Abstract
Acute kidney failure is the main cause of death among patients with severe trauma due to massive blood loss and hemorrhagic shock (HS). Renal cell injury is caused by tissue ischemia. Renal ischemia initiates a complex and interconnected chain of events resulting in cell injury and renal cell necrosis. Nitric oxide plays a crucial role in renal function and can be inhibited by aminoguanidine (AG). We studied whether AG can ameliorate pathological renal changes associated with HS syndrome in a rat model and explored the AG protection mechanism. Rats were intraperitoneally injected with heparin sodium and mean arterial blood pressure was monitored. Animals were divided into three groups: control (without hemorrhage), with or without intra-arterially injected AG; HS (blood continuously withdrawn or reinfused to maintain an MABP of 35–40 mmHg); and HS with AG. We found that AG decreased plasma concentrations of urea, creatinine, and nitrates; ameliorated histological changes of HS-induced rats; and decreased the expressions of inducible nitrogen oxide synthase (iNOS), proapoptotic protein (BAX), and vitamin D receptors (VDR). AG ameliorated kidney injury by inhibiting iNOS resulting in decreased BAX and VDR expressions. Therefore, a therapeutic strategy targeting AG may provide new insights into kidney injury during severe shock.
Keywords: hemorrhagic shock, kidney, aminoguanidine, histology, immunohistochemistry
I. Introduction
Hemorrhagic shock (HS) is the main cause of death among severe trauma patients due to massive blood loss leading to multiple organ failure, including kidney failure [22]. HS is characterized by two phases. The first phase is a compensatory phase, in which the body attempts to maintain a normal blood pressure by releasing endogenous vasoconstrictors such as norepinephrine and angiotensin II. The second phase is a decompensatory phase, which occurs after the development of hypo-reactivity to the vasoconstrictors, and is characterized by a progressive vasodilatation that ultimately results in death [4]. The kidney is the organ that suffers the most and the earliest in HS owing to the drop in oxygen saturation at a much earlier stage than in other organs such as the gut or heart [32]. Therefore, acute renal failure (ARF) is a frequent complication of trauma that is associated with high mortality from HS [25, 29]. Thus, the development of novel pharmacological intervention therapies to reduce renal injury mediated by HS are considered an important area of study in view of the fact that dialysis is currently the only effective therapy [25].
Tissue ischemia, insufficient evacuation of metabolic degradation products, and energy depletion of renal tissue are the main factors leading to renal cell injury from HS [40]. Moreover, renal ischemia initiates a complex and interconnected chain of events resulting in cell injury and renal cell necrosis [29]. Furthermore, reperfusion, which is crucial for ischemic renal tissue survival, can cause additional damage. Therefore, ischemia/reperfusion (I/R) injury affecting mainly proximal tubules contributes to the damage of renal cells and complicates the prognosis of ARF [29].
Nitric oxide (NO) plays a crucial role in renal function both in normal and pathophysiological conditions [13]. Three different isoforms of the enzyme nitric oxide synthase (NOS); neuronal NOS (nNOS; NOS I), inducible NOS (iNOS; NOS II), and endothelial NOS (eNOS; NOS III), can produce NO [13]. The nNOS and eNOS have been identified in the macula densa and renal vasculature, respectively [5]. In addition, iNOS has been reported to play a role during renal I/R insult [5]. Moreover, tumor necrosis factor-alpha released due to I/R renal injury enhances production of iNOS and subsequently ARF during HS [16].
I/R causes an imbalance between free radical generation (hydrogen peroxide, superoxide, and hydroxyl radicals) and cell defense mechanisms leading to the accumulation of reactive oxygen species and acute renal I/R injury [5]. In addition, NO reacts with superoxide anions to form peroxynitrite, which has been implicated in renal I/R injury [20, 33]. Furthermore, NO overproduction contributes to vascular hyporeactivity and vascular decompensation following HS [30]. Therefore, it has been shown that the inhibition of iNOS expression ameliorates or prevents renal I/R injury in both in vivo and in vitro studies [17].
Vitamin D receptors (VDRs) function to maintain calcium homeostasis through a well-defined mechanism by binding to vitamin D3 [11]. Further, the VDR/D3 complex controls cell proliferation, differentiation, and apoptosis and down-regulates the renal inflammatory response in lipopolysaccharide-induced acute kidney injury [38]. However, it is not known whether VDRs alone exhibit any of these actions in the absence of vitamin D3. In addition, to our knowledge the relationship between VDRs and renal injury in HS has not been investigated.
In addition to having antioxidant effects, aminoguanidine (AG) is an inhibitor of NOS, with high selectivity for iNOS [19]. The purpose of this study was to determine whether AG can ameliorate the pathological renal changes associated with HS in a rat model and to explore the protection mechanism.
