Abstract
Key points
Ion channels are transmembrane proteins that are synthesized within the cells but need to be trafficked to the cell membrane for the channels to function.
Small‐conductance, Ca2+‐activated K+ channels (SK, KCa2) are unique subclasses of K+ channels that are regulated by Ca2+ inside the cells; they are expressed in human atrial myocytes and responsible for shaping atrial action potentials.
We have previously shown that interacting proteins of SK2 channels are important for channel trafficking to the membrane.
Using total internal reflection fluorescence (TIRF) and confocal microscopy, we studied the mechanisms by which the surface membrane localization of SK2 (KCa2.2) channels is regulated by their interacting proteins.
Understanding the mechanisms of SK channel trafficking may provide new insights into the regulation controlling the repolarization of atrial myocytes.
Abstract
The normal function of ion channels depends critically on the precise subcellular localization and the number of channel proteins on the cell surface membrane. Small‐conductance, Ca2+‐activated K+ channels (SK, KCa2) are expressed in human atrial myocytes and are responsible for shaping atrial action potentials. Understanding the mechanisms of SK channel trafficking may provide new insights into the regulation controlling the repolarization of atrial myocytes. We have previously demonstrated that the C‐ and N‐termini of SK2 channels interact with the actin‐binding proteins α‐actinin2 and filamin A, respectively. However, the roles of the interacting proteins on SK2 channel trafficking remain incompletely understood. Using total internal reflection fluorescence (TIRF) microscopy, we studied the mechanisms of surface membrane localization of SK2 (KCa2.2) channels. When SK2 channels were co‐expressed with filamin A or α‐actinin2, the membrane fluorescence intensity of SK2 channels increased significantly. We next tested the effects of primaquine and dynasore on SK2 channels expression. Treatment with primaquine significantly reduced the membrane expression of SK2 channels. In contrast, treatment with dynasore failed to alter the surface membrane expression of SK2 channels. Further investigations using constitutively active or dominant‐negative forms of Rab GTPases provided additional insights into the distinct roles of the two cytoskeletal proteins on the recycling processes of SK2 channels from endosomes. α‐Actinin2 facilitated recycling of SK2 channels from both early and recycling endosomes while filamin A probably aids the recycling of SK2 channels from recycling endosomes.
Keywords: Ca‐activated K channel, Alpha‐actinin2, Filamin A, SK channels, Ion channel interacting proteins, Rab GTPase
Key points
Ion channels are transmembrane proteins that are synthesized within the cells but need to be trafficked to the cell membrane for the channels to function.
Small‐conductance, Ca2+‐activated K+ channels (SK, KCa2) are unique subclasses of K+ channels that are regulated by Ca2+ inside the cells; they are expressed in human atrial myocytes and responsible for shaping atrial action potentials.
We have previously shown that interacting proteins of SK2 channels are important for channel trafficking to the membrane.
Using total internal reflection fluorescence (TIRF) and confocal microscopy, we studied the mechanisms by which the surface membrane localization of SK2 (KCa2.2) channels is regulated by their interacting proteins.
Understanding the mechanisms of SK channel trafficking may provide new insights into the regulation controlling the repolarization of atrial myocytes.
Abbreviations
- A.U.
arbitrary unit
- CA
constitutively active
- DN
dominant‐negative
- FLNA
filamin A
- HA
human influenza hemagglutinin
- HEK 293 cells
human embryonic kidney 293 cells
- IK,Ca
Ca2+‐activated K+ current
- SK channels
small conductance Ca2+‐activated K+ channels
- TIRF
total internal reflection fluorescence microscopy
Introduction
Cellular excitability may be regulated by controlling the number of ion channel proteins and receptors on the cell membrane. Gene regulation represents one of the key mechanisms controlling the number of channel proteins that are available within the cells over a period of time. However, for excitable cells to adapt to changes within a short period of time or on a beat‐to‐beat basis as in cardiac myocytes, a faster mechanism may need to be employed (e.g. trafficking or translocation of the membrane proteins). Specifically, anterograde trafficking brings ion channels from subcellular organelles, such as the trans‐Golgi network to the plasma membrane, while retrograde trafficking reduces the number of ion channels by translocating them from plasma membrane to lysosomes or endosomes for further degradation or recycling. Recycling following retrograde trafficking provides a dynamic means to fine‐tune the number of ion channel proteins residing on the membrane.
One critical aspect of the retrograde trafficking of ion channels includes internalization and subsequent sorting to either late endosomes for degradation or recycling endosomes for delivery of the channels to the surface membrane. Rab proteins, also known as monomeric GTPases, constitute the largest branch of the small G protein superfamily and use the guanine nucleotide‐dependent switch mechanism to regulate each of the major steps in vesicular transport (Maxfield & McGraw, 2004; McEwen et al. 2007; Zadeh et al. 2008). Indeed, different Rab proteins have been shown to be critically involved in K+ channels, transient receptor potential channels, and cystic fibrosis transmembrane conductance regulator internalization and recycling (Saxena & Kaur, 2006; McEwen et al. 2007; Pochynyuk et al. 2007; Zadeh et al. 2008).
Several isoforms of small conductance Ca2+‐activated K+ (SK or KCa2) channels have recently been identified in the heart and have been shown to underlie Ca2+‐activated K+ current (I K,Ca) in human and mouse atrial myocytes (Xu et al. 2003; Tuteja et al. 2005, 2010). Further studies show that different isoforms of SK channels form heteromultimers in native cardiac tissues (Tuteja et al. 2010). Indeed, these early findings have been supported by several subsequent studies (Sosunov et al. 2005; Ozgen et al. 2007; Diness et al. 2010, 2011; Chua et al. 2011; Skibsbye et al. 2011). Moreover, null mutation of the SK2 channels results in atrial arrhythmias and atrioventricular node dysfunction (Zhang et al. 2008; Li et al. 2009). The critical roles of these subclasses of Ca2+‐activated K+ (KCa) channels in the heart are only beginning to emerge (Zhang et al. 2015) including recent genome‐wide association analyses that have provided a genetic link between SK channel polymorphisms and lone atrial fibrillation in humans (Ellinor et al. 2010).
We have previously shown using high throughput screening that several interacting proteins of cardiac SK2 channel, namely different cytoskeletal actin‐binding proteins are involved in SK2 channel trafficking. These include α‐actinin2 (Lu et al. 2007, 2009) and filamin A (FLNA) (Rafizadeh et al. 2014), which we have shown to interact with the C‐ and N‐termini of the channel, respectively. Both α‐actinin2 and FLNA are critical for the surface membrane localization of SK2 channels. FLNA is a scaffolding cytoskeletal protein with two calponin homology domains that has been shown to be critical for trafficking of a number of membrane proteins (Petrecca et al. 2000; Sampson et al. 2003; Gravante et al. 2004; Thelin et al. 2007; Minsaas et al. 2010). Here, using a combination of live‐cell imaging and cellular electrophysiological recordings, we tested the mechanistic basis by which SK2 expression is regulated by α‐actinin2 and FLNA. We took advantage of different constitutively‐active (CA) or dominant‐negative (DN) Rab GTPases. Our findings provide new insights into the possible beat‐to‐beat regulation of SK channels in atria and may help in the development of new treatment strategies for atrial arrhythmias.
Methods
All animal care and procedures were approved by the University of California, Davis, Institutional Animal Care and Use Committee. Animal use was in accordance with National Institutes of Health and institutional guidelines. All reagents were obtained from Sigma‐Aldrich (St Louis, MO, USA) unless stated otherwise.
