Abstract
Myelofibrosis (MF) is a bone marrow disorder characterized by clonal myeloproliferation, aberrant cytokine production, extramedullary hematopoiesis, and bone marrow fibrosis. Although somatic mutations in JAK2, MPL, and CALR have been identified in the pathogenesis of these diseases, inhibitors of the Jak2 pathway have not demonstrated efficacy in ameliorating MF in patients. TGF-β family members are profibrotic cytokines and we observed significant TGF-β1 isoform overexpression in a large cohort of primary MF patient samples. Significant overexpression of TGF-β1 was also observed in murine clonal MPLW515L megakaryocytic cells. TGF-β1 stimulated the deposition of excessive collagen by mesenchymal stromal cells (MSCs) by activating the TGF-β receptor I kinase (ALK5)/Smad3 pathway. MSCs derived from MPLW515L mice demonstrated sustained overproduction of both collagen I and collagen III, effects that were abrogated by ALK5 inhibition in vitro and in vivo. Importantly, use of galunisertib, a clinically active ALK5 inhibitor, significantly improved MF in both MPLW515L and JAK2V617F mouse models. These data demonstrate the role of malignant hematopoietic stem cell (HSC)/TGF-β/MSC axis in the pathogenesis of MF, and provide a preclinical rationale for ALK5 blockade as a therapeutic strategy in MF.
TGFβ receptor I kinase (ALK5) inhibition improves myelofibrosis in animal models and reduces deposition of excessive collagen by mesenchymal stromal cells.
Introduction
Philadelphia-negative myeloproliferative neoplasms (MPNs) comprise multipotent stem cell disorders including polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF) (1). These disorders are characterized by clonal expansion of myeloid lineage cells due to constitutive activation of the JAK/STAT pathway driven by several somatic mutations in JAK2, MPL, and CALR genes (2–4). Myelofibrosis (MF), either presenting as PMF or evolving from PV or ET secondarily, is exemplified by remodeling of bone marrow stroma towards excessive deposition of extracellular matrix (ECM), neoangiogenesis, and osteosclerosis. These changes collectively result in substitution of normal bone marrow niche proteins and factors with dense fibrillar matrix (5, 6). MF patients suffer from a series of symptoms such as splenomegaly, hepatomegaly, severe anemia, bleeding, and thrombosis caused by bone marrow fibrosis and extramedullary hematopoiesis, which decrease both quality of life and survival. Currently there are limited treatment options for MF, although allogeneic stem cell transplantation provides remissions in younger patients (7, 8). Curative therapies may be lacking, owing, in part, to the poorly understood bone marrow niche–related pathogenesis of MF.
It has been hypothesized that the development of MF is attributable to aberrant interactions between neoplastic hematopoietic clones and bone marrow niche–comprising mesenchymal stromal cells (MSCs) and their differentiated counterparts, adipocytes, chondrocytes, and osteoblasts. Neoplastic clones might modify the bone marrow niche by releasing fibrotic and angiogenic cytokines such as platelet factor 4 (PF4), TGF-β1, PDGF, and VEGF (6, 9–13). However, it is still not fully understood what the pathologic changes are in bone marrow stromal cells and how they participate in MF pathogenesis and survival. In our previous studies, MSC cultures have been informative in understanding the mechanisms leading to excessive collagen production in patients’ bone marrow (14), which may be useful to evaluate therapeutics that target these pathways specifically.
Among the abnormally expressed cytokines in MF, TGF-β1 has received attention due to its critical role in inducing fibrosis not only in bone marrow (15–18), but other organs (19–21). TGF-β1 may be produced by abnormal megakaryocytes or monocytes, and this in turn may remodel the MSCs that promote the changes illustrated by MF (22–25). In this study, galunisertib (LY2157299) (26), a TGF-β receptor I kinase (ALK5) inhibitor, was used to show that TGF-β1 indeed alters the bone marrow microenvironment through modulation of MSCs. Since galunisertib is an ALK5 inhibitor, it is possible that TGF-β1 or other TGF-β–family proteins induce the fibrotic phenotype in MF mouse models. Collectively, blockade of TGF-β signaling by galunisertib reverses the fibrotic MSC phenotype and restores the microenvironment in animal models of MF.
Results
TGF-β1 is overexpressed in human PMF samples and MPLW515L mice.
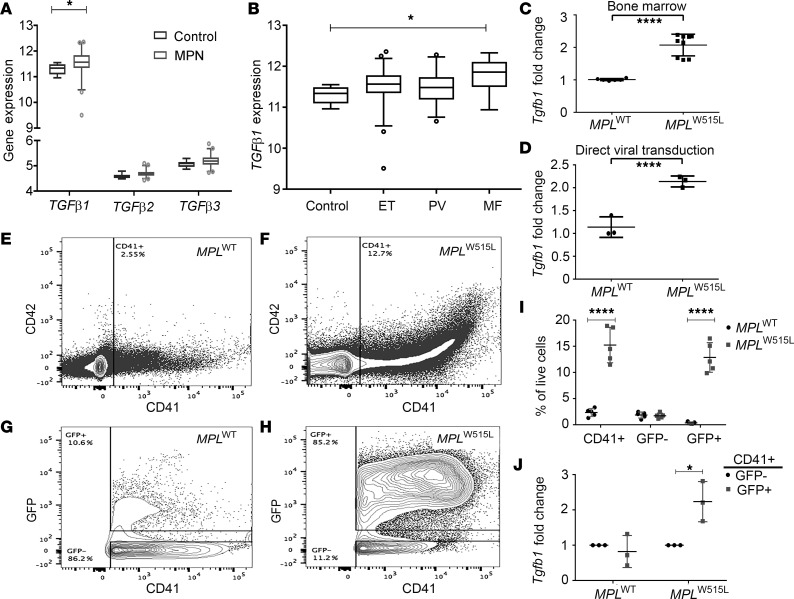
Expression patterns of various TGF ligands in human MPN neutrophil samples were first examined in a large cohort of samples representing PV, ET, and MF (27). TGFβ1 mRNA was significantly overexpressed compared with TGFβ2 and TGFβ3 mRNA when compared with healthy controls (P = 0.03) (Figure 1A). A subgroup analysis in the MPN cohort demonstrated that TGFβ1 overexpression relative to control was highest in MF samples (n = 18, P = 0.002), followed by samples from patients with PV (n = 28) and ET (n = 47) (Figure 1B). Expression of the Tgfb1 isoform was also markedly increased in bone marrow mononuclear cells from mice on day 17 after transplantation with MPLW515L bone marrow compared with those from MPLWT recipients (P < 0.0001) (Figure 1C). Furthermore, mouse bone marrow hematopoietic cells transduced in vitro with MPLW515L retrovirus displayed significantly higher Tgfb1 mRNA expression compared with those expressing MPLWT virus (P = 0.0024) (Figure 1D).
Figure 1. TGF-β1 is overexpressed in human primary myelofibrosis samples and MPLW515L mice.
(A and B) Neutrophils derived from myeloproliferative neoplasm (MPN) patients were evaluated for expression of TGFβ family members by gene expression analysis (using Gapdh as reference gene). *P < 0.05 by unpaired Student’s t test. (A) Analysis of TGFβ1, TGFβ2, and TGFβ3 mRNA expression in an MPN cohort compared with healthy donors (Control). *P < 0.05 as compared with control group by unpaired Student’s t test. (B) TGFβ1 mRNA expression was determined in MPN patients stratified by disease subtype including essential thrombocytosis (ET, n = 47), polycythemia vera (PV, n = 28), and myelofibrosis (MF, n = 18). Data represent values relative to healthy control cells (Control). (C) qRT-PCR was performed for Tgfb1 mRNA using bone marrow mononuclear cells from MPLWT and MPLW515L mice (n = 6). ****P < 0.0001 by unpaired 2-sided Student’s t test with Welch’s correction. (D) Normal mouse bone marrow cells were directly transduced with retroviruses overexpressing either MPLWT or MPLW515L, following which cells were collected and Tgfb1 mRNA was measured by qRT-PCR. ****P < 0.0001 by unpaired 2-sided Student’s t test. (E–H) Representative contour flow cytometry plots of cells stained with antibodies against CD41 (clone HIP8) and CD42 (clone 1C2). GFP was simultaneously determined by flow cytometry and represents expression of GFP+ retroviral constructs (y axis, G and H). The total CD41+ cells (E and F) and GFP+CD41+ cells (G and H) in MPLW515L spleens compared to MPLWT controls. (I) Graph represents percentage of each population (CD41+, GFP–, and GFP+) among live cells in the sample. Graphs (I and J) represent cells from spleens of 5 individual mice with the mean of the population indicated as a line (n = 5 MPLWT and n = 5 MPLW515L). Statistical significance determined using the Holm-Sidak method, with α = 5.000%. ****P < 0.0001. (J) Fold change in normalized Tgfβ1 mRNA expression of CD41+GFP+ cells vs. Tgfβ1 expression in CD41+GFP– population from the same mouse (n = 3 MPLWT and n = 3 MPLW515L). *P < 0.05 by unpaired 2-sided Student’s t test. All error bars indicate the mean ± SD.
