Abstract
Brain function relies on the ability of neural networks to maintain stable levels of activity, while experiences sculpt them. In neocortex, the balance between activity and stability relies on the co-regulation of excitatory and inhibitory inputs onto principal neurons. Shifts of excitation or inhibition result in altered excitability impaired processing of incoming information.
In many neurodevelopmental and neuropsychiatric disorders, the excitability of local circuits is altered, suggesting that their pathophysiology may involve shifts in synaptic excitation, inhibition or both. Most studies focused on identifying the cellular and molecular mechanisms controlling network excitability to assess whether may be altered in animal models of disease. The impact of changes in excitation/inhibition (E/I) balance on local circuit and network computations is not clear. Here we report findings on the integration of excitatory and inhibitory inputs in healthy cortical circuits and discuss how shifts in E/I balance may relate to pathological phenotypes.
Keywords: neocortex, excitation, inhibition, synapses, neurodevelopment, disease
Introduction
Neocortical neurons integrate thousands of inputs to produce appropriate outputs. These computations occur while local circuits and networks maintain their stability. The preservation of balanced excitatory and inhibitory synaptic drive onto cortical neurons is thought to be crucial for preserving circuit function (1). How stringent the regulation of the excitation/inhibition balance needs to be to allow flexibility while preserving stability is unknown.
In this review we will not focus on how the mechanisms controlling the E/I balance, but will discuss published data in the context of possible effects of shifts in E/I balance on network function. We propose that to fully understand the impact of the E/I balance on neural circuits it is not sufficient to identify how it is established. An investigation of the interdependence of changes in excitatory and inhibitory circuits is necessary, as this may offer clues about how they may be coordinated and co-regulated throughout life.
Excitation and inhibition in neocortical circuits
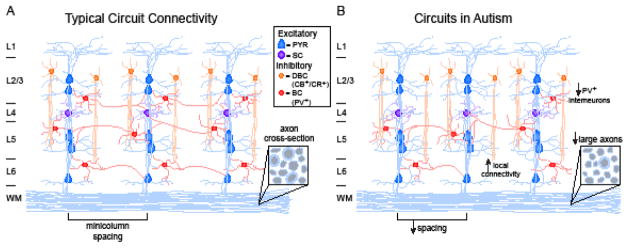
The foundation for balanced excitation and inhibition is the establishment of local and long-range cortical circuits. In neocortex, most neurons are glutamatergic excitatory neurons that synapse locally or project to distant cortical, subcortical, or brainstem targets (2). GABAergic inhibitory neurons, up ~20% of neocortical neurons, project locally, and regulate ongoing activity. Glutamatergic neurons in neocortex are primarily pyramidal neurons, and can be identified by the expression of transcription factors (3) and the source of their inputs and the target region of their projections (4). GABAergic interneurons have been classified by firing type, expression of calcium-binding proteins or neuropeptides, and postsynaptic targets (5). Together, excitatory and inhibitory neurons form local and long-range circuits whose connectivity is specialized for signal processing. Common structural features of neocortex are narrow radial arrays of neurons known as minicolumns that are considered the elemental units of signal processing. Minicolumns are present in prefrontal and sensory cortices, and are important for cognitive functions like working memory and sensory processing (6–8). Vertically aligned pyramidal and excitatory stellate cells constitute the core of a minicolumn. Pyramidal neurons in a minicolumn are connected both within and between layers. Flanking inhibitory neurons provide lateral inhibition, modulating signal propagation across minicolumns (Fig. 1A). The activity of excitatory neurons within a minicolumn is balanced by multiple types of inhibition: parvalbumin-positive (PV+) neurons are wide-arbor basket or chandelier cells that inhibit nearby minicolumns, while calbindin- or calretinin-positive (CB+/CR+) double bouquet cells provide inhibition through translaminar synapses (9, 10). The connection probability and strength of synaptic connections between neurons within and between minicolumns provide the substrate for establishing balanced activity. Thanks to this modular structure, excitatory and inhibitory neurons can be tuned to the same stimulus features and work concurrently to process incoming signals (6, 11). Integration of excitation and inhibition onto cortical neurons within minicolumns regulate network gain, tune responses, and stabilize cortical activity by preventing runaway cortical excitation (12–15). Failure to establish connectivity motifs can lead to imbalanced activity across neocortex and may provide a neurophysiological basis for the cognitive symptoms of several neurological disorders.
Figure 1. Cortical circuit connectivity and E/I balance.
A. Minicolumn structure and long-range connectivity in healthy brains. B. Reduced minicolumn spacing, decreased PV+ interneuron immunoreactivity, local hyperconnectivity, and decreased large axons in white matter characterize circuit structure in ASD.
Impaired connectivity and its consequences for neocortical circuits
Pathological changes in neuron connectivity impact function within and across neocortical regions. Patients with autism spectrum disorder (ASD) show narrowed minicolumns and reduced proportions of large axons in the white matter underlying certain cortical areas (16–18). In the valproic acid (VPA) rodent model of autism, pyramidal neurons and inhibitory interneurons show increased connection probability (19, 20). These findings suggest that ASD may be characterized by neurons that are locally hyperconnected, with reduced communication with distant cortical regions (Fig. 1B). This, and evidence for reduced PV+ interneurons in prefrontal cortex, may provide an anatomical substrate for dysregulated E/I balance and underly the hypersensitivity and hyper-reactivity to stimuli observed in ASD (17). Minicolumn pathology was also observed in schizophrenia, especially in auditory cortex, where structural abnormalities are thought to correlate with the incidence of auditory hallucinations in patients (21, 22). Minicolumns typically thin with age due to plastic changes in the neurons’ dendrites and axons. The brains of schizophrenic patients lack this age-dependent thinning (22), especially in regions that rely on plasticity to perform associative functions (22). Inhibitory interneurons are also impacted in schizophrenia (23, 24) and can show a reduced levels of the GABA-synthesizing enzyme GAD-67 (25–27) and of the GABA transported GAT1(28). In animal models of schizophrenia, reduction of inhibition has been associated with reduced cortical oscillations that are thought to mediate important cognitive processes (29–31). Diminished GABAergic inhibition together with minicolumn pathology may underlie a number of circuit alterations associated with ASD and schizophrenia. Specifically, these factors may provide an explanation for how local circuit changes may result in hyperexcitability and hyperplasticity.
Synaptic plasticity and the remodeling of neocortical circuits
Connectivity of long-range and local circuits is refined and remodeled by experience and learning. Hebbian plasticity, a form of long term plasticity based on correlative pre- and postsynaptic activity is one of the plasticity mechanisms involved in circuit reorganization. Plasticity can alter the strength of both excitatory and inhibitory synaptic inputs through multiple presynaptic and postsynaptic mechanisms. Different factors can influence the rules governing Hebbian plasticity in neocortex, including the developmental maturation of GABAergic circuits, patterning of synaptic activity, subtype of pre- or postsynaptic neurons, laminar circuits, cortical areas, and neuromodulatory influences (32–36).
Hebbian-like experience-dependent modifications of synaptic strength or local connectivity can affect the excitability of excitatory and inhibitory neurons (37, 38). Plasticity can also be induced at afferent inputs (39), altering how local excitatory and inhibitory circuits become engaged by an incoming stimulus (40). Feedforward projections such as thalamocortical afferents synapse on both excitatory and inhibitory neurons (41). The feedforward inhibitory circuit activated by afferent inputs in turn provides inhibition onto nearby excitatory neurons (42, 43). The delay between the arrival of a direct thalamocortical input onto an excitatory cell and the feedforward inhibitory signal determines a temporal window for the integration of thalamocortical and intracortical activity (44). Plasticity at cortical synapses can widen or shorten temporal windows for inputs’ integration (14), possibly modulating further induction of plasticity (36, 45). Thus, neural plasticity not only influences the state of excitability of a circuit, but can prime neurons so that future patterns of incoming activity will favor one set of changes over another, a process known as metaplasticity (46).
