Abstract
Although estrogen is implicated in the regulation of cell growth and differentiation in many organs, the exact mechanism for liver regeneration is not completely understood. We investigated the effect of estrogen on liver regeneration in male and female Wistar rats after 70% partial hepatectomy (PHx) and performed immunohistochemistry, western blotting and Southwestern histochemistry. 17β-estradiol (E2) and ICI 182,780 were injected into male rats on the day before PHx. The proliferating cell nuclear antigen (PCNA) labeling index reached a maximum at 48 hr after PHx in males, and at 36 hr in females and E2-treated male rats. Estrogen receptor α (ERα) was expressed in zones 1 and 2 in male rats, but was found in all zones in female rats. Interestingly, ERα was not detected at 6–12 hr after PHx but was found at 24–168 hr in male rats. However, ERα expression was found at all sampling time-points in female and E2-treated male rats. The activity of estrogen responsive element binding proteins was detected from 12 hr after PHx in male rats but was found from 6 hr in female and E2-treated male rats. ERα was co-expressed with PCNA during liver regeneration. These results indicate that estrogen may play an important role in liver regeneration through ERα.
Keywords: estrogen, estrogen receptor α, liver regeneration, partial hepatectomy, cell proliferation
I. Introduction
Although hepatocytes in adult livers rarely divide under normal conditions, the liver possesses a remarkable ability to restore its original mass and size following surgical removal or after various chemical injuries [7, 29]. This regenerative capacity allows the removal of tumor masses from the liver without impairment of its function. However, the potential for liver regeneration is limited in chronic liver diseases such as cirrhosis, hepatocellular carcinoma (HCC), non-alcoholic steatohepatitis, and excessive resection leads to liver failure [8, 28]. Recently there has been much interest in rational therapies to reduce the factors which inhibit liver regeneration or to stimulate remnant liver regeneration [10].
In a clinical setting, there are substantial sex-based differences such as enzyme activity, gene expressions and steroid hormone responsiveness which can modulate the liver’s capacity to metabolize certain drugs and hormones [27, 33, 37]. Chronic liver diseases are more severe and occur more frequently in males compared to females, and women have a significantly lower incidence of HCC than men [23, 30]. Similar sex-based differences are also observed in rodents. It was reported that the survival rate following partial hepatectomy or portal branch ligation was significantly higher in female compared to male rodents [14, 18, 43]. The liver is protected from injury due to reduced-size ischemia and reperfusion by 17β-estradiol (E2). The survival rate in male mice after hepatic surgery is significantly improved by treatment with E2 [14]. Although several factors are suggested to influence gender-based differences, sex steroid hormones such as estrogen and androgen may be closely associated with sex-based differences in the liver [42]. It was reported that estrogen prevents and androgen enhances DNA damage and oxidative stress during hepatocarcinogenesis [23, 26]. Interestingly, most sex-based differences in the liver are diminished after menopause [3] suggesting that female sex hormones, especially estrogen might have an important role in the differences.
Estrogen receptor alpha (ERα) and ERβ bind to the estrogen response element (ERE), which is present in the promoter region of estrogen-target genes and regulates the transcriptional activity of various genes [4, 5, 21]. ERα, but not ERβ, is expressed in hepatocytes, and involved in regulation of glucose and lipid metabolism in the liver [9, 11, 40]. Although estrogen has been implicated in cell proliferation in the intestine and skin [6, 38], its effect on liver regeneration is not completely understood.
In this study, we investigated the effect of estrogen on liver regeneration using a 70% partial hepatectomy (PHx) model in rats. Cell proliferation activity was determined using proliferating cell nuclear antigen (PCNA) and ERα expression in immunohistochemistry and western blot analysis. The activity of estrogen responsive elements (ERE) was analyzed using Southwestern histochemistry (SWH). Finally, to confirm the effect of estrogen on liver regeneration, we treated male rats with E2 or estrogen receptor antagonist ICI 182,780 and analyzed cell proliferation activity and ERα expression after PHx.
II. Materials and Methods
Chemicals and biochemicals
Paraformaldehyde (PFA) was purchased from Merck (Darmstadt, Germany). ICI 182,780, 17β-estradiol (E2), bovine serum albumin (BSA), 2-mercaptoethanol, 3-aminopropyl-triethoxysilane, Brij L23 were from Sigma Chemical Co. (St Louis, MO). Diethyl ether, sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) reagents were from Wako Pure Chemical Industries, Ltd. Protein marker was from Bio Dynamics Laboratory Inc, Ltd. Polyvinylidene fluoride membrane (PVDF) was from Millipore (Bedford, MA). 3,3'-Diaminobenzidine–4 HCl (DAB) was from Dojindo Chemicals (Kumamoto, Japan) and 4-Cl-1-naphthol was from Tokyo Kasei Kogyo (Tokyo, Japan). All other chemicals used in this study were from Wako Pure Chemicals (Osaka, Japan).
Animals and tissue preparation
Eight-week-old male (240–260 g) and female (190–210 g) Wistar rats were used in the study. Rats were kept under constant 12 hr dark/light conditions and fed normal chow and drinking water ad libitum. The experimental protocol was approved by the Animal Ethics Review Committee of the University of Miyazaki (2012-502-5). After sacrifice, liver tissue was cut into several small pieces and some were snap frozen and kept at −80°C until use in western blot analysis. The remaining pieces of liver tissue were fixed in 4% PFA in phosphate-buffered saline (PBS) at room temperature (RT) overnight and paraffin-embedded using standard methods. Each rat was anesthetized by inhalation of isoflurane and 70% PHx was performed using the technique described by Higgins and Anderson [15]. After PHx, rats were sacrificed at 0, 6, 12, 24, 36, 48, 72, 120 and 168 hr and tissues were sampled as described above. Male, female, E2 and E2+ICI 182,780 treated groups consisting of 3–5 rats for each time-point were used in these experiments.
Estrogen and estrogen receptor antagonist administration
Male rats were given a single intraperitoneal injection of E2 at a dose of 9 μg/g bodyweight [14] and ICI 182,780 at a dose of 2 μg/g bodyweight [19, 39]. E2 and E2+ICI 182,780 were dissolved in 500 μl of corn oil and injected on the day before PHx.
Antibodies
Mouse monoclonal antibody against PCNA (PC-10; 2 μg/ml) and normal mouse IgG were purchased from Dako (Glostrup, Denmark). Mouse monoclonal antibody against ERα (6F11; dilution 1:50) and horseradish peroxidase (HRP)-goat anti-mouse IgG (dilution 1:100) were from Thermo scientific (Rockford, IL), and mouse monoclonal antibody against β-actin (AC-15; dilution 1:12,000) and normal goat IgG were from Sigma.