Since megakaryocytes are known to be major source of TGF-β secretion (24), we analyzed whether numbers of megakaryocytes were increased in the mice transplanted with MPLW515L-transduced cells that coexpressed GFP. Megakaryocytes were determined by anti-CD41 antibody staining and platelets were stained with anti-CD42 (glycoprotein Ib, GP1b). We observed a significantly higher number of CD41+ megakaryocytes in the MPLW515L mice when compared with controls (Figure 1, E and F). The CD41+ cells expressed GFP, indicating derivation from the mutant MPLW515L stem and progenitor population (Figure 1, G–I). Sorting and expression analysis of these megakaryocytes demonstrated that GFP+CD41+ cells that arose from the mutant clone displayed a significantly higher expression of Tgfb1 mRNA when compared with WT GFP– megakaryocytes (Figure 1J). These data confirm that TGFβ1 is increased in human MF as well as in a mouse model based on expression of the mutant MPLW515L oncogene. The increased expression in Tgfb1 in the mouse model is collectively due to higher numbers of megakaryocytes as well as greater secretion of Tgfb1 by mutant megakaryocytes. This then prompted us to assess the therapeutic effect of TGF-β blockade in mouse models of MF.
Galunisertib antagonizes TGF-β1–stimulated collagen production by normal MSCs.
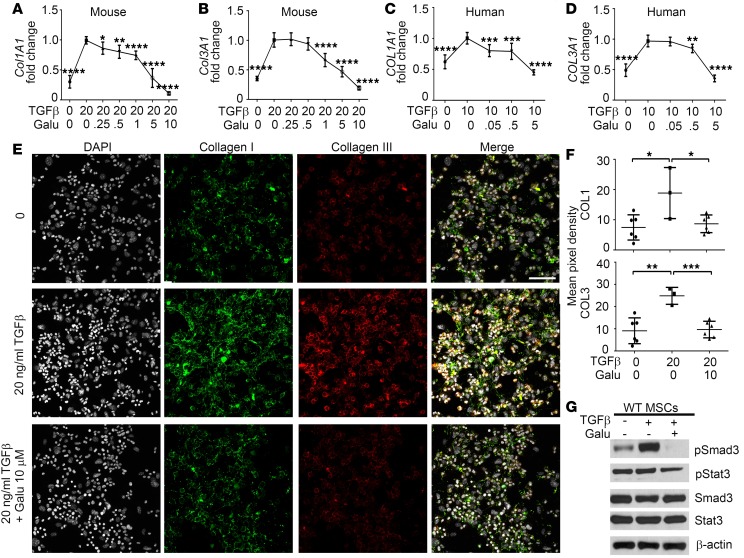
Next, studies were performed on MSCs that were cultured from bone marrow of mice transplanted with MPLWT- and MPLW515L-transduced grafts. MSCs derived from MPLWT mice and normal human bone marrow were cultured with recombinant murine or human TGF-β1 together with variable doses of the clinical ALK5 inhibitor, galunisertib (28–30), for 72 hours and collagen (Col1A1 and Col3A1) mRNA expression was determined. Collagen I and III are clinically relevant since they are recognized by trichrome and reticulin staining, respectively. Our results show that TGF-β1 led to induction of both Col1A1 and Col3A1 production in MSCs that was suppressed by the ALK5 inhibitor galunisertib in a dose-dependent manner (Figure 2, A and B). A similar result was seen using MSCs from normal human bone marrow (Figure 2, C and D). To confirm ECM deposition from cultured MSCs, immunofluorescence staining was also performed using multispectral fluorescence. Specificity of this staining with collagenase pretreatment was shown previously (14). We determined that excessive collagen deposition after TGF-β1 treatment was reduced by ALK5 inhibition (Figure 2, E and F). TGF-β1 also led to activation of p-Smad3 in MSCs that was abrogated by galunisertib (Figure 2G). Since STAT3 has also been indicated in tissue fibrosis and potentially interacts with the TGF-β pathway (31–33), STAT3 was also tested. TGF-β1 failed to induce p-STAT3, but galunisertib slightly decreased this activation signal which could result from the modulation of a signaling network. These results indicate that galunisertib selectively suppresses TGF-β signaling that is associated with the synthesis and deposition of collagen ECM in vitro in MSCs.
Figure 2. Galunisertib antagonizes the stimulatory effect of TGF-β1 on collagen production by normal murine and human MSCs in vitro.
(A and B) Mesenchymal stromal cells (MSCs) derived from MPLWT mice (n = 6) were cultured in the presence of TGF-β1 and different concentrations of galunisertib (Galu) in vitro for 72 hours, following which collagen I (Col1A1) and collagen III (Col3A1) mRNA levels were assessed by qRT-PCR with TATA sequence–binding protein (TBP), as reference gene (70). *P < 0.05, **P < 0.01, ****P < 0.0001 by ANOVA, multiple comparisons using 20 ng/ml TGF-β group as control. (C and D) Human COL1A1 and COL3A1 mRNA levels were measured by qRT-PCR in MSCs that were derived from normal human bone marrow (n = 3) and treated with human TGF-β (hTGF-β) and galunisertib for 72 hours. **P < 0.01, ***P < 0.001, ****P < 0.0001 by ANOVA, multiple comparisons using 10 ng/ml hTGF-β group as control. Results are representative of 3 independent experiments. (E) Representative immunofluorescence images showing collagen I (red), collagen III (green), and DAPI (white) in MSCs derived from MPLWT mice with different treatments in vitro. A merged image is shown on the right. Scale bar: 75 μm. (F) Mean pixel intensity was acquired from the immunofluorescence images (n = 3–6) to quantify collagen I and collagen III deposition. *P < 0.05, **P < 0.01, ***P < 0.001 by ANOVA, followed by Dunnett’s multiple comparison test. All dot plots represent randomly taken images with means indicated. (G) Western blot showing p-Smad3 and p-STAT3 in MSCs derived from MPLWT mice under indicated treatments, which represents 1 of 3 independent experiments conducted. See complete unedited blots in the supplemental material.
Elevated collagen production by MSCs derived from MPLW515L mice is decreased by galunisertib in vitro.
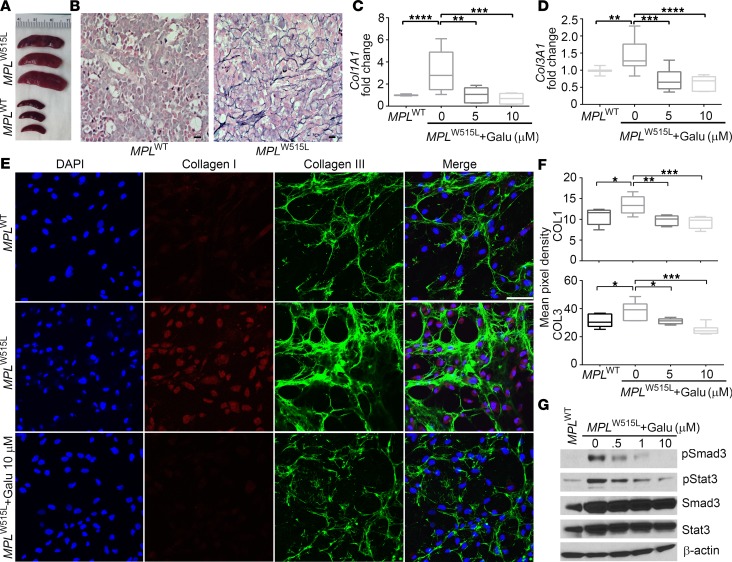
MPLW515L mice developed enlarged spleens (Figure 3A) and severe MF (Figure 3B) within 17 days after transplantation with MPLW515L-transduced bone marrow. Increased collagen production was detected in MSCs derived from MPLW515L mice compared with those from MPLWT mice, as determined by quantitative reverse transcription PCR (qRT-PCR) (Figure 3, C and D) and immunofluorescence (Figure 3E). Since TGF-β blockade suppresses collagen production, MSCs from MPLW515L-transplanted mice were treated with galunisertib in vitro. Galunisertib significantly decreased Col1A1 and Col3A1 production by these MSCs to levels that approached or were reduced compared with mice transplanted with MPLWT-expressing virus (Figure 3, C and D). Collagen I and collagen III ECM deposition was also markedly diminished by galunisertib treatment, as shown by immunofluorescence staining (Figure 3, E and F), which is consistent with a role for TGF-β in MF and suggests that galunisertib is a possible therapy for MF. Furthermore, p-Smad3 and p-STAT3 were suppressed by galunisertib in a dose-dependent manner in MPLW515L bone marrow–derived MSCs, suggesting that this pathway is susceptible to inhibition with this drug (Figure 3G). These findings indicate that TGF-β blockade by the ALK5 inhibitor galunisertib may suppress the fibrotic phenotype of MPLW515L-derived MSCs.