Studies of excitatory synaptic plasticity have revealed some common mechanisms for the activity-dependent strengthening or weakening of connections between neocortical neurons. Postsynaptic NMDA receptor (NMDAR) - dependent plasticity requires presynaptic glutamate release coupled with postsynaptic depolarization to relieve the magnesium block and allow calcium (Ca2+) influx through NMDARs (47–49). The timing of presynaptic versus postsynaptic activity (50, 51), the amount of postsynaptic depolarization (36), and additional recruitment of postsynaptic voltage-gated Ca2+ channels (VDCCs) (35) can affect the magnitude of the Ca2+ influx (52), which determines the sign of plasticity (53, 54). Rapid and large increases in postsynaptic Ca2+ activate CAMKII, and trigger a cascade of events leading to an increased number of AMPA receptors (AMPARs) in the postsynaptic membrane, inducing long term potentiation of excitatory synaptic responses (LTPe). Slow and small increases of Ca2+ influx engage protein phosphatases and promote the removal membrane AMPARs resulting in long term depression (LTDe) (54, 55).
Another postsynaptic form of long-term synaptic plasticity at neocortical excitatory synapses depends on group I metabotropic glutamate receptors (mGluRs), and/or other G-protein coupled receptors. Here, receptor activation triggers G-protein mediated signaling cascades. Receptors coupled to Gs-proteins activate the adenyl cyclase pathway and promote LTPe, whereas Gq11-coupled receptors drive the phospholipase-C pathway and promote LTDe (56). Activity-dependent excitatory neocortical plasticity can also engage changes in presynaptic terminals. Presynaptic NMDARs mediate some forms of cortical LTDe, either alone or in conjunction with the activation of presynaptic cannabinoid receptors type 1 (CB1-R) (57).
A growing body of literature demonstrated that inhibitory synapses in neocortex undergo bidirectional plasticity too, however the mechanisms underlying these changes are less clear. Postsynaptic Ca2+ plays a role in some forms of inhibitory plasticity (58–60). At some synapses the relative contribution of different subtypes of VDCCs to Ca2+ influx favors the insertion or removal of GABAA receptors (GABAARs), and subsequent LTP or LTD of inhibition (LTPi, LTDi) (61). Postsynaptic activation of GABABR also contribute to different forms of LTPi, one that engages Ca2+ release from intracellular stores (62) and a different type that is Ca2+-independent but depends on Gi/o protein signaling (36). Inhibitory plasticity can also engage presynaptic mechanisms (63), although these often require coincident release of glutamate, and therefore represent heterosynaptic forms of plasticity (64). The activation of mGluRs and coincident activation of presynaptic CB1Rs by retrograde cannabinoid signaling decreases presynaptic protein kinase A (PKA) activity mediated by Gi/o proteins and results in LTDi (65, 66).
The induction of neocortical plasticity can vary greatly across areas and developmental windows. In periods of heightened plasticity for sensory neocortices, critical periods, similar patterns of activity can engage distinct mechanisms depending on the specific developmental window. For instance, in layer (L) 4 of V1 during the pre-critical period, LTDe of unitary connections can be induced by an mGluR mediated spike timing dependent paradigm, or via an NMDAR-mediated presynaptic bursting paradigm. However, during the critical period, LTDe is no longer inducible with spike-timing, while presynaptic bursting leads to NMDAR-dependent LTPe (35). Similar developmental changes in plasticity rules have also been documented for inhibitory synapses (67). This developmental regulation of plasticity correlates with the maturation of glutamatergic synapses (68–70) and GABAergic circuitry (71–73). Developmentally regulated changes in receptor expression or subunit composition can contribute to switches in the sign of plasticity, and/or changes in the underlying mechanisms recruited by different activity patterns (35, 69, 74). It is therefore important to consider the specific period in development when comparing the capacity for plasticity of different circuits across cortex.
The capacity for plasticity of a neuron can also be affected by its previous activity that can either prime or occlude certain signaling cascades (46). This metaplasticity may not always result in altered neuronal output or significant changes in synaptic strength, but may affect the state of a neuron and its ability to respond to future inputs (75). While conceptually metaplasticity is intuitive, what accounts for metaplastic mechanisms in neurons or circuits is currently unclear. Given the diversity of induction parameters and mechanisms underlying activity-dependent cortical plasticity, future work is needed to elucidate how these factors may subserve plasticity in vivo, in healthy brains and disease states.
Altered plasticity and loss of healthy circuit function disease
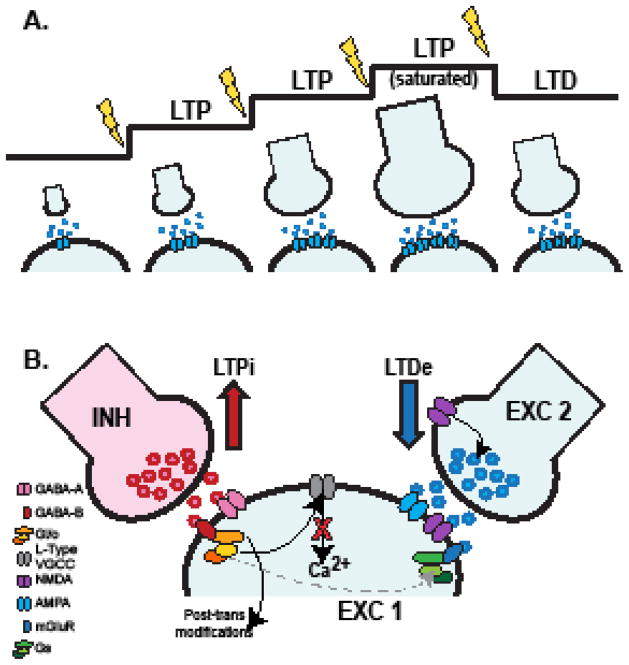
During postnatal development and throughout life, Hebbian plasticity plays a fundamental role in how organisms respond to their environment. If unconstrained, Hebbian plasticity can lead to profound circuit instability (76). Based on principles taken from the Bienenstock, Cooper, Munro (BCM) theory (77, 78) (Fig. 2A), the “sliding threshold” hypothesis of plasticity was formulated to propose a framework for how the destabilizing effects of Hebbian plasticity may be prevented (79). The prediction of the “sliding threshold” hypothesis is that previous induction of plasticity shifts the threshold for induction of additional Hebbian changes (75). According to this theory, the magnitude of plasticity in the form of LTP or LTD is constrained between a maximum (ceiling effect) and a minimum (floor effect). If potentiation reached a maximum, future potentiation is prevented and incoming activity would result in a de-potentiation so that the induction threshold can be adjusted (79). Conversely, synapses that have been maximally depressed will not be depressed further, and potentiation will be favored. The framework proposed by the BCM/sliding threshold theory sets the basis for plasticity occlusion experiments according to the principle that if a manipulation or a genetic mutation has changed the strength of a synapse by Hebbian plasticity, the threshold for induction of plasticity has shifted. Thus, further induction of plasticity will either be impaired, or even trigger plasticity with the opposite sign (80). This set of mechanisms can be at play in healthy circuits to maintain circuit stability in the face of changes induced by learning and experience.
Figure 2. Cortical plasticity models and mechanisms.
A. Diagram representation of the sliding threshold for Hebbian plasticity. B. Summary diagram of crosstalk between excitatory and inhibitory mechanisms for plasticity (adapted from Wang and Maffei, 2014).
If the events regulating circuit development are altered due to mutations in risk genes, or stressors, the capacity for plasticity of a synapse may be impaired. For example, the FMR1KO mouse, a model of Fragile X syndrome, is characterized by hyperexcitability and impaired cortical LTPe (81–83), although LTDe not only is effectively induced but enhanced (84, 85). It is currently unknown whether the altered capacity for plasticity of the FMR1KO mouse is causally related to the change in circuit excitability. In view of the BCM/sliding threshold theory, it was proposed that in the FMR1KO mouse mechanisms for LTPe induction may be impaired or saturated (85), thus this form of plasticity cannot be induced; differently LTDe can be induced as the induction threshold for depression has been shifted.
