Abstract
Objective
Understanding individual differences in the psychobiology of the stress response is critical to grasping how psychosocial factors contribute to racial and ethnic health disparities. However, the ways in which environmentally sensitive biological systems coordinate in response to acute stress is not well understood. We employed a social-evaluative stressor task to investigate coordination among the autonomic nervous system (ANS), hypothalamic-pituitary-adrenal (HPA) axis, immune/inflammatory system, and neurotrophic response system in a community sample of 85 healthy African American men and women.
Methods
Six saliva samples – two collected before and four collected during and after the stressor – were assayed for cortisol and dehydroepiandrosterone-sulfate (DHEAs; HPA-axis markers), salivary α amylase (sAA; ANS marker), salivary c-reactive protein (sCRP; inflammatory/immune marker), and salivary nerve growth factor (sNGF; neurotrophic marker). Individual differences in perceived discrimination and racial identity were also measured.
Results
Factor analysis demonstrated that stress systems were largely dissociated before stressor exposure, but became aligned during event and recovery phases into functional biological stress responses (factor loadings .71to.96). Coordinated responses were related to interactions of perceived discrimination and racial identity: when racial identity was strong, high perceived discrimination was associated with low hypothalamic-pituitary-adrenal (HPA) axis arousal at baseline (B’s = .68 to.72, p < .001) and during the task (B’s =.46 to .62, p ≤ .049), and a robust inflammatory response (sCRP) during recovery (B’s =.72 to.94, p ≤ .002).
Conclusion
Culturally-relevant social perceptions are linked to a specific pattern of changing alignment in biological stress responses. Better understanding these links may significantly advance understanding of stress-related illnesses and health disparities.
Keywords: social stress, perceived discrimination, racial identity, multisystem stress response
Psychosocial factors contribute to a broad range of racial and ethnic health disparities, including cardiovascular disease, metabolic illness, and cancer (1–3). These disparities persist even after accounting for structural and economic differences, such as exposure to harmful environments and access to preventative care (4). Although several pathways have been identified (5), the effects of psychosocial factors on health and illness can be especially traced to their influence on biological stress responses known to link social and environmental factors to disease processes (6). The mechanistic capacity of stress underscores that characterizing adaptive biological reactivity and regulation is critical to better understanding how psychosocial factors influence the health and well-being of minority groups and individuals (7, 8).
A number of environmentally sensitive biological systems are implicated in acute stress response, including the autonomic nervous system (ANS), the hypothalamic-pituitary-adrenal (HPA) axis, the immune/inflammatory system, and also the neurotrophic response system (9). These biological systems govern physiological responses that are fundamental to adaptively regulating stress. For example, the ANS controls catecholamines that regulate rapid fight-or-flight behavioral responses, whereas glucocorticoids controlled by the HPA axis guide sustained behavioral responses to stressors (9, 10). In similar fashion, immune and neurotrophic responses control physiological processes that promote biological protection against harmful environmental stressors, including fighting infection and repairing injured tissue (11). Together, these systems contribute to and protect against the physiological wear and tear of allostatic load (5).
Valuable insight into the links between psychosocial factors, biological stress processes, and illness has been achieved by separately examining ANS, HPA, immune, and neurotrophic stress systems. However, recent perspectives emphasize that changes in any one system, studied individually, may offer an incomplete picture of biological stress processes, and that attending to coordinated change across multiple systems may be necessary to fully grasp how psychosocial factors contribute to stress-related illness (12, 13). Enthusiasm for multisystem approaches coincides with technical advances that have enabled simultaneous and non-invasive measurement of multiple stress systems in oral fluids (14–16). In turn, multisystem approaches have gained momentum as emerging research has further revealed the interconnected nature of biological stress systems, including their co-occurring responses to social stressors (17).
Typically, multisystem responses have been operationalized as parallel change in two intra-individual stress response systems, especially including HPA and ANS responses (17). Although assessing dual alignments has proven valuable, the capacity to simultaneously assess inflammatory and neurotrophic stress responses in addition to HPA and ANS responses illuminates a broader potential for multisystem measurement. The next step in this research involves testing dynamic alignment of many biological stress responses, encompassing temporary, functional, and shifting coordination of an array of biological systems across time and in response to stressful episodes. Available literature suggests that attending to changing alignments of stress systems across time is both possible and potentially useful (18, 19). For example, prior research has demonstrated changes in the structure of stress-related illnesses such as post-traumatic stress disorder across time (18), which suggests that underlying biological stress systems may be similarly dynamic. To date however, such an approach has not been applied to multisystem biological stress responses.
Of critical importance, attending to the dynamic nature of stress system alignment may be needed to reveal function-oriented features of temporary alignments. For example, outputs of HPA, ANS, inflammatory, and neurotrophic systems might momentarily synchronize at different times for specific purposes, such as actively mobilizing against a stressor or aiding in post-exposure recovery. Considering dynamic alignment also may be timely given increasing awareness of the multiple adaptive functions that many biological stress responses serve. For example, Dehydroepiandrosterone-sulfate (DHEAs) may be thought of not only as indicating general HPA axis arousal, but also as exerting neuroprotective effects via antiglucocorticoid action (20). Dynamic alignment suggests that DHEAs might functionally align with cortisol early in an acute stress response, but with recovery-oriented biological factors during later phases, including neurotrophic responses that also aid in stress recovery and neuroprotection (21–23).
In parallel to developing methods to operationalize dynamic alignments, there is a specific need to comprehend how biological systems align in ways that ultimately contribute to health disparities. A deeper understanding of dynamic alignment may be especially critical in linking stress responses to psychosocial factors, including individual difference characteristics. For African American men and women, one such individual difference is perceived racial discrimination, or feelings of interpersonal discrimination attributed to one’s race or ethnicity (24). Perceived discrimination is a strong determinant of minority physical and mental health (23), and racial discrimination is recognized as a major contributing factor in African American health disparities (26). Perceived racial discrimination is also implicated in chronically deregulated stress response among African American men and women, including overexpressed HPA, autonomic, and inflammatory arousal (27, 28). Despite known links to basal activity in individual stress systems, the ways in which perceived racial discrimination affects acute stress reactivity are less well understood (29), including whether perceived discrimination contributes to deregulated multisystem alignments.
Another important individual difference is racial identity. Racial identity among African American individuals has been conceptualized as an individual difference in the significance to the self-concept of being Black (30, 31). Being strongly identified as a member of one’s race or ethnicity has been shown to buffer against health-harming racially-based stressors (32, 33), and in this respect, racial identity may confer a health benefit (34). However, strong racial identity may augment attention to discrimination, which some have suggested could be health-harming (35, 36). It is not yet known how racial identity relates to acute stress response, both as a stand-alone influence and in conjunction with perceived discrimination.
This study examines coordination among biological systems at baseline, in response to a social stressor, and during recovery. The alignment of autonomic, adrenocortical, inflammatory, and neurotrophic measures was examined and associations with psychosocial perceptions implicated in stress-related health disparities were evaluated. The present research addressed the following questions:
How do autonomic, adrenocortical, inflammatory, and neurotrophic systems align with one another in response to acute stress?
Do alignments of autonomic, adrenocortical, inflammatory, and neurotrophic systems change across phases of an acute stress response, and do changing alignments reveal functional aspects of stress system coordination?
Are biological system alignments linked to perceived discrimination and racial identity among African-American individuals, and do these individual differences conjointly affect stress reactivity?
Method
Sample and Procedure
Participants were recruited between April, 2011 and May 2013 from the metropolitan Detroit area via advertisements and snowball sampling. After completing a Wayne State University IRB-approved online prescreen to determine eligibility, participants were contacted by phone or email and invited to participate. Individuals were eligible to participate if they were over 18, African American, and if they did not report a pre-existing mental health condition that would prohibit undertaking a mild stress induction, specifically including medically diagnosed anxiety or depression. Individuals were also excluded if they reported poor oral health, any type of endocrine disorder, or if they were using steroid based anti-inflammatory medication or adrenergic agonists or antagonists (i.e., beta blockers).
