Abstract
Although napping has received attention because of its associations with health and use as a method to understand the function of sleep, to our knowledge no study has systematically and statistically assessed reasons for napping. Using factor analysis, we determined the underlying structure of reasons for napping in diverse undergraduates (N=430, 59% female) and examined their relationships with self-reported sleep, psychological, and physical health. The 5 reasons for napping can be summarized using the acronym DREAM (Dysregulative, Restorative, Emotional, Appetitive, and Mindful). Only Emotional reasons for napping were uniformly related to lower well-being. The use of factor analysis raises possibilities for future research, including examining the stability, structure, and psychological and physical health processes related to napping throughout the lifespan.
Keywords: napping, normal sleep, individual differences, mental health, factor analysis
Napping – deliberate periods of sleep lasting from three minutes to three hours (Mednick & Drummond, 2008) – is a culturally embedded, lifespan-developmental phenomenon (Jenni & O’Connor, 2005). Napping in infants and young children is very common cross-culturally (Owens, 2004), but by adulthood, cultural practices influence napping behaviors, with the frequency of napping at least once per week varying between 36% to 80% (Dinges, 1989). Recent estimates indicate that 41%–74% (National Sleep Foundation, 2005, 2008, 2011, 2014; McDevitt, Alaynick, & Mednick, 2012; Pilcher, Michalowski, & Carrigan, 2001) of healthy American adults nap at least once per week.
Napping has recently received increased attention because of its associations with health and its use as a tool to understand the function of sleep, with both areas of research showing conflicting associations with well-being. In the epidemiology and public health literature, some studies show that napping is associated with increased mortality risk (Bursztyn, Ginsberg, Hammerman-Rozenberg, & Stressman, 1999; Jung, Song, Ancoli-Israel, & Barrett-Connor, 2013; Leng et al., 2014; Stone et al., 2009), but these studies are limited by confounds, disparate approaches to controlling for comorbid illnesses, and different definitions for napping, which make it difficult to compare results. For example, one recent study showed that there was an increase in mortality with frequent napping (Leng et al., 2014), but it used an extreme definition of napping where participants were asked to choose between napping every day for 60 minutes or never napping. In another study, frequent nappers who reported getting so sleepy throughout the day or evening that they needed a nap were at 1.73 times greater mortality risk (Hays, Blazer, & Foley, 1996), but they were also more likely to report depressive symptoms and be overweight. On the other hand, a study in healthy Greek individuals showed that people who took naps of any frequency or duration were at lower mortality risk 6 years later (Naska, Oikonomou, Trichopoulou, Psaltopoulou, & Trichopoulos, 2007).
Napping is also used as a methodological tool in psychology and neuroscience to understand the function of sleep. In healthy populations, studies show benefits of napping for perceptual learning (Mednick, Nakayama, & Stickgold, 2003), motor memory (Nishida & Walker, 2007), declarative memory (Tucker et al., 2006), creativity (Cai, Mednick, Harrison, Kanady, & Mednick, 2009), and vigilance (Milner & Cote, 2009). In addition, naps help promote homeostasis and recovery of alertness and immune functioning after sleep deprivation (Faraut et al., 2015; Macchi, Boulos, Ranney, Simmons, & Campbell, 2002). In contrast with epidemiology studies, individuals with mental or physical health problems are typically excluded from these experimental, laboratory studies. Furthermore, naps in the lab are in structured environments and are often optimized for length and time of day to take advantage of or control for circadian confounds (McDevitt, Rowe, Brady, Duggan, & Mednick, 2014); naps in the “real-world” may vary in terms of length, time of day, location, and psychological motivation. Thus, results from these studies may not generalize to other populations, and experimental naps may not be ecologically valid or representative of participants’ ordinary napping behaviors.
Despite the multidisciplinary nature of napping research, little is understood about the causal interrelationships between napping and health risk, such as whether changing napping frequency will directly change health, whether napping is a result of disruptions in physical or mental health, or whether napping and health are correlated because of biological, psychological, or social third (confounding) variables. Many participants in epidemiological studies are older adults with comorbid chronic illnesses (Goldman et al., 2008), and thus may be napping due to other health problems. For example, Tanabe and colleagues (2010) noted that associations between napping and mortality could potentially be explained by comorbid health factors, such as high body weight. On the other hand, although some studies suggest that frequent napping may interfere with nighttime sleep (Owens et al., 2010), studies of napping in healthier populations show markedly reduced or no associations with nighttime sleep or general health (Dautovich, McCrae, & Rowe, 2008; McDevitt et al., 2012).
How can we rectify these discrepant findings in the epidemiological and cognitive literatures regarding the consequences of napping? In a recent study of napping and mortality, Leng and colleagues (2014) report “Voluntary naps and naps as a result of underlying pathology have different implications for health, and identification of the reasons for the naps is crucial.” Furthermore, people who nap for one hour or more daily (Leng et al., 2014, p. 1120), or nap due to excessive sleepiness (Hays et al., 1996), are likely psychologically, socially, and physiologically different from people who take short naps a few times per week (Naska et al., 2007). Thus, there may be differences in psychological and physical health between individuals who nap voluntarily for relatively short periods of time versus individuals who frequently nap for long periods of time.
Understanding the reasons why people nap, as well as the correlates of these napping behaviors, can provide insights into normal and maladaptive nap behaviors in healthy and unhealthy populations. Most research on reasons for napping (Dinges, 1992) has categorized nappers into three categories: Appetitive (napping for enjoyment), Restorative (napping in response to subjective fatigue), and Prophylactic (napping in preparation for future sleep loss; see Milner and Cote, 2009 for a review). Studies in this area typically categorize people post-hoc based on other measures, such as daytime sleepiness ratings (Macchi et al., 2002) or frequency of napping (categorized as Appetitive/Habitual nappers; Milner, Fogel, & Cote, 2006). Experimental studies, on the other hand, often categorize naps based on study design. For example, when participants are randomly assigned to nap after sleep loss, these naps are termed “Restorative” or “Replacement” naps (Brooks & Lack, 2006). However, given the literature in epidemiology and public health on napping in populations with chronic illness (Patel et al., 2014; Picarsic et al., 2008; Xu et al., 2010), as well as research on napping and depression (Foley et al., 2007), there are likely other reasons individuals may choose to nap. To our knowledge, no previous study has aimed to statistically examine associations among Appetitive, Restorative, and Prophylactic reasons for napping, as well as other reasons for napping that may be associated with physical and psychological well-being.
Because of the discrepancies between the epidemiological and experimental psychology literature, as well as the lack of assessments of ordinary napping behavior in the psychological literature, we systematically assessed the reasons people nap by creating an inventory of 29 reasons for napping by determining the underlying structure using factor analysis. These results are summarized in our five-factor model (DREAM, see Figure 2). Finally, we demonstrate that use of the DREAM model shows differential associations between reasons for napping and psychological, social, and physical health variables in a college sample, thus helping to clarify discrepancies in the literature. Importantly, the DREAM model can be tested and extended in populations that vary in age, cultural or socioeconomic background, and health.
Figure 2.
