Abstract
Background
It is uncertain whether measurement of circulating total atherogenic lipoprotein particle cholesterol mass (nonHDLc) or particle concentration (apoB and LDLp) more accurately reflects risk of incident coronary heart disease (CHD). We evaluated CHD risk among women in whom these markers where discordant.
Methods
Among 27,533 initially-healthy women in the Women's Health Study (NCT00000479), using residuals from linear regression models, we compared risk among women with higher or lower observed particle concentration relative to nonHDLc (highest and lowest residual quartiles, respectively) to individuals with agreement between markers (middle quartiles) using Cox proportional hazards models.
Results
Although all 3 biomarkers were correlated (r ≥0.77), discordance occurred in up to 20.2% of women. Women with discordant high particle concentration were more likely to have metabolic syndrome (MetS) and diabetes (both P<0.001). Over median follow-up of 20.4 years, 1,246 CHD events occurred (514725 person-years). Women with high particle concentration relative to nonHDLc had increased CHD risk: for apoB (age-adjusted HR [95% CI] = 1.77 [1.56, 2.00]) and for LDLp (1.70 [1.50, 1.92]). After adjusting for clinical risk factors including MetS, these risks attenuated to 1.22 (1.07, 1.39) for apoB and 1.13 (0.99, 1.29) for LDLp. Discordant low apoB or LDLp relative to nonHDLc was not associated with lower risk.
Conclusions
Discordance between atherogenic particle cholesterol mass and particle concentration occurs in a sizeable proportion of apparently healthy women, and should be suspected clinically among women with cardiometabolic traits. In such women, direct measurement of lipoprotein particle concentration might better inform CHD risk assessment.
Keywords: Apolipoprotein B, Cardiovascular disease, Coronary heart disease, Discordance analysis, LDLp, Prevention, nonHDLc
Background
Recent evidence suggests that the total cholesterol carried by atherogenic lipoprotein particles (estimated as non-high density lipoprotein cholesterol; nonHDLc) or the concentration of these atherogenic lipoproteins (measured as apolipoproteinB [apoB] or low density lipoprotein particle concentration [LDLp]) may be superior markers of longitudinal risk of coronary heart disease (CHD) events compared with LDL cholesterol concentration (LDLc).1-5 nonHDLc (calculated as total cholesterol minus HDLc) reflects LDLc, but also the cholesterol in very low density lipoproteins (VLDL) and intermediate density lipoproteins (IDL), and therefore may represent a more complete view of risk related to circulating plasma cholesterol content. Beyond cholesterol content, though, it has been hypothesized that the number [concentration] of atherogenic lipoprotein particles (the lipid-protein assemblies which transport cholesterol in circulation) might better reflect the potential for these cholesterol transporters to be taken up into the neointima of atheromatous lesions, depositing cholesterol which becomes esterified, inciting and then propagating atherosclerotic coronary disease.6, 7
Although clinical practice has generally favored the use of LDLc for identifying CHD risk,8-11 interest in the clinical applicability of these alternate measures of risk has grown in recent years, as the number of CHD events occurring among individuals with low or normal LDLc remains unacceptably high,12 with 1 in 10 individuals without known cardiovascular disease (CVD), and 1 in 5 individuals with known CVD, experiencing a CVD event over a 5-year period in clinical trials of statin therapy.13 Additionally, as novel lipid-modulating therapies become available, identifying optimal lipid/lipoprotein markers for patient selection and assessment of therapeutic efficacy is of critical importance.
To compare the utility of these risk markers, we recently observed that when LDLc is discordant with (that is, in disagreement with) either nonHDLc, apoB, or LDLp in women, risk tracked more closely with the latter three markers than it did with LDLc.14 In the present study, we sought to extend these observations by examining risk when nonHDLc is discordant with apoB or LDLp – to determine if CHD risk is more closely related to total atherogenic lipoprotein particle cholesterol (nonHDLc) or rather to atherogenic particle number (apoB or LDLp). We hypothesized that circulating atherogenic lipoprotein particle concentration could provide more accurate insight into risk when nonHDLc and these markers of particle concentration were discordant.
Methods
Study Population
The study population is drawn from the Women's Health Study (WHS; NCT00000479) – a primary prevention trial of vitamin E, β-carotene, or aspirin versus placebo in the prevention of cardiovascular disease and cancer.15-17 From April 30th, 1993, through January 24th, 1996, the study enrolled 39,876 women aged 45 years or older and free of self-reported CVD or cancer at study entry. There was no significant effect of the study drugs on CHD events during the trial.15-17 All participants were also asked to provide a voluntary baseline blood sample, of whom 28,345 did so. Blood measurements were subsequently performed on these stored samples, as below. For the current study, we excluded women with missing data on baseline nonHDLc, apoB, or LDLp, which left 27,533 women for analysis. The study was approved by the institutional review board of the Brigham and Women's Hospital (Boston, Massachusetts, USA).
Biomarkers
At the outset of the original trial, blood samples were collected in EDTA tubes and stored in vapor-phase liquid nitrogen (-170°C). In a laboratory certified by the National Heart, Lung, and Blood Institute/Centers for Disease Control and Prevention Lipid Standardization Program (Dr. Nader Rifai, Children's Hospital, Boston, Massachusetts, USA), baseline samples were thawed and analyzed for standard lipids and apolipoproteins. Standard lipids were measured directly with reagents from Roche Diagnostics. nonHDLc was calculated as total cholesterol (TC) minus HDLc. ApoB was measured with immunoturbidometric technique on the Hitachi 917 analyzer (Roche Diagnostics), using reagents and calibrators from Wako Chemicals. Coefficients of variation (CV) for standard lipids, nonHDLc, and apoB were all ≤3.3%.18 LDLp was measured by 400 MHz proton nuclear magnetic resonance (1H NMR) spectroscopy (LipoScience, now LabCorp) using the LipoProfile III assay, an assay with CVs generally ≤4.1%.
