Abstract
We investigated the association between five factor model personality traits (neuroticism, extraversion, openness, agreeableness, and conscientiousness) and risk of dementia, cognitive impairment not dementia (CIND), and conversion from CIND to dementia in a large national cohort. Participants from the Health and Retirement Study (N>10,000) completed a personality scale in 2006–2008 and their cognitive status was tracked for up to 8 years using the modified Telephone Interview for Cognitive Status (TICSm). Adjusting for age, sex, education, race, and ethnicity, lower conscientiousness and agreeableness and higher neuroticism were independently associated with increased risk of dementia. These associations remained significant after adjusting for other risk factors for dementia, including income, wealth, smoking, physical inactivity, obesity, diabetes, hypertension, and blood biomarkers. These associations were not modified by age, sex, race, ethnicity, and education, suggesting that the associations of personality with risk of dementia were similar across demographic groups. Neuroticism and conscientiousness were also associated with risk of CIND. Low conscientiousness predicted conversion from CIND to dementia. Using brief assessments of personality and cognition, we found robust evidence that personality is associated with risk of cognitive impairment and dementia in a large national sample.
Keywords: personality, dementia, cognitive impairment, prospective study
Introduction
Dementia is a leading cause of disability and death among older adults, and it is associated with considerable burden in the US and other world regions. A broad range of clinical and psychosocial factors, including personality traits (Terracciano et al., 2014), may modulate dementia risk (Prince et al., 2014). Personality traits are relevant risk factors for dementia because of their widespread impact on individuals’ behaviors, lifestyle, and health (Friedman, 2000; Hampson, 2012; McCrae and John, 1992). Research on personality can advance knowledge on the etiology of dementia and aid in prevention and risk-reduction efforts. Of the five major dimensions of personality (McCrae and John, 1992), low conscientiousness and high neuroticism have had the most consistent associations with increased risk of incident dementia (Duberstein et al., 2011; Johansson et al., 2014; Terracciano et al., 2014; Wilson et al., 2005; Wilson et al., 2007). The evidence is more mixed for the other traits, but a meta-analysis suggested that scoring high on agreeableness and openness may also reduce dementia risk (Terracciano et al., 2014). Most studies to date have relied on well-characterized samples, with high quality personality measures, and in-depth clinical evaluations to ascertain dementia diagnosis. However, these studies were based on relatively small and selective samples. The primary objective of this study was to examine the association between personality traits and risk of incident dementia in a larger national sample that assessed personality and cognition with brief measures. Further, we examined the association of personality traits with incident cognitive impairment not dementia (CIND), and conversion from CIND to dementia to gain knowledge on the role of personality traits in people at higher risk of dementia.
Methods
Participants
We used data from the Health and Retirement Study (HRS), a large population-based, longitudinal study of Americans aged 50 years or older. The University of Michigan Institutional Review Board approved the HRS research protocol and the informed consent procedure, and the data are publicly available at http://hrsonline.isr.umich.edu. The investigation was carried out in accordance with the latest version of the Declaration of Helsinki. Cognition was assessed every two years, and data was available up to 2014. Personality traits were first assessed in 2006 as part of a psychosocial and lifestyle questionnaire in a random half of the HRS participants; the other half completed the personality questionnaire in 2008. Personality at baseline was based on the combined 2006–2008 data. Among those who received the psychosocial questionnaire, the response rate was 90%–89% in 2006–2008. Among those who completed the questionnaire, 97%–98% answered the personality items (http://hrsonline.isr.umich.edu/sitedocs/userg/HRS2006-2010SAQdoc.pdf).