A sample of 118 African American adults met criteria and enrolled in this research. Participants were excluded from current analyses if any of the five subsequently described biological assay values were missing or out of range (i.e., above or below acceptable detection thresholds for any assay). Missing values resulted when a participant did not provide a sufficient amount of oral fluid to conduct the full assay panel. Listwise deletion based on these parameters resulted in a final sample of 85 participants (64 women and 21 men). All participants received modest financial compensation for participating in a single laboratory session that lasted about three hours. The laboratory protocol was IRB-approved and took place at least one week after completing the prescreen measure.
The Trier Social Stress Test (TSST) was used to induce mild psychosocial stress and associated physiological responses (37). All sessions took place between 11:30 and 13:30 to minimize diurnal influence. Sessions were conducted using two adjacent testing rooms. After providing informed consent, participants were given 10 minutes to acclimate. The remaining TSST protocol was then employed and included a task description phase, a 10-minute speech preparation period, and a 10-minute performance (5-minute speech and 5-minute arithmetic task) given in front of a 2-person panel (one male and one female). Participants remained standing in front of this panel for both tasks. Participants were allotted a 1-hour recovery period following task performance.
Six salivary samples were collected from each participant. An initial sample was collected following the 10-minute acclimation period. The second and third samples were collected immediately before and after the TSST performance. Samples 4 through 6 were collected during the recovery period 15, 30 and 60 minutes after task completion. Participants drank 2.5ml of water upon arrival to the laboratory, as well as after each salivary collection. Participants were asked to refrain from consuming food, caffeine, citric drinks and dairy, to avoid exercise or brushing teeth in the 30 minutes prior to saliva collection, and to report adherence to these guidelines (15). Participants provided 2 mls whole saliva by passive drool at each timepoint (16). Saliva samples were divided into approximately 1 mL aliquots to minimize the impact of freeze-thaw cycles on salivary analyte data. Aliquoted samples were stored at −80 C until shipped frozen overnight to Salimetrics laboratories (State College, PA). Samples were assayed for salivary α amylase (sAA; ANS marker), cortisol and DHEAs (HPA axis markers), salivary C-reactive protein (sCRP; inflammatory/immune marker), and salivary nerve growth factor (sNGF; neurotrophic marker) at each of the six collection timepoints for all participants. The time required to collect 2 mls whole saliva was recorded for each participant in order to correct for flow rate for both sAA and DHEAs.
Measures
Perceived discrimination
Perceived racial discrimination was assessed using the Everyday Discrimination Scale (38). This nine-item measure assesses perceptions of everyday discrimination. Items are rated on a scale ranging from 1 (Almost every day) to 6 (Never). All items were reverse-scored so that higher scores indicated greater perceived discrimination. A total score was calculated by averaging scale items (α = .85).
Racial identity
Racial identity was measured using the eight-item centrality subscale of the Multdimensional Inventory of Black Identity (39). The centrality subscale measures the extent to which race is a core component of self-concept. Responses were rated from 1 (Strongly Agree) to 7 (Strongly Disagree). After reverse-scoring three items, an overall score was calculated by averaging all subscale items, with higher scores indicating stronger racial identity (α =.78).
Biological Stress Measures
Oral fluids were assayed for five biological stress markers. The mean concentration for each marker was within an acceptable range. Moreover, the current concentrations were similar to other stress reactivity studies (17), suggesting that the current iteration of the TSST was comparable.
Alpha-Amylase (sAA)
All samples were assayed for sAA in singlet using commercially available kinetic reaction assays (Salimetrics, State College, PA) without modification to the manufacturers recommended protocols. The assay employs a chromagenic substrate, 2-chloro-4-nitrophenol, linked to maltotriose. The enzymatic action of sAA on this substrate yields 2-chloro-p-nitrophenol, which can be spectrophotometrically measured at 405 nm using a standard laboratory plate reader. The amount of sAA activity present in the sample is directly proportional to the increase (over a 2 minute period) in absorbance at 405 nm. Intra-assay variation (CV) computed for the mean of 30 replicate tests was less than 7.5 percent. Inter-assay variation computed for the mean of average duplicates for 16 separate runs was less than 6 percent. All sAA scores were corrected for salivary flow rate prior to analysis. The mean concentration of sAA across the six collection points was 157.97 U/ml (SD = 159.74).
Cortisol
Saliva samples were assayed for cortisol in duplicate using a highly sensitive enzyme immunoassay (Salimetrics, State College, PA). The test used 25 μL of saliva per determination, has a lower limit of sensitivity of 0.007 μg/dL, standard curve range from 0.012 μg/dL to 3.0 μg/dL, an average intra-assay coefficient of variation of 5.32 percent and an average inter-assay coefficient of variation less than 10 percent. The mean concentration of cortisol across the six collection points was 0.18 μg/dL (SD = 0.12).
Dehydroepiandrosterone-sulfate (DHEAs)
Saliva samples were assayed in duplicate for DHEAs using a highly sensitive enzyme immunoassay (Salimetrics, State College, PA). The test used 100 μl of saliva per determination, has a lower limit of sensitivity of 43 pg/mL, standard curve range from188.9 pg/mL to 15,300 pg/mL, an average intra-assay coefficient of variation of 5.20 percent and an inter-assay coefficient of variation less than 10 percent. DHEAs scores were also corrected for salivary flow rate. The mean concentration of DHEAs across the six collection points was 4058.56 pg/mL (SD = 3622.78).
C-reactive Protein (sCRP)
Samples were assayed for salivary sCRP in duplicate using a highly sensitive enzyme immunoassay (Salimetrics, State College, PA). The test used 50 ìl of a 10× dilution of saliva per determination (15 ìl of saliva), has a lower limit of sensitivity of 10 pg/mL, a standard curve range from 93.75 to 3000 pg/mL, an average intra-assay coefficient of variation of 4.00 percent and an average inter-assay coefficient of variation less than 10 percent. The mean concentration of sCRP across the six collection points was 2282.27 pg/mL (SD = 2779.01).
Nerve Growth Factor (sNGF)
Saliva samples were assayed for NGF in triplicate using a commercially available enzyme immunoassay kit (Promega NGF Emax immunoassay system Cat.# G7631; Madison, WI) modified for use with saliva.1 All saliva samples were diluted 1:4 before testing. The assay standard curve range is 3.9–250 pg/mL, average intra-assay coefficient of variation 12.60 percent, average inter-assay coefficient of variation less than 15 percent. The mean concentration of sNGF across the six collection points was 223.58 pg/mL (SD = 169.54).
Analytic Strategy
Factor analysis was used to explore alignment of biological measures across the six collection timepoints. Standardized scores were first computed at all timepoints. This ensured that biological responses were described by a common metric, and that unique biological response measurement scales did not affect to-be-derived factor structures (Figure 1). Two sets of factor analysis were then conducted on non-log transformed data. First, we empirically derived the reactivity structure of each individual biomarker across the task (i.e., across-timepoint structures). Although we considered these analyses exploratory, structural equation modeling was used because many different across-timepoint factor structures could be specified and formally compared. These analyses were performed using LISREL 8.80 (40). In all instances, the covariance structure was analyzed using maximum likelihood. Because it was assayed in singlet, models for sAA were evaluated using a manifest-level indicator at each timepoint. Accordingly, a six factor model for sAA was fully saturated and fit perfectly. Models for all other biomarkers were specified using latent variables formed from treating the multiple assays at each timepoint as indicators. Degrees of freedom for cortisol, DHEAs and sCRP reflected assays performed in duplicate, whereas degrees of freedom for sNGF reflected that this assay was performed in triplicate. Model fit was assessed using the non-normed fit index (NNFI), the comparative fix index (CFI), and the standardized root mean square residual (SRMR). Acceptable fit was indicated by values approaching or above .90 for the NNFI and CFI, and below .08 for SRMR (41). Importantly, several possible factor structures were directly compared. As a starting point, and informed by literature indicating that biological stress response processes may be characterized by baseline, event, and recovery phases (42), our theoretical model specified a tripartite factor structure in which a baseline factor was indicated by the first and second measurement timepoint variables, an event factor was indicated by the third and fourth measurement timepoint variables, and a recovery factor was indicated by the fifth and sixth measurement timepoint variables (for measurement model see Figure S1, Supplemental Digital Content 1). With the exception of sAA, which was assayed in singlet and thus used manifest level indicators of timepoints, higher-order baseline, event, and recovery factors were specified from lower-order measurement timepoint latent variables. The tripartite model was compared to several alternatives, and a significant chi square difference or change in CFI > .01 indicated a meaningful difference between two models (43). For all biomarkers, the tripartite and alternative model latent variables were always correlated.