II. Materials and Methods
Thirty adult male Sprague-Dawley rats weighing 300–350 g were allowed water and food at constant humidity (55 ± 10%), temperature (25 ± 2°C), and light/dark cycle (12/12 hr). The animals were housed with 5 rats/cage, and were given free access to standard diet and water. The animals were acclimatized to the laboratory conditions for one week before conducting the experiment.
Rats were intraperitoneally (i.p.) injected with heparin sodium (2000 IU) 15 min prior to anesthesia, which was achieved with i.p. urethane (125 mg/kg). The left carotid artery was cannulated, and a three-way stopcock was attached in-line for monitoring mean arterial blood pressure (MABP) using a blood pressure transducer and then the animals were allowed to stabilize for a period of 30 min [24]. The animals were assigned to one of three experimental groups (n = 10 per group). Group I (control) rats were subdivided into rats that underwent carotid artery cannulation without hemorrhage insult to exclude any effects from the carotid artery cannulation (control A; n = 5) and rats that were treated similarly to control A and then injected with 1 mL of 60 mg/kg of AG (Sigma, St Louis, MO), which was dissolved in a 0.9% sodium chloride solution (Sigma) intra-arterially [24] (control B; n = 5). Group II (HS) rats were hemorrhaged using a reservoir (10 mL syringe) that was connected to the carotid artery with a three-way stopcock. Blood was aspirated at a rate of 1 mL/min over 60 min to mimic hemorrhage by opening the stopcock and aspirating gently and gradually with the syringe. MABP was maintained at approximately 35–40 mmHg by continuously withdrawing or reinfusing blood. The rats were then resuscitated in vivo by reinfusion of the shed blood to restore normotension, and MABP was monitored for 30 min [24]. Group III (HS with AG) rats were treated similarly to group II and then injected with AG as described for the control B group. The rats were then resuscitated in vivo by reinfusion of the shed blood to restore normotension, and MABP was monitored for 30 min [24].
The manipulations were performed at the Laboratory Animal Center of the College of Medicine, King Saud University (Riyadh, KSA) in accordance with institutional and national guidelines for the care and use of laboratory animals. The experiment was approved by the Ethical Committee at the National Plan for Science, Technology and Innovation, King Saud University.
Biochemical analysis
Blood samples were collected during aspiration, and the separated plasma samples were used to assess NO concentration and renal function by measuring urea and creatinine levels. These levels were assessed using an automatic biochemical analyzer (Aeroset, Abbott, Chicago, IL) with Randox kits (Randox Laboratories Ltd., London, UK). Total plasma concentrations of NO were performed with Total Nitric Oxide and Nitrate/Nitrite Assay Kits (R&D Systems Europe, Ltd., Abingdon, UK). Urea and creatinine values were expressed (mg/dL) and (μmol/L) respectively; NOS was expressed as μmol/L.
Histological and immunohistochemical evaluation
Tissue samples from the right kidney were fixed in 10% neutral buffered formalin solution and processed to produce 4-μm-thick paraffin sections. The sections were stained for histological and immunohistochemical studies. Hematoxylin and eosin (H&E) [23] and periodic acid-Schiff (PAS) [9] staining were used to verify histological details and the presence of tubular basement membrane and brush borders, respectively, in the renal tissue sections.
For the immunohistochemistry studies, paraffin sections were deparaffinized in xylene and processed for BAX, iNOS, and VDR antibody immunohistochemistry. Immunohistochemistry was performed using a three-step indirect process based on the labeled avidin-biotin-peroxidase complex (ABC) method. Sections were rehydrated in descending grades of alcohol. Following blocking of endogenous peroxidase activity with 3% H2O2 in methanol and non-specific binding sites with a protein blocker, the sections were incubated for 32 min with a 1:100 dilution of Bax, iNOS (rabbit anti-rats Bax and iNOS polyclonal antibody, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and VDR (rabbit monoclonal, Novus Biologicals, Abingdon, UK) primary antibodies. Next, a biotinylated secondary antibody was added at a concentration of 2% for 30 min (37°C) followed by the addition of the ABC. Visualization of the reaction was performed using 3,3'-diaminobenzidine (DAB) as the chromogen, which produces a dark brown precipitate that is readily detected by light microscopy. The sections were then counterstained with Mayer’s hematoxylin, dehydrated in ascending grades of alcohol, cleared in xylene, and mounted with distyrene (a polystyrene), a plasticizer (tricresyl phosphate), and xylene. BAX cytoplasmic and membrane reaction sites stained brown and nuclei stained blue/brown; iNOS cytoplasmic and nuclear reaction sites stained brown; VDR nuclear, membranous, and cytoplasmic sites stained brown. The negative control included sections that were incubated in the absence of BAX, iNOS, and VDR primary antibodies.