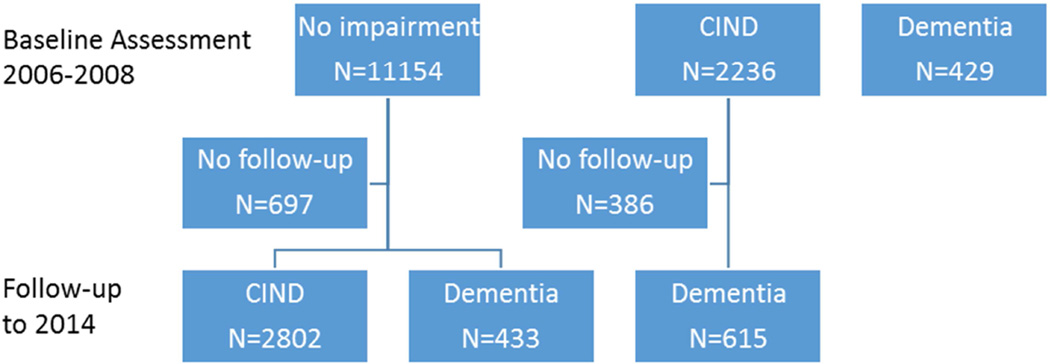
Of the 13,882 individuals with both cognitive and personality assessments at baseline, 492 (3.5%) scored in the dementia range, 2,236 (16.1%) in the CIND range, and 11,154 (80.3%) in the normal range (Figure 1). Of the 11,154 individuals with normal cognition at baseline, 697 were excluded because of lack of follow-up assessments (66% died before the first follow-up and the rest did not respond to contact requests or refused to participate at follow-up). Compared to the 10,457 with follow-up data, the 697 without follow-up data were older, more likely to be male, Hispanic, had lower education, and scored lower on all personality traits (p < 0.05) except for neuroticism (p > 0.05). The primary analyses focused on the 10,457 participants with normal cognition at baseline and follow-up data. The normal to CIND analysis included 10,265 individuals with normal cognition at baseline and follow-up data, but we excluded those who developed dementia before CIND (N=192) (i.e., we excluded those who transitioned directly from normal to dementia range, but included those who transitioned from normal to CIND, whether or not they developed dementia at a later wave). For the CIND to dementia conversion analysis, we examined whether individuals with cognitive scores in the CIND range at baseline converted to dementia status at follow-up. Of the 2,236 individuals who were classified as CIND at baseline, 1,850 had follow-up assessments.
Figure 1.
Classification of the study participants at baseline and incident cognitive impairment not dementia (CIND) and dementia at follow-up.
Assessments
Personality
Personality traits were assessed with the Midlife Development Inventory (MIDI) (Lachman et al., 1997) a brief measure of neuroticism, extraversion, openness, agreeableness, and conscientiousness. Respondents used a Likert scale (1=not at all; to 4=a lot) to rate how much 26 adjectives describe themselves (see adjectives in Table S1). Raw scores were transformed into z-scores (Mean=0, SD=1). The MIDI is a self-report measure that was completed on paper as part of a psychosocial questionnaire.
Cognitive status
Consistent with other HRS-based studies (Clark et al., 2013; Crimmins et al., 2011; Davydow et al., 2015; Saczynski et al., 2015), we classified individual’s cognitive status based on the modified Telephone Interview for Cognitive Status (TICSm), which was administered at baseline and at every 2-year follow-up. The TICSm included measures of short-term memory, working memory, attention, and processing speed. A 27-point composite score was computed from performance on three tests: immediate and delayed recall of 10 nouns (0 to 20 points), serial 7 subtraction (0 to 5 points), and backward counting (0 to 2 points). Individuals who scored 6 or less were classified in the dementia group, those who scored 7 to 11 were classified in the CIND group, and those who scored 12 to 27 were classified in the normal cognitive group (Crimmins et al., 2011). These cut-offs have been validated against a diagnosis based on DSM-III-R criteria made by a consensus expert panel that evaluated a detailed neuropsychological and clinical assessment in a subsample of the HRS, the Aging, Demographics, and Memory Study (ADAMS). Using TICSm to predict ADAMS diagnosis, it was possible to correctly classify 74% of the individuals who completed the HRS survey (Crimmins et al., 2011).
Covariates
Age (in years), sex, race (African-American vs. other and White; other vs African-American and White), ethnicity (Hispanic vs Not Hispanic), and educational level (in years) were included as demographic covariates in all analyses. In follow-up analyses, we also controlled for baseline self-reported measures of physician-diagnosed diabetes and hypertension (yes/no), obesity (body mass index >=30), current smoking (yes/no), and moderate physical activity (Question: How often do you take part in sports or activities that are moderately energetic such as, gardening, cleaning the car, walking at a moderate pace, dancing, floor or stretching exercises? Response: 1=more than once a week, to 4=hardly ever or never) because these factors have been related to dementia risk (Baumgart et al., 2015). Financial wealth and household income at baseline were also included as covariates given the role of socioeconomic disparities on the risk of dementia (Yaffe et al., 2013). We also controlled for cardiovascular, inflammation, and metabolic biomarkers: Total cholesterol, High-Density Lipoprotein (HDL), Cystatin C, C-Reactive Protein (CRP), and Hemoglobin A1c. The assessment of these biomarkers from a dried blood spot has been described in detail elsewhere: http://hrsonline.isr.umich.edu/sitedocs/userg/Biomarker2006and2008.pdf.
