Abstract
AIM
To examine the effects of Acanthopanax senticosus polysaccharides (ASPS) on intestinal tight junction (TJ) disruption and nuclear factor-kappa B (NF-κB)/myosin light chain kinase (MLCK) activation in endotoxemia.
METHODS
BALB/C mice (6-8-weeks-old) received continuous intragastric gavage of ASPS for 7 d before injection of lipopolysaccharide (LPS), or received ASPS once after LPS injection. Blood and intestinal mucosal samples were collected 6 h after LPS challenge. Clinical symptoms, histological injury, intestinal permeability, TJ ultrastructure, and TJ protein expression were determined.
RESULTS
Compared with mice in the LPS group, pretreatment with ASPS improved clinical and histological scores by 390.9% (P < 0.05) and 57.89% (P < 0.05), respectively, and gut permeability change in endotoxemic mice was shown by a 61.93% reduction in reduced leakage of fluorescein isothiocyanate-dextran 6 h after LPS injection (P < 0.05). ASPS pretreatment also prevented LPS-induced TJ ultrastructure breakdown supported by increased electron dense materials between adjoining cells, sustained redistribution and expression of occludin (0.597 ± 0.027 vs 0.103 ± 0.009, P < 0.05) and zonula occludens-1 (0.507 ± 0.032 vs 0.125 ± 0.019, P < 0.05), and suppressed activation of the NF-κB/MLCK pathway indicated by reduced expression of NF-κB, phospho-inhibitor kappa B-alpha, MLCK and phospho-myosin light-chain-2 by 16.06% (P < 0.05), 54.31% (P < 0.05), 66.10% (P < 0.05) and 64.82% (P < 0.05), respectively.
CONCLUSION
ASPS pretreatment may be associated with inhibition of the NF-κB/MLCK pathway and concomitant amelioration of LPS-induced TJ dysfunction of intestinal epithelium in endotoxemia.
Keywords: Acanthopanax senticosus polysaccharide, Intestinal permeability, Tight junction, Nuclear factor-kappa B, Myosin light chain kinase
Core tip: Acanthopanax senticosus polysaccharides (ASPS) effectively protect against gastric tight junction (TJ) injury in sepsis. ASPS pretreatment significantly improved intestinal histological appearance and gut permeability, increased electron dense between adjoining cells, sustained the expression and redistribution of occludin and zonula occludens-1, suppressed the expression of nuclear factor-kappa B p65 (NF-κBp65) and phospho-inhibitor kappa B-alpha and myosin light chain kinase (MLCK), as well as phospho-myosin light-chain-2 in endotoxemia. These findings suggest that ASPS pretreatment may be associated with inhibition of the NF-κB/MLCK pathway and concomitant amelioration of gastric TJ dysfunction in the mouse model of endotoxemia.
INTRODUCTION
Sepsis and resulting organ system dysfunction are the most frequent causes of death in intensive care patients worldwide[1], and were identified to occur mainly in response to lipopolysaccharide (LPS) from Gram-negative bacteria and to develop rapidly into fatal systemic infections[2]. The gastrointestinal tract is involved in the initial response to the systemic inflammatory reaction[3]. Impaired intestinal barrier function or increased epithelial permeability may promote the translocation of bacteria and the entry of allergenic compounds from the gut into the body, increasing susceptibility to infections[4,5], and this process has been implicated in the development of sepsis and septic multiple organ dysfunction[6].
Tight junctions (TJs) and their associated proteins, such as zonula occludens (ZO), occludin and claudins, are critical in maintenance of the intact intestinal epithelial barrier[7], which can regulate the entry of nutrients, ions and water, while restricting the entry of luminal pathogens and antigenic molecules into the mucosa[8]. TJ breakdown occurs in polymicrobial sepsis when TJ proteins are remodeled due to interactions with external stimuli, such as pathogenic bacteria[9]. Signaling molecules, such as myosin light chain kinase (MLCK) have been implicated in the assembly and regulation of TJs via phosphorylation of myosin light chain (MLC)[10]. MLCK can be mediated according to transcriptional increase by nuclear factor-kappa B (NF-κB) in the inflammatory response, and thus results in TJ barrier breakdown[11]. In vivo and in vitro models have demonstrated that inhibition of MLCK[12] and NF-κB[13] can prevent the deleterious effects of LPS-induced sepsis and leads to TJ preservation.
In recent years, there has been growing interest in the development of new therapeutic strategies in sepsis. Acanthopanax senticosus (AS) has been widely used for thousands of years in China as a traditional herbal medicine to regulate hypoxia, fatigue and appetite loss without side effects[14,15]. Polysaccharides extracted from AS (ASPS) are major active ingredients with multiple pharmacologic and biological characteristics, including immune regulation[16] and anti-inflammation[17]. A recent in vivo study suggested that ASPS could exert positive effects on intestinal mucosal integrity and suppress NF-κB activation[18]. However, the mechanisms by which ASPS exert these effects on TJ disruption in a mouse model of endotoxemia have not yet been elucidated. In the present study, we determined the effects of ASPS on MLCK activation and TJ barrier breakdown in LPS-induced endotoxemia to evaluate whether the administration of ASPS alleviates endotoxemia-induced epithelial TJ breakdown by suppressing the NF-κB/MLCK signaling pathway.