A similar interpretation could be applied to data obtained from a mouse model of Rett Syndrome. Rett Syndrome is a neurodevelopmental disorder due to a mutation in the X-linked gene, methyl CpG binding protein-2 (MeCP2) (86–88) that is characterized by significant changes in circuit excitability. MeCP2 KO mice show increased GABAergic transmission and reduced glutamatergic transmission in L5 pyramidal neurons of the somatosensory cortex (89). These effects are consistent with an overall shift of the E/I balance toward inhibition. According to the BCM/sliding threshold theory, the MeCP2KO mouse should show impaired LTDe, since excitation is already reduced, and an increased capacity for LTPe. However, experimental data show that the capacity for LTPe at recurrent synapses remains unchanged, (90) suggesting that in the MeCP2KO model of Rett syndrome, the relationship between shifts in excitability and capacity for plasticity is more complex than previously appreciated.
Altered GABAergic inhibition is a common feature of many neuropsychiatric diseases. GABAergic synapses are plastic and can be modified by experience (91–95), however alterations in inhibitory plasticity have not yet been investigated as a possible mechanism for the pathophysiology of psychiatric disorders. Since excitatory and inhibitory forms of plasticity share some common signaling mechanisms (64, 65, 96–98), the possibility arises for crosstalk between signaling pathways that may affect how a neuron responds to incoming inputs. This is particularly relevant for cortical circuits that are recurrently connected, and excitatory and inhibitory synapses can occupy overlapping areas of a postsynaptic neuron (99).
During acute induction of inhibitory (LTPi) and excitatory (LTPe) forms of LTP, signaling mechanisms for excitatory and inhibitory synaptic plasticity can interact (Fig. 2B) (36). In view of results showing cooperative interactions between excitatory and inhibitory forms of plasticity, we propose a number of testable hypotheses regarding how altered inhibition may contribute to the circuit changes observed in psychiatric diseases. For instance, changes in inhibitory drive alter cortical excitability and this may be sufficient to affect further induction of plasticity. Alternatively, changes inhibition may affect the capacity for plasticity due to impaired crosstalk of signaling pathways for excitatory and inhibitory plasticity, changing how signals may be processed.
Circuit perturbations and compensatory mechanisms
Hebbian plasticity could potentially destabilize cortical circuits, as connections between neurons with correlated activity are strengthened and those between neurons with uncorrelated activity are weakened. However, in healthy brains changes in synaptic strength occur without resulting in pathological conditions. To maintain circuit stability, mechanisms are in place for neurons to sense their own excitability (100, 101) or the excitability of the circuit (102) and modulate their intrinsic properties and/or synaptic inputs to maintain functional levels of activity (71, 102–104). These mechanisms, known as homeostatic plasticity, are crucial for healthy brain function (105). Different forms of homeostatic plasticity have been identified, the best studied being synaptic scaling, a form of homeostatic plasticity in which a neuron modulates its input/output curve by globally adjusting the strength of its inputs via insertion or removal of synaptic receptors (101, 106). Thus, a neuron can maintain relative differences in synaptic strength induced by Hebbian plasticity while preserving functional states of excitability (103). Postsynaptic neurons can also retrogradely control the strength of their inputs through signaling molecules like retinoic acid (RA) (107) or brain-derived neurotrophic factor (BDNF)(108), which modulate neurotransmitter release (109).
Since homeostatic plasticity occurs in multiple neuron populations, it can constrain individual neuron’s activity while coordinating the excitability of excitatory and inhibitory neurons within a circuit (102, 110, 111). Perturbations of homeostatic plasticity could leave a circuit vulnerable to destabilizing swings in excitability without means of compensation (105, 109). Although homeostatic plasticity is an understudied area of neural regulation, experimental evidence strongly suggests that these mechanisms are crucial for offsetting shifts in synaptic transmission due to experience (92, 93, 112), and for maintaining healthy E/I balance (113, 114).
Disruption of the E/I balance in models of neurodevelopmental disorders
Even in properly connected circuits, neuronal and local circuit excitability can be altered due to: inappropriate differentiation of neuronal phenotypes (115), improper regulation of neurotransmitter release (116), impaired expression of excitatory (117) or inhibitory postsynaptic receptors (118) and/or their scaffolding proteins (119, 120). As our understanding of the mechanisms for homeostatic plasticity is still limited, most experimental work on disease models focused on identifying changes in synaptic transmission within circuits, but has not yet delved deeply into investigating possible defects in compensatory mechanisms.
Experimental work in animal models has been instrumental to identify some of the mechanisms contributing to the alteration of neocortical excitability. In rodent models of epilepsy altered circuit activity may result from changes in intrinsic properties that in turn affect neurotransmission, or may depend on changes in synaptic transmission only.
In animal models of Dravet’s Syndrome, a severe form of myoclonic childhood epilepsy, a loss-of-function mutation in one allele of SCN1A encoding the voltage-gated, type 1, α-subunit NaV1.1 prevents the development of fast spiking behavior in PV+ neurons of the neocortex (121), a feature that may have significant consequences for how inhibition regulates the circuit in this model. It is currently unknown whether the circuit instability in models of Dravet’s syndrome depends on loss or incorrect engagement of compensatory mechanisms. Recently identified additional mutations in the gene for the α-1 subunit of the GABAA receptor in Dravet’s syndrome adds to the complexity of the circuit defects in this disease (122).
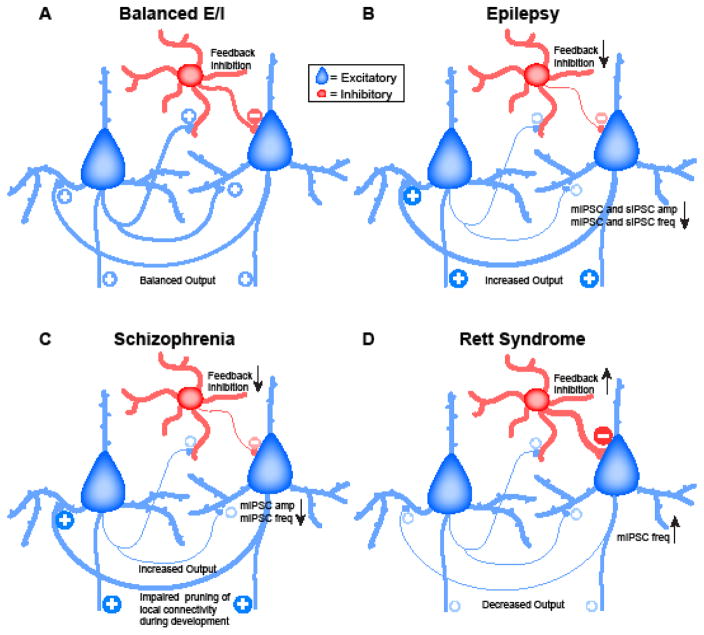
An important aspect of many forms of epilepsy is the increase in circuit excitability. In many models decreased amplitude, frequency, or both, of spontaneous and miniature post-synaptic inhibitory currents (sIPSCs and mIPSCs, Fig. 3A) is reported, suggesting dis-inhibition as a mechanism for increased excitability (123, 124). Studies from animal models of intractable epilepsy suggest that the dis-inhibition may result from altered excitatory drive onto inhibitory neurons (125, 126). Thus, one may speculate that altered excitability in epilepsy may result from alteration or loss of compensatory forms of plasticity, or resulting from perturbations of circuit excitability that exceed the circuits’ compensation capabilities.
Figure 3. Examples of changes in circuit excitability due to shifts in E/I balance.