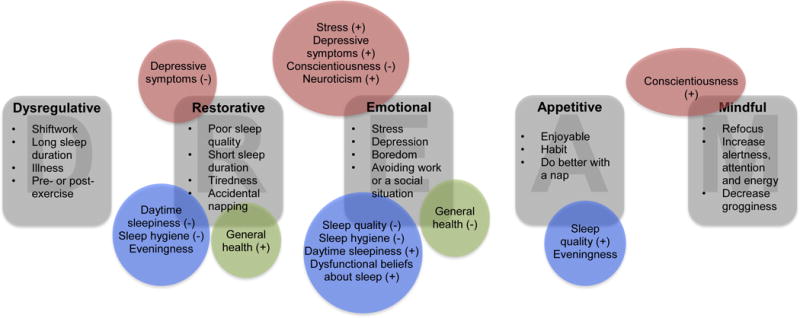
The DREAM model of reasons for napping and a summary of the results
Methods
Participants
A total of 438 undergraduate students enrolled at the University of California, Riverside volunteered to participate in a research study for course credit. Consent and survey responses were documented anonymously online using SurveyMonkey (http://www.surveymonkey.com). The survey took approximately 30 minutes to complete. Participants who started the survey but did not complete the majority of measures (n = 5), as well as those who did not endorse at least one reason for napping but otherwise completed the other sleep, psychological, and health scales (n = 3) were eliminated, leaving a final sample size of 430 (Mage = 19.91, SD = 1.47, range [17.9, 30.9]; 59% female). Participants were ethnically diverse (49% Asian, 34% Hispanic, 11% White, 4% Black, 1% other), and most were second-generation immigrants (15% first generation, 69% second generation, 15% third generation or higher). Participants were also diverse in terms of perceived socioeconomic status (M = 6.89, SD = 1.69, range [2, 10]), which was assessed from low (1) to high (10) using a modified ladder scale (Adler, Epel, Castellazzo, & Ickovics, 2000).
Measures
Napping
Participants were asked “When you nap, even if only very rarely, why do you choose to nap? Choose all that apply.” Reasons for napping were developed based on previous literature on the psychological and physical health correlates of napping, theory about appetitive, prophylactic, and restorative napping, and open-ended questions about reasons for napping from our previous research studies. A total of 29 reasons were listed, and participants were able to select “other” to specify a different reason. Additionally, participants rated their frequency of napping using a 4-point categorical scale (0 = never nap, 1 = nap once or twice a month, 2 = nap once or twice a week, 3 = nap every day; see Figure 1). Finally, participants rated their typical levels of post-nap sleep inertia by responding to the single item question, “How do you feel when you wake up from a nap?” using a 9-point scale (9 = extremely sleepy, fighting sleep, 5 = neither alert nor sleepy, and 1 = extremely alert; Åkerstedt & Gillberg, 1990).
Figure 1.
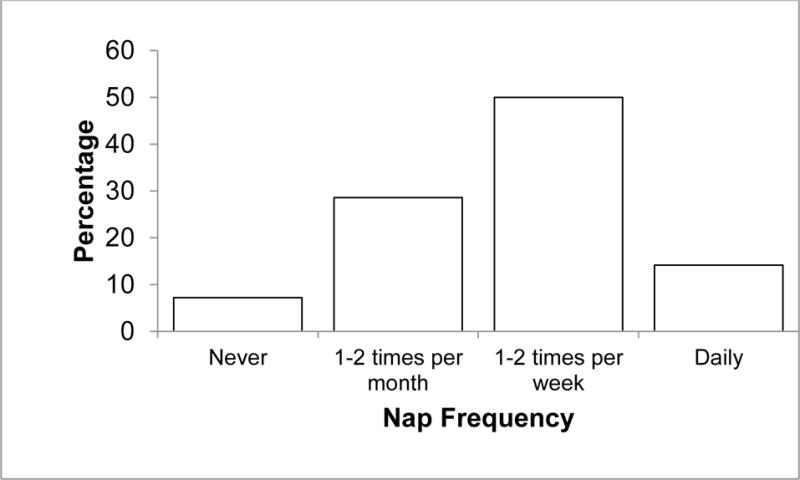
Frequency of Napping
Sleep
In addition to reasons for napping, participants also provided other information about their sleep. Nighttime sleep quality was assessed using the Pittsburgh Sleep Quality Index (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989) which measures global sleep quality using seven components: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleeping medication, and daytime dysfunction. The Pittsburgh Sleep Quality Index has high internal consistency reliability (α = .83) and is sensitive and specific enough to discriminate healthy patients free of sleep complaints from patients with sleep disorders (Buysse et al., 1989). Items were answered either using a 4-point rating scale or by indicating time. Sample items include “During the last month, how often have you had trouble sleeping because you wake up in the middle of the night or early morning?” and “During the past month, how would you rate your sleep quality overall?” Higher scores indicate worse sleep quality.
Trait daytime sleepiness was assessed using the Epworth Sleepiness Scale (Johns, 1991), which asks participants how likely they are to doze off or fall asleep in particular situations. The Epworth Sleepiness Scale has high internal consistency (α = .73–.88) and high test-retest reliability in situations where sleepiness is expected to remain constant (r = .82), and scores decrease when patients are treated for sleep disturbance (Johns, 1992). Items were answered using a 4-point rating scale (0 = would never doze and 3 = high chance of dozing). Sample situations include “sitting and reading” and “lying down to rest in the afternoon when circumstances permit.” Higher scores indicate higher trait sleepiness.
Sleep hygiene was measured using the Sleep Hygiene Index (Mastin, Bryson, & Corwyn, 2006). The Sleep Hygiene Index has acceptable internal consistency reliability (α = .66) and test-retest reliability (r = .71) and is positively correlated with associated features of inadequate sleep hygiene (Mastin, Bryson, & Corwyn, 2006). Items were answered using a 6-point rating scale (1 = never and 6 = always). Sample items include “I go to bed at different times from day to day” and “I use alcohol, tobacco, or caffeine within 4 h of going to bed or after going to bed.” Higher scores indicate worse sleep hygiene.
Chronotype was measured using the Horne-Östberg Morningness-Eveningness Questionnaire (Horne & Östberg, 1976). The Morningness-Eveningness Questionnaire has high internal consistency reliability (α = .86), high test-retest reliability (r = .89), and correlates with rising time and circadian variation in oral temperature (Horne & Ӧstberg, 1976; Neubauer, 1992). Items were answered either by indicating a time preference or making ratings on a 4-point scale. Sample items include “Considering only your own ‘feeling best’ rhythm, at what time would you get up if you were entirely free to plan your day?” and “One hears about ‘morning’ and ‘evening’ types of people. Which ONE of these types do you consider yourself to be?” Higher scores indicate morningness whereas lower scores indicate eveningness.
Dysfunctional beliefs about sleep were measured using the Dysfunctional Beliefs About Sleep Scale (Morin, Vallières, & Ivers, 2007). The Dysfunctional Beliefs About Sleep Scale has adequate internal consistency (α = .79) and temporal stability (r = .83), and correlates with measures of insomnia severity, anxiety, and depression, but not specific sleep parameters (Morin, Valliѐres, & Ivers, 2007). Items were answered using an 11-point rating scale (0 = strongly disagree and 10 = strongly agree). Sample items include “After a poor night’s sleep, I know that it will interfere with my daily activities on the next day” and “When I sleep poorly on one night, I know it will disturb my sleep schedule for the whole week.” Higher scores indicate more dysfunctional beliefs and attitudes about sleep.
Psychological, social, and physical health
Information was also collected on participant well-being. Depression symptoms were measured using the Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977). The CES-D has high internal consistency (α = .85–.90) and acceptable test-retest reliability (r = .57). Scores differentiate between psychiatric inpatients and the general population, and they improve after psychiatric treatment (Radloff, 1977). Participants were asked to indicate how often they have felt a particular way during the past week on a 4-point rating scale (0 = rarely or none of the time (less than 1 day) and 3 = most or all of the time (5–7 days)). Sample items include “I felt depressed” and “I felt that I could not shake off the blues even with help from my family and friends.” Higher scores indicate greater depression symptoms.
