Abstract
AIM
To determine whether oral administration of Bifidobacterium infantis CGMCC313-2 (B. infantis CGMCC313-2) inhibits allergen-induced airway inflammation and food allergies in a mouse model.
METHODS
Ovalbumin (OVA)-induced allergic asthma and β-lactoglobulin-induced food allergy mouse models were used in this study. Following oral administration of B. infantis CGMCC313-2 during or after allergen sensitization, histopathologic changes in the lung and intestine were evaluated by hematoxylin and eosin (HE) staining. In the allergic asthma mouse model, we evaluated the proportion of lung-infiltrating inflammatory cells. OVA-specific IgE and IgG1 levels in serum and cytokine levels in bronchoalveolar lavage fluid (BALF) were also assessed. In the food allergy mouse model, the levels of total IgE and cytokines in serum were measured.
RESULTS
Oral administration of B. infantis CGMCC313-2 during or after allergen sensitization suppressed allergic inflammation in lung and intestinal tissues, while the proportion of infiltrating inflammatory cells was significantly decreased in the BALF of allergic asthma mice. Moreover, B. infantis CGMCC313-2 decreased the serum levels of total IgE in food allergy mice, and reductions in IgE and IgG1 were also observed in OVA-induced allergic asthma mice. The expression of interleukin-4 (IL-4) and IL-13 in both serum and BALF was suppressed following the administration of B. infantis CGMCC313-2, while an effect on serum IL-10 levels was not observed.
CONCLUSION
B. infantis CGMCC313-2 inhibits the secretion of allergen-induced IgE, IL-4 and IL-13, and attenuates allergic inflammation.
Keywords: Bifidobacterium infantis, Asthma, Allergy, Ovalbumin, β-lactoglobulin
Core tip: Bifidobacterium infantis CGMCC313-2 significantly decreased the serum concentration of IgE and IgG1 in asthma and food allergy mouse models. The number of infiltrating cells in bronchoalveolar lavage fluid was reduced, and eosinophil infiltration in lungs was relieved by B. infantis CGMCC313-2 in allergic asthma mice. Body weight was regained in food allergy mice, and intestinal inflammation was attenuated by B. infantis CGMCC313-2. Following administration of B. infantis CGMCC313-2, the concentrations of interleukin-4 (IL-4) and IL-13 decreased in both allergic asthma and food allergy mice.
INTRODUCTION
The prevalence of asthma, food allergies, eczema, and allergic rhinitis in developed countries has increased over the last three decades. In China, childhood allergic diseases are generally lower than those in Western countries; however, the prevalence of asthma, allergic rhinitis, and eczema in children has increased markedly during the past two decades[1-4]. A number of environmental factors including air pollution, cigarette smoking, and allergen exposure have been proposed to explain the changes in the prevalence of allergic diseases; however, no major risk factors have been identified. A common explanation for the increased incidence rates of childhood allergy and asthma observed in industrialized countries during the past few decades is the “hygiene hypothesis,” which states that a lack of early childhood exposure to infectious agents, symbiotic microorganisms, and parasites increase susceptibility to allergic diseases by suppressing the natural development of the immune system[5,6]. Recent epidemiological and experimental studies have both renewed the “hygiene hypothesis” and extended it to a more specific theorem, the “microflora hypothesis”[6-8].
Probiotics are live microorganisms that confer a health benefit to the host when administered in adequate numbers[9]. In other words, ingested probiotics can modify microbial flora, which benefit the host[10,11]. Previous studies have shown that probiotics can reduce allergic diseases by modifying the immune system of the host. Some probiotic genera including Lactobacilli and Bifidobacteria are intensively investigated as novel alternative options for the management of allergic diseases including asthma and food allergy[12,13].
Experimental studies have shown that probiotics have strain-specific effects. In the present study, mice received nebulized ovalbumin and were used as an asthma model, while mice fed with β-lactoglobulin were used as a food allergy model (details in Materials and Methods). The effects of Bifidobacterium infantis CGMCC313-2, which is extensively used as a probiotic drug in China, were investigated in these two mouse models during (prevention) or after allergen sensitization (pre-treatment).
MATERIALS AND METHODS
Mice
Male BALB/c mice aged 6-8 wk were obtained from the Laboratory Animal Center of the Fourth Military Medical University. All experimental procedures involving animals were approved by the Ethics Committee for Animal Studies of the Fourth Military Medical University and performed in accordance with their guidelines (approval ID: 20150902).
Probiotic bacterial preparations
Bifidobacterium infantis CGMCC313-02 powder (Kexing Biotech Company Limited, Shenzhen, China) was stored at -20 °C. Solutions were prepared using normal saline only or normal saline plus B. infantis CGMCC313-2. B. infantis CGMCC313-2 preparations were adjusted at concentrations of 5 × 1010 colony-forming units (CFU)/mL.