MATERIALS AND METHODS
Preparation and analysis of ASPS
Details on the preparation of ASPS, which were taken from the root of AS, using an ethanol precipitation method have been reported previously[18]. Proteins were removed by the Sevag method[19] and polysaccharide content after purification using the phenol-sulfuric acid method was 92.7%[20]. Analysis of monosaccharide composition in ASPS was by ion chromatography according to a previously described method[21], which showed that it is a heteropolysaccharide composed of glucose, galactose, arabinose, mannose, rhamnose and xylopyranose.
Experimental animals
Male BALB/C mice (Changsheng Life Sciences Co., Ltd., Changchun, China), weighing 20-25 g, aged 6-8 wk, were housed individually in a temperature (22 ± 2 °C) and humidity (53% ± 2%) controlled room with a 12-h light/dark cycle and ad libitum access to chow and water. All animal experiments conformed to the guidelines on caring for and use of laboratory animals which were reviewed by the Animal Ethics Committee of College of Animal Science & Veterinary Medicine, Shenyang Agricultural University (Permit No. SYXK (Liao) 2011-0001).
Experimental protocols
Following acclimation for 1 wk, all animals were randomly assigned to 4 groups (7-8 mice per group): control, LPS, ASPS + LPS, and LPS + ASPS. Mice in the ASPS + LPS group were administered continuous intragastric gavage of ASPS dissolved in normal saline at the dose of 300 mg/kg daily for 7 d, and mice in the control, LPS, and LPS + ASPS groups were given an equivalent amount of normal saline. After 1 h of intragastric treatment on day 7, mice in the LPS, ASPS + LPS and LPS + ASPS groups were injected intraperitoneally with LPS from Escherichia coli serotype (055:B5; Sigma, St Louis, MO, United States) at 10 mg/kg dissolved in 1 mL normal saline, and the control group was given an equivalent amount of normal saline. The ASPS dose was determined in accordance with our previous study[17]. Mice in the LPS + ASPS group received 300 mg/kg ASPS intragastrically 30 min after LPS injection. All animals were anesthetized with pentobarbital sodium (60 mg/kg, intraperitoneally), killed by cervical dislocation and samples were collected 6 h after LPS treatment. All efforts were made to minimize animal suffering.
Clinical symptom score
Clinical symptom scores of severity of conjunctiva secretion, stool consistency, messy fur, and inactivity were determined at specified time points using a 3-point scale according to a method described previously[22] with slight modifications. The scoring system is presented in Table 1. Clinical symptoms in each mouse were evaluated at 2, 4 and 6 h after LPS injection and scored blindly by three independent researchers. The means of three assessments were obtained for grading.
Table 1.
Clinical scoring system
| Variables |
Score |
||
| 0 | 1 | 2 | |
| Conjunctiva secretion | Closed eyes or opened with serious secretion | Opened eyes with moderate discharge | Normal eye without conjunctivitis |
| Stool consistency | Watery stool | Loose stool | Normal stool |
| Fur appearance | Rough and dull fur | Reduced grooming fur | Smooth and shiny fur |
| Stimulation activity | Lethargy and raising head after moderate stimulation | Inactive and reduced alert, < 2 steps after moderate stimulation | Normal action and reaction, > 2 steps after moderate stimulation |
Histopathological evaluation of the intestine
After sacrifice and excision of ileal and colonic segments near to the cecum for observation of intestinal macroscopic features, ileal segments measuring approximately 2-cm were stained with hematoxylin and eosin (HE) for morphological observation. The details of this process were as follows: intestinal segments were transferred into 4% paraformaldehyde and embedded in paraffin. Sections measuring 5-μm thick were sliced, deparaffinized, rehydrated and stained with HE to observe the degree of intestinal mucosal damage using a biomicroscope (Axio Scope A1; Zeiss, Oberkochen, Germany) and scored according to the method by Chiu, as follows: score of (1) normal mucosal villi without damage; (2) broadened subepithelial Gruenhagen’s space at villous tip; (3) further extension of subepithelial space from the epithelial layer to the lamina propria; (4) detachment of less than half of the villous epithelium; (5) detachment of more than half of the villous epithelium and exposed villi with lamina propria; and (6) disintegration and detachment of the lamina propria. Five images in each slice were blindly assessed by three pathologists.
Determination of intestinal permeability
At 2, 4 and 6 h after LPS injection, 3 mice from each group were anesthetized with pentobarbital sodium and a midline laparotomy was performed to expose the intestinal tract. Lengths of distal ileum measuring 5 cm were isolated and ligated at both ends. A solution of 100 μL PBS containing 20 mg of 4-kDa fluorescein isothiocyanate (FITC)-dextran (Sigma) was injected into the lumen and then the midline skin was sutured. A 100 μL blood sample was collected via cardiac puncture 30 min after FITC-dextran injection and was diluted with 1.9 mL of 50 mmol/L Tris-buffered saline (TBS) and centrifuged at 10000 × g for 10 min to obtain plasma. The concentration of FITC-dextran in plasma was assayed using a fluorescence spectrophotometer (970CRT; Shanghai Lengguang Technology Co, Shanghai, China) with excitation and emission wavelengths of 480 and 520 nm, respectively.
TJ transmission electron microscopy
After rinsing with cold PBS, distal ileal sections measuring 1 mm × 1 mm × 2 mm were cut on ice and immediately transferred into 4% glutaraldehyde to fix for 2 h, post-fixed with 1% osmium tetroxide, and embedded in Epon 812. Thin slices measuring 500 nm were cut and double stained with uranyl acetate and lead citrate, and then examined with an transmission electron microscope (TEM) (HT-7700; Hitachi, Tokyo, Japan) operated at 100 kV.