Stress was measured using the 14-item Perceived Stress Scale (Cohen, Kamarck, & Mermelstein, 1983). The Perceived Stress Scale is reliable (α = .84–.86), stable across times (r = .55), and correlates with stressful life events, health care utilization, and social anxiety (Cohen, Kamarck, & Mermelstein, 1983). Items were answered using a 5-point rating scale (0 = never and 4 = very often). Sample items include “In the last month, how often have you felt nervous and ‘stressed’?” and “In the last month, how often have you felt difficulties were piling up so high that you could not overcome them?” Higher scores indicate greater levels of stress.
Personality (conscientiousness and neuroticism) was measured using the 44-item Big Five Inventory (John, Donahue, & Kentle, 1991), which has high test-retest reliability (ravg = .74) and maps well with peer reports of personality (ravg = .56; Rammstedt & John, 2007). Items were answered using a 5-point rating scale (1 = disagree strongly and 5 = agree strongly7). Sample items include “Makes plans and follows through with them” (conscientiousness) and “Can be moody” (neuroticism). Higher scores indicate higher levels of each personality trait.
General health was measured using the RAND Short Form-36 (only the general health subscale is reported here; Ware & Sherbourne, 1992). The Short Form-36 is reliable (αs ≥ .70) and correlates with frequency and severity of physical health symptoms, morbidity, and mortality (Lowrie, Curtin, LePain, & Schatell, 2003; Ware & Sherbourne, 1992). Items were answered using a 5-point rating scale. Sample items include “In general, would you say your health is…” (rated using a 5-point scale from excellent to poor) and “I am as healthy as anybody I know” (rated using a 5-point scale from definitely true to definitely false). Higher scores indicate better self-rated health. For participant descriptives on these measures, see Table 1 and Figure 1.
Table 1.
Descriptives.
| Measure | Higher scores indicate… | N | M (SD) | Range |
|---|---|---|---|---|
| Nap Experience | ||||
| Post-Nap Sleep Inertia | More sleep inertia | 430 | 5.28 (1.99) | 1–9 |
| Sleep Variables | ||||
| Sleep quality | Worse sleep quality | 430 | 7.16 (2.96) | 0–16 |
| Daytime sleepiness | More sleepiness | 430 | 8.6 (3.58) | 0–20 |
| Sleep hygiene | Worse sleep hygiene | 430 | 37.7 (6.33) | 13–58 |
| Chronotype | Morningness | 430 | 43.46 (8.55) | 23–65 |
| Dysfunctional beliefs about sleep | More dysfunctional beliefs | 399 | 4.31 (1.61) | 0.38–9.19 |
| Psychological and Health Variables | ||||
| Depression | More depression symptoms | 430 | 23.80 (10.07) | 4–53 |
| Stress | More stress | 425 | 27.32 (6.97) | 0–53 |
| Conscientiousness | Higher conscientiousness | 430 | 3.44 (0.63) | 1.67–5 |
| Neuroticism | Higher neuroticism | 430 | 2.94 (0.73) | 1–4.88 |
| General health | Better health | 419 | 61.03 (19.16) | 0–100 |
Analyses
First, we examined descriptive statistics for napping habits. Frequency of napping and endorsement rates for reasons for napping were analyzed using frequency distributions. Reasons for napping were coded dichotomously, with a 1 indicating that the participant endorsed that reason, and a 0 indicating the participant did not endorse that reason. For endorsement rates of the 29 reasons for napping, see Table 2. We examined whether nap habits are significantly associated with sex, age, and nap experience using Pearson, Spearman, and phi correlation coefficients, as appropriate (see Table 3).
Table 2.
Endorsement rates and factor structure.
| Item | Percentage Endorsed | Dysregulative Factor E = 1.30 α = .63 |
Restorative Factor E = 2.43 α = .70 |
Emotional Factor E = 2.84 α = .76 |
Appetitive Factor E = 1.77 α = .68 |
Mindful Factor E = 10.79 α = .78 |
|---|---|---|---|---|---|---|
| Because I have taken medication that makes me drowsy | 13.02% | .73 | .33 | .44 | .17 | .30 |
| I slept a lot the night before; I slept too much the night before | 10.93% | .69 | .39 | .44 | .42 | .33 |
| I work a nightshift for my job | 6.74% | .65 | .17 | .18 | .25 | .33 |
| I have been sick; I am not feeling well | 47.21 % | .55 | .62 | .46 | .01 | .30 |
| I nap to prepare for strenuous physical activity (i.e., exercise) | 17.67% | .54 | .21 | .25 | .39 | .43 |
| I nap after strenuous physical activity (i.e., exercise) | 33.95% | .52 | .38 | .45 | .45 | .38 |
| I am experiencing pain | 17.44% | .52 | .53 | .51 | .19 | .35 |
| I didn’t get enough sleep the night before | 70.23% | .22 | .95 | .40 | .25 | .38 |
| I didn’t sleep well the night before | 60.23% | .32 | .75 | .35 | .26 | .29 |
| I am tired | 81.16% | .23 | .71 | .36 | .23 | .42 |
| I know I have to stay up late (e.g., for school or work) that night | 43.95% | .38 | .64 | .53 | .34 | .54 |
| I fell asleep even though I didn’t intend to (i.e., accidentally dozed off) | 40% | .39 | .51 | .66 | .12 | .20 |
| Because I am avoiding a social situation | 10.23% | .42 | .29 | .88 | .41 | .29 |
| Because I am avoiding work; procrastinating | 31.16% | .21 | .46 | .82 | .33 | .23 |
| I am sad or depressed | 34.42% | .31 | .62 | .71 | .46 | .37 |
| Because I am bored | 25.81% | .35 | .26 | .70 | .45 | .16 |
| I am stressed or overwhelmed | 42.79% | .25 | .69 | .68 | .43 | .34 |
| To improve my mood | 36.05% | .27 | .58 | .58 | .55 | .56 |
| Because napping is a habit for me | 18.37% | .30 | .25 | .46 | .82 | .21 |
| Because napping is part of my schedule; I plan to nap | 12.56% | .27 | .26 | .38 | .80 | .31 |
| I enjoy napping; it feels good | 59.30% | .27 | .27 | .39 | .77 | .39 |
| I feel I do better with a nap; I feel that naps are beneficial | 55.35% | .18 | .33 | .27 | .75 | .67 |
| Because I have free time | 35.81% | .30 | .40 | .63 | .54 | .27 |
| To increase my attention | 44.65% | .33 | .36 | .27 | .32 | .93 |
| To increase alertness | 42.79% | .35 | .28 | .22 | .28 | .91 |
| To give me more energy | 60.47% | .35 | .41 | .24 | .34 | .85 |
| To help me refocus; Because I have been thinking a lot and need to refocus | 47.67% | .30 | .51 | .34 | .31 | .71 |
| To decrease grogginess | 37.67% | .33 | .41 | .26 | .19 | .56 |
| I have heard that people do better with a nap; I have heard that napping is beneficial | 30.70% | .26 | .27 | .23 | .32 | .49 |
Table 3.