Statistical analyses
We present descriptive statistics as means and SD or proportions for the demographic variables at baseline. Multivariate analyses of variance, controlling for demographic variables, were used to compare the personality profile of the normal, CIND, and dementia groups at baseline. We focused on measures of effect size, such as Cohen’s d (d=(M1–M2)/SD). For the prospective analysis, we used separate Cox regression models with the five personality traits assessed in 2006–2008 as main predictors of incident dementia in the follow-up waves (2008 to 2014 for the 2006 cohort and 2010 to 2014 for the 2008 cohort). Time was coded in years, starting from the year of personality assessment. Cases were censored at the last available cognitive assessment at which the participant did not score in the dementia range. The proportional hazard assumption was met. All analyses controlled for demographic variables linked to the risk of dementia (age, sex, race, ethnicity, and education). Follow-up analyses were further adjusted for household income, wealth, current smoking, physical activity, obesity, diabetes, and hypertension. In a subsample with available biomarkers, we further included total cholesterol, HDL, Cystatin C, CRP, and Hemoglobin A1c along with the covariates listed above.
In a set of sensitivity analyses, we explored the robustness of our main models. We tested all five factors included in the analyses simultaneously to examine the extent to which a significant association of any trait was independent from the others. We repeated the analyses by excluding participants younger than 65 at baseline, who were less likely to develop dementia over the 6 to 8 year follow-up. Consistent with past research, we also examined whether there was an interaction between neuroticism and conscientiousness (Terracciano et al., 2014; Wilson et al., 2007) and between neuroticism and extraversion (Johansson et al., 2014), entering the interaction terms in the models as appropriate. Further, because a previous study suggested that the association between personality traits and dementia risk may be weaker in African-Americans (Wilson et al., 2005), we examined whether the association was significantly different across race and ethnicity by testing interaction terms. Similarly, we tested the interactions of personality traits with age, sex, and education (Duberstein et al., 2011; Skogen et al., 2015; Terracciano et al., 2014; Wilson et al., 2005; Wilson et al., 2007).
The same model specifications were used to test the associations between personality traits and risk of developing CIND, and the risk of converting from CIND to dementia. The IBM-SPSS statistical software was used for the analyses.
Results
Baseline demographic characteristics for the entire sample and for the cognitive status groups are presented in Table 1. Individuals classified into CIND and dementia groups were more likely to be older, African-American, other race, Hispanic, and to have fewer years of education (p < 0.001). The internal consistency coefficients of the personality scales were slightly lower in the impaired groups, but the coefficients were acceptable in all three groups (Table 1). Figure 2 presents baseline personality mean scores adjusted for the demographic variables. Compared to the group with normal cognition, individuals classified in the dementia group were more likely to score higher on neuroticism (d = 0.20) and lower on extraversion (d = 0.19), openness (d = 0.17), agreeableness (d = 0.27), and conscientiousness (d = 0.46) (p < 0.05). . The CIND group had a personality profile similar to the dementia group. Except for scoring the highest on neuroticism, the CIND group scored in the middle between normal and dementia groups.
Table 1.
Demographic characteristics and internal consistency of the personality scales of the full sample and by cognitive status at baseline.
| Normal | CIND | Dementia | Total | |
|---|---|---|---|---|
| N | 11,154 | 2,236 | 429 | 13,882 |
| Age (years), mean (SD) | 67.52 (9.44) | 73.23 (10.28) | 76.17 (10.41) | 68.74 (9.94) |
| Education (years), mean (SD) | 13.16 (2.67) | 10.71 (3.47) | 8.97 (3.96) | 12.62 (3.08) |
| Female, N (%) | 6,656 (60%) | 1,275 (57%) | 300 (61%) | 8,231 (59%) |
| Hispanic, N (%) | 725 (6%) | 305 (14%) | 77 (16%) | 1,107 (8%) |
| African-American, N (%) | 1,092 (10%) | 544 (24%) | 149 (30%) | 1,785 (13%) |
| Other race | 258 (2%) | 78 (4%) | 26 (5%) | 362 (3%) |
| White | 9,804 (88%) | 1,614 (72%) | 317 (64%) | 11,735(85%) |
| Internal Consistency (Cronbach α) | ||||
| Neuroticism | 0.73 | 0.65 | 0.67 | 0.71 |
| Extraversion | 0.75 | 0.71 | 0.72 | 0.74 |
| Openness | 0.78 | 0.77 | 0.80 | 0.79 |
| Agreeableness | 0.78 | 0.76 | 0.80 | 0.78 |
| Conscientiousness | 0.66 | 0.61 | 0.64 | 0.66 |
Notes: In parentheses are SD for age and education, % for sex, ethnicity, and race. CIND = Cognitive impairment not dementia.