Mouse model of OVA-induced allergic asthma
The mice were divided into four experimental groups, and each group consisted of 10 mice. Four groups of mice were treated as follows: (Group 1) the normal control group received normal saline plus 1.5 mg alum intraperitoneally. The mice were placed in an atomizing chamber (20 cm × 20 cm × 35 cm), and 8 mL saline was administered by nebulization. The mice were incubated for 30 min each time for 7 continuous days; (Group 2) the positive group (as shown in Figure 1A) received 100 μg ovalbumin (OVA) (Sigma, Buchs, Switzerland) plus 1.5 mg alum intraperitoneally from Day 0 to Day 7, and subsequently challenged with 1% OVA inhaled by nebulizer from Day 21 to Day 28; and (Group 3) the prevention and (Group 4) pre-treatment groups received 100 μg OVA plus alum intraperitoneally and 1% OVA inhaled, and were fed 0.2 mL/d (5 × 1010 CFU/mL) of B. infantis CGMCC313-2 from Day 0 to Day 14 (prevention group, as shown in Figure 1B), or from Day 15 to Day 28 (pre-treatment group, as shown in Figure 1C). Serum and BALF samples were collected from mice at sacrifice on Day 29.
Figure 1.
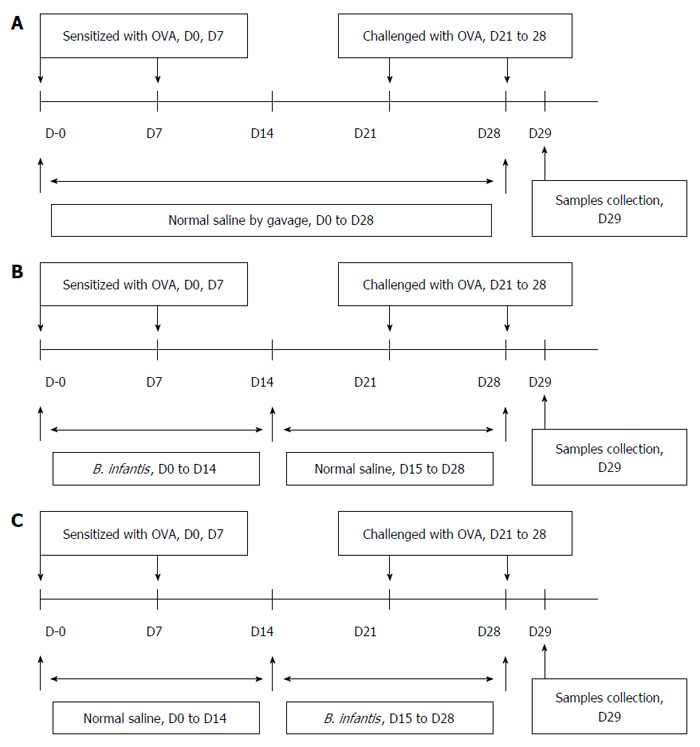
Protocols used for establishment of the mouse models. A: Allergic asthma; B: Prevention; C: Pre-treatment of OVA-induced airway allergy with B. infantis CGMCC313-2.
Mouse model of β-lactoglobulin-induced food allergy
The mice were divided into four experimental groups, and each group consisted of 10 mice. Four groups of mice were treated as follows: (Group 1) the normal control group was fed normal saline (2 mL each time for 7 continuous days); (Group 2) the positive group (as shown in Figure 2A) received the mixture of 20 mg β-lactoglobulin (BLG) (Sigma, Buchs, Switzerland) and 10 μg CTX (Cholera toxin, List Biological Laboratories, Campbell, CA, United States) on days 0, 7, and 14 by intragastric gavage (2 mL of the mixture was used each time). Subsequently, the mice were challenged with 100 mg BLG (3 mL) on day 21 by intragastric gavage; and (Group 3) the prevention and (Group 4) pre-treatment groups received 20 mg BLG plus 10 μg CTX and challenged with 100 mg BLG by intragastric gavage, and were fed 0.2 mL/d (5 × 1010 CFU/mL) of B. infantis CGMCC313-2 from Day 0 to Day 21 (prevention group, as shown in Figure 2B), or from Day 22 to Day 28 (pre-treatment group, as shown in Figure 2C). Body weight was measured on Day 29, and then serum samples were collected after the mice were sacrificed.
Figure 2.
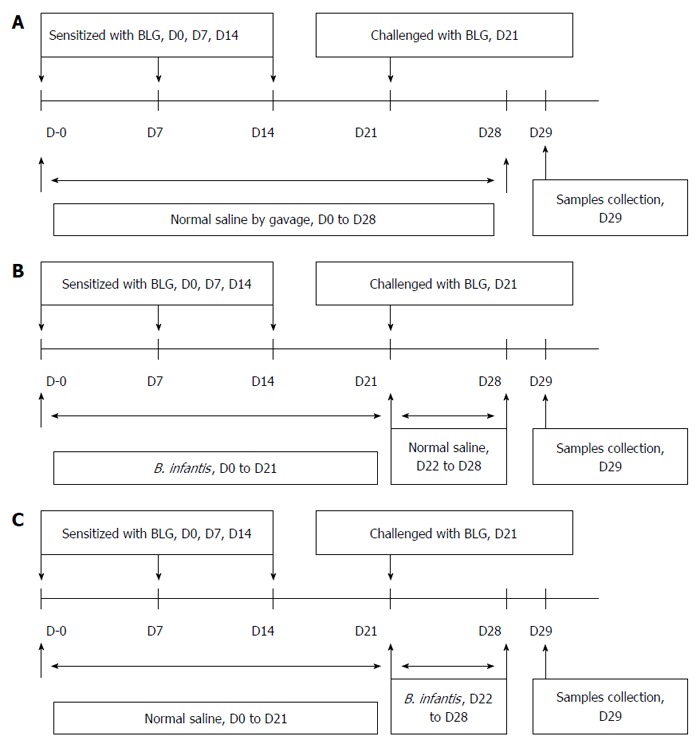
Protocols used for establishment of the mouse models. A: Food allergy; B: Prevention; C: Pre-treatment of β-lactoglobulin-induced food allergies with B. infantis CGMCC313-2.