All histopathological examinations were performed by the same histologist and pathologist who were blinded to the group definitions. The H&E-stained sections were investigated for abnormalities such as pyknotic nuclei, dilated tubular lumina, swelling of tubular cells, the presence of tubular red blood cells, tubular necrosis, and cellular detachment. Ten fields for each animal were randomly selected for evaluation at 40× magnification using a semi-quantitative scale. This scale graded the histopathological abnormalities from 0 to 4+ (0, no abnormalities; 1+, changes affecting <25% of the sample; 2+, changes affecting 25%–50% of the sample; 3+, changes affecting 50%–75% of the sample; 4+, changes affecting >75% of the sample) [7].
Quantitative measurements of the mean optical density (OD) of cytoplasmic BAX, iNOS, and nuclear VDR brown color reactions of the adrenal cortex were measured using an image analyzer (Super eye-Heidi soft, Anatomy Department, Faculty of Medicine, King Saud University, Saudi Arabia). Ten randomly selected high-power fields (400× magnifications) in each slide were captured for each group. The image analyzer was calibrated for color measurement before use.
Statistical analysis
All data were analyzed using SPSS statistical software version 19. Values are expressed as mean ± SEM. A chi-square test and an ANOVA were used to compare histopathological changes and the OD of the immune-positive immune-histochemical reactions, respectively, among the groups. The significance level was set at p ≤ 0.05.
III. Results
Biochemical analysis
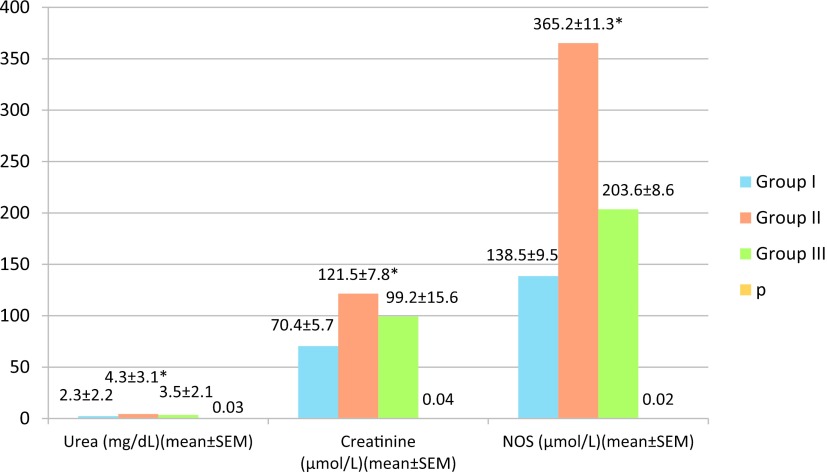
Urea and creatinine were significantly higher in group II (4.3 ± 3.1 mg/dL and 121.5 ± 7.8 μmol/L, respectively) compared to group I (2.3 ± 2.2 mg/dL and 70.4 ± 5.7 μmol/L, respectively) and group III (3.5 ± 2.1 mg/dL and 99.2 ± 15.6 μmol/L, respectively) (Fig. 1). The concentration of total NO was also significantly higher in group II (365.2 ± 11.3 μmol/L) than in groups I (138.5 ± 9.5 μmol/L) and III (203.6 ± 8.6 μmol/L) (Fig. 1).
Fig. 1.
Plasma concentrations of creatinine, urea, and nitric oxide synthase (NOS) in rats under different hemorrhage shock treatments. * = p ≤ 0.05 as determined from an ANOVA; Group I consisted of control rats that had been cannulated but not hemorrhaged. Half of these rats also received aminoguanidine (AG). Group II consisted of rats that had been hemorrhaged. Group III consisted of rats that had been hemorrhaged and then administered AG.
