Abstract
Brain atrophy occurs at a faster rate in patients with multiple sclerosis (MS) than in healthy individuals. In three randomized, controlled, phase III trials, fingolimod reduced the annual rate of brain volume loss (BVL) in patients with relapsing MS (RMS) by approximately one-third relative to that in individuals receiving placebo or intramuscular interferon beta-1a. Analysis of brain volume changes during study extensions has shown that this reduced rate of BVL is sustained in patients with RMS receiving fingolimod continuously. Subgroup analyses of the core phase III and extension studies have shown that reductions in the rate of BVL are observed irrespective of levels of inflammatory lesion activity seen by magnetic resonance imaging at baseline and on study; levels of disability at baseline; and treatment history. The rate of BVL in these studies was predicted independently by T2 lesion and gadolinium-enhancing lesion burdens at baseline, and correlations observed between BVL and increasing levels of disability strengthened over time. In another phase III trial in patients with primary progressive MS (PPMS), fingolimod did not reduce BVL overall relative to placebo; however, consistent with findings in RMS, there was a treatment effect on BVL in patients with PPMS with gadolinium-enhancing lesion activity at baseline. The association between treatment effects on BVL and future accumulation of disability argues in favor of measuring BVL on a more routine basis and with a more structured approach than is generally the case in clinical practice. Despite several practical obstacles, progress is being made in achieving this goal.
Key Points
| Fingolimod reduces the rate of brain volume loss in patients with relapsing multiple sclerosis (RMS), and this effect is independent of disease status and previous treatment history. |
| Brain volume is clinically relevant in RMS because it tracks disease progression, and the rate of brain volume loss predicts long-term disability. |
| Routine measurement of brain volume in RMS could be valuable in informing treatment decisions. |
| Although reliable, longitudinal measurement of brain volume to determine rate of loss is difficult in routine clinical practice; developments in magnetic resonance imaging analysis are beginning to address the challenges faced. |
Introduction
Brain atrophy is a characteristic feature of multiple sclerosis (MS) that occurs throughout the disease course [1–3]. On magnetic resonance imaging (MRI), brain volume loss (BVL) between two or more time points can be accurately determined by co-registration-based analysis, using algorithms such as ‘Structural Image Evaluation, using Normalization, of Atrophy’ (SIENA) [1, 4–6]. Patients with MS lose brain volume (BV) at a considerably faster rate than age-matched individuals who do not have MS [1, 3, 7, 8], and data from studies using the SIENA method have shown that the annual percentage BV change (PBVC) among healthy adults is approximately −0.1 to −0.3% [8–11], compared with −0.4 to −1.0% in patients with MS [2, 3, 7, 8, 11]. Furthermore, a threshold annual rate of −0.4% can distinguish healthy individuals from patients with MS, with a specificity of 80% and a sensitivity of 65% [8]. BVL has been shown to correlate with and predict disability [1, 12–15], representing a relevant measure of central nervous system damage associated with MS disease progression. As such, BVL is a useful outcome measure for evaluating the relative efficacy of MS therapies [16–18].
Fingolimod (Gilenya®, FTY720; Novartis Pharma AG, Basel, Switzerland) is a sphingosine 1-phosphate receptor modulator and was the first oral therapy (0.5 mg once daily) available for patients with relapsing MS (RMS) [19]. In three randomized, double-blind, controlled, phase III studies of fingolimod in patients with RMS (FREEDOMS [FTY720 Research Evaluating Effects of Daily Oral therapy in Multiple Sclerosis] [20], FREEDOMS II [21], and TRANSFORMS [Trial Assessing injectable interferon vs FTY720 Oral in Relapsing-remitting Multiple Sclerosis] [22]), consistent reductions in the rate of BVL were observed with fingolimod compared with placebo or intramuscular interferon beta-1a (IFNβ-1a IM) [20–22]. Reductions in the rate of BVL have also been reported for a number of MS disease-modifying therapies [11]. Treatment with IFNβ or glatiramer acetate reduced the rate of BVL compared with no treatment in patients with relapsing–remitting MS (RRMS), and over 5 years BVL was lower with glatiramer acetate than with either low-dose or high-dose IFNβ [23]. More recently, a retrospective analysis of natalizumab therapy in patients with MS showed that although significant BVL occurred during the first year of therapy, the rate was reduced in the second and third years [24], and compared with subcutaneous IFNβ, alemtuzumab slowed the rate of BVL in patients with RRMS over 2 years in the two CARE-MS (Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis) trials [25].
The precise mechanism by which fingolimod reduces the rate of BVL in patients with RMS has not been fully elucidated, but may be a consequence of direct anti-inflammatory effects and of indirect effects on neurodegenerative processes triggered by inflammation [26]. Data reviewed here will focus on the fingolimod 0.5-mg dose, although significant effects on BVL were also seen among patients receiving the 1.25-mg dose in clinical trials. A substantial proportion of participants in FREEDOMS, FREEDOMS II and TRANSFORMS continued to receive fingolimod in the respective extensions to these studies [27–29], and most continue to be followed up under the umbrella of the LONGTERMS study, a single-arm, open-label extension following up patients with relapsing MS receiving fingolimod, who were enrolled in the various phase II, III and IIIb fingolimod trials [30]. The effect of fingolimod in patients with primary progressive MS (PPMS) has also been evaluated in the phase III placebo-controlled INFORMS trial (Oral Fingolimod in Primary Progressive Multiple Sclerosis) [31].