Measurement of serum immunoglobulins
Serum samples from the mouse model of OVA-induced allergic asthma were assayed for OVA-specific IgE and IgG1 levels using ELISA kits (Chondrex Inc., United States) following the manufacturer’s protocol. The serum level of total IgE was assayed in BLG-induced food allergy mice using ELISA kits (Chondrex, Inc., United States).
Measurement of cytokines
IL-4, IL-10, IL-13, and IFN-γ levels in serum (from the BLG-induced food allergy mouse model) or in BALF (from the OVA-induced allergic asthma mouse model) were assayed using ELISA Kits (R&D Systems, Boston, MA, United States) according to the manufacturer’s protocol.
Cell counts of BALF
BALF was isolated in 1 mL of phosphate buffered saline (PBS) from the mouse model of OVA-induced allergic asthma. The BALF cellularity was determined using a hemocytometer. A 10 μL aliquot of centrifuged cells (4000 rpm, 5 min) was transferred onto slides, and all leukocytes were fixed for staining using Giemsa. The observer counted 200-300 cells per slide, and standard morphological criteria were adopted to identify the individual leukocyte populations. The number of leukocytes was counted twice, and the average value was calculated.
Histological analysis
To assess the pathological changes, samples from either lungs (OVA-induced allergic asthma) or intestine (BLG-induced food allergy) were collected. The samples were fixed in neutrally buffered 10% formaldehyde and embedded in paraffin. Sections 4 μm thick were stained with HE to detect inflammatory cell infiltration in intestinal tissue (BLG-induced food allergy), or to assess the extent of inflammation in the lungs (OVA-induced asthma) at 200 × magnification.
Statistical analysis
All data points represent the mean ± SEM in each mouse group. Analysis was performed using SPSS 19.0 software for Windows. Variance analysis of single factor and multi factor was conducted to determine the statistical significance. A P value lower than 0.05 was considered statistically significant.
RESULTS
B. infantis decreased the levels of IgE and IgG1 in OVA-induced asthma and BLG-induced food allergy mouse models
We determined whether oral B. infantis CGMCC313-2 affected serum levels of allergen-induced specific IgE and IgG1, and ELISA was used for data analysis in the OVA-induced allergic asthma mouse model. The serum levels of OVA-specific IgE and IgG1 were significantly elevated in the OVA sensitization/challenge (Group 2) compared with the normal control group (Group 1). In groups which received B. infantis CGMCC313-2 for prevention (Group 3) and pre-treatment (Group 4) during the OVA sensitization/challenge, the serum levels of IgE and IgG1 were significantly decreased (P < 0.05; Figure 3A and B). Moreover, the levels of serum IgE in the prevention group were also significantly decreased compared with the pre-treatment group (P < 0.05; Figure 3A).
Figure 3.
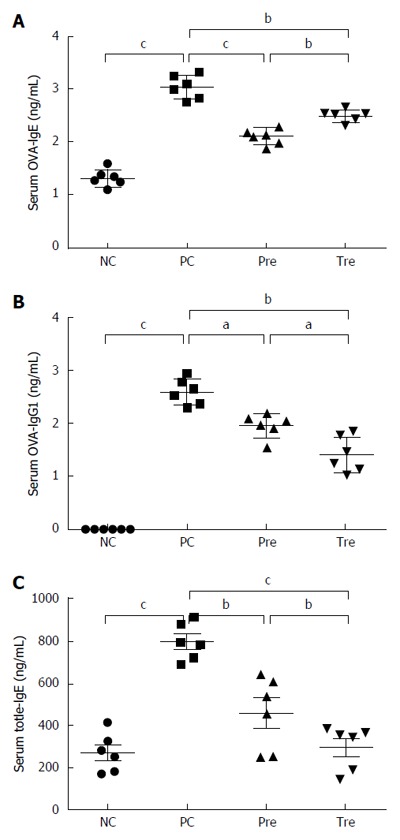
Effect of B. infantis CGMCC313-2 on the reversal of IgE and IgG1 in ovalbumin-induced asthma and β-lactoglobulin-induced food allergy mouse models. A and B: There were significant increases in OVA-specific IgE and IgG1 expression in the positive control (PC; Group 2) group compared with the normal control (NC; Group 1) group in the allergic asthma mouse model. The prevention (pre; Group 3) and pre-treatment (tre; Group 4) groups following B. infantis CGMCC313-2 administration showed decreased expression; C: A significant increase in total IgE expression was seen in the positive control (PC; Group 2) group compared with the normal control (NC; Group 1) group in the BLG-induced food allergy mouse model. The prevention (pre; Group 3) and pre-treatment (tre; Group 4) groups following B. infantis CGMCC313-2 administration showed decreased expression. The statistical differences are represented as follows: aP < 0.05; bP < 0.01, and cP < 0.001.