Figure 3. Galunisertib suppresses collagen production by MSCs derived from MPLW515L mice in vitro.
(A) Photographs of excised spleens from MPLWT (n = 3) and MPLW515L (n = 3) mice to demonstrate the exemplary increase in size of MPLW515L mice consistent with the presence of an myeloproliferative neoplasm–like (MPN-like) disease. (B) Reticulin staining was performed with bones isolated from MPLWT and MPLW515L mice. Scale bars: 10 μm. (C and D) Col1A1 and Col3A1 mRNA levels were assessed by qRT-PCR in mesenchymal stromal cells (MSCs) derived from MPLWT and MPLW515L mice. Results are representative of 3 independent experiments. **P < 0.01, ***P < 0.001, ****P < 0.0001 by ANOVA, followed by Dunnett’s multiple comparison test. (E) Representative immunofluorescence images of collagen I (red), collagen III (green), and DAPI (blue) in MSCs derived from MPLW515L mice and treated with galunisertib (Galu) in vitro. A merged image is shown on the right. Scale bar: 75 μm. (F) Mean pixel fluorescent intensity was acquired for the images in D (n = 5) to compare collagen deposition. *P < 0.05, **P < 0.01, ***P < 0.001 by ANOVA, followed by Dunnett’s multiple comparison test. In C, D, F, and G, graphs represent the mean and 95% confidence interval represented as a box-and-whisker blot of 5 individual mice or images per group. (G) Signaling pathways including p-Smad3 and p-STAT3 were detected by Western blot in MSCs. See complete unedited blots in the supplemental material.
In vivo treatment with galunisertib significantly improves myelofibrosis in MPLW515L mice.
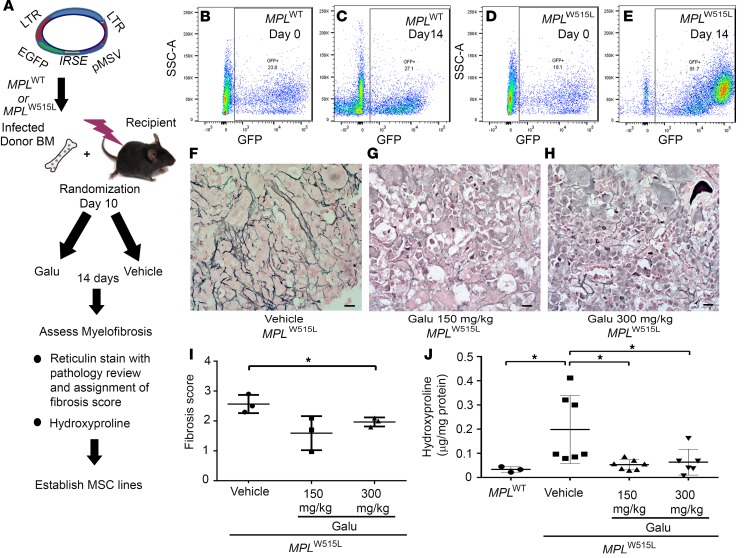
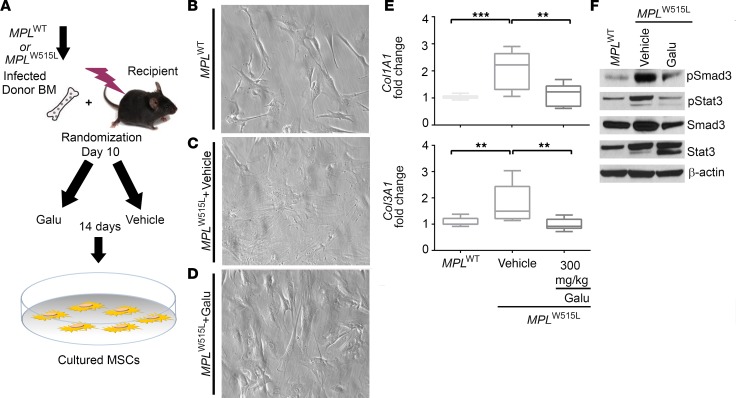
Next, the antifibrotic effect of galunisertib was determined after in vivo treatment, as outlined in Figure 4A. MPLW515L mice were randomized to receive vehicle or galunisertib at 150 mg/kg or 300 mg/kg daily from day 12 after bone marrow transplantation for 14 days by oral administration. Representative flow cytometry plots show GFP+ cells in mice transduced with MPLWT (Figure 4, B and C) and MPLW515L (Figure 4, D and E) on day 0 and on day 14 after therapy. After treatment on day 14, MF was assessed by reticulin stain (Figure 4, F–H) and assigned a pathology score of 1 to 3 based on the European consensus grading scale used to assign fibrosis severity to patients with MF (34) (Figure 4I). Additionally, hydroxyproline quantification was used to quantify these results, as shown in Figure 4J. Compared with severe fibrosis in the vehicle-treated group, reduced reticulin staining was seen in MPLW515L mice receiving the ALK5 inhibitor (Figure 4, G and H). Fibrosis grade showed a reduced trend in mice treated with 150 mg/kg, but was significantly reduced in mice treated with 300 mg/kg galunisertib (Figure 4C). Since hydroxyproline is a direct measure of the amount of collagen in tissues (35), hydroxyproline content in femurs was markedly reduced to near baseline levels in both the 150 mg/kg galunisertib and the 300 mg/kg galunisertib group compared with vehicle control–treated animals, providing quantitative assessment of decreased fibrosis with ALK5 inhibition (Figure 4J).
Figure 4. Galunisertib in vivo treatment significantly improves myelofibrosis of MPLW515L mice.
(A) Diagram showing the procedures of the experiments. Bones isolated from MPLW515L mice were randomized on day 10 to receive 1 of 2 doses of galunisertib (Galu) or vehicle control. Then, the mice were treated by oral gavage once daily for 14 days. (B–E) Representative flow cytometry staining of GFP+ retrovirally transduced bone marrow cells from MPLWT (B and C) and MPLW515L (D and E) on day 0 (representative mouse randomly selected and sacrificed) and on day 14 after treatment when all mice were sacrificed and further examined for pathological changes and for hydroxyproline quantification. Reticulin staining was then performed to evaluate fibrosis. Representative images of the reticulin stains are shown after treatment with vehicle (F), 150 mg/kg galunisertib (G), and 300 mg/kg galunisertib (H) to assess myelofibrosis in MPLW515L mice. Scale bars: 10 μm. (I) Fibrosis was scored according to European consensus on grading myelofibrosis (n = 3). *P < 0.05 by ANOVA, followed by Dunnett’s multiple comparison test. (J) Hydroxyproline amount was determined in the bones to quantify myelofibrosis in MPLWT-transduced mouse bone marrow, vehicle (n = 7), 150 mg/kg galunisertib (n = 7), and 300 mg/kg galunisertib (n = 6) groups. *P < 0.05 by ANOVA, followed by Dunnett’s multiple comparison test.
Galunisertib in vivo treatment suppresses the fibrotic phenotype of the MSCs from MPLW515L mice.
Based on the collective findings, the effect of galunisertib treatment in vivo was assessed on abnormal MSCs. Cultured MSCs were established from MPLW515L mice after galunisertib or vehicle in vivo treatment with the 300 mg/kg dose (Figure 5A). The morphological appearance of profibrotic MSCs has previously been shown to adopt a fattened shape relative to normal MSCs (14). Compared with MPLWT-transduced mice (Figure 5B), MSCs from MPLW515L mice displayed an abnormal morphological shape (Figure 5C), which was reversed by galunisertib treatment (Figure 5D). MSCs from galunisertib-treated mice produced significantly less Col1A1 and Col3A1 mRNA compared with those from vehicle-treated mice measured by qRT-PCR (Figure 5E), and both p-Smad3 and p-STAT3 were downregulated in the MSCs from galunisertib-treated mice compared with vehicle-treated mice (Figure 5F). These results suggest that galunisertib suppresses the fibrotic phenotype associated with malignant hematopoiesis by blocking the TGF-β signaling pathway activated in MSCs. Subsequent decrease in collagen production is consistent with the mechanism for improved MF in galunisertib-treated MPLW515L transplanted mice.