Immunofluorescence microscopy
Ileal segments were fixed with 4% paraformaldehyde and then cut into 3-μm thick slices. The slices were dewaxed and dehydrated with xylene and ethanol, respectively, and then incubated in 3% hydrogen peroxide and the antigens repaired in citrate buffer. The resulting tissue samples were blocked with 5% normal goat serum in PBS. After incubation with antibodies against occludin (1:100; Proteintech, Chicago, IL, United States), ZO-1 (1:100; Proteintech), and MLCK (1:200; Abcam, Cambridgeshire, United Kingdom) in 1% fetal bovine serum overnight at 4 °C, the sections were washed and incubated with Cy3-conjugated secondary antibodies for 1 h. Sample images were obtained using a BX43 (Olympus, Tokyo, Japan) microscope.
Protein extraction from the nucleus and cytoplasm of intestinal mucosa
Protein extracts were prepared according to a previously described method[23] with some modifications. Ileal mucosa samples were collected near the cecocolonic junction and ground with liquid nitrogen. The powder was incubated on ice for 10 min with a buffer containing KCl at 10 mmol/L, HEPES at 10 mmol/L (pH 7.9), MgCl2 at 1.5 mmol/L, dithiothreitol at 1 mmol/L and benzene methyl sulfuryl fluoride at 1 mmol/L, and then centrifuged at 5000 × g for 3 min. The precipitate was resuspended in this buffer and centrifuged again to obtain the supernatant as the cytoplasmic extract for protein expression assay of occludin, ZO-1, phospho-MLC2, and phospho-IκBa, and the resulting precipitate was lysed by incubation for 30 min in 0.2 mL buffer containing HEPES at 20 mmol/L, glycerol at 25%, NaCl at 420 mmol/L, MgCl2 at 1.5 mmol/L and EDTA at 0.2 mmol/L. Following centrifugation at 12 000 for 15 min, the supernatant (nuclear extract) was obtained and the expression of NF-κB p65 and MLCK was analyzed. The extracted proteins were quantified using the bicinchoninic acid assay and stored at -80 °C for subsequent assay.
Western blot assay
An equal amount of protein exact (20-40 μg) was electrophoresed on a 10% reducing polyacrylamide gel and transferred onto polyvinylidene difluoride membranes. Immunoblots were blocked with 3% bovine serum albumin (BSA) in TBS for 70 min at room temperature and incubated overnight at 4 °C with specific primary antibodies including rabbit anti-occludin (1:1000; Proteintech), rabbit anti-ZO-1 (1:1000; Proteintech), rabbit anti-NF-κB p65 (1:5000; Abcam), rabbit anti-MLCK (1:5000; Abcam), phospho-MLC2 (1:1000; Cell Signaling Technology, Danvers, MA, United States), and phospho-IκBα (1:1000; Cell Signaling Technology) in TBS and 0.05% Tween-20 containing 1% BSA.
Blots were washed and then incubated with anti-rabbit horseradish peroxidase-conjugated secondary antibodies for 120 min at room temperature. The bands were detected by enhanced chemiluminescence and quantified (relative to β-actin expression) using Scion Image 4.03 analysis software.
Statistical analysis
Data were statistically analyzed by one-way analysis of variance (ANOVA) using IBM SPSS statistical software, version 22.0, and differences among the groups were compared using Duncan’s multiple test. The results were expressed as mean ± SE, and a 5% level of probability was considered significant for all analyses.
RESULTS
Clinical symptom score and morphological and histopathologic evaluation of the intestine
The clinical symptoms and morphological and histopathologic changes following ASPS treatment were assessed in this model of endotoxemia induced by LPS challenge. The LPS group showed a pronounced decline in the clinical symptom score compared with the control group (P < 0.05). The clinical symptom score in mice pretreated with ASPS was significantly improved by 390.9% (P < 0.05) (Figure 1A), and showed less edema in the cecum and a thicker colon with more and larger stool pellets compared with the LPS-treated mice (Figure 1B).
Figure 1.