Figure 2.
Personality scores by cognitive status.
Notes: n = 13,882 total sample; n = 11,154 normal, n = 2,236 cognitive impairment not dementia (CIND), n = 492 dementia at baseline. The personality scores were z-transformed, so for the total sample the Mean=0 and SD=1. The bars represent estimated marginal means from models that accounted for demographic variables. Error bars are SE.
Personality and incident dementia
At baseline, the prospective sample (N=10,457) was aged 50 to 98 years (Mean=67.17, SD=9.23), had mean of 13.19 years of education (SD=2.66), and was 60% female, 7% Hispanic, and 10% African-American. Follow-up time ranged from 2 to 8 years, with a mean of 6.29 years (SD=1.78), and a total of 65,790 person-years. During the follow-up period, 433 (4.1%) had test scores that fell below the dementia threshold, on average five years after the baseline assessment (Mean=4.99; SD=2.05).
In Cox regression analyses adjusted for demographic variables (age, sex, education, race, and ethnicity), scoring 1 SD higher on neuroticism, lower on agreeableness, and lower on conscientiousness was associated with about a 20% higher risk of incident dementia (Table 2). In regression models that included all five traits simultaneously, neuroticism (HR=1.16, 95%CI=1.05–1.29), agreeableness (HR=0.80, 95%CI=0.70–0.90), and conscientiousness (HR=0.82, 95%CI=0.73–0.91) remained significant (p < 0.005), indicating that these effects were independent. In regression models that further adjusted for household income, wealth, smoking, physical activity, obesity, diabetes, and hypertension, the effect of neuroticism (HR=1.16, 95%CI=1.05–1.27), agreeableness (HR=0.83, 95%CI=0.75–0.91), and conscientiousness (HR=0.82, 95%CI=0.74–0.90) were mostly unchanged. In a subsample with available biomarkers (N=6,989), adding total cholesterol, HDL, Cystatin C, CRP, and Hemoglobin A1c to all other covariates did not change the results: neuroticism (HR=1.23, 95%CI=1.09–1.38), agreeableness (HR=0.81, 95%CI=0.72–0.91), and conscientiousness (HR=0.81, 95%CI=0.72–0.91). Similar results were obtained when we excluded participants younger than 65 years old at baseline from the analyses: neuroticism (HR=1.18, 95%CI=1.06–1.31), agreeableness (HR=0.79, 95%CI=0.72–0.89), and conscientiousness (HR=0.81, 95%CI=0.73–0.90).
Table 2.
Personality and risk of incident dementia, and cognitive impairment.
| Risk of dementia in those with normal cognition at baseline |
Risk of CIND in those with normal cognition at baseline |
Risk of progression to dementia in those with CIND at baseline |
|
|---|---|---|---|
| Baseline sample | 10,457 | 10,265 | 1,850 |
| Incident cases | 433 | 2,802 | 615 |
| Trait | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| Neuroticism | 1.18 (1.07 – 1.30)* | 1.14 (1.10 – 1.19)* | 1.07 (.99 – 1.17) |
| Extraversion | .98 (.89 – 1.09) | 1.00 (.96 – 1.04) | .97 (.89 – 1.04) |
| Openness | .94 (.86 – 1.04) | .97 (.93 – 1.00) | .95 (.88 – 1.02) |
| Agreeableness | .83 (.75 –.91)* | .98 (.94 –1.02) | .96 (.90 – 1.04) |
| Conscientiousness | .80 (.73 –.88)* | .88 (.85 –.92)* | .92 (.86 – .99)* |
Notes: HR = Hazard ratio; CI = Confidence interval; CIND = Cognitive impairment not dementia. Cox regression analyses controlling for age, sex, education, race, and ethnicity.
indicates p < .05
Interactions between personality and age were not significant (p > 0.10), except for openness (p = 0.02): the association between openness with dementia risk was slightly weaker among older individuals. There were no significant interactions between personality traits and either race or ethnicity (p ≥ 0.10), which suggested that the associations in African-Americans and Hispanics were not significantly different from the associations observed in the rest of the sample. We found no interactions between each of the five personality traits and sex or education (p ≥ 0.10), which suggested that the associations were not significantly different in men and women or across the educational spectrum. Furthermore, we found no significant interactions between neuroticism and conscientiousness or between neuroticism and extraversion (p > 0.10).