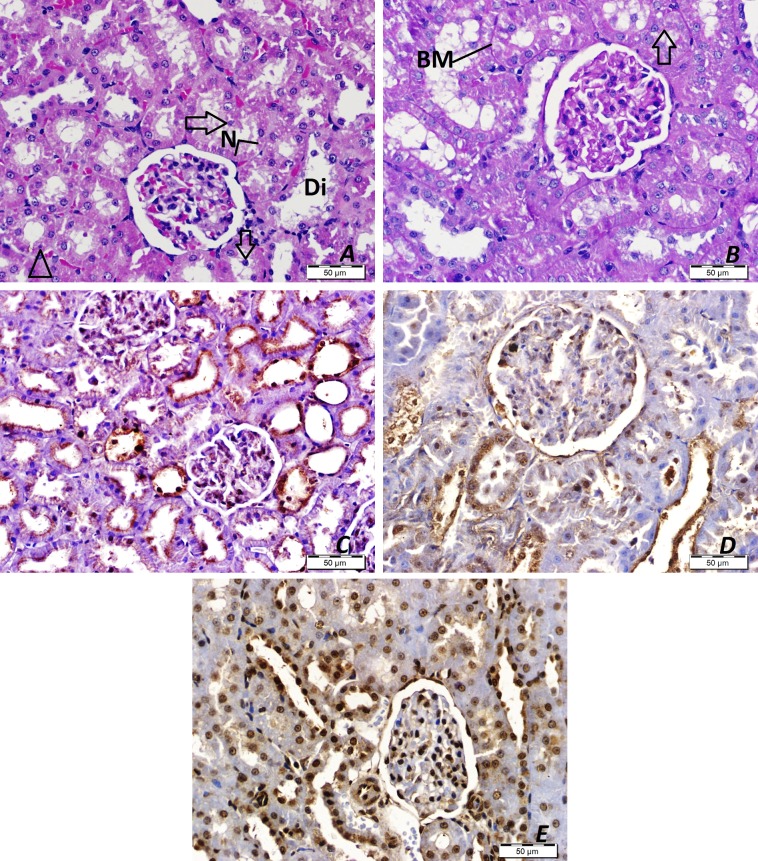
The H&E histological staining of renal tissue from rats in control A and B showed normal kidney structure with Malpighian corpuscles that were made up of a tuft of capillaries (the glomerulus) surrounded by Bowman’s capsule. The proximal convoluted tubules were lined with pyramidal epithelial cells with eosinophilic cytoplasm and central rounded nuclei, and the distal convoluted tubules were lined with a relatively large number of cuboidal epithelial cells. The lumens of the distal tubules were wider than those of the proximal tubules; their cytoplasm was less acidophilic; and the nuclei were round (Fig. 2A). The PAS stained sections revealed a PAS-positive reaction (magenta red stained) on the brush borders of the proximal renal tubules (Fig. 2B) and basement membranes of the proximal and distal convoluted tubules. The immunohistochemical-stained sections revealed a weak iNOS immune-positive reaction in the glomeruli but an immune-negative reaction in the proximal and distal convoluted tubules (Fig. 2C). BAX staining showed weak and moderate nuclear immune-positive reactions in the proximal and distal convoluted tubules respectively (Fig. 2D). VDR staining showed weak cytoplasmic and moderate nuclear immune-positive reactions in the proximal and distal convoluted tubules (Fig. 2E).
Fig. 2.
Results of hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), and immunohistochemical staining (3,3'-diaminobenzidine and hematoxylin, DAB&H) of renal tissue from control rats. A. H&E staining. D, distal tubules; G, glomerulus; P, proximal tubules. B. PAS staining. Arrows, brush borders; BM, tubular basement membranes; D, distal tubules; P, proximal tubules. C. iNOS immunostaining (DAB&H). D. BAX immunostaining (DAB&H). E. VDR immunostaining (DAB&H).
The H&E histological staining of group II revealed dilated tubular lumina, cellular detachment, and an absence of nuclei indicating tubular necrosis in the majority of renal tubules. Other histological changes such as the swelling of tubular cells, the presence of tubular red blood cells, and pyknotic nuclei in some tubules were also seen (Fig. 3A). The PAS-stained sections indicated that the brush borders of most of the proximal tubules were completely lost, and their basement membranes were faintly stained (Fig. 3B). The immunohistochemical-stained sections revealed marked iNOS immune-positive reactions in most of the proximal and distal convoluted tubules (Fig. 3C), and BAX and VDR staining showed marked nuclear and cytoplasmic immune-positive reactions in the proximal and distal convoluted tubules (Fig. 3D, E).
Fig. 3.
Results of hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), and immunohistochemical staining (3,3'-diaminobenzidine and hematoxylin, DAB&H) of renal tissue from rats in which HS was induced. A. H&E staining. Arrowheads, pyknotic nuclei; Arrows, detached tubular cells; Di, dilated renal tubules; N, tubular necrosis; S, swollen tubular cells; Star, blood containing renal tubules. B. PAS staining. BM, tubular basement membranes. C. iNOS immunostaining (DAB&H). D. BAX immunostaining (DAB&H). E. VDR immunostaining (DAB&H).
The H&E histological staining of group III revealed some areas of necrosis and detached renal tubular epithelium, but the majority of tubules were normal (Fig. 4A). The PAS-stained sections showed that the brush borders and basement membranes were preserved in some tubules (Fig. 4B). The immunohistochemical-stained sections revealed mild iNOS immune-positive reactions in most of the proximal and distal convoluted tubules (Fig. 4C). BAX staining showed moderate immune-positive reactions in the proximal and distal convoluted tubules (Fig. 4D), and VDR staining showed mild to moderate immune-positive reactions in the proximal and distal convoluted tubules (Fig. 4E).
Fig. 4.
Results of hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), and immunohistochemical staining (3,3'-diaminobenzidine and hematoxylin, DAB&H) of renal tissue from rats in which HS was induced and then aminoguanidine (AG) was administered. A. H&E staining. Arrowheads, pyknotic nuclei; Arrows, detached tubular cells; Di, dilated renal tubules; N, tubular necrosis. B. PAS staining. Arrows, brush borders; BM, tubular basement membranes. C. iNOS immunostaining (DAB&H). D. BAX immunostaining (DAB&H). E. VDR immunostaining (DAB&H).