Plasmid construction
Human FLNA in pREP4 vector (Life Technologies, Carlsbad, CA, USA) was a kind gift from Dr. Paramita M. Ghosh (UC Davis, Davis, CA, USA). α‐Actinin2 cDNA in pcDNA3 vector was a kind gift from Dr. David Fedida (University of British Columbia, Canada; Maruoka et al. 2000; Cukovic et al. 2001). Construction of SK2 expression plasmids for heterologous expression in human embryonic kidney cells (HEK 293) was as follows: full length human cardiac SK2 cDNA was subcloned into pIRES2‐EGFP (Takara Bio USA, Inc., Mountain View, CA, USA) to obtain pSK2‐IRES‐EGFP plasmid. The CA and DN constructs of Rab proteins were kind gifts from Dr. José A. Esteban (Centro de Biología Molecular ‘Severo Ochoa’, Consejo Superior de Investigaciones Científicas, Universidad Autónoma de Madrid, Madrid 28049, Spain) and Dr. Jeffrey Martens (University of Michigan, Ann Arbor, MI 48109, USA).
To study the subcellular localization of SK2 channel subunit, modified human influenza haemagglutinin (HA) tag was inserted into the extracellular S1–S2 loop of the channel. Specifically, modified HA epitope was flanked with the ClC‐5 Cl− channel D1–D2 loop to increase accessibility and inserted in the end of the S1–S2 loop of SK2 channel subunit as described previously, in the Kv7.2/7.3 (Schwake et al. 2000) and Kv7.4 (Mencia et al. 2008) channels. The inserted amino acid sequences were NSEHYPYDVPDYAVTFEERDKCPEWNC. The epitopes are in bold. Epitope tags were generated by recombination polymerase chain reaction and verified by automated sequencing. For TIRF microscopy, the cardiac SK2 channel containing a C‐terminal tdTomato fusion protein was used in ptdTomato‐N1 vector (Takara Bio USA, Inc.) as previously described (Fig. 1 A, Rafizadeh et al. 2014).
Figure 1. Both FLNA and α‐actinin2 cytoskeletal proteins increase SK2 channel membrane expression in HEK293 cells.

A, a schematic diagram of the human SK2 channel fused with tdTomato used in the study. B, epifluorescence and TIRF images of SK2 channels expressed alone or co‐expressed with FLNA or α‐actinin2; scale bar is 20 μm. C, summary data for averaged mean fluorescence intensity from TIRF images, when SK2 channels were expressed alone or co‐expressed with FLNA or with α‐actinin2. * P < 0.05 (n = 13 cells). D, immunofluorescence confocal microscopic imaging of HEK 293 cells transfected with human cardiac SK2‐HA alone or co‐transfected with SK2‐HA and FLNA or α‐actinin2. Cells were immunostained using anti‐HA antibody followed by chicken anti‐mouse Alexa Fluor 555 secondary antibody with no permeabilization (NP). Cells were then permeabilized (P) and immunostained using anti‐HA antibody followed by rabbit anti‐mouse Alexa Fluor 633 secondary antibody. E, summary data of the fluorescence ratios (555/633) from the three groups of cells (* P < 0.05, n = 15–18 cells).
HEK 293 cells and plasmids transfection
HEK 293 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin. Cell lines were maintained at 37°C in a humidified atmosphere containing 5% CO2. All cell culture reagents were purchased from Life Technologies. HEK 293 cells were transfected using the following plasmid compositions: pSK2‐IRES‐EGFP in combination with (1) pREP4‐FLNA, or (2) pcDNA3‐α‐actinin2, or (3) pIRES‐EGFP2 empty vector (1 μg for each plasmid) using Lipofectamine 2000 (Life Technologies, Catalogue no. 11668–019) according to the manufacturer's protocol. The 1:1 ratio of the plasmids was determined to be most optimal for the SK2 current expression in our prior published studies (Lu et al. 2007, 2009; Rafizadeh et al. 2014).
Primaquine has been shown to block recycling from recycling endosome and early endosome (van Weert et al. 2000; Chung et al. 2009) while dynasore is a small molecule GTPase inhibitor that blocks dynamin‐dependent endocytosis (Hong et al. 2012). Primaquine and dynasore were purchased from Sigma‐Aldrich and the solutions were prepared fresh before use in DMSO.
Cardiac myocytes isolation
Single mouse atrial myocytes were isolated from male C57Bl/6J mice (Jackson Laboratory, Sacramento, CA, USA) at 12–14 weeks of age as per approved protocol. Briefly, mice were injected 0.1 ml heparin (1,000 units ml−1) 10 min prior to heart excision, then anaesthetized with pentobarbital i.p. (80 mg kg−1). Hearts were removed, placed into ice‐cold Tyrode solution (mmol l−1) (NaCl 140, KCl 5.4, MgCl2 1, Hepes 10 and glucose 10; pH 7.4 with NaOH), cannulated under a dissecting microscope and mounted on a Langendorff apparatus. Hearts were perfused with Tyrode solution gassed with 100% O2 at 37 °C. The perfusion pressure was monitored and the flow rate (∼2 ml min−1) was adjusted to maintain perfusion pressure at ∼80 mmHg. After 5 min, the solution was switched to 30 ml of Tyrode solution containing 13 mg collagenase (type 2, 322 units mg−1, Worthington Biochemical Corporation, Lakewood, NJ, USA) and 1 mg protease (type XIV, 4.5 units mg−1, Sigma Chemicals). After 30–45 min of enzyme perfusion, hearts were removed from the perfusion apparatus. Atrial tissue was collected into high‐K+ solution (mmol l−1) (potassium glutamate 120, KCl 20, MgCl2 1, EGTA 0.3, glucose 10 and Hepes 10, pH 7.4 with KOH), gently teased using pipettes for 3 min. Cells were allowed to rest for 2 h before use for electrophysiologic recording. This isolation procedure yields ∼80% of Ca2+‐tolerant atrial myocytes with clear striation. Electrophysiological recordings were performed within 8 h of cell isolation.
Patch‐clamp recordings
Whole‐cell I K,Ca was recorded from transfected HEK293 cells and mouse atrial myocytes at room temperature using conventional patch‐clamp techniques as previously described (Hamill et al. 1981; Xu et al. 2003). The extracellular solution contained (in mmol l−1): N‐methylglucamine (NMG) 140, KCl 4, MgCl2 1, glucose 5 and Hepes 10 (pH 7.4 using methane sulfonic acid). The internal solution consisted of (in mmol l−1): potassium gluconate 144, MgCl2 1.15, EGTA 5, Hepes 10, and CaCl2 yielding a free (unchelated) [Ca2+ i] of 500 nmol l−1 using Calcium Titration Software (Robertson & Potter, 1984) to calculate free [Ca2+ i], bound and dissociated. The pH was adjusted to 7.25 using KOH. The pipettes had resistances of 2—3 MΩ when filled with the pipette solution. Whole‐cell I K,Ca was calculated as the apamin‐sensitive component using 100 pmol l−1 of apamin. The cell capacitance was calculated by integrating the area under an uncompensated capacitive‐transient elicited by a 20 mV hyperpolarizing pulse from a holding potential of −40 mV. Whole‐cell current records were filtered at 2 kHz and sampled at 10 kHz. Liquid junction potentials were measured and corrected as previously described (Neher, 1992).
Total internal reflection fluorescence (TIRF) microscopy
Human cardiac SK2 channel fused with tdTomato fluorescent protein (Fig. 1 A) and expressed in HEK 293 cells was used for TIRF microscopy (Carl Zeiss, Oberkochen, Germany) to examine the effects of FLNA and α‐actinin2 co‐expression on the membrane expression of SK2 channels as previously described (Rafizadeh et al. 2014). The fluorescence intensity originating from the channels on the surface membrane was quantified by obtaining the background‐subtracted mean fluorescence intensity from the whole cell under the TIRF mode in arbitrary unit. All experiments were performed using the same concentration of plasmids for transfection and imaged under identical settings.