Figure 5. Galunisertib in vivo treatment reverses the fibrotic phenotype of the MSCs in MPLW515L mice.
(A) Diagram showing the procedures of the experiments. After randomization on either day 10 or day 12, groups of mice were treated for 14 days with of vehicle or galunisertib (Galu) in vivo. Mesenchymal stromal cells (MSCs) were then isolated from MPLW515L mice and cultured for collagen production analysis. (B–D) Representative images of MSCs from MPLWT (B) and MPLW515L mice treated with vehicle (C) or 300 mg/kg galunisertib (D) are shown. Original magnification, ×250. (E) Col1A1 and Col3A1 mRNA production was measured by qRT-PCR relative to TATA sequence–binding protein (TBP) in the MSCs (n = 6). **P < 0.01, ***P < 0.001 by ANOVA, followed by Dunnett’s multiple comparison test. (F) Signaling pathways in the MSCs were detected by Western blot after galunisertib in vivo treatment. See complete unedited blots in the supplemental material.
In vivo treatment with galunisertib does not significantly change blood counts and spleen size in MPLW515L-transduced mice.
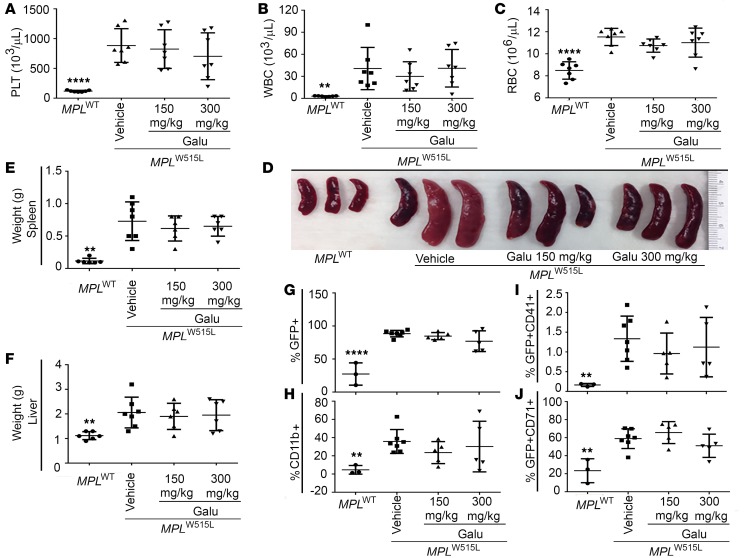
There have been concerns that TGF-β suppression may promote myeloid proliferation, thus increasing disease burden. Therefore, we evaluated tumor burden in MPLW515L mice after galunisertib treatment. Peripheral blood cell counts were similar in vehicle- and galunisertib-treated animals (Figure 6, A–C). Although not significant, mice receiving galunisertib showed some inconsistency, but slightly decreased spleen weights (Figure 6, D and E) compared with those receiving vehicle. No difference was observed in liver weight among vehicle- or galunisertib-treated mice (Figure 6F). Bone marrow populations were also assessed using flow cytometry. The percentages of GFP+ cells (Figure 6G), myeloid cells (CD11b+, Figure 6H), GFP+CD41+ megakaryocytes (Figure 6I), and GFP+CD71+ erythrocytes (Figure 6J) show no significant differences in response to galunisertib treatment. These data support the safety of TGF-β blockade in an aggressive MPN animal model driven by the MPLW515L mutation.
Figure 6. In vivo treatment with galunisertib does not increase tumor burden of MPLW515L mice.
(A–C) Peripheral blood cell counts of MPLW515L mice after 14 days treatment with either vehicle or 150 mg/kg or 300 mg/kg galunisertib (Galu) in vivo. Values of MPLWT mice served as normal control. n = 7. Results are representative of 3 independent experiments. **P < 0.01, ****P < 0.0001 by ANOVA, followed by Dunnett’s multiple comparison test using vehicle group as control. PLT, platelets. (D) Photographs of excised spleens from MPLWT and MPLW515L mice in A–C. (E) Spleens and (F) livers were isolated from the mice and weights were compared (n = 6). **P < 0.01 by ANOVA, followed by Dunnett’s multiple comparison test using vehicle group as control. Flow cytometry for bone marrow cell populations was conducted after gating on viable cells (using DAPI) including percentages of (G) GFP+, (H) CD11b+, (I) GFP+CD41+, and (J) GFP+CD71+. Percentages were assessed in 5 individual mice and repeated in 3 independent experiments (data points represent individual mice with means indicated). **P < 0.01, ****P < 0.0001 by ANOVA, followed by Dunnett’s multiple comparison test using vehicle group as control.
MF in JAK2V617F transgenic mice is significantly reduced by galunisertib in vivo treatment.
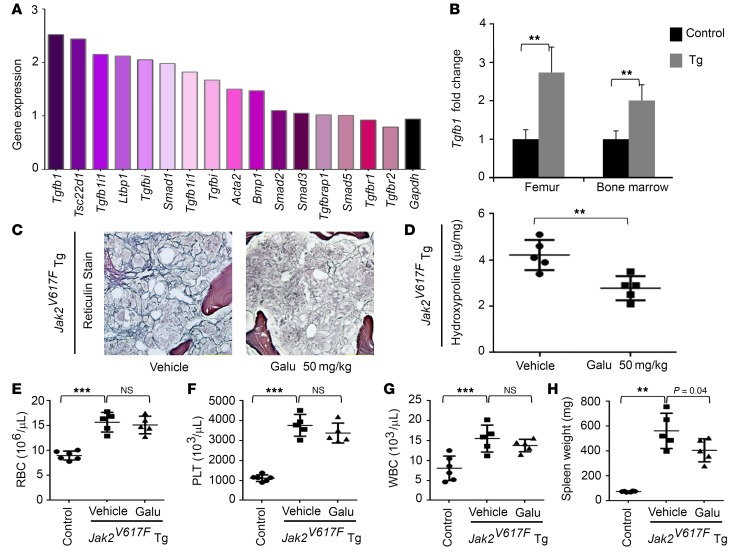
Further evaluation of the therapeutic effect of galunisertib was conducted using JAK2V617F transgenic mice (36). These mice display high levels of peripheral blood cell counts at early stages, and begin to develop MF at 20 weeks old and by 30 weeks present with severe splenomegaly and progressive MF (36). Transcriptome analysis of total RNAs from whole femurs revealed that Tgfb1 and several TGF-β–induced genes were overexpressed in 56-week-old JAK2V617 mice with severe MF (Figure 7, A–C). The reason for using femur RNAs for the analysis is that it was difficult to collect bone marrow cells from mice with severe MF. Nonetheless, qRT-PCR analysis confirmed the overexpression of Tgfb1 in both femur and bone marrow cells (Figure 7B). The data suggest that TGF-β1 may also contribute to MF in JAK2V617 transgenic mice. Since these mice show relatively chronic disease progression compared with MPLW515L mice, a lower dose of galunisertib was administered orally for an extended period of time. Galunisertib was orally administered to 30-week-old JAK2V617F transgenic mice at 50 mg/kg daily for 4 weeks. Bone marrow reticulin stains demonstrated improvement of MF in the galunisertib-treated group compared with vehicle-treated mice (Figure 7C). Hydroxyproline content was also quantified from the femurs of the mice, confirming that collagen production is decreased by drug treatment (P = 0.005) (Figure 7D). Note that the drug treatment did not cause changes in blood cell counts (Figure 7, E–G) but showed significantly reduced spleen size (P = 0.04) (Figure 7H).
Figure 7. TGF-β is elevated in JAK2V617F transgenic mice and galunisertib treatment markedly reduces myelofibrosis.