Due to the unavailability of reagents for BLG-specific IgE and IgG1 detection, the serum levels of total IgE were evaluated in the BLG-induced food allergy mouse model. The serum levels of total IgE were significantly increased after the BLG sensitization/challenge (Group 2) compared with the normal control group (Group 1). In the groups challenged with B. infantis CGMCC313-2 for prevention (Group 3) and pre-treatment (Group 4), the levels of total IgE were significantly decreased. Moreover, the total IgE serum levels in the pre-treatment group were also significantly decreased compared with the prevention group (P < 0.05; Figure 3C).
B. infantis administration increases body weight in BLG-induced food allergy mice
Compared with the normal control group, mice in the BLG-sensitization/challenge group showed weight loss. However, the prevention and pre-treatment groups showed weight gain following B. infantis CGMCC313-2 (Figure 4), and the pre-treatment group gained more weight than the prevention group.
Figure 4.
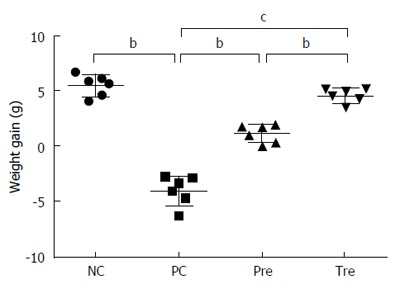
Effect of B. infantis CGMCC313-2 on body weight in BLG-induced food allergy mice. Average body weight decreased significantly in the positive control (PC; Group 2) group compared with the normal control (NC; Group 1) group. The prevention (pre; Group 3) and pre-treatment (tre; Group 4) groups following B. infantis CGMCC313-2 administration showed an increase in body weight. The statistical differences are represented as follows: aP < 0.05; bP < 0.01, and cP < 0.001.
B. infantis alters the proportion of lung-infiltrating cells in OVA-induced allergic asthma mice
In order to evaluate the degree of inflammatory cell infiltration in the lungs of OVA-induced allergic asthma mice, leukocyte counts were conducted in BALF tissue. Inflammatory cell number was significantly increased in the OVA-sensitized/challenged mice compared to the control group. However, the proportion of infiltrating cells in the lung was significantly decreased in the groups treated with B. infantis CGMCC313-2 (Figure 5). Differential cell counts using Giemsa staining failed to identify cell types in our study.
Figure 5.
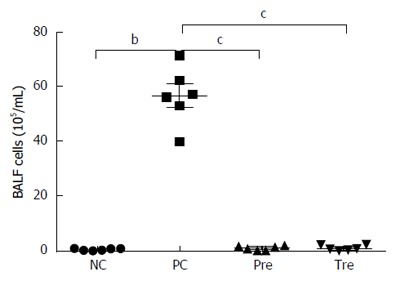
Effects of B. infantis CGMCC313-2 on infiltrating cells in the lungs of ovalbumin-induced allergic asthma mice. Total cell number in BALF increased significantly in the positive control (PC; Group 2) group compared with the normal control (NC; Group 1) group. The prevention (pre; Group 3), and pre-treatment (tre; Group 4) groups following B. infantis CGMCC313-2 administration showed a decrease. The statistical differences are represented as: aP < 0.05; bP < 0.01, and cP < 0.001.
Impact of B. infantis on allergic inflammation in OVA-induced asthma and BLG-induced food allergy mouse models
The effect of B. infantis CGMCC313-2 in the OVA or BLG-sensitized/challenged mice was evaluated from the perspective of overall lung or intestinal inflammation using histological HE staining (Figures 6 and 7, respectively). Compared with normal control mice (Figure 6A and Figure 7A), allergen sensitized/challenged mice (Figure 6B and Figure 7B) had severe inflammation; while the prevention (Figure 6C and Figure 7C) and pre-treatment (Figure 6D and Figure 7D) mice showed significantly diminished signs of inflammation following B. infantis CGMCC313-2 treatment.
Figure 6.
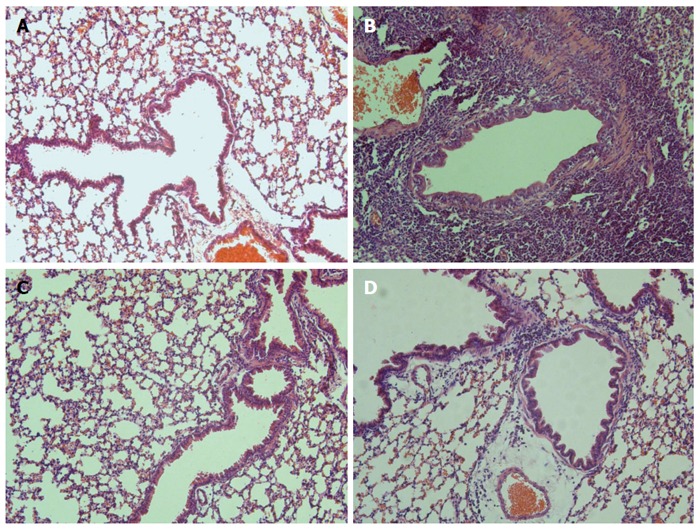
Effects of B. infantis CGMCC313-2 on OVA-induced airway inflammation. Lung tissues were obtained from the (C) prevention group and (D) pre-treatment group treated with B. infantis CGMCC313-2, and from (A) the normal control group and (B) the ovalbumin sensitized/challenged group on Day 29. The tissues were stained and observed under × 200 magnification. The positive control group showed severe airway inflammation, while the groups treated with B. infantis CGMCC313-2 showed attenuation of airway inflammation.