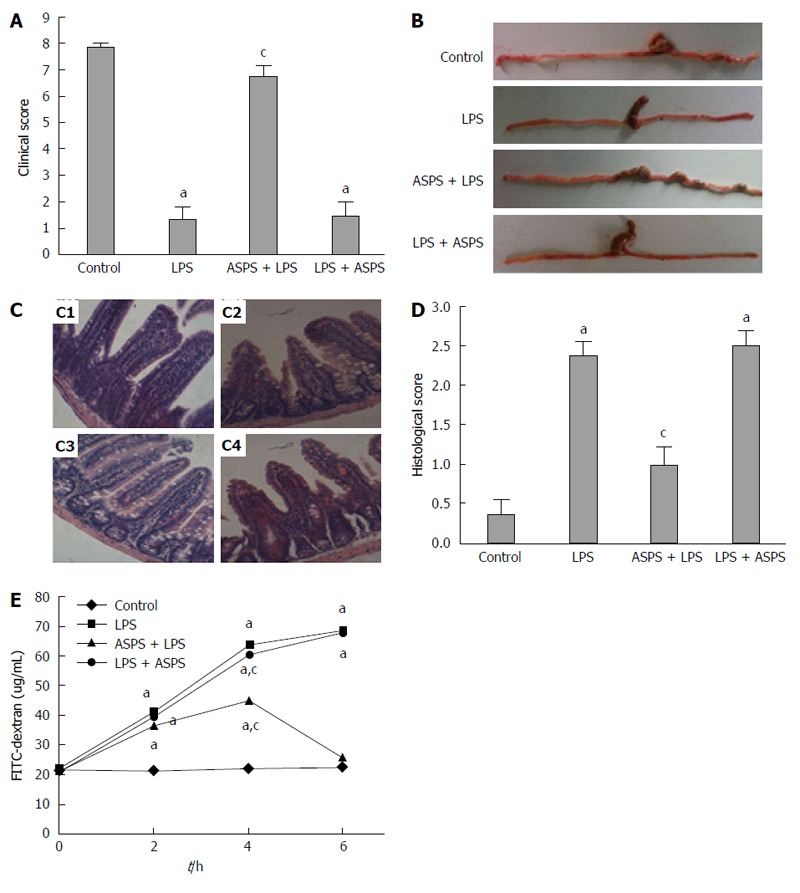
Effects of acanthopanax senticosus polysaccharides on clinical score, macroscopic features of distal ileum and colon, histological appearance and score of distal ileum in lipopolysaccharide-induced mice. A: Mice were assessed for clinical score at designated time points after lipopolysaccharide (LPS) challenge (n = 8); B: Representative photographs of the distal ileums and colons at 6 h after LPS injection (n = 8); C: Effects of acanthopanax senticosus polysaccharides (ASPS) on LPS-induced intestinal histopathologic changes. Ileum was processed for morphological and histopathologic evaluation at 6 h after LPS induction (n = 3). The representative photomicrographs of ileal segments stained with hematoxylin and eosin at 200 × magnification of C1, control group; C2, LPS group; C3, ASPS + LPS group; and C4, LPS + ASPS group; D: Intestinal histopathologic score was determined at 6 h after LPS challenge (n = 3); E: Effects of ASPS on LPS-induced increase in iliac mucosal permeability. The intestinal permeability of 4 kDa fluorescein isothiocyanate (FITC)-dextran in ileal pouch was measured at 2, 4 and 6 h after LPS administration (n = 8). aP < 0.05, vs the control group; cP < 0.05, vs the LPS group.
The histological examination using HE staining showed marked damage characterized by atrophic villi with a discontinuous brush border and irregular epithelium in endotoxemic mice in the LPS group. As expected, these negative histologic changes in the LPS group were significantly alleviated by pretreatment with ASPS rather than subsequent administration of ASPS following LPS injection (Figure 1C). The intestinal histological score in the LPS group was significantly increased compared with the control group (P < 0.05). ASPS pretreatment markedly reversed the effect of LPS by 57.89% (P < 0.05). However, oral administration of ASPS following LPS injection did not reverse the damage induced by LPS (P > 0.05) (Figure 1D).
Intestinal permeability assay
At 2, 4 and 6 h after LPS administration, gut mucosal permeability was evaluated ex vivo by measuring the leakage of FITC-dextran from the intestinal epithelium into the systemic circulation. The concentration of FITC-dextran was significantly increased after LPS administration compared with the control group (P < 0.05). A marked reduction (61.93%) in the amount of FITC-dextran in the circulation was observed in the ASPS pretreatment group (P < 0.05) rather than post-treatment in the ASPS group (P > 0.05) (Figure 1E).
TJ protein location and expression and TJ ultrastructure
The localization and expression of occludin and ZO-1 proteins were evaluated by immunofluorescence to determine the influence of ASPS on TJ disruption induced by LPS. Mice in the LPS group exhibited less staining of occludin and ZO-1 in the ileum. Correspondingly, ASPS pretreatment attenuated the redistribution of TJ proteins with the presence of continuous bands along the epithelial sheet. However, in the LPS + ASPS group, the loss of both proteins was not attenuated, and TJ distribution was similar to that in the LPS group (Figure 2A). Similarly, the expression of both proteins using immunoblotting was decreased in ileal epithelium in endotoxemic mice (P < 0.05). Pretreatment with ASPS partially up-regulated LPS-induced loss of occludin (0.597 ± 0.027 vs 0.103 ± 0.009, P < 0.05) and ZO-1 (0.507 ± 0.032 vs 0.125 ± 0.019, P < 0.05). In contrast, the administration of ASPS after LPS injection did not ameliorate the loss of these proteins (P > 0.05) (Figure 2B). The intact structure and electron dense materials between the adjoining cells observed in the control group decreased 6 h after LPS treatment. As expected, ASPS pretreatment significantly attenuated the negative changes induced by LPS induction. However, these pathologic changes were not reversed following the administration of ASPS after LPS injection (Figure 2C).
Figure 2.
