Abstract
Positive physiologic and cognitive responses to aerobic exercise have resulted in a proposed cardiorespiratory (CR) fitness hypothesis in which fitness gains drive changes leading to cognitive benefit. The purpose of this study was to directly assess the CR fitness hypothesis. Using data from an aerobic exercise trial, we examined individuals who completed cardiopulmonary and cognitive testing at baseline and 26 weeks. Change in cognitive test performance was not related to CR fitness change (r2=0.06, p=0.06). However, in the subset of individuals who gave excellent effort during exercise testing, change in cognitive test performance was related to CR fitness change (r2=0.33, p<0.01). This was largely due to change in the cognitive domain of Attention (r2=0.36, p<0.01). The magnitude of change was not explained by duration of exercise. Our findings support further investigation of the CR fitness hypothesis and mechanisms by which physiologic adaptation may drive cognitive change.
Keywords: Cognition, cardiorespiratory fitness, exercise, attention
Aerobic exercise results in well-documented physiologic adaptations that support overall function and health (Garber et al., 2011). Human and animal studies have documented changes in the brain that are associated with improved cardiorespiratory (CR) fitness after aerobic exercise. This is evidenced by increased frontal and parietal cerebral blood flow (Ainslie et al., 2008; Brown et al., 2010), cerebral angiogenesis (Bullitt et al., 2009; Isaacs, Anderson, Alcantara, Black, & Greenough, 1992; Kleim, Cooper, & VandenBerg, 2002; Swain et al., 2003), upregulation of neurotrophic factors (Erickson et al., 2011; Neeper, Gomez-Pinilla, Choi, & Cotman, 1995), and neurogenesis (Pereira et al., 2007; van Praag, Christie, Sejnowski, & Gage, 1999). Further, the benefits of aerobic exercise for supporting cognitive function in older adults have been consistently demonstrated. This is true of both large epidemiologic trials (Buchman et al., 2012; Weuve et al., 2004; Yaffe, Barnes, Nevitt, Lui, & Covinsky, 2001) and smaller randomized controlled trials (Colcombe et al., 2004; Dustman et al., 1984; Erickson et al., 2011; Kramer et al., 1999; Vidoni et al., 2015). Executive functions, including planning, sequencing, attention and inhibition, appear to selectively benefit from aerobic exercise (Colcombe & Kramer, 2003; Smiley-Oyen, Lowry, Francois, Kohut, & Ekkekakis, 2008), and episodic memory may benefit as well.(Smith et al., 2010)
The neurophysiologic responses observed following aerobic exercise have resulted in a proposed “cardiorespiratory fitness hypothesis” (Kramer et al., 1999). Specifically, improved CR fitness as a result of aerobic exercise is hypothesized as a driving force behind cognitive benefit. We recently reported a dose response effect of aerobic exercise on components of visuospatial function in a group of older adults without cognitive impairment (Vidoni et al., 2015). We enrolled individuals into one of 3 ascending aerobic exercise doses (75 mins/wk, 150 mins/wk, or 225 mins/wk) or a no-change in activity control group for 26 weeks. We found cognitive benefits at all exercise doses in attention and visuospatial function, and evidence of a dose-response relationship between increasing doses of aerobic exercise and visuospatial function. Mediation analyses suggested that gains in CR fitness over the 26-week trial best predicted gains in cognition.
Recognizing the need to more directly assess the CR fitness hypothesis we performed a secondary analysis of those same data for the present report. We expected that change in CR fitness (measured as percent change in peak oxygen consumption, peak VO2) over the course of an aerobic exercise trial would be directly related to observed cognitive changes. Given that many factors can influence maximal achieved effort (Balady et al., 2010), we hypothesized that a relationship between change in fitness and cognition would be most apparent in those individuals who gave a physiological maximum or “excellent effort”, defined as a respiratory exchange ratio (RER) ≥ 1.1 (Balady et al., 2010) which we defined as Top Performers.