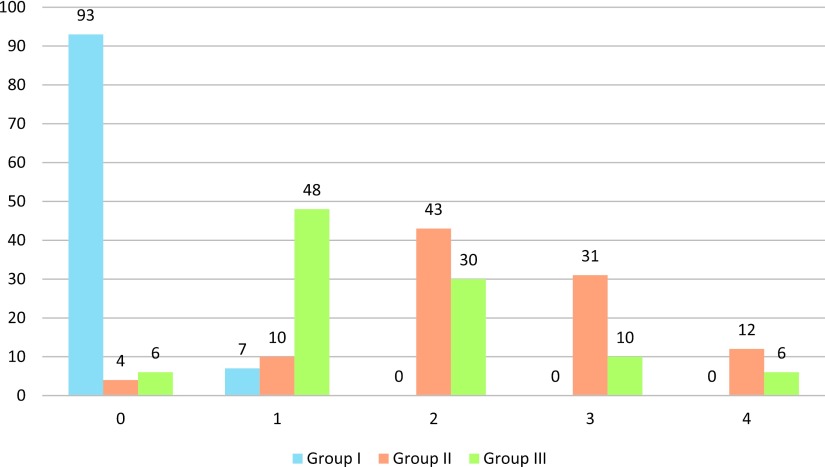
The semi-quantitative grade score of renal histological changes was significantly higher in group II than in the other groups (χ2 = 275.132, df = 8, p = 0.0001) (Fig. 5).
Fig. 5.
Semi-quantitative analysis of renal histological changes in groups of rats under different hemorrhage shock treatments. Results expressed in number of the studied fields in each group (100 fields/each). Group I consisted of control rats that had been cannulated but not hemorrhaged. Half of these rats also received aminoguanidine (AG). Group II consisted of rats that had been hemorrhaged. Group III consisted of rats that had been hemorrhaged and then administered AG.
Quantitative analysis of the immunohistochemistry (Fig. 6) of the renal tissue samples showed that the iNOS, BAX, and VDR expression was significantly higher in group II (0.26 ± 0.05, 0.28 ± 0.04, and 0.25 ± 0.03, respectively) compared to groups I (0.009 ± 0.0008, 0.02 ± 0.0003, and 0.1 ± 0.003, respectively) and III (0.05 ± 0.002, 0.15 ± 0.008, and 0.19 ± 0.003, respectively).
Fig. 6.
Quantitative comparison of optical density (OD) scores of rats under different HS treatments. * = p ≤ 0.05 as determined from an ANOVA; Group I consisted of control rats that had been cannulated but not hemorrhaged. Half of these rats also received aminoguanidine (AG). Group II consisted of rats that had been hemorrhaged. Group III consisted of rats that had been hemorrhaged and then administered AG.
IV. Discussion
The HS model used in the current study mimicked clinical reality to test a potential treatment modality after severe trauma and blood loss in humans [23]. Previous studies showed that HS is accompanied by NO overproduction [2]. In addition, renal impairment, as demonstrated by increased urea and creatinine in the plasma, is a common outcome of HS [35]. Similarly, the results of the current study showed that HS syndrome induced impaired renal functions in the form of increased plasma urea and creatinine. In addition, it increased plasma levels of iNOS. However, urea, creatinine, and NOS levels were significantly lower in rats that were induced into HS and then given AG (a highly selective iNOS inhibitor), thus preventing injury to the kidneys. Similarly, it has been reported that AG improves renal function and is associated with improvement of the glomerular filtration rate and renal plasma flow [34]. Moreover, our results are in agreement with other studies that showed that the infusion of AG reduces serum nitrite concentration through the inhibition of iNOS [10].
Peroxynitrite formed as a result of NO overproduction and its subsequent reaction with superoxide anions. Peroxynitrite is a direct inhibitor of mitochondrial respiratory chain enzymes leading to DNA damage, inhibition of membrane Na+, K+-ATPase activity, and the activation of caspases resulting in cell death [3]. In addition, peroxynitrite induces damage to membrane phospholipids leading to an elevated concentration of intracellular Ca2+ also resulting in cell death [26]. Therefore, the protection effect of AG observed in this study may be attributable to the prevention of peroxynitrite generation. Similarly, it has been shown that iNOS inhibitors prevent peroxynitrite generation that leads to the prevention of vascular decomposition in the late phase of shock and the significant reduction of local tissue damage caused by I/R [10]. In addition, it has been shown that AG inhibits iNOS resulting in a decrease of stress proteins, such as c-Jun, which is responsible for cell death [27].