Figure 7.
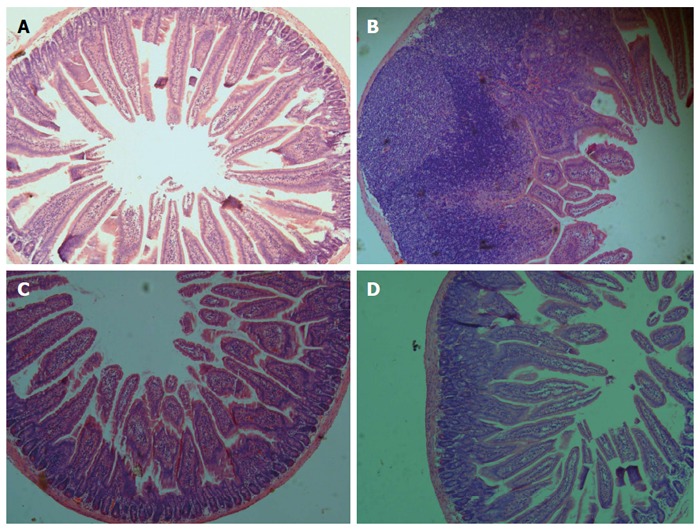
Effects of B. infantis CGMCC313-2 on BLG-induced intestinal inflammation. Intestinal tissues were obtained from (A) the normal control group and (B) the BLG-sensitized/challenged group on Day 29, and from the (C) prevention group and (D) pre-treatment group which were treated with B. infantis CGMCC313-2. The tissues were stained and observed under 200 × magnification. The positive control group showed severe intestinal inflammation, while the groups treated with B. infantis CGMCC313-2 showed attenuated intestinal inflammation.
Effect of B. infantis on cytokines in serum and BALF
To further elucidate possible mechanisms responsible for the effects of B. infantis CGMCC313-2 on systemic sensitization and allergic inflammation, ELISA was used to determine the expression of IL-4, IL-10, IL-13, and IFN-γ in serum and BALF was collected from BLG-induced food allergy mice and OVA-induced allergic asthma mice, respectively. IL-4 and IL-13 in serum (Figure 8A and B) or BALF (Figure 8D and E) increased significantly in the positive control (PC; Group 2) group compared with the normal control (NC; Group 1) group. In the groups that received B. infantis CGMCC313-2, the serum levels of IL-4 and IL-13 in the prevention group were significantly decreased. In addition, a reduction in IL-4 and IL-13 in the prevention and treatment groups was also observed in OVA-induced allergic asthma mice. Serum IL-10 (Figure 8C) decreased significantly in the positive control (PC; Group 2) group, prevention (Pre; Group 3) group, and pre-treatment (Tre; Group 4) group compared with the normal control (NC; Group 1) group. IL-10 was not detected in BALF from OVA-induced allergic asthma mice, and IFN-γ was not detected in either serum or BALF from any of the mice.
Figure 8.
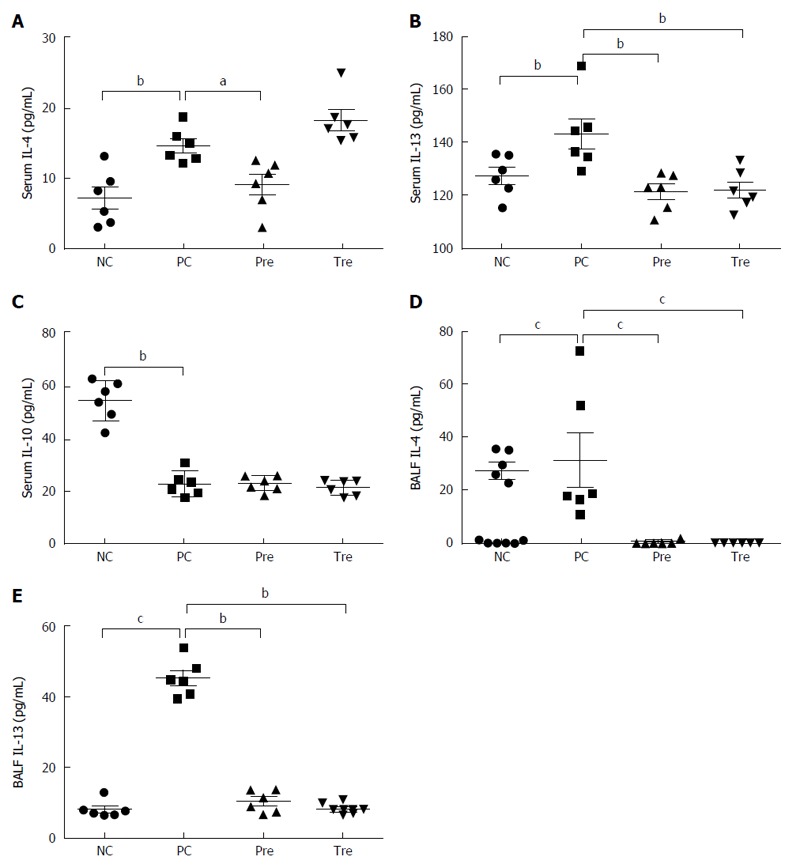
Effects of B. infantis CGMCC313-2 on cytokines in serum and bronchoalveolar lavage fluid. IL-4, IL-10, and IL-13 in serum and BALF were determined in BLG-induced food allergy mice and OVA-induced allergic asthma mice, respectively. A: Serum IL-4 in the prevention (pre; Group 3) group; B: serum IL-13 in the prevention (pre; Group 3) and pre-treatment (tre; Group 4) groups were significantly decreased compared with the positive control (PC; Group 2) group; C: There was no significant difference in IL-10 between the positive control group (PC; Group 2), prevention group (pre; Group 3), and pre-treatment (tre; Group 4), which was significantly decreased when compared with the normal control (NC; Group 1) group; D: The concentrations of BALF IL-4 and (E) BALF IL-13 were significantly decreased in the prevention (pre; Group 3) group and the pre-treatment (tre; Group 4) group treated with B. infantis CGMCC313-2. The statistical differences are represented as follows: aP < 0.05; bP < 0.01, and cP < 0.001.