Immunofluorescence confocal microscopy of HEK 293 cells
HEK 293 cells were co‐transfected with human cardiac SK2‐HA channel together with α‐actinin or FLNA using lipofectamine (Life Technologies) as previously described (Rafizadeh et al. 2014). After blocking with 1% bovine serum albumin (Sigma, Catalogue no. A7030) and no permeabilization (NP), SK2 channels localized on the cell membrane were labelled with monoclonal anti‐HA antibody (Covance Inc., Los Angeles, CA, USA, Catalogue no. MMS‐101P, 1:100 dilution) by incubating overnight in the humidified chamber (4°C) followed by treatment with chicken anti‐mouse Alexa Fluor 555 secondary antibody (Life Technologies, Catalogue no. A‐21200, 1:500 dilution) for 1 h at room temperature. Cells were then permeabilized (P) with 0.01% Triton X (Fisher Scientific, Hampton, NH, USA) and blocked with 1% bovine serum albumin. Intracellular SK2 channels were labelled with anti‐HA antibody (Covance, 1:500 dilution) at 4°C overnight and a rabbit anti‐mouse Alexa Fluor 633 secondary antibody (Life Technologies, Catalogue no. 21427, 1:500 dilution). Coverslips were mounted using mounting medium containing 4′,6‐diamidino‐2‐phenylindole (DAPI, VectaMount, Catalogue no. H‐5000, Vector Laboratories, Inc. Burlingame, CA, USA) and imaged under Zeiss LSM 700 confocal laser scanning microscope (Carl Zeiss).
Specifically, to quantify the 555/633 fluorescence ratio, anti‐HA antibody and Alexa Fluor 555 secondary antibody were used to label SK2 channels with extracellular haemagglutinin (HA) tag (SK2‐HA) prior to permeabilization. The cells were then permeabilized using Triton‐X. Intracellular SK2 channels were then labelled with anti‐HA antibody and Alexa Fluor 633 secondary antibody. Fluorescence ratio of 555/633 then represents the ratio of SK2 channel numbers on cell membrane over those inside the cells as previously described (Rafizadeh et al. 2014). Cells transfected with CA or DN forms of Rab11 were immunolabelled in parallel and all the microscopic settings were kept the same between control and treated groups.
Data analysis
Curve fits and data analysis was performed by using Origin software (MicroCal Inc.). Current density was obtained by normalizing the current with cell capacitance. Where appropriate, pooled data are presented as means ± S.E.M. Statistical comparisons were performed using statistical package in the Origin software with P < 0.05 considered significant. For multiple comparisons, one‐way analysis of variance combined with Dunnett's test was used.
Results
Cytoskeletal proteins FLNA and α‐actinin2 increase the membrane expression of SK2 channels
The cell surface membrane expression of SK2 channels was evaluated using total internal reflection fluorescence (TIRF) microscopy. SK2 channels fused with tdTomato fluorescent protein (tdTomato, Fig. 1 A) were expressed in HEK 293 cells alone or co‐expressed with FLNA or α‐actinin2. We have previously used this construct and have shown that the fusion protein expressed normally in HEK 293 cells (Rafizadeh et al. 2014). Epifluorescence images (EPI) demonstrate the overall expression level of SK2 channels from the cells, while under TIRF mode, the fluorescence signals are selectively obtained from the channels residing on the cell surface membrane (Fig. 1 B). The fluorescence intensity originating from the channels on the surface membrane was quantified by obtaining the background‐subtracted mean fluorescence intensity from the whole cell under the TIRF mode in arbitrary units (A.U.). All experiments were performed using the same concentration of plasmids for transfection and imaged under identical settings. When SK2 were co‐expressed with either FLNA or α‐actinin2, there was a significant increase in the fluorescent intensity in the TIRF mode compared to that obtained when the channels were expressed alone (Fig. 1 B). Summary data are presented in Fig. 1 C (* P < 0.05). Indeed, the findings are consistent with our previous studies using immunofluorescence confocal microscopic imaging (Lu et al. 2007, 2009; Rafizadeh et al. 2014). The results suggest that the cytoskeletal proteins FLNA and α‐actinin2 augment forward trafficking or decrease retrograde trafficking of SK2 channels.
Complementary experiments using immunofluorescence confocal microscopy
To further support the data obtained from TIRF imaging, we performed immunofluorescence confocal microscopy in HEK 293 cells co‐transfected with human cardiac SK2 channels harbouring extracellular haemagglutinin (HA) tag in the S1–S2 linker (SK2‐HA) as used in our prior work (Rafizadeh et al. 2014) together with α‐actinin2 or FLNA. Cells were immunostained using anti‐HA antibody followed by chicken anti‐mouse Alexa Fluor 555 secondary antibody with no permeabilization (NP, Fig. 1 D and E). Cells were then permeabilized (P) and immunostained using anti‐HA antibody followed by rabbit anti‐mouse Alexa Fluor 633 secondary antibody. Consistent with data in Fig. 1 B and C, co‐expression of SK2 channels with either FLNA or α‐actinin2 resulted in a significant increase in the fluorescence ratios (555/633) compared to that obtained when the channels were expressed alone (Fig. 1 D and E, * P < 0.05).
Primaquine blocks recycling of SK2 channels from endosomes
To investigate possible mechanisms involved in the trafficking pathways of SK2 channels, primaquine and dynasore were used (Fig. 2 A and C). Primaquine has been shown to block recycling from recycling and early endosomes, (van Weert et al. 2000; Chung et al. 2009) while dynasore is known to be a specific inhibitor of dynamin which is involved in endocytosis (see schematic in Fig. 2 E) (Hong et al. 2012). Pretreatment with primaquine (50 μm for 5 h) significantly reduced surface membrane expression of SK2 channels co‐transfected with either FLNA or α‐actinin2 in HEK 293 cells compared with cells treated with vehicle (labelled as Control in Fig. 2). In contrast, pretreatment with dynasore (1 μm for 5 h) failed to alter the surface membrane expression of SK2 channels, suggesting that dynamin is not involved in the retrieval of SK2 channels from the plasma membrane. Summary data are shown in Fig. 2 B and D.
Figure 2. Primaquine blocks the recycling of SK2 channels from endosomes.
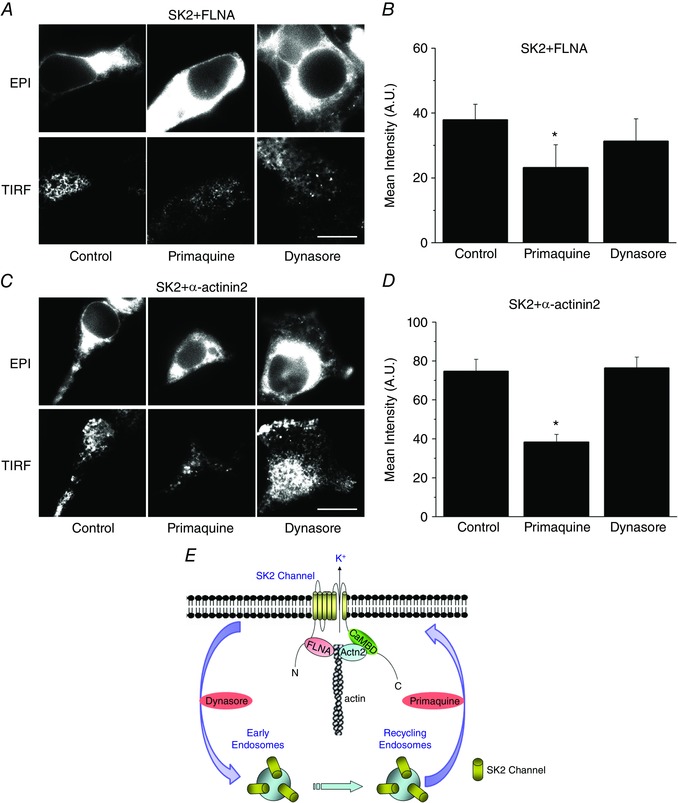
A and C, epifluorescence and TIRF images of HEK 293 cells expressing SK2 channels and FLNA (A) or α‐actinin2 (C), pretreated with vehicle, primaquine or dynasore. B and D, summary data for averaged mean fluorescence intensity from A and C, * P < 0.05 (n = 10–12 cells). Scale bar is 20 μm. E, a schematic diagram of SK2 channels recycling and endocytosis from plasma membrane to recycling or early endosomes and the known actions of primaquine and dynasore. CaMBD in the diagram refers to calmodulin binding domain within the C terminus of SK2 channels.