Figure 1.
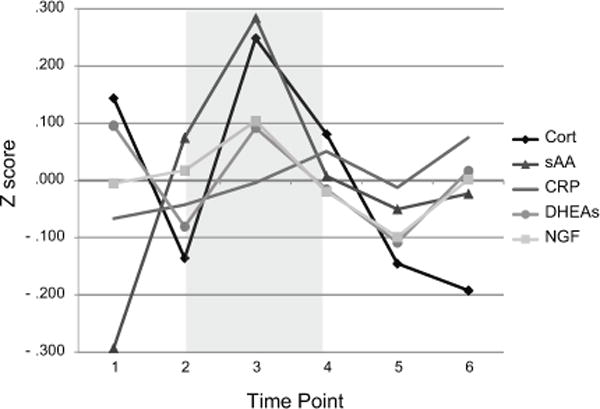
Mean standardized biological response values for study participants across sample timepoints. Grey portion represents stress induction period of task.
Second, we used exploratory factor analysis to functionally characterize coordination among all five biomarkers at each collection timepoint (within-timepoint structures). Due to subsequently described variation in the across-timepoint factor structures, we conducted a separate exploratory factor analysis at each of the six collection timepoints. Based on literature suggesting distinct HPA axis, autonomic, and inflammatory systems (9, 44), we used Varimax rotation to consider uncorrelated three factor solutions, and we examined Pearson correlations among to-be-derived factors to verify their orthogonal structure. Finally, we used hierarchical multiple regression to consider unique and interactive associations of perceived discrimination and racial identity with empirically-derived biological structures at each timepoint.
Results
Demographic characteristics are presented in Table 1 for 85 participants who provided adequate assays of all five analytes needed for the current analysis. Participants ranged in age from 18 to 63 (M = 31.28; SD = 13.60). Information to calculate body mass index was supplied by 18 male (M = 24.47; SD = 3.58) and 50 female participants (M = 25.95; SD = 4.74). Thirty-nine participants were employed, 18 were unemployed, and 28 were students. Thirty-nine participants reported a high school education, while 27 reported some college or technical training, 18 reported a bachelor’s degree or higher, and one participant reported not graduating high school. The median reported annual income was $15,000 to $25,000.
Table 1.
Sample Demographics (N=85).
| Gender | |
| Male | 21 (24.7) |
| Female | 64 (75.3) |
| Age | |
| 18–20 | 19 (22.35) |
| 21–30 | 33 (38.82) |
| 31–40 | 9 (10.59) |
| 41–50 | 10 (11.76) |
| 51–60 | 13 (15.29) |
| Over 60 | 1 (1.18) |
| Missing | 3 (3.53) |
| Income | |
| Less than $15,000 | 31 (36.47) |
| $15,000–$24,999 | 16 (18.82) |
| $25,000–$34,999 | 11 (12.94) |
| $35,000–$49,999 | 9 (10.59) |
| $50,000–$74,999 | 8 (9.41) |
| $75,000–$99,999 | 7 (8.24) |
| $100,000 and above | 2 (2.35) |
| Missing | 1 (1.18) |
| Education | |
| Less than High School | 1 (1.18) |
| High School/GED | 39 (45.88) |
| Some College or Trade School | 27 (31.76) |
| College Graduate | 10 (11.76) |
| Professional/Advanced Degree | 8 (9.41) |
Notes. Percentages parentheses (may add to less than 100 due to rounding).
Across-Timepoint Factor Structures
Table 2 presents means, standard deviations, and bivariate associations of each salivary marker across the task. To evaluate across-timepoint factor structure of each individual biomarker, we specified and compared a single-factor structure to two-factor (event, recovery), three-factor (baseline, event, recovery), four-factor (baseline, transition, event, recovery), and six-factor structures (see Table S1, Supplemental Digital Content 1). For two- and three-factor structures, we assessed two alternative models by considering the potential for some timepoints to load with an alternate underlying factor (e.g., timepoint 4 potentially indicating event or recovery). A three-factor structure provided the best fit for DHEAs (χ2(45, N = 85) = 135.58, p < .001, NNFI = .95, CFI = .96, SRMR = .05) and sNGF (χ2(126, N = 85) = 166.10, p = .009, NNFI = .99, CFI = .99, SRMR = .02). This model comprised baseline (T1,T2), event (T3,T4) and recovery (T5,T6) task phases. This three-factor structure was also supported for cortisol (χ2(45, N = 85) = 91.41, p < .001, NNFI = .92, CFI = .95, SRMR = .06) though alternative six-factor structure fit somewhat comparably (Δχ2(6, N = 85) = 16.05, p< .001, ΔCFI= .01). Likewise, the three-factor structure was plausible for sAA (χ2(6, N = 85) = 34.80, p < .001, NNFI = .87, CFI = .95, SRMR = .03), though a six-factor structure also fit well (Δχ2(6, N = 85) = 34.80, p< .001, ΔCFI= .05). Fit indices and model comparison both suggested that sCRP could be adequately characterized only by a six-factor structure (χ2(39, N = 85) = 384.92, p < .001, NNFI = .81, CFI = .89, SRMR = .01). For all selected models, factor loadings were large and significant at p <.010 or better.
Table 2.