A. Representative diagram of E/I balance in a healthy circuit. B. Representation of E/I balance changes in animal models of epilepsy. C. Summary of local circuit changes reported for animal models of schizophrenia. D. Diagram of synaptic changes reported in a model of Rett syndrome. Blue neuron: excitatory pyramidal neurons. Red neuron: inhibitory interneuron. Inhibitory neurons types are not specified here, as studies from disease models show primarily spontaneous inhibitory currents, of unidentified presynaptic origin. Line thickness and plus/minus signs indicate the sign of changes in synaptic strength and activity, respectively.
Circuit disinhibition by reduced excitation onto inhibitory neurons has been reported in a number of neurodevelopmental disorders. In animal models of schizophrenia, the decrease in inhibition is correlated with a reduction in the expression of the GluNR2A subunit of the NMDARs on inhibitory neurons (127–131). Ketamine administration in rodents, a paradigm that in healthy human subjects induces schizophrenic-like symptoms (132–135), decreases the strength of inhibitory inputs onto pyramidal neurons (136) by reducing both frequency and amplitude of mIPSCs (Fig. 3C) (137). Thus ketamine mimics the disinhibition observed in the schizophrenia models and has led investigators to associate dis-inhibition to some aspects of the disease.
The FMR1KO model of Fragile X syndrome, a disease characterized by delayed cognitive development and autistic traits, is also characterized by hyperactive neocortical circuits. The mechanisms underlying increased excitability in this model are only beginning to be unraveled. In the somatosensory cortex of FMR1KO mice, pyramidal neurons display firing rates 3 fold-higher than controls (138). This hyperexcitability may be due to an increase in pyramidal neurons density in the early postnatal neocortex (139) that produces hyperconnected excitatory circuits. In addition, FMR1KO mice show reduced density of PV+ neurons in the somatosensory cortex (140) and diminished excitatory drive onto fast spiking inhibitory neurons (141). Both these factors likely disrupt the function of inhibitory circuits early in development and may shift in E/I balance of neocortical circuits. The state of hyperexcitability of pyramidal neurons in the FMR1KO mice can alter sensory perception, consistent with results from the auditory cortex where pyramidal neurons are hyper-responsive to sound (142).
Hyperexcitability of pyramidal neurons and impaired cortical inhibition are hallmarks of other models of ASD. As there is a significant degree of comorbidity between ASD and epilepsy (143), it is possible that the defects occurring in these diseases may lead to common functional alterations at the level of cortical circuits. Some of the shared features between epilepsy and ASD are: reduction in the density of interneurons expressing the GABA synthetizing enzyme GAD67 (144–148), reduction in the expression of several GABAAR subunits (149–152), and reduction in the number of PV+ neurons (1, 146, 153).
The outcome of altered circuit excitability is quantified as changes in firing rates. However, the underlying mechanisms may be quite different. In fact, even different models of the same disease can produce opposite effects on circuit excitability depending on the specific manipulation of the same gene. Mutations in neuroligin-1 and 3 occur in a subset of ASD patients (1, 154). In animal models, mutations in these genes can produce very different effects. A point mutation of the neuroligin-3 gene is associated with increased inhibition in the somatosensory cortex (155). Double knock-out mice for neuroligin-1 and 3 show decreased spontaneous GABAergic activity in respiratory centers (156). These results suggest that mutations that affect the E/I balance may result in alteration that neurons and circuits are not capable of compensating, or in activation of compensatory mechanisms that result in loss of circuit function (105).
Another example of complex resulting from mutations of a single gene comes from the MeCP2 mouse models of Rett syndrome. While defects in inhibitory circuits were consistently reported, experimental results differ significantly depending on genetic models. MeCP2KO mice show increased inhibitory and decreased excitatory transmission onto L5 pyramidal neurons of the somatosensory cortex (89) (Fig. 3D), and reduced connection probability between cortical pyramidal neurons (90), shifting the E/I balance toward inhibition and altering plasticity (89, 90). Selective mutations of MeCP2 in specific groups of neurons reported different outcomes. Cre-dependent deletion of MeCP2 from all GABAergic neurons decreases inhibitory quantal size in L2/3 pyramidal neurons of the somatosensory cortex and reduced levels of GABA, GAD65 and 67 (157), without changes in glutamatergic transmission. A similar selective reduction in inhibition has been reported if MeCP2 was deleted selectively in cortical pyramidal neurons (158). Selective deletion of MeCP2 in a subset of L2/3 pyramidal neurons reduced GABAergic transmission only onto MeCP2-deficient neurons (159), suggesting that the defects in synaptic transmission is specific for neurons carrying the mutation. If MeCP2 expression is suppressed selectively in L2/3 neurons using short hairpin (sh) RNA, excitatory synaptic transmission is reduced while inhibition remains unaffected (160), suggesting that the location of genetic defects matters for the dysregulation of local excitability.
A common thread of neurodevelopmental disorders seems to the occurrence of defects in GABAergic synaptic transmission that go uncompensated and result altered circuit excitability. Inappropriate differentiation of inhibitory circuits shifts the E/I balance and may result in loss of tuning, especially if changes in inhibition occur during critical periods early in development (1, 161). Given the different effects of mutations in selected population of neurons it is also possible that coordinated interactions between signaling pathways for excitatory and inhibitory synaptic transmission become impaired resulting in alterations of circuit excitability and capacity for plasticity. This last hypothesis arises from recent studies in healthy brains (36), and has not been tested yet in animal models of psychiatric diseases.
Conclusions
Many neurodevelopmental and neuropsychiatric disorders are characterized by changes in synaptic transmission (Table 1). Most studies focused on investigating mechanisms regulating excitation or inhibition independently. However, there is now sufficient evidence that signaling pathways for GABAergic and glutamatergic transmission may act in a coordinated fashion (36, 162). In view of this evidence, investigating whether factors contributing to the co-regulation of excitatory and inhibitory synaptic transmission are affected in models of neuropsychiatric disorders may lead to the identification of new targets for therapeutic interventions.
Table 1.
Examples of changes in excitation and/or inhibition in mouse models of diseases characterized by altered E/I balance.
| Disease | Cell type | Parameters | Animal model | Reference # |
|---|---|---|---|---|
|
| ||||
| Epilepsy | Pyr |
 mIPSCs and sIPSCs mIPSCs and sIPSCs |
Pilocarpine treatment and cortical dysplasia | 73 |
 sEPSCs sEPSCs |
Cortical dysplasia | 75 | ||
|
| ||||
| FS |
 sEPSCs and mEPSCs. sEPSCs and mEPSCs. sIPSCs and mIPSCs sIPSCs and mIPSCs |
Cortical dysplasia | 74, 75 | |
|
| ||||
| Schizophrenia | Pyr |
 mIPSCs and sIPSCs. No change in mEPSCs but increase intrinsic excitability mIPSCs and sIPSCs. No change in mEPSCs but increase intrinsic excitability |
Ketamine treatment | 86 |
|
| ||||
| Fragile X syndrome | Pyr |
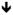 firing rate firing rate |
FM1 knock-out | 87 |
|
| ||||
| FS |
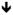 uEPSCs uEPSCs |
FM1 knock-out | 90 | |
|
| ||||
| Pyr | Unaltered uIPSCs | |||
|
| ||||
| Rett Syndrome | Pyr |
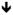 spontaneous firing rate spontaneous firing rate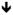 sEPSCs, sEPSCs,
 sIPSCs sIPSCs mEPSCs, no change mIPSCs mEPSCs, no change mIPSCs |
60 | |
| Pyr |
 mIPSCs, no change in mEPSCs mIPSCs, no change in mEPSCs |
Viaat-Mecp2−/y MeCP2 Knock-out | 105 | |
| Pyr |
 mIPSCs, no change in mEPSCs mIPSCs, no change in mEPSCs |
MeCP2−/y; Emx1-Cre | 106 | |
| Pyr |
 EPSCs EPSCs |
Sh-RNA to knock-down MeCP2 | 107 | |
Acknowledgments
Funding sources: Whitehall Foundation Award and NIH – DC013770 to A.M.; SNSF Post Doc mobility fellowship to R.T.