Method
Participants
We have previously reported on the results of The Trial of Exercise on Aging and Memory (TEAM: ClinicalTrials.gov, NCT01129115), a 26-week pilot randomized, controlled trial of aerobic exercise dose in nondemented individuals 65 years and older (Vidoni et al., 2015). Briefly, we recruited a convenience sample of participants who were sedentary or underactive as defined by the Telephone Assessment of Physical Activity (Mayer, Steinman, Williams, Topolski, & LoGerfo, 2008), and free of cognitive impairment. Participants could not be insulin-dependent, have uncorrected hearing or vision problems, uncontrolled hypertension, or have had recent history (<2 years) of major cardiac, pulmonary, musculoskeletal or neuropsychiatric impairment (e.g. major depression or bipolar disorder). We obtained written, informed consent approved by our Institutional Review Board. For the present analysis, we included only those individuals who participated “per protocol” and had all measures at baseline and follow-up (n=59).
Assessments and Measures
A trained psychometrician administered a comprehensive cognitive testing battery. Several tests in this battery overlap with those in the Uniform Data Set 2.0 (UDS), a test battery administered by all NIH-designated Alzheimer’s Disease Centers. These UDS tests can be combined into an age, sex and education normed score that represents Global Cognition or separate cognitive domains of Attention [Digit Span (Wechsler, 1987b)], Memory [Logical Memory IA and IIA (Wechsler, 1987b)], Executive Function [Trailmaking B (Reitan & Wolfson, 1985)], Processing Speed [Trailmaking A (Reitan & Wolfson, 1985) & Digit Symbol Substitution (Wechsler, 1987a)],] and Language [Semantic Fluency (Morris et al., 1989) & Boston Naming Test (Goodglass & Kaplan, 1983)]. The psychometrician also administered the Community Healthy Activities Model Program for Seniors physical activity survey (Stewart et al., 2001).
Participants performed a cardiopulmonary exercise test (CPX) using a modified Bruce protocol (Cornell treadmill test), which is designed for older adults (Hollenberg, Ngo, Turner, & Tager, 1998). Participants were attached to a 12-lead electrocardiograph to continuously monitor HR and rhythm. A 2-way non-rebreathing valve, headgear, mouthpiece, and nose clip were worn to continuously capture oxygen and carbon dioxide. Blood pressure and RPE were acquired during the last 30 seconds of each stage. The CPX was terminated if the participant reached volitional exhaustion or met absolute test termination criteria according to the American College of Sports Medicine guidelines (2014). We required that participants achieve greater than submaximal effort (RER>=1.0) on the baseline exercise test to be randomized (Balady et al., 2010). We tested the cognitive effects of 26 weeks of moderate aerobic exercise using 1 of 3 weekly durations: 75 min/wk, 150 min/wk, or 225 min/wk. We also included a no treatment control group who were asked not to change their current level of physical activity. For those in the aerobic exercise intervention, the duration of exercise was distributed over 3–5 sessions each week. Participants monitored their heart rate (F4 or FT4, Polar Electro, Inc, Lake Success, NY) and exercised in a target heart rate zone that increased over the course of the study. In weeks 1–4 the target heart rate zone was 40–55% of heart rate reserve (HRR) as calculated by the Karvonen formula. In Weeks 5–18, the target heart rate zone was 50–65 % of HRR. In weeks 19–26, the target heart rate zone was 60–75% of HRR. Participants primarily walked on a treadmill, adjusting speed and incline to achieve the desired heart rate zone. Initially, certified personal trainers provided supervision at every exercise session. Supervision was provided once a week for weeks 7 through 26, as individuals became more independent. Following 26 weeks of training, CPX was again performed with the same methodology as baseline testing.
Cognition, physical activity, and CR fitness were measured at baseline and post-intervention. Immediately after baseline testing, participants were block randomized into aerobic exercise dose assignments based on age and sex. For those participants randomized to an exercise group, the intervention was conducted in the community under the guidance of certified personal trainers who were trained and monitored by study staff.
Analysis
To test the CR fitness hypothesis we assessed the association of percent change in peak VO2 with our primary outcome of change in Global Cognition (the change in the average age, sex and education normalized cognitive test scores across the intervention) using linear regression, setting α=0.01 to protect against Type I error for our multiple comparisons and because the peak VO2 values have been used before. We planned to test the 5 cognitive domains only if change in Global Cognition was significantly associated with change in peak VO2.
We performed these analyses in two groups. First, we looked at all participants beginning with our cohort of individuals who finished the study, adhered to the intervention protocol, and gave similar effort at follow-up testing (Balady et al., 2010). We defined “similar effort” as RER +/− 0.1 units at follow-up testing. The use of similar RER values at pre- and post-exercise testing with improvements in peak VO2 “provides strong support for the assertion that observed changes are secondary to the aerobic exercise intervention” (Balady et al., 2010). We then looked only at the top performers who reached an RER>=1.1 on both CPX, which has been used in older adults (Hollenberg et al., 1998) and is used by the American Heart Association as a threshold for a excellent effort during an exercise test. Finally, we assessed the relationship of change in Global Cognition and measures of physical activity in the same manner.