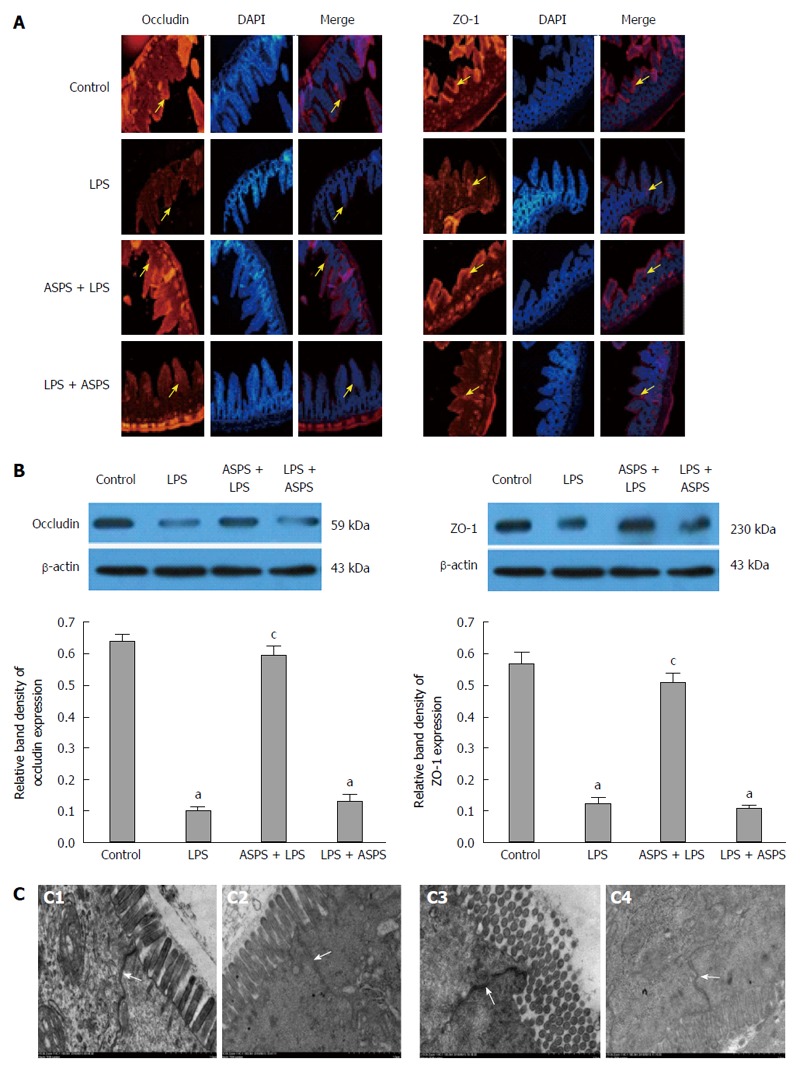
Localization and expression of tight junction proteins, and tight junction proteins ultrastructure in ileum were evaluated 6 h after lipopolysaccharide administration in mice of four groups. A: Effects of Acanthopanax senticosus polysaccharides (ASPS) on distribution of occludin and ZO-1. Staining of both proteins along the villous epithelium at a 200 × magnification (red fluorescence) were observed by immunofluorescence. Nuclei were stained by DAPI (blue fluorescence). Arrows indicate the location of tight junction (TJ) proteins staining; B: Effects of ASPS on intestinal TJ proteins expression of occludin and ZO-1 (n = 3). Protein samples were analyzed by western blotting, and β-actin was used as an internal control. The values are presented as mean ± SE. aP < 0.05, vs the control group; cP < 0.05, vs the LPS group; C: Effects of ASPS on intestinal TJ ultrastructure in ileum viewed under transmission electron microscope of C1, control group; C2, LPS group; C3, ASPS + LPS group; and C4, LPS + ASPS group. Arrows indicate the location of the TJ (scale bar = 1 μm).
NF-κB/MLCK pathway response
Figure 3A shows the nuclear expression of NF-κB p65 and MLCK, and the cytoplasmic expression of phospho-IκBα and phospho-MLC2 in intestinal epithelium analyzed by western blotting in the 4 experimental groups. Furthermore, staining of MLCK in the distal ileal epithelium was shown by immunofluorescence to determine the distribution of MLCK (Figure 3B). The expression of NF-κB p65 and MLCK in the nucleus, and phospho-IκBα and phospho-MLC2 in the cytoplasm were markedly increased in LPS-challenged mice, which was concordant with localization of MLCK at the periphery of the cells (P < 0.05). ASPS pretreatment significantly reversed the effects of endotoxemia induced by LPS on the expression of these proteins 6 h after LPS challenge (P < 0.05). However, administration of ASPS following LPS injection did not improve these effects.
Figure 3.
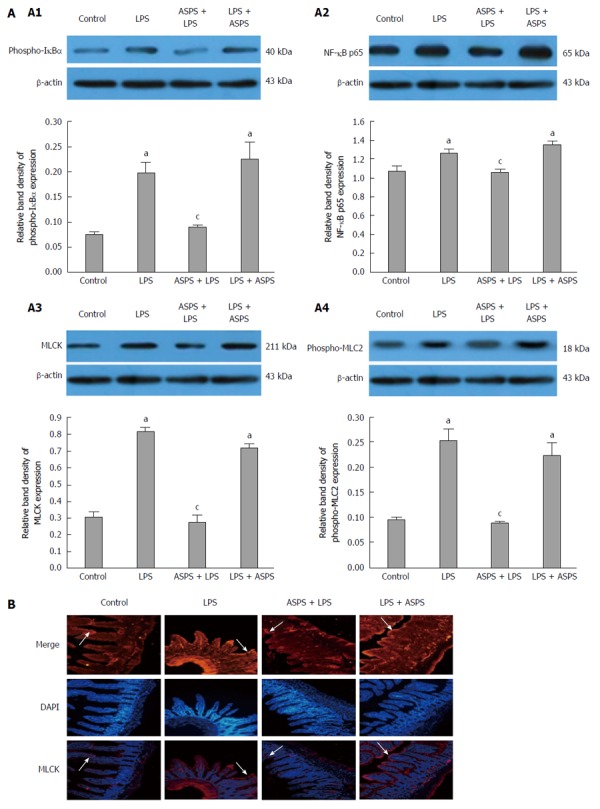
Protein expression of phospho-IκBα, nuclear factor-kappa B p65, myosin light chain kinase and phospho-MLC2 (A) and MLCK localization (B) in ileum epithelium. A: Protein expression of phospho-IκBα (A1), nuclear factor-kappa B (NF-κB) p65 (A2), myosin light chain kinase (MLCK) (A3) and phospho-MLC2 (A4) were analyzed by western blotting at 6 h after lipopolysaccharide (LPS) induction, and β-actin was used as internal control (n = 3). Data are shown as mean ± SE (n = 3). aP < 0.05, vs the control group; cP < 0.05, vs the LPS group; B: MLCK location was observed by immunofluorescence at 6 h after LPS administration at 200 × magnification (red fluorescence) (n = 3). Nuclei were stained by DAPI (blue fluorescence).
