Abstract
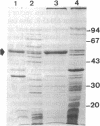
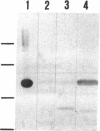
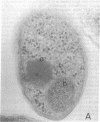
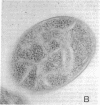
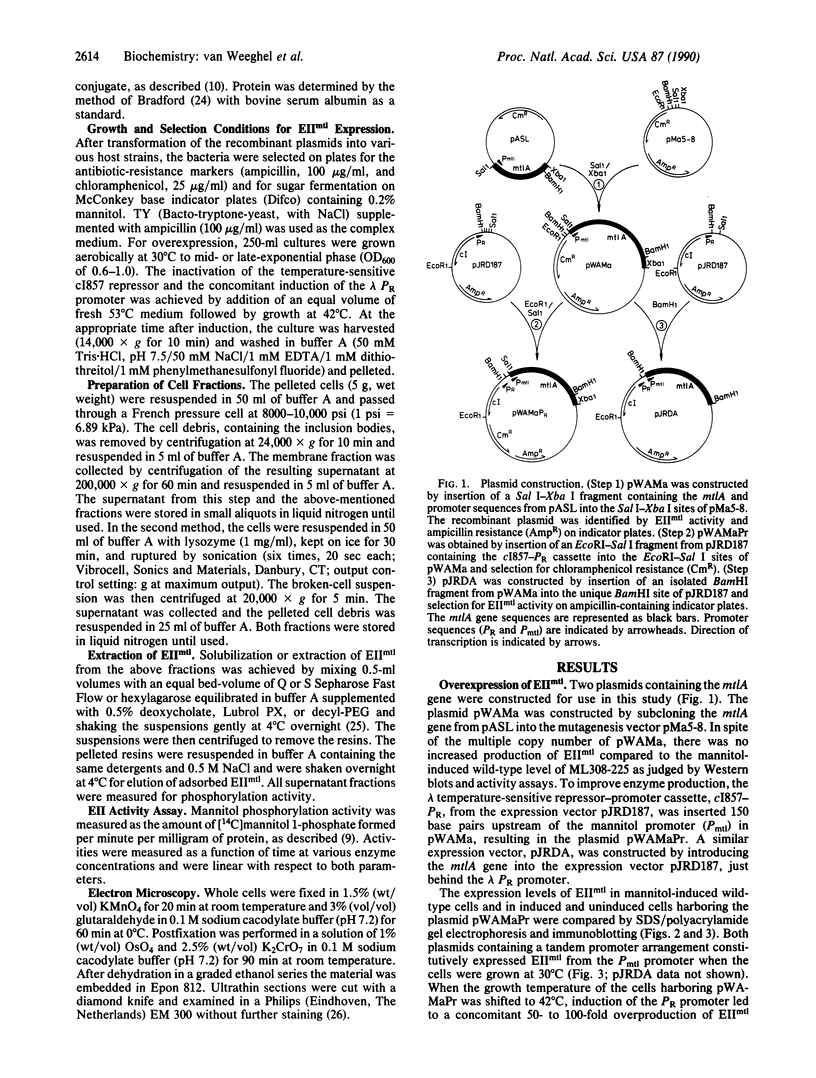
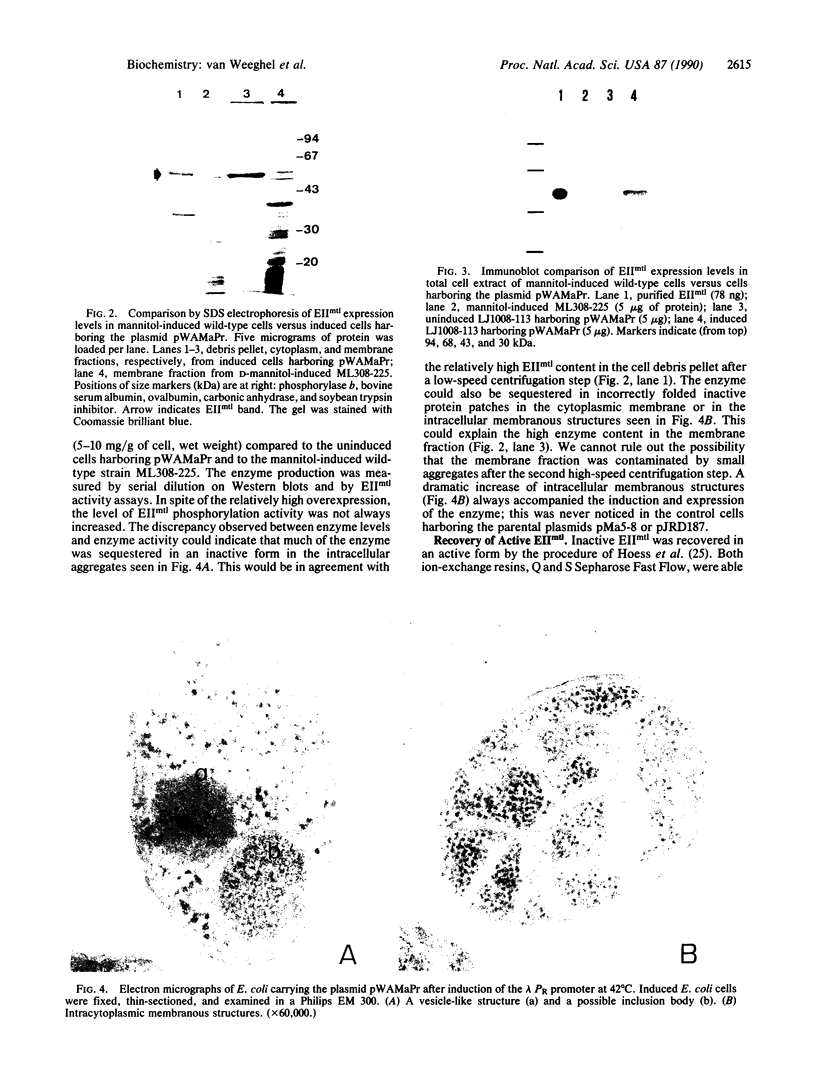
The structural gene (mtlA) of the Escherichia coli phosphoenolpyruvate-dependent mannitol-transport protein (EIImtl) and its upstream promoter region (Pmtl) were subcloned approximately 150 base pairs downstream of a lambda PR promoter on a multicopy mutagenesis/expression vector and used to transform a mutant (MtlA-) E. coli strain. Induction at 42 degrees C led to 50 to 100-fold overproduction of EIImtl (5-10 mg/g of cell wet weight) relative to mannitol-induced levels in a wild-type (Mtl+) strain. Most of the overproduced protein was sequestered as an inactive form in inclusion bodies and cytoplasmic membranous structures. The protein could be extracted in an active form by rupturing the cells with lysozyme and sonication or with a passage through a French pressure cell and incubating the inclusion bodies and membranous structures with detergent (Lubrol PX or deoxycholate) in the presence of Q or S Sepharose ion-exchange resin for several hours. This procedure resulted in a 20- to 25-fold overproduction of active EIImtl compared with mannitol-induced wild-type levels.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bramley H. F., Kornberg H. L. Nucleotide sequence of bglC, the gene specifying enzymeIIbgl of the PEP:sugar phosphotransferase system in Escherichia coli K12, and overexpression of the gene product. J Gen Microbiol. 1987 Mar;133(3):563–573. doi: 10.1099/00221287-133-3-563. [DOI] [PubMed] [Google Scholar]
- Davison J., Heusterspreute M., Chevalier N., Brunel F. A 'phase-shift' fusion system for the regulation of foreign gene expression by lambda repressor in gram-negative bacteria. Gene. 1987;60(2-3):227–235. doi: 10.1016/0378-1119(87)90231-9. [DOI] [PubMed] [Google Scholar]
- Dooijewaard G., Roossien F. F., Robillard G. T. Escherichia coli phosphoenolpyruvate dependent phosphotransferase system. Copurification of HPr and alpha 1-6 glucan. Biochemistry. 1979 Jul 10;18(14):2990–2996. doi: 10.1021/bi00581a013. [DOI] [PubMed] [Google Scholar]