Outcomes
The primary outcome was an incident CHD event, defined as the composite of myocardial infarction, coronary revascularization (coronary artery bypass graft surgery or percutaneous coronary intervention), or CHD death. Events were ascertained annually in extended follow-up through 2014 via questionnaire, letter, and phone calls, and confirmed by medical record review.19, 20
Statistical Analyses
We modeled discordance in two ways: (1) using residuals from linear regression models to reflect the discordance between expected and observed (measured) apoB or LDLp based on nonHDLc, and (2) by dividing the population into concordant/discordant groups based on median marker concentrations, as previously done. We chose to use two methods to assess the robustness of the potential findings. For Method 1, modeling risk based on residuals, each individual's degree of discordance was measured using the residuals from linear regression models to identify the distance between the “observed” (measured) and the “expected” apoB or LDLp concentration based on the individual's nonHDLc (e.g. apoB = B0 + [B1]nonHDLc + [B2, etc.]covariables]). This method identifies positive residuals (higher apoB or LDLp than expected based on nonHDLc) and negative residuals (lower apoB or LDLp than expected based on nonHDLc), in each individual. To compare risk, we examined risk among those with very positive residuals (>75th percentile; i.e. observed apoB or LDLp much higher than expected based on nonHDLc) or very negative residuals (<25th percentile; i.e. observed apoB or LDLp much lower than expected), compared to those with intermediate residuals (25th - 75th percentiles; i.e. observed apoB or LDLp similar to expected). Examination of the highest and lowest quartiles was chosen in order to evaluate risk among women with more extreme lipid discordance phenotypes.
For Method 2, modeling risk based on medians, discordance was defined as nonHDLc < median and the alternative measure (apoB or LDLp) ≥ its median, or vice versa. The median values for nonHDLc, apoB, and LDLp were 154.4 mg/dL (4.0 mmol/L), 100.2 mg/dL (2.6 mmol/L), and 1,215.9 nmol/L, respectively. A comparison of individuals selected by these two methods is shown for apoB and nonHDLc in Figure 1a and for LDLp and nonHDLc in Figure 1b.
Figure 1.
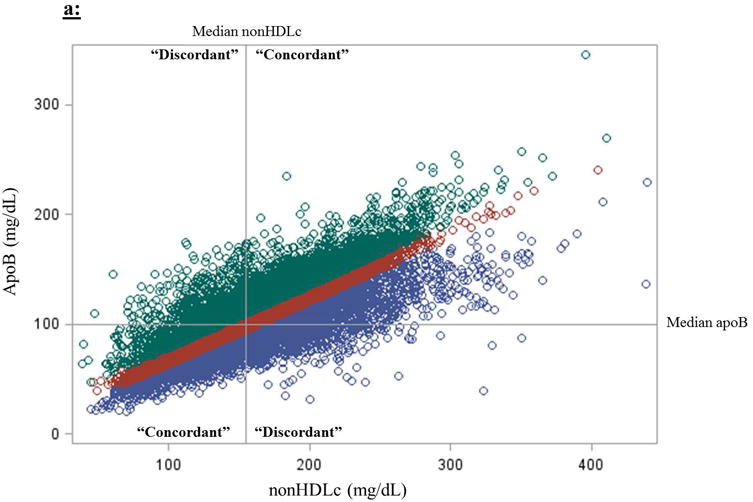
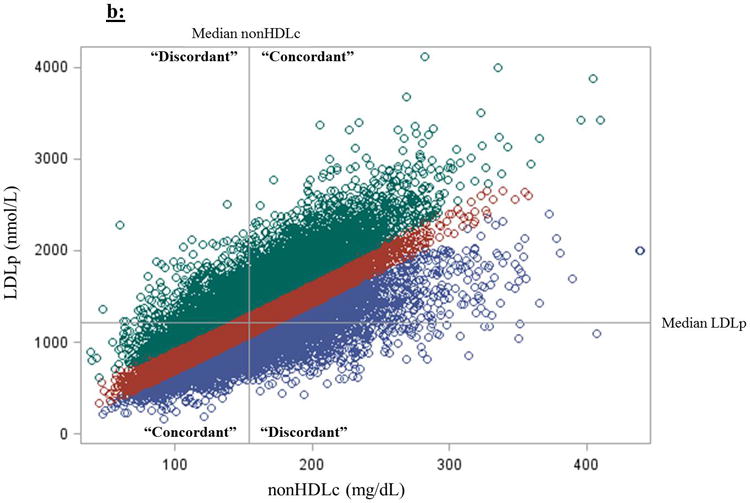
Two methods for defining discordance between (a) nonHDLc and apoB and (b) nonHDLc and LDLp. (1) Discordance can be defined as one marker above and another below the median marker cut-points. (2) Discordance could also be defined based on residual differences from linear regression models estimating the difference between expected (based on the marker in comparison) and observed marker concentrations; here, those in the lowest residual quartile (purple circles; “apoB lower than expected based on nonHDLc”) are compared with those on the highest residual quartile (green circles; “apoB higher than expected”), using those in the intermediate two quartiles (red circles; “apoB close to excepted”) as the reference group. Median concentrations of nonHDLc, apoB, and LDLp are 154.4 mg/dL (4.0 mmol/L), 100.2 mg/dL (2.6 mmol/L), and 1,215.9 nmol/L, respectively.