Immunohistochemistry
Immunohistochemistry was performed as reported previously [2, 4, 31, 36]. Paraffin-embedded tissues were cut into 5 μm sections and placed onto silane-coated glass slides. The sections were deparaffinized with toluene, and rehydrated through a graded ethanol series, and then heated to 120°C for 15 min in 10 mM citrate buffer (pH 6.0). After inhibition of endogenous peroxidase activity with 0.3% H2O2 in methanol for 15 min, the sections were pre-incubated with 500 μg/ml normal goat IgG and 1% BSA in PBS for 1 hr to block non-specific antibody binding. Unless otherwise specified, all reactions were conducted at RT. The sections were then reacted with the primary antibodies for overnight. After washing with 0.075% Brij L23 in PBS, slides were reacted with HRP-goat anti-mouse IgG for 1 hr. After washing in 0.075% Brij L23 in PBS, the HRP site was visualized with DAB and DAB, Ni, Co, and H2O2 according to the method of Adams [1]. Normal mouse IgG was used at the same concentration instead of the primary antibodies for each experiment as a negative control.
Double-staining for ERα and PCNA
For simultaneous detection of ERα and PCNA, double-staining was performed as described previously [4]. Briefly, after antigen retrieval, the sections were stained with anti-ERα overnight and HRP sites were visualized as brown deposits of DAB and H2O2. The slides were then immersed and stirred in 0.1 M glycine-HCl buffer (pH 2.2) to remove immuno-complexes. After washing with double distilled water (DDW) once and PBS three times, the sections were reacted with anti-PCNA overnight. HRP sites were visualized by the purple-blue product of 4-Cl-1-naphthol and H2O2 solution.
Western blot analysis
Lysate containing 20 μg of protein was mixed with loading solution [200 mM Tris–HCl (pH 8.0), 0.5 M sucrose, 5 mM EDTA, 0.01% bromophenol blue, 10% 2-mercaptoethanol, and 2.5% SDS], boiled for 5 min, separated by SDS-PAGE with 8% polyacrylamide gel and electrophoretically transferred onto PVDF membranes. The membranes were blocked with 5% nonfat milk in Tris-buffered saline (TBS; 20 mM Tris buffer, pH 7.6, and 150 mM NaCl) for 1 hr at RT and then incubated overnight with mouse monoclonal antibody anti-ERα diluted 1:500 in TBS buffer. As a secondary antibody, HRP-goat anti-mouse IgG was diluted with TBS buffer for 1 hr and membranes were washed six times for 15 min each with TBS/0.05% Triton X-100 buffer. The bands were visualized with DAB, Ni, Co, and H2O2.
Southwestern histochemistry (SWH) for localization of ERE binding proteins
To confirm the localization of ERE binding proteins, SWH was performed as described previously [16]. Briefly, paraffin sections from male, female, E2 and E2+ICI 182,780 treated rats were deparaffinized and heated to 120°C for 15 min in 0.01 M citrate buffer (pH 6.0). The sections were then reacted with a digoxigenin labeled double-stranded DNA probe which contained a complete palindromic estrogen responsive element (vERE: 5'-GATCCAGGTCACAGTGACCTGGATC-3') of the chicken vitellogenin gene, and a mutated estrogen responsive element (mERE: 5'-GATCCAGATCACAGTGATCTGGATC-3') with 2 base mutations and a digoxigenin label at the 3'-end. For the detection of hybridized oligo-DNA probes, the sections were immunohistochemically stained with HRP-conjugated sheep anti-digoxigenin antibody using a chromogen solution with DAB, Ni, Co, and H2O2, as described previously [1].
Quantitative analysis
At least 2000 cells were counted at ×400 magnification in random fields, and the percentage of positive cells per total number of counted cells was represented by a labeling index (LI).
Statistical analysis
All data were expressed as mean ± SE. Differences between experimental groups were assessed by Student’s t-test. P < 0.05 was considered statistically significant. All analyses were performed with The Statistical Package for Social Sciences (version 11.5; Chicago, IL, USA).
III. Results
Liver weight/bodyweight (LW/BW) ratio in male and female rats after PHx
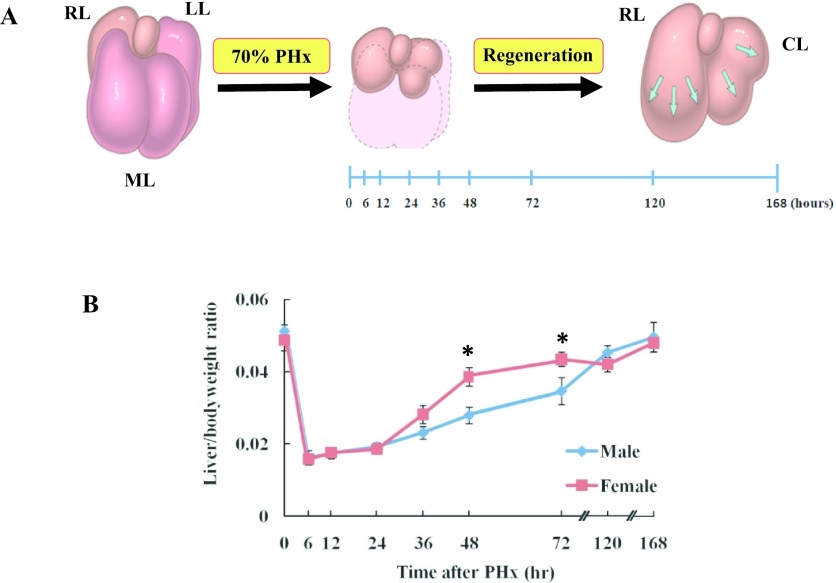
A model of liver regeneration [13] and the liver weight/bodyweight ratio of male and female Wistar rats after PHx was shown in Fig. 1. Liver weight/bodyweight ratio was evaluated following 70% PHx. The results indicated that liver weight in females was increased significantly at 48 and 72 hr after PHx compared to the males. The liver weight was fully restored at 168 hr after PHx in both male and female rats (Fig. 1B).
Fig. 1.
Model of liver regeneration and the liver weight/bodyweight ratio of male and female rats after PHx. A: Schema of 70% PHx in Wistar rats [13]. RL: right lobe, LL: left lobe, ML: median lobe, CL: caudate lobe. B: Liver weight/body weight ratio of male and female rats after PHx. Asterisks indicate statistically significant differences (*p < 0.05). Data represent the mean ± SE of 3–5 rats.