- Grisafi P. L., Scholle A., Sugiyama J., Briggs C., Jacobson G. R., Lengeler J. W. Deletion mutants of the Escherichia coli K-12 mannitol permease: dissection of transport-phosphorylation, phospho-exchange, and mannitol-binding activities. J Bacteriol. 1989 May;171(5):2719–2727. doi: 10.1128/jb.171.5.2719-2727.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson G. R., Lee C. A., Leonard J. E., Saier M. H., Jr Mannitol-specific enzyme II of the bacterial phosphotransferase system. I. Properties of the purified permease. J Biol Chem. 1983 Sep 10;258(17):10748–10756. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. A., Saier M. H., Jr Mannitol-specific enzyme II of the bacterial phosphotransferase system. III. The nucleotide sequence of the permease gene. J Biol Chem. 1983 Sep 10;258(17):10761–10767. [PubMed] [Google Scholar]
- Lee C. A., Saier M. H., Jr Use of cloned mtl genes of Escherichia coli to introduce mtl deletion mutations into the chromosome. J Bacteriol. 1983 Feb;153(2):685–692. doi: 10.1128/jb.153.2.685-692.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler J. Mutations affecting transport of the hexitols D-mannitol, D-glucitol, and galactitol in Escherichia coli K-12: isolation and mapping. J Bacteriol. 1975 Oct;124(1):26–38. doi: 10.1128/jb.124.1.26-38.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J. E., Saier M. H., Jr Mannitol-specific enzyme II of the bacterial phosphotransferase system. II. Reconstitution of vectorial transphosphorylation in phospholipid vesicles. J Biol Chem. 1983 Sep 10;258(17):10757–10760. [PubMed] [Google Scholar]
- Manayan R., Tenn G., Yee H. B., Desai J. D., Yamada M., Saier M. H., Jr Genetic analyses of the mannitol permease of Escherichia coli: isolation and characterization of a transport-deficient mutant which retains phosphorylation activity. J Bacteriol. 1988 Mar;170(3):1290–1296. doi: 10.1128/jb.170.3.1290-1296.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pas H. H., Ellory J. C., Robillard G. T. Bacterial phosphoenolpyruvate-dependent phosphotransferase system: association state of membrane-bound mannitol-specific enzyme II demonstrated by inactivation. Biochemistry. 1987 Oct 20;26(21):6689–6696. doi: 10.1021/bi00395a019. [DOI] [PubMed] [Google Scholar]
- Pas H. H., Robillard G. T. S-phosphocysteine and phosphohistidine are intermediates in the phosphoenolpyruvate-dependent mannitol transport catalyzed by Escherichia coli EIIMtl. Biochemistry. 1988 Aug 9;27(16):5835–5839. doi: 10.1021/bi00416a002. [DOI] [PubMed] [Google Scholar]
- Pas H. H., ten Hoeve-Duurkens R. H., Robillard G. T. Bacterial phosphoenolpyruvate-dependent phosphotransferase system: mannitol-specific EII contains two phosphoryl binding sites per monomer and one high-affinity mannitol binding site per dimer. Biochemistry. 1988 Jul 26;27(15):5520–5525. doi: 10.1021/bi00415a020. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C. A vector that uses phage signals for efficient synthesis of proteins in Escherichia coli. J Mol Appl Genet. 1983;2(1):1–10. [PubMed] [Google Scholar]