Within concordant/discordant groups, baseline descriptive clinical and biochemical characteristics were summarized as medians (25th, 75th percentiles) for quantitative variables and as percentages for qualitative variables. Comparisons across concordant/discordant groups were assessed with the Kruskal-Wallis test for quantitative variables and with the chi-square or Fisher exact test for qualitative variables. Scatter plots were used to depict the distribution of biomarker values in the overall study population. Correlations were measured with Spearman rank correlation coefficient (r).
We compared risks between concordant and discordant groups with Cox proportional hazards models (hazard ratio [HR] and 95% confidence intervals [CI]) and Kaplan-Meier survival-free-of-CHD curves. For Method 1 (residuals) we used the intermediate residual group (25th – 75th percentiles) as the reference group, and for Method 2 (medians) we used those with concordant low nonHDLc and apoB or LDLp as the reference group. Consistent with previous analyses,14 we performed three regression models for each group/outcome: 1) an age-adjusted model (Model 1); 2) a model additionally incorporating randomized treatment assignment, hormone use, postmenopausal status, smoking (baseline history or not), and baseline history of hypertension (Model 2); and 3) a model additionally incorporating cardiometabolic traits, including diabetes mellitus, metabolic syndrome (MetS), body mass index (BMI), HDLc, triglycerides, high-sensitivity C-reactive protein (hsCRP), and parental history of premature myocardial infarction (Model 3). MetS was defined as having 3 or more of the following traits: (1) BMI >26.7 (as a surrogate for waist circumference, which was not measured at baseline in WHS), (2) blood pressure > 130/85 mmHg or history of hypertension or treatment for hypertension, (3) triglycerides ≥ 150 mg/dL, (4) HDL-c < 50 mg/dL (1.29 mmol/L), and (5) HbA1c ≥ 5.7% (as a surrogate for fasting glucose, which was not measured in WHS) or history of diabetes. Finally, as we observed post hoc that women in the discordant high apoB and LDLp groups also had higher concentrations of nonHDLc than the referent group, to assess potential confounding by baseline nonHDLc concentration we performed (4) a fourth regression model (Model 4) which included the variables in Model 3 above plus nonHDLc concentration. All P-values were two-tailed using α=0.05. Analyses were done using SAS v9.4 (SAS Institute).
Results
The two methods for defining discordance differed in the patients whom they identified (Figure 1). Although all three biomarkers were highly correlated – nonHDLc and apoB (Spearman r=0.86, P<0.0001), nonHDLc and LDLp (r=0.77, P<0.0001), and apoB and LDLp (r=0.85, P<0.0001) – discordance, defined based on medians, between nonHDLc and apoB or LDLp, overall occurred in 13.9% and 20.2% of women, respectively. As concentrations of nonHDLc deviated from the median, the prevalence of discordance decreased progressively, with discordance between nonHDLc and apoB or LDLp decreasing to 5.3% and 7.9%, respectively, for those in the 90th percentile of nonHDLc, and to 2.7 and 3.9%, respectively, for those in the 10th percentile of nonHDLc (Figure 2). Using residuals, for both comparisons with nonHDLc to apoB (Table 1) and to LDLp (Table 2), women with discordant high apoB or LDLp generally had a higher baseline prevalence of CHD risk factors, particularly cardiometabolic risk factors such as hypertension, diabetes, and increased hsCRP, compared with those in the concordant and discordant low apoB or LDLp groups, and MetS was significantly more prevalent among women with discordant high apoB and LDLp (P<0.001).
Figure 2.
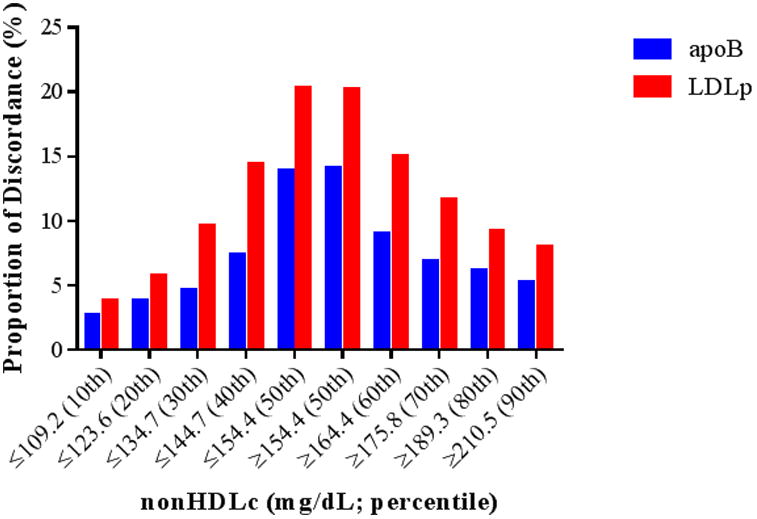
Proportion of individuals with discordant nonHDLc and lipoprotein particle concentration (apoB and LDLp), for those at or above (≥50th percentile) and below (≤50th percentile) decile cutoffs of nonHDLc.
Table 1.
