijn J, Hofman A, Jolles J, Breteler MM. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol. 2002;52:335–341. doi: 10.1002/ana.10294. [DOI] [PubMed] [Google Scholar]
- 19.Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, Romero JR, Kase CS, Wolf PA, Seshadri S. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke. 2010;41:600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgmans S, van Boxtel MP, Smeets F, Vuurman EF, Gronenschild EH, Verhey FR, Uylings HB, Jolles J. Prefrontal cortex atrophy predicts dementia over a six-year period. Neurobiol Aging. 2009;30:1413–1419. doi: 10.1016/j.neurobiolaging.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 21.Van Elderen SS, Zhang Q, Sigurdsson S, Haight TJ, Lopez O, Eiriksdottir G, Jonsson P, de Jong L, Harris TB, Garcia M, Gudnason V, van Buchem MA, Launer LJ. Brain volume as an integrated marker for the risk of death in a community-based sample: age gene/environment susceptibility – Reykjavik Study. J Gerontol A Bio Sci Med Sci. 2014 doi: 10.1093/gerona/glu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox AM, McKevitt C, Rudd AG, Wolfe CDA. Socioeconomic status and stroke. Lancet Neurol. 2006;5:181–188. doi: 10.1016/S1474-4422(06)70351-9. [DOI] [PubMed] [Google Scholar]
- 23.Goldbourt U, Schnaider-Beeri M, Davidson M. Socioeconomic status in relationship to death of vascular disease and late-life dementia. J Neurol Sci. 2007;257:177–181. doi: 10.1016/j.jns.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Turrell G, Lynch J, Kaplan GA, Everson SA, Helkala EL, Kauhanen J, Salonen JT. Socioeconomic position across the lifecourse and cognitive function in late middle age. J Gerontol: Social sciences. 2002;57B:S43–S51. doi: 10.1093/geronb/57.1.s43. [DOI] [PubMed] [Google Scholar]
- 25.Butterworth P, Cherbuin N, Sachdev P, Anstey K. The association between financial hardship and amygdala and hippocampal volumes: results from the PATH through life project. Soc Cog Affect Neurosci. 2012;7:548–556. doi: 10.1093/scan/nsr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gianaros PJ, Marsland AL, Sheu LK, Erickson KI, Verstynen TD. Inflammatory pathways link socioeconomic inequalities to white matter architecture. Cerebral Cortex. 2013;23:2058–2071. doi: 10.1093/cercor/bhs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorelick PB. Cerebrovascular disease in African Americans. Stroke. 1998;29:2656–2664. doi: 10.1161/01.str.29.12.2656. [DOI] [PubMed] [Google Scholar]
- 28.Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann NY Acad Sci. 2012;1186:69–101. doi: 10.1111/j.1749-6632.2009.05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, Akshoomoff N, Amaral DG, Bloss CS, Libiger O, Schork NJ, Murray SS, Casey BJ, Chang L, Ernst TM, Frazier JA, Gruen JR, Kennedy DN, Van Zijl P, Mostofsky S, Kaufmann WE, Kenet T, Dale AM, Jernigan TL, Sowell ER. Family income, parental education, and brain structure in children and adolescents. Nat Neuroscience. 2015;18:773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adler N, Bush NR, Panell MS. Rigor, vigor, and the study of health disparities. P Natl A Sci. 2012;109(Suppl 2):17154–17159. doi: 10.1073/pnas.1121399109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, Zonderman AB. Healthy aging in neighborhoods of diversity across the life span (HANDLS): overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethn Dis. 2010;20:267–275. [PMC free article] [PubMed] [Google Scholar]
- 32.Doshi J, Erus G, Ou Y, Gaonkar B, Davatzikos C. Multi-Atlas Skull-Stripping. Acad Radiol. 2013;20:1566–1576. doi: 10.1016/j.acra.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li C, Gore JC, Davatzikos C. Multiplicative intrinsic component optimization (MICO) for MRI bias field estimation and tissue segmentation. Magn Res Imaging. 2014;32:913–923. doi: 10.1016/j.mri.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ou Y, Sotiras A, Paragios N, Davatzikos C. DRAMMS: Deformable registration via attribute matching and mutual-saliency weighting. Med Image Anal. 2011;15:622–639. doi: 10.1016/j.media.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davatzikos C, Genc A, Xu D, Resnick SM. Voxel-based morphometry using the RAVENS maps: methods and validation using simulated longitudinal atrophy. Neuroimage. 2001;14:1361–1369. doi: 10.1006/nimg.2001.0937. [DOI] [PubMed] [Google Scholar]
- 36.Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, Resnick SM. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72:1906–1913. doi: 10.1212/WNL.0b013e3181a82634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lao Z, Shen D, Liu D, Jawad AF, Melhem ER, Launer LJ, Bryan RN, Davatzikos C. Computer-assisted segmentation of white matter lesions in 3D MR images using support vector machine. Acad Radiol. 2008;15:300–313. doi: 10.1016/j.acra.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doshi J, Erus G, Ou Y, Davatzikos C. Ensemble-based medical image labeling via sampling morphological appearance manifolds. MICCAI Challenge Workshop on Segmentation: Algorithms, Theory and Applications. 2013 [Google Scholar]
- 39.Paradies Y. A systematic review of empirical research of self-reported racism and health. Journal of Epidemiol. 2006;35:888–901. doi: 10.1093/ije/dyl056. [DOI] [PubMed] [Google Scholar]
- 40.Mills KL, Tamnes CK. Methods and considerations for longitudinal structural brain imaging analysis across development. Dev Cogn Neurosci. 2014;9(172):190. doi: 10.1016/j.dcn.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malat J, Oh HJ, Hamilton MA. Poverty experience, race, and child health. Public Health Rep. 2005;120:442–447. doi: 10.1177/003335490512000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lawson GM, Duda JT, Avants BB, Wu J, Farah MJ. Associations between children's socioeconomic status and prefrontal cortical thickness. Developmental Science. 2013;16:641–652. doi: 10.1111/desc.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noble KG, Houston SM, Kan E, Sowell ER. Neural correlates of socioeconomic status in the developing human brain. Developmental Science. 2012;15:516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Staff RT, Murray AD, Ahearn TS, Mustafa N, Fox HC, Whalley LJ. Childhood socioeconomic status and adult brain size: childhood socioeconomic status influences adult hippocampal size. Ann Neurol. 2012;71:653–660. doi: 10.1002/ana.22631. [DOI] [PubMed] [Google Scholar]
- 45.Tomalski P, Johnson MH. The effects of early life adversity on the adult and developing brain. Curr Opin Psychiatry. 2010;23:233–238. doi: 10.1097/YCO.0b013e3283387a8c. [DOI] [PubMed] [Google Scholar]
- 46.Diez-Roux AV, Nieto FJ, Tyroler HA, Crum LD, Szklo M. Social inequalities and atherosclerosis: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1995;141:960–972. doi: 10.1093/oxfordjournals.aje.a117363. [DOI] [PubMed] [Google Scholar]
- 47.Fuller-Rowell TE, Curtis DS, Doan SN, Coe CL. Racial disparities in the health benefits of educational attainment: a study of inflammatory trajectories among African American and White adults. Psychosom Med. 2015;77:33–40. doi: 10.1097/PSY.0000000000000128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.