The renal histopathological investigations in our study revealed renal tubular swelling, detached epithelia, dilatation, pyknosis, necrosis, and loss of the brush borders as a result of HS. However, these histological changes were ameliorated by AG. Our results were in agreement with other studies that have shown the same histopathological results in different HS models [23].
The HS induced group in our study exhibited significant increases of iNOS, BAX, and VDR immunohistochemical reactions. Our results were in agreement with those of other studies that demonstrated an increase in iNOS expression as detected by immunohistochemistry in the kidneys of HS rat models [30]. Increased expression of iNOS enhances the formation of NO and may contribute to circulatory failure and organ dysfunction associated with HS. In addition, it has been reported that NO promotes apoptosis via activation of c-Jun [1, 39].
Increased BAX expression (a proapoptotic protein) in our study was in agreement with other studies that found the same result in rats experiencing HS [6, 8]. Tait and Green [28] demonstrated that apoptosis is completely dependent on BAX/Bak at the level of the mitochondria because the lack of genes encoding these two proteins results in cells that resist apoptosis by preventing release of cytochrome c. BAX induces apoptosis through the formation of ion channels in ER membranes causing the release of Ca2+ from the ER [14]. Moreover, the essential role of BAX in mediating apoptosis via c-JNK has been demonstrated [21]. However, other studies have shown an indirect role of Bax/Bak in apoptosis and necrosis through release of cytochrome c and activation of caspases 3 and 8 [36]. Moreover, previous studies have suggested a role for Bax/Bak in the regulation or formation of mitochondrial permeability transition pores [18]. In addition, Bax/Bak is responsible for the formation of these permeability pores leading to cell necrosis [12]. Therefore, the increased expression and activation of BAX as a result of I/R in HS-induced group may induce both apoptosis and necrosis.
Vitamin D has classical actions in calcium uptake and bone metabolism and non-classical actions including the modulation of innate immune, the regulation of cell proliferation [15]. In non-classical action, VDR interacts with other proteins such as Iκ B kinase β, nuclear factor kappa B (NF-κB) and c-Jun thereby regulating its transcription activity independent of vitamin D3 [31]. The increased expression of VDR in the HS model examined in the current study may be attributed to increased c-Jun, which leads to cell death via non-classical pathways [15].
The presence of AG significantly decreased the number of iNOS, BAX, and VDR immunohistochemical reactions in renal tissue samples compared to those from rats that had been induced into HS but did not receive AG. In addition to iNOS inhibition, AG has many pharmacological properties, including the inhibition of advanced glycosylation end product formation; catalase, polyamine catabolism, and histamine metabolism thereby, protects the renal injury against I/R injury in HS induced model. Therefore, the beneficial effect of AG might not only be due to inhibition of iNOS activity [37].
Our results demonstrated that a NOS inhibitor, AG, is a potential therapeutic agent for the treatment of HS in an experimental rat model. Further studies are needed to investigate the physiological and immunohistochemical basis for the beneficial role of AG in preventing renal functional and histological impairment, such as studying the role of c-Jun and cytochrome c. In summary, AG ameliorated kidney injury by inhibiting iNOS, which resulted in decreased BAX and VDR expression. Therefore, a therapeutic strategy targeting AG may provide new insights into kidney injury during severe shock.
V. Conflicts of Interest
The authors have no financial conflicts of interest.
VI. Authors’ Contributions
AAD conceived the study, designed and conducted the experiments, performed statistical analyses, and finalized the paper. MSK conducted the experiments, analyzed the histological and immunohistochemical data, and wrote the first draft of the manuscript. MS conducted the experiments and finalized the paper.
VII. Acknowledgments
The authors extend their gratitude to College of Medicine Research Centre, King Saud University, Riyadh, Saudi Arabia for the grant support of this work.