Subcellular mechanisms of the anterograde trafficking of SK2 channels using constitutively active (CA) or dominant‐negative (DN) forms of Rab GTPases
Rab proteins, also known as monomeric GTPases, have been shown to be involved in various trafficking pathways and provide energy for cellular organelles. To investigate the anterograde and retrograde pathways involved in SK2 channels trafficking, Rab GTPases were used as markers for different intracellular organelles. We co‐expressed SK2 channels with CA and DN forms of Rab4, 5, 8, and 11 proteins. The upper, middle and lower panels of Fig. 3 A show results obtained in HEK 293 cells expressing SK2 channel alone or SK2 channels co‐expressing with FLNA or α‐actinin2, respectively. Summary data for the mean fluorescence intensity of the corresponding panels are shown to the right (Fig. 3 B).
Figure 3. Effects of CA and DN forms of Rab4, 5, 8, and 11 on the membrane expression of SK2 channels.
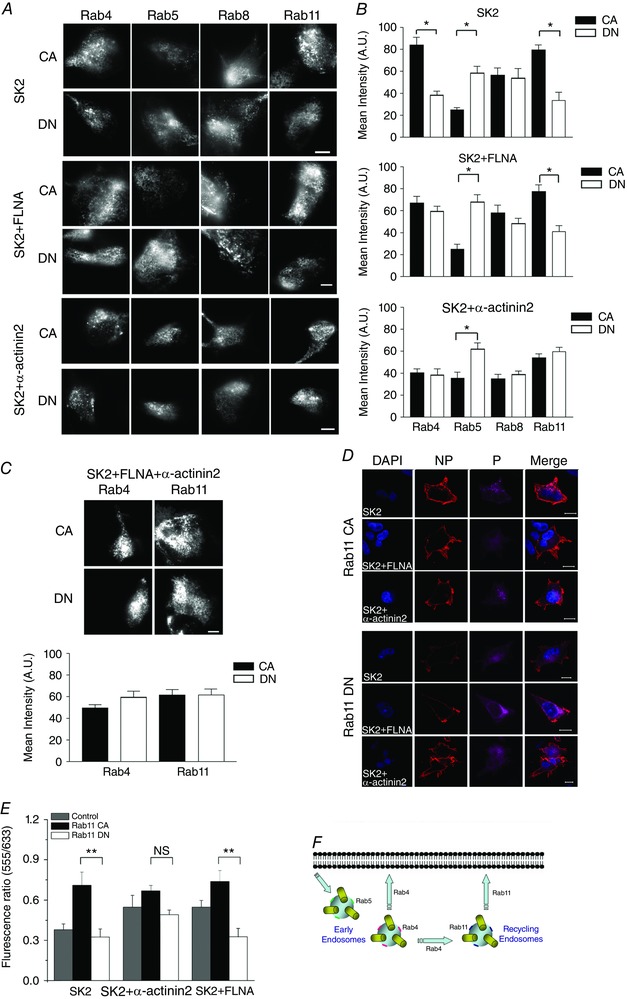
A, TIRF images of SK2 channels in HEK 293 cells when expressed alone or co‐expressed with FLNA or α‐actinin2. Different forms of Rab proteins including CA or DN forms of Rab4, 5, 8, and 11 were co‐expressed. B, corresponding summary data for averaged mean fluorescence intensity under TIRF imaging are presented. Statistical comparisons were made between CA and DN forms. C, SK2 channels were co‐expressed with FLNA and α‐actinin2, and with the CA or DN forms of Rab4 or Rab11 proteins. TIRF images and averaged mean fluorescence intensity are shown. Scale bar is 20 μm, * P < 0.05 (n = 14 cells for each group). D, immunofluorescence confocal microscopic imaging of HEK 293 cells transfected with human cardiac SK2‐HA or co‐transfected with SK2‐HA and α‐actinin2 or FLNA as shown in Fig. 1 D. Cells were further co‐transfected CA or DN forms of Rab11. Cells were immunostained as described in Fig. 1 D. E, summary data of the fluorescence ratios (555/633) from the three groups of cells (* P < 0.05, n = 15–18 cells per group). F, a schematic diagram of the roles of different Rab proteins in the recycling and endocytosis of ion channel proteins.
When SK2 channels were expressed alone, DN forms of Rab4 and Rab11 significantly reduced SK2 surface membrane expression compared to the corresponding CA forms (Fig. 3 A, top two panels). Indeed, Rab4 and Rab11 are involved in recycling through early and recycling endosomes, respectively. Therefore, our results suggest that both early and recycling endosomes are involved in the retrograde trafficking of SK2 channels. However, there were no significant differences in the SK2 surface membrane expression between cells co‐expressing the CA or DN forms of Rab8. Since Rab8 is responsible for forward trafficking from the trans‐Golgi network to the plasma membrane, the results obtained suggest that Rab8 may not be the rate limiting steps in the forward trafficking of SK2 channel to the surface membrane even when the channels were expressed alone.
Since the two different cytoskeletal interacting proteins α‐actinin2 or FLNA increase the surface membrane expression of SK2 channels (Fig. 1), we next tested the subcellular mechanisms underlying the observed effects of the two interacting proteins on SK2 channel trafficking. In contrast to cells expressing SK2 channels alone, co‐expression of SK2 channels with FLNA or α‐actinin2 resulted in significantly different outcomes (Fig. 3 A, middle and lower panels). The DN form of Rab4 protein no longer resulted in decreased SK2 cell surface expression. The results suggest that α‐actinin2 or FLNA facilitate SK2 channel recycling through early endosomes and Rab4 is no longer a rate limiting step. Nonetheless, since Rab4 also participates in the transport from early to recycling endosomes, blocking Rab4 with DN construct does not allow us to distinguish the pathway directly from early endosomes vs. that of early to recycling endosomes (see diagram in Fig. 3 F).
Moreover, α‐actinin2 and FLNA facilitate the surface membrane expression of SK2 channels via distinct intracellular organelles. Specifically, the effects of the DN or CA forms of Rab11 were dependent on whether the channels were co‐expressed with FLNA or α‐actinin2 (Fig. 3 A, middle and lower panels). Cells co‐expressing SK2 and FLNA remained affected by the DN form of Rab11. In contrast, when SK2 channels were co‐expressed with α‐actinin2, the surface membrane expression of SK2 channels was independent of the CA or DN forms of Rab11 (Fig. 3 A, two lower panels). Similarly, the surface membrane expression of SK2 channels was not affected by the CA or DN forms of Rab4 or Rab11 when the channels were co‐expressed with both FLNA and α‐actinin2 (Fig. 3 C). Taken together, the data suggest that the mechanism by which α‐actinin2 increases membrane expression of SK2 channels is likely to be via facilitated recycling from recycling endosomes as well as early endosomes as described above.
Complementary experiments were performed using SK2‐HA channels and immunofluorescence confocal microscopy as described for Fig. 1 D and E. Consistent with the data presented above, co‐expression of the DN form of Rab11 resulted in a significant decrease in the fluorescence ratios (555/633) in HEK 293 cells expressing SK2 alone or SK2 with FLNA but not in cells co‐expressing SK2 and α‐actinin2 (Fig. 3 D and E, * P < 0.05).