Footnotes
Financial disclosures. All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gogolla N, Leblanc JJ, Quast KB, Sudhof TC, Fagiolini M, Hensch TK. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J Neurodev Disord. 2009;1:172–181. doi: 10.1007/s11689-009-9023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- 3.Sugino K, Hempel CM, Miller MN, Hattox AM, Shapiro P, Wu C, et al. Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nat Neurosci. 2006;9:99–107. doi: 10.1038/nn1618. [DOI] [PubMed] [Google Scholar]
- 4.Hattox AM, Nelson SB. Layer V neurons in mouse cortex projecting to different targets have distinct physiological properties. J Neurophysiol. 2007;98:3330–3340. doi: 10.1152/jn.00397.2007. [DOI] [PubMed] [Google Scholar]
- 5.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu CZ. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 6.Rao SG, Williams GV, Goldman-Rakic PS. Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: Evidence for microcolumnar organization in PFC. J Neurophysiol. 1999;81:1903–1916. doi: 10.1152/jn.1999.81.4.1903. [DOI] [PubMed] [Google Scholar]
- 7.Casanova MF, Switala AE, Trippe J, Fitzgerald M. Comparative minicolumnar morphometry of three distinguished scientists. Autism. 2007;11:557–569. doi: 10.1177/1362361307083261. [DOI] [PubMed] [Google Scholar]
- 8.Opris I, Casanova MF. Prefrontal cortical minicolumn: from executive control to disrupted cognitive processing. Brain. 2014;137:1863–1875. doi: 10.1093/brain/awt359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raghanti MA, Spocter MA, Butti C, Hof PR, Sherwood CC. A comparative perspective on minicolumns and inhibitory GABAergic interneurons in the neocortex. Front Neuroanat. 2010:4. doi: 10.3389/neuro.05.003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casanova MF, Buxhoeveden D, Gomez J. Disruption in the inhibitory architecture of the cell minicolumn: implications for autism. Neuroscientist. 2003;9:496–507. doi: 10.1177/1073858403253552. [DOI] [PubMed] [Google Scholar]
- 11.Tan AYY, Zhang LI, Merzenich MM, Schreiner CE. Tone-evoked excitatory and inhibitory synaptic conductances of primary auditory cortex neurons. J Neurophysiol. 2004;92:630–643. doi: 10.1152/jn.01020.2003. [DOI] [PubMed] [Google Scholar]
- 12.van Vreeswijk C, Sompolinsky H. Chaos in neuronal networks with balanced excitatory and inhibitory activity. Science. 1996;274:1724–1726. doi: 10.1126/science.274.5293.1724. [DOI] [PubMed] [Google Scholar]
- 13.Hansel D, Sompolinsky H. Chaos and synchrony in a model of a hypercolumn in visual cortex. J Comput Neurosci. 1996;3:7–34. doi: 10.1007/BF00158335. [DOI] [PubMed] [Google Scholar]
- 14.Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- 15.Priebe NJ, Ferster D. Inhibition, spike threshold, and stimulus selectivity in primary visual cortex. Neuron. 2008;57:482–497. doi: 10.1016/j.neuron.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Zikopoulos B, Barbas H. Changes in Prefrontal Axons May Disrupt the Network in Autism. J Neurosci. 2010;30:14595–14609. doi: 10.1523/JNEUROSCI.2257-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- 19.Rinaldi T, Silberberg G, Markram H. Hyperconnectivity of local neocortical microcircuitry induced by prenatal exposure to valproic acid. Cereb Cortex. 2008;18:763–770. doi: 10.1093/cercor/bhm117. [DOI] [PubMed] [Google Scholar]
- 20.Rinaldi T, Perrodin C, Markram H. Hyper-connectivity and hyper-plasticity in the medial prefrontal cortex in the valproic Acid animal model of autism. Front Neural Circuits. 2008;2:4. doi: 10.3389/neuro.04.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casanova MF, de Zeeuw L, Switala A, Kreczmanski P, Korr H, Ulfig N, et al. Mean cell spacing abnormalities in the neocortex of patients with schizophrenia. Psychiatry Res. 2005;133:1–12. doi: 10.1016/j.psychres.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Chance SA, Casanova MF, Switala AE, Crow TJ. Auditory cortex asymmetry, altered minicolumn spacing and absence of ageing effects in schizophrenia. Brain. 2008;131:3178–3192. doi: 10.1093/brain/awn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tseng KY, Lewis BL, Hashimoto T, Sesack SR, Kloc M, Lewis DA, et al. A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. J Neurosci. 2008;28:12691–12699. doi: 10.1523/JNEUROSCI.4166-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volk DW, Matsubara T, Li S, Sengupta EJ, Georgiev D, Minabe Y, et al. Deficits in transcriptional regulators of cortical parvalbumin neurons in schizophrenia. Am J Psychiatry. 2012;169:1082–1091. doi: 10.1176/appi.ajp.2012.12030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 26.Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, et al. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168:921–929. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujihara K, Miwa H, Kakizaki T, Kaneko R, Mikuni M, Tanahira C, et al. Glutamate Decarboxylase 67 Deficiency in a Subset of GABAergic Neurons Induces Schizophrenia-Related Phenotypes. Neuropsychopharmacol. 2015;40:2475–2486. doi: 10.1038/npp.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volk D, Austin M, Pierri J, Sampson A, Lewis D. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am J Psychiatry. 2001;158:256–265. doi: 10.1176/appi.ajp.158.2.256. [DOI] [PubMed] [Google Scholar]
- 29.Lodge DJ, Behrens MM, Grace AA. A Loss of Parvalbumin-Containing Interneurons Is Associated with Diminished Oscillatory Activity in an Animal Model of Schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 31.Lewis DA. Cortical circuit dysfunction and cognitive deficits in schizophrenia - implications for preemptive interventions. Eur J Neurosci. 2012;35:1871–1878. doi: 10.1111/j.1460-9568.2012.08156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baroncelli L, Bonaccorsi J, Milanese M, Bonifacino T, Giribaldi F, Manno I, et al. Enriched experience and recovery from amblyopia in adult rats: impact of motor, social and sensory components. Neuropharmacology. 2012;62:2388–2397. doi: 10.1016/j.neuropharm.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Bartoletti A, Medini P, Berardi N, Maffei L. Environmental enrichment prevents effects of dark-rearing in the rat visual cortex. Nat Neurosci. 2004;7:215–216. doi: 10.1038/nn1201. [DOI] [PubMed] [Google Scholar]
- 34.Hensch TK, Fagiolini M. Excitatory-inhibitory balance and critical period plasticity in developing visual cortex. Prog Brain Res. 2005;147:115–124. doi: 10.1016/S0079-6123(04)47009-5. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Fontanini A, Maffei A. Experience-Dependent Switch in Sign and Mechanisms for Plasticity in Layer 4 of Primary Visual Cortex. J Neurosci. 2012;32:10562–10573. doi: 10.1523/JNEUROSCI.0622-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Maffei A. Inhibitory Plasticity Dictates the Sign of Plasticity at Excitatory Synapses. J Neurosci. 2014;34:1083–1093. doi: 10.1523/JNEUROSCI.4711-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saraga F, Balena T, Wolansky T, Dickson CT, Woodin MA. Inhibitory synaptic plasticity regulates pyramidal neuron spiking in the rodent hippocampus. Neuroscience. 2008;155:64–75. doi: 10.1016/j.neuroscience.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 38.D’Amour JA, Froemke RC. Inhibitory and excitatory spike-timing-dependent plasticity in the auditory cortex. Neuron. 2015;86:514–528. doi: 10.1016/j.neuron.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chun S, Bayazitov IT, Blundon JA, Zakharenko SS. Thalamocortical long-term potentiation becomes gated after the early critical period in the auditory cortex. J Neurosci. 2013;33:7345–7357. doi: 10.1523/JNEUROSCI.4500-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takesian AE, Kotak VC, Sharma N, Sanes DH. Hearing loss differentially affects thalamic drive to two cortical interneuron subtypes. J Neurophysiol. 2013;110:999–1008. doi: 10.1152/jn.00182.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol. 2003;90:2987–3000. doi: 10.1152/jn.00283.2003. [DOI] [PubMed] [Google Scholar]