Immunohistochemical detection of PCNA in male and female rat liver after PHx
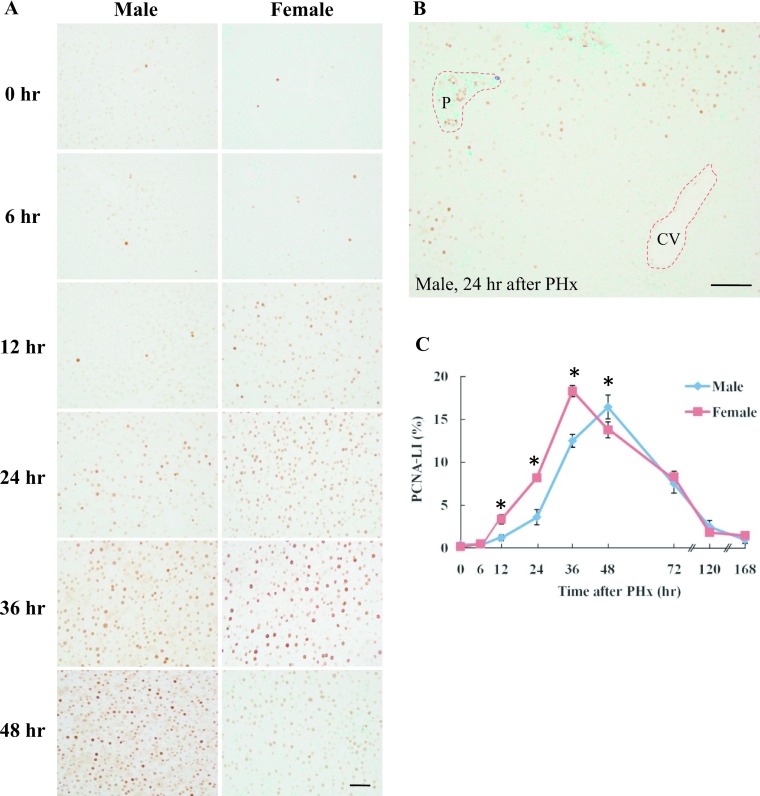
PCNA expression was a marker of late G1 to S phase in regenerating liver and was investigated using immunohistochemistry. PCNA positive cells were found in zones 1 and 2 at 24–36 hr after PHx in male rats, but at 12–24 hr in female rats (Fig. 2A, 2B). PCNA positive cells were increased in all zones at 48 hr after PHx in male rats, but at 36 hr in female rats (Fig. 2A). PCNA-LI indicates that the number of positive cells reached a peak at 48 hr in male rats, while the peak in female rats occurred at 36 hr after PHx. PCNA-LI was significantly higher at 12–36 hr after PHx in female rats compared to male rats (Fig. 2C). These results indicated that after PHx, cell proliferation starts 12 hr earlier in female rats compared to male rats. PCNA expression was significantly reduced at 72–168 hr after PHx and significant differences were not found between male and female rats (data not shown).
Fig. 2.
Immunohistochemical detection of PCNA in male and female rat liver after PHx. A: Liver tissue was collected from male and female rats at 0, 6, 12, 24, 36 and 48 hr after PHx. Paraffin-embedded rat liver sections were analyzed by immunohistochemistry. Magnification ×400. Bar = 50 μm. B: Zonal distribution of PCNA-positive cells at 24 hr in male rat liver after PHx. P: portal area, CV: central vein. Magnification ×200. Bar = 100 μm. C: PCNA-LI in male and female rats after PHx. The number of PCNA positive hepatocytes was counted at each time-point after PHx. Blue and red lines represent male and female, respectively. Asterisks indicate statistically significant differences (*p < 0.05). Data represent the mean ± SE of three independent experiments.
Immunohistochemical detection of ERα in male and female rat liver after PHx
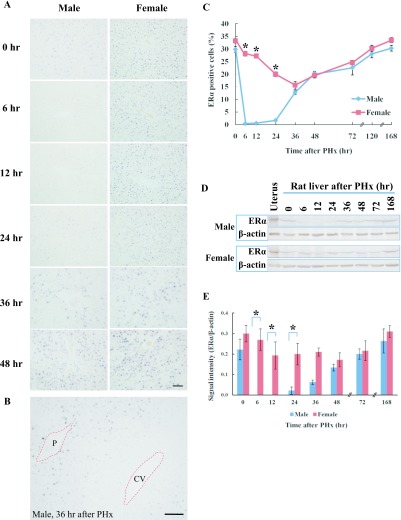
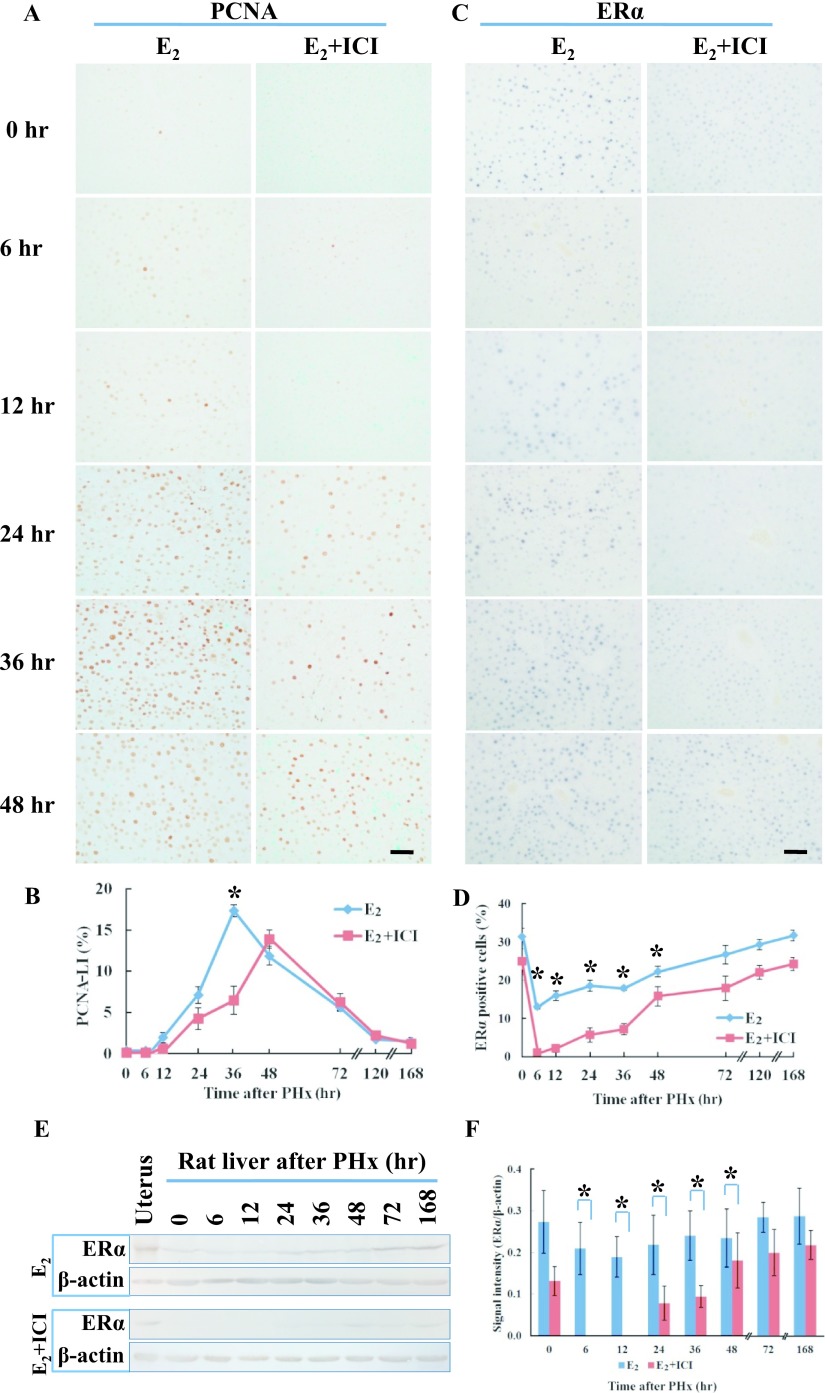
The expression of ERα was examined in the regenerating liver using immunohistochemistry and western blot analysis. In normal liver, ERα was only expressed in zones 1 and 2 in male rats, but was found in all zones in female rats (Fig. 3A). In male rats, ERα was not detected from 6–12 hr after PHx but was weakly found at 24 hr in zone 1. ERα expression was increased in zones 1 and 2 at 36 hr after PHx (Fig. 3B), and was found