Descriptives for factor analytically-derived reasons for napping and their relationships with demographics and nap experience.
| Dysregulative | Restorative | Emotional | Appetitive | Mindful | |
|---|---|---|---|---|---|
| Descriptives | |||||
| Median | 1 | 3 | 1 | 2 | 3 |
| M (SD) | 1.47 (1.50) | 2.96 (1.57) | 1.80 (1.80) | 1.81 (1.47) | 2.64 (2.02) |
| Range | 0–7 | 0–5 | 0–6 | 0–5 | 0–6 |
| Correlations among reasons for napping | |||||
| Restorative |
r = .46 p < .0001 |
||||
| Emotional |
r = .48 p < .0001 |
r = .54 p < .0001 |
|||
| Appetitive |
r = .37 p < .0001 |
r = .34 p < .0001 |
r = .53 p < .0001 |
||
| Mindful |
r = .41 p < .0001 |
r = .41 p < .0001 |
r = .36 p < .0001 |
r = .39 p < .0001 |
|
| Correlations between reasons for napping, demographics, and nap experience | |||||
| Sex |
r = .12 p = .48 |
r = .17 p = .03 |
r = .18 p = .04 |
r = .16 p = .06 |
r = .14 p = .25 |
| Age |
r = .02 p = .72 |
r = −.08 p = .09 |
r = −.04 p = .41 |
r = −.07 p = .14 |
r = .005 p = .92 |
| Frequency of Napping |
r = .17 p = .0004 |
r = .15 p = .002 |
r = .27 p < .0001 |
r = .49 p < .0001 |
r = .26 p < .0001 |
| Sleep Inertia |
r = −.004 p = .94 |
r = .04 p = .38 |
r = .07 p = .16 |
r = −.11 p = .02 |
r = −.16 p = .001 |
| Differences in reasons for napping based on ethnicity | |||||
| Ethnicity |
F(4, 417) = 1.01 p = .40 |
F(4, 417) = 1.56 p = .18 |
F(4, 417) = 1.49 p = .21 |
F(4, 417) = 3.22 p = .01 |
F(4, 417) = 0.24 p = .92 |
Next, we reduced the 29 reasons for napping into meaningful and interpretable groups while still retaining much of the original variance in the items by doing an exploratory factor analysis (Cohen, Cohen, West, & Aiken, 2003; Rosenthal & Rosnow, 2008) using a tetrachoric correlation matrix due to the dichotomous nature of the items in SAS 9.3. Due to the fact that reasons for napping could theoretically correlate (i.e., individuals may endorse multiple reasons for napping, and those reasons may be related to each other), we used oblique rotation. This is advantageous because the resulting factors can correlate, but also makes it more difficult to interpret the resulting factors and the item loadings. Thus, we used a holistic approach to select the number of factors to retain, considering factors with Eigenvalues > 1.0, but also considering the change in Eigenvalue across factors and the reliability of the resulting factors in order to retain meaningful dimensions. Items that loaded highly on only one factor were retained for that factor. If an item loaded within multiple factors by ±.15, we selected the final assignment based on a combination of interpretability, previous theory, and reliability of that factor. Finally, nap preferences (factor) scores were computed for each participant using unit weighting, which assigns each item to only one factor and adds up the scores on the items that compose each factor. All factor analytic procedures were done a priori, before examining the associations between nap preferences and the other survey data.
Finally, we used the factor scores to examine napping profiles. To determine whether the reasons for napping varied based on ethnicity, we used one-way ANOVAs (with Bonferroni corrections for multiple comparisons in post-hoc tests). To determine whether individuals tend to endorse multiple reasons for napping, participant scores on the nap preferences were correlated with each other. Because results showed that reasons for napping are correlated with each other (rs > .34, ps < .0001; see Table 3), and individuals that nap more frequently may be more likely to endorse multiple reasons for napping, correlations are not ideal for determining whether specific reasons for napping are associated with sleep, psychological, or health variables. Thus, multiple linear regressions were used, with nap preferences as the independent variables and sleep, psychological, or health status as the dependent variable. This allows us to examine the overall contribution of reasons for napping (using model fit statistics) as well as independent contribution of each reason for napping, controlling for all other reasons for napping (using the individual parameter estimates and their associated p values). Nap preference scales were centered at 0 to aid in interpretability of the parameter estimates. Parameter estimates are interpreted as the unit change in psychosocial health for every 1-unit change in each reason for napping beyond 0 reasons, controlling for the other reasons in the model.
Results
Why do people nap? Descriptive results
Most participants endorsed napping at least once per month: 14% reported napping every day (N = 61), 50% napped at least once per week (N = 215), 29% napped at least once per month (N = 123), and 7% reported never napping (N = 31). On average, participants report feeling neither alert nor sleepy when waking up from a nap (M = 5.27, SD = 1.99), and participants who nap more frequently report lower rates of sleep inertia after napping than participants who nap less frequently (r = −.14, p = .005).
There was much variability in the number of reasons for napping endorsed by participants (M = 10.68, SD = 6.16, median = 10, mode = 4, range [1, 29]). Because naps reduce fatigue, we were not surprised that the most frequently reported reason for napping was “I am tired” (81% of participants endorsed this reason), followed by “I didn’t get enough sleep the night before (70%), “To give me more energy” (60%), and “I didn’t sleep well the night before” (60%). Appetitive reasons for napping were also endorsed by over half of participants, including “I enjoy napping; it feels good” (59%) and “I feel I do better with a nap; I feel that naps are beneficial” (55%). The three least frequently reported reasons were “I work a nightshift for my job” (7%), “Because I am avoiding a social situation” (10%), and “I slept a lot the night before; I slept too much the night before” (11%).
We investigated whether nap frequency, sleep inertia, or the number or type of reasons endorsed differed by sex or age. Women endorsed more reasons for napping (r = .15, p = .002) and napped more frequently (r = .16, p = .0007) than men. Women were also more likely to endorse napping to give them energy (r = .14, p = .003) and help them refocus (r = .11, p = .02); because they were stressed or overwhelmed (r = .17, p = .0004), sad or depressed (r = .12, p = .01); due to not enough sleep (r = .13, p = .009), being tired (r = .12, p = .01); when they have free time (r = .11, p = .03), because it is a habit (r = .11, p = .02); and when they are in pain (r = .12, p = .01) or sick (r = .16, p = .001). Men were more likely to endorse napping due to working a nightshift (r = −.10, p = .05). Age was only significantly correlated with sleep inertia post-nap (r = −.12, p = .01), with older participants reporting less sleep inertia after their naps. Age was not significantly associated with individual reasons for napping, though there was a restriction of range on age. Overall, these results suggest that women nap more frequently and report more reasons for napping than men.
Can reasons for napping be reduced to theoretically meaningful, interpretable factors?
We used exploratory factor analysis to reduce the 29 reasons for napping into interpretable factors. The factor analysis yielded 7 factors with Eigenvalues > 1.0. Aside from the first and second factors (Es = 10.79 and 2.84, respectively), the change in Eigenvalues across factors 1–5 was between 0.42–0.65. However, the difference between Factors 5 and 6, as well as Factors 6 and 7, was ΔE = 0.1–.15. Thus, even though factors 6 and 7 had Eigenvalues > 1.0, we chose to retain 5 factors for analysis. Item endorsement rates, factor loadings, eigenvalues, and reliability coefficients are presented in Table 2. These 5 factors can be summarized by the acronym DREAM: Dysregulative, Restorative, Emotional, Appetitive, and Mindful (see Figure 2).