Responses of Biological Measures Across Timepoints Prior to and Following Social Challenge
| M | SD | 1. | 2. | 3. | 4. | 5. | 6. | |
|---|---|---|---|---|---|---|---|---|
| sAA | ||||||||
| 1. Time 1 | 36.444 | 25.935 | – | |||||
| 2. Time 2 | 58.861 | 54.008 | .705*** | – | ||||
| 3. Time 3 | 67.868 | 65.952 | .738*** | .875*** | – | |||
| 4. Time 4 | 52.870 | 55.915 | .782*** | .822*** | .799*** | – | ||
| 5. Time 5 | 50.167 | 57.190 | .738*** | .773*** | .771*** | .930*** | – | |
| 6. Time 6 | 51.994 | 60.836 | .692*** | .624*** | .710*** | .779*** | .886*** | – |
| Cortisol | ||||||||
| 1. Time 1 | .199 | .0128 | 5.200 | |||||
| 2. Time 2 | .165 | 0.105 | .750*** | 5.351 | ||||
| 3. Time 3 | .213 | 0.166 | .341*** | .736*** | 4.591 | |||
| 4. Time 4 | .192 | 0.124 | .350*** | .669*** | .826*** | 6.099 | ||
| 5. Time 5 | .164 | 0.093 | .259*** | .532*** | .536*** | .700*** | 4.537 | |
| 6. Time 6 | .158 | 0.109 | .283*** | .371*** | .231* | .411*** | .759*** | 6.133 |
| DHEAs | ||||||||
| 1. Time 1 | 1452.974 | 1329.762 | 4.778 | |||||
| 2. Time 2 | 1247.333 | 1152.718 | .857*** | 5.512 | ||||
| 3. Time 3 | 1456.000 | 1611.560 | .654*** | .643*** | 5.649 | |||
| 4. Time 4 | 1324.272 | 1245.300 | .792*** | .869*** | .710*** | 5.185 | ||
| 5. Time 5 | 1214.287 | 901.334 | .721*** | .820*** | .583*** | .822*** | 5.346 | |
| 6. Time 6 | 1371.024 | 1034.660 | .664*** | .746*** | .574*** | .766*** | .879*** | 4.691 |
| sCRP | ||||||||
| 1. Time 1 | 2636.667 | 2238.735 | 3.987 | |||||
| 2. Time 2 | 2703.578 | 2586.028 | .813*** | 3.876 | ||||
| 3. Time 3 | 2810.904 | 2536.557 | .789*** | .950*** | 4.320 | |||
| 4. Time 4 | 2963.850 | 3009.568 | .631*** | .634*** | .649*** | 4.080 | ||
| 5. Time 5 | 2787.710 | 3161.209 | .510*** | .438*** | .446*** | .945*** | 4.042 | |
| 6. Time 6 | 3030.887 | 3141.976 | .501*** | .481*** | .438*** | .909*** | .924*** | 3.681 |
| sNGF | ||||||||
| 1. Time 1 | 222.754 | 159.927 | 10.106 | |||||
| 2. Time 2 | 226.531 | 176.757 | .719*** | 13.539 | ||||
| 3. Time 3 | 241.173 | 183.106 | .721*** | .655*** | 13.201 | |||
| 4. Time 4 | 220.258 | 154.167 | .683*** | .693*** | .800*** | 12.726 | ||
| 5. Time 5 | 206.852 | 157.845 | .720*** | .648*** | .832*** | .839*** | 12.726 | |
| 6. Time 6 | 223.898 | 185.439 | .604*** | .548*** | .650*** | .729*** | .806*** | 12.740 |
Notes. Data show means, standard deviations, and bivariate correlations across the tasks. Six salivary samples were obtained: Time 1 = 10 minutes following the acclimation period, Time 2 = immediately before the TSST, Time 3 = immediately after the TSST performance, Time 4 = 15 min into recovery, Time 5 = 30 min into recovery, Time 6 = 60 min into recovery.
sAA=salivary alpha amylase (in U/m), cortisol=salivary cortisol (in μg/dL), DHEAs=dehydroepiandrosterone-sulfate (in pg/mL), sCRP=salivary C-reactive protein (in pg/mL), sNGF=salivary nerve growth factor (in (pg/mL). Values on diagonals are intra-assay coefficients of variation (not available for sAA which was assayed in singlet). sNGF was assayed in triplicate.
p<.001,
p<.05.
Dynamic Alignment: Within-Timepoint Factor Structures
Overall, exploratory factor analysis supported the proposed hypothesis in that factor structures changed across baseline, event, and recovery phases (Table 3). At the first and second timepoints (baseline phase) we extracted an HPA axis factor indicated by cortisol and DHEAs, an autonomic system factor indicated by sAA, and a coordinated inflammatory factor indicated by sNGF and sCRP. At the third and fourth timepoints (event phase), we again derived a coordinated inflammatory factor indicated by sNGF and sCRP. However, cortisol and sAA loaded together during this phase, whereas DHEAs alone indicated a third biological response factor. We functionally characterized the emergent cortisol and sAA factor as an active mobilization stress response. Factor structure once again shifted over timepoints 5 and 6 (recovery phase). Cortisol and sAA continued to indicate an active mobilization factor. However, in contrast to earlier phases, sNGF and DHEAs loaded together during the recovery phase, whereas sCRP alone continued to indicate an inflammation-oriented factor. Based on literature that suggests NGF and DHEAs both may aid in stress recovery and neuroprotection, we characterized the emerging coordinated factor as indicating a recovery-oriented biological response.
Table 3.
Factor Loadings of the Biological Measures for Each of the Timepoints Throughout the Study
| Baseline Phase | Time 1 | Time 2 | ||||
| Factors | 1 | 2 | 3 | 1 | 2 | 3 |
| 1.sAA | .06 | .06 | .97 | .15 | .06 | .97 |
| 2.Cortisol | .85 | .16 | .11 | .81 | .11 | .14 |
| 3.DHEAs | .85 | .13 | .20 | .83 | .16 | .04 |
| 4.sCRP | .11 | .79 | .12 | .02 | .73 | .17 |
| 5.sNGF | .08 | .80 | .21 | .06 | .81 | .04 |
| Event Phase | Time 3 | Time 4 | ||||
| Factors | 1 | 2 | 3 | 1 | 2 | 3 |
| 1.sAA | .01 | .71 | .26 | .08 | .79 | .05 |
| 2.Cortisol | .07 | .83 | .10 | .06 | .80 | .10 |
| 3.DHEAs | .06 | .09 | .95 | .03 | .14 | .96 |
| 4.sCRP | .87 | .16 | .14 | .83 | .13 | .18 |
| 5.sNGF | .85 | .08 | .24 | .82 | .11 | .23 |
| Recovery Phase | Time 5 | Time 6 | ||||
| Factors | 1 | 2 | 3 | 1 | 2 | 3 |
| 1.sAA | .82 | .03 | .10 | .06 | .78 | .06 |
| 2.Cortisol | .82 | .01 | .08 | .10 | .78 | .02 |
| 3.DHEAs | .21 | .76 | .35 | .87 | .15 | .19 |
| 4.sCRP | .02 | .02 | .92 | .03 | .01 | .96 |
| 5.sNGF | .14 | .79 | .34 | .77 | .12 | .38 |
Notes. (N=85)
sAA=alpha amylase, cortisol=salivary cortisol, DHEAs=dehydroepiandrosterone-sulfate, sNGF=salivary nerve growth factor, sCRP=salivary c-reactive protein. Reported values are varimax rotated factor loadings.
Although factor loadings were large (>.70) and no cross-loadings were observed, third factor eigenvalues less than 1.0 at second (0.85), third (0.97), fourth (0.92), and sixth (0.97) timepoints suggested a plausible cortisol-DHEAs-sAA and sCRP-sNGF two-factor structure. We used structural equation modeling to specify and formally compare this alternative factor solution. We also computed bivariate correlations among empirically derived factors to verify that within-timepoint factors were uncorrelated. The derived three-factor solution was parsimonious and fit comparably to the suggested two-factor structure at the second (Δχ2(1, N = 85) = 3.00, p = .083, ΔCFI = .00), (Δχ2(1, N = 85) = 47.13, p< .001, ΔCFI= .00), third (Δχ2(1, N = 85) = 0.47, p= .49, ΔCFI= .00), and fourth timepoints (Δχ2(1, N = 85) = 2.03, p = .15, ΔCFI= .00), and was superior at the sixth timepoint (Δχ2(1, N = 85) = −4.04, p = .044, ΔCFI= −.46). Nonsignificant factor correlations corroborated that within-timepoint structures were orthogonal (r’s = −.06– .12, p’s > .25).
Influence of Racial Identity and Perceived Discrimination on Coordinated Reactivity
Pearson correlations were considered to initially assess links between perceived discrimination, racial identity and each of the five biomarkers (see Tables S2 and S3, Supplemental Digital Content 1). At baseline, perceived discrimination was significantly positively associated with cortisol (r = .269, p = .013) and DHEAs (r = .227, p = .038). Positive associations with cortisol (r = .273, p = .012) and sCRP (r = .276, p = .011) were significant during the event phase, whereas positive associations with DHEAs (r = .229, p = .036) and sCRP (r = .258, p = .018) were significant over the recovery phase. For racial identity, there were significant negative associations with DHEAs (r = −.305, p = .005) at baseline and with DHEAs (r = −.315, p = .003) and sCRP (r = .272, p = .012) at recovery. Racial identity was also related to a positive change in DHEAs across the event (r = .258, p = .017).
To consider links to coordinated multisystem responses, we computed unweighted average linear composites of biological responses derived through exploratory analysis at each timepoint. We then conducted hierarchical multiple regressions to assess whether perceived discrimination and racial identity, which were independent of one another (r = −.04, p = .72), predicted biological response factors (Table 4).