DISCUSSION
There is increasing evidence to show that intestinal microbiota and ingested probiotics may induce important metabolic and physiological reactions in the host, and drive maturation of the immune system in early life. Of these diverse probiotics, Lactobacilli and Bifidobacteria, which are part of the gut flora in infants, are the most promising candidates that naturally affect immune system development[14,15]. For the same reason, Lactobacilli and Bifidobacteria are the most frequently used probiotics for clinical intervention studies[16-20]. However, the most important characteristic of probiotics is their strain-specificity effect[21]. In this study, we investigated the role of Bifidobacterium infantis CGMCC313-2 in allergic disease prevention and treatment in two mouse models, as B. infantis CGMCC313-2 has been extensively used in the treatment and prevention of diarrhea including antibiotic-associated diarrhea in China. In OVA-sensitized/challenged mice, severe lung inflammation and infiltrating cells in the lungs were observed, and the administration of B. infantis CGMCC313-2 significantly diminished inflammation. Similarly, in β-lactoglobulin-induced food allergy mice, B. infantis CGMCC313-2 decreased intestinal inflammation, and ameliorated weight loss in BLG-sensitized/challenged mice. These results demonstrate that oral administration of B. infantis CGMCC313-2 during or after allergen sensitization may relieve allergic inflammation in the airway and intestine.
In the allergen sensitized/challenged mice, IL-4, IL-13, total IgE, and allergen-induced serum specific IgE and IgG1 levels were highly expressed. Based on the immunological basis of allergy, the overexpression of IL-4 and IL-13, which is modulated by type 2 T helper cells, could promote IgE production and eosinophil infiltration in target organs. In the present study, following the oral administration of B. infantis CGMCC313-2, the levels of IL-13 and total IgE were significantly decreased, which was accompanied by the attenuation of inflammatory symptoms. We deduced that the metabolites of B. infantis CGMCC313-2, including butyrate and short-chain fatty acids, can suppress the inflammatory responses triggered by Th2 cytokines[22-28]. However, the level of IL-4 was higher in the treatment group, which was opposite to the results of IL-13 and IgE. Due to the complexity of the immune system and response, the role of IL-4 as an allergic disorder marker requires further investigation in our future study. In addition, the oral administration of this probiotic helped in the prevention and treatment of airway and intestine allergy.
On the other hand, there was a decrease in IL-10 serum levels in mice sensitized/challenged with BLG. There is strong evidence to indicate that the production of IL-10, which is affected by antigens exposure, is associated with T cell tolerance and Treg secretion, which in turn plays important roles in controlling allergic diseases. However, the administration of B. infantis CGMCC313-2 did not promote the secretion of IL-10. This phenomenon was inconsistent with previous preclinical studies in which probiotic strains promoted Treg responses[29,30]. We deduced that the different probiotic strains adopted in different studies may have strain-specific effects, or the immunomodulatory effect of B. infantis CGMCC313-2 suppressed Th2 responses. In our study, the levels of IFN-γ in both serum and BALF were too low to be detected in all mice, and this may have been due to the poor sensitivity of the measurement technique. This is a limitation of our study.
In the present study, which included allergic asthma and food allergy mouse models, we found that B. infantis CGMCC313-2 inhibited the secretion of allergen-induced IgE and Th2 cytokines, and further attenuated allergic inflammation. Our study also suggested that the modulatory activity of B. infantis CGMCC313-2 was not only confined to intestinal allergic diseases, but also to allergic airway disease. Therefore, B. infantis CGMCC313-2 may be regarded as a candidate probiotic strain in the prevention and treatment of allergic diseases. However, further clinical and experimental studies are required to delineate the potential preventive and treatment effects of B. infantis CGMCC313-2.
ACKNOWLEDGMENTS
We would like to thank the staff at WeHealthGene who contributed greatly to this work and the laboratory technicians at Shenzhen Children’s Hospital who provided valuable assistance with the animal experiments.
COMMENTS
Background
Probiotics exhibit beneficial effects on allergies based on the “microflora hypothesis”, and experimental studies have also shown that probiotics have strain-specific effects. In this study, the specific effects of B. infantis CGMCC313-2, which is widely used as a probiotic drug in China, on allergic asthma and food allergy mouse models were determined.