Subcellular mechanisms of the retrograde trafficking of SK2 channels using constitutively active (CA) or dominant‐negative (DN) forms of Rab5
We next tested the effects of retrograde trafficking on SK2 channel surface membrane expression. The effects of the DN compared to CA form of Rab5 were examined (Fig. 3 A). In contrast to the DN forms of Rab4 and Rab11, the DN form of Rab5 significantly increased rather than decreased the SK2 surface membrane expression, consistent with the notion that Rab5 is involved in endocytosis instead of the recycling process. Rab5 has been shown to be essential for clathrin‐coated vesicle endocytosis from plasma membrane to early endosomes. More importantly, the effects of the DN form of Rab5 were independent of the co‐expression with the two interacting proteins, α‐actinin2 or FLNA, suggesting that both cytoskeletal proteins increase the surface membrane expression via recycling pathways and not by decreasing the endocytosis of the SK2 channels. The findings are consistent with the effects of primaquine in Fig. 2.
Functional assessment of the effects of interacting proteins on SK2 channel current density
To further confirm the findings obtained from TIRF imaging and immunofluorescence confocal imaging, we next tested the effects of different Rab proteins using functional analyses. Using whole‐cell patch‐clamp recordings, apamin‐sensitive Ca2+‐activated K+ current (I K,Ca) density was recorded from HEK 293 cells co‐expressing SK2 and FLNA (Fig. 4 A and B) or α‐actinin2 (Fig. 4 C and D). Cells were also co‐transfected with (1) the CA or DN forms of Rab4 (Fig. 4 A and C, left and right panels, respectively), or (2) the CA or DN forms of Rab11 (Fig. 4 B and D, left and right panels, respectively). Traces shown represent currents before (continuous lines) and after 100 pm of apamin (dotted lines). Summary data for the apamin‐sensitive I K,Ca density at the test potentials of −120 and +60 mV are shown on the right.
Figure 4. Functional analyses of SK2 channel co‐expression with CA or DN forms of Rab4 and Rab11 proteins.
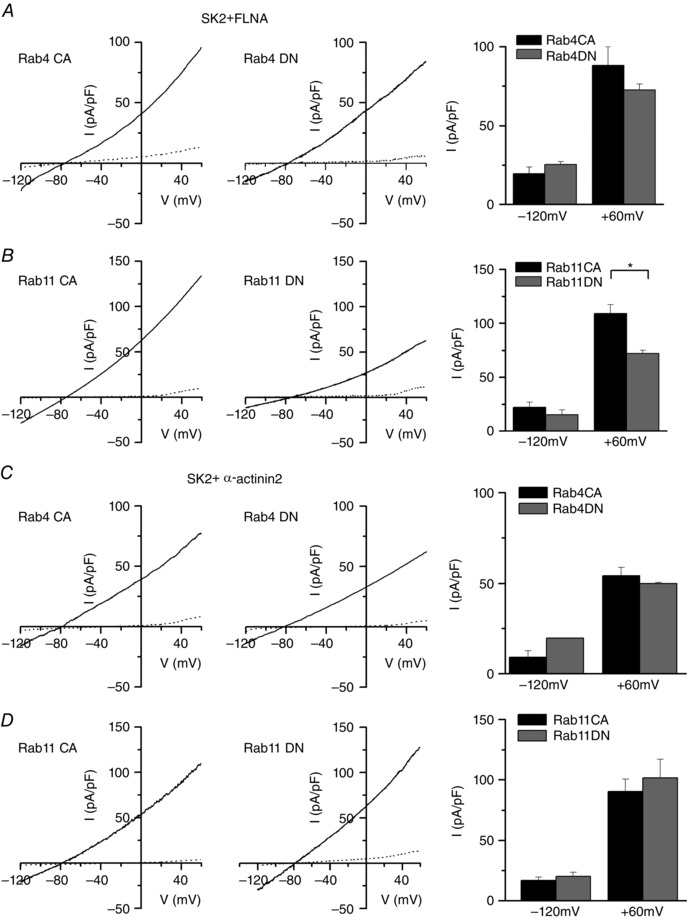
Current–voltage relationship of SK2 channels before apamin (continuous line) and after apamin (dotted line). Voltage‐ramp protocol was used to elicit the currents from a holding potential of −55 mV from −120 mV to +60 mV using a slope of 0.36 mV ms−1. A, SK2 channels and FLNA are co‐expressed with either CA or DN forms of Rab4 (Rab4CA or Rab4DN). B, SK2 channels and FLNA are co‐expressed with either CA or DN forms of Rab11 (Rab11CA or Rab11DN). C, SK2 channels and α‐actinin2 are co‐expressed with either CA or DN forms of Rab4. D, SK2 channels and α‐actinin2 are co‐expressed with either CA or DN forms of Rab11. The corresponding summary data for apamin‐sensitive I K,Ca density recorded at −120 and +60 mV were plotted in bar graphs on the right. * P < 0.05 (n = 7–8 cells for each group).
Consistent with the data obtained using TIRF imaging, there were no significant differences in current density between the CA or DN forms of Rab4 (Fig. 4 A and C). In contrast, the DN form of Rab11 significantly decreased I K,Ca density compared to the CA form when SK2 channels were co‐expressed with FLNA (Fig. 4 B) but not when SK2 channels were co‐expressed with α‐actinin2 (Fig. 4 D). These functional measurements are consistent with the findings we observed using TIRF imaging with live cells and immunofluorescence confocal microscopy (Fig. 3). Taken together, we conclude that both cytoskeletal proteins FLNA and α‐actinin2 increase SK2 channels expression, but probably through different subcellular mechanisms.
Primaquine reduces apamin‐sensitive currents in adult mouse atrial myocytes
To test the cellular mechanisms of SK channels trafficking in cardiomyocytes, we isolated adult mouse atrial cardiomyocytes and recorded apamin‐sentive I K,Ca density before and after treatment with primaquine. Treatment with primaquine for 5 h at room temperature resulted in a significant decrease in apamin‐sensitive I K,Ca density compared to control (Fig. 5 A and B). This result is consistent with our findings in heterologous system, where primaquine decreases SK2 channel expression on the membrane. The data support the critical roles of channel protein recycling in regulating the number of functional SK2 channels on the surface membrane in atrial myocytes.
Figure 5. Primaquine reduces apamin‐sensitive I K,Ca in mouse atrial myocytes.