Clinical (% or N [%]) and biochemical (median [25th, 75th percentiles]) variables among women with discordant or concordant values of nonHDL-c and apoB.
| Discordant | Concordant | Discordant | |
|---|---|---|---|
| apoB Lower Than Expected (Low Negative Residual) (n=6,884) | apoB Close to Expected (Intermediate Residual) (n=13,765) | apoB Higher Than Expected (High Positive Residual) (n=6,884) | |
| Age, years | 51.9 (48.2, 57.5) | 52.7 (48.9, 58.6) | 54.7 (50.1, 61.0) |
| Family history premature MI*, % | 13.3 | 14.3 | 15.7 |
| Smoker**, % | 47.3 | 48.0 | 49.9 |
| Post-menopausal, % | 48.9 | 52.9 | 62.6 |
| HRT use, % | 36.5 | 41.9 | 50.1 |
| Cardiometabolic Traits | |||
| Metabolic syndrome, % | 16.5 | 21.1 | 40.0 |
| BMI, kg/m2 | 24.2 (22.0, 27.4) | 24.8 (22.3, 28.3) | 25.8 (23.2, 29.6) |
| Hypertension, % | 20.8 | 23.2 | 33.3 |
| Diabetes, % | 2.0 | 2.1 | 4.7 |
| hsCRP, mg/L | 1.57 (0.62, 3.59) | 1.89 (0.76, 4.07) | 2.84 (1.28, 5.51) |
| Triglycerides, mg/dL | 109 (77, 162) | 110 (79, 158) | 154 (108.5, 217.0) |
| Lipid/Lipoprotein Profile | |||
| HDL-c, mg/dL | 57.7 (48.2, 68.8) | 52.6 (44.3, 62.4) | 45.2 (37.7, 54.2) |
| LDL-c, mg/dL | 116.2 (94.0, 142.9) | 118.1 (99.6, 139.2) | 132.3 (112.2, 154.3) |
| nonHDL-c, mg/dL | 149.0 (123.3, 182.4) | 148.2 (126.5, 173.8) | 170.6 (147.8, 195.9) |
| apoB, mg/dL | 83.7 (70.2, 97.9) | 97.5 (84.9, 114.0) | 126.9 (112.8, 143.4) |
| LDL size, nm | 21.3 (20.9, 21.6) | 21.2 (20.8, 21.6) | 20.9 (20.3, 21.3) |
| LDL-p, μmol/L | 1,030 (832, 1,263) | 1,178 (975, 1,409) | 1,527 (1,280, 1,800) |
| VLDL-p, μmol/L | 54 (40, 73) | 57 (41, 75) | 68 (49, 87) |
Abbreviations: CABG = coronary artery byupass grafting, hsCRP = high sensitivity C-reactive protein, HbA1c = glycated hemoglobin A1c, HRT = hormone replacement therapy, LDLp = low density lipoprotein particle concentration, MI = myocardial infarction, nm = nanometer, PTCA = percutaneous transluminal coronary angioplasty.
Family history premature MI <60 years of age.
Current or Former.
To convert mg/dL to mmol/L multiply by 0.02586 for cholesterol and by 0.01129 for triglycerides.
Table 2.
Clinical (% or N [%]) and biochemical (median [25th, 75th percentiles]) variables among women with discordant or concordant values of nonHDL-c and LDLp.
| Discordant | Concordant | Discordant | |
|---|---|---|---|
| LDLp Lower Than Expected (Low Negative Residual) (n=6,884) | LDLp Close to Expected (Intermediate Residual) (n =13,766) | LDLp Higher Than Expected (High Positive Residual) (n=6,883) | |
| Age, years | 52.7 (48.8, 58.5) | 52.6 (48.8, 58.6) | 53.9 (49.6, 60.2) |
| Family history premature MI*, % | 13.7 | 14.0 | 16.0 |
| Smoker**, % | 47.5 | 47.7 | 50.4 |
| Post-menopausal, % | 53.3 | 52.4 | 59.2 |
| HRT use, % | 38.3 | 41.9 | 48.5 |
| Cardiometabolic Traits | |||
| Metabolic syndrome, % | 14.3 | 20.9 | 42.7 |
| BMI, kg/m2 | 24.0 (21.9, 27.1) | 24.6 (22.3, 28.2) | 26.5 (23.6, 30.2) |
| Hypertension, % | 21.3 | 23.0 | 33.2 |
| Diabetes, % | 2.0 | 2.0 | 4.8 |
| hsCRP, mg/L | 1.56 (0.62, 3.57) | 1.82 (0.73, 3.96) | 3.04 (1.40, 5.70) |
| Triglycerides, mg/dL | 110 (79, 160) | 110 (77, 158) | 154 (107, 217) |
| Lipid/Lipoprotein Profile | |||
| HDL-c, mg/dL | 59.5 (50.8, 69.8) | 52.5 (44.5, 62.3) | 43.9 (37.6, 51.7) |
| LDL-c, mg/dL | 124.0 (101.5, 149.6) | 116.7 (97.6, 137.6) | 129.1 (107.8, 151.5) |
| apoB, mg/dL | 93.6 (78.6, 113.4) | 96.3 (81.3, 115.2) | 118.8 (99.9, 136.9) |
| nonHDL-c, mg/dL | 157.1 (131.2, 187.9) | 147.3 (124.2, 172.2) | 167.5 (141.4, 193.8) |
| LDL size, nm | 21.5 (21.1, 21.8) | 21.2 (20.8, 21.5) | 20.7 (20.2, 21.1) |
| LDL-p, μmol/L | 965 (794, 1166) | 1175 (989, 1374) | 1635 (1419, 1880) |
| VLDL-p, μmol/L | 58.9 (44.2, 77.0) | 56.8 (40.4, 75.8) | 62.6 (45.1, 81.9) |
Abbreviations: CABG = coronary artery byupass grafting, hsCRP = high sensitivity C-reactive protein, HbA1c = glycated hemoglobin A1c, HRT = hormone replacement therapy, LDLp = low density lipoprotein particle concentration, MI = myocardial infarction, nm = nanometer, PTCA = percutaneous transluminal coronary angioplasty.