Dysregulative Nappers
Dysregulative Nappers (E = 1.30, α = .63) reported napping due to shiftwork (occupational dysregulation), long sleep duration (homeostatic dysregulation), or due to illness, pain, preparing for exercise, or after exercise (physical or physiological dysregulation). As would be expected in this relatively healthy (see Table 1) young adult population, this factor was endorsed the least frequently relative to the other factors (M = 1.47, SD = 1.50, median = 1, range [0, 7]).
Individuals scoring high on Restorative Napping (E = 2.43, α = .70) primarily endorsed napping because of poor sleep, including short sleep duration, poor sleep quality, tiredness, prophylactically napping before a night of short sleep, and accidental napping. Consistent with research showing women report worse nighttime sleep quality (Reyner & Horne, 1995), restorative nappers were significantly more likely to be women (r = .17; see Table 3). This factor was endorsed more frequently than the other factors (M = 2.96, SD = 1.57, median = 3, range [0, 5]).
Emotional Nappers
Emotional Nappers (E = 2.84, α = .75) reported napping because they want to improve their mood due to stress, depression, or boredom, or because they are avoiding work or a social situation. Consistent with previous research showing that women report higher rates of psychological distress (Nolen-Hoeksema, 2001), women are more likely to endorse Emotional reasons for napping (r = .18; see Table 3). The strong reliability of this factor, coupled with the dissociation of Emotional Factor from the Restorative (poor sleep) or the Dysregulative (physiological) Factors in the factor analysis, suggests that napping due to psychological distress may be a key underexplored reason for napping (M = 1.80, SD = 1.80, median = 1, range [0, 6]).
Appetitive Nappers
Appetitive Nappers (E = 1.77, α = .68) enjoy napping, make it a habit, incorporate it into their schedules, and report doing better with a nap. People who endorse appetitive reasons for napping are more likely to have lower levels of sleep inertia after a nap (r = −.11). Appetitive reasons for napping are also the most strongly associated with frequency of napping (r = .49). Appetitive napping was the only factor significantly related to ethnicity (F(4, 417) = 3.22, p = .01); with Asian participants reporting slightly more Appetitive reasons (M = 2.03, SD = 1.52) then White participants (M = 1.23, SD = 1.21). Relative to the other factors, this factor was endorsed moderately (M = 1.81, SD = 1.47, median = 2, range [0, 5]).
Finally, individuals scoring high on Mindful Napping (E = 10.79, α = .78) reported napping to refocus, increase alertness, attention, and energy, to decrease grogginess, and because they have heard that people do better with a nap. It is interesting that the hearing people do better with a nap loaded more strongly on Mindful than Appetitive Napping, whereas believing one personally does better with a nap loaded more strongly on Appetitive than Mindful napping. Thus, Mindful Nappers may nap due to cognitive benefits they may have heard about from others, whereas Appetitive Nappers nap due to personal experience. Compared to the other reasons for napping, this factor was endorsed relatively frequently (M = 2.64, SD = 2.02, median = 3, range [0, 6]).
Are these factors differentially related to sleep, psychological, and physical health indices?
As expected, correlation coefficients revealed that individuals tend to endorse multiple reasons for napping (see Table 3). This is expected because more frequent nappers are likely to endorse more reasons for napping, and also because the factor analytic procedure used oblique rotation, which allowed the resulting factors to correlate. Thus, we used multiple regression to examine the unique contribution of each reason for napping, controlling for all other reasons for napping in the model, to assess the relationship between each factor-analytically derived reason for napping with self-reported sleep, psychosocial, and physical health variables.
Using multiple regressions, reasons for napping explained between 2.83%–11.87% of the variance in sleep, psychological, and health variables (see Table 4), and they also revealed distinct profiles associated with specific reasons for napping. Independent of the other reasons for napping, Dysregulative reasons were not associated with significantly worse sleep, psychological status, or general health. This is consistent with the relatively high levels of poor sleep quality (M = 7.16) but relatively good general health (M = 61.03) in our sample, and suggests that college students may nap for Dysregulative reasons only when they are sick or exhausted due to external factors (e.g., shiftwork) which may occur relatively infrequently.
Table 4.
Multiple linear regressions
| Dependent Variable | Model Statistics (F, p, R2) |
Constant | Dysregulative (b, p) |
Restorative (b, p) |
Emotional (b, p) |
Appetitive (b, p) |
Mindful (b, p) |
|---|---|---|---|---|---|---|---|
| Sleep quality |
F(5, 424) = 3.50 p = .004 R2 = 2.83% |
7.31 |
b = 0.21 p = .07 |
b = −0.19 p = .10 |
b = 0.34 p = .001 |
b = −0.25 p = .04 |
b = −0.03 p = .74 |
| Daytime sleepiness |
F(5, 424) = 5.53 p < .0001 R2 = 5.01% |
8.07 |
b = 0.08 p = .58 |
b = −0.29 p = .03 |
b = 0.42 p = .0009 |
b = 0.20 p = .16 |
b = 0.06 p = .55 |
| Sleep hygiene |
F(5, 424) = 12.55 p < .0001 R2 = 11.87% |
34.17 |
b = 0.24 p = .30 |
b = 0.57 p = .01 |
b = 0.68 p = .002 |
b = 0.38 p = .12 |
b = −0.16 p = .35 |
| Chronotype |
F(5, 424) = 8.44 p < .0001 R2 = 7.98% |
47.61 |
b = 0.61 p = .06 |
b = −1.17 p = .0002 |
b = −0.33 p = .26 |
b = −1.01 p = .002 |
b = 0.33 p = .15 |
| Dysfunctional beliefs about sleep |
F(5, 393) = 4.02 p = .001 R2 = 3.65% |
3.76 |
b = −0.06 p = .32 |
b = 0.11 p = .08 |
b = 0.14 p = .02 |
b = 0.003 p = .97 |
b = 0.02 p = .59 |
| Depression |
F(5, 424) = 8.83 p < .0001 R2 = 8.37% |
23.02 |
b = 0.14 p = .72 |
b = −0.87 p = .02 |
b = 2.05 p <.0001 |
b = −0.18 p = .64 |
b = −0.09 p = .73 |
| Stress |
F(5, 419) = 11.60 p < .0001 R2 = 11.11% |
24.62 |
b = −0.08 p = .75 |
b = 0.17 p = .50 |
b = 1.29 p < .0001 |
b = 0.04 p = .89 |
b = −0.04 p = .83 |
| Conscientiousness |
F(5, 424) = 8.67 p < .0001 R2 = 8.21% |
3.54 |
b = 0.01 p = .57 |
b = 0.004 p = .86 |
b = −0.10 p < .0001 |
b = −0.04 p = .12 |
b = 0.05 p = .005 |
| Neuroticism |
F(5, 424) = 7.46 p < .0001 R2 = 7.00% |
2.76 |
b = −0.03 p = .25 |
b = 0.01 p = .61 |
b = 0.12 p < .0001 |
b = 0.003 p = .92 |
b = −0.02 p = .44 |
| General health |
F(5, 413) = 4.70 p = .0003 R2 = 4.24% |
60.72 |
b = −0.20 p = .78 |
b = 1.72 p = .02 |
b = −3.06 p < .0001 |
b = 0.62 p = .41 |
b = −0.03 p = .95 |
Restorative reasons for napping were associated with significantly lower daytime sleepiness (b = −0.29), worse sleep hygiene (b = 0.57), evening chronotype (b = −1.17), lower depression symptoms (b = −0.87), and better general health (b = 1.72). This is consistent with the content of the Restorative factor, and suggests that individuals are endorsing these items due to a bout of a poor night of sleep rather than poor physical or psychological health.