(A) cDNA microarray was performed with total RNAs from femurs of 56-week-old JAK2V617F transgenic and control mice. Changes in expression levels of 16 probe sets representing 13 distinct genes are shown that are involved in the TGF-β pathway together with the housekeeping gene Gapdh. (B) Real-time PCR confirmed elevation of Tgfb1 expression in femurs from 56-week-old mice and bone marrow cells from 30-week-old mice (4 mice used in each group). **P < 0.01 by unpaired 2-sided Student’s t test, control vs. transgenic (Tg). (C–H) JAK2V617F transgenic mice (30-week-old males, 5 in each group) were treated through oral gavage with vehicle control or galunisertib (Galu) at a daily dose of 50 mg/kg for 4 weeks. Data represent images of reticulin staining of femurs from vehicle- and drug-treated JAK2V617F transgenic mice (C; original magnification, ×200), quantification of fibrosis by determining hydroxyproline contents in femurs (D), and blood cell counts (E–G) together with spleen weights (H). Untreated nontransgenic mice of comparable ages are provided for comparison (n = 6). PLT, platelets. Error bars indicate the mean ± SD. **P < 0.01, ***P < 0.001 by ANOVA, followed by Dunnett’s multiple comparison test. n.s., not significant.
Discussion
TGF-β is a well-established fibrotic cytokine that plays a vital role in the pathophysiology of MF as well as other fibrotic diseases (15, 17, 19–21, 37–42). Elevated levels of the TGF-β1 isoform were detected in plasma and bone marrow serum in MF mouse models. Overexpression of thrombopoietin generates typical features of MF in WT mice, but this phenotype is prevented by the homozygous genetic deletion of TGF-β (16, 17, 43). However, targeting TGF-β for the treatment of MF has not been accomplished clinically owing to the lack of clinical inhibitors of this pathway. In addition, TGF-β1–mediated changes to the bone marrow niche remain to be fully elucidated. In this study, we first show that normal MSCs produce increased ECM collagen components after TGF-β1 treatment, mimicking the fibrotic features of MSCs derived from MPLW515L mice. The TGF-β1 isoform is also overexpressed in both MF patients and MPLW515L mouse samples, further reinforcing that TGF-β1 is responsible for reprogramming the MSCs present in the bone marrow niche, which induces them to adopt the fibrotic phenotype. Most importantly, we show the efficacy of a clinical inhibitor of ALK5 in mouse models of MF.
Our results further the understanding of the bone marrow niche in MF. MSCs are multipotent stromal cells that are critical components of bone marrow niche that regulate proliferation, differentiation, and homing of hematopoietic stem cells (HSCs). They possess self-renewal and immunomodulatory capacities and could differentiate into osteoblasts, adipocytes, and chondrocytes under specific conditions (44–46). Evidence indicates that neoplastic HSCs lead to profound modification of the bone marrow niche, which in turn affects the proliferation and trafficking of HSCs in MF (6, 9, 11, 47–49). Although the mechanism for these profound changes in the bone marrow niche are poorly defined, recent studies reported that MSCs in MPN patients have elevated fibronectin levels and exhibit both genetic and functional aberrations (50, 51). Martinaud et al. showed that MF-MSCs have increased osteogenic potential contributing to MF development (52). Our previous results show that cultured MSCs do not originate from HSCs and do not have MPLW515L mutations (53). In patient-derived xenografts, Verstovsek et al. showed that fibrosis may also be caused by fibrocytes which are morphologically distinct from MSCs and are derived directly from a CD14+ monocyte mutant precursor cell population (54). In our study based on the finding that MSCs from MF mice produced elevated collagen I and collagen III, which accounted for osteosclerosis and fibrillar ECM, respectively, we further confirm the role of TGF-β1 in MSC modulation and bone marrow niche remodeling in MF. Although malignant fibrocytes were not studied here, traditional MSCs from recipient mice are indirectly modified by HSCs and are notable contributors to fibrosis.
Based on these observations, the effect of TGF-β blockade with galunisertib was further tested in MF mouse models. It has been challenging to develop TGF-β inhibitors for clinical use as they can have cardiac-adverse effects, can affect bone development, and also alter inflammatory responses in the skin or gut of rats and dogs. It has also been shown that blocking TGF-β signaling can cause chronic inflammation in certain models (26). Significant improvement in the severity of fibrosis was observed in 2 different models of MPN driven by MPLW515L and JAK2V617F. The observation that ALK5 inhibition reverses the abnormal morphology and fibrotic characteristics of MSCs both in vitro and in vivo provides a mechanistic explanation for the rational development of TGF-β pathway inhibitors for the treatment of MF. Galunisertib is an ALK5 inhibitor that has been shown to be safe in human studies and is a potential therapeutic agent in MF owing to good specificity, tolerability, and oral administration (28). The ALK5/Smad3 pathway is considered to be critical in the profibrotic activities mediated by TGF-β1 (55, 56). Loss of Smad3 interrupts TGF-β1–mediated induction of collagen genes (57). Smad3 null mice displayed attenuated fibrosis in several experimental models such as bleomycin-induced pulmonary fibrosis (58), irradiation-induced dermal fibrosis (59), and cardiac fibrosis (60, 61). In our findings, we observed elevated Smad3 signaling in MPLW515L-derived MSCs and normal MSCs treated with TGF-β1, both of which were markedly suppressed by galunisertib. Collectively, galunisertib treatment effectively inhibits the intrinsically elevated ALK5/Smad3 pathway, leading to a diminution in collagen production by abnormal MSCs. Interestingly, we also observed decreased p-STAT3 by TGF-β blockade, which may result from the modulation of interacting pathways (62, 63) important for regulation of collagen production and inflammation related to IL-6 (64).
Detecting TGF-β protein levels in human blood samples has been problematic owing to patient heterogeneity, issues with sample collection, and the need for platelet depletion for adequate estimation of levels. Thus, we analyzed TGF-β1 levels at the expression level in purified neutrophils and found that PMF patients specifically had significant overexpression of this cytokine. Previous efforts to test TGF-β1 suppression in MF (65) were conducted by Shehata et al. They found that TGF-β1 enhances collagen production by fibroblasts, which is reduced by TGF-β1–neutralizing antibodies in vitro (43). Regarding in vivo treatment, there has been 1 clinical trial with anti–TGF-β antibody (GC1008) reported, which enrolled 3 subjects before it was terminated. However, transfusion independence was observed in 1 of the 3 subjects and 12 cycles of treatment did not induce disease progression or leukemic transformation, which was consistent with our findings in this study (66). Furthermore, there are data suggesting that stroma-derived cytokines might protect JAK2V617F-mutated cells against JAK2 inhibitor therapy (67). This highlights the importance of targeting the interaction between neoplastic HSCs and the bone marrow niche to rescue the abnormal niche and reverse stroma-mediated protection of the malignant clone.
Collectively, in this study using 2 distinct MF mouse models, the profibrotic role of TGF-β1 was confirmed and a direct role in modulating MSC-derived ECM was established. Therapeutic effects of TGF-β pathway blockade appears to reverse, not only prevent, fibrosis severity in the JAK2V617F MPN model, which is currently observed only after HSC transplantation. Therapies targeting the interplay between HSCs and MSCs in MF may improve the clinical outcome of MF patients.
Methods
Animal experiments.
The MPLW515L transplantation model of MPN was established using procedures as described previously with minor modifications (68). Bone marrow cells were harvested from C57BL/6 donor mice 7 days after 5-fluorouracil injection (150 mg/kg). Cells were then treated with red blood cell lysis buffer and cultured overnight in transplantation medium (RPMI + 10% FBS + 6 ng/ml IL-3, 10 ng/ml IL-6, and 10 ng/ml stem-cell factor) at 37°C and 5% CO2. The next day cells were transduced with recombinant retroviruses overexpressing either MPLW515L or MPLWT by spin infection at 1,140 g for 90 minutes at 30°C. The spin infection was repeated 24 hours later. Cells were then resuspended in PBS and injected into tail veins of lethally irradiated (2 × 450 cGy) C57BL/6 recipient mice at 0.8 × 106 to 1.0 × 106 cells/mouse. Viral constructs used included MSCV-human-MPLW515L-GFP and MSCV-human-MPLWT-GFP (68). Retroviral vectors were obtained from Ross Levine at the Memorial Sloan-Kettering Cancer Center. Recipient mice receiving MPLW515L-transduced bone marrow developed leukocytosis, thrombocytosis, and MF in 2 to 3 weeks. In the interest of brevity, mice transplanted with MPLW515L- or MPLWT-transduced bone marrow cells will be referred to as MPLW515L and MPLWT mice, respectively. For drug studies, MPLW515L mice were randomized to the vehicle or treatment group and peripheral blood cell counts among groups were comparable at the starting point. MPLWT mice served as nontumor controls. Vehicle (NaCMC/SLS/PVP/antifoam solution) or galunisertib was administered to MPLW515L mice by oral gavage daily from day 12 after bone marrow transplantation for 14 consecutive days. Stable homozygous JAK2V617F transgenic mice were generated using a vav gene promoter, as previously described (36). The transgenic mice exhibited a stable phenotype that closely resembled PV at an early stage and developed MF as they aged, exhibiting a relatively chronic disease status. Thus, a lower dose of galunisertib at 50 mg/kg was administered to 30-week-old mice orally once per day for 4 weeks after onset of fibrosis. Mice were sacrificed at the end of treatment and organs were harvested for analysis. Spleens were homogenized through a 100-μm mesh, and splenocytes were frozen in FBS with 10% DMSO until sorting.