Family history premature MI <60 years of age.
Current or Former.
To convert mg/dL to mmol/L multiply by 0.02586 for cholesterol and by 0.01129 for triglycerides.
A total of 1,246 CHD events occurred over a median (maximum) of 20.4 (21.6) years of follow-up, representing 514,725 person-years of follow-up. The proportion of CHD subtypes comprising the primary endpoint was similar across the discordant and concordant groups (Supplemental Tables 1 and 2). Defining discordance based on residual differences, women with discordant high apoB were at higher risk of CHD relative to those with concordant apoB and nonHDLc (Table 3; Figure 3a). This risk association persisted after adjustment for clinical variables plus MetS and related variables (Model 3 HR [95% CI] = 1.22 [1.07, 1.39]). Women with discordant low apoB were not at lower risk of coronary events (Model 3 HR [95%] = 0.97 [0.83, 1.14]) relative to those with concordant values. Women with discordant high LDLp were at higher risk of CHD relative to those with concordant LDLp and nonHDLc in models adjusting for age and basic clinical variables (Table 3), but no longer in the model adjusting for clinical variables plus MetS and related cardiometabolic variables (Model 3 1.13 [0.99, 1.29]; Figure 3b). Although median nonHDLc was higher among women with discordant high lipoprotein particle concentration, risk estimates were not appreciably changed after adjustment for baseline nonHDLc (Table 3). Although a larger proportion of women with MetS had discordant high particle concentration, the magnitude of associated increased risk of CHD was similar among women with and without MetS for both apoB (MetS present: Model 2 HR [95% CI] = 1.45 [1.22, 1.72]; MetS absent: 1.43 [1.19, 1.72]; Supplemental Table 3) and LDLp (MetS present: 1.24 [1.05, 1.48]; MetS absent: 1.42 [1.17, 1.72]). In sensitivity analyses restricted to those with nonHDL-c below the median, we observed a similar pattern of results to those in the overall cohort. Among women with nonHDLc and apoB or LDLp above their medians (those in the right upper quadrant of Figures 1a and 1b), 66% and 65%, respectively, of women had an estimated 10-year ASCVD risk <5% by Pooled Cohort Risk Assessment Equations, and thus might not necessarily qualify for statin therapy.
Table 3.
Hazard ratios (95% confidence intervals) from Cox proportional hazards models of incident CHD associated with discordant and concordant concentrations of nonHDL-c and apoB or LDL-p, based on residual differences.
| Group | HR (95% CI) CHD* from Cox Proportional Hazards Regression | |||
|---|---|---|---|---|
| apoB and nonHDLc | ||||
| Model 1** | Model 2** | Model 3** | Model 4** | |
| Discordant High apoB | 1.77 (1.56, 2.00) | 1.67 (1.48, 1.89) | 1.22 (1.07, 1.39) | 1.14 (1.00, 1.30)† |
| Concordant | ref. | ref. | ref. | ref. |
| Discordant Low apoB | 0.87 (0.74, 1.01) | 0.88 (0.75, 1.03) | 0.97 (0.83, 1.14) | 0.93 (0.79, 1.10) |
| Group | LDLp and nonHDLc | |||
| Model 1** | Model 2** | Model 3** | Model 4** | |
| Discordant High LDLp | 1.70 (1.50, 1.92) | 1.57 (1.39, 1.78) | 1.13 (0.99, 1.29) | 1.06 (0.93, 1.21) |
| Concordant | ref. | ref. | ref. | ref. |
| Discordant Low LDLp | 0.93 (0.80, 1.08) | 0.93 (0.80, 1.09) | 1.04 (0.89, 1.22) | 0.98 (0.83, 1.15) |
Abbreviations: CI = confidence interval; HR = hazard ratio.
Model 1 = randomization age-adjusted; Model 2 = Model 1 + randomized treatment assignment, hormone use, postmenopausal status, smoking (baseline history or not), and history of baseline hypertension; Model 3 = Model 2 + metabolic syndrome, diabetes mellitus (pre-randomization), body mass index, HDLc, triglycerides, high-sensitivity C-reactive protein (hsCRP), and parental history of premature myocardial infarction; Model 4 = Model 3 + baseline concentration of nonHDLc.
For apoB: P<0.001 for Models 1 and 2, P=0.002 for Model 3, P=0.012 for Model 4; for LDLp: P<0.001 for Models 1 and 2, P=0.23 for Model 3, P= 0.29 for Model 4.
P=0=0.046.
Figure 3.