Table 4.
Perceived Discrimination and Racial Identity Predicting Biomarker Factors Across Time
| Baseline Phase | ||||||
| HPA Axis | First Timepoint Inflammatory | ANS | HPA Axis | Second Timepoint Inflammatory | ANS | |
| Cortisol, DHEAs | sCRP, sNGF | sAA | Cortisol, DHEAs | sCRP, sNGF | sAA | |
| Step1 Model r2 | .12*** | .01 | .00 | .12** | .07+ | .01 |
| Perceived Discrimination | . 27* | .09 | .01 | .27* | .26* | .04 |
| Racial Identity | −.21* | .02 | .06 | −.20+ | .00 | −.05 |
| Step 2Model Δ r2 | .10** | .02 | .09 | .08** | .01 | .00 |
| Discrimination x Identity | −.32** | .13 | −.09 | −.28** | .10 | −.06 |
| Event Phase | ||||||
| Mobilization | Third Timepoint Inflammatory | Recovery | Mobilization | Fourth Timepoint Inflammatory | Recovery | |
| Cortisol, sAA | sCRP, sNGF | DHEAs | Cortisol, sAA | sCRP, sNGF | DHEAs | |
| Step1 Model r2 | .03 | .06+ | .01 | .10* | .10** | .07* |
| Perceived Discrimination | .17 | .25* | .07 | .27* | .27* | .18+ |
| Racial Identity | .00 | .01 | −.09 | .02 | .19+ | −.19+ |
| Step 2Model Δ r2 | .01 | .01 | .11** | .00 | .06* | .07** |
| Discrimination x Identity | −.07 | .09 | −.33** | −.03 | .24* | −.27** |
| Recovery Phase | ||||||
| Mobilization | Fifth Timepoint Repair/Recovery | Inflammatory | Mobilization | Sixth Timepoint Repair/Recovery | Inflammatory | |
| Cortisol, sAA | DHEAs, sNGF | sCRP | Cortisol, sAA | DHEAs, sNGF | sCRP | |
| Step1 Model r2 | .02 | .08* | .11** | .01 | .11** | .19*** |
| Perceived Discrimination | .14 | .19+ | .21* | .09 | .27** | .32** |
| Racial Identity | −.02 | −.20+ | .27* | −.03 | −.19+ | .29** |
| Step 2Model Δ r2 | .00 | .03 | .08** | .00 | .03 | .09** |
| Discrimination x Identity | −.04 | −.16 | .29** | −.01 | −.16 | .30** |
(N=85).
Coefficients are standardized regression weights.
p<.001,
p<.01,
p<.05, +p<.10.
At the first and second timepoints, the HPA axis factor was associated positively with perceived discrimination, but negatively with racial identity. These divergent main effects were qualified by a significant discrimination x identity interaction, which we probed by modeling effects of perceived discrimination separately for participants high and low (± 1 SD) in racial identity (45). As seen left in Figure 2, perceived discrimination was not associated with HPA axis arousal among high racial identity participants (BHPAAxis1 = .16, S.E.= .19, p = .40; BHPAAxis2 = .19, S.E. = .19, p = .31). However, perceived discrimination was associated with greater HPA-axis arousal among low racial identity participants (BHPAAxis1 = .72, S.E. = .18 p < .001; BHPAAxis2 = .68, S.E. = .18, p < .001).
Figure 2.

Perceived discrimination and racial identity predicting multisystem stress responses.
At the third and fourth timepoints, perceived discrimination was again positively associated with the sCRP/sNGF inflammatory factor. In addition, perceived discrimination was positively associated with cortisol/sAA mobilization and DHEAs factors at the fourth timepoint only. For racial identity, a moderate positive association with the inflammatory factor and a moderate negative association with the DHEAs recovery factor were observed at the fourth timepoint. In addition to main effects, a significant interaction emerged for the DHEAs recovery factor at both the third and fourth timepoints, and also the aligned sCRP/sNGF inflammatory factor at the fourth timepoint. As seen center in Figure 2, perceived discrimination was not associated with DHEAs among high racial identity participants (BDHEAs3 = −.22, S.E. = .23, p = .35; BDHEAs4 = .06, S.E. = .23, p = .78); however, perceived discrimination was positively associated with DHEAs among low racial identity participants (BDHEAs3 = .46, S.E. = .23, p = .049; BDHEAs4 = .62, S.E. = .23, p = .004). Additionally, perceived discrimination was positively associated with the coordinated sCRP/sNGF stress response among high racial identity participants (BInflammatory4 = .63, S.E. = .19, p < .001), but was not associated with inflammatory response among low racial identity participants (BInflammatory4 = .23, S.E. = .18, p = .21).
At the fifth and sixth timepoints, the emergent NGF/DHEAs recovery factor was positively associated with perceived discrimination, but negatively with racial identity. In parallel, the sCRP inflammatory factor at both timepoints was positively associated with perceived discrimination and racial identity. In addition to main effects, a significant discrimination x identity interaction emerged for the sCRP inflammatory factor at both the fifth and sixth timepoints. As seen to the right in Figure 2, perceived discrimination was positively associated with inflammatory stress response among high racial identity participants (BInflammatory5 = .72, S.E. = .23, p = .002; BInflammatory6 = .94, S.E. = .21, p < .001), but was not associated with inflammatory response among low racial identity participants (BInflammatory5 = .11, S.E. = .22, p = .61; BInflammatory6 = .33, S.E. = .21, p = .12).
To ensure that associations of perceived discrimination and racial identity with coordinated stress reactivity were robust, multiple regressions were also conducted while covarying trait measures of positive and negative affectivity, as well as gender. Perceived discrimination x racial identity interactions were robust to including positive and negative affectivity covariates (all p’s < .050), and no new significant interactions emerged (all p’s > .10). Additionally, the prior notable (p’s < .10) and significant (p’s < .050) main effects of racial identity were also unaffected. Only the main effect of perceived discrimination was slightly diminished by including affectivity covariates. This attenuation was observed for the inflammatory factor at timepoints two through five (βInflammatory2 = .20, p = .079; βInflammatory3 = .20, p = .077; βInflammatory4 = .22, p = .051; βInflammatory5 = .16, p = .15). All discrimination x racial identity interactions were robust to including gender as a covariate (all p’s < .050), and no new significant interactions emerged (all p’s > .10). Additionally, the prior notable (p’s < .10) and significant (p’s < .050) main effects of perceived discrimination and racial identity were also unaffected by gender.
To assess whether reported associations of biological responses with perceived discrimination and racial identity were affected by socioeconomic characteristics, multiple regressions were also repeated while including age, education and income as covariates. The reported results and statistical significance were generally unaffected. One exception was that the perceived discrimination x racial identity time 3 interaction was enhanced for inflammatory response (βDCRP/NGF3 = .21, p = .060). The pattern of this interaction was identical to the pattern reported for the time 4 inflammatory response. In addition, the initially marginal main effect of perceived discrimination for time 5 repair/recovery was attenuated (βDHEAs/NGF5 = .16, p = .14).
This study also included two minor variations to the traditional Trier Social Stress Test (TSST) protocol. One variation led participants to believe that their individual performance during the TSST was judged to be either satisfactory or unsatisfactory by a speech expert. A second variation called for a laboratory assistant to treat participants either politely or slightly impolitely just prior to the post-task recovery portion of the session. These variations were fully crossed and simultaneously implemented ten minutes prior to the fourth salivary collection timepoint. A substantive consideration of these manipulations is provided elsewhere (46). Of current interest, hierarchical multiple regressions were also repeated while controlling for both protocol variations to ensure that links between perceived discrimination, racial identity and coordinated biological responses were robust. On no occasion did we observe a significant main effect of either protocol variation (all p’s > .15). More importantly, perceived discrimination x racial identity interactions were robust to including TSST protocol covariates (all p’s < .050), and no new significant interactions emerged (all p’s > .10). Additionally, prior notable (p’s < .10) and significant (p’s < .050) main effects of perceived discrimination and racial identity were largely unaffected, although the marginal association between racial identity and DHEAs response was further reduced at the fourth timepoint (βDHEAs4 = −.16, p = .16).