MSC isolation and culture.
MSC cultures were established as previously described by adhesion to plastic (14, 69). Cells were cultured in αMEM (Life Technologies) supplemented with 10% FBS, 100 U/ml penicillin, and 100 U/ml streptomycin under hypoxic conditions (5% CO2, 93% nitrogen, and 2% O2) at 37°C. Bone marrow aspirates from healthy human volunteers were obtained commercially (Lonza) and MSCs were established using similar methods. Culture medium was replaced at least once per week and cells were trypsinized and passaged when 90% confluence was reached. Cells from the second or third passages were used for experiments since there was no evidence of viral infection or hematopoietic contamination at this time (data not shown).
Virus production.
For virus production, 293T cells (2.6 × 106) were seeded in a 10-cm dish the day before transfection and then transfected with MSCV-human-MPLW515L-GFP or MSCV-human-MPLWT-GFP plasmids together with envelope plasmids and 2.5 M CaCl2. Forty-eight hours after transfection, viral supernatant was collected and frozen until use.
Human MPN expression data.
Expression patterns of various TGF ligands in human MPN neutrophil samples were obtained from a publically available gene expression cohort of samples representing PV, ET, and PMF (27).
qRT-PCR analysis.
Total RNAs were extracted from cultured MSCs using an RNeasy Micro Kit (Qiagen), and converted into cDNA using iScript cDNA synthesis kit (Bio-Rad) according to the manufacturers’ indications. cDNA was added to Taqman PCR mix (Life Technologies) in a final volume of 25 μl containing forward and reverse primers. All primers were purchased from Life Technologies (Col1a1 Mm00801666_g1, Col3a1 Mm01254476_m1, Tgfβ1 Mm01178820_m1, TATA-binding protein (TBP) Mm00446971_m1). Amplification cycles (n = 40) were performed on a 7900HT Real-time PCR System (Applied Biosystems). Data were normalized to the reference gene TBP by a relative quantification using the ΔΔCt method.
Total RNA from sorted splenocytes was isolated using an RNeasy Micro Kit and reverse transcribed with the SuperScript III First-Strand Synthesis System (Invitrogen). Multiplex qPCR was performed with TaqMan hydrolysis probes for Tgfβ1 (Mm01178820_m1, FAM) and glyceraldehyde-3-phosphate dehydrogenase (Gapdh, Mm99999915_g1, VIC, primer limited) in TaqMan Multiplex Master Mix (all Life Technologies) on a CFX 96 touch thermocycler (Bio-Rad) in 45 amplification cycles. TGFβ1 levels were normalized to the reference gene Gapdh by a relative quantification using the ΔΔCt method.
cDNA microarray analysis.
Total RNAs were isolated from bone marrow cells or whole femurs using the RNeasy Plus Mini Kit (Qiagen). The Illumina mouse ref-8 microarray was employed for analysis of gene expression in whole femurs from 56-week-old control and JAK2V617F transgenic mice using the core facility at the Oklahoma Medical Research Foundation. The data were normalized by using MATLAB and analyzed by using BRB ArrayTools. For real-time PCR confirmation, single-strand cDNAs were synthesized with equal amounts of total RNAs using the QuantiTect reverse transcription kit (Qiagen). Real-time PCR was performed with iQ SYBR Green Supermix (Bio-Rad) and Tgfβ1 primers 5′-GCGCTTGCAGAGATTAAAATCAA and 5′-GTAACGCCAGGAATTGTTGCTATA. Gapdh served as control as previously described (36).
Immunofluorescence staining.
MSCs were cultured in Lab-Tek chamber slides (Nalgene Nunc) for 3 days to allow collagen production and formation of fibrillar structures. Polychromatic collagen stains were performed with antibodies against collagen types I (Abcam, ab6308) and III (Abcam, ab7778). Secondary stains were conducted using Alexa Fluor 647 F (ab′)2 donkey anti-mouse and Alexa Fluor 488 F (ab′)2 donkey anti-rabbit (Jackson ImmunoResearch Laboratories). Vectashield mounting medium containing DAPI (Vector Laboratories) was used to stain nuclei. To visualize the fibrillar structure, a Leica DMI6000 inverted microscope, TCS SP5 confocal scanner, and a 20×/0.7NA Plan Apochromat oil immersion objective (Leica Microsystems) were used. LAS AF lite version 2.6 was used to analyze fluorescence images and mean pixel intensity was calculated for each field.
Western blot.
MSCs were lysed in RIPA buffer (Sigma-Aldrich) supplemented with protease inhibitors (Roche) and phosphatase inhibitors (Sigma-Aldrich, P5726). Proteins were separated by SDS-PAGE and transferred onto a nitrocellulose membrane. Membranes were blotted with antibodies detecting p-Smad3 (Abcam, ab52903), Smad3 (Abcam, ab40854), p-STAT3 (Cell Signaling Technology, 9145) and STAT3 (Cell Signaling Technology, 9139).
Histology.
Femurs were fixed in 10% neutral-buffered formalin, decalcified in 5% nitric acid, and embedded in paraffin. Sections (2–4 μm) were mounted for Gomori’s silver impregnation (reticulin). MF was scored according to European consensus grading scale (34).
Hydroxyproline quantification.
Hydroxyproline assays were performed based on an established protocol (35). After fixation and decalcification, bone marrow tissues were dissected from the bones under a stereo microscope and hydrolyzed with 6 M HCl at 120°C for 3 hours. The hydrolysates were then dried in a speed vacuum concentrator and oxidized with chloramine T for 5 minutes at room temperature. Ehrlich’s reagent was then added and incubated for 90 minutes at 60°C. The absorbance was then measured at 560 nm using a spectrophotometer. Standard hydroxyproline was measured in the same way and hydroxyproline contents in the samples were calculated based on standard curves and normalized to total protein concentrations, which were determined by measuring OD280nm and OD260nm of the hydrolysates.
Flow cytometry.
Single-cell suspensions of bone marrow cells were prepared by resuspending the cells in PBS with 1% BSA (Sigma-Aldrich) and 2 mM EDTA (Life Technologies). Surface marker staining was performed using the following antibodies: APC-CD11b (clone M1/70) (BD Biosciences, BDB561690); BV605-CD41 (clone MWReg30) (BioLegend, 133921); and PE/Cy7-CD71 (clone RI7217) (BioLegend, 113812). Viability was determined using DAPI (Invitrogen). Acquisition was achieved on an LSRII cytometer (BD Biosciences). Aggregates were excluded using the height and width of forward scatter and side scatter parameters. Analysis was performed using FlowJo software version 10.0.8 (Tree Star).
Frozen splenocytes were thawed and dead cells were depleted using the Dead Cell Removal Kit with LS columns (Miltenyi Biotec). Negative fractions were stained in 1% BSA/2 mM EDTA buffer with Fixable Viability Dye eFluor 780 (eBioscience), PE-CD41 (BD Biosciences, clone HIP8), and APC-CD42 (Biolegend, clone 1C2) antibody conjugates and sorted on an Aria II Special Order flow cytometer (Beckton Dickinson). Data were analyzed and graphed using FlowJo software version 10.0.8.
Statistics.
Statistical analyses were conducted using GraphPad Prism software v6.04 and the R software package (https://www.r-project.org). Differences between groups were compared using the unpaired 2-tailed Student’s t test with Welch’s correction for unequal variance. One-way ANOVA, followed by Tukey’s honest significant difference (HSD) multiple comparison test, was performed on datasets when comparing multiple groups. ANOVA, followed by Dunnett’s multiple comparison test, was performed when comparing multiple groups with a single group in a dataset. All tests were evaluated at a statistical significance of 0.05, if not otherwise specified.
Study approval.
Animal experiments regarding MPLW515L mice were conducted in the H. Lee Moffitt Cancer Center and Research Institute in accordance with IACUC protocols (IS00001729) approved by University of South Florida IACUC. Animal experiments regarding JAK2V617F mice were conducted in Oklahoma University Health Sciences Center, and the experiments were performed in accordance with animal protocols (15-122-HW) approved by Oklahoma University IACUC.
