Abstract
Objective
To investigate fatigue and cognitive impairments in systemic lupus erythematous (SLE) in relation to diffuse white matter microstructural brain damage.
Methods
Diffusion tensor MRI, used to generate biomarkers of brain white matter microstructural integrity, was obtained in patients with SLE and age-matched controls. Fatigue and cognitive function were assessed and related to SLE activity, clinical data and plasma biomarkers of inflammation and endothelial dysfunction.
Results
Fifty-one patients with SLE (mean age 48.8 ± 14.3 years) were included. Mean diffusivity (MD) was significantly higher in all white matter fibre tracts in SLE patients versus age-matched healthy controls (p<0.0001). Fatigue in SLE was higher than a normal reference range (p<0.0001) and associated with lower MD (β = −0.61, p=0.02), depression (β = 0.17, p=0.001), anxiety (β = 0.13, p=0.006) and higher body mass index (β = 0.10, p=0.004) in adjusted analyses. Poorer cognitive function was associated with longer SLE disease duration (p=0.003) and higher MD (p=0.03) and, in adjusted analysis, higher levels of IL-6 (β = −0.15, p=0.02) but not with MD. Meta-analysis (10 studies, n=261, including the present study) confirmed that patients with SLE have higher MD than controls.
Conclusion
Patients with SLE have more microstructural brain white matter damage for age than the general population, but this does not explain increased fatigue or lower cognition in SLE. The association between raised IL-6 and worse current cognitive function in SLE should be explored in larger datasets.
Keywords: autoimmune diseases, cytokines, inflammation, Systemic Lupus Erythematosus
INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease. It is associated with increased risk of stroke1,2 and cognitive decline has been reported.3 Fatigue is a common4 but unexplained feature of SLE which increases distress and lost work days and has been associated with increased burden of brain white matter hyperintensities (WMH),5 suggestive of cerebral small vessel disease (SVD).6
Diffusion tensor MRI (DT-MRI) can map the brain’s white matter tracts (bundles of individual white matter fibres) and provide biomarkers for microstructural integrity. Water molecules preferentially diffuse along the principal fibre direction in healthy white matter, while loss of structural integrity, which has a deleterious effect on brain function, increases free water movement. The magnitude and directionality of water molecule diffusion can be quantified within segmented tracts using mean diffusivity (MD) and fractional anisotropy (FA),7 with low MD and high FA indicating structurally intact white matter.
Nine small (average n=24 patients) studies8–16 show microstructural tract damage in SLE and neuropsychiatric SLE (NPSLE) versus healthy controls. However, none of these studies has investigated whether sub-visible DT-MRI-detected brain damage associates with fatigue or cognitive function.
Our aims were: (1) compare water diffusion values in major white matter tracts in SLE patients with age-matched healthy controls, (2) identify if fatigue or cognitive function in SLE were related to DT-MRI parameters, SLE disease activity or plasma biomarkers of inflammation and endothelial dysfunction and (3) to consider the totality of the data on DTI-MRI in SLE by meta-analysing the current and all prior studies.
METHODS
Subjects
From April to December 2014, patients with SLE were prospectively recruited into a neuroimaging study and underwent DT-MRI to quantify white matter tract integrity. We invited patients consecutively as they attended clinic, of whom 31/51 (60.7%) were already members of the prospective Scottish Lupus Exchange registry project (http://public.ukcrn.org.uk/search/StudyDetail.aspx?StudyID=15489). All patients were seen by a consultant rheumatologist. SLE was diagnosed according to updated American College of Rheumatology 1997 criteria17. We excluded patients with concurrent infection. The project received research ethics committee approval (South East Scotland REC 01, 14/SS/0003) and all participants gave written informed consent. We compared DT-MRI biomarkers in the SLE group with control data obtained from a group of consenting healthy volunteers scanned on the same scanner with the same sequences under a study approved by the Lothian Research Ethics Committees (REC 05/S1104/45).
Fatigue
We assessed fatigue using the Fatigue Severity Scale (FSS)18 with higher scores indicating more severe fatigue. The mean (± SD) from normal healthy adults in the standardisation sample was 2.3 (± 0.7).18
Cognitive assessments
We used Hospital Anxiety and Depression Scale (HADS),19 Montreal Cognitive Assessment (MoCA),20 Addenbrooke’s Cognitive Examination – Revised (ACER)21 and Mini Mental State Examination (MMSE)22 to assess anxiety, depression and cognitive function, while the National Adult Reading Test (NART)23 was used to adjust for premorbid intelligence. The NART is a validated24 estimate of childhood peak premorbid intelligence as it appears broadly resilient to age-related cognitive decline.
Clinical data
Medical history including cardiovascular risk factors such as smoking status, prior cerebrovascular events, hypertension and diabetes were recorded together with height, weight and body mass index (BMI).
Disease activity
Current SLE disease activity was assessed using the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K)25 and British Isles Lupus Assessment Group 2004 (BILAG)26 tools. Accumulated permanent damage was assessed with the Systemic Lupus International Collaborating Clinics (SLICC)27,28 tool.