DISCUSSION
The gastrointestinal epithelium which forms a boundary effectively provides a selective permeable barrier that prevents pathogenic bacteria and their effectors entering the mucosal tissues from the intestinal lumen. This selective permeable barrier is achieved by intercellular TJ structures[8]. A TJ is a multi-protein complex comprised of the transmembrane proteins occludin, the claudin family proteins, as well as the cytoplasmic protein ZO-1, and forms a seal between adjacent intestinal epithelial cells[24]. However, opening of the TJ is primarily dependent on the composition and organization of these TJ proteins[6], which is not static but a highly dynamic structure that is constantly being remodeled due to interactions with pathogenic bacteria. These bacteria cause TJ damage and further increase intestinal permeability and the systemic inflammatory response syndrome, which is characterized by a whole body inflammatory state and multiple organ failure[9]. Therapy is conceivable by regulating TJ integrity to trigger decreased permeability via the paracellular pathway.
Although the underlying mechanism by which intestinal TJ is damaged in endotoxemia is not fully elucidated, the altered localization of TJ proteins due to activation of the NF-κB/MLCK signaling pathway is believed to play a vital role in TJ disruption in intestinal inflammation. NF-κB is a transcription factor and has long been considered the central mediator of the inflammatory process, with the main heterodimer consisting of NF-κBp65 and regulating the genes involved in many aspects of the inflammatory response[25]. NF-κBp65 can be induced to undergo cytoplasmic-to-nuclear translocation when its inhibitory factor I-κB is phosphorylated and degraded in intestinal mucosa during endotoxemia[26], and can bind to the MLCK promoter region to cause MLCK-mediated MLC phosphorylation and concomitant remodeling of the localization of TJ proteins and functional opening by contracting actin-myosin filaments[27,28]. Thus, it is becoming increasingly evident that inhibiting activation of the NF-κB/MLCK signaling pathway may potentially lead to repair of the compromised intestinal TJ barrier in endotoxemia.
ASPS are widely used as therapy for immune regulation and anti-inflammation in China. ASPS have been demonstrated to ameliorate LPS-induced inflammatory response in piglets[15] and appear to have beneficial effects against LPS-induced intestinal mucosal injury and integrity loss in the mouse model of endotoxemia by suppressing over-activation of the NF-κB signaling pathway[17]. Although NF-κB and MLCK-mediated MLC phosphorylation are clearly involved in TJ regulation in inflammation, the beneficial effects of ASPS on intestinal TJ disruption in endotoxemia and whether this signaling pathway is involved in the opening of TJ following administration of ASPS are poorly elucidated.
In the current study, a well-documented mouse model of endotoxemia induced by LPS injection was successfully used. The mice appeared to have typical clinical symptoms characterized by watery stools, increased secretion, somnolence and inactivity, as well as histopathologic macroscopic and microscopic changes, including edematous and thin intestine, villus atrophy, and epithelial shedding. In addition, 6 h after injection of LPS was chosen as the sampling time, according to previous studies which had suggested that an acute intestinal inflammatory response was observed 3-6 h after LPS injection[29,30].
HE staining and the FITC-dextran assay of distal ileum showed that ASPS alleviated mucosal integrity loss in mice with endotoxemia, as demonstrated by an improvement in morphological appearance and a decline in the concentration of FITC-dextran in plasma. These findings were consistent with our previous results regarding improved intestinal integrity by ASPS in LPS-challenged mice. ASPS also prevented LPS-induced TJ ultrastructural breakdown, supported by increased electron dense materials between adjoining cells using TEM. In addition, ASPS pretreatment positively reversed the distribution and expression of occludin and ZO-1 in mice with endotoxemia. Collectively, these results indicate that pretreatment with oral ASPS may be a preventive option for decreasing TJ disruption in endotoxemia. However, our study on the effects of ASPS administration subsequent to LPS injection demonstrated their unavailability in endotoxemia.
Gut-associated systemic infection resulting in systemic diseases is associated with increased mucosal permeability[31]. TJ opening involved in permeability regulation is primarily dependent on MLCK-mediated MLC phosphorylation during the pathophysiology of endotoxemia. In order to determine the underlying mechanism involved in the beneficial effect of ASPS on TJ opening in endotoxemic mice, activity of the NF-κB/MLCK signaling pathway in intestinal epithelium was determined and the results showed that ASPS modulated the expression of NF-κBp65 and MLCK in the nucleus and phospho-IκBα and phospho-MLC2 in the cytoplasm. The results of our study demonstrated that ASPS pretreatment suppressed the activation of related signaling molecules of the NF-κB/MLCK pathway rather than post-administration, which is consistent with the results of attenuated TJ dysfunction and decreased intestinal permeability in endotoxemic mice. This may be attributed to the pharmacokinetic features of ASPS, although little is understood regarding these features. Interestingly, our recent work may provide some clues as to whether regulatory expression of TLR4 and EGF/EGFR occurred following pretreatment with ASPS[18,32]. We suggest that ASPS administration prior to endotoxemia functions via EGF/EGFR-dependent regulation of TLR4[33], whereby EGFR mediates intestinal epithelium growth and differentiation. More attention should be paid to the relationship between EGFR and TJ proteins. However, in the case of endotoxemia, ASPS are unavailable due to LPS combining with TLR4 to activate NF-κB rather than EGF/EGFR.