- Robillard G. T., Lolkema J. S. Enzymes II of the phosphoenolpyruvate-dependent sugar transport systems: a review of their structure and mechanism of sugar transport. Biochim Biophys Acta. 1988 Oct 11;947(3):493–519. doi: 10.1016/0304-4157(88)90005-6. [DOI] [PubMed] [Google Scholar]
- Roossien F. F., Blaauw M., Robillard G. T. Kinetics and subunit interaction of the mannitol-specific enzyme II of the Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system. Biochemistry. 1984 Oct 9;23(21):4934–4939. doi: 10.1021/bi00316a017. [DOI] [PubMed] [Google Scholar]
- Roossien F. F., Robillard G. T. Mannitol-specific carrier protein from the Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system can be extracted as a dimer from the membrane. Biochemistry. 1984 Nov 20;23(24):5682–5685. doi: 10.1021/bi00319a003. [DOI] [PubMed] [Google Scholar]
- Stanssens P., Opsomer C., McKeown Y. M., Kramer W., Zabeau M., Fritz H. J. Efficient oligonucleotide-directed construction of mutations in expression vectors by the gapped duplex DNA method using alternating selectable markers. Nucleic Acids Res. 1989 Jun 26;17(12):4441–4454. doi: 10.1093/nar/17.12.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan M. M., Jacobson G. R. Membrane disposition of the Escherichia coli mannitol permease: identification of membrane-bound and cytoplasmic domains. Biochemistry. 1986 Dec 16;25(25):8230–8234. doi: 10.1021/bi00373a016. [DOI] [PubMed] [Google Scholar]
- Stephan M. M., Jacobson G. R. Subunit interactions of the Escherichia coli mannitol permease: correlation with enzymic activities. Biochemistry. 1986 Jul 15;25(14):4046–4051. doi: 10.1021/bi00362a009. [DOI] [PubMed] [Google Scholar]
- Veenhuis M., Sulter G., van der Klei I., Harder W. Evidence for functional heterogeneity among microbodies in yeasts. Arch Microbiol. 1989;151(2):105–110. doi: 10.1007/BF00414422. [DOI] [PubMed] [Google Scholar]
- Williams D. C., Van Frank R. M., Muth W. L., Burnett J. P. Cytoplasmic inclusion bodies in Escherichia coli producing biosynthetic human insulin proteins. Science. 1982 Feb 5;215(4533):687–689. doi: 10.1126/science.7036343. [DOI] [PubMed] [Google Scholar]
- von Meyenburg K., Jørgensen B. B., van Deurs B. Physiological and morphological effects of overproduction of membrane-bound ATP synthase in Escherichia coli K-12. EMBO J. 1984 Aug;3(8):1791–1797. doi: 10.1002/j.1460-2075.1984.tb02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]