in all zones from 48 to 168 hr (Fig. 3A). On the other hand, ERα expression was found in zones 1, 2 and 3 at all time-points after PHx in female rats (Fig. 3A). The number of ERα positive cells was significantly higher at 6–24 hr after PHx in female rats compared to male rats (Fig. 3C). ERα expression was increased at 72–168 hr in male and female rats but significant differences were not found (data not shown). The amount of ERα protein in the regenerating liver was analyzed by western blot at various time-points (Fig. 3D) using mouse uterus as a positive control. In male rats, ERα protein was detectable at 0 hr, but not at 6–12 hr after PHx and gradually increased from 24–168 hr. In female rats, ERα was continuously expressed at all time-points after PHx (Fig. 3D). Densitometry analysis revealed that the amount of the ERα protein was significantly higher at 6–24 hr after PHx in female rats compared to male rats (Fig. 3E).
Fig. 3.
ERα expression in rat liver after PHx. A: Immunohistochemical detection of ERα in male and female rat liver at various time-points after PHx. Paraffin-embedded liver sections were analyzed by immunohistochemistry. Magnification ×400. Bar = 50 μm. B: Zone dependent ERα expression at 36 hr in male rat liver after PHx. P: portal area, CV: central vein. Magnification ×200. Bar = 100 μm. C: The number of ERα positive cells in the liver sections of male and female rats at various time-points after PHx. Blue and red lines represent male and female, respectively. D: Western blot analysis of ERα in male and female rats. ERα (66 kDa) and β-actin (42 kDa). E: Densitometry analysis of western blot. Asterisks indicate statistically significant differences (*p < 0.05). Data represent the mean ± SE of three independent experiments.
Localization of ERE binding proteins in male and female rat liver after PHx
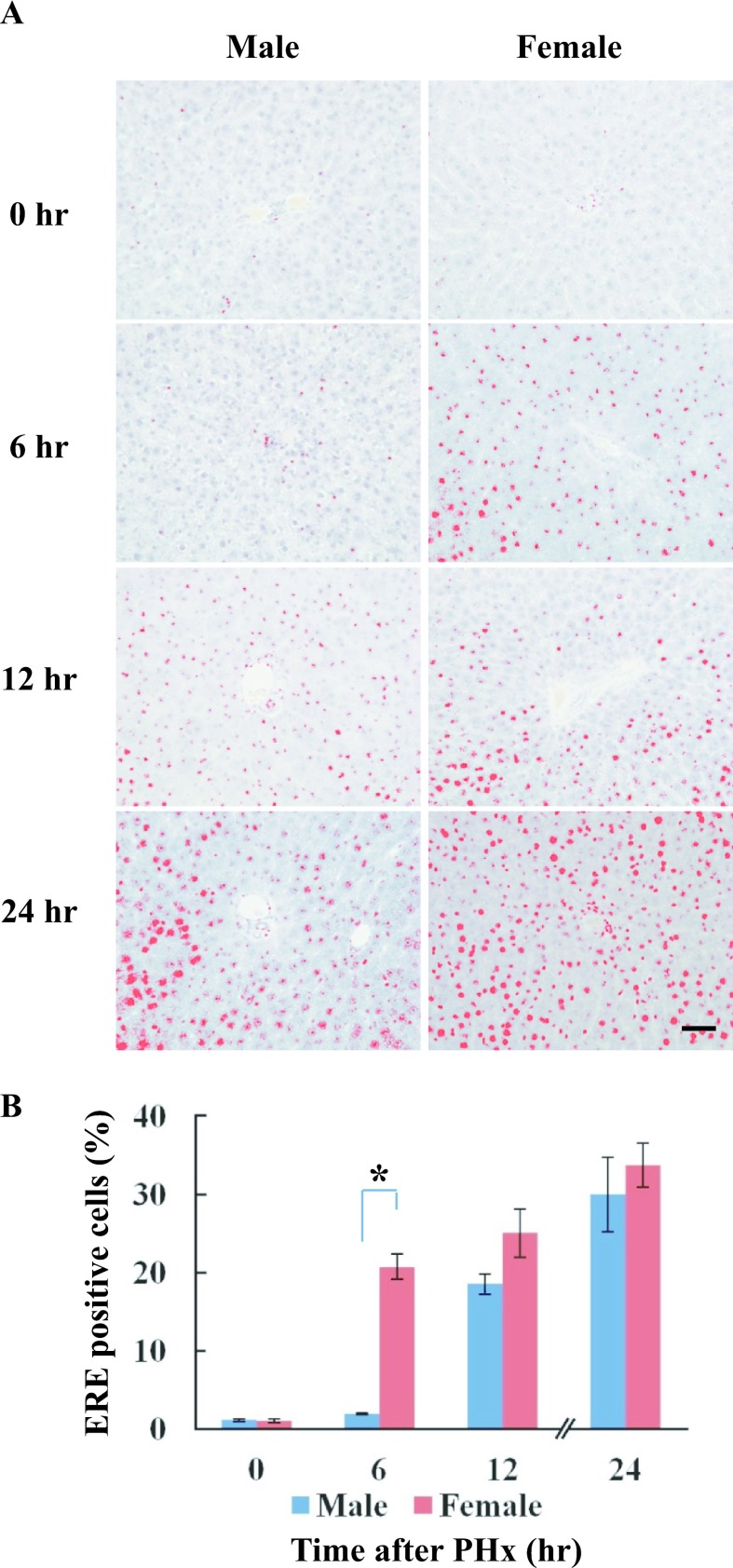
ERE binding proteins were detected from 12 hr after PHx in male rats, but were found from 6 hr in female rats. Positive staining was processed using a DAB-image analyzer (Fig. 4A). The activity of ERE binding proteins was significantly higher at 6 hr after PHx in female rats compared to male rats (Fig. 4B). Interestingly, the staining pattern and localization of ERE binding proteins by SWH were highly similar to the ERα expression in regenerating rat liver after PHx.