It is worth noting that our present study provides a new understanding of the influencing mechanism of ASPS on TJ damage in relation to the MLCK/NF-κB pathway. Further attention to other modulations between TJ damage and the protein kinase pathway, calcium ion pathway, G proteins and so on will allow the comprehensive identification of ASPS action. In addition, ASPS intake preceding any upcoming stressful and infectious conditions is likely to be applied in routine clinical practice. Further clinical research should be carried out to accumulate evidence to support treatment with ASPS.
In conclusion, the present study demonstrates that pretreatment with oral ASPS prior to the development of endotoxemia can mitigate intestinal epithelial TJ breakdown in the mouse model of endotoxemia. The underlying mechanism may be associated with inhibition of activation of the NF-κB/MLCK signaling pathway. These results suggest that ASPS may be a potential therapeutic strategy for intestinal permeability loss in sepsis.
COMMENTS
Background
Sepsis and subsequent organ system dysfunction are the most frequent causes of death in intensive care patients worldwide, and have been identified to have a close relationship with intestinal tight junction damage induced by systemic infections. However, it is unclear whether tight junction disruption and its modulatory nuclear factor-kappa B (NF-κB)/myosin light chain kinase (MLCK) signaling pathway are influenced by Acanthopanax senticosus polysaccharides (ASPS) in endotoxemia.
Research frontiers
Understanding and regulating intestinal epithelial barrier function via relevant inflammatory signaling pathways using a safe and effective substance is an important area of future research.
Innovations and breakthroughs
The present study demonstrates that ASPS pretreatment may be associated with inhibition of the NF-κB/MLCK pathway and concomitant amelioration of intestinal epithelium tight junction dysfunction in endotoxemia.
Applications
Further clinical research should be carried out to provide evidence to support treatment with ASPS, and ASPS intake preceding any upcoming stressful and infectious conditions should be applied in routine clinical practice.
Terminology
Acanthopanax senticosus polysaccharides - a major active extract isolated from Acanthopanax senticosus, which is a well-known shrub native to far eastern areas of Russia and northern regions of Japan, Korea and China. Tight junction - a multi-protein complex that forms a seal between adjacent intestinal epithelial cells.
Peer-review
Han et al try to understand the signaling pathway involved in the beneficial effects of ASPS against LPS-induced mouse intestinal injury, which is a logical follow-up of their recent article. Essentially, the paper pointed out that pretreatment of mice with ASPS inhibited the NF-κB/ MLCK pathway.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of College of Animal Science & Veterinary Medicine, Shenyang Agricultural University, China, Protocol No. SYXK (Liao) 2011-0001.
Conflict-of-interest statement: The authors declare that there are no conflicts of interest related to this study.
Data sharing statement: The data referred to in this manuscript have been generated solely by the authors. No other party has been involved. Therefore, no additional unpublished data are available.
Peer-review started: December 11, 2016
First decision: January 10, 2017
Article in press: February 17, 2017
P- Reviewer: Amornyotin S, Marie JC S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Wang CH
References
- 1.Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–S116. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 2.Polderman KH, Girbes AR. Drug intervention trials in sepsis: divergent results. Lancet. 2004;363:1721–1723. doi: 10.1016/S0140-6736(04)16259-4. [DOI] [PubMed] [Google Scholar]
- 3.Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15:1–10. doi: 10.1097/00024382-200115010-00001. [DOI] [PubMed] [Google Scholar]
- 4.Lee SH. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res. 2015;13:11–18. doi: 10.5217/ir.2015.13.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De-Souza DA, Greene LJ. Intestinal permeability and systemic infections in critically ill patients: effect of glutamine. Crit Care Med. 2005;33:1125–1135. doi: 10.1097/01.ccm.0000162680.52397.97. [DOI] [PubMed] [Google Scholar]
- 6.Vogt A, Reuken PA, Stengel S, Stallmach A, Bruns T. Dual-sugar tests of small intestinal permeability are poor predictors of bacterial infections and mortality in cirrhosis: A prospective study. World J Gastroenterol. 2016;22:3275–3284. doi: 10.3748/wjg.v22.i11.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Zhang Q, Wang C, Liu X, Li N, Li J. Disruption of tight junctions during polymicrobial sepsis in vivo. J Pathol. 2009;218:210–221. doi: 10.1002/path.2525. [DOI] [PubMed] [Google Scholar]
- 10.Yu M, Yang S, Qiu Y, Chen G, Wang W, Xu C, Cai W, Sun L, Xiao W, Yang H. Par-3 modulates intestinal epithelial barrier function through regulating intracellular trafficking of occludin and myosin light chain phosphorylation. J Gastroenterol. 2015;50:1103–1113. doi: 10.1007/s00535-015-1066-z. [DOI] [PubMed] [Google Scholar]