Results
For this analysis, we focused on the 59 individuals completing follow-up testing that adhered to their intervention assignment (completed 80% of minutes prescribed in their assigned treatment arm or did not change their routine), and gave a similar effort on the baseline and follow-up CPX. Table 1 provides demographics and CR fitness change for our two cohorts of interest; all participants (n=59), and the subset who gave good effort on the CPX (Top Performers, defined as RER >=1.1 at both timepoints, n=26).
Table 1.
Demographics, cognitive testing and peak VO2 of all individuals and those of the top performers on the CPX (RER>1.1).
| All Individuals (n=59) | Top CPX Performers (n=26) | |||||
|---|---|---|---|---|---|---|
| Age (y) | 72.7 (5.2) | 71.5 (5.5) | ||||
| Females (%) | 59.3 | 53.4 | ||||
| Education (y) | 16.0 (2.6) | 16.1 (2.7) | ||||
| Group Assignment | ||||||
| No treatment control | 19 | 11 | ||||
| 75min/wk | 16 | 9 | ||||
| 150min/wk | 15 | 4 | ||||
| 225min/wk | 9 | 2 | ||||
| Cognitive Test Measures | Baseline | Week 26 | Change | Baseline | Week 26 | Change |
| Memory | 0.52 (0.86) | 0.93 (0.74) | 0.41 (0.74) | 0.50 (0.87) | 0.99 (0.85) | 0.50 (0.77) |
| Speed | 0.26 (0.57) | 0.37 (0.56) | 0.12 (0.39) | 0.24 (0.55) | 0.38 (0.51) | 0.14 (0.40) |
| Attention | −0.17 (0.77) | −0.07 (0.81) | 0.09 (0.55) | −0.05 (0.81) | 0.04 (0.85) | 0.09 (0.57) |
| Executive Function | −0.05 (0.91) | 0.14 (0.84) | 0.18 (0.87) | 0.35 (0.96) | 0.26 (0.83) | 0.22 (0.41) |
| Language | 0.36 (0.64) | 0.44 (0.61) | 0.08 (0.42) | 0.34 (0.67) | 0.48 (0.65) | 0.13 (0.41) |
| Global Cognition | 0.20 (0.49) | 0.33 (0.46) | 0.13 (0.29) | 0.23 (0.53) | 0.39 (0.50) | 0.16 (0.29) |
| Peak VO2, mL*kg−1*min−1 | 22.2 (4.5) | 23.3 (4.9) | 1.0 (2.0) | 23.2 (4.4) | 24.3 (5.0) | 1.1 (2.1) |
| Percent change in peak VO2 | 4.8 (9.0) | 4.8 (9.0) | ||||
Note. CPX = cardiopulmonary exercise test; RER = respiratory exchange ratio
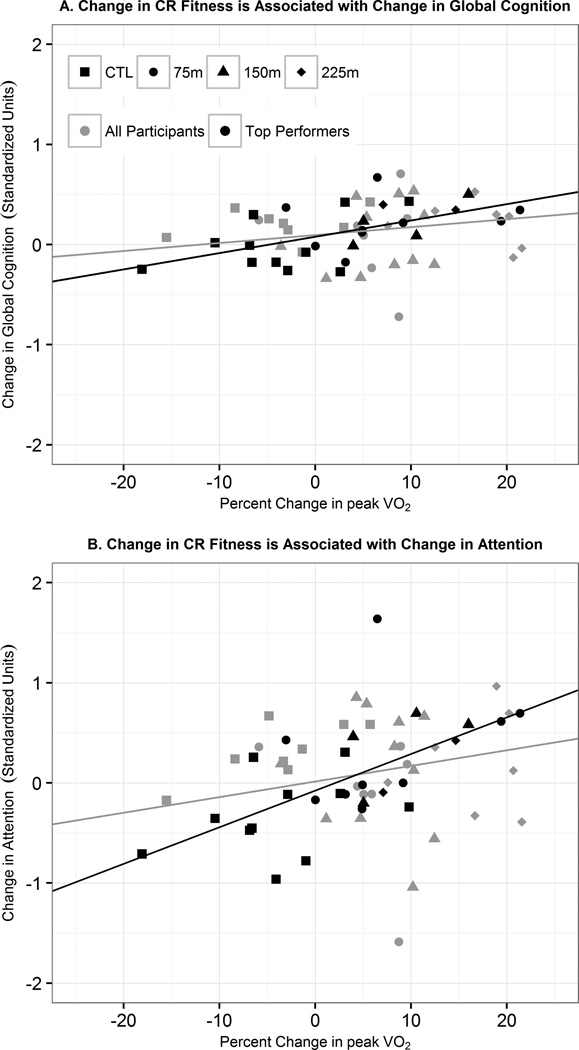
To test our hypothesis that change in Global Cognition would be associated with percent change in VO2, we first performed linear regression of change in Global Cognition against percent change in VO2 in all 59 subjects. Change in Global Cognition was not associated with percent change in VO2 (r2=0.06, p=0.06, Table 2). We then performed linear regression of change in Global Cognition against percent change in VO2 in only the 26 Top Performers. In this more restricted sample, change in Global Cognition was associated with percent change in VO2 (r2=0.33, p<0.01, Figure 1A). In the cognitive domain analysis, only Attention was significantly related to percent change in VO2 (r2=0.36, p<0.01, Figure 1B).
Table 2.
Correlation of percent peak VO2 change over the intervention period VO2 mL/kg/min with UDS domains
| All Participants (n=59) | Top CPX Performers (n=26) |
|||
|---|---|---|---|---|
| Domain | r2 | p | r2 | p |
| Global | 0.06 | 0.06 | 0.33 | <0.01 |
| Memory | 0.02 | 0.49 | ||
| Speed | 0.04 | 0.32 | ||
| Attention | 0.36 | <0.01 | ||