Emotional reasons for napping were associated with significantly worse sleep quality (b = 0.34) and sleep hygiene (b = 0.68)1, as well as higher daytime sleepiness (b = 0.42) and dysfunctional beliefs about sleep (b = 0.14). Emotional reasons for napping were also associated with self-reported psychological and physical health, including higher levels of depression (b = 2.05), stress (b = 1.29), and poor general health (b = −3.06). Additionally, emotional reasons for napping correlated with personality: emotional nappers tend to score higher on neuroticism (b = 0.12) and lower on conscientiousness (b = −0.10). Emotional napping was the only factor significantly correlated with poor sleep, psychological functioning, and physical health, regardless of the indicator used.
In contrast with Emotional reasons for napping, Appetitive reasons were associated with significantly better sleep quality (b = −0.25) despite their frequent napping (r = .49) and evening chronotype (b = −1.01). Finally, mindful napping was associated with high conscientiousness (b = 0.05), but not with any of the other sleep, psychological functioning, or physical health variables1. Thus, these results suggest that it is not frequent napping per se that is associated with poor physical and psychological health. Rather, considering the psychological motivation underlying napping behaviors can shed light on theoretically meaningful sleep behaviors that differentially relate to psychological and physical well-being.
Discussion
To our knowledge, this study is the first to statistically examine individuals’ reasons for napping, as well as their associated self-reported sleep, psychological, and physical health profiles. Using previous theoretical models of reasons for napping (Appetitive, Restorative, and Prophylactic; Dinges, 1992) as well as reviewing the empirical literature on the correlates of napping (e.g., psychological and physical health; Milner & Cote, 2009), we developed 29 reasons for napping. We then used factor analysis to reduce our 29 reasons for napping into five interpretable factors, thus providing insight into the psychological motivation for nap behaviors. We also used regression to construct sleep, psychological, and physical health profiles associated with reasons for napping, which have clinical and theoretical implications for the identification of nappers at risk of having health problems.
Why do people nap?
We developed a comprehensive list of 29 reasons for napping and examined their associations in a college population. Then, we used factor analysis to reduce these reasons for napping into 5 meaningful factors, summarized by the acronym DREAM (see Figure 2). Previous literature (see Milner and Cote, 2009 for a review) has primarily focused on Appetitive (napping for enjoyment), Restorative (napping to make up for sleep loss), and Prophylactic napping (napping in preparation for sleep loss). Although we found evidence for Appetitive reasons as their own factor, we found that Restorative and Prophylactic reasons were part of the same factor (termed “Restorative” in the factor analysis), suggesting that they share similar motivational processes. Furthermore, our factor analysis suggests the presence of three additional factors that have largely been ignored in the literature: Dysregulative (napping due to occupational, homeostatic or physiological dysregulation), Emotional (napping due to stress, boredom, depression, or avoiding work or a social situation), and Mindful (napping to increase alertness, attention, and energy). These reasons for napping should be incorporated in future research.
Sleep, psychological, and physical health correlates of napping
We also found distinct sleep, psychological, and health profiles associated with the different reasons for napping. While some studies have found that napping is associated with poor sleep quality and increased mortality risk (Hays et al., 1996), not all studies find that frequent napping is associated with poor outcomes (Fichten et al., 1995; Pilcher, Ginter, & Sadowsky, 1997), especially in healthier samples (Dautovich et al., 2008). In fact, some kinds of napping were associated with positive outcomes (e.g., Appetitive reasons were associated with significantly better nighttime sleep quality). Our data show only Emotional reasons for napping were uniformly associated with poor sleep, psychological, and physical health across all regression models. People that nap for Emotional reasons do so because they want to improve their mood due to stress, depression, or boredom, or to avoid social situations. Because depression and stress are associated with poor physical health status (Cassano & Fava, 2002; Cohen, Janicki-Deverts, & Miller, 2007), assessing whether individuals are napping for Emotional reasons may explain some of the discrepancies in the literature.
Personality traits that correlate with well-being also correlate with reasons for napping
Our results for associations between reasons for napping and personality traits build on previous research on personality, sleep, and health. Neuroticism is a personality trait that describes individuals who are emotionally labile and tend to experience more negative emotions such as anxiety, hostility, nervousness, and depression (John & Srivastava, 1999). Like depression and stress, high neuroticism is also associated with poor health (Lahey, 2009), but this may be due to increased sensitivity to somatic complaints (Costa & McCrae, 1987; Watson & Pennebaker, 1989). Individuals high in neuroticism also report poor sleep hygiene and sleep quality as well as high levels of daytime sleepiness (Duggan, Friedman, McDevitt, & Mednick, 2014). The current results add to this literature by demonstrating that individuals high in Neuroticism are more likely to report napping for Emotional reasons, which was the only factor associated with poor physical and mental health.
On the other hand, high levels of Conscientiousness were negatively associated with napping for Emotional reasons, and positively associated with napping for Mindful reasons. Conscientiousness is a personality trait that describes socially-prescribed impulse control, task- and goal-oriented behavior, planfulness, persistence, and dependability (John & Srivastava, 1999), and has been associated with health-promoting behaviors and decreased mortality risk (Bogg & Roberts, 2004; Kern & Friedman, 2008). Individuals high in conscientiousness also report good sleep hygiene and sleep quality as well as decreased daytime sleepiness (Duggan et al., 2014). The current results add to the literature on conscientiousness, sleep, and health by showing that conscientious individuals do not nap to compensate for poor nighttime sleep or physical health. Instead, consistent with their goal-oriented, persistent nature, conscientious individuals seem to use naps as a tool to increase their productivity by helping them increase alertness, attention, and energy.
Conclusions, Limitations, and Future Directions
To our knowledge, this is the first study to use factor analysis to distill people’s reasons for napping into interpretable categories. This allows the examination of the psychological motivation underlying napping behaviors, and sheds light on the psychosocial correlates of sleep that are often obscured by not separating napping behaviors into theoretically meaningful subtypes. However, the correlational nature of the study design does not permit causal or directional conclusions about the relationships between reasons for napping and well-being, nor does it suggest that individuals must have an awareness of the consequences of their sleep in order for their sleep to serve a particular function.
The analytic approach we chose has some limitations. Reasons for napping were answered dichotomously, which necessitated the use of a tetrachoric correlation matrix for the factor analysis. Consequently, this may artificially inflate the variance explained by the factor analysis, but would not change the interpretation of the resulting factors (Cohen et al., 2003). Furthermore, content and structure of the factor analysis could change if other potential reasons for napping were not included. We utilized a relatively healthy, diverse young adult sample. It is possible that we have not captured all possible reasons for or functions of napping. The number and content of the factors, as well as the associations between the factors and well-being, might change in other samples. Finally, relatively little variance in sleep, psychological, social, and physical health was accounted for by reasons for napping in the regressions (2.8%–11.9%), which highlights a need for further study, including refining the reasons for napping and expanding the response format in other samples. Future research should consider using a rating scale response format, examine reasons for napping in other groups, including older adults and individuals with health problems, and study relationships between reasons for napping and well-being across time. In conclusion, this novel application of factor analysis to reasons for napping raises exciting possibilities for future research, such as examining the stability and structure of reasons for napping throughout the lifespan, as well as the psychological, social, and health processes associated with napping behaviors.
Acknowledgments
This research was supported by grant 1R01AG046646-01 to S.C.M. from the National Institute on Aging.