MRI
All subjects were scanned at 1.5T with an 8-channel phased-array head coil (GE, Milwaukee, WI). The scan protocol included axial T2, gradient-recalled echo, fluid-attenuated inversion recovery, sagittal T2, high-resolution coronal 3D T1 volume, and whole brain DT-MRI sequences. The DT-MRI sequence consisted of thee T2-weighted and 32 diffusion-weighted (b=1000 s mm−2) axial single-shot spin-echo echo-planar imaging volumes (field of view 240×240 mm, matrix 128×128, TR 13.75s, TE 78.4ms). Each volume comprised 56 contiguous 2.5 mm thick axial slices with 1.875 mm in-plane resolution. Detailed scanning parameters are shown in Supplementary Table 1.
Tractography
Tractography analysis was performed blind to all other data. Major white matter tracts were identified using probabilistic neighbourhood tractography (PNT), an automatic tract segmentation method based on modelling tract topology, as implemented in the TractoR package for fibre tracking analysis (http://www.tractor-mri.org.uk). Further details are provided in Supplementary Material.
Plasma markers
All participants had blood drawn on the day of MRI scanning. We measured the pro-inflammatory cytokine interleukin-6 (IL-6), endothelial dysfunction (von Willebrand Factor antigen (vWF Ag) and two measures of vWF activity: factor VIIIc (fVIIIc) and ristocetin cofactor (RCOF)), endothelial toxicity (homocysteine), cholesterol (total, high- (HDL) and low-density (LDL) lipoprotein) and anti-phospholipid antibodies (anti-cardiolipin Immunoglobulin G (IgG) and M (IgM)). We also had access to blood data from recent clinic visits (most within one month; all within six months) including SLE disease activity (C3, C4 and anti-double stranded DNA) and routine inflammatory markers (C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR)). Additionally, interferon beta (IFN) signatures were measured by analysis of messenger ribonucleic acid in a subset of 25 (49%) patients.
Statistical analysis
Data distributions were checked graphically for normality and presented as means (± SD) or medians (Q1–Q3) as appropriate. Principal components analysis (PCA) was used to create a ‘general factor’ of MD and FA representing all tracts (‘tract-averaged’) from the individual tracts. PCA is a data reduction technique that extracts a latent variable calculated from several measured variables. Tract-averaged MD and FA were compared between SLE patients and healthy controls using Student’s t test. Mean fatigue in patients was compared with a normal reference range18 using the Welch two-sample t test. Linear regression was used to examine the association between fatigue and other variables by univariate analyses. Multiple linear regression was used to adjust for age alone, then age + disease duration + steroids. PCA was used to create a general factor of cognitive function (g)29 from the three cognitive tools (MoCA, ACER, MMSE). Linear regression was used to examine the association between g and other variables, and included use of NART to adjust for a person’s prior (peak) cognitive function before the deleterious effect of age and SLE disease duration, if any, and any impact from current steroid use. For all regression models, we checked multicollinearity, independence, constancy of variance and normality in the residuals. A p value of <0.05 was considered significant. In the interests of transparency, we report results from all analyses regardless of the p value as this aids interpretation of the entire study and we did not adjust the p values for multiple comparisons30. All analyses were performed with the statistical programming language R version 3.0.1 (http://www.r-project.org/).31
Literature review and meta-analysis
We searched MEDLINE and EMBASE (from 1990) on 15th January 2015 using the terms “diffusion-tensor imaging” and “diffusion-weighted imaging” in conjunction with the terms “lupus”, “systemic lupus erythematosus” and “neuropsychiatric systemic lupus erythematosus”. Acronyms and different combinations of the main search terms were also used. We checked reference lists of relevant papers for additional studies. We extracted data on study population (demographics, sample size, disease duration), imaging parameters, part of the brain measured (whole brain, specific tracts) and the reported diffusion measures. Standardised mean differences were calculated using the Cochrane Collaboration’s Review Manager 5 software and used to compare DT-MRI findings in the meta-analysis.
RESULTS
Subjects
Fifty-one patients with SLE were recruited with mean age 48.8±14.3 years (range 20 to 76 years), including 47 women (92%) which is consistent with community prevalence. Clinical, fatigue and cognitive data are given in Table 1. MD, FA and NART scores were available for 51 age- and sex-matched healthy controls of mean age 44.9±11.1 years, including 39 women (76%). A third of patients (18/51 (35%)) were currently prescribed corticosteroids.
Table 1.