Fig. 4.
Localization of ERE binding proteins in male and female rats after PHx. Paraffin-embedded liver sections were used for detection of ERE binding proteins by Southwestern histochemistry at various time-points after PHx. A: The localization of ERE binding proteins was detected from 12 hr after PHx in male rats, but was found from 6 hr in female rats. Positive staining was processed using a DAB-image analyzer. B: The number of ERE-positive cells in the liver sections of male and female rats at various time-points after PHx. Blue and red columns represent male and female, respectively. Asterisk indicates statistically significant difference (*p < 0.05). Magnification ×400. Bar = 50 μm.
Proliferating cells express ERα in regenerating liver
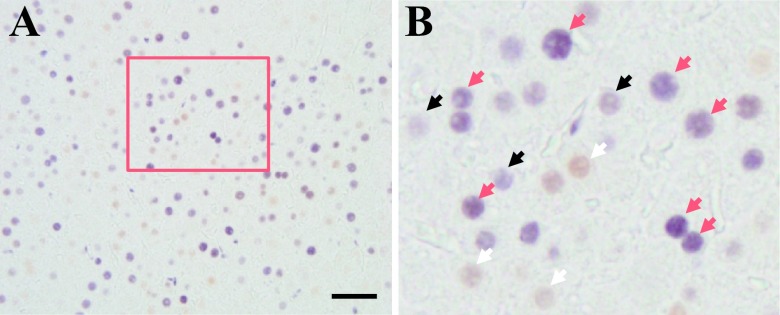
To determine whether ERα affects liver cell proliferation, double staining was performed for ERα and PCNA using immunohistochemistry. The results revealed that ERα was co-expressed with PCNA during the liver regeneration especially at the peak time-points of liver regeneration after PHx (Fig. 5A). Arrows indicated black (PCNA), white (ERα) and red (double staining), respectively (Fig. 5B).
Fig. 5.
Double staining for PCNA and ERα in male and female rat liver after PHx. The paraffin-embedded sections were analyzed by immunohistochemistry. A: ERα-positive cells were stained brown (DAB), whereas PCNA positive cells were stained purple-blue (4-Cl-1-Naphtol). Boxed area is enlarged in B. Arrows indicate white (ERα), black (PCNA) and red (double staining), respectively. Magnification ×400. Scale Bar = 50 μm.
Estrogen induces and ICI 182,780 inhibits liver regeneration in rats after PHx
Male rats were treated with E2 or E2+ICI 182,780 on the day before PHx. PCNA positive cells were found in zones 1 and 2 at 12–24 hr after PHx and the number of positive cells reached a peak at 36 hr in E2-treated male rats. On the other hand, in E2+ICI 182,780 treated male rats, PCNA-positive cells were found in zone 1 at 24–36 hr and peaked at 48 hr after PHx (Fig. 6A, B). PCNA-LI indicated that the positive cell number was significantly higher at 36 hr in E2-treated male rats compared to rats treated with E2+ICI 182,780. These results indicated that the peak number of proliferating hepatocytes at S phase in E2-treated male rats after PHx occurred 12 hr earlier than in male rats treated with E2+ICI 182,780 (Fig. 6B). ERα expression was found in zones 1 and 2 at 6–12 hr after PHx and the number of positive cells was increased in zone 3 from 24–168 hr in E2 treated male rats. However, in E2+ICI 182,780 treated male rats, ERα expression was not detected at 6–12 hr, but was found at 24–168 hr after PHx (Fig. 6C). In E2-treated male rats, ERα-positive cells were significantly higher at 6–48 hr after PHx compared to rats treated with E2+ICI 182,780 (Fig. 6D). The results of immunohistochemistry were confirmed by western blot analysis. ERα protein was continuously expressed in E2-treated male rats at all time points after PHx (Fig. 6E). Densitometry analysis revealed that the amount of the ERα protein was significantly higher at 6–48 hr after PHx in E2-treated rats compared to E2+ICI 182,780 treated male rats (Fig. 6F).
Fig. 6.
PCNA and ERα expression in E2 and E2+ICI treated male rat liver after PHx. A: Immunohistochemical detection of PCNA in E2 and E2+ICI treated male rat liver after PHx. B: PCNA-LI in E2 and E2+ICI treated rats after PHx. The number of PCNA-positive hepatocytes was counted at each time-point after PHx. Blue and red lines represent E2 or E2+ICI treated male rats, respectively. Data represent the mean ± SE of three independent experiments. C: Immunohistochemical detection of ERα in E2 and E2+ICI treated rats at various time-points after PHx. Magnification ×400. Bar = 50 μm. D: The number of ERα-positive cells in the liver sections of E2 and E2+ICI treated rats at various time-points after PHx. Blue and red lines represent E2 or E2+ICI treated male rats, respectively. E: Western blot analysis of ERα in E2 or E2+ICI treated male rats after PHx. ERα (66 kDa) and β-actin (42 kDa). F: Densitometry analysis of western blot. Asterisks indicate statistically significant differences (*p < 0.05). Data represent the mean ± SE of three independent experiments.