- 42.Kloc M, Maffei A. Target-specific properties of thalamocortical synapses onto layer 4 of mouse primary visual cortex. J Neurosci. 2014;34:15455–15465. doi: 10.1523/JNEUROSCI.2595-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L, Kloc M, Gu Y, Ge S, Maffei A. Layer-specific experience-dependent rewiring of thalamocortical circuits. J Neurosci. 2013;33:4181–4191. doi: 10.1523/JNEUROSCI.4423-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan Z, Hu H, Huang ZJ, Agmon A. Robust but delayed thalamocortical activation of dendritic-targeting inhibitory interneurons. Proc Natl Acad Sci U S A. 2008;105:2187–2192. doi: 10.1073/pnas.0710628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang B, Huang ZJ, Morales B, Kirkwood A. Maturation of GABAergic transmission and the timing of plasticity in visual cortex. Brain Res Brain Res Rev. 2005;50:126–133. doi: 10.1016/j.brainresrev.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- 47.Artola A, Singer W. The Involvement of N-Methyl-D-Aspartate Receptors in Induction and Maintenance of Long-Term Potentiation in Rat Visual Cortex. Eur J Neurosci. 1990;2:254–269. doi: 10.1111/j.1460-9568.1990.tb00417.x. [DOI] [PubMed] [Google Scholar]
- 48.Artola A, Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987;330:649–652. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
- 49.Kampa BM, Clements J, Jonas P, Stuart GJ. Kinetics of Mg2+ unblock of NMDA receptors: implications for spike-timing dependent synaptic plasticity. J Physiol. 2004;556:337–345. doi: 10.1113/jphysiol.2003.058842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Markram H, Gerstner W, Sjostrom PJ. A history of spike-timing-dependent plasticity. Front Synaptic Neurosci. 2011;3:4. doi: 10.3389/fnsyn.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Letzkus JJ, Kampa BM, Stuart GJ. Learning rules for spike timing-dependent plasticity depend on dendritic synapse location. J Neurosci. 2006;26:10420–10429. doi: 10.1523/JNEUROSCI.2650-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kampa BM, Letzkus JJ, Stuart GJ. Requirement of dendritic calcium spikes for induction of spike-timing-dependent synaptic plasticity. J Physiol. 2006;574:283–290. doi: 10.1113/jphysiol.2006.111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sjostrom PJ, Rancz EA, Roth A, Hausser M. Dendritic excitability and synaptic plasticity. Physiol Rev. 2008;88:769–840. doi: 10.1152/physrev.00016.2007. [DOI] [PubMed] [Google Scholar]
- 54.Lisman JE. Three Ca2+ levels affect plasticity differently: the LTP zone, the LTD zone and no man’s land. J Physiol. 2001;532:285. doi: 10.1111/j.1469-7793.2001.0285f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lisman J. A Mechanism for the Hebb and the Anti-Hebb Processes Underlying Learning and Memory. P Natl Acad Sci USA. 1989;86:9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang SY, Trevino M, He KW, Ardiles A, de Pasquale R, Guo YT, et al. Pull-Push Neuromodulation of LTP and LTD Enables Bidirectional Experience-Induced Synaptic Scaling in Visual Cortex. Neuron. 2012;73:497–510. doi: 10.1016/j.neuron.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castillo PE, Younts TJ, Chavez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76:70–81. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haas JS, Nowotny T, Abarbanel HDI. Spike-timing-dependent plasticity of inhibitory synapses in the entorhinal cortex. J Neurophysiol. 2006;96:3305–3313. doi: 10.1152/jn.00551.2006. [DOI] [PubMed] [Google Scholar]
- 59.D’amour JA, Froemke RC. Inhibitory and Excitatory Spike-Timing-Dependent Plasticity in the Auditory Cortex. Neuron. 2015;86:514–528. doi: 10.1016/j.neuron.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holmgren CD, Zilberter Y. Coincident spiking activity induces long-term changes in inhibition of neocortical pyramidal cells. J Neurosci. 2001;21:8270–8277. doi: 10.1523/JNEUROSCI.21-20-08270.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kurotani T, Yamada K, Yoshimura Y, Crair MC, Komatsu Y. State-dependent bidirectional modification of somatic inhibition in neocortical pyramidal cells. Neuron. 2008;57:905–916. doi: 10.1016/j.neuron.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Komatsu Y. GABAB receptors, monoamine receptors, and postsynaptic inositol trisphosphate-induced Ca2+ release are involved in the induction of long-term potentiation at visual cortical inhibitory synapses. J Neurosci. 1996;16:6342–6352. doi: 10.1523/JNEUROSCI.16-20-06342.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nahmani M, Turrigiano GG. Deprivation-induced strengthening of presynaptic and postsynaptic inhibitory transmission in layer 4 of visual cortex during the critical period. J Neurosci. 2014;34:2571–2582. doi: 10.1523/JNEUROSCI.4600-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- 65.Younts TJ, Chevaleyre V, Castillo PE. CA1 pyramidal cell theta-burst firing triggers endocannabinoid-mediated long-term depression at both somatic and dendritic inhibitory synapses. J Neurosci. 2013;33:13743–13757. doi: 10.1523/JNEUROSCI.0817-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chevaleyre V, Heifets BD, Kaeser PS, Sudhof TC, Castillo PE. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron. 2007;54:801–812. doi: 10.1016/j.neuron.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lefort S, Gray AC, Turrigiano GG. Long-term inhibitory plasticity in visual cortical layer 4 switches sign at the opening of the critical period. Proc Natl Acad Sci U S A. 2013;110:E4540–4547. doi: 10.1073/pnas.1319571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quinlan EM, Olstein DH, Bear MF. Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci U S A. 1999;96:12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Corlew R, Wang Y, Ghermazien H, Erisir A, Philpot BD. Developmental switch in the contribution of presynaptic and postsynaptic NMDA receptors to long-term depression. J Neurosci. 2007;27:9835–9845. doi: 10.1523/JNEUROSCI.5494-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sanz-Clemente A, Nicoll RA, Roche KW. Diversity in NMDA receptor composition: many regulators, many consequences. Neuroscientist. 2013;19:62–75. doi: 10.1177/1073858411435129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maffei A, Turrigiano G. The age of plasticity: developmental regulation of synaptic plasticity in neocortical microcircuits. Prog Brain Res. 2008;169:211–223. doi: 10.1016/S0079-6123(07)00012-X. [DOI] [PubMed] [Google Scholar]
- 72.Bosman LW, Rosahl TW, Brussaard AB. Neonatal development of the rat visual cortex: synaptic function of GABAA receptor alpha subunits. J Physiol. 2002;545:169–181. doi: 10.1113/jphysiol.2002.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Datta D, Arion D, Lewis DA. Developmental Expression Patterns of GABAA Receptor Subunits in Layer 3 and 5 Pyramidal Cells of Monkey Prefrontal Cortex. Cereb Cortex. 2015;25:2295–2305. doi: 10.1093/cercor/bhu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abraham WC, Mason-Parker SE, Bear MF, Webb S, Tate WP. Heterosynaptic metaplasticity in the hippocampus in vivo: a BCM-like modifiable threshold for LTP. Proc Natl Acad Sci U S A. 2001;98:10924–10929. doi: 10.1073/pnas.181342098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci. 2000;3:1178–1183. doi: 10.1038/81453. [DOI] [PubMed] [Google Scholar]
- 77.Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clothiaux EE, Bear MF, Cooper LN. Synaptic plasticity in visual cortex: comparison of theory with experiment. J Neurophysiol. 1991;66:1785–1804. doi: 10.1152/jn.1991.66.5.1785. [DOI] [PubMed] [Google Scholar]