| Executive Function |
0.07 | 0.20 | ||
| Language | 0.001 | 0.87 | ||
Figure 1.
To confirm that the association was due to CR fitness gains and not frequency of exercise, we assessed the relationship of change in estimated calories expended during monthly physical activity, as measured by the CHAMPS physical activity survey, to our variables of interest. In all 59 individuals, change in monthly physical activity was not associated with change in Global Cognition (r2=0.08, p=0.03), though it was associated with percent change in VO2 (r2=0.11, p<0.01). In our Top Performers change in monthly physical activity was also not associated with change in Global Cognition (r2=0.04, p<0.35), but it was more strongly associated with percent change in VO2 (r2=0.26, p<0.01).
Discussion
Our findings in this secondary data analysis support the CR fitness hypothesis. Our clinical trial (Vidoni et al., 2015) provides some evidence that cognitive benefit is linked to fitness change in the form of a mediation analysis. However, the purpose of that association was to test the effect of dose duration on cognition, with fitness as an indicator of duration effect. In the present analysis, we were able to treat CR fitness change as the primary effect of interest. In doing so, were able to more directly test the CR fitness hypothesis by focusing on a subset of participants who gave excellent effort on the exercise test before and after the intervention (Balady et al., 2010; Hollenberg et al., 1998).
Our results did not support simple engagement in physical activities as a driver of cognitive benefit. If the socialization, or general health benefits of increased physical activity explained change in cognition, we would expect that increased physical activity would be correlated with change in cognition, regardless of performance on the CPX. Instead we found that while duration of exercise and change in peak VO2 were closely related, duration of exercise was not related to our global cognitive outcome measure and further supports the CR fitness hypothesis.
Not all studies have supported the CR fitness hypothesis. Prior meta-analysis has failed to demonstrate a dose response of aerobic exercise on cognitive function.(Colcombe & Kramer, 2003) Another study attempted to directly test the hypothesis and found no evidence that improved CR fitness was directly associated with improved executive function (Smiley-Oyen et al., 2008). However, in that study, peak VO2 was not measured but rather estimated from a submaximal exercise test. In the present analysis, we used specific RER thresholds and also ensured that individuals were giving similar effort at pre- and post-testing. In doing so, we minimized other sources of test variability and increase confidence that the observed changes in peak VO2 were the direct result of aerobic exercise intervention (Balady et al., 2010). It is therefore possible that the relationship between cognitive and fitness change is best detected when variability in either is reduced.