Discussion
Building on recent attempts to define multisystem coordination of biological stress systems, we predicted that HPA, ANS, autoimmune, and neurotrophic systems would display dynamic alignment, defined as a temporary, function-oriented, and shifting alignment of system outputs across time in response to social evaluative threat. We also expected that coordinated biological responses would be predicted by perceived racial discrimination and racial identity – two culturally important individual differences implicated in stress regulation processes among African American men and women.
Overall, the across-timepoint structure of individual stress system outputs was largely characterized by a three-factor structure that indicated baseline, event, and recovery phases of biological response, particularly for DHEAs and sNGF. However, evidence also supported a viable six-factor structure for cortisol, sAA, and especially sCRP. This suggests that individual timepoints may characterize a functionally unique biological phase for some biomarkers, while highlighting the potential for differences in response patterns to underlie multisystem alignments. Better recognizing the potential for reactivity patterns to diverge may inform attempts to examine multisystem coordination through this and other methodological approaches. For example, non-identical reactivity patterns could suggest caution is warranted in operationalizing paired reactivity responses as aggregated ratios (47, 48).
The current research took an important step by revealing the dynamic alignment of four unique stress systems. Prior to stressor exposure, biological measures were largely characterized by non-aligned system structures, although we did observe an initially coordinated sCRP and sNGF factor, suggesting a preexisting and cooperative self-protection function of the immune and neurotrophic systems, which is consistent with some available research (49, 50). In response to stress, biological systems further aligned in ways that revealed function-oriented stress regulation processes. Over the event phase, sCRP and sNGF continued to indicate a common factor, whereas cortisol and sAA benchmarked the emergence of an active mobilization coordinated response. Alignment of cortisol and sAA is consistent with prior studies that suggest coordinated ANS and HPA responses may be an especially important facet of adaptive stress regulation (17, 51–53). Alignments observed during the event phase further illuminated a change in the role of DHEAs, which no longer functionally coordinated with cortisol. A temporary dissociation at this phase is consistent with evidence that other biological processes relate in divergent ways to cortisol and DHEAs during stressor exposure. For example, DHEAs is positively associated with testosterone during acute stress response (54), whereas cortisol and testosterone responses may be unrelated or negatively associated (55, 56). Positive associations with testosterone further underscore that DHEAs may be implicated in resiliency-oriented biological reactivity processes, as increased testosterone in response to acute stress has also been linked to sustained competitiveness and dominance (57).
The emergence of a common sNGF and DHEAs factor suggested a new alignment over the recovery phase. This late phase re-alignment is consistent with emerging literature that suggests DHEAs and NGF may work in concert to exert neuroprotective and recovery-oriented functions in an acute stress response. Animal studies suggest that the DHEAs–NGF interface may be initiated when DHEAs binds with transmembrane NGF receptors tyrosine kinase-A (TrkA) and p75 neurotrophin receptor (p75NTR) on target cells to alter the function of proteins governing cell death (22). The current research may support ongoing attempts to isolate similar mechanistic pathways in humans. We also observed that a self-protection and inflammatory response was maintained over the recovery phase, but this facet was indicated only by sCRP at this stage. Attending to late phase acute stress response processes could bolster attempts to link psychosocial factors to isolated immune responses.
Our findings also provide new insight into two culturally important individual difference variables that are highly relevant to stress-related illness among African American individuals. Importantly, these insights came from examining acute stress reactivity–an area that is underdeveloped in ethnic minority stress research (58). Significant main effects observed at every collection timepoint suggested divergent associations of perceived discrimination and racial identity with stress reactivity; higher levels of perceived discrimination generally predicted greater biological arousal, and a stronger racial identity predicted less. However, these main effects were often qualified by interactions between perceived discrimination and racial identity. When racial identity was weak, perceived discrimination was associated with greater HPA (cortisol and DHEAs) activity over the baseline phase of the task, and greater DHEAs response over the event phase. When racial identity was strong, however, perceived discrimination was associated with greater inflammatory response over the recovery phase. Thus, a strong racial identity appeared to confer a stress-buffering effect, which attenuated early overexpression of the HPA response, and which facilitated self-protection inflammatory processes, but only when accompanied by a high level of perceived discrimination. Although a strong racial identity appears to be salutogenic, it should be noted that we do not know what level of biological arousal constitutes a functionally beneficial or adaptive response. For example, inflammatory responses to acute social stress have been characterized as potentially deleterious (59), even though immunoenhancement reduces susceptibility to disease when individuals are exposed to naturalistic stressors (60).
Some limitations suggest a cautious interpretation of our results, as well as directions for future research. First, the correlational nature of the current study does not establish a definitive causal role of perceived discrimination and racial identity in multisystem stress response. This concern is attenuated by prospectively administering individual difference measures prior to stress induction, and by a considerable body of literature that suggests perceived discrimination and racial identity are causally implicated in racial minority stress and well-being (26, 34). Second, this study focused on a single racial group. Although theory and research suggest many universal biological and cognitive stress response adaptations (5, 44, 61, 62), future research will also be needed to address whether the present multisystem alignments occur in other racial or ethnic groups. Multiracial comparison will especially be needed to more fully consider the ways in which stress-related health disparities relate to differences in multisystem alignments. Third, although we selected widely used and well-validated individual difference measures, alternative conceptualizations and measurement of both constructs are available and could reveal additional nuance. Similarly, although our multiple regression results were generally robust to a comprehensive set of covariates, results for perceived discrimination and racial identity could also be influenced by sociodemographic or other individual difference characteristics that were not currently considered.
A final potential limitation concerns measurement of stress responses via oral fluids. One issue is that the dynamic alignments that were presently considered may be conflated with the physiological kinetics of each marker in oral fluids. Related, although oral fluid measurement of cortisol and sAA is well established, the assessment of sCRP and sNGF in oral fluids is newer and less validated. The Promega assay used in this study to estimate sNGF is the current state of the art and was selected because it is the most commonly employed assay to date for measuring NGF in saliva. Our subsequent work with this assay revealed an issue overlooked by prior research—the antibody used in this assay system cross-links with sIgA. We suspect that current results were not driven by this immune system marker, particularly because sNGF and sCRP did not load onto a common factor at either of the recovery phase within-timepoint structures. To fully explore this potential confound, future research will be needed to improve assay strategies and to explore basic questions about the nature and timing of connections across salivary and blood levels of NGF. Until future research is available, we are encouraged in that some initial research suggests that salivary measures of CRP and NGF may effectively indicate a biological stress response of the inflammatory and neurotrophic system, respectively. For example, research has shown that salivary and blood measures are positively associated for both CRP (63) and NGF (64), and other recent studies have successfully linked sCRP and sNGF responses to interpersonal perception (17, 59, 65). However, the measurement and meaning of sCRP and sNGF reactivity are not yet well understood. Moreover, evidence for the effectiveness of measuring sCRP in stress reactivity research continues to be debated (65, 66). Thus, caution should be used when interpreting biological measurements of stress in oral fluids, and results pertaining to sCRP and sNGF are best considered suggestive at this stage.
These limitations notwithstanding, this research suggests that attending to multisystem stress responses may further clarify pathways linking psychosocial factors to stress-related health disparities. This research also illustrates an analytic framework for tracking dynamic and function-oriented alignments of stress systems. Such integrative approaches will be increasingly important as multisystem measurement via oral fluids gains momentum in social and behavioral research. Finally, this research suggests that dynamic alignments can be linked to psychosocial factors (i.e., perceived discrimination and racial identity) specifically relevant to the health of African American men and women.