Localization of ERE binding proteins in rats treated with E2 or E2+ICI 182,780 after PHx
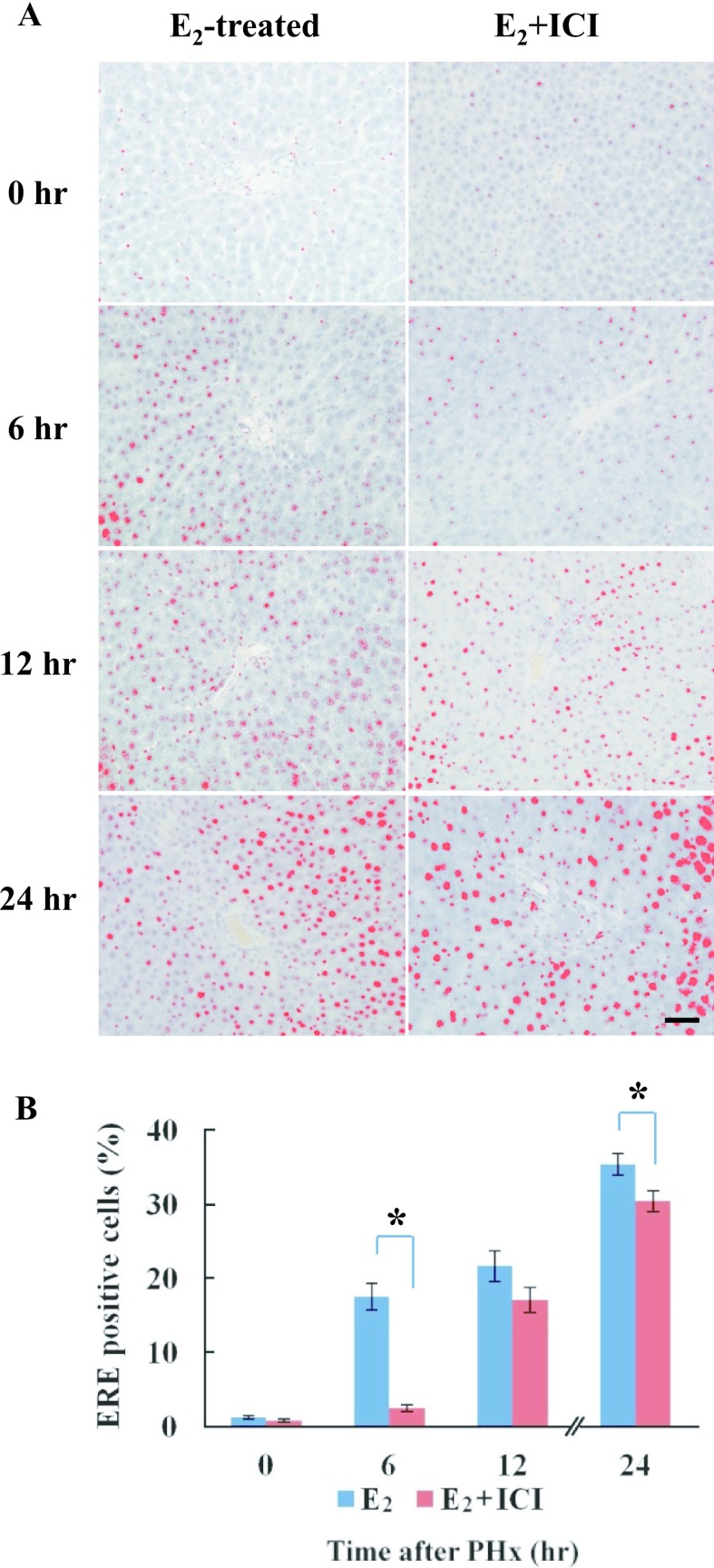
The localization of ERE binding proteins was detected from 12 hr after PHx in E2+ICI treated rats, but was found from 6 hr in rats treated with E2. Positive staining was processed by a DAB-image analyzer (Fig. 7A). The activity of ERE binding proteins was significantly higher at 6 hr after PHx in E2-treated male rats compared to rats treated with E2+ICI 182,780 (Fig. 7B). The staining pattern and localization of ERE binding proteins by SWH were highly similar to the ERα expression in regenerating rat liver after PHx.
Fig. 7.
Localization of ERE binding proteins in E2 or E2+ICI treated male rats after PHx. A: The localization of ERE binding proteins was detected from 12 hr after PHx in E2+ICI treated rats, but was found from 6 hr in rats treated with E2. Positive staining was processed using a DAB-image analyzer. B: The number of ERE positive cells in the liver sections of E2 and E2+ICI treated male rats at various time-points after PHx. Blue and red columns represent E2 or E2+ICI treated male rats, respectively. Asterisks indicate statistically significant differences (*p < 0.05). Magnification ×400. Bar = 50 μm.
IV. Discussion
In this study, we found that estrogen accelerates liver regeneration in rats after PHx. Our results indicate that the peak number of proliferating hepatocytes in S phase in female and E2-treated male rats occurred 12 hr earlier than in male rats after PHx. Although aging affects liver regeneration, the peak number of PCNA, Ki-67 and 5-bromo-2'-deoxyuridine positive cells was previously shown to occur at 48 hr in young (7–8 weeks) male rats and mice after 70% PHx [2, 25, 35]. Our data suggests that female and E2-treated male rat livers have a higher regenerative potential than livers from untreated male rats and that estrogen may have an essential role in liver regeneration after PHx.
In this study, ERα was the predominant ER type in rat liver and was found in zones 1 and 2 in male rats, and in all zones in female rats. Surprisingly, ERα expression was not detected at 6–12 hr after PHx in male rats but was observed at 24–168 hr. In female and E2-treated male rats, ERα expression was found in all zones during the liver regeneration after PHx. It has been reported that the plasma estradiol level was 2.5-fold higher in female compared to male mice [32]. Moreover, in male rats, estrogen was mainly produced in testicular Sertoli cells and was also converted from testosterone by aromatase. However, the serum testosterone level was reduced to 25–80%, but the estradiol level increased and the peak occurred in male rats at 3 days after PHx. Similar changes in plasma estradiol and testosterone levels were observed in male patients after PHx [12, 22, 24]. Alteration of ERα expression in male rats after PHx indicates that conversion from testosterone to estradiol might be important in the initiation of the liver regenerative response in male rats after PHx. Our findings suggest that significantly different expression of ERα might affect the hepatic regenerative response in male and female rats after PHx. Moreover, ERα expression was up-regulated by treatment with E2 in male rats and was inhibited in rats treated with E2+ICI 182,780 after PHx. Our findings suggest that E2-treatment accelerates liver regeneration after PHx through ERα expression.