Subject charactersitics
| N (%) or mean ± SD or median (Q1–Q3) | Reference data from controls or standard ranges | |
|---|---|---|
| SLE patients, n = 51 | ||
| Female | 47 (92%) | 39 (76%); p=0.06 |
| Age (years) | 48.8 ± 14.3 | 44.9 ± 11.1; p=0.12 |
| Disease duration (months) | 50 (24.5–148) | |
| Members of SLEx registry | 31 (61%) | |
| NPSLE | 4 (8%) | |
| BMI (kg/m2) | 29 (6.5) | 20–25 normal, 25–30 overweight, 30–35 obese, 35+ clinically obese |
| Current smoker | 6 (12%) | |
| Hypertension | 9 (18%) | ≥140/90 mm Hg |
| Fatigue (score) | 5.0 ± 1.7 | 2.3 ± 0.7; p<0.0001 |
| Anxiety (score) | 6 (3–12) | 0–7 non-case, 8–10 borderline, 11+ case |
| Depression (score) | 8 (6–12) | As anxiety |
| Current steroids | 18 (35%) | |
| MoCA (score) (n=50) | 26 (24–28) | 0–30. Normal ≥26 |
| ACER (score) (n=50) | 91 (87–94) | 0–100. Normal ≥88 |
| MMSE (score) (n=50) | 28 (27–30) | 0–30. Normal ≥27 |
| NART (score) (n=50) | 34 (27–39) | 40 (34–42); p=0.0008 |
ACER = Addenbrooke’s Cognitive Examination – Revised, BMI = body mass index, MoCA = Montreal Cognitive Assessment, MMSE = Mini Mental State Examination, NART = National Adult Reading Test
General factors for MD and FA
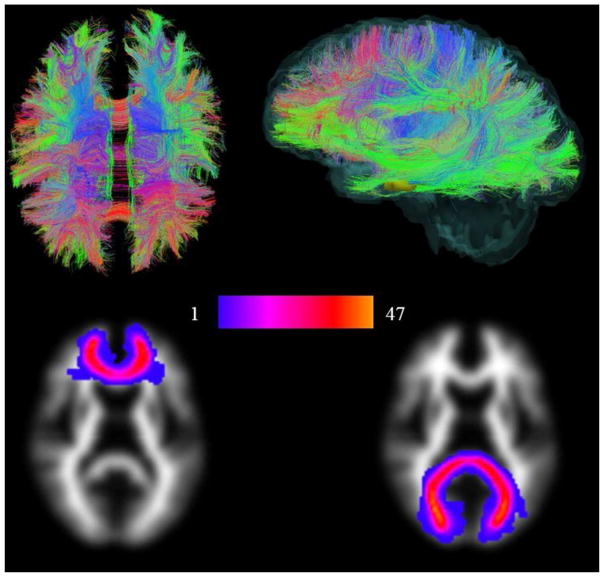
DT-MRI failed in four patients so the following analyses are based on 47 patients. In SLE, the general factors MD and FA accounted for 40% and 27% of the variance among tracts, respectively. A similar level of variance was explained in the control group. Factor loadings of each tract are given in Supplementary Table 2. An image illustrating whole brain white matter tractography from a representative patient along with a group map illustrating two tracts (the genu and splenium of corpus callosum) is shown in Figure 1.
Figure 1.
Whole brain white matter tractography and group maps. The first row shows maps of whole brain white matter structure from a representative patient. White matter tracts running predominantly anterior/posterior are coloured green, superior/inferior blue and right/left red. The second row shows group maps (all patients superimposed on each other) of two major tracts, the genu and splenium of corpus callosum for all 47 patients. Note the close correspondence of these tracts, indicated by the lighter red/yellow colours, which allows the integrity of the same structure to be measured across the cohort.
Comparing MD and FA to healthy controls
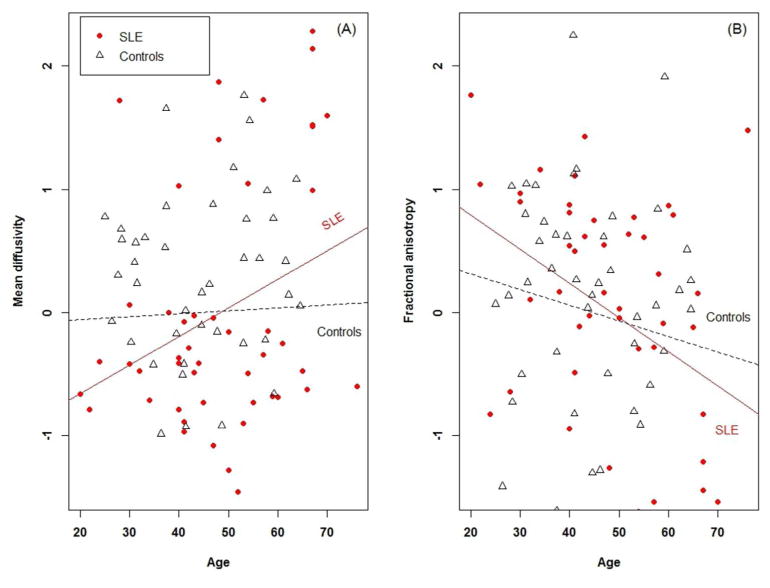
MD was significantly higher in all tracts (p<0.0001 for all tracts) in SLE patients versus controls (Table 2). FA was significantly higher in SLE patients than controls in the genu (p<0.0001) and left corticospinal tract (p<0.0001), and significantly lower in the splenium (p=0.008), and left (p=0.04) and right (p=0.01) cingulum bundles (Table 2). The expected increase in MD and decrease in FA with age appeared accelerated in SLE patients versus controls (Figure 2). However, the patients’ and controls’ regression slopes (for both MD and FA) did not differ when tested via an interaction term in a linear regression model.
Table 2.