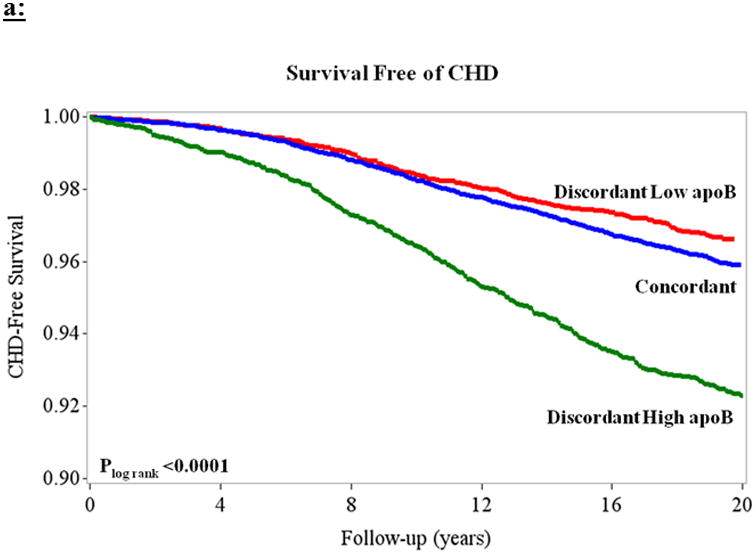
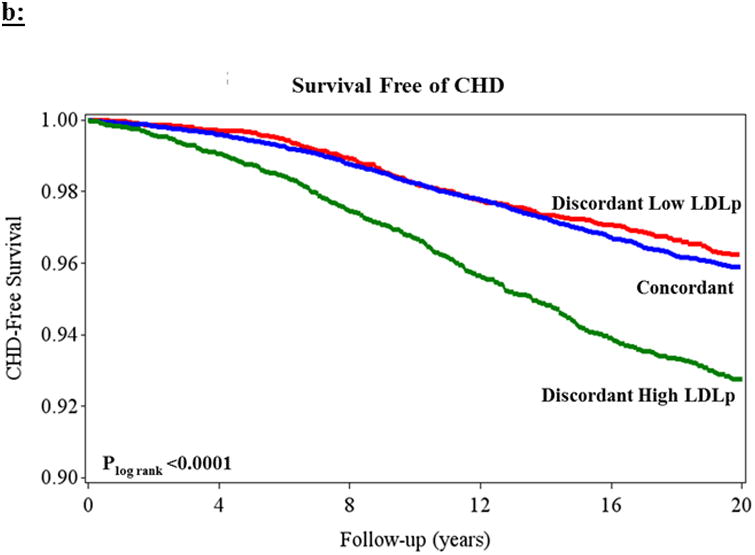
Kaplan-Meier curves demonstrating survival free of CHD based on residual differences among women with concordant (blue) and discordant high (green) and low (red) (a) apoB and (b) LDL-p, both relative to nonHDLc. Plog rank< 0.0001 for both.
Defining discordance based on medians, women with discordant high apoB were at greater risk of CHD events (age-adjusted HR [95% CI] = 1.90 [1.50, 2.40]) than those with discordant low apoB (age-adjusted HR [95% CI] = 1.33 [1.01, 1.75]), although these differences were no longer apparent after adjustment for clinical covariables including MetS (Supplemental Table 4). Using medians to define discordance, no major differences in risk among nonHDLc-LDLp discordance groups were observed.
Discussion
Several highly correlated measures of atherogenic lipoproteins have been proposed by prevention guidelines for CHD risk assessment, and there is uncertainty regarding when and if clinicians should order tests to measure lipoprotein particle concentrations (e.g. apoB or LDLp) or simply calculate the cholesterol (e.g. nonHDLc) that is obtained from a simple lipid panel. This study aimed to better understand the magnitude and contribution to CHD risk that is related to atherogenic particle cholesterol mass versus particle concentration in apparently healthy women. We found that despite high correlations, a sizable proportion (∼20%) of these women had discordant nonHDLc and apoB or LDLp. Women with discordant high apoB or LDLp tended to also have higher prevalence of baseline cardiometabolic comorbidities, notably MetS. When nonHDLc and lipoprotein particle concentration (particularly apoB) were discordant, risk tracked better with lipoprotein particle concentration, irrespective of the presence of MetS. These findings could inform CHD risk discussions, in particular for individuals with cardiometabolic traits, in whom it may be reasonable to directly measure lipoprotein particle number for better risk assessment.
Accurate identification and profiling of risk related to circulating atherogenic lipids and lipoproteins remains a priority in clinical practice. First, it has been observed that the number of CHD events occurring among individuals with low or normal LDLc remains unacceptably high,12 with 1 in 10 individuals without known cardiovascular disease (CVD), and 1 in 5 individuals with known CVD, experiencing a CVD event over a 5-year period in clinical trials of statin therapy.13 Such residual risk may reflect alternative pathways of atherogenesis, such as inflammation, but also the imprecision of current lipid/lipoprotein approaches to identify risk. Additionally, as novel primary and adjunctive lipid-modulating therapies become available,21, 22 identifying optimal lipid/lipoprotein markers for patient selection and assessment of therapeutic efficacy is of critical importance.23
Although clinical practice has generally favored the use of LDLc as the first-line marker of CHD risk related to circulating atherogenic lipoproteins, recent evidence suggests that measures of total atherogenic lipoprotein cholesterol concentration (nonHDLc) and atherogenic lipoprotein particle concentration (apoB or LDLp) may be superior markers of longitudinal risk of CHD.1, 2, 4, 5, 14 Theoretic benefits of measuring nonHDLc beyond LDLc include more comprehensive assessment of total circulating atherogenic cholesterol (beyond that associated with LDL). Even so, both nonHDLc and LDLc reflect circulating lipoprotein cholesterol mass, whereas concentration of atherogenic lipoprotein particles represents an alternate view of circulating lipoprotein burden which may better reflect risk.7 Biologically, atherogenic lipoprotein particle concentration might better reflect the potential for cholesterol-carrying lipoproteins to be taken up into the neointima of atheromatous lesions, inciting and then propagating atherosclerotic coronary disease.6, 7
Building on our previous observations that nonHDLc, apoB, and LDLp better captured risk compared with LDLc in situations of discordance,14 the current study examined risk of CHD events when apoB and LDLp were discordant with nonHDLc. The findings herein support the hypothesis that in many women, nonHDLc and apoB or LDLp are discordant, and in such cases lipoprotein particle concentration (primarily apoB) better reflects their true risk. An interesting observation was that women with discordant high apoB or LDLp generally had a higher proportion of MetS and worse cardiometabolic risk factor profiles. Notably, though, the associations observed among those with discordant apoB remained significant after adjustment for MetS and covariables. There was also no evidence of effect modification among women with compared to those without MetS in terms of the magnitude of risk associated with discordant apoB and LDLp. Taken together, these data suggest that women with MetS traits might be a group in whom potential for discordance should be suspected in clinical practice, although among those with and without MetS alike, particle number better captures true CHD risk. Equally interesting and likely to represent a challenge to clinicians was the observation that discordance remained relatively prevalent even at the extremes of nonHDLc. Hence, it could be difficult to endorse a cut-point where the chances of discordance are “too low to worry about.”