Author contributions
L. Yue conducted the MSC studies and experiments with MPLW515L mice, analyzed data, and wrote the manuscript. Y. Han established the transplantation model. M. Bartenstein performed human sample analysis, megakaryocyte sorting, and TGF-β production and wrote the corresponding part of the manuscript. W. Zhao and W. T. Ho conducted experiments with JAK2V617F mice and wrote the corresponding part. C. Murdun performed virus production and drug treatment in MPLW515L mice. A. Mailloux assisted with MSC culture and IF staining. L. Zhang performed histological evaluation. X. Wang conducted statistical analyses. A. Budhathoki and K. Pradhan performed experiments and statistical analysis. F. Rapaport and RL Levine provided human sample data. U. Steidl, H. Wang, X. Ren and Z. Shao assisted with the studies. Z. Zhao, A. Verma, and P. Epling-Burnette together directed the project, analyzed data, provided conceptual input, and wrote the manuscript.
Supplementary Material
Acknowledgments
We would like to thank Gary W. Reuther for critical assistance in retrovirus production. We also thank the Microscopy Core and Flow Cytometry Core at the H. Lee Moffitt Cancer Center for assistance with experiments. This work has been supported in part by the Biostatistics Core Facility at the H. Lee Moffitt Cancer Center and Research Institute (P30-CA076292). The studies were supported by the MPN Foundation and Leukemia Lymphoma Society. Amit Verma was supported by the Gottesman Stem Institute.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:JCI Insight. 2017;2(6):e90932. https://doi.org/10.1172/jci.insight.90932.
Contributor Information
Lanzhu Yue, Email: yuelan1110@hotmail.com.
Matthias Bartenstein, Email: Matthias.bartenstein@phd.einstein.yu.edu.
Wanke Zhao, Email: wanke-zhao@ouhsc.edu.
Ying Han, Email: 13612190539@163.com.
Cem Murdun, Email: Cem.Murdun@moffitt.org.
Ling Zhang, Email: Ling.Zhang@moffitt.org.
Xuefeng Wang, Email: Xuefeng.Wang@moffitt.org.
Anjali Budhathoki, Email: abudhath@montefiore.org.
Kith Pradhan, Email: kith.pradhan@einstein.yu.edu.
Franck Rapaport, Email: rapaport@cbio.mskcc.org.
Huaquan Wang, Email: floing@126.com.
Zonghong Shao, Email: shaozonghong@sina.com.
Xiubao Ren, Email: renxiubao@tjmuch.com.
Ulrich Steidl, Email: ulrich.steidl@einstein.yu.edu.
Ross L. Levine, Email: leviner@mskcc.org.
Amit Verma, Email: amit.verma@einstein.yu.edu.
References
- 1.Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22(1):14–22. doi: 10.1038/sj.leu.2404955. [DOI] [PubMed] [Google Scholar]
- 2.Kralovics R, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 3.Tefferi A. Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia. 2010;24(6):1128–1138. doi: 10.1038/leu.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klampfl T, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369(25):2379–2390. doi: 10.1056/NEJMoa1311347. [DOI] [PubMed] [Google Scholar]
- 5.Tefferi A. Pathogenesis of myelofibrosis with myeloid metaplasia. J Clin Oncol. 2005;23(33):8520–8530. doi: 10.1200/JCO.2004.00.9316. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt-Graeff AH, Nitschke R, Zeiser R. The hematopoietic niche in myeloproliferative neoplasms. Mediators Inflamm. 2015;2015:347270. doi: 10.1155/2015/347270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tefferi A, et al. One thousand patients with primary myelofibrosis: the mayo clinic experience. Mayo Clin Proc. 2012;87(1):25–33. doi: 10.1016/j.mayocp.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerquozzi S, Farhadfar N, Tefferi A. Treatment of myelofibrosis: a moving target. Cancer J. 2016;22(1):51–61. doi: 10.1097/PPO.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 9.Lataillade JJ, et al. Does primary myelofibrosis involve a defective stem cell niche? From concept to evidence. Blood. 2008;112(8):3026–3035. doi: 10.1182/blood-2008-06-158386. [DOI] [PubMed] [Google Scholar]
- 10.Walkley CR, Shea JM, Sims NA, Purton LE, Orkin SH. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007;129(6):1081–1095. doi: 10.1016/j.cell.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schepers K, et al. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell. 2013;13(3):285–299. doi: 10.1016/j.stem.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreipe H, Büsche G, Bock O, Hussein K. Myelofibrosis: molecular and cell biological aspects. Fibrogenesis Tissue Repair. 2012;5(Suppl 1):S21. doi: 10.1186/1755-1536-5-S1-S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varricchio L, Mancini A, Migliaccio AR. Pathological interactions between hematopoietic stem cells and their niche revealed by mouse models of primary myelofibrosis. Expert Rev Hematol. 2009;2(3):315–334. doi: 10.1586/ehm.09.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mailloux AW, et al. Fibrosis and subsequent cytopenias are associated with basic fibroblast growth factor-deficient pluripotent mesenchymal stromal cells in large granular lymphocyte leukemia. J Immunol. 2013;191(7):3578–3593. doi: 10.4049/jimmunol.1203424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ponce CC, de Lourdes Lopes Ferrari Chauffaille M, Ihara SS, Silva MR. Increased angiogenesis in primary myelofibrosis: latent transforming growth factor-β as a possible angiogenic factor. Rev Bras Hematol Hemoter. 2014;36(5):322–328. doi: 10.1016/j.bjhh.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chagraoui H, Komura E, Tulliez M, Giraudier S, Vainchenker W, Wendling F. Prominent role of TGF-beta 1 in thrombopoietin-induced myelofibrosis in mice. Blood. 2002;100(10):3495–3503. doi: 10.1182/blood-2002-04-1133. [DOI] [PubMed] [Google Scholar]
- 17.Zingariello M, et al. Characterization of the TGF-β1 signaling abnormalities in the Gata1low mouse model of myelofibrosis. Blood. 2013;121(17):3345–3363. doi: 10.1182/blood-2012-06-439661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ceglia I, et al. Preclinical rationale for TGF-β inhibition as a therapeutic target for the treatment of myelofibrosis. Exp Hematol. 2016;44(12):1138–1155.e4. doi: 10.1016/j.exphem.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18(7):816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 20.Pohlers D, et al. TGF-beta and fibrosis in different organs - molecular pathway imprints. Biochim Biophys Acta. 2009;1792(8):746–756. doi: 10.1016/j.bbadis.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Meindl-Beinker NM, Matsuzaki K, Dooley S. TGF-β signaling in onset and progression of hepatocellular carcinoma. Dig Dis. 2012;30(5):514–523. doi: 10.1159/000341704. [DOI] [PubMed] [Google Scholar]
- 22.Vannucchi AM, et al. A pathobiologic pathway linking thrombopoietin, GATA-1, and TGF-beta1 in the development of myelofibrosis. Blood. 2005;105(9):3493–3501. doi: 10.1182/blood-2004-04-1320. [DOI] [PubMed] [Google Scholar]
- 23.Le Bousse-Kerdilès MC, Martyré MC, French INSERM research network on Idiopathic Myelofibrosis Involvement of the fibrogenic cytokines, TGF-beta and bFGF, in the pathogenesis of idiopathic myelofibrosis. Pathol Biol. 2001;49(2):153–157. doi: 10.1016/S0369-8114(00)00021-3. [DOI] [PubMed] [Google Scholar]
- 24.Ciurea SO, et al. Pivotal contributions of megakaryocytes to the biology of idiopathic myelofibrosis. Blood. 2007;110(3):986–993. doi: 10.1182/blood-2006-12-064626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitt A, Jouault H, Guichard J, Wendling F, Drouin A, Cramer EM. Pathologic interaction between megakaryocytes and polymorphonuclear leukocytes in myelofibrosis. Blood. 2000;96(4):1342–1347. [PubMed] [Google Scholar]