Comparison of MD and FA values for white matter tracts between SLE patients and healthy controls
| SLE (n=47) | Controls (n=47) | p value | Cohen’s d | |
|---|---|---|---|---|
| Female n (%) | 43 (91.5%) | 41 (87.2%) | p=0.74 | NA |
| Age (years) | 48.5 ± 13.7 | 44.6 ± 11.5 | p=0.14 | NA |
| MD (10−6 mm2/s) | ||||
| Genu | 833.18 ± 68.13 | 751.29 ± 57.36 | p<0.0001 | 1.33 |
| Splenium | 1072.33 ± 162.98 | 761.49 ± 129.37 | p<0.0001 | 2.19 |
| Left cingulum | 740.69 ± 44.08 | 624.21 ± 35.41 | p<0.0001 | 3.01 |
| Right cingulum | 721.06 ± 43.33 | 619.70 ± 35.74 | p<0.0001 | 2.62 |
| Left CST | 729.25 ± 30.76 | 667.24 ± 39.4 | p<0.0001 | 1.82 |
| Right CST | 740.96 ± 30.18 | 683.32 ± 35.31 | p<0.0001 | 1.79 |
| Left ILF | 781.68 ± 49.53 | 728.42 ± 66.48 | p<0.0001 | 0.96 |
| Right ILF | 818.16 ± 136.72 | 703.82 ± 63.03 | p<0.0001 | 1.29 |
| Average | 804.6 ± 39.8 | 692.4 ± 32.2 | p<0.0001 | 3.20 |
| FA (unitless, values lie in the range 0 to 1) | ||||
| Genu | 0.49 ± 0.06 | 0.42 ± 0.04 | p<0.0001 | 1.42 |
| Splenium | 0.52 ± 0.07 | 0.55 ± 0.06 | p=0.008 | −0.57 |
| Left cingulum | 0.44 ± 0.05 | 0.46 ± 0.04 | p=0.04 | −0.45 |
| Right cingulum | 0.42 ± 0.04 | 0.44 ± 0.03 | p=0.01 | −0.57 |
| Left CST | 0.49 ± 0.04 | 0.44 ± 0.04 | p<0.0001 | 1.26 |
| Right CST | 0.47 ± 0.04 | 0.48 ± 0.04 | p=0.24 | −0.25 |
| Left ILF | 0.45 ± 0.05 | 0.43 ± 0.04 | p=0.08 | 0.36 |
| Right ILF | 0.42 ± 0.05 | 0.43 ± 0.04 | p=0.59 | −0.11 |
| Average | 0.46 ± 0.02 | 0.46 ± 0.02 | p=0.25 | 0.24 |
CST = corticospinal tract, FA = fractional anisotropy, ILF = inferior longitudinal fasciculus, MD = mean diffusivity.
Figure 2.
General factors for (A) mean diffusivity and (B) fractional anisotropy in relation to age among SLE patients and healthy controls.
Fatigue
Fatigue in SLE was significantly higher than the expected normal range18 (5.0±1.7 v 2.3±0.7, p<0.0001). Fatigue was non-significantly negatively correlated with age (r = −0.24, p=0.09) (Supplementary Table 3; Supplementary Figure 5, panel (A)).
In the univariate analysis, higher fatigue was associated with higher depression (β = 0.17, r = 0.47, p=0.0004), higher anxiety (β = 0.13, r = 0.41, p=0.002), higher BMI (β = 0.09, r = 0.36, p=0.01), lower MD (β = −0.58, r = −0.35, p=0.01), lower IFN (β = −0.09, r = −0.54, p=0.006) and lower vWF (RCOF (β = −1.43, r = −0.34, p=0.02) and fVIIIc (β = −1.17, r = −0.30, p=0.04)) (Supplementary Tables 3 and 4). Except vWF (RCOF), all associations remained after adjusting for age only. Except vWF (RCOF) and IFN all associations remained after adjusting for age, disease duration and steroids (Table 3).
Table 3.
Multiple linear regression models of fatigue and cognitive function in SLE patients
| Unadjusted | Age adjusted | Fully adjusteda | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | SE B | p value | B | SE B | p value | B | SE B | p value | |
| Fatigue | |||||||||
| BMI (kg/m2) | 0.093 | 0.034 | 0.01 | 0.106 | 0.033 | 0.003 | 0.102 | 0.033 | 0.004 |
| Anxiety | 0.132 | 0.041 | 0.003 | 0.119 | 0.044 | 0.01 | 0.127 | 0.044 | 0.006 |
| Depression | 0.175 | 0.046 | 0.0004 | 0.162 | 0.048 | 0.001 | 0.170 | 0.047 | 0.001 |
| MD (10−6 mm2/s) | −0.580 | 0.234 | 0.02 | −0.556 | 0.249 | 0.03 | −0.610 | 0.253 | 0.02 |
| IFN (RQ value) | −0.090 | 0.030 | 0.006 | −0.091 | 0.029 | 0.005 | −0.080 | 0.040 | 0.06 |
| vWF (fVIIIc) (IU/mL) | −1.168 | 0.550 | 0.04 | −1.121 | 0.539 | 0.04 | −1.111 | 0.533 | 0.04 |
| vWF (RCOF) (IU/mL) | −1.426 | 0.599 | 0.02 | −1.212 | 0.623 | 0.06 | −1.154 | 0.616 | 0.07 |
| Cognitive function | |||||||||
| Disease duration (months) | −0.003 | 0.001 | 0.003 | −0.003 | 0.001 | 0.005 | −0.001 | 0.000 | 0.07 |
| MD (10−6 mm2/s) | −0.271 | 0.126 | 0.04 | −0.264 | 0.135 | 0.06 | −0.012 | 0.108 | 0.91 |
| vWF (Ag) (IU/mL) | −0.800 | 0.204 | 0.0003 | −0.794 | 0.215 | 0.0006 | −0.286 | 0.208 | 0.18 |
| vWF (RCOF) (IU/mL) | −0.889 | 0.358 | 0.02 | −0.848 | 0.380 | 0.03 | −0.296 | 0.317 | 0.36 |
| IL-6 (pg/mL) | −0.234 | 0.080 | 0.006 | −0.241 | 0.080 | 0.005 | −0.151 | 0.063 | 0.02 |
Fatigue adjusted for age, disease duration and seroids; Cognitive function adusted for age, disease duration, steroids and NART.