- 79.Bear MF. Bidirectional synaptic plasticity: from theory to reality. Philos Trans R Soc Lond B Biol Sci. 2003;358:649–655. doi: 10.1098/rstb.2002.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- 81.Wilson BM, Cox CL. Absence of metabotropic glutamate receptor-mediated plasticity in the neocortex of fragile X mice. Proc Natl Acad Sci U S A. 2007;104:2454–2459. doi: 10.1073/pnas.0610875104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang S, Yang S, Park JS, Kirkwood A, Bao S. Failed stabilization for long-term potentiation in the auditory cortex of FMR1 knockout mice. PLoS One. 2014;9:e104691. doi: 10.1371/journal.pone.0104691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao MG, Toyoda H, Ko SW, Ding HK, Wu LJ, Zhuo M. Deficits in trace fear memory and long-term potentiation in a mouse model for fragile X syndrome. J Neurosci. 2005;25:7385–7392. doi: 10.1523/JNEUROSCI.1520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Desai NS, Casimiro TM, Gruber SM, Vanderklish PW. Early postnatal plasticity in neocortex of Fmr1 knockout mice. J Neurophysiol. 2006;96:1734–1745. doi: 10.1152/jn.00221.2006. [DOI] [PubMed] [Google Scholar]
- 85.Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 87.Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 88.Shahbazian M, Young J, Yuva-Paylor L, Spencer C, Antalffy B, Noebels J, et al. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- 89.Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2005;102:12560–12565. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dani VS, Nelson SB. Intact long-term potentiation but reduced connectivity between neocortical layer 5 pyramidal neurons in a mouse model of Rett syndrome. J Neurosci. 2009;29:11263–11270. doi: 10.1523/JNEUROSCI.1019-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maffei A, Lambo ME, Turrigiano GG. Critical period for inhibitory plasticity in rodent binocular V1. J Neurosci. 2010;30:3304–3309. doi: 10.1523/JNEUROSCI.5340-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- 93.Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- 94.Takesian AE, Kotak VC, Sanes DH. Developmental hearing loss disrupts synaptic inhibition: implications for auditory processing. Future Neurol. 2009;4:331–349. doi: 10.2217/FNL.09.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takesian AE, Kotak VC, Sanes DH. Age-dependent effect of hearing loss on cortical inhibitory synapse function. J Neurophysiol. 2012;107:937–947. doi: 10.1152/jn.00515.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nusser Z, Hajos N, Somogyi P, Mody I. Increased number of synaptic GABA(A) receptors underlies potentiation at hippocampal inhibitory synapses. Nature. 1998;395:172–177. doi: 10.1038/25999. [DOI] [PubMed] [Google Scholar]
- 97.Kurotani T, Yoshimura Y, Komatsu Y. Postsynaptic firing produces long-term depression at inhibitory synapses of rat visual cortex. Neurosci Lett. 2003;337:1–4. doi: 10.1016/s0304-3940(02)01160-6. [DOI] [PubMed] [Google Scholar]
- 98.Komatsu Y, Iwakiri M. Long-term modification of inhibitory synaptic transmission in developing visual cortex. Neuroreport. 1993;4:907–910. doi: 10.1097/00001756-199307000-00017. [DOI] [PubMed] [Google Scholar]
- 99.Chiu CQ, Lur G, Morse TM, Carnevale NT, Ellis-Davies GC, Higley MJ. Compartmentalization of GABAergic inhibition by dendritic spines. Science. 2013;340:759–762. doi: 10.1126/science.1234274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 101.Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron. 2008;57:819–826. doi: 10.1016/j.neuron.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 102.Maffei A, Fontanini A. Network homeostasis: a matter of coordination. Curr Opin Neurobiol. 2009;19:168–173. doi: 10.1016/j.conb.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Turrigiano GG. The Self-Tuning Neuron: Synaptic Scaling of Excitatory Synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wierenga CJ, Ibata K, Turrigiano GG. Postsynaptic expression of homeostatic plasticity at neocortical synapses. J Neurosci. 2005;25:2895–2905. doi: 10.1523/JNEUROSCI.5217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ramocki MB, Zoghbi HY. Failure of neuronal homeostasis results in common neuropsychiatric phenotypes. Nature. 2008;455:912–918. doi: 10.1038/nature07457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 107.Maghsoodi B, Poon MM, Nam CI, Aoto J, Ting P, Chen L. Retinoic acid regulates RARalpha-mediated control of translation in dendritic RNA granules during homeostatic synaptic plasticity. Proc Natl Acad Sci U S A. 2008;105:16015–16020. doi: 10.1073/pnas.0804801105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rutherford LC, Nelson SB, Turrigiano GG. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21:521–530. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- 109.Wondolowski J, Dickman D. Emerging links between homeostatic synaptic plasticity and neurological disease. Front Cell Neurosci. 2013;7:223. doi: 10.3389/fncel.2013.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- 111.O’Leary T, Williams AH, Franci A, Marder E. Cell types, network homeostasis, and pathological compensation from a biologically plausible ion channel expression model. Neuron. 2014;82:809–821. doi: 10.1016/j.neuron.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maffei A, Turrigiano GG. Multiple modes of network homeostasis in visual cortical layer 2/3. J Neurosci. 2008;28:4377–4384. doi: 10.1523/JNEUROSCI.5298-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Petrus E, Anguh TT, Pho H, Lee A, Gammon N, Lee HK. Developmental switch in the polarity of experience-dependent synaptic changes in layer 6 of mouse visual cortex. J Neurophysiol. 2011;106:2499–2505. doi: 10.1152/jn.00111.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Whitt JL, Petrus E, Lee HK. Experience-dependent homeostatic synaptic plasticity in neocortex. Neuropharmacology. 2014;78:45–54. doi: 10.1016/j.neuropharm.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Patel B, Patel J, Cho JH, Manne S, Bonala S, Henske E, et al. Exosomes mediate the acquisition of the disease phenotypes by cells with normal genome in tuberous sclerosis complex. Oncogene. 2016;35:3027–3036. doi: 10.1038/onc.2015.358. [DOI] [PubMed] [Google Scholar]
- 116.Takada Y, Hirano M, Kiyonaka S, Ueda Y, Yamaguchi K, Nakahara K, et al. Rab3 interacting molecule 3 mutations associated with autism alter regulation of voltage-dependent Ca(2)(+) channels. Cell Calcium. 2015;58:296–306. doi: 10.1016/j.ceca.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 117.Catts VS, Lai YL, Weickert CS, Weickert TW, Catts SV. A quantitative review of the postmortem evidence for decreased cortical N-methyl-D-aspartate receptor expression levels in schizophrenia: How can we link molecular abnormalities to mismatch negativity deficits? Biol Psychol. 2016;116:57–67. doi: 10.1016/j.biopsycho.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 118.Tong XJ, Hu Z, Liu Y, Anderson D, Kaplan JM. A network of autism linked genes stabilizes two pools of synaptic GABA(A) receptors. Elife. 2015;4:e09648. doi: 10.7554/eLife.09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sala C, Vicidomini C, Bigi I, Mossa A, Verpelli C. Shank synaptic scaffold proteins: keys to understanding the pathogenesis of autism and other synaptic disorders. J Neurochem. 2015;135:849–858. doi: 10.1111/jnc.13232. [DOI] [PubMed] [Google Scholar]