ER-ligand complexes bind to specific consensus sequences known as EREs which are located in various target gene promoters and stimulate gene transcription [16]. Our results demonstrated that the activity of ERE binding proteins was significantly higher at 6 hr after PHx in female and E2-treated male rats compared to male and rats treated with E2+ICI. Emmerson et al. [6] reported that ERα induces ERE-mediated signaling in skin regeneration in female mice. Thus, our results suggest that ERα may exert transcriptional activation in regenerating rat liver after PHx.
ERα and PCNA were co-expressed during liver regeneration, especially at peak time points of cell proliferation after PHx. It has been reported that the PCNA gene contains half-palindromic ERE sequences (TGACC) that can bind to ERs and regulate the transcriptional activity of various genes [21, 41]. Moreover, Schultz-Norton et al. [34] reported that PCNA interacts with ERα and enhances receptor-DNA interaction in a breast cancer cell line. Taken together, these findings suggest that estrogen might be involved in the initiation of DNA synthesis through the transcriptional activation of the PCNA gene, which harbors ERE in the promoter region.
Orphan nuclear receptors such as estrogen-related receptor alpha (ERRα), ERRβ and ERRγ are also involved in estrogen signaling. Increased liver damage through impaired mitochondrial energy production has been associated with hepatocytes lacking ERRα [17]. Moreover, ERRγ is upregulated in HCC and its inhibition suppressed cancer cell proliferation [20]. Although the similar expression pattern of ERE binding proteins paralleled to ERα in regenerating rat liver, indicating that ERα may be the main receptor for estrogen pathway, it is also possible that orphan nuclear receptors may have the potential effects during liver regeneration.
In conclusion, we found that estrogen may play an important role in liver regeneration through ERα expression and that cell proliferation in male and female rats after PHx is differently affected. Taken together, these results suggest that estrogen treatment can induce liver regeneration after PHx.
V. Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 24590255 and No. 16K08471 to Y. Hishikawa).
VI. References
- 1.Adams J. C. (1981) Heavy metal intensification of DAB-based HRP reaction product. J. Histochem. Cytochem. 29; 775. [DOI] [PubMed] [Google Scholar]
- 2.An S., Soe K., Akamatsu M., Hishikawa Y. and Koji T. (2012) Accelerated proliferation of hepatocytes in rats with iron overload after partial hepatectomy. Histochem. Cell Biol. 138; 773–786. [DOI] [PubMed] [Google Scholar]
- 3.Brady C. W. (2015) Liver disease in menopause. World J. Gastroenterol. 21; 7613–7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choijookhuu N., Sato Y., Nishino T., Endo D., Hishikawa Y. and Koji T. (2012) Estrogen-dependent regulation of sodium/hydrogen exchanger-3 (NHE3) expression via estrogen receptor β in proximal colon of pregnant mice. Histochem. Cell Biol. 137; 575–587. [DOI] [PubMed] [Google Scholar]
- 5.El Marzouk S., Gahattamaneni R., Joshi S. R. and Scovell W. M. (2008) The plasticity of estrogen receptor-DNA complexes: binding affinity and specificity of estrogen receptors to estrogen response element half-sites separated by variant spacers. J. Steroid Biochem. Mol. Biol. 110; 186–195. [DOI] [PubMed] [Google Scholar]
- 6.Emmerson E., Rando G., Meda C., Campbell L., Maggi A. and Hardman M. J. (2013) Estrogen receptor-mediated signaling in female mice is locally activated in response to wounding. Mol. Cell. Endocrinol. 375; 149–156. [DOI] [PubMed] [Google Scholar]
- 7.Fausto N., Campbell J. S. and Riehle K. J. (2012) Liver regeneration. J. Hepatol. 57; 692–694. [DOI] [PubMed] [Google Scholar]
- 8.Fausto N. (2004) Liver regeneration and repair: hepatocytes, progenitor cells and stem cells. Hepatology 39; 1477–1487. [DOI] [PubMed] [Google Scholar]
- 9.Fernández-Pérez L., Guerra B., Díaz-Chico J. C. and Flores-Morales A. (2013) Estrogens regulate the hepatic effects of growth hormone, a hormonal interplay with multiple fates. Front. Endocrinol. (Lausanne) 4; 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forbes S. J. and Newsome P. N. (2016) Liver regeneration-mechanisms and models to clinical application. Nat. Rev. Gastroenterol. Hepatol. 13; 473–485. [DOI] [PubMed] [Google Scholar]
- 11.Foryst-Ludwig A. and Kintscher U. (2009) Metabolic impact of estrogen signaling through ERalpha and ERbeta. J. Steroid Biochem. Mol. Biol. 122; 74–81. [DOI] [PubMed] [Google Scholar]
- 12.Francavilla A., Gavaler J. S., Makowka L., Barone M., Mazzaferro V., Ambrosino G., Iwatsuki S., Guglielmi F. W., Dileo A. and Balestrazzi A. (1989) Estradiol and testosterone levels in patients undergoing partial hepatectomy. A possible signal for hepatic regeneration? Dig. Dis. Sci. 34; 818–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goss, R. G. (1992) Regeneration versus repair. In “Wound Healing: Biochemical and Clinical Aspects” ed. by I. K. Cohen, R. F. Diegelmann and W. J. Lindblad, W. B. Saunders Co., Philadelphia, pp. 20–39. [Google Scholar]
- 14.Harada H., Bharwani S., Pavlick K. P., Korach K. S. and Grisham M. B. (2004) Estrogen receptor-alpha, sexual dimorphism and reduced-size liver ischemia and reperfusion injury in mice. Pediatr. Res. 55; 450–456. [DOI] [PubMed] [Google Scholar]
- 15.Higgins G. M. and Anderson R. M. (1931) Experimental pathology of the liver. 1. Restoration of the liver of the white rat following partial surgical removal. Arch. Pathol. 12; 186–202. [Google Scholar]
- 16.Hishikawa Y., Damavandi E., Izumi S. and Koji T. (2003) Molecular histochemical analysis of estrogen receptor alpha and beta expressions in the mouse ovary: in situ hybridization and Southwestern histochemistry. Med. Electron Microsc. 36; 67–73. [DOI] [PubMed] [Google Scholar]
- 17.Hong E. J., Levasseur M. P., Dufour C. R., Perry M. C. and Giguere V. (2013) Loss of estrogen-related receptor α promotes hepatocarcinogenesis development via metabolic and inflammatory disturbances. Proc. Natl. Acad. Sci. U S A 110; 17975–17980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inaba K., Suzuki S., Ihara H., Sakaguchi T., Baba S., Urano T., Konno H. and Nakamura S. (2005) Sexual dimorphism in endotoxin susceptibility after partial hepatectomy in rats. J. Hepatol. 42; 719–727. [DOI] [PubMed] [Google Scholar]