Footnotes
To verify that the napping item on the Sleep Hygiene Index did not affect our results, we re-ran the regression with the five reasons for napping predicting sleep hygiene, after subtracting out the napping item on the Sleep Hygiene Index. Model fit remained the same (p < .0001), but variance explained decreased (from 11.87% to 9.31%). The parameters’ effect sizes and statistical significance are virtually unchanged: Restorative (originally b = 0.57, p = .01 to b = 0.55, p = .01) and Emotional (originally b = 0.68, p = .002 to b = 0.63, p = .002) remained significant; Dysregulative, Appetitive, and Mindful remained non-significant. Thus, the napping item on the Sleep Hygiene Index has not substantially influenced our results.
Some participants (n=31) reported never napping (or napping less frequently than once per month), but still checked off at least one reason for napping. To see whether this `influenced the regression results, we re-ran the regressions while removing these participants. Model fit, variance explained, and parameter effect sizes and statistical significance were essentially unchanged for sleep quality, sleepiness, sleep hygiene, depression, stress, conscientiousness, and neuroticism. For chronotype, variance explained increased to 8.86%, and dysregulative became significant (b=0.69, p=.03). For dysfunctional beliefs about sleep, variance explained increased to 4.96%, and restorative became statistically significant (b=0.15, p=.02). For general health, variance explained decreased to 3.89%, and restorative dipped below traditional statistical significance levels (b=1.46, p=.054).
Contributor Information
Katherine A. Duggan, Department of Psychology, University of California, Riverside
Elizabeth A. McDevitt, Department of Psychology, University of California, Riverside
Lauren N. Whitehurst, Department of Psychology, University of California, Riverside
Sara C. Mednick, Department of Psychology, University of California, Riverside.
References
- Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: Preliminary data in healthy white women. Health Psychology. 2000;19(6):586–592. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. The International Journal of Neuroscience. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- Bogg T, Roberts BW. Conscientiousness and health-related behaviors: A meta-analysis of the leading behavioral contributors to mortality. Psychological Bulletin. 2004;130(6):887–919. doi: 10.1037/0033-2909.130.6.887. [DOI] [PubMed] [Google Scholar]
- Brooks A, Lack L. A brief afternoon nap following nocturnal sleep restriction: Which nap duration is most recuperative? Sleep. 2006;29(6):831–840. doi: 10.1093/sleep/29.6.831. [DOI] [PubMed] [Google Scholar]
- Bursztyn M, Ginsberg G, Hammerman-Rozenberg R, Stressman J. The siesta in the elderly: Risky factor for mortality? Archives of Internal Medicine. 1999;159:1582–1586. doi: 10.1001/archinte.159.14.1582. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cai DJ, Mednick S, Harrison EM, Kanady JC, Mednick SC. REM, not incubation, improves creativity by priming associative networks. Proceedings of the National Academy of Sciences. 2009;106(25):10130–10134. doi: 10.1073/pnas.0900271106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassano P, Fava M. Depression and public health: an overview. Journal of Psychosomatic Research. 2002;53(4):849–57. doi: 10.1016/s0022-3999(02)00304-5. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. 3rd. Mahwah, NJ: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA : the journal of the American Medical Association. 2007;298(14):1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social behavior. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Costa P, McCrae R. Neuroticism, somatic complaints, and disease: Is the bark worse than the bite? Journal of Personality. 1987;55(2):299–316. doi: 10.1111/j.1467-6494.1987.tb00438.x. [DOI] [PubMed] [Google Scholar]
- Dautovich ND, McCrae CS, Rowe M. Subjective and objective napping and sleep in older adults: Are evening naps “bad” for nighttime sleep? Journal of the American Geriatrics Society. 2008;56(9):1681–1686. doi: 10.1111/j.1532-5415.2008.01822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinges DF. Napping patterns and effects in human adults. In: Dinges DF, Broughton RJ, editors. Sleep and Alertness: Chronobiological, Behavioural, and Medical Aspects of Napping. New York, NY: Raven Press; 1989. pp. 171–204. [Google Scholar]
- Dinges DF. Adult napping and its effects on ability to function. In: Stampi C, editor. Why we nap: Evolution, chronobiology, and functions of polyphasic and ultrashort sleep. Boston: Birkhauser; 1992. pp. 118–134. [Google Scholar]
- Duggan KA, Friedman HS, McDevitt EA, Mednick SC. Personality and healthy sleep: The importance of conscientiousness and neuroticism. PloS one. 2014;9(3):e90628. doi: 10.1371/journal.pone.0090628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraut B, Nakib S, Drogou C, Elbaz M, Sauvet F, De Bandt J, Léger D. Napping reverses the salivary interleukin-6 and urinary norepinephrine changes induced by sleep restriction. Journal of Clinical Endocrinology & Metabolism. 2015;100(3):E416–E426. doi: 10.1210/jc.2014-2566. [DOI] [PubMed] [Google Scholar]
- Fichten CS, Creti L, Amsel R, Brender W, Weinstein N, Libman E. Poor sleepers who do not complain of insomnia: Myths and realities about psychological and lifestyle characteristics of older good and poor sleepers. Journal of Behavioral Medicine. 1995;18(2):189–223. doi: 10.1007/BF01857869. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Vitiello MV, Bliwise DL, Ancoli-Israel S, Monjan AA, Walsh JK. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: Findings from the National Sleep Foundation “2003 Sleep in America” Poll. The American Journal of Geriatric Psychiatry. 2007;15(4):344–350. doi: 10.1097/01.JGP.0000249385.50101.67. [DOI] [PubMed] [Google Scholar]
- Goldman SE, Hall M, Boudreau R, Matthews KA, Cauley JA, Ancoli-Israel S, Newman SB. Association between nighttime sleep and napping in older adults. Sleep. 2008;31(5):733–740. doi: 10.1093/sleep/31.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays JC, Blazer DG, Foley DJ. Risk of napping: Excessive daytime sleepiness and mortality in an older community population. Journal of the American Geriatrics Society. 1996;44(6):693–698. doi: 10.1111/j.1532-5415.1996.tb01834.x. [DOI] [PubMed] [Google Scholar]
- Horne JA, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology. 1976;4:97–110. [PubMed] [Google Scholar]
- Jenni OG, O’Connor BB. Children’s sleep: An interplay between culture and biology. Pediatrics. 2005;115(1):204–216. doi: 10.1542/peds.2004-0815B. [DOI] [PubMed] [Google Scholar]
- John OP, Donahue EM, Kentle RL. The Big Five Inventory - versions 4a and 54. Berkeley: 1991. [Google Scholar]
- John OP, Srivastava S. The Big Five Trait Taxonomy: History, Measurement, and Theoretical Perspectives. In: Pervin LA, John OP, editors. Handbook of Personality, Theory and Research. 2nd. New York: Guilford Press; 1999. pp. 102–138. [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Johns MW. Reliability and factor analysis fo the Epworth Sleepiness Scale. Sleep. 1992;14:540–545. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- Jung K, Song CH, Ancoli-Israel S, Barrett-Connor E. Gender differences in nighttime sleep and daytime napping as predictors of mortality in older adults: The Rancho Bernardo Study. Sleep Medicine. 2013;14(1):12–19. doi: 10.1016/j.sleep.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern ML, Friedman HS. Do conscientious individuals live longer? A quantitative review. Health Psychology. 2008;27(5):505–512. doi: 10.1037/0278-6133.27.5.505. [DOI] [PubMed] [Google Scholar]