B = beta coefficient, IL-6 = interleukin-6, MD = mean difussivity, NART = national adult reading test, SE B = standard error for beta coefficient, vWF Ag = von Willebrand Factor antigen, vWF fVIIIc = von Willebrand Factor VIII, vWF RCOF = von Willebrand Factor ristocen co-factor
Cognitive function
One patient was excluded from the analysis of cognitive results as English was not his first language. On average, patients met the minimum levels required for normal cognition by the three individual tests (Table 1). However, 19/50 (38%) (MoCA), 13/50 (26%) (ACER) and 5/50 (10%) (MMSE) patients scored below the tests’ commonly-used cut-offs indicating clinical levels of cognitive impairment. The mean NART score in SLE was significantly lower than age-matched healthy controls (34 v 40, p=0.0008).
In the univariate analysis, poorer cognitive function (g) was associated with longer SLE disease duration (β = −0.003, r = −0.41, p=0.003), higher MD (β = −0.27, r = −0.31, p=0.03), higher vWF (Ag (β = −0.80, r = −0.51, p=0.0003) and RCOF (β = −0.89, r = −0.35, p=0.02)) and higher IL-6 (β = −0.23, r = −0.43, p=0.006) (Supplementary Tables 3 and 4). Except MD, all associations remained after adjusting for age (Table 3). After adjusting for age, disease duration, steroids and NART, only higher IL-6 (β = −0.15, p=0.02) remained independently and significantly associated with poorer cognitive function (Table 3).
Literature review and meta-analysis
The search uncovered 19 papers of which 10 were excluded as not relevant (no measure of MD or FA in SLE or NPSLE). We meta-analysed results in 3 groups: (1) SLE versus healthy controls, (2) NPSLE versus healthy controls and (3) SLE versus NPSLE.
Nine8–16 prior studies were reviewed (n=214 patients; n=24 average per study) and added to the current study for meta-analysis (Table 4 and Supplementary Figures 1–4). Four studies9–12 did not provide data to permit inclusion in the forest plots and are summarised with the other studies in Table 4 only.
Table 4.
Diffusion imaging studies in SLE and NPSLE
| Study | Sequence, gradient directions | Field | Technique | n (mean age in years) | Comparison | Measurement | Region | Findings |
|---|---|---|---|---|---|---|---|---|
| SLE versus healthy controls | ||||||||
| Zhang, 2007 | DTI, 26 | 1.5T | MTR and ADC histogram, and ROI | 34 (47) | SLE v HC | FA and MD | Whole brain and Various regions | ↑ MD in brain in SLE ↓ FA in SLE in most regions ↑ MD in SLE in most regions |
| Emmer, 2010 | DTI, 6 | 3T | TBSS | 12 (42) | SLE v HC | FA | Various tracts | ↓ FA in SLE in most tracts |
| Jung, 2010 | DTI, 12 | 1.5T | TBSS | 16 (37) | SLE v HC | FA and MD | Various tracts | No difference in FA No difference in MD |
| Schmidt-Wilcke, 2014 | DTI, 15 | 3T | TBSS & TFCE | 19 (38) | SLE v HC | FA | Voxels and clusters across WM | ↓ FA in SLE in prefrontal WM |
| Zimny, 2014 | DTI, 25 | 1.5T | ROI | 13 (34) | SLE v HC | FA | 14 WM tracts | ↓ FA in SLE in most tracts (see FP) |
| Wiseman, 2015 | DTI, 32 | 1.5T | Quantitative tractography | 47 (48) | SLE v HC | FA and MD | 8 WM tracts | ↓ FA in SLE in most tracts (see FP) ↑ MD in SLE in all tracts (see FP) |
| NPSLE versus healthy controls | ||||||||
| Bosma, 2003 | DWI, 3 | 1.5T | ADC histogram | 11 (35) | NPSLE v HC | mean ADC | Whole brain | ↑ mean ADC in brain in NPSLE |
| Welsh, 2007 | DWI, 3 | 1.5T | ADC histogram | 17 (43) | NPSLE v HC | mean ADC | Whole brain and segmented tissues | ↑ mean ADC in brain in NPSLE ↑ mean ADC in GM & WM in NPSLE |
| Hughes, 2007 | DTI, 9 | 1.5T | ROI | 8 (43) | NPSLE v HC | FA and MD | Various regions | ↓ FA in NPSLE in various regions ↑ MD in NPSLE in various regions |
| Jung, 2010 | DTI, 12 | 1.5T | TBSS | 17 (39) | NPSLE v HC | FA and MD | Various tracts | ↓ FA in NPSLE in some tracts ↑ MD in NPSLE in most tracts |
| Zivadinov, 2013 | DTI, 39 | 3T | Voxel based with tissue segmentation | 26 (48) | NPSLE v HC | MD | NAWM | ↑ MD in NAWM in NPSLE |
| Schmidt-Wilcke, 2014 | DTI, 15 | 3T | TBSS | 19 (39) | NPSLE v HC | FA | Voxels and clusters across WM | ↓ FA in SLE in prefrontal WM |
| Zimny, 2014 | DTI, 25 | 1.5T | ROI in WM tracts | 22 (35) | NPSLE v HC | FA | 14 WM tracts | ↓ FA in NPSLE in most tracts (see FP) |
| SLE versus NPSLE | ||||||||
| Jung, 2010 | DTI, 12 | 1.5T | TBSS | 16 (37) | SLE v NPSLE | FA and MD | Various tracts | ↑ FA in SLE in 2 tracts ↓ MD in SLE in 3 tracts |
| Schmidt-Wilcke, 2014 | DTI, 15 | 3T | TBSS | 19 (38) | SLE v NPSLE | FA | Voxels and clusters across WM | No difference in FA between SLE and NPSLE |
| Zimny, 2014 | DTI, 25 | 1.5T | ROI | 13 (34) | SLE v NPSLE | FA | 14 WM tracts | Generally no difference in FA between SLE and NPSLE (see FP) |
ADC = average diffusion coefficient, FA = fractional anisotropy, FP = forest plot (see Supplementary Material), GM = grey matter, MD = mean diffusivity, MTR = mean transit ratio, NPSLE = neuropsychiatric SLE, NAT = normal appearing tissue, NAWM = normal appearing white matter, ROI = region of interest, SLE = systemic lupus erythematosus, TBSS = tract based spatial statistics, WM = white matter