As reported in Table 2, controlling for demographic covariates, we found that high neuroticism and low conscientiousness were associated with greater risk of incident CIND (N=2,802; 27%). We also examined whether personality traits were significant predictors of conversion from CIND to dementia. The sample included 1,850 individuals with CIND at baseline, of whom 615 (33%) developed dementia. Controlling for demographics, we found that lower conscientiousness was a significant predictor of conversion to dementia. The other traits were not associated with risk of conversion, but there was a non-significant trend (p = 0.07) in the expected direction for neuroticism.
Discussion
The results indicate that low conscientiousness, high neuroticism, and low agreeableness are significantly, and independently, related with higher risk of incident dementia. For each trait, a difference of 1 SD was associated with about 20% difference in risk of dementia, even after accounting for demographics, socioeconomic status, health behaviors, and clinical risk factors. This study extends the research on personality and dementia by examining one of the most representative and largest samples to date, larger than the combined sample of a previous meta-analysis (Terracciano et al., 2014).
The main analyses indicate that personality is associated with risk of dementia independent of potential demographic (e.g., age, sex) confounders. The follow-up analyses indicate that personality is still associated with dementia risk after accounting for a number of factors that could also be mediators of the association. That is, personality traits are associated with sedentary lifestyle, cigarette smoking, and other behavioral, cardiovascular, and metabolic risk factors for dementia that could mediate the association between personality and dementia (Friedman, 2000; Hampson, 2012; Jokela et al., 2014; Sutin et al., 2011; Sutin et al., 2016; Terracciano and Costa, 2004). The results suggest, however, that the links between personality and dementia are not fully accounted by these behavioral and clinical characteristics. Similar findings have emerged in studies of personality as predictors of the rate of cognitive decline in older adults (Luchetti et al., 2016). Personality may modulate dementia risk through other pathways. Personality traits are associated with inflammatory markers (Luchetti et al., 2014) that may contribute to the underlying dementia neuropathology (Heneka et al., 2015). Neuroticism and conscientiousness may be directly related to brain structure and markers of neurodegeneration (Jackson et al., 2011; Terracciano et al., 2011). In a population-based cohort of over 500 older adults, for example, low conscientiousness was associated with measures of whole brain integrity, including brain-tissue loss and white matter hyperintensities (Booth et al., 2014). In samples with mild cognitive impairment, lower levels of conscientiousness and higher levels of neuroticism have been associated with white matter lesions (Duron et al., 2014). A prospective study with an autopsy component (Terracciano et al., 2013) found that higher neuroticism and lower agreeableness were significantly associated with advanced neurofibrillary tangles stages, one hallmark of Alzheimer’s neuropathology. Other studies have found the level of tangle formations to be associated with depression (Rapp et al., 2008) and aggressive behaviors (Lai et al., 2010), although not all have found an association between personality and neuropathology (Wilson et al., 2007).
The large HRS sample provided a powerful test of whether demographic variables, including age, sex, race, ethnicity, and education, moderate the association between personality and risk of dementia. Previous studies have reported mixed evidence on the interactions between neuroticism and race (Wilson et al., 2005) or age (Skogen et al., 2015) in predicting dementia. In the HRS sample these interactions were not statistically significant, suggesting that the associations of personality and dementia risk may be similar across diverse demographic groups. Of interest, education level was high in some previous samples (Terracciano et al., 2014; Wilson et al., 2007), but education did not modify the association between personality and dementia in the HRS.
This study examined the rarely addressed question of whether personality traits are associated with risk of incident CIND and conversion from CIND to dementia. Mild forms of cognitive impairment are considered a pre-morbid stage of dementia, but there is considerable variation in the timing to conversion, and not all individuals with cognitive impairment progress to develop dementia (Ritchie et al., 2001). We found that neuroticism and conscientiousness were significant predictors of the risk of developing CIND, consistent with a previous study (Wilson et al., 2007). Among those with CIND at baseline, we found that conscientiousness was a significant predictor of conversion to dementia, but there was only a non-significant trend for neuroticism. These HRS findings are consistent with a European study of 215 individuals with mild cognitive impairment (Ramakers et al., 2015). The study found that high scores on rigidity, a scale related to conscientiousness, were associated with a lower risk of conversion to dementia. Thus, even among individuals with compromised cognition, self-reported conscientiousness predicts conversion to dementia. The effect size observed in the conversion analyses (CIND to dementia) was substantially smaller than the effect observed in the primary analysis (normal to dementia). The smaller effect might be due to more measurement error in the individuals with compromised cognition (but see Table 1). However, this finding is contrary to expectations from the reverse causality hypothesis. Assuming that personality is changed by the underlying neuropathology in the prodromal phase of the disease, we would expect the association of personality and risk of dementia to be stronger among individuals with CIND. For example, based on the reverse causality hypothesis, neuroticism would be expected to increase in those progressing toward the onset of dementia. However, we found a weaker and non-significant association between neuroticism and risk of progression from CIND to dementia.