- 19.Kawai T., Yokoyama Y., Kawai S., Yokoyama S., Oda K., Nagasaka T., Nagino M., Chaudry I. H. and Nimura Y. (2007) Does estrogen contribute to the hepatic regeneration following portal branch ligation in rats? Am. J. Physiol. Gastrointest. Liver Physiol. 292; G582–589. [DOI] [PubMed] [Google Scholar]
- 20.Kim J. H., Choi Y. K., Byun J. K., Kim M. K., Kang Y. N., Kim S. H., Lee S., Jang B. K. and Park K. G. (2016) Estrogen-related receptor γ is upregulated in liver cancer and its inhibition suppresses liver cancer cell proliferation via induction of p21 and p27. Exp. Mol. Med. 48; 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klinge C. M., Jernigan S. C., Mattingly K. A., Risinger K. E. and Zhang J. (2004) Estrogen response element-dependent regulation of transcriptional activation of estrogen receptors alpha and beta by coactivators and corepressors. J. Mol. Endocrinol. 33; 387–410. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi H., Yoshida S., Sun Y. J., Shirasawa N. and Naito A. (2013) Gastric estradiol-17β (E2) and liver ERα correlate with serum E2 in the cholestatic male rat. J. Endocrinol. 219; 39–49. [DOI] [PubMed] [Google Scholar]
- 23.Li Z., Tuteja G., Schug J. and Kaestner K. H. (2012) Foxa1 and Foxa2 are essential for sexual dimorphism in liver cancer. Cell 148; 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liddle C., Hollands M., Little J. M. and Farrell G. C. (1992) The effect of partial hepatectomy on serum sex steroids in humans. Hepatology 15; 623–628. [DOI] [PubMed] [Google Scholar]
- 25.Lin T., Ibrahim W., Peng C. Y., Finegold M. J. and Tsai R. Y. (2013) A novel role of nucleostemin in maintaining the genome integrity of dividing hepatocytes during mouse liver development and regeneration. Hepatology 58; 2176–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma W. L., Lai H. C., Yeh S., Cai X. and Chang C. (2014) Androgen receptor roles in hepatocellular carcinoma, cirrhosis, and hepatitis. Endocr. Relat. Cancer 21; R165–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcos R., Lopes C., Malhão F., Correia-Gomes C., Fonseca S., Lima M., Gebhardt R. and Rocha E. (2016) Stereological assessment of sexual dimorphism in the rat liver reveals differences in hepatocytes and Kupffer cells but not hepatic stellate cells. J. Anat. 228; 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meier M., Andersen K. J., Knudsen A. R., Nyengaard J. R., Hamilton-Dutoit S. and Mortensen F. V. (2016) Liver regeneration is dependent on the extent of hepatectomy. J. Surg. Res. 205; 76–84. [DOI] [PubMed] [Google Scholar]
- 29.Michalopoulos G. K. (2014) Advances in liver regeneration. Expert. Rev. Gastroenterol. Hepatol. 8; 897–907. [DOI] [PubMed] [Google Scholar]
- 30.Montagner A., Rando G., Degueurce G., Leuenberger N., Michalik L. and Wahli W. (2011) New insights into the role of PPARs. Prostaglandins Leukot. Essent. Fatty Acids 85; 235–243. [DOI] [PubMed] [Google Scholar]
- 31.Nakajima K., Shibata Y., Hishikawa Y., Suematsu T., Mori M., Fukuhara S., Koji T., Sawase T. and Ikeda T. (2014) Coexpression of ang1 and tie2 in odontoblast of mouse developing and mature teeth-a new insight into dentinogenesis. Acta Histochem. Cytochem. 47; 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oury F., Sumara O., Ferron M., Chang H., Smith C. E., Hermo L., Saurez S., Roth B. L., Ducy P. and Karsenty G. (2011) Endocrine regulation of male fertility by the skeleton. Cell 144; 796–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rando G. and Wahli W. (2011) Sex differences in nuclear receptor-regulated liver metabolic pathways. Biochim. Biophys. Acta 1812; 964–973. [DOI] [PubMed] [Google Scholar]
- 34.Schultz-Norton J. R., Gabisi V. A., Ziegler Y. S., McLeod I. X., Yates J. R. and Nardulli A. M. (2007) Interaction of estrogen receptor alpha with proliferating cell nuclear antigen. Nucleic Acids Res. 35; 5028–5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shang N., Chitsike L., Wang F., Viswakarma N., Breslin P. and Qiu W. (2016) FAK deletion accelerates liver regenartion after two-thirds partial hepatectomy. Sci. Rep. 6; 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shirendeb U., Hishikawa Y., Moriyama S., Win N., Thu M. M., Mar K. S., Khatanbaatar G., Masuzaki H. and Koji T. (2009) Human papillomavirus infection and its possible correlation with p63 expression in cervical cancer in Japan, Mongolia and Myanmar. Acta Histochem. Cytochem. 42; 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tadic S. D., Elm M. S., Li H. S., Van Londen G. J., Subbotin V. M., Whitcomb D. C. and Eagon P. K. (2002) Sex differences in hepatic expression in rat model of ethanol induced liver injury. J. Appl. Physiol. 93; 1057–1068. [DOI] [PubMed] [Google Scholar]
- 38.Thomas M. L., Xu X., Norfleet A. M. and Watson C. S. (1993) The presence of functional estrogen receptors in intestinal epithelial cells. Endocrinology 132; 426–430. [DOI] [PubMed] [Google Scholar]
- 39.Umeda M., Hiramoto M. and Imai T. (2015) Partial hepatectomy induces delayed hepatocyte proliferation and normal liver regeneration in ovariectomized mice. Clin. Exp. Gastroenterol. 8; 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villa E., Colantoni A., Grotolla A., Ferreti I., Buttafoco P., Bertani H., De Maria N. and Manenti F. (2002) Varient estrogen receptors and their role in liver disease. Mol. Cell. Endocrinol. 193; 65–69. [DOI] [PubMed] [Google Scholar]
- 41.Wang C., Yu J. and Kallen C. B. (2008) Two estrogen response element sequences near the PCNA gene are not responsible for its estrogen-enhanced expression in MCF7 cells. PLoS One 3; 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yokoyama Y., Nagino M. and Nimura Y. (2007) Which gender is better positioned in the process of liver surgery? Male or female? Surg. Today 37; 823–830. [DOI] [PubMed] [Google Scholar]
- 43.Yokoyama Y., Nimura Y., Nagino M., Bland K. I. and Chaudry I. H. (2007) Current understanding of gender dimorphism in hepatic pathophysiology. J. Surg. Res. 128; 147–156. [DOI] [PubMed] [Google Scholar]