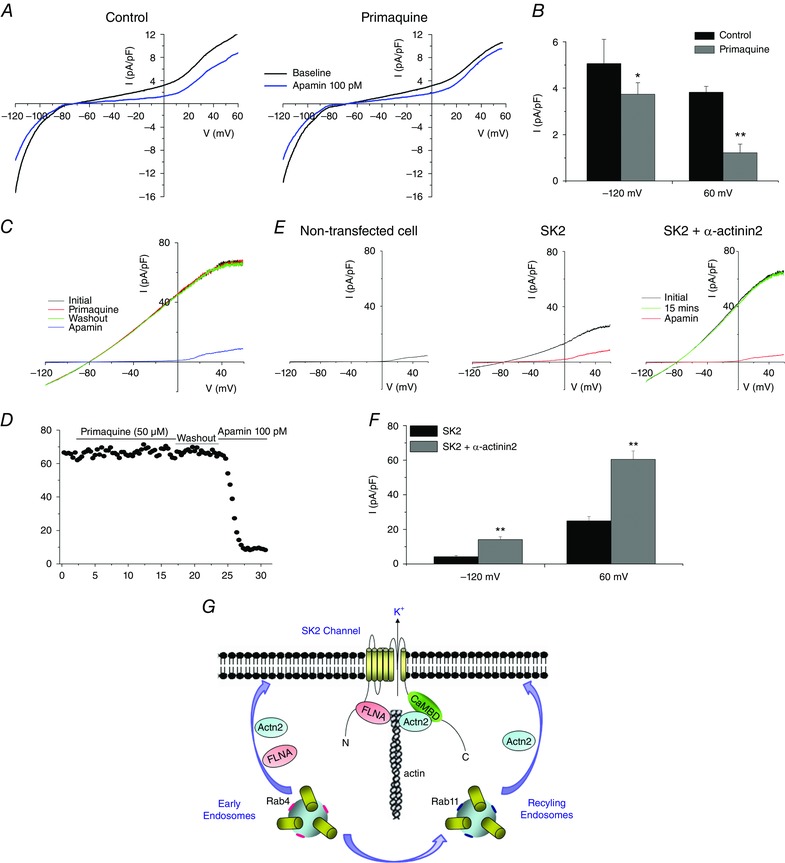
A, Ca2+‐activated K+ currents recorded from mouse atrial myocytes, before and after application of 100 pm apamin. Current–voltage relationship of Ca2+‐activated K+ currents before (black line) and after apamin (blue line). Atrial myocytes were pretreated with 50 μm primaquine or control. Currents were elicited using a voltage‐ramp protocol from a holding potential of −55 mV from −120 mV to +60 mV using a slope of 0.09 mV ms−1. B, summary data for apamin‐sensitive current density recorded at −120 and +60 mV were plotted in a bar graph. * P = 0.01, ** P < 0.0000001, (n = 6). C and D, acute effects of primaquine (50 μm) in HEK 293 cells co‐expressing SK2 and α‐actinin2. There was no change in the apamin‐sensitive currents after 15 min application of primaquine. Similar data were obtained from 5 separate cells. E, control experiments were performed by recording the currents from non‐transfected HEK293 cells. In addition, consistent with our previous findings, co‐expression of SK2 channels with α‐actinin2 significantly increased the apamin‐sensitive currents (100 pm of apamin). F, summary data showing I K,Ca density at −120 and +60 mV from HEK 293 cells expressing SK2 alone or SK2 and α‐actinin2 (n = 6, * P < 0.05). G, a schematic diagram depicting the possible steps where α‐actinin2 and FLNA may enhance the anterograde trafficking of SK2 channels to the membrane.
To determine the acute effects of primaquine, we recorded SK2 currents from HEK 293 cells at baseline, 15 min after perfusion with primaquine, after washout, and after 100 pm apamin. As shown in Fig. 5 C and D, application of premaquine for 15 min did not alter the apamin‐sensitive currents. Finally, control experiments were performed by recording SK2 currents from non‐transfected HEK293 cells (Fig. 5 E). No significant current was observed. In addition, as we have previously demonstrated (Lu et al. 2007, 2009), co‐expression of SK2 channels with α‐actinin2 significantly increased the apamin‐sensitive currents using 100 pm of apamin (Fig. 5 E and F).
Discussion
In the current study, we directly investigated the subcellular mechanisms regulating SK2 channel trafficking. We took advantage of live‐cell imaging combined with eight different forms of Rab GTPases to directly test the mechanistic basis for the enhancement of SK2 channel expression by two cytoskeletal interacting proteins, FLNA and α‐actinin2. Both FLNA and α‐actinin2 increase SK2 channel membrane expression. Treatment with primaquine significantly reduces membrane expression of SK2 channels, supporting the roles of recycling from intracellular endosomes. Indeed, similar effects from primaquine on apamin‐sensitive I K,Ca were documented in adult mouse atrial myocytes, suggesting the critical roles of channel protein recycling on the number of functional channels on the plasma membrane.
FLNA and α‐actinin2 facilitate the anterograde trafficking of SK2 channels via distinct intracellular endosomes
We further investigated the specific trafficking pathways that are involved in the SK2 channel recycling process enhanced by two cytoskeletal interacting proteins. FLNA and α‐actinin2 facilitate SK2 channel trafficking via distinct intracellular organelles. Specifically, the effects of the DN or CA forms of Rab11 were dependent on whether the channels were co‐expressed with α‐actinin2 or FLNA. Cells co‐expressing SK2 and FLNA remained affected by the DN form of Rab11. In contrast, when SK2 channels were co‐expressed with α‐actinin2 interacting protein, the surface membrane expression of SK2 channels was independent of the CA or DN forms of Rab11. The data support the notion that α‐actinin2 increases membrane expression of SK2 channels by facilitating the recycling of the channels from both early and recycling endosomes (Fig. 5 G).
Both cytoskeletal proteins increase the surface membrane expression via recycling pathways and not by decreasing endocytosis
The subcellular mechanisms of the retrograde trafficking of SK2 channels were tested using CA and DN forms of Rab5, which is known to be involved in clathrin‐coated vesicle endocytosis from plasma membrane to early endosomes. The DN form of Rab5 significantly increased SK2 surface membrane expression. However, the effects of the DN form of Rab5 were independent of the co‐expression with the two interacting proteins α‐actinin2 or FLNA, suggesting that both cytoskeletal proteins increase the surface membrane expression via recycling pathways and not by decreasing the endocytosis of the SK2 channels. The findings are consistent with the effects of primaquine in Fig. 2 A. However, acute applications of premaquine for 15 min did not alter apamin‐sensitive I K,Ca in our experiments and future studies are needed to provide additional temporal resolution for the channel recycling.
Roles of cytoskeletal proteins in SK2 channel trafficking
Ion channels are tightly regulated through their life cycle in cardiac cells: starting from their synthesis and transport in the endoplasmic reticulum, trafficking through vesicular machinery to the plasma membrane, anchoring to the membrane and later re‐uptake into endosomes, where they undergo further degradation or recycling back to the membrane (Steele & Fedida, 2014). The expression levels of ion channels on the membrane therefore are dynamically and constantly modulated.
Cardiac myocytes contain intracellular scaffolds or cytoskeletal proteins (Rogers & Gelfand, 2000; Calaghan et al. 2004). The three main components of the cytoskeletal proteins are actin microfilaments, microtubules and desmin filaments. Ion channels have been shown to physically interact with the cytoskeletal proteins allowing for the precise anchoring of the ion channel proteins within specialized subcellular microdomains or compartments. Therefore, alterations in the cytoskeletal proteins can directly affect ion channel function (Johnson, 1999). Indeed, mutations in cytoskeletal adaptor proteins have been shown to be linked in cardiac arrhythmias in human, e.g. mutations in ankyrin B can lead to mistrafficking of Na+ channel responsible for LQT syndrome (Chauhan et al. 2000; Le Scouarnec et al. 2008). In addition, the cytoskeletal proteins actin and filamin enhance both the voltage‐gated and voltage‐independent K+ channels, Kv4.2 and Kir2.1, respectively, by direct protein interaction with the channels (Petrecca et al. 2000; Sampson et al. 2003).
We have previously demonstrated that cytoskeletal proteins FLNA and α‐actinin2 directly interact with SK2 channels and are crucial for SK2 channel membrane localization (Lu et al. 2007, 2009; Rafizadeh et al. 2014). Indeed, we have demonstrated that direct protein–protein interaction is required in the observed enhancement of membrane localization (Lu et al. 2009). However, the mechanisms by which cytoskeletal proteins facilitate SK2 channel trafficking remain unclear.
We employed live‐cell TIRF microscopic imaging to address the regulatory mechanisms of SK2 channel trafficking to and from the plasma membrane in living cells. The results provide new insights into the regulation of SK2 channel trafficking by the cytoskeletal proteins FLNA and α‐actinin2, involving distinct recycling pathways. Compared to methods such as immunocytochemistry and biochemical analyses, live‐cell imaging provides direct measurement and quantification, and less dependency on antibodies and other intermediate steps. Importantly, we provide the evidence that FLNA and α‐actinin2 facilitate SK2 recycling through different pathways by interfering with different Rab GTPases (Fig. 5 G). The current study provides new insights into the mechanisms by which SK2 channel trafficking is being regulated, and may shed light on future therapeutic avenues for SK2 targeting and cardiomyocyte excitability (Balut et al. 2012). Future experiments in cardiomyocytes are required using gene silencing or genetically targeted animal models to directly establish that similar mechanisms are involved in the regulation of SK2 channel expression in cardiac myocytes.