Compared to the normal group, individuals with dementia had higher neuroticism and lower scores on the other traits at baseline (Figure 2). The observed pattern was consistent with other studies that relied on self-reports (Duchek et al., 2007) or structured interviews (Pocnet et al., 2011), but the magnitude of the difference was substantially smaller than the differences found with observer ratings (Pocnet et al., 2011; Rankin et al., 2005). The neurodegenerative disorder may impair the capacity to update self-image, and it has been argued that individuals with dementia, to the extent that their memory function is sufficiently preserved to be assessed, may report on their former personality rather than their current personality traits (Rankin et al., 2005). As such, the relatively smaller differences with self-reports might be due to anosognosia and relatively positive self-appraisals in those with dementia (Orfei et al., 2010). However, it is also possible that observer ratings may be biased by labelling effects, such as knowledge of the disease status and associated symptoms.
The limitations of this study included the brief measures of personality and cognitive status. In particular, dementia ascertainment relied on brief cognitive tests instead of the clinical and neuropsychological evaluations used in previous studies (Terracciano et al., 2014; Wilson et al., 2007). However, the validity of these brief assessments of personality and cognitive status had been previously demonstrated (Clark et al., 2013; Crimmins et al., 2011; Davydow et al., 2015; Lachman et al., 1997; Luchetti et al., 2014; Saczynski et al., 2015; Stephan et al., 2016; Sutin et al., 2016), and the magnitude and direction of the associations observed in the HRS were consistent with results of studies that used more in-depth measures of personality and cognitive status (Duberstein et al., 2011; Johansson et al., 2014; Terracciano et al., 2014; Wilson et al., 2005; Wilson et al., 2007). Another limitation was the relatively short follow-up, but studies with longer time frame have found similar results (Johansson et al., 2014; Terracciano et al., 2014).
In conclusion, this study strengthens the evidence that low conscientiousness and high neuroticism are significant risk factors for the development of dementia. In the HRS, we also found strong evidence that low agreeableness (i.e., antagonism) may increase dementia risk. Neuroticism and conscientiousness were also associated with incident CIND, and among individuals with CIND at baseline, conscientiousness was a significant predictor of progression to dementia. This finding expands knowledge on key psychological risk factors for the development of dementia. This knowledge can potentially inform diagnostic paradigms and help in the targeting of prevention and treatment efforts (Kolanowski et al., 2011).
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2015;11(6):718–726. doi: 10.1016/j.jalz.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Booth T, Mottus R, Corley J, Gow AJ, Henderson RD, Maniega SM, Murray C, Royle NA, Sprooten E, Hernandez MC, Bastin ME, Penke L, Starr JM, Wardlaw JM, Deary IJ. Personality, health, and brain integrity: the Lothian birth cohort study 1936. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2014;33(12):1477–1486. doi: 10.1037/hea0000012. [DOI] [PubMed] [Google Scholar]
- Clark DO, Stump TE, Tu W, Miller DK, Langa KM, Unverzagt FW, Callahan CM. Hospital and nursing home use from 2002 to 2008 among U.S. older adults with cognitive impairment, not dementia in 2002. Alzheimer disease and associated disorders. 2013;27(4):372–378. doi: 10.1097/WAD.0b013e318276994e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. The journals of gerontology. Series B, Psychological sciences and social sciences. 2011;66(Suppl 1):i162–i171. doi: 10.1093/geronb/gbr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davydow DS, Levine DA, Zivin K, Katon WJ, Langa KM. The association of depression, cognitive impairment without dementia, and dementia with risk of ischemic stroke: a cohort study. Psychosomatic medicine. 2015;77(2):200–208. doi: 10.1097/PSY.0000000000000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duberstein PR, Chapman BP, Tindle HA, Sink KM, Bamonti P, Robbins J, Jerant AF, Franks P. Personality and risk for Alzheimer's disease in adults 72 years of age and older: a 6- year follow-up. Psychology and aging. 2011;26(2):351–362. doi: 10.1037/a0021377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchek JM, Balota DA, Storandt M, Larsen R. The power of personality in discriminating between healthy aging and early-stage Alzheimer's disease. The journals of gerontology. Series B, Psychological sciences and social sciences. 2007;62(6):P353–P361. doi: 10.1093/geronb/62.6.p353. [DOI] [PubMed] [Google Scholar]