- 26.Herbertz S, et al. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des Devel Ther. 2015;9:4479–4499. doi: 10.2147/DDDT.S86621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rampal R, et al. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood. 2014;123(22):e123–e133. doi: 10.1182/blood-2014-02-554634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodon J, et al. First-in-human dose study of the novel transforming growth factor-β receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin Cancer Res. 2015;21(3):553–560. doi: 10.1158/1078-0432.CCR-14-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhola NE, et al. TGF-β inhibition enhances chemotherapy action against triple-negative breast cancer. J Clin Invest. 2013;123(3):1348–1358. doi: 10.1172/JCI65416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou L, et al. Reduced SMAD7 leads to overactivation of TGF-beta signaling in MDS that can be reversed by a specific inhibitor of TGF-beta receptor I kinase. Cancer Res. 2011;71(3):955–963. doi: 10.1158/0008-5472.CAN-10-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C, et al. Noncanonical STAT3 activation regulates excess TGF-β1 and collagen I expression in muscle of stricturing Crohn’s disease. J Immunol. 2015;194(7):3422–3431. doi: 10.4049/jimmunol.1401779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laklai H, et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat Med. 2016;22(5):497–505. doi: 10.1038/nm.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prêle CM, Yao E, O’Donoghue RJ, Mutsaers SE, Knight DA. STAT3: a central mediator of pulmonary fibrosis? Proc Am Thorac Soc. 2012;9(3):177–182. doi: 10.1513/pats.201201-007AW. [DOI] [PubMed] [Google Scholar]
- 34.Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005;90(8):1128–1132. [PubMed] [Google Scholar]
- 35.Zhao W, Ho WT, Zhao ZJ. Quantitative analyses of myelofibrosis by determining hydroxyproline. Stem Cell Investig. 2015;2:2. doi: 10.3978/j.issn.2306-9759.2015.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xing S, et al. Transgenic expression of JAK2V617F causes myeloproliferative disorders in mice. Blood. 2008;111(10):5109–5117. doi: 10.1182/blood-2007-05-091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wen QJ, et al. Targeting megakaryocytic-induced fibrosis in myeloproliferative neoplasms by AURKA inhibition. Nat Med. 2015;21(12):1473–1480. doi: 10.1038/nm.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agarwal A, Morrone K, Bartenstein M, Zhao ZJ, Verma A, Goel S. Bone marrow fibrosis in primary myelofibrosis: pathogenic mechanisms and the role of TGF-β. Stem Cell Investig. 2016;3:5. doi: 10.3978/j.issn.2306-9759.2016.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spangrude GJ, et al. P-selectin sustains extramedullary hematopoiesis in the Gata1 low model of myelofibrosis. Stem Cells. 2016;34(1):67–82. doi: 10.1002/stem.2229. [DOI] [PubMed] [Google Scholar]
- 40.Zingariello M, et al. A novel interaction between megakaryocytes and activated fibrocytes increases TGF-β bioavailability in the Gata1 (low) mouse model of myelofibrosis. Am J Blood Res. 2015;5(2):34–61. [PMC free article] [PubMed] [Google Scholar]
- 41.Hong SH, et al. Rescue of a primary myelofibrosis model by retinoid-antagonist therapy. Proc Natl Acad Sci USA. 2013;110(47):18820–18825. doi: 10.1073/pnas.1318974110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ponce CC, de Lourdes F Chauffaille M, Ihara SS, Silva MR. The relationship of the active and latent forms of TGF-β1 with marrow fibrosis in essential thrombocythemia and primary myelofibrosis. Med Oncol. 2012;29(4):2337–2344. doi: 10.1007/s12032-011-0144-1. [DOI] [PubMed] [Google Scholar]
- 43.Shehata M, Schwarzmeier JD, Hilgarth M, Hubmann R, Duechler M, Gisslinger H. TGF-beta1 induces bone marrow reticulin fibrosis in hairy cell leukemia. J Clin Invest. 2004;113(5):676–685. doi: 10.1172/JCI19540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013;31:285–316. doi: 10.1146/annurev-immunol-032712-095919. [DOI] [PubMed] [Google Scholar]
- 45.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 46.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10(6):709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 47.Méndez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.García-García A, de Castillejo CL, Méndez-Ferrer S. BMSCs and hematopoiesis. Immunol Lett. 2015;168(2):129–135. doi: 10.1016/j.imlet.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 49.Shen Y, Nilsson SK. Bone, microenvironment and hematopoiesis. Curr Opin Hematol. 2012;19(4):250–255. doi: 10.1097/MOH.0b013e328353c714. [DOI] [PubMed] [Google Scholar]
- 50.Schneider RK, et al. Activated fibronectin-secretory phenotype of mesenchymal stromal cells in pre-fibrotic myeloproliferative neoplasms. J Hematol Oncol. 2014;7:92. doi: 10.1186/s13045-014-0092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Avanzini MA, et al. Functional and genetic aberrations of in vitro-cultured marrow-derived mesenchymal stromal cells of patients with classical Philadelphia-negative myeloproliferative neoplasms. Leukemia. 2014;28(8):1742–1745. doi: 10.1038/leu.2014.97. [DOI] [PubMed] [Google Scholar]
- 52.Martinaud C, et al. Osteogenic potential of mesenchymal stromal cells contributes to primary myelofibrosis. Cancer Res. 2015;75(22):4753–4765. doi: 10.1158/0008-5472.CAN-14-3696. [DOI] [PubMed] [Google Scholar]
- 53.Han Y, et al. Mesenchymal cell reprogramming in experimental MPLW515L mouse model of myelofibrosis. PLoS One. 2017;12(1):e0166014. doi: 10.1371/journal.pone.0166014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verstovsek S, et al. Role of neoplastic monocyte-derived fibrocytes in primary myelofibrosis. J Exp Med. 2016;213(9):1723–1740. doi: 10.1084/jem.20160283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-β signaling in fibrosis. Growth Factors. 2011;29(5):196–202. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flanders KC. Smad3 as a mediator of the fibrotic response. Int J Exp Pathol. 2004;85(2):47–64. doi: 10.1111/j.0959-9673.2004.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schnabl B, Kweon YO, Frederick JP, Wang XF, Rippe RA, Brenner DA. The role of Smad3 in mediating mouse hepatic stellate cell activation. Hepatology. 2001;34(1):89–100. doi: 10.1053/jhep.2001.25349. [DOI] [PubMed] [Google Scholar]
- 58.Zhao J, et al. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol. 2002;282(3):L585–L593. doi: 10.1152/ajplung.00151.2001. [DOI] [PubMed] [Google Scholar]
- 59.Flanders KC, et al. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am J Pathol. 2002;160(3):1057–1068. doi: 10.1016/S0002-9440(10)64926-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dobaczewski M, et al. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107(3):418–428. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bujak M, et al. Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation. 2007;116(19):2127–2138. doi: 10.1161/CIRCULATIONAHA.107.704197. [DOI] [PubMed] [Google Scholar]
- 62.Saitoh M, et al. STAT3 integrates cooperative Ras and TGF-β signals that induce Snail expression. Oncogene. 2016;35(8):1049–1057. doi: 10.1038/onc.2015.161. [DOI] [PubMed] [Google Scholar]
- 63.Liu RY, et al. JAK/STAT3 signaling is required for TGF-β-induced epithelial-mesenchymal transition in lung cancer cells. Int J Oncol. 2014;44(5):1643–1651. doi: 10.3892/ijo.2014.2310. [DOI] [PubMed] [Google Scholar]
- 64.Liu M, et al. Immunomodulation by mesenchymal stem cells in treating human autoimmune disease-associated lung fibrosis. Stem Cell Res Ther. 2016;7(1):63. doi: 10.1186/s13287-016-0319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gastinne T, et al. Adenoviral-mediated TGF-beta1 inhibition in a mouse model of myelofibrosis inhibit bone marrow fibrosis development. Exp Hematol. 2007;35(1):64–74. doi: 10.1016/j.exphem.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 66.Mascarenhas J, et al. Anti-transforming growth factor-β therapy in patients with myelofibrosis. Leuk Lymphoma. 2014;55(2):450–452. doi: 10.3109/10428194.2013.805329. [DOI] [PubMed] [Google Scholar]
- 67.Manshouri T, et al. Bone marrow stroma-secreted cytokines protect JAK2 (V617F)-mutated cells from the effects of a JAK2 inhibitor. Cancer Res. 2011;71(11):3831–3840. doi: 10.1158/0008-5472.CAN-10-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pikman Y, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3(7):e270. doi: 10.1371/journal.pmed.0030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lennon DP, Caplan AI. Isolation of human marrow-derived mesenchymal stem cells. Exp Hematol. 2006;34(11):1604–1605. doi: 10.1016/j.exphem.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 70.Santos AR, Duarte CB. Validation of internal control genes for expression studies: effects of the neurotrophin BDNF on hippocampal neurons. J Neurosci Res. 2008;86(16):3684–3692. doi: 10.1002/jnr.21796. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.