In general, among most tracts, MD was significantly increased (standardised mean difference (SMD) 1.07 (95% CI, 0.62 to 1.52)) and FA non-significantly reduced (SMD −0.16 (−0.48 to 0.17)) in SLE patients versus healthy controls (Supplementary Figures 1 and 2). A similar pattern was seen in NPSLE versus healthy controls. Data comparing SLE to NPSLE were limited: only one study11 provided data on MD, and whereas three studies8,9,11 reported on FA, there were no data suitable for pooling although the general observation was little difference in water diffusion measures between SLE and NPSLE (Table 4).
DISCUSSION
The main findings in the present study, the largest to date on DT-MRI in SLE adding ~25% more to the total available data and the only one to use quantitative tractography, were that: (i) MD was higher in eight major white matter tracts in SLE versus healthy controls but the MD levels did not account for either fatigue or cognitive function in adjusted analyses; (ii) fatigue in patients was higher than a normal reference range and (iii) patients with SLE had poorer current cognitive function which was independently associated with higher levels of the pro-inflammatory biomarker IL-6.
The direction of the regression slopes relating MD (rising) and FA (declining) with age were as expected but steeper in SLE patients versus controls, indicating accelerated decline in white matter microstructure with age. However, when we tested the slopes using an interaction term in a linear regression model there was no significant difference between patients and controls and a larger sample will be needed to confirm a modest difference in accelerated ageing. Nonetheless, significantly higher MD levels were found in all white matter tracts in patients versus controls which is in accordance with prior literature. This indicates a diffuse increase in brain water mobility in SLE, possibly indicating a subtle decline in white matter microstructural integrity. We recently demonstrated increased stroke risk in SLE versus the general population with greatest risk in those <50 years2; again possibly indicative of accelerated ageing.
In SLE, higher fatigue was associated with lower MD, although this likely reflects higher fatigue scores being more common in younger participants. A much larger study will be required to determine if fatigue in SLE is associated with reduced MD levels for a given age. Higher fatigue was also associated with lower IFN, lower vWF (fVIIIc and RCOF) and approached significance with higher CRP.
Higher levels of fatigue were associated with higher BMI in the current study, and has been established previously.32 Higher levels of fatigue were also associated with lower levels of endothelial dysfunction (vWF). The plasma marker that had the highest association with fatigue was IFN, independent of age, although the direction was unexpected. Fatigue is a common side effect of IFN therapy; meanwhile, prior studies of endogenous IFN in SLE did not show associations with fatigue.33,34
Lower levels of current cognitive function were associated with longer SLE disease duration and higher MD and between 10% and 38% of patients had cognitive test scores indicating clinical levels of cognitive impairment. Our finding that lower levels of cognitive function were associated with higher MD is in agreement with Bosma et al. 35 who examined 24 patients diagnosed with NPSLE. Bosma and colleagues also correlated lower levels of neurological functioning (essentially motor skills) with higher levels of magnetization transfer MRI parameters.
Poorer current cognitive function was also associated with higher levels of the pro-inflammatory cytokine IL-6, independent of age and prior cognitive abilities. The PROSPER36 study (randomised controlled trial; n=5,653) associated higher IL-6 with worse executive function (p<0.001), independent of age. At follow-up (mean 39 months), higher IL-6 was independently associated with an increased rate of cognitive decline in both executive function (p=0.002) and memory (p=0.002).36 The mean age of participants in the PROSPER study was 75 years, considerably older than the mean age of participants in the current study, yet the similarity in findings could indicate that SLE patients are experiencing aged-related effects on the brain at younger ages. The PROSPER study corrected for educational level but not premorbid intelligence using NART.
In contrast to the findings of Nishimura et al. (n=43 steroid-naïve SLE patients)37 we did not find an association between current cognitive impairment and SLE activity (in univariate analysis or after correcting for steroid use), possibly due to different measures of psychological assessment used between studies. Other variables associated with cognitive function on univariate analysis became nonsignificant when NART was added to the regression models. Current cognitive impairment is thus mostly explained by premorbid IQ, although inflammation also plays a role.