Supplementary Material
Acknowledgments
We are very thankful to Mercedes Hendrickson, Nathan Weidner, Stefan Goetz, Lenwood Hayman, Edyta Debowska, Kaitlyn Simmonds, Kevin Wynne, and the entire staff of the Clinical Research Center at Wayne State University for assistance with data collection. Finally, we very much appreciate the biotechnical support with the salivary assays provided by Carla Slike, Becky Zavacky, and Jessica Acevedo.
FUNDING/DISCLOSURES
This research was supported by Award Number R21HL097191 from the National Heart, Lung, And Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health.
Glossary
- HPA
hypothalamic-pituitary-adrenal axis
- ANS
autonomic nervous system
- sNGF
salivary nerve growth factor
- sAA
salivary α-amylase
- DHEAs
Dehydroepiandrosterone-sulfate
- sCRP
salivary C-reactive protein
- TSST
Trier Social Stress Test
Footnotes
Douglas A. Granger is Founder and Chief Strategy and Scientific Advisor at Salimetrics LLC (State College, PA) and Salivabio LLC (Baltimore, MD). DAG’s relationships with these entities are managed by the policies of the Conflict of Interest Committee at the Johns Hopkins University and the Office of Research Integrity and Assurance at Arizona State University.
References
- 1.Major B, Mendes WB, Dovidio JF. Intergroup relations and health disparities: A social psychological perspective. Health Psychology. 2013;32:514–24. doi: 10.1037/a0030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penner LA, Albrecht TL, Orom H, Coleman DK, Underwood W., III . Health and health care disparities. London, UK: Sage; 2010. [Google Scholar]
- 3.Williams DR, Jackson PB. Social sources of racial disparities in health. Health Affairs. 2005;24:325–34. doi: 10.1377/hlthaff.24.2.325. [DOI] [PubMed] [Google Scholar]
- 4.Smedley BD, Stith AY, Nelson AR. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care (full printed version) The National Academies Press; 2003. [PubMed] [Google Scholar]
- 5.McEwen BS. Brain on stress: How the social environment gets under the skin. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17180–5. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 7.Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Annual Review of Psychology. 2011;62:501. doi: 10.1146/annurev.psych.031809.130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. Journal of Behavioral Medicine. 2009;32:20–47. doi: 10.1007/s10865-008-9185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiner H. Perturbing the organism: The biology of stressful experience. University of Chicago Press; 1992. [Google Scholar]
- 10.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. JAMA. 1992;267:1244–52. [PubMed] [Google Scholar]
- 11.Levi-Montalcini R, Aloe L, Alleva E. A role for nerve growth factor in nervous, endocrine and immune systems. Prog Neuro Endocrin Immunol. 1990;3:1–10. [Google Scholar]
- 12.Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: Advantages of a multisystem approach. Journal of Developmental & Behavioral Pediatrics. 2002;23:102–13. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Hastings PD, Shirtcliff EA, Klimes-Dougan B, Allison AL, Derose L, Kendziora KT, Usher BA, Zahn-Waxler C. Allostasis and the development of internalizing and externalizing problems: Changing relations with physiological systems across adolescence. Development and Psychopathology. 2011;23:1149. doi: 10.1017/S0954579411000538. [DOI] [PubMed] [Google Scholar]
- 14.Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and alpha-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31:976–87. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Granger DA, Kivlighan KT, El-Sheikh M, Gordis EB, Stroud LR. Salivary α-amylase in biobehavioral research. Annals of the New York Academy of Sciences. 2007;1098:122–44. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- 16.Granger DA, Fortunato CK, Beltzer EK, Virag M, Bright MA, Out D. Focus on Methodology: Salivary bioscience and research on adolescence: An integrated perspective. Journal of Adolescence. 2012;35:1081–95. doi: 10.1016/j.adolescence.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Laurent HK, Powers SI, Granger DA. Refining the multisystem view of the stress response: Coordination among cortisol, alpha-amylase, and subjective stress in response to relationship conflict. Physiology & Behavior. 2013 doi: 10.1016/j.physbeh.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King DW, Orazem RJ, Lauterbach D, King LA, Hebenstreit CL, Shalev AY. Factor structure of posttraumatic stress disorder as measured by the Impact of Event Scale–Revised: Stability across cultures and time. Psychological Trauma: Theory, Research, Practice, and Policy. 2009;1:173. [Google Scholar]
- 19.McArdle JJ, Nesselroade JR. Factor invariance in longitudinal research. In: McArdle JJ, Nesselroade JR, editors. Longitudinal data analysis using structural equation models. Washington D.C: American Psychological Association; 2014. pp. 345–50. [Google Scholar]
- 20.Kimonides V, Spillantini M, Sofroniew M, Fawcett J, Herbert J. Dehydroepiandrosterone antagonizes the neurotoxic effects of corticosterone and translocation of stress-activated protein kinase 3 in hippocampal primary cultures. Neuroscience. 1999;89:429–36. doi: 10.1016/s0306-4522(98)00347-9. [DOI] [PubMed] [Google Scholar]
- 21.Gubba E, Fawcett J, Herbert J. The effects of corticosterone and dehydroepiandrosterone on neurotrophic factor mRNA expression in primary hippocampal and astrocyte cultures. Molecular Brain Research. 2004;127:48–59. doi: 10.1016/j.molbrainres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Lazaridis I, Charalampopoulos I, Alexaki V-I, Avlonitis N, Pediaditakis I, Efstathopoulos P, Calogeropoulou T, Castanas E, Gravanis A. Neurosteroid dehydroepiandrosterone interacts with nerve growth factor (NGF) receptors, preventing neuronal apoptosis. PLoS Biology. 2011;9:e1001051. doi: 10.1371/journal.pbio.1001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor MK. Dehydroepiandrosterone and Dehydroepiandrosterone Sulfate: Anabolic, Neuroprotective, and Neuroexcitatory Properties in Military Men. Military Medicine. 2013;178:100–6. doi: 10.7205/milmed-d-12-00296. [DOI] [PubMed] [Google Scholar]
- 24.Harrell S, Merchant M, Young S. Psychometric properties of the racism and life experiences scales (RaLES) 1997 Unpublished manuscript. [Google Scholar]
- 25.Pascoe EA, Smart Richman L. Perceived discrimination and health: A meta-analytic review. Psychological Bulletin. 2009;135:531–54. doi: 10.1037/a0016059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.A supplement In: Services USDoHaH. Washington, DC: 2001. Mental health: Culture, race, and ethnicity. [PubMed] [Google Scholar]
- 27.Clark R, Anderson NB, Clark VR, Williams DR. Racism as a stressor for African Americans: A biopsychosocial model. American Psychologist. 1999;54:805–16. doi: 10.1037//0003-066x.54.10.805. [DOI] [PubMed] [Google Scholar]
- 28.Brondolo E, ver Halen NB, Pencille M, Beatty D, Contrada RJ. Coping with racism: A selective review of the literature and a theoretical and methodological critique. Journal of Behavioral Medicine. 2009;32:64–88. doi: 10.1007/s10865-008-9193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merritt MM, Bennett GG, Jr, Williams RB, Edwards CL, Sollers JJ., III Perceived racism and cardiovascular reactivity and recovery to personally relevant stress. Health Psychology. 2006;25:364–9. doi: 10.1037/0278-6133.25.3.364. [DOI] [PubMed] [Google Scholar]
- 30.Sellers RM, Shelton JN. The role of racial identity in perceived racial discrimination. Journal of Personality and Social Psychology. 2003;84:1079–92. doi: 10.1037/0022-3514.84.5.1079. [DOI] [PubMed] [Google Scholar]
- 31.Sellers RM, Smith MA, Shelton JN, Rowley SA, Chavous TM. Multidimensional model of racial identity: A reconceptualization of African American racial identity. Personality and Social Psychology Review. 1998;2:18–39. doi: 10.1207/s15327957pspr0201_2. [DOI] [PubMed] [Google Scholar]