- Duron E, Vidal JS, Bounatiro S, Ben Ahmed S, Seux ML, Rigaud AS, Hanon O, Viollet C, Epelbaum J, Martel G. Relationships between Personality Traits, Medial Temporal Lobe Atrophy, and White Matter Lesion in Subjects Suffering from Mild Cognitive Impairment. Frontiers in aging neuroscience. 2014;6:195. doi: 10.3389/fnagi.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman HS. Long-term relations of personality and health: dynamisms, mechanisms, tropisms. J Pers. 2000;68(6):1089–1107. doi: 10.1111/1467-6494.00127. [DOI] [PubMed] [Google Scholar]
- Hampson SE. Personality processes: mechanisms by which personality traits "get outside the skin". Annual review of psychology. 2012;63:315–339. doi: 10.1146/annurev-psych-120710-100419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM. Neuroinflammation in Alzheimer's disease. The Lancet Neurology. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J, Balota DA, Head D. Exploring the relationship between personality and regional brain volume in healthy aging. Neurobiology of aging. 2011;32(12):2162–2171. doi: 10.1016/j.neurobiolaging.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson L, Guo X, Duberstein PR, Hallstrom T, Waern M, Ostling S, Skoog I. Midlife personality and risk of Alzheimer disease and distress: a 38-year follow-up. Neurology. 2014;83(17):1538–1544. doi: 10.1212/WNL.0000000000000907. [DOI] [PubMed] [Google Scholar]
- Jokela M, Elovainio M, Nyberg ST, Tabak AG, Hintsa T, Batty GD, Kivimaki M. Personality and risk of diabetes in adults: pooled analysis of 5 cohort studies. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2014;33(12):1618–1621. doi: 10.1037/hea0000003. [DOI] [PubMed] [Google Scholar]
- Kolanowski A, Litaker M, Buettner L, Moeller J, Costa PT., Jr A randomized clinical trial of theory-based activities for the behavioral symptoms of dementia in nursing home residents. Journal of the American Geriatrics Society. 2011;59(6):1032–1041. doi: 10.1111/j.1532-5415.2011.03449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman ME, Weaver SL, Waltham MA. The Midlife Development Inventory (MIDI) personality scales: Scale construction and scoring. Brandeis University; 1997. [Google Scholar]
- Lai MK, Chen CP, Hope T, Esiri MM. Hippocampal neurofibrillary tangle changes and aggressive behaviour in dementia. Neuroreport. 2010;21(17):1111–1115. doi: 10.1097/WNR.0b013e3283407204. [DOI] [PubMed] [Google Scholar]
- Luchetti M, Barkley JM, Stephan Y, Terracciano A, Sutin AR. Five-factor model personality traits and inflammatory markers: new data and a meta-analysis. Psychoneuroendocrinology. 2014;50:181–193. doi: 10.1016/j.psyneuen.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchetti M, Terracciano A, Stephan Y, Sutin AR. Personality and Cognitive Decline in Older Adults: Data From a Longitudinal Sample and Meta-Analysis. The journals of gerontology. Series B, Psychological sciences and social sciences. 2016;71:591–601. doi: 10.1093/geronb/gbu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae RR, John OP. An introduction to the Five-Factor Model and its applications. J Pers. 1992;60(2):175–215. doi: 10.1111/j.1467-6494.1992.tb00970.x. [DOI] [PubMed] [Google Scholar]
- Orfei MD, Varsi AE, Blundo C, Celia E, Casini AR, Caltagirone C, Spalletta G. Anosognosia in mild cognitive impairment and mild Alzheimer's disease: frequency and neuropsychological correlates. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2010;18(12):1133–1140. doi: 10.1097/JGP.0b013e3181dd1c50. [DOI] [PubMed] [Google Scholar]
- Pocnet C, Rossier J, Antonietti JP, von Gunten A. Personality changes in patients with beginning Alzheimer disease. Can J Psychiatry. 2011;56(7):408–417. doi: 10.1177/070674371105600704. [DOI] [PubMed] [Google Scholar]
- Prince M, Albanese E, Guerchet M, Prina M World Alzheimer Report 2014. Dementia and risk reduction: an analysis of protective and modifiable factors. Londres: Alzheimers Disease International; 2014. [Google Scholar]