Strengths of the present study include use of a continuum of fatigue and cognitive scores rather than dichotomised data, the 32 diffusion-encoding gradient directions in the DT-MRI exam which increases the precision of the imaging data, a large sample size relative to existing DT-MRI studies (although we note our sample is still small, limiting study power) in SLE and use of quantitative tractography rather than ‘region of interest’ or ‘voxel-based’ methods. PNT has the advantage that it segments tracts automatically in native space, avoiding brain distortion by use of registration to standard space and thereby providing objective measures of tract microstructure that can be correlated with phenotypic data. Additionally, our study did not solely focus on patients that were neurologically symptomatic or diagnosed with NPSLE but instead included a range of SLE patients making our findings relevant to the wider SLE patient population. In the multiple linear regression models we corrected for age, disease duration, and where current cognitive function was the outcome, NART, to adjust for an inferred prior (peak) IQ, but had limited power to adjust for other variables.
The study also had weaknesses. Although being larger than all prior studies and adding 25% more data to the available literature, the sample size limited statistical testing and, although we report all results for transparency, we urge caution in the interpretation and confirmation in larger studies. We did not assess reaction times, information processing speeds or motor skills and so are unable to comment on these aspects of neurological function. Although subjects were asked to consider their fatigue over the prior week, they were seen at different times of the day and this could have impacted the self-reported fatigue scores via diurnal variation.
The main observation from the literature review and meta-analysis (increased MD and decreased FA in SLE versus controls) was in agreement with findings in this study. Studies of other inflammatory autoimmune diseases show a similar pattern of findings, for example, in Sjögren Syndrome (n=19; method = TBSS) there was increased MD and decreased FA in several tracts compared to controls.38
Conclusion
Patients with SLE have more microstructural brain white matter damage for age than the general population, but this does not explain increased fatigue or lower cognition in SLE. Worse current cognitive function in SLE is related to lower prior cognitive ability although inflammation also plays a detrimental role and the association with raised IL-6 should be explored in larger datasets.
Supplementary Material
Acknowledgments
We acknowledge support from the Scottish Lupus Exchange registry. We thank Dr Francesca Chappell for expert statistical advice, Gayle Barclay for helping to develop the scan protocol and Gayle Barclay, Charlotte Jardine, Iona Hamilton and Elaine Sandeman (all Brain Research Imaging Centre, University of Edinburgh, UK) for administering some of the cognitive tests. We acknowledge Gillian Rice (University of Manchester) and Yanick Crow (Institut Imagine, Paris, France) for the interferon analysis.
Funding:
This study was funded by Lupus UK. Stewart Wiseman is supported by a Principal’s Career Development PhD Scholarship from the University of Edinburgh.
Footnotes
Conflicts of Interest/Disclosures:
None
References
- 1.Holmqvist M, Simard JF, Asplund K, Arkema EV. Stroke in systemic lupus erythematosus: a meta-analysis of population-based cohort studies. RMD Open. 2015;1:e000168. doi: 10.1136/rmdopen-2015-000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiseman SJ, Ralston SH, Wardlaw JM. Cerebrovascular disease in rheumatic diseases: A systematic review and meta-analysis. Stroke. 2016;47:0–0. doi: 10.1161/STROKEAHA.115.012052. [DOI] [PubMed] [Google Scholar]
- 3.Carlomagno S, Migliaresi S, Ambrosone L, Sannino M, Sanges G, Di Lorio G. Cognitive impairment in systemic lupus erythematosus: a follow-up study. J Neurol. 2000;247:273–279. doi: 10.1007/s004150050583. [DOI] [PubMed] [Google Scholar]
- 4.Hewlett S, Nicklin J, Treharne G. Fatigue in musculoskeletal conditions. J Rheumatol Suppl. 2003;67(Series 6):42–44. [PubMed] [Google Scholar]
- 5.Harboe E, Greve OJ, Beyer M, et al. Fatigue is associated with cerebral white matter hyperintensities in patients with systemic lupus erythematosus. J Neurol Neurosurg Psychiatry. 2008;79:199–201. doi: 10.1136/jnnp.2007.120626. [DOI] [PubMed] [Google Scholar]
- 6.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12:483–497. doi: 10.1016/S1474-4422(13)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penke L, Maniega SM, Bastin ME, et al. Brain white matter tract integrity as a neural foundation for general intelligence. Mol Psychiatry. 2012;17:1026–1030. doi: 10.1038/mp.2012.66. [DOI] [PubMed] [Google Scholar]
- 8.Zimny A, Szewczyk P, Bladowska J, et al. In vivo evaluation of brain damage in the course of systemic lupus erythematosus using magnetic resonance spectroscopy, perfusion-weighted and diffusion-tensor imaging. Lupus. 2014;23:10–19. doi: 10.1177/0961203313511556. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt-Wilcke T, Cagnoli P, Wang P, et al. Diminished white matter integrity in patients with systemic lupus erythematosus. NeuroImage Clin. 2014;5:291–297. doi: 10.1016/j.nicl.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zivadinov R, Shucard JL, Hussein S, et al. Multimodal imaging in systemic lupus erythematosus patients with diffuse neuropsychiatric involvement. Lupus. 2013;22:675–683. doi: 10.1177/0961203313486193. [DOI] [PubMed] [Google Scholar]