- Lahey BB. Public health significance of neuroticism. American Psychologist. 2009;64(4):241–256. doi: 10.1037/a0015309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Wainwright NWJ, Cappuccio FP, Surtees PG, Hayat S, Luben R, Brayne C, et al. Daytime Napping and the Risk of All-Cause and Cause-Specific Mortality: A 13-Year Follow-up of a British Population. American Journal of Epidemiology. 2014;179(9):1115–24. doi: 10.1093/aje/kwu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrie EG, Curtin RB, LePain N, Schatell D. Medical outcoes study short form-36: A consistent and powerful predictor of morbidity and mortality in dialysis patients. American Journal of Kidney Diseases. 2003;41(6):1286–1292. doi: 10.1016/s0272-6386(03)00361-5. [DOI] [PubMed] [Google Scholar]
- Macchi MM, Boulos Z, Ranney T, Simmons L, Campbell SS. Effects of an afternoon nap on nighttime alertness and performance in long-haul drivers. Accident Analysis and Prevention. 2002;34(6):825–834. doi: 10.1016/s0001-4575(01)00089-6. [DOI] [PubMed] [Google Scholar]
- Mastin DF, Bryson J, Corwyn R. Assessment of sleep hygiene using the Sleep Hygiene Index. Journal of Behavioral Medicine. 2006;29(3):223–227. doi: 10.1007/s10865-006-9047-6. [DOI] [PubMed] [Google Scholar]
- McDevitt EA, Rowe KM, Brady M, Duggan KA, Mednick SC. The benefit of offline sleep and wake for novel object recognition. Experimental Brain Research. 2014;232(5):1487–96. doi: 10.1007/s00221-014-3830-3. [DOI] [PubMed] [Google Scholar]
- McDevitt EA, Alaynick WA, Mednick SC. The effect of nap frequency on daytime sleep architecture. Physiology & Behavior. 2012;107(1):40–44. doi: 10.1016/j.physbeh.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick SC, Drummond SPA. Napping. In: Stickgold R, Walker M, editors. The New Encyclopedia of Neuroscience. London: Elsevier; 2008. pp. 254–269. [Google Scholar]
- Mednick S, Nakayama K, Stickgold R. Sleep-dependent learning: A nap is as good as a night. Nature Neuroscience. 2003;6(7):697–698. doi: 10.1038/nn1078. [DOI] [PubMed] [Google Scholar]
- Milner CE, Cote KA. Benefits of napping in healthy adults: impact of nap length, time of day, age, and experience with napping. Journal of Sleep Research. 2009;18(2):272–281. doi: 10.1111/j.1365-2869.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- Milner CE, Fogel SM, Cote KA. Habitual napping moderates motor performance improvements following a short daytime nap. Biological Psychology. 2006;73(2):141–156. doi: 10.1016/j.biopsycho.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Morin CM, Vallières A, Ivers H. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16) Sleep. 2007;30(11):1547–54. doi: 10.1093/sleep/30.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naska A, Oikonomou E, Trichopoulou A, Psaltopoulou T, Trichopoulos D. Siesta in healthy adults and coronary mortality in the general population. Archives of Internal Medicine. 2007;167(3):296–301. doi: 10.1001/archinte.167.3.296. [DOI] [PubMed] [Google Scholar]
- National Sleep Foundation. 2005 Sleep in America Poll. Washington: 2005. [Google Scholar]
- National Sleep Foundation. 2008 Sleep in America Poll. Washington: 2008. [Google Scholar]
- National Sleep Foundation. 2011 Sleep in America Poll - Communications Technology in the Bedroom. Washington: 2011. [Google Scholar]
- National Sleep Foundation. 2014 Sleep in America Poll - Sleep In The Modern Family. Arlington: 2014. [Google Scholar]
- Neubauer AC. Psychometric comparison of two circadian rhythm questionnaires and their relationship with personality. Personality and Individual Differences. 1992;13:125–131. [Google Scholar]
- Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS ONE. 2007;(4):e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Gender differences in depression. Current Directions in Psychological Sciencce. 2001;10(5):173–176. [Google Scholar]
- Owens JA. Sleep in children: Cross-cultural perspectives. Sleep and Biological Rhythms. 2004;2:165–173. [Google Scholar]
- Owens JF, Buysse DJ, Hall M, Kamarck TW, Lee L, Strollo PJ, Matthews KA. Napping, nighttime sleep, and cardiovascular risk factors in mid-life adults. Journal of Clinical Sleep Medicine. 2010;6(4):330–335. [PMC free article] [PubMed] [Google Scholar]
- Patel SR, Hayes AL, Blackwell T, Ancoli-Israel S, Wing YK, Stone KL. The association between sleep duration and obesity in older adults. International Journal of Obesity. 2014;38:1159–1164. doi: 10.1038/ijo.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picarsic JL, Glynn NW, Taylor CA, Katula JA, Goldman SE, Studenski SA, Newman AB. Self-reported napping and duration and quality of sleep in the lifestyle interventions and independence for elders pilot study. Journal of the American Geriatrics Society. 2008;56(9):1674–1680. doi: 10.1111/j.1532-5415.2008.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilcher JJ, Ginter DR, Sadowsky B. Sleep quality versus sleep quantity: Relationships between sleep and measures of health, well-being and sleepiness in college students. Journal of Psychosomatic Research. 1997;42(6):583–596. doi: 10.1016/s0022-3999(97)00004-4. [DOI] [PubMed] [Google Scholar]
- Pilcher JJ, Michalowski KR, Carrigan RD. The prevalence of daytime napping and its relationship to nighttime sleep. Behavioral Medicine. 2001;27(2):71–76. doi: 10.1080/08964280109595773. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Rammstedt B, John OP. Measuring personality in one minute or less: A 10-item short version of the Big Five Inventory in English and German. Journal of Research in Personality. 2007;41:203–212. [Google Scholar]
- Reyner LA, Horne JA. Gender- and age-related differences in sleep determined by home-recorded sleep logs and actimetry from 400 adults. Sleep. 1995;18(2):127–134. [PubMed] [Google Scholar]
- Rosenthal R, Rosnow RL. In: Essentials of Behavioral Research: Methods and Data Analysis. 3rd. McGraw-Hill, editor. New York, NY: 2008. [Google Scholar]
- Stone KL, Ewing SK, Ancoli-Israel S, Ensrud KE, Redline S, Bauer DC, Cummings SR. Self-reported sleep and nap habits and risk of mortality in a large cohort of older women. Journal of the American Geriatrics Society. 2009;57(4):604–611. doi: 10.1111/j.1532-5415.2008.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe N, Iso H, Seki N, Suzuki H, Yatsuya H, Toyoshima H, Tamakoshi A. Daytime napping and mortality, with a special reference to cardiovascular disease: The JACC study. International Journal of Epidemiology. 2010;39(1):233–243. doi: 10.1093/ije/dyp327. [DOI] [PubMed] [Google Scholar]
- Tucker MA, Hirota Y, Wamsley EJ, Lau H, Chaklader A, Fishbein W. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiology of Learning and Memory. 2006;86(2):241–247. doi: 10.1016/j.nlm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- Watson D, Pennebaker JW. Health complaints, stress, and distress: Exploring the central role of negative affectivity. Psychological Review. 1989;96(2):234–254. doi: 10.1037/0033-295x.96.2.234. [DOI] [PubMed] [Google Scholar]
- Xu Q, Song Y, Hollenbeck A, Blair A, Schatzkin A, Chen H. Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes Care. 2010;33(1):78–83. doi: 10.2337/dc09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]