Additional information
Competing interests
The authors declare no competing interests.
Author contributions
Z.Z., H.A.L., S.P., W.W., H.J.K., L.L., A.A.K., X‐D.Z., E.N.Y. and N.C. contributed towards the conception and design of the experiments. Z.Z., H.A.L., S.P., W.W., S.R., H.J.K., W.X., L.L., V.C.L., A.A.K., X‐D.Z., E.N.Y. and N.C. contributed towards the collection, assembly, analysis and interpretation of data. All authors assisted in the preparation and revision of the article critically for important intellectual content. All authors approved the final version of the manuscript and all authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by NIH grants (R01 HL085727 and R01 HL085844 to N.C., R01 DC003826, R01 DC007592, and R01 DC010386 to E.N.Y., and R01 HL079071 to AAK), the VA Merit Review Grant I01 BX000576 (N.C.), and American Heart Association Western States Affiliate Beginning Grant‐in‐Aid (14BGIA18870087 to X‐D.Z.).
Acknowledgements
The authors would like to sincerely thank Dr. José A. Esteban (Centro de Biología Molecular ‘Severo Ochoa’, Consejo Superior de Investigaciones Científicas, Universidad Autónoma de Madrid, Madrid 28049, Spain) and Dr. Jeffrey Martens (University of Michigan, Ann Arbor, MI 48109, USA) for graciously sharing the constitutively active and dominant negative constructs of Rab proteins. Human filamin A in pREP4 vector (Life Technologies, Grand Island, NY, USA) was a kind gift from Dr. Paramita M. Ghosh (UC Davis, Davis, CA, USA). α‐Actinin2 cDNA in pcDNA3 vector was a kind gift from Dr. David Fedida (University of British Columbia, Canada). Dr. Chiamvimonvat and Dr. Knowlton are part‐time staff physicians at VA Northern California Health Care System, Mather, CA. Dr. Chiamvimonvat is the holder of the Roger Tatarian Endowed Professor in Cardiovascular Medicine at University of California, Davis.
Z. Zhang and H. A. Ledford contributed equally to this work.
References
- Balut CM, Hamilton KL & Devor DC (2012). Trafficking of intermediate (KCa3.1) and small (KCa2.x) conductance, Ca2+‐activated K+ channels: a novel target for medicinal chemistry efforts? ChemMedChem 7, 1741–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calaghan SC, Le Guennec JY & White E (2004). Cytoskeletal modulation of electrical and mechanical activity in cardiac myocytes. Prog Biophys Mol Biol 84, 29–59. [DOI] [PubMed] [Google Scholar]
- Chauhan VS, Tuvia S, Buhusi M, Bennett V & Grant AO (2000). Abnormal cardiac Na+ channel properties and QT heart rate adaptation in neonatal ankyrinB knockout mice. Circ Res 86, 441–447. [DOI] [PubMed] [Google Scholar]
- Chua SK, Chang PC, Maruyama M, Turker I, Shinohara T, Shen MJ, Chen Z, Shen C, Rubart‐von der Lohe M, Lopshire JC, Ogawa M, Weiss JN, Lin SF, Ai T & Chen PS (2011). Small‐conductance calcium‐activated potassium channel and recurrent ventricular fibrillation in failing rabbit ventricles. Circ Res 108, 971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Qian X, Ehlers M, Jan YN & Jan LY (2009). Neuronal activity regulates phosphorylation‐dependent surface delivery of G protein‐activated inwardly rectifying potassium channels. Proc Natl Acad Sci USA 106, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukovic D, Lu GW, Wible B, Steele DF & Fedida D (2001). A discrete amino terminal domain of Kv1.5 and Kv1.4 potassium channels interacts with the spectrin repeats of α‐actinin‐2. FEBS Lett 498, 87–92. [DOI] [PubMed] [Google Scholar]
- Diness JG, Skibsbye L, Jespersen T, Bartels ED, Sorensen US, Hansen RS & Grunnet M (2011). Effects on atrial fibrillation in aged hypertensive rats by Ca2+‐activated K+ channel inhibition. Hypertension 57, 1129–1135. [DOI] [PubMed] [Google Scholar]
- Diness JG, Sorensen US, Nissen JD, Al‐Shahib B, Jespersen T, Grunnet M & Hansen RS (2010). Inhibition of small‐conductance Ca2+‐activated K+ channels terminates and protects against atrial fibrillation. Circ Arrhythm Electrophysiol 3, 380–390. [DOI] [PubMed] [Google Scholar]
- Ellinor PT, Lunetta KL, Glazer NL, Pfeufer A, Alonso A, Chung MK, Sinner MF, de Bakker PI, Mueller M, Lubitz SA, Fox E, Darbar D, Smith NL, Smith JD, Schnabel RB, Soliman EZ, Rice KM, Van Wagoner DR, Beckmann BM, van Noord C, Wang K, Ehret GB, Rotter JI, Hazen SL, Steinbeck G, Smith AV, Launer LJ, Harris TB, Makino S, Nelis M, Milan DJ, Perz S, Esko T, Kottgen A, Moebus S, Newton‐Cheh C, Li M, Mohlenkamp S, Wang TJ, Kao WH, Vasan RS, Nothen MM, MacRae CA, Stricker BH, Hofman A, Uitterlinden AG, Levy D, Boerwinkle E, Metspalu A, Topol EJ, Chakravarti A, Gudnason V, Psaty BM, Roden DM, Meitinger T, Wichmann HE, Witteman JC, Barnard J, Arking DE, Benjamin EJ, Heckbert SR & Kaab S (2010). Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet 42, 240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravante B, Barbuti A, Milanesi R, Zappi I, Viscomi C & DiFrancesco D (2004). Interaction of the pacemaker channel HCN1 with filamin A. J Biol Chem 279, 43847–43853. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B & Sigworth FJ (1981). Improved patch‐clamp techniques for high‐resolution current recording from cells and cell‐free membrane patches. Pflugers Arch 391, 85–100. [DOI] [PubMed] [Google Scholar]
- Hong TT, Smyth JW, Chu KY, Vogan JM, Fong TS, Jensen BC, Fang K, Halushka MK, Russell SD, Colecraft H, Hoopes CW, Ocorr K, Chi NC & Shaw RM (2012). BIN1 is reduced and Cav1.2 trafficking is impaired in human failing cardiomyocytes. Heart Rhythm 9, 812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD (1999). The company they keep: ion channels and their intracellular regulatory partners. Adv Second Messenger Phosphoprotein Res 33, 203–228. [DOI] [PubMed] [Google Scholar]
- Le Scouarnec S, Bhasin N, Vieyres C, Hund TJ, Cunha SR, Koval O, Marionneau C, Chen B, Wu Y, Demolombe S, Song LS, Le Marec H, Probst V, Schott JJ, Anderson ME & Mohler PJ (2008). Dysfunction in ankyrin‐B‐dependent ion channel and transporter targeting causes human sinus node disease. Proc Natl Acad Sci USA 105, 15617–15622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Timofeyev V, Tuteja D, Xu D, Lu L, Zhang Q, Zhang Z, Singapuri A, Albert TR, Rajagopal AV, Bond CT, Periasamy M, Adelman J & Chiamvimonvat N (2009). Ablation of a Ca2+‐activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. J Physiol 587, 1087–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Timofeyev V, Li N, Rafizadeh S, Singapuri A, Harris TR & Chiamvimonvat N (2009). α‐Actinin2 cytoskeletal protein is required for the functional membrane localization of a Ca2+‐activated K+ channel (SK2 channel). Proc Natl Acad Sci USA 106, 18402–18407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Zhang Q, Timofeyev V, Zhang Z, Young JN, Shin HS, Knowlton AA & Chiamvimonvat N (2007). Molecular coupling of a Ca2+‐activated K+ channel to L‐type Ca2+ channels via α‐actinin2. Circ Res 100, 112–120. [DOI] [PubMed] [Google Scholar]