- 120.Grabrucker AM. A role for synaptic zinc in ProSAP/Shank PSD scaffold malformation in autism spectrum disorders. Dev Neurobiol. 2014;74:136–146. doi: 10.1002/dneu.22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, et al. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci. 2007;27:5903–5914. doi: 10.1523/JNEUROSCI.5270-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gallagher MJ, Song L, Arain F, Macdonald RL. The juvenile myoclonic epilepsy GABA(A) receptor alpha1 subunit mutation A322D produces asymmetrical, subunit position-dependent reduction of heterozygous receptor currents and alpha1 subunit protein expression. J Neurosci. 2004;24:5570–5578. doi: 10.1523/JNEUROSCI.1301-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhu WJ, Roper SN. Reduced inhibition in an animal model of cortical dysplasia. J Neurosci. 2000;20:8925–8931. doi: 10.1523/JNEUROSCI.20-23-08925.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kobayashi M, Wen X, Buckmaster PS. Reduced inhibition and increased output of layer II neurons in the medial entorhinal cortex in a model of temporal lobe epilepsy. J Neurosci. 2003;23:8471–8479. doi: 10.1523/JNEUROSCI.23-24-08471.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xiang H, Chen HX, Yu XX, King MA, Roper SN. Reduced excitatory drive in interneurons in an animal model of cortical dysplasia. J Neurophysiol. 2006;96:569–578. doi: 10.1152/jn.01133.2005. [DOI] [PubMed] [Google Scholar]
- 126.Zhou FW, Chen HX, Roper SN. Balance of inhibitory and excitatory synaptic activity is altered in fast-spiking interneurons in experimental cortical dysplasia. J Neurophysiol. 2009;102:2514–2525. doi: 10.1152/jn.00557.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Akbarian S, Sucher NJ, Bradley D, Tafazzoli A, Trinh D, Hetrick WP, et al. Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J Neurosci. 1996;16:19–30. doi: 10.1523/JNEUROSCI.16-01-00019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dracheva S, Marras SA, Elhakem SL, Kramer FR, Davis KL, Haroutunian V. N-methyl-D-aspartic acid receptor expression in the dorsolateral prefrontal cortex of elderly patients with schizophrenia. Am J Psychiatry. 2001;158:1400–1410. doi: 10.1176/appi.ajp.158.9.1400. [DOI] [PubMed] [Google Scholar]
- 129.Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61:649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- 130.Kehrer C, Maziashvili N, Dugladze T, Gloveli T. Altered Excitatory-Inhibitory Balance in the NMDA-Hypofunction Model of Schizophrenia. Front Mol Neurosci. 2008;1:6. doi: 10.3389/neuro.02.006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cohen SM, Tsien RW, Goff DC, Halassa MM. The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr Res. 2015;167:98–107. doi: 10.1016/j.schres.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 133.Adler CM, Malhotra AK, Elman I, Goldberg T, Egan M, Pickar D, et al. Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am J Psychiat. 1999;156:1646–1649. doi: 10.1176/ajp.156.10.1646. [DOI] [PubMed] [Google Scholar]
- 134.Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G, Melson AK, Hershey T, et al. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacol. 1999;20:106–118. doi: 10.1016/S0893-133X(98)00067-0. [DOI] [PubMed] [Google Scholar]
- 135.Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiat Res. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 136.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang Y, Behrens MM, Lisman JE. Prolonged exposure to NMDAR antagonist suppresses inhibitory synaptic transmission in prefrontal cortex. J Neurophysiol. 2008;100:959–965. doi: 10.1152/jn.00079.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Goncalves JT, Anstey JE, Golshani P, Portera-Cailliau C. Circuit level defects in the developing neocortex of Fragile X mice. Nat Neurosci. 2013;16:903–909. doi: 10.1038/nn.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tervonen TA, Louhivuori V, Sun X, Hokkanen ME, Kratochwil CF, Zebryk P, et al. Aberrant differentiation of glutamatergic cells in neocortex of mouse model for fragile X syndrome. Neurobiol Dis. 2009;33:250–259. doi: 10.1016/j.nbd.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 140.Selby L, Zhang C, Sun QQ. Major defects in neocortical GABAergic inhibitory circuits in mice lacking the fragile X mental retardation protein. Neurosci Lett. 2007;412:227–232. doi: 10.1016/j.neulet.2006.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gibson JR, Bartley AF, Hays SA, Huber KM. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J Neurophysiol. 2008;100:2615–2626. doi: 10.1152/jn.90752.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rotschafer S, Razak K. Altered auditory processing in a mouse model of fragile X syndrome. Brain Res. 2013;1506:12–24. doi: 10.1016/j.brainres.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 143.Jeste SS, Tuchman R. Autism Spectrum Disorder and Epilepsy: Two Sides of the Same Coin? J Child Neurol. 2015;30:1963–1971. doi: 10.1177/0883073815601501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 145.Volk DW, Lewis DA. Impaired prefrontal inhibition in schizophrenia: relevance for cognitive dysfunction. Physiol Behav. 2002;77:501–505. doi: 10.1016/s0031-9384(02)00936-8. [DOI] [PubMed] [Google Scholar]
- 146.Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12:335–344. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, et al. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci U S A. 1998;95:15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gilby KL, Da Silva AG, McIntyre DC. Differential GABA(A) subunit expression following status epilepticus in seizure-prone and seizure-resistant rats: A putative mechanism for refractory drug response. Epilepsia. 2005;46:3–9. doi: 10.1111/j.1528-1167.2005.01001.x. [DOI] [PubMed] [Google Scholar]
- 150.Adusei DC, Pacey LK, Chen D, Hampson DR. Early developmental alterations in GABAergic protein expression in fragile X knockout mice. Neuropharmacology. 2010;59:167–171. doi: 10.1016/j.neuropharm.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 151.Oblak AL, Gibbs TT, Blatt GJ. Reduced GABAA receptors and benzodiazepine binding sites in the posterior cingulate cortex and fusiform gyrus in autism. Brain Res. 2011;1380:218–228. doi: 10.1016/j.brainres.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD. GABA(A) Receptor Downregulation in Brains of Subjects with Autism. J Autism Dev Disord. 2009;39:223–230. doi: 10.1007/s10803-008-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fukuda T, Itoh M, Ichikawa T, Washiyama K, Goto Y. Delayed maturation of neuronal architecture and synaptogenesis in cerebral cortex of Mecp2-deficient mice. J Neuropath Exp Neur. 2005;64:537–544. doi: 10.1093/jnen/64.6.537. [DOI] [PubMed] [Google Scholar]
- 154.Burrows EL, Laskaris L, Koyama L, Churilov L, Bornstein JC, Hill-Yardin EL, et al. A neuroligin-3 mutation implicated in autism causes abnormal aggression and increases repetitive behavior in mice. Mol Autism. 2015;6:62. doi: 10.1186/s13229-015-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, et al. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 157.Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang W, Peterson M, Beyer B, Frankel WN, Zhang ZW. Loss of MeCP2 from forebrain excitatory neurons leads to cortical hyperexcitation and seizures. J Neurosci. 2014;34:2754–2763. doi: 10.1523/JNEUROSCI.4900-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kishi N, Macklis JD. MeCP2 functions largely cell-autonomously, but also non-cell-autonomously, in neuronal maturation and dendritic arborization of cortical pyramidal neurons. Exp Neurol. 2010;222:51–58. doi: 10.1016/j.expneurol.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wood L, Gray NW, Zhou Z, Greenberg ME, Shepherd GM. Synaptic circuit abnormalities of motor-frontal layer 2/3 pyramidal neurons in an RNA interference model of methyl-CpG-binding protein 2 deficiency. J Neurosci. 2009;29:12440–12448. doi: 10.1523/JNEUROSCI.3321-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hirono M, Yoshioka T, Konishi S. GABA(B) receptor activation enhances mGIuR-mediated responses at cerebellar excitatory synapses. Nature Neuroscience. 2001;4:1207–1216. doi: 10.1038/nn764. [DOI] [PubMed] [Google Scholar]