VIII. References
- 1.Amir M., Liu K., Zhao E. and Czaja M. J. (2012) Distinct functions of JNK and c-Jun in oxidant induced hepatocyte death. J. Cell Biochem. 113; 3254–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ates E., Yalcin A. U., Yilmaz S., Koken T. and Tokyol C. (2005) Protective effect of erythropoietin on renal ischemia and reperfusion injury. ANZ J. Surg. 75; 1100–1105. [DOI] [PubMed] [Google Scholar]
- 3.Barmaki B. and Khazaei M. (2015) Effect of aminoguanidine on cardiovascular responses and survival time during blood loss: A study in normotensive and deoxycorticosterone acetate-salt hypertensive rats. Int. J. Appl. Basic Med. Res. 5; 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bond R. F. and Johnson G. (1985) Vascular adrenergic interactions during hemorrhagic shock. Fed. Proc. 44; 281–289. [PubMed] [Google Scholar]
- 5.Chatterjee P. K., Hawksworth G. M. and McLay J. S. (1999) Cytokine-stimulated nitric oxide production in human renal proximal tubule and its modulation by natriuretic peptides: a novel immunomodulatory mechanism? Exp. Nephrol. 7; 438–448. [DOI] [PubMed] [Google Scholar]
- 6.Choi B. M., Pae H. O., Jang S. I., Kim Y. M. and Chung H. T. (2002) Nitric oxide as a pro-apoptotic as well as anti-apoptotic modulator. BMB Rep. 35; 116–126. [DOI] [PubMed] [Google Scholar]
- 7.Cook, H. C. (1974) Manual of Histological Demonstration Techniques. Butterworths, London. [Google Scholar]
- 8.Deng G. R., Ling Q., Wu B. H., Dong Y. Y., Gao X., Li T. Q., Miu X. and Li Z. F. (2016) Protection mechanism of deacetylase inhibitor on spleen of rats with severe hemorrhagic shock. Asian Pac. J. Trop. Med. 9; 572–576. [DOI] [PubMed] [Google Scholar]
- 9.Drury, R. A. B., Wallington, E. A. and Carleton, H. M. (1980) Carleton’s Histological Technique. 5th ed., Oxford University Press, Oxford. [Google Scholar]
- 10.Heemskerk S., Masereeuw R., Russel F. G. M. and Pickkers P. (2009) Selective iNOS inhibition for the treatment of sepsis-induced acute kidney injury. Nat. Rev. Nephrol. 5; 629–640. [DOI] [PubMed] [Google Scholar]
- 11.Jones G., Strugnell S. A. and DeLuca H. F. (1998) Current understanding of the molecular actions of vitamin D. Physiol. Rev. 78; 1193–1231. [DOI] [PubMed] [Google Scholar]
- 12.Karch J., Kwong J. Q., Burr A. R., Sargent M. A., Elrod J. W., Peixoto P. M., Martinez-Caballero S., Osinska H., Cheng E. H., Robbins J., Kinnally K. W. and Molkentin J. D. (2013) Bax and Bak function as the outer membrane component of the mitochondrial permeability pore in regulating necrotic cell death in mice. Elife 2; e00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kone B. C. and Baylis C. H. (1997) Biosynthesis and homeostatic roles of nitric oxide in the normal kidney. Am. J. Physiol. Renal Physiol. 272; F561–F578. [DOI] [PubMed] [Google Scholar]
- 14.Lam M., Dubyak G., Chen L., Nuñez G., Miesfeld R. L. and Distelhorst C. W. (1994) Evidence that BCL-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc. Natl. Acad. Sci. U S A 91; 6569–6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q. P., Qi X., Pramanik R., Pohl N. M., Loesch M. and Chen G. (2007) Stress-induced c-Jun-dependent Vitamin D receptor (VDR) activation dissects the non-classical VDR pathway from the classical VDR activity. J. Biol. Chem. 19; 1544–1551. [DOI] [PubMed] [Google Scholar]
- 16.Linderholm M., Groeneveld E. H. E. and Tanvik A. (1996) Increased production of nitric oxide in patients with hemorrhagic fever with renal syndrome-relation to arterial hypotension and tumor necrosis factor. Infection 24; 337–340. [DOI] [PubMed] [Google Scholar]
- 17.Ling H., Edelstein C., Gengaro P., Meng X., Lucia S., Knotek M., Wangsiripaisan A., Shi Y. and Schrier R. (1999) Attenuation of renal ischemia-reperfusion injury in inducible nitric oxide synthase knockout mice. Am. J. Physiol. 277; F383–F390. [DOI] [PubMed] [Google Scholar]
- 18.Marzo I., Brenner C., Zamzami N., Jürgensmeier J. M., Susin S. A., Vieira H. L., Prévost M. C., Xie Z., Matsuyama S., Reed J. C. and Kroemer G. (1998) Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science 281; 2027–2031. [DOI] [PubMed] [Google Scholar]
- 19.Misko T. P., Moore W. M., Kasten T. P., Nickols G. A., Corbett J. A., Tilton R. G., McDaniel M. L., Williamson J. R. and Currie M. G. (1993) Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur. J. Pharmacol. 233; 119–125. [DOI] [PubMed] [Google Scholar]
- 20.Paller M. S., Weber K. and Patten M. (1998) Nitric oxide-mediated renal epithelial cell injury during hypoxia and reoxygenation. Ren. Fail. 20; 459–469. [DOI] [PubMed] [Google Scholar]