Evidence for the CR fitness hypothesis is important because it suggests possible physiologic mechanisms underlying exercise-related gains in cognition. Aerobic exercise improves peak oxygen uptake capacity, cerebral and peripheral vascular regulation and energy metabolism, all of which may engender cerebrovascular (Ainslie et al., 2008; Albinet, Mandrick, Bernard, Perrey, & Blain, 2014; Brown et al., 2010) or neuroendocrine (Cotman & Berchtold, 2002) change that supports brain health and cognitive function. For example, one recent study showed that prefrontal cerebral blood flow mediated the relationship of CR fitness and executive function (Albinet et al., 2014). It is possible that exercise-mediated cerebral blood flow changes support brain tissue health and thus cognition as we age (Pereira et al., 2007)
Our study has important limitations. First, this was a secondary analysis of previously reported data. To protect ourselves against multiple comparisons bias, we used a corrected threshold for significance and alternative measures of cognitive performance, i.e. a normalized cognitive aggregate score rather than latent residuals. In our original report, we noted an exercise effect on Attention, and a dose response effect on Visuospatial Processing. These domains echo previous work that has been classified within the scope of Executive Function (Colcombe & Kramer, 2003; Colcombe et al., 2004; Kramer et al., 1999). In the present report, using a separate system for aggregating and normalizing test scores, we found that our global cognitive effects were driven mostly by a linear association of CR fitness and Attention. Second, unlike a clinical trial, we limited our sample to individuals who adhered to our exercise protocol and participated in all testing, thus limiting the generalizability of our findings to those who are willing and motivated to participate in exercise. The potential for bias due to dropout, especially at the highest dose of exercise, further complicates interpretation. However dose-response groups demonstrated similar changes in peak heart rate, RER and CR fitness changes (Supplementary Tables 1–3) suggesting a limited effect on our measures. And it is important to note that both cognitive testing and CPX may be influenced by motivation and we cannot discount this potential confound. Thus our data cannot be interpreted as definitive evidence for the CR fitness hypothesis.
In conclusion, we found an association between change CR fitness and change in Global Cognition largely driven by the Attention domain of our cognitive tests. The magnitude of the change was explained by change in CR fitness and not duration of exercise. Those individuals who were willing to give an excellent effort by reaching the physiologic max (RER ≥ 1.1) had a stronger association with cognition. The American Heart Association states that peak RER is the “most accurate and reliable” measure for an individual’s effort on the CPX (Balady et al., 2010). Using CPX to measure CR fitness, not estimating peak VO2 as done by (Smiley-Oyen et al., 2008), and using recommended RER values pre- and post-peak VO2 testing reveals cognitive improvement that we can confidently attribute to the exercise intervention and variability of participant effort. Our findings support the further investigation of the CR fitness hypothesis and mechanisms by which physiologic adaptation may drive cognitive change.
Supplementary Material
Acknowledgments
This research was supported by NIH grants P30AG035982, UL1TR000001, K01HD02528, KL2TR000119, F32AG044953, R01DK088940 and R01AG034614 through the National Institutes of Health.
Contributor Information
Sandra A. Billinger, Departments of Physical Therapy and Rehabilitation Sciences, University of Kansas Medical Center
Eric D. Vidoni, Department of Neurology and the KU Alzheimer’s Disease Center, University of Kansas Medical Center
Jill K. Morris, Department of Neurology and the KU Alzheimer’s Disease Center, University of Kansas Medical Center
John P. Thyfault, Department of Molecular and Integrative Physiology, University of Kansas Medical Center
Jeffrey M. Burns, Department of Neurology and the KU Alzheimer’s Disease Center, University of Kansas Medical Center
References
- Ainslie PN, Cotter JD, George KP, Lucas S, Murrell C, Shave R, Atkinson G. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol. 2008;586(16):4005–4010. doi: 10.1113/jphysiol.2008.158279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albinet CT, Mandrick K, Bernard PL, Perrey S, Blain H. Improved cerebral oxygenation response and executive performance as a function of cardiorespiratory fitness in older women: a fNIRS study. Front Aging Neurosci. 2014;6:272. doi: 10.3389/fnagi.2014.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescatello LS, editor. American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 9th. Baltimore, MD: Lippincott Williams & Wilkins; 2014. [DOI] [PubMed] [Google Scholar]
- Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF Outcomes, Research. Clinician's Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2):191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- Brown AD, McMorris CA, Longman RS, Leigh R, Hill MD, Friedenreich CM, Poulin MJ. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiol Aging. 2010;31(12):2047–2057. doi: 10.1016/j.neurobiolaging.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78(17):1323–1329. doi: 10.1212/WNL.0b013e3182535d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullitt E, Ewend M, Vredenburgh J, Friedman A, Lin W, Wilber K, Reardon D. Computerized assessment of vessel morphological changes during treatment of glioblastoma multiforme: report of a case imaged serially by MRA over four years. Neuroimage. 2009;47(Suppl 2):T143–T151. doi: 10.1016/j.neuroimage.2008.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe Stanley J, Kramer Arthur F, Erickson Kirk I, Scalf Paige, McAuley Edward, Cohen Neal J, Elavsky Steriani. Cardiovascular fitness, cortical plasticity, and aging. Proc Nat Acad Sci U S A. 2004;101(9):3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. doi: S0166223602021434 [pii] [DOI] [PubMed] [Google Scholar]
- Dustman RE, Ruhling RO, Russell EM, Shearer DE, Bonekat HW, Shigeoka JW, Bradford DC. Aerobic Exercise Training and Improved Neuropsychological Function of Older Individuals. Neurobiol Aging. 1984;5(1):35–42. doi: 10.1016/0197-4580(84)90083-6. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Nat Acad Sci U S A. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. doi: 1015950108 [pii] 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM American College of Sports, Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Boston Naming Test scoring booklet. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Hollenberg M, Ngo LH, Turner D, Tager IB. Treadmill Exercise Testing in an Epidepiologic Study of Elderly Subjects. J Gerontol Biol Sci. 1998;53A(4):259–267. doi: 10.1093/gerona/53a.4.b259. [DOI] [PubMed] [Google Scholar]
- Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metabol. 1992;12(1):110–119. doi: 10.1038/jcbfm.1992.14. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Cooper NR, VandenBerg PM. Exercise induces angiogenesis but does not alter movement representations within rat motor cortex. Brain Res. 2002;934(1):1–6. doi: 10.1016/s0006-8993(02)02239-4. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400(6743):418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Mayer CJ, Steinman L, Williams B, Topolski TD, LoGerfo J. Developing a Telephone Assessment of Physical Activity (TAPA) questionnaire for older adults. Prev Chronic Dis. 2008;5(1):A24. [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373(6510):109–109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(13):5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. 2nd. Tuscon, AZ: Neuropsychology Press; 1985. [Google Scholar]
- Smiley-Oyen AL, Lowry KA, Francois SJ, Kohut ML, Ekkekakis P. Exercise, fitness, and neurocognitive function in older adults: the "selective improvement" and "cardiovascular fitness" hypotheses. Ann Behav Med. 2008;36(3):280–291. doi: 10.1007/s12160-008-9064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Sherwood A. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart Anita L, Verboncoeur Carol J, McLellan Barbara Y, Gillis Dawn E, Rush Stephanie, Mills Kris M, Bortz Walter M., II Physical Activity Outcomes of CHAMPS II: A Physical Activity Promotion Program for Older Adults. J Gerontol A Biol Sci Med Sci. 2001;56(8):M465–M470. doi: 10.1093/gerona/56.8.m465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, Greenough WT. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117(4):1037–1046. doi: 10.1016/s0306-4522(02)00664-4. doi: S0306452202006644 [pii] [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Nat Acad Sci U S A. 1999;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidoni ED, Johnson DK, Morris JK, Van Sciver A, Greer CS, Billinger SA, Burns JM. Dose-Response of Aerobic Exercise on Cognition: A Community-Based, Pilot Randomized Controlled Trial. PLoS One. 2015;10(7):e0131647. doi: 10.1371/journal.pone.0131647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. San Antonio, TX: Psychological Corp; 1987a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised. San Antonio, TX: Psychological Corp; 1987b. [Google Scholar]
- Weuve Jennifer, Kang Jae Hee, Manson JoAnn E, Breteler Monique MB, Ware James H, Grodstein Francine. Physical Activity, Including Walking, and Cognitive Function in Older Women. JAMA. 2004;292(12):1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- Yaffe Kristine, Barnes Deborah, Nevitt Michael, Lui Li Yung, Covinsky Kenneth. A Prospective Study of Physical Activity and Cognitive Decline in Elderly Women: Women Who Walk. Arch Intern Med. 2001;161(14):1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.