- Ramakers IH, Honings ST, Ponds RW, Aalten P, Sebastian K, Verhey FR, Visser PJ. The Effect of Psychological Distress and Personality Traits on Cognitive Performances and the Risk of Dementia in Patients with Mild Cognitive Impairment. Journal of Alzheimer's disease : JAD. 2015;46(3):805–812. doi: 10.3233/JAD-142493. [DOI] [PubMed] [Google Scholar]
- Rankin K, Baldwin E, Pace-Savitsky C, Kramer J, Miller B. Self awareness and personality change in dementia. Journal of Neurology, Neurosurgery & Psychiatry. 2005;76(5):632–639. doi: 10.1136/jnnp.2004.042879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp MA, Schnaider-Beeri M, Purohit DP, Perl DP, Haroutunian V, Sano M. Increased neurofibrillary tangles in patients with Alzheimer disease with comorbid depression. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2008;16(2):168–174. doi: 10.1097/JGP.0b013e31816029ec. [DOI] [PubMed] [Google Scholar]
- Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment A population-based validation study. Neurology. 2001;56(1):37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- Saczynski JS, Rosen AB, McCammon RJ, Zivin K, Andrade SE, Langa KM, Vijan S, Pirraglia PA, Briesacher BA. Antidepressant Use and Cognitive Decline: The Health and Retirement Study. Am J Med. 2015;128(7):739–746. doi: 10.1016/j.amjmed.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogen JC, Bergh S, Stewart R, Knudsen AK, Bjerkeset O. Midlife mental distress and risk for dementia up to 27 years later: the Nord-Trondelag Health Study (HUNT) in linkage with a dementia registry in Norway. BMC geriatrics. 2015;15:23. doi: 10.1186/s12877-015-0020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan Y, Sutin AR, Luchetti M, Terracciano A. Feeling Older and the Development of Cognitive Impairment and Dementia. The journals of gerontology. Series B, Psychological sciences and social sciences. 2016 doi: 10.1093/geronb/gbw085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin AR, Ferrucci L, Zonderman AB, Terracciano A. Personality and obesity across the adult life span. Journal of personality and social psychology. 2011;101(3):579–592. doi: 10.1037/a0024286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin AR, Stephan Y, Luchetti M, Artese A, Oshio A, Terracciano A. The five-factor model of personality and physical inactivity: A meta-analysis of 16 samples. J Res Pers. 2016;63:22–28. doi: 10.1016/j.jrp.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Costa PT., Jr Smoking and the Five-Factor Model of personality. Addiction. 2004;99:472–481. doi: 10.1111/j.1360-0443.2004.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Iacono D, O'Brien RJ, Troncoso JC, An Y, Sutin AR, Ferrucci L, Zonderman AB, Resnick SM. Personality and resilience to Alzheimer's disease neuropathology: a prospective autopsy study. Neurobiology of aging. 2013;34:1045–1050. doi: 10.1016/j.neurobiolaging.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Lobina M, Piras MG, Mulas A, Cannas A, Meirelles O, Sutin AR, Zonderman AB, Uda M, Crisponi L, Schlessinger D. Neuroticism, depressive symptoms, and serum BDNF. Psychosomatic medicine. 2011;73(8):638–642. doi: 10.1097/PSY.0b013e3182306a4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Sutin AR, An Y, O'Brien RJ, Ferrucci L, Zonderman AB, Resnick SM. Personality and risk of Alzheimer's disease: New data and meta-analysis. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2014;10:179–186. doi: 10.1016/j.jalz.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Bennett DA, Li Y, Bienias JL, Mendes de Leon CF, Evans DA. Proneness to psychological distress and risk of Alzheimer disease in a biracial community. Neurology. 2005;64(2):380–382. doi: 10.1212/01.WNL.0000149525.53525.E7. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Arnold SE, Bienias JL, Bennett DA. Conscientiousness and the incidence of Alzheimer disease and mild cognitive impairment. Archives of general psychiatry. 2007;64(10):1204–1212. doi: 10.1001/archpsyc.64.10.1204. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Falvey C, Harris TB, Newman A, Satterfield S, Koster A, Ayonayon H, Simonsick E. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: Prospective study. Bmj. 2013;347:f7051. doi: 10.1136/bmj.f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.