Among women who would be expected to be at highest risk based on cholesterol and lipoprotein markers above their medians (those in the right upper quadrant of Figure 1), we observed that approximately 2/3 of women had a Pooled Cohort Risk Assessment Equations estimated 10-year ASCVD risk <5%, and thus might not necessarily qualify for statin therapy. Within this group, we did observe differential patterns of risk when these individuals were further stratified by a measure of lipoprotein particle concentration. Thus, in the further development of risk-stratifying algorithms, more personalized tailoring of therapy could be achieved by incremental knowledge of atherogenic lipoprotein particle concentration beyond measuring atherogenic cholesterol concentration (nonHDLc) alone.
Several other studies have used discordance analyses24 to compare risks when nonHDLc and apoB are discordant, including in the Framingham Offspring Cohort and the INTERHEART studies.5, 25 Strengths of our study include the prospective design, longitudinal (>20 years) follow-up, inclusion of multiple measures of lipoprotein particle concentration (apoB and NMR-measured LDLp), and focus on women. Overall, our findings are generally in agreement with those of these prior studies, and support the hypothesis that CHD risk may be more closely related to the number of atherogenic lipoprotein particles, rather than to the mass of cholesterol carried in them.
We evaluated two approaches for conceptualizing and defining discordance, which labeled different groups of women as “discordant” (Figures 1a and 1b). We chose to emphasize the residual difference method of identifying discordance over the method using median cut-points. Although the method using medians is informative and potentially more clinically accessible, splitting around medians carries the risk of misclassification. For example, the analysis based on medians would categorize a woman as “discordant” if her nonHDLc was in the 49th percentile and her apoB was in the 51st percentile. Conversely, a woman with nonHDLc in the 1st percentile and apoB in the 49th percentile would be categorized as “concordant.” This misclassification would, if anything, be expected to bias the results towards the null. Indeed, in fully adjusted models based on medians, CHD risk estimates attenuated considerably. Furthermore, in dichotomizing the population around medians, participants with very high biomarker concentrations (hence at higher risk) or very low biomarker concentrations (hence at lower risk) were more likely to be labeled as “concordant” than those with intermediate marker concentrations (likely at more intermediate risk; Figure 2). Therefore, baseline concentration of the referent biomarker (nonHDLc) could potentially be a confounder in assessing risk associations when the “concordant” group (characterized by more extreme marker concentrations) is used as the reference group. In other words, using the “concordant” group as the reference group in a median-based analysis could inherently lead to the perception of over- or under-estimation of risk due to baseline referent biomarker (e.g. nonHDLc) concentrations being more extreme in the “concordant” groups, an inherent result of this method. To minimize this potential confounding, we modeled risk for all four concordant/discordant groups together, using the “concordant low” group as the reference group. Doing so, although the comparisons between discordant and concordant groups may continue to be affected by this confounding, direct comparisons between the two discordant groups should be less affected. Nonetheless, taken together, defining discordance based on the residual differences method could avoid many of these misclassification and confounding limitations inherent to the median cut-point method, at the expense of being more difficult to determine in clinical practice.
Several potential limitations bear mention. By dividing the cohort into groups based on biomarker concentrations, our study should be considered a subgroup analysis. Additionally, our study examined CHD risk only among women. However, previous studies – including the Health Professionals Follow-up Study – found that in men, lipoprotein particle concentration better predicted CVD risk than nonHDLc,26 and hence our findings may be generalizable to broader populations including men.
In conclusion, among women with discordant concentrations of nonHDLc and apoB or LDLp, CHD risk is more strongly associated with lipoprotein particle concentration (particularly apoB). Overall, our findings support the hypothesis that CHD risk may be more closely related to the concentration of atherogenic lipoprotein particles rather than to the mass of cholesterol carried by them. In clinical practice, discordance might be suspected among women with cardiometabolic traits of metabolic syndrome and diabetes. In such women (particularly those with a normal or low nonHDL-c, who might not otherwise be targeted for lipid-lowering primary prevention), direct measurement of lipoprotein particle concentration (particularly with apoB) might help better inform clinical risk assessment and guide clinical decision making.
Supplementary Material
Acknowledgments
P.R. Lawler receives support from NIH T32 (HL007575), NIH LRP, and Brigham and Women's Hospital. A.O. Akinkoulie receives support from NIH T32 (HL007575). WHS was funded by grants CA047988, HL043851, HL080467, HL099355, and UM1 CA182913. The research for this article was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R01HL117861 to S. Mora, as well as by the Molino Family Trust. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures: S. Mora: Received research grant support from Atherotech Diagnostics for research outside the current work, and served as a consultant to Lilly, Pfizer, Cerenis Therapeutics, and Quest Diagnostics. R.J.Glynn: Received research grant support from AstraZeneca. P. M. Ridker: Received research grant support from AstraZeneca, Novartis, Amgen, Pfizer, and NHLBI, and is listed as a co-inventor on patents held by the Brigham and Women's Hospital related to the use of inflammatory biomarkers in CVD (licensed to AstraZeneca and Siemens). All other authors report no disclosures.