Research frontiers
Previous studies have indicated that ingested Lactobacilli and Bifidobacteria can modify microbial flora, and can reduce the symptoms of allergic diseases by modifying the host immune system. Some probiotic drugs have been intensively investigated as novel alternative options for the management of allergic diseases including asthma and food allergy.
Innovations and breakthroughs
In this study, B. infantis CGMCC313-2 was administered to allergic asthma and food allergy mouse models. Following B. infantis CGMCC313-2 treatment, the serum concentrations of IgE and IgG1 significantly decreased, and the concentrations of interleukin-4 (IL-4) and IL-13 also reduced in mice with allergic asthma and food allergy. Inflammatory cell infiltration and inflammation were also attenuated.
Applications
B. infantis CGMCC313-2 can inhibit the secretion of allergen-induced IgE and Th2 cytokines, and can attenuate allergic inflammation. B. infantis CGMCC313-2 provides an important reference for the prevention and treatment of intestinal and airway allergic diseases.
Terminology
IgE and IgG, which are closely related with anaphylaxis, are higher in patients with allergies. IL-4 and IL-13, which are secreted by Th2 cells, are involved in the humoral immune response and indicate the degree of allergic disease.
Peer-review
The authors performed clear experiments on asthma mouse model and food allergy mouse model to detect the effects of B. infantis on allergy diseases. They found that B. infantis could inhibit the secretion of allergens induced IgG, IgE, IL-4 and IL-13, and allergic inflammation were also attenuated. The study shed the light on the prevention and treatment of intestinal and airway allergic diseases. However, further clinical studies are still required.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Institutional Review Board (IRB) in Shenzhen Children’s Hospital.
Institutional animal care and use committee statement: The experimental procedures involving animals in this study were reviewed and approved by the Animal Welfare and Ethical Committee in The Fourth Military Medical University (approval ID: 20150902).
Conflict-of-interest statement: The authors declare that they have no competing interests.
Data sharing statement: No additional data are available.
Peer-review started: November 28, 2016
First decision: December 28, 2016
Article in press: February 17, 2017
P- Reviewer: Classen CF, Islek A, Watanabe T S- Editor: Qi Y L- Editor: Webster JR E- Editor: Wang CH
References
- 1.Zhao J, Bai J, Shen K, Xiang L, Huang S, Chen A, Huang Y, Wang J, Ye R. Self-reported prevalence of childhood allergic diseases in three cities of China: a multicenter study. BMC Public Health. 2010;10:551. doi: 10.1186/1471-2458-10-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yangzong Y, Shi Z, Nafstad P, Håheim LL, Luobu O, Bjertness E. The prevalence of childhood asthma in China: a systematic review. BMC Public Health. 2012;12:860. doi: 10.1186/1471-2458-12-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, Zhang L. Prevalence of allergic rhinitis in china. Allergy Asthma Immunol Res. 2014;6:105–113. doi: 10.4168/aair.2014.6.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li F, Zhou Y, Li S, Jiang F, Jin X, Yan C, Tian Y, Zhang Y, Tong S, Shen X. Prevalence and risk factors of childhood allergic diseases in eight metropolitan cities in China: a multicenter study. BMC Public Health. 2011;11:437. doi: 10.1186/1471-2458-11-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strachan DP. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax. 2000;55 Suppl 1:S2–10. doi: 10.1136/thorax.55.suppl_1.s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strannegård O, Strannegård IL. The causes of the increasing prevalence of allergy: is atopy a microbial deprivation disorder? Allergy. 2001;56:91–102. doi: 10.1034/j.1398-9995.2001.056002091.x. [DOI] [PubMed] [Google Scholar]
- 7.Kalliomäki M, Isolauri E. Role of intestinal flora in the development of allergy. Curr Opin Allergy Clin Immunol. 2003;3:15–20. doi: 10.1097/00130832-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Björkstén B. Effects of intestinal microflora and the environment on the development of asthma and allergy. Springer Semin Immunopathol. 2004;25:257–270. doi: 10.1007/s00281-003-0142-2. [DOI] [PubMed] [Google Scholar]
- 9.Food and Agriculture Organization of the United Nations and World Health Organization (FAO-WHO) (2002) Guideline for the evaluation of probiotics in food. FAO of the United Nations and WHO working group report; Online: Available from: http://who.int/foodsafety/publications.fs-management/probiotics/en/
- 10.Zuccotti G, Meneghin F, Aceti A, Barone G, Callegari ML, Di Mauro A, Fantini MP, Gori D, Indrio F, Maggio L, et al. Probiotics for prevention of atopic diseases in infants: systematic review and meta-analysis. Allergy. 2015;70:1356–1371. doi: 10.1111/all.12700. [DOI] [PubMed] [Google Scholar]