- 32.Sellers RM, Copeland-Linder N, Martin PP, Lewis RH. Racial identity matters: The relationship between racial discrimination and psychological functioning in African American adolescents. Journal of Research on Adolescence. 2006;16:187–216. [Google Scholar]
- 33.Wong CA, Eccles JS, Sameroff A. The influence of ethnic discrimination and ethnic identification on African American adolescents’ school and socioemotional adjustment. Journal of Personality. 2003;71:1197–232. doi: 10.1111/1467-6494.7106012. [DOI] [PubMed] [Google Scholar]
- 34.Smith TB, Silva L. Ethnic identity and personal well-being of people of color: a meta-analysis. Journal of Counseling Psychology. 2011;58:42–60. doi: 10.1037/a0021528. [DOI] [PubMed] [Google Scholar]
- 35.Cross WE., Jr . Shades of black: Diversity in African-American identity. Philadelphia: Temple University Press; 1991. [Google Scholar]
- 36.Romero AJ, Roberts RE. Perception of discrimination and ethnocultural variables in a diverse group of adolescents. Journal of Adolescence. 1998;21:641–56. doi: 10.1006/jado.1998.0185. [DOI] [PubMed] [Google Scholar]
- 37.Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’: A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 38.Forman TA, Williams DR, Jackson JS. Race, place, and discrimination. Jai Press; 1997. [Google Scholar]
- 39.Sellers RM, Rowley SA, Chavous TM, Shelton JN, Smith MA. Multidimensional Inventory of Black Identity: A preliminary investigation of reliability and constuct validity. Journal of Personality and Social Psychology. 1997;73:805–15. [Google Scholar]
- 40.Jöreskog KG, Sörbom D. Computer Software Lincolnwood. IL: Scientific Software International, Inc; 2006. LISREL 8.80 for Windows. [Google Scholar]
- 41.Hoyle RH. Structural equation modeling: Concepts, issues, and applications. Sage; 1995. [Google Scholar]
- 42.Glynn LM, Christenfeld N, Gerin W. The role of rumination in recovery from reactivity: Cardiovascular consequences of emotional states. Psychosomatic Medicine. 2002;64:714–26. doi: 10.1097/01.psy.0000031574.42041.23. [DOI] [PubMed] [Google Scholar]
- 43.Cheung GW, Rensvold RB. Testing factorial invariance across groups: A reconceptualization and proposed new method. Journal of Management. 1999;25:1–27. [Google Scholar]
- 44.Cacioppo JT. Social neuroscience: Autonomic, neuroendocrine, and immune responses to stress. Psychophysiology. 1994;31:113–28. doi: 10.1111/j.1469-8986.1994.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 45.Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage; 1991. [Google Scholar]
- 46.Lucas T, Lumley MA, Flack JM, Wegner R, Pierce J, Goetz S. Justice and injustice as worldview verification: The consistency of justice beliefs with externally imposed justice affects perceived racism and stress reactivity in African Americans. Unpublished Manuscript. [Google Scholar]
- 47.Ali N, Pruessner JC. The salivary alpha amylase over cortisol ratio as a marker to assess dysregulations of the stress systems. Physiology & Behavior. 2012;106:65–72. doi: 10.1016/j.physbeh.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Saczawa ME, Graber JA, Brooks-Gunn J, Warren MP. Methodological considerations in use of the cortisol/DHEA (S) ratio in adolescent populations. Psychoneuroendocrinology. 2013;38:2815–9. doi: 10.1016/j.psyneuen.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aloe L, Bracci-Laudiero L, Alleva E, Lambiase A, Micera A, Tirassa P. Emotional stress induced by parachute jumping enhances blood nerve growth factor levels and the distribution of nerve growth factor receptors in lymphocytes. Proceedings of the National Academy of Sciences. 1994;91:10440–4. doi: 10.1073/pnas.91.22.10440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aloe L, Bracci-Laudiero L, Bonini S, Manni L, Aloe L. The expanding role of nerve growth factor: From neurotrophic activity to immunologic diseases. Allergy. 1997;52:883–994. doi: 10.1111/j.1398-9995.1997.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 51.Berry D, Blair C, Willoughby M, Granger DA. Salivary alpha-amylase and cortisol in infancy and toddlerhood: Direct and indirect relations with executive functioning and academic ability in childhood. Psychoneuroendocrinology. 2012;37:1700–11. doi: 10.1016/j.psyneuen.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Gordis EB, Granger DA, Susman EJ, Trickett PK. Salivary alpha amylase–cortisol asymmetry in maltreated youth. Hormones and Behavior. 2008;53:96–103. doi: 10.1016/j.yhbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laurent HK, Ablow JC, Measelle J. Risky shifts: How the timing and course of mothers’ depressive symptoms across the perinatal period shape their own and infant’s stress response profiles. Development and Psychopathology. 2011;23:521. doi: 10.1017/S0954579411000083. [DOI] [PubMed] [Google Scholar]
- 54.Taylor MK, Padilla GA, Stanfill KE, Markham AE, Khosravi JY, Dial Ward MD, Koehler MM. Effects of dehydroepiandrosterone supplementation during stressful military training: A randomized, controlled, double-blind field study. Stress. 2012;15:85–96. doi: 10.3109/10253890.2011.585189. [DOI] [PubMed] [Google Scholar]
- 55.Filaire E, Bernain X, Sagnol M, Lac G. Preliminary results on mood state, salivary testosterone: Cortisol ratio and team performance in a professional soccer team. European Journal of Applied Physiology. 2001;86:179–84. doi: 10.1007/s004210100512. [DOI] [PubMed] [Google Scholar]
- 56.Hoogeveen AR, Zonderland ML. Relationships between testosterone, cortisol and performance in professional cyclists. Int J Sports Med. 1996;17:423–8. doi: 10.1055/s-2007-972872. [DOI] [PubMed] [Google Scholar]
- 57.Carré J, Campbell J, Lozoya E, Goetz S, Welker K. Changes in testosterone mediate the effect of winning on subsequent aggression. Psychoneuroendocrinology. 2013;38:2034–41. doi: 10.1016/j.psyneuen.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 58.Keenan K, Gunthorpe D, Young D. Patterns of cortisol reactivity in African-American neonates from low-income environments. Developmental Psychobiology. 2002;41:265–76. doi: 10.1002/dev.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chiang JJ, Eisenberger NI, Seeman TE, Taylor SE. Negative and competitive social interactions are related to heightened proinflammatory cytokine activity. Proceedings of the National Academy of Sciences. 2012;109:1878–82. doi: 10.1073/pnas.1120972109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen S, Hamrick N. Stable individual differences in physiological response to stressors: Implications for stress-elicited changes in immune related health. Brain, Behavior, and Immunity. 2003;17:407–14. doi: 10.1016/s0889-1591(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 61.Lazarus RS. Psychological stress and the coping process. New York: McGraw-Hill; 1966. [Google Scholar]
- 62.Selye H. The physiology and pathology of exposure to stress. Oxford, England: Acta, Inc; 1950. [Google Scholar]
- 63.Out D, Hall RJ, Granger DA, Page GG, Woods SJ. Assessing salivary C-reactive protein: longitudinal associations with systemic inflammation and cardiovascular disease risk in women exposed to intimate partner violence. Brain, Behavior, and Immunity. 2012;26:543–51. doi: 10.1016/j.bbi.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aloe L, Alleva E, Böhm A, Levi-Montalcini R. Aggressive behavior induces release of nerve growth factor from mouse salivary gland into the bloodstream. Proceedings of the National Academy of Sciences. 1986;83:6184–7. doi: 10.1073/pnas.83.16.6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain, Behavior, and Immunity. 2007;21:901–12. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 66.Slavish DC, Graham-Engeland JE, Smyth JM, Engeland CG. Salivary markers of inflammation in response to acute stress. Brain, Behavior, and Immunity. 2014 doi: 10.1016/j.bbi.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