References
- 1.Boekholdt SM, Arsenault BJ, Mora S, Pedersen TR, LaRosa JC, Nestel PJ, et al. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: A meta-analysis. JAMA. 2012;307:1302–1309. doi: 10.1001/jama.2012.366. [DOI] [PubMed] [Google Scholar]
- 2.Sniderman AD, Williams K, Contois JH, Monroe HM, McQueen MJ, de Graaf J, Furberg CD. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337–345. doi: 10.1161/CIRCOUTCOMES.110.959247. [DOI] [PubMed] [Google Scholar]
- 3.Thanassoulis G, Williams K, Ye K, Brook R, Couture P, Lawler PR, et al. Relations of change in plasma levels of LDL-C, non-HDL-C and apoB with risk reduction from statin therapy: A meta-analysis of randomized trials. J Am Heart Assoc. 2014;3:e000759. doi: 10.1161/JAHA.113.000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mudd JO, Borlaug BA, Johnston PV, Kral BG, Rouf R, Blumenthal RS, Kwiterovich PO., Jr Beyond low-density lipoprotein cholesterol: Defining the role of low-density lipoprotein heterogeneity in coronary artery disease. J Am Coll Cardiol. 2007;50:1735–1741. doi: 10.1016/j.jacc.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 5.Pencina MJ, D'Agostino RB, Zdrojewski T, Williams K, Thanassoulis G, Furberg CD, et al. Apolipoprotein B improves risk assessment of future coronary heart disease in the framingham heart study beyond LDL-C and non-HDL-C. Eur J Prev Cardiol. 2015;22:1321–7. doi: 10.1177/2047487315569411. [DOI] [PubMed] [Google Scholar]
- 6.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 7.Sniderman AD, Lawler PR, Williams K, Thanassoulis G, de Graaf J, Furberg CD. The causal exposure model of vascular disease. Clin Sci (Lond) 2012;122:369–373. doi: 10.1042/CS20110449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Association for Cardiovascular Prevention & Rehabilitation; Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, et al. ESC Committee for Practice Guidelines (CPG) 2008-2010 and 2010-2012 Committees. ESC/EAS guidelines for the management of dyslipidaemias: The task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) European Heart Journal. 2011;32:1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 9.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF American College of Cardiology/American Heart Association Task Force on Practice G. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 11.Anderson TJ, Gregoire J, Hegele RA, Couture P, Mancini GB, McPherson R, et al. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2013;29:151–167. doi: 10.1016/j.cjca.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 12.Law MR, Wald NJ. Risk factor thresholds: Their existence under scrutiny. BMJ. 2002;324:1570–1576. doi: 10.1136/bmj.324.7353.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Cholesterol Treatment Trialists Consortium. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 14.Mora S, Buring JE, Ridker PM. Discordance of low-density lipoprotein (LDL) cholesterol with alternative LDL-related measures and future coronary events. Circulation. 2014;129:553–561. doi: 10.1161/CIRCULATIONAHA.113.005873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 16.Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Low-dose aspirin in the primary prevention of cancer: The Women's Health Study: A randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 17.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: The Women's Health Study: A randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 18.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119:931–939. doi: 10.1161/CIRCULATIONAHA.108.816181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook NR, Ridker PM. Further insight into the cardiovascular risk calculator: The roles of statins, revascularizations, and underascertainment in the women's health study. JAMA Intern Med. 2014;174:1964–1971. doi: 10.1001/jamainternmed.2014.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawler PR, Akinkuolie AO, Chandler PD, Moorthy MV, VanDenburgh MJ, Schaumberg DA, et al. Circulating N-linked glycoprotein acetyls and longitudinal mortality risk. Circ Res. 2016;118:1106–15. doi: 10.1161/CIRCRESAHA.115.308078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarcho JA, Keaney JF., Jr Proof that lower is better - LDL cholesterol and IMPROVE-IT. N Engl J Med. 2015;372:2448–2450. doi: 10.1056/NEJMe1507041. [DOI] [PubMed] [Google Scholar]
- 22.Giugliano RP, Sabatine MS. Are PCSK9 inhibitors the next breakthrough in the cardiovascular field? J Am Coll Cardiol. 2015;65:2638–2651. doi: 10.1016/j.jacc.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Everett BM, Smith RJ, Hiatt WR. Reducing LDL with PCSK9 inhibitors - the clinical benefit of lipid drugs. N Engl J Med. 2015;373:1588–1591. doi: 10.1056/NEJMp1508120. [DOI] [PubMed] [Google Scholar]
- 24.Sniderman AD, Lamarche B, Contois JH, de Graaf J. Discordance analysis and the gordian knot of LDL and non-HDL cholesterol versus apoB. Curr Opin Lipidol. 2014;25:461–467. doi: 10.1097/MOL.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 25.Sniderman AD, Islam S, Yusuf S, McQueen MJ. Discordance analysis of apolipoprotein B and non-high density lipoprotein cholesterol as markers of cardiovascular risk in the INTERHEART study. Atherosclerosis. 2012;225:444–449. doi: 10.1016/j.atherosclerosis.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 26.Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer MJ, Rimm EB. Non-high-density lipoprotein cholesterol and apolipoprotein B in the prediction of coronary heart disease in men. Circulation. 2005;112:3375–3383. doi: 10.1161/CIRCULATIONAHA.104.532499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