- 11.Jung RE, Caprihan A, Chavez RS, et al. Diffusion tensor imaging in neuropsychiatric systemic lupus erythematosus. BMC Neurol. 2010;10:65. doi: 10.1186/1471-2377-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emmer BJ, Veer IM, Steup-Beekman GM, Huizinga TWJ, van der Grond J, van Buchem MA. Tract-based spatial statistics on diffusion tensor imaging in systemic lupus erythematosus reveals localized involvement of white matter tracts. Arthritis Rheum. 2010;62:3716–3721. doi: 10.1002/art.27717. [DOI] [PubMed] [Google Scholar]
- 13.Welsh RC, Rahbar H, Foerster B, Thurnher M, Sundgren PC. Brain diffusivity in patients with neuropsychiatric systemic lupus erythematosus with new acute neurological symptoms. J Magn Reson Imaging. 2007;26:541–551. doi: 10.1002/jmri.21036. [DOI] [PubMed] [Google Scholar]
- 14.Hughes M, Sundgren PC, Fan X, et al. Diffusion tensor imaging in patients with acute onset of neuropsychiatric systemic lupus erythematosus: a prospective study of apparent diffusion coefficient, fractional anisotropy values, and eigenvalues in different regions of the brain. Acta Radiol. 2007;48:213–222. doi: 10.1080/02841850601105825. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Harrison M, Heier LA, et al. Diffusion changes in patients with systemic lupus erythematosus. Magn Reson Imaging. 2007;25:399–405. doi: 10.1016/j.mri.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 16.Bosma GPT, Huizinga TWJ, Mooijaart SP, van Buchem MA. Abnormal brain diffusivity in patients with neuropsychiatric systemic lupus erythematosus. AJNR Am J Neuroradiol. 2003;24:850–854. [PMC free article] [PubMed] [Google Scholar]
- 17.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 18.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 19.Zigmond A, Snaith R. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 20.Narseddine Z, Phillips N, Bedirian V, et al. The Montreal Cognitive Assessment, MoCAX: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh S, Schubert S, Hoon C, Mioshi E, Hodges JR. Validation of the Addenbrooke’s Cognitive Examination III in frontotemporal dementia and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2013;36:242–250. doi: 10.1159/000351671. [DOI] [PubMed] [Google Scholar]
- 22.Folstein M, Folstein S, McHugh P. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiat Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Nelson H, Willison J. National Adult Reading Test (NART):Test Manual. NFER_Nelson Publ; 1982. [Google Scholar]
- 24.McGurn B, Starr JM, Topfer JA, et al. Pronunciation of irregular words is preserved in dementia, validating premorbid IQ estimation. Neurology. 2004;62:1184–1187. doi: 10.1212/01.wnl.0000103169.80910.8b. [DOI] [PubMed] [Google Scholar]
- 25.Gladman D, Ibanez D, Urowitz M. Systemc lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29:288–291. [PubMed] [Google Scholar]
- 26.Isenberg DA, Rahman A, Allen E, et al. BILAG 2004. Development and initial validation of an updated version of the British Isles Lupus Assessment Group’s disease activity index for patients with systemic lupus erythematosus. Rheumatology. 2005;44:902–906. doi: 10.1093/rheumatology/keh624. [DOI] [PubMed] [Google Scholar]
- 27.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the systemic lupus international collaborating clinics/America College of Rheumatology Damage Index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–369. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 28.Gladman D, Goldsmith C, Urowitz M, et al. The Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index for Systemic Lupus Erythematosus International Comparison. J Rheumatol. 2000;27:373–376. [PubMed] [Google Scholar]
- 29.Jensen A. The g Factor: The science of mental ability. London: Praeger; 1998. [Google Scholar]
- 30.Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316:1236. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Au: 2013. [Google Scholar]
- 32.Chaiamnuay S, Bertoli A, Fernandez M, et al. The impact of increased body mass index on systemic lupus erythematosus: Data from LUMINA, a multiethnic cohort. J Clin Rheumatol. 2007;13:128–133. doi: 10.1097/RHU.0b013e3180645865. [DOI] [PubMed] [Google Scholar]
- 33.Kellner ES, Lee PY, Li Y, et al. Endogenous type-I interferon activity is not associated with depression or fatigue in systemic lupus erythematosus. J Neuroimmunol. 2010;223:13–19. doi: 10.1016/j.jneuroim.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Omdal R, Mellgren S, Koldingsness W, Jacobsen EA, Husby G. Fatigue in patients with systemic lupus erythematosus: lack of associations to serum cytokines, antiphospholipid antibodies, or other disease characteristics. J Rheumatol. 2002;29:482–486. [PubMed] [Google Scholar]
- 35.Bosma GPT, Middelkoop HAM, Rood MJ, Bollen ELEM, Huizinga TWJ, van Buchem MA. Association of global brain damage and clinical functioning in neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 2002;46:2665–2672. doi: 10.1002/art.10574. [DOI] [PubMed] [Google Scholar]
- 36.Mooijaart SP, Sattar N, Trompet S, et al. Circulating interleukin-6 concentration and cognitive decline in old age: the PROSPER study. J Intern Med. 2013;274:77–85. doi: 10.1111/joim.12052. [DOI] [PubMed] [Google Scholar]
- 37.Nishimura K, Omori M, Katsumata Y, et al. Neurocognitive impairment in corticosteroid-naive patients with active systemic lupus erythematosus: a prospective study. J Rhematol. 2015;42:441–448. doi: 10.3899/jrheum.140659. [DOI] [PubMed] [Google Scholar]
- 38.Tzarouchi LC, Zikou AK, Tsifetaki N, et al. White matter water diffusion changes in Primary Sjogren Syndrome. AJNR Am J Neuroradiol. 2014;35:680–685. doi: 10.3174/ajnr.A3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.