- 11.Ye D, Ma I, Ma TY. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2006;290:G496–G504. doi: 10.1152/ajpgi.00318.2005. [DOI] [PubMed] [Google Scholar]
- 12.Moriez R, Salvador-Cartier C, Theodorou V, Fioramonti J, Eutamene H, Bueno L. Myosin light chain kinase is involved in lipopolysaccharide-induced disruption of colonic epithelial barrier and bacterial translocation in rats. Am J Pathol. 2005;167:1071–1079. doi: 10.1016/S0002-9440(10)61196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye D, Ma TY. Cellular and molecular mechanisms that mediate basal and tumour necrosis factor-alpha-induced regulation of myosin light chain kinase gene activity. J Cell Mol Med. 2008;12:1331–1346. doi: 10.1111/j.1582-4934.2008.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen R, Liu Z, Zhao J, Chen R, Meng F, Zhang M, Ge W. Antioxidant and immunobiological activity of water-soluble polysaccharide fractions purified from Acanthopanax senticosu. Food Chem. 2011;127:434–440. doi: 10.1016/j.foodchem.2010.12.143. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Liu LM, Song ZQ, Dong YZ, Du ZY, Ning ZC, Liu YY, Liu ZL. Survey of active components in commonly-used Chinese material medica injections and related Chinese material medica for cardiovascular disease. Zhong Cao Yao. 2015;46:2315–2328. [Google Scholar]
- 16.Han SB, Yoon YD, Ahn HJ, Lee HS, Lee CW, Yoon WK, Park SK, Kim HM. Toll-like receptor-mediated activation of B cells and macrophages by polysaccharide isolated from cell culture of Acanthopanax senticosus. Int Immunopharmacol. 2003;3:1301–1312. doi: 10.1016/S1567-5769(03)00118-8. [DOI] [PubMed] [Google Scholar]
- 17.Han J, Bian L, Liu X, Zhang F, Zhang Y, Yu N. Effects of Acanthopanax senticosus Polysaccharide Supplementation on Growth Performance, Immunity, Blood Parameters and Expression of Pro-inflammatory Cytokines Genes in Challenged Weaned Piglets. Asian-Australas J Anim Sci. 2014;27:1035–1043. doi: 10.5713/ajas.2013.13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han J, Liu L, Yu N, Chen J, Liu B, Yang D, Shen G. Polysaccharides from Acanthopanax senticosus enhances intestinal integrity through inhibiting TLR4/NF-κB signaling pathways in lipopolysaccharide-challenged mice. Anim Sci J. 2016;87:1011–1018. doi: 10.1111/asj.12528. [DOI] [PubMed] [Google Scholar]
- 19.Staub AM. Removal of protein-sevag method. In: Whistler RL, Wolfrom ML, editors. Methods in carbohydrate chemistry. New York: Academic Press; 1965. pp. 5–6. [Google Scholar]
- 20.DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 21.Ou YF, Yin PH, Zhao L, Huang XS. Determination of monsaccharides in garlic polysaccharide using ion chromatography. Spectrographic Lab. 2006;23:629–632. [Google Scholar]
- 22.Kadl A, Pontiller J, Exner M, Leitinger N. Single bolus injection of bilirubin improves the clinical outcome in a mouse model of endotoxemia. Shock. 2007;28:582–588. doi: 10.1097/shk.0b013e31804d41dd. [DOI] [PubMed] [Google Scholar]
- 23.Gu L, Li N, Gong J, Li Q, Zhu W, Li J. Berberine ameliorates intestinal epithelial tight-junction damage and down-regulates myosin light chain kinase pathways in a mouse model of endotoxinemia. J Infect Dis. 2011;203:1602–1612. doi: 10.1093/infdis/jir147. [DOI] [PubMed] [Google Scholar]
- 24.Watts T, Berti I, Sapone A, Gerarduzzi T, Not T, Zielke R, Fasano A. Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc Natl Acad Sci USA. 2005;102:2916–2921. doi: 10.1073/pnas.0500178102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawrence T, Fong C. The resolution of inflammation: anti-inflammatory roles for NF-kappaB. Int J Biochem Cell Biol. 2010;42:519–523. doi: 10.1016/j.biocel.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Arita Y, Ito T, Oono T, Kawabe K, Hisano T, Takayanagi R. Lysophosphatidic acid induced nuclear translocation of nuclear factor-kappaB in Panc-1 cells by mobilizing cytosolic free calcium. World J Gastroenterol. 2008;14:4473–4479. doi: 10.3748/wjg.14.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol. 2005;288:G422–G430. doi: 10.1152/ajpgi.00412.2004. [DOI] [PubMed] [Google Scholar]
- 29.Al-Sadi R, Ye D, Dokladny K, Ma TY. Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability. J Immunol. 2008;180:5653–5661. doi: 10.4049/jimmunol.180.8.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mercer DW, Smith GS, Cross JM, Russell DH, Chang L, Cacioppo J. Effects of lipopolysaccharide on intestinal injury; potential role of nitric oxide and lipid peroxidation. J Surg Res. 1996;63:185–192. doi: 10.1006/jsre.1996.0245. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Huang J, Hou Y, Zhu H, Zhao S, Ding B, Yin Y, Yi G, Shi J, Fan W. Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br J Nutr. 2008;100:552–560. doi: 10.1017/S0007114508911612. [DOI] [PubMed] [Google Scholar]
- 32.Han J, Xu Y, Yang D, Yu N, Bai Z, Bian L. Effect of Polysaccharides from Acanthopanax senticosus on Intestinal Mucosal Barrier of Escherichia coli Lipopolysaccharide Challenged Mice. Asian-Australas J Anim Sci. 2016;29:134–141. doi: 10.5713/ajas.15.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu K, Anderson GP, Bozinovski S. DNA vector augments inflammation in epithelial cells via EGFR-dependent regulation of TLR4 and TLR2. Am J Respir Cell Mol Biol. 2008;39:305–311. doi: 10.1165/rcmb.2007-0458OC. [DOI] [PubMed] [Google Scholar]