- McEwen DP, Schumacher SM, Li Q, Benson MD, Iniguez‐Lluhi JA, Van Genderen KM & Martens JR (2007). Rab‐GTPase‐dependent endocytic recycling of Kv1.5 in atrial myocytes. J Biol Chem 282, 29612–29620. [DOI] [PubMed] [Google Scholar]
- Maruoka ND, Steele DF, Au BP, Dan P, Zhang X, Moore ED & Fedida D (2000). alpha‐actinin‐2 couples to cardiac Kv1.5 channels, regulating current density and channel localization in HEK cells. FEBS Lett 473, 188–194. [DOI] [PubMed] [Google Scholar]
- Maxfield FR & McGraw TE (2004). Endocytic recycling. Nat Rev Mol Cell Biol 5, 121–132. [DOI] [PubMed] [Google Scholar]
- Mencia A, Gonzalez‐Nieto D, Modamio‐Hoybjor S, Etxeberria A, Aranguez G, Salvador N, Del Castillo I, Villarroel A, Moreno F, Barrio L & Moreno‐Pelayo MA (2008). A novel KCNQ4 pore‐region mutation (p.G296S) causes deafness by impairing cell‐surface channel expression. Hum Genet 123, 41–53. [DOI] [PubMed] [Google Scholar]
- Minsaas L, Planaguma J, Madziva M, Krakstad BF, Masia‐Balague M, Katz AA & Aragay AM (2010). Filamin a binds to CCR2B and regulates its internalization. PLoS One 5, e12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E (1992). Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol 207, 123–131. [DOI] [PubMed] [Google Scholar]
- Ozgen N, Dun W, Sosunov EA, Anyukhovsky EP, Hirose M, Duffy HS, Boyden PA & Rosen MR (2007). Early electrical remodeling in rabbit pulmonary vein results from trafficking of intracellular SK2 channels to membrane sites. Cardiovasc Res 75, 758–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrecca K, Miller DM & Shrier A (2000). Localization and enhanced current density of the Kv4.2 potassium channel by interaction with the actin‐binding protein filamin. J Neurosci 20, 8736–8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochynyuk O, Stockand JD & Staruschenko A (2007). Ion channel regulation by Ras, Rho, and Rab small GTPases. Exp Biol Med (Maywood) 232, 1258–1265. [DOI] [PubMed] [Google Scholar]
- Rafizadeh S, Zhang Z, Woltz RL, Kim HJ, Myers RE, Lu L, Tuteja D, Singapuri A, Bigdeli AA, Harchache SB, Knowlton AA, Yarov‐Yarovoy V, Yamoah EN & Chiamvimonvat N (2014). Functional interaction with filamin A and intracellular Ca2+ enhance the surface membrane expression of a small‐conductance Ca2+‐activated K+ (SK2) channel. Proc Natl Acad Sci USA 111, 9989–9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S & Potter JD (1984). The regulation of free Ca2+ ion concentration by metal chelators In Methods in Pharmacology, ed Schwartz A, pp. 63–75. Plenum Press, New York. [Google Scholar]
- Rogers SL & Gelfand VI (2000). Membrane trafficking, organelle transport, and the cytoskeleton. Curr Opin Cell Biol 12, 57–62. [DOI] [PubMed] [Google Scholar]
- Sampson LJ, Leyland ML & Dart C (2003). Direct interaction between the actin‐binding protein filamin‐A and the inwardly rectifying potassium channel, Kir2.1. J Biol Chem 278, 41988–41997. [DOI] [PubMed] [Google Scholar]
- Saxena SK & Kaur S (2006). Regulation of epithelial ion channels by Rab GTPases. Biochem Biophys Res Commun 351, 582–587. [DOI] [PubMed] [Google Scholar]
- Schwake M, Pusch M, Kharkovets T & Jentsch TJ (2000). Surface expression and single channel properties of KCNQ2/KCNQ3, M‐type K+ channels involved in epilepsy. J Biol Chem 275, 13343–13348. [DOI] [PubMed] [Google Scholar]
- Skibsbye L, Diness JG, Sorensen US, Hansen RS & Grunnet M (2011). The duration of pacing‐induced atrial fibrillation is reduced in vivo by inhibition of small conductance Ca2+‐activated K+ channels. J Cardiovasc Pharmacol 57, 672–681. [DOI] [PubMed] [Google Scholar]
- Sosunov EA, Anyukhovsky EP, Hefer D, Rosen TS, Danilo P Jr, Janse MJ & Rosen MR (2005). Region‐specific, pacing‐induced changes in repolarization in rabbit atrium: an example of sensitivity to the rare. Cardiovasc Res 67, 274–282. [DOI] [PubMed] [Google Scholar]
- Steele DF & Fedida D (2014). Cytoskeletal roles in cardiac ion channel expression. Biochim Biophys Acta 1838, 665–673. [DOI] [PubMed] [Google Scholar]
- Thelin WR, Chen Y, Gentzsch M, Kreda SM, Sallee JL, Scarlett CO, Borchers CH, Jacobson K, Stutts MJ & Milgram SL (2007). Direct interaction with filamins modulates the stability and plasma membrane expression of CFTR. J Clin Invest 117, 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja D, Rafizadeh S, Timofeyev V, Wang S, Zhang Z, Li N, Mateo RK, Singapuri A, Young JN, Knowlton AA & Chiamvimonvat N (2010). Cardiac small conductance Ca2+‐activated K+ channel subunits form heteromultimers via the coiled‐coil domains in the C termini of the channels. Circ Res 107, 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja D, Xu D, Timofeyev V, Lu L, Sharma D, Zhang Z, Xu Y, Nie L, Vazquez AE, Young JN, Glatter KA & Chiamvimonvat N (2005). Differential expression of small‐conductance Ca2+‐activated K+ channels SK1, SK2, and SK3 in mouse atrial and ventricular myocytes. Am J Physiol Heart Circ Physiol 289, H2714–H2723. [DOI] [PubMed] [Google Scholar]
- van Weert AW, Geuze HJ, Groothuis B & Stoorvogel W (2000). Primaquine interferes with membrane recycling from endosomes to the plasma membrane through a direct interaction with endosomes which does not involve neutralisation of endosomal pH nor osmotic swelling of endosomes. Eur J Cell Biol 79, 394–399. [DOI] [PubMed] [Google Scholar]
- Xu Y, Tuteja D, Zhang Z, Xu D, Zhang Y, Rodriguez J, Nie L, Tuxson HR, Young JN, Glatter KA, Vazquez AE, Yamoah EN & Chiamvimonvat N (2003). Molecular identification and functional roles of a Ca2+‐activated K+ channel in human and mouse hearts. J Biol Chem 278, 49085–49094. [DOI] [PubMed] [Google Scholar]
- Zadeh AD, Xu H, Loewen ME, Noble GP, Steele DF & Fedida D (2008). Internalized Kv1.5 traffics via Rab‐dependent pathways. J Physiol 586, 4793–4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Timofeyev V, Lu L, Li N, Singapuri A, Long MK, Bond CT, Adelman JP & Chiamvimonvat N (2008). Functional roles of a Ca2+‐activated K+ channel in atrioventricular nodes. Circ Res 102, 465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XD, Lieu DK & Chiamvimonvat N (2015). Small‐conductance Ca2+‐activated K+ channels and cardiac arrhythmias. Heart Rhythm 12, 1845–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]