- 21.Papadakis E. S., Finegan K. G., Wang X., Robinson A. C., Guo C., Kayahara M. and Tournier C. (2006) The regulation of Bax by c-Jun N-terminal protein kinase (JNK) is a prerequisite to the mitochondrial-induced apoptotic pathway. FEBS Lett. 580; 1320–1326. [DOI] [PubMed] [Google Scholar]
- 22.Rossaint R., Cerny V., Coats T. J., Duranteau J., Fernandez-Mondejar E., Gordini G., Stahel P. F., Hunt B. J., Neugebauer E. and Spahn D. R. (2006) Key issues in advanced bleeding care in trauma. Shock 26; 322–331. [DOI] [PubMed] [Google Scholar]
- 23.Rönn T., Lendemans S., de Groot H. and Petrat F. (2011) A new model of severe hemorrhagic shock in rats. Comp. Med. 61; 419–426. [PMC free article] [PubMed] [Google Scholar]
- 24.Soliman M. M. and Arafah M. M. (2012) Treatment with dipyridamole improves cardiac function and prevent injury in a rat model of hemorrhage. Eur. J. Pharmacol. 678; 26–31. [DOI] [PubMed] [Google Scholar]
- 25.Star R. A. (1998) Treatment of acute renal failure. Kidney Int. 54; 1817–1831. [DOI] [PubMed] [Google Scholar]
- 26.Szabó C. (2003) Multiple pathways of peroxynitrite cytotoxicity. Toxicol. Lett. 140; 105–112. [DOI] [PubMed] [Google Scholar]
- 27.Szabó C. and Módis K. (2010) Pathophysiological roles of peroxynitrite in circulatory shock. Shock 34; 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tait S. W. G. and Green D. R. (2010) Mitochondria and cell death: outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 11; 621–632. [DOI] [PubMed] [Google Scholar]
- 29.Thadhani R., Pascual M. and Bonventre J. V. (1996) Acute renal failure. N. Engl. J. Med. 334; 1448–1460. [DOI] [PubMed] [Google Scholar]
- 30.Thiemermann C., Szabo C., Mitchell, J. A., and Vane J. R. (1993) Vascular hyporeactivity to vasoconstrictor agents and hemodynamic decompensation in hemorrhagic shock is mediated by nitric oxide. Proc. Natl. Acad. Sci. U S A 90; 267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tolón R. M., Castillo A. I., Jiménez-Lara A. M. and Aranda A. (2000) Association with Ets-1 causes ligand-and AF2-independent activation of nuclear receptors. Mol. Cell. Biol. 20; 8793–8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Bommel J., Siegemund M., Henny Ch. P. and Ince C. (2008) Heart, kidney and intestine have different tolerances for anemia. Transl. Res. 151; 110–107. [DOI] [PubMed] [Google Scholar]
- 33.Walker L. M., Walker P. D., Imam S. Z., Ali S. F. and Mayeux P. R. (2000) Evidence for peroxynitrite formation in renal ischemia-reperfusion injury: studies with the inducible nitric oxide synthase inhibitor L-N 6-(1-Iminoethyl)lysine. J. Pharmacol. Exp. Ther. 295; 417–422. [PubMed] [Google Scholar]
- 34.Wang Y., Yan J., Xi L., Qian Z., Wang Z. and Yang L. (2012) Protective effect of crocetin on hemorrhagic shock-induced acute renal failure in rats. Shock 38; 63–67. [DOI] [PubMed] [Google Scholar]
- 35.Warke V. G., Nambiar M. P., Krishnan S., Tenbrock K., Geller D. A., Koritschoner N. P., Atkins J. L., Farber D. L. and Tsokos G. C. (2003) Transcriptional activation of the human inducible nitric-oxide synthase promoter by Krüppel-like factor 6. J. Biol. Chem. 278; 14812–14819. [DOI] [PubMed] [Google Scholar]
- 36.Whelan R. S., Konstantinidis K., Wei A. C., Chen Y., Reyna D. E., Jha S., Yang Y., Calvert J. W., Lindsten T., Thompson C. B., Crow M. T., Gavathiotis E., Dorn G. W. 2nd, O’Rourke B. and Kitsis R. N. (2012) Bax regulates primary necrosis through mitochondrial dynamics. Proc. Natl. Acad. Sci. U S A 109; 6566–6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu C., Chen S., Szabo C., Thiemermann C. and Vane J. R. (1995) Aminoguanidine attenuates the delayed circulatory failure and improves survival in rodent models of endotoxic shock. Br. J. Pharmacol. 11; 1666–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu S., Chen Y. H., Tan Z. X., Xie D. D., Zhang C., Zhang Z. H., Wang H., Zhao H. and De-Xin Y. (2015) Vitamin D3 pretreatment regulates renal inflammatory responses during lipopolysaccharide-induced acute kidney injury. Sci. Rep. 5; 18687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamaguchi M., Abiko Y., Nishimura M., Saitoh M. and Kaku T. (2004) An immunohistochemical study of the localization of inducible nitric oxide synthase (iNOS) and heat shock protein (HPS) in pleomorphic adenoma. Acta Histochem. Cytochem. 37; 267–271. [Google Scholar]
- 40.Zager R. A. (1991) Adenine nucleotide changes in kidney, liver, and small intestine during different forms of ischemic injury. Circ. Res. 68; 185–196. [DOI] [PubMed] [Google Scholar]