- 11.Floch MH, Walker WA, Sanders ME, Nieuwdorp M, Kim AS, Brenner DA, Qamar AA, Miloh TA, Guarino A, Guslandi M, et al. Recommendations for Probiotic Use--2015 Update: Proceedings and Consensus Opinion. J Clin Gastroenterol. 2015;49 Suppl 1:S69–S73. doi: 10.1097/MCG.0000000000000420. [DOI] [PubMed] [Google Scholar]
- 12.Cuello-Garcia CA, Brożek JL, Fiocchi A, Pawankar R, Yepes-Nuñez JJ, Terracciano L, Gandhi S, Agarwal A, Zhang Y, Schünemann HJ. Probiotics for the prevention of allergy: A systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2015;136:952–961. doi: 10.1016/j.jaci.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 13.Szajewska H, Shamir R, Turck D, van Goudoever JB, Mihatsch WA, Fewtrell M. Recommendations on probiotics in allergy prevention should not be based on pooling data from different strains. J Allergy Clin Immunol. 2015;136:1422. doi: 10.1016/j.jaci.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Saavedra JM. Use of probiotics in pediatrics: rationale, mechanisms of action, and practical aspects. Nutr Clin Pract. 2007;22:351–365. doi: 10.1177/0115426507022003351. [DOI] [PubMed] [Google Scholar]
- 15.Ringel-Kulka T, Cheng J, Ringel Y, Salojärvi J, Carroll I, Palva A, de Vos WM, Satokari R. Intestinal microbiota in healthy U.S. young children and adults--a high throughput microarray analysis. PLoS One. 2013;8:e64315. doi: 10.1371/journal.pone.0064315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weston S, Halbert A, Richmond P, Prescott SL. Effects of probiotics on atopic dermatitis: a randomised controlled trial. Arch Dis Child. 2005;90:892–897. doi: 10.1136/adc.2004.060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viljanen M, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, Kuitunen M. Probiotics in the treatment of atopic eczema/dermatitis syndrome in infants: a double-blind placebo-controlled trial. Allergy. 2005;60:494–500. doi: 10.1111/j.1398-9995.2004.00514.x. [DOI] [PubMed] [Google Scholar]
- 18.Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076–1079. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 19.Kalliomäki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet. 2003;361:1869–1871. doi: 10.1016/S0140-6736(03)13490-3. [DOI] [PubMed] [Google Scholar]
- 20.Rautava S, Kalliomäki M, Isolauri E. Probiotics during pregnancy and breast-feeding might confer immunomodulatory protection against atopic disease in the infant. J Allergy Clin Immunol. 2002;109:119–121. doi: 10.1067/mai.2002.120273. [DOI] [PubMed] [Google Scholar]
- 21.Hougee S, Vriesema AJ, Wijering SC, Knippels LM, Folkerts G, Nijkamp FP, Knol J, Garssen J. Oral treatment with probiotics reduces allergic symptoms in ovalbumin-sensitized mice: a bacterial strain comparative study. Int Arch Allergy Immunol. 2010;151:107–117. doi: 10.1159/000236000. [DOI] [PubMed] [Google Scholar]
- 22.Schouten B, van Esch BC, Hofman GA, van Doorn SA, Knol J, Nauta AJ, Garssen J, Willemsen LE, Knippels LM. Cow milk allergy symptoms are reduced in mice fed dietary synbiotics during oral sensitization with whey. J Nutr. 2009;139:1398–1403. doi: 10.3945/jn.109.108514. [DOI] [PubMed] [Google Scholar]
- 23.Thang CL, Baurhoo B, Boye JI, Simpson BK, Zhao X. Effects of Lactobacillus rhamnosus GG supplementation on cow’s milk allergy in a mouse model. Allergy Asthma Clin Immunol. 2011;7:20. doi: 10.1186/1710-1492-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frossard CP, Steidler L, Eigenmann PA. Oral administration of an IL-10-secreting Lactococcus lactis strain prevents food-induced IgE sensitization. J Allergy Clin Immunol. 2007;119:952–959. doi: 10.1016/j.jaci.2006.12.615. [DOI] [PubMed] [Google Scholar]
- 25.Forsythe P, Inman MD, Bienenstock J. Oral treatment with live Lactobacillus reuteri inhibits the allergic airway response in mice. Am J Respir Crit Care Med. 2007;175:561–569. doi: 10.1164/rccm.200606-821OC. [DOI] [PubMed] [Google Scholar]
- 26.Li CY, Lin HC, Hsueh KC, Wu SF, Fang SH. Oral administration of Lactobacillus salivarius inhibits the allergic airway response in mice. Can J Microbiol. 2010;56:373–379. doi: 10.1139/w10-024. [DOI] [PubMed] [Google Scholar]
- 27.Wu CT, Chen PJ, Lee YT, Ko JL, Lue KH. Effects of immunomodulatory supplementation with Lactobacillus rhamnosus on airway inflammation in a mouse asthma model. J Microbiol Immunol Infect. 2016;49:625–635. doi: 10.1016/j.jmii.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Fujimura KE, Demoor T, Rauch M, Faruqi AA, Jang S, Johnson CC, Boushey HA, Zoratti E, Ownby D, Lukacs NW, et al. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proc Natl Acad Sci USA. 2014;111:805–810. doi: 10.1073/pnas.1310750111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berin MC. Bugs versus bugs: probiotics, microbiome and allergy. Int Arch Allergy Immunol. 2014;163:165–167. doi: 10.1159/000357946. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida T, Fujiwara W, Enomoto M, Nakayama S, Matsuda H, Sugiyama H, Shimojoh M, Okada S, Hattori M. An increased number of CD4+CD25+ cells induced by an oral administration of Lactobacillus plantarum NRIC0380 are involved in antiallergic activity. Int Arch Allergy Immunol. 2013;162:283–289. doi: 10.1159/000354924. [DOI] [PubMed] [Google Scholar]