In this review, we will focus on the effects of fingolimod in reducing the rate of BVL in these studies, and in various patient subgroups, based on demographic, clinical, or MRI characteristics at baseline. We will also discuss the clinical significance of, and factors that may be predictive of, increased rates of BVL in patients with MS. Finally, we will summarize guideline recommendations and challenges for the routine assessment of changes in BV in clinical practice.
Effect of Fingolimod on BVL in Patients with RMS
Reductions in BVL with Fingolimod: Evidence from Randomized, Double-Blind, Controlled Phase III Studies
The three phase III studies of fingolimod in patients with RMS were FREEDOMS [20], FREEDOMS II [21] and TRANSFORMS [22] (see Table 1 for an overview of the study designs). Across these trials, BVL was measured by estimating PBVC using SIENA. Reductions in BVL with fingolimod were rapid (detected at 6 months) compared with placebo in the FREEDOMS trials [20, 21], and were demonstrated consistently over 2 years in these trials, and over 1 year compared with IFNβ-1a IM in TRANSFORMS [22].
Table 1.
Study designs of the pivotal phase III and IV trials of daily oral fingolimod in patients with MS
| Study name and identifier | Design | Duration | Patients | Intervention/s | Brain volume assessment |
|---|---|---|---|---|---|
| RMS studies | |||||
| FREEDOMS NCT00289978 [20] |
Multicenter, phase III, randomized, double-blind, placebo-controlled, parallel-group | 2 years | Aged 18–55 years; RMS according to 2005 revised McDonald criteria; ≥1 confirmed relapses during the preceding year (or ≥2 during the previous 2 years); EDSS score of 0.0–5.5; treatment-naïve or previously treated; no relapse or steroid treatment within 30 days of randomization (N = 1272) | Fingolimod 1.25 mg (n = 429) Fingolimod 0.5 mg (n = 425) Placebo (n = 418) |
MRI scan at screening, 6, 12, and 24 months; PBVC evaluated with SIENA |
| FREEDOMS II NCT00355134 [21] |
Multicenter, phase III, randomized, double-blind, placebo-controlled, double-dummy, parallel-group | 2 years | Aged 18–55 years; RMS according to 2005 revised McDonald criteria; ≥1 confirmed relapses during the preceding year (or ≥2 during the previous 2 years); EDSS score of 0.0–5.5; treatment-naïve or previously treated; no relapse or steroid treatment within 30 days of randomization (N = 1083) | Fingolimod 1.25 mg (n = 370) Fingolimod 0.5 mg (n = 358) Placebo (n = 355) |
MRI scan at screening, 6, 12, and 24 months; PBVC evaluated with SIENA |
| TRANSFORMS NCT00340834 [22] |
Multicenter, phase III, randomized, double-blind, active-controlled, double-dummy, parallel-group | 1 year | Aged 18–55 years; RMS according to 2005 revised McDonald criteria; ≥1 confirmed relapses during the preceding year (or ≥2 during the previous 2 years); EDSS score of 0.0–5.5; treatment-naïve or previously treated with IFNβ or GA; no relapse or steroid treatment within 30 days of randomization (N = 1292) | Fingolimod 1.25 mg (n = 426) Fingolimod 0.5 mg (n = 431) IFNβ-1a IM 30 μg/week (n = 435) |
MRI scan at screening and 12 months; PBVC evaluated with SIENA |
| FREEDOMS extension NCT00662649 [27] |
Dose-blinded, parallel-group extension | 2 years | Completed FREEDOMS; did not discontinue study owing to an AE; did not experience onset of chronic immune system disease requiring immunosuppressive treatment (N = 920) |
Continuous
Fingolimod 1.25 mg (n = 289) Fingolimod 0.5 mg (n = 331) Switch Fingolimod 1.25 mg (n = 145) Fingolimod 0.5 mg (n = 155) |
MRI scan every 12 months; PBVC evaluated with SIENA |
| FREEDOMS II extension [29, 72] | Randomized, dose-blinded, then open-label extension | 2 years | Completed FREEDOMS II (N = 632) |
Continuous
Fingolimod 1.25 mg (n = 203) Fingolimod 0.5 mg (n = 217) Switch Fingolimod 1.25 mg (n = 105) Fingolimod 0.5 mg (n = 107) |
MRI scan at baseline, month 12, month 24, and EOS; PBVC evaluated with SIENA |
| TRANSFORMS extension NCT00340834 [28, 36] |
Randomized, dose-blinded, double-blinded, then open-label extension | 2 years, then up to 4.5 years | Completed TRANSFORMS (N = 1027) |
Continuous
Fingolimod 1.25 mg (n = 330) Fingolimod 0.5 mg (n = 356) Switch Fingolimod 1.25 mg (n = 174) Fingolimod 0.5 mg (n = 167) |
MRI scan at screening, month 12, and month 24, at study discontinuation and at a 3-month follow-up visit; PBVC evaluated with SIENA |
| LONGTERMS NCT01281657 [30, 34, 73] |
Single-arm, open-label, long-term follow-up extension | Up to 7 years | Completed phase II, III and IIIb trials or extension studies (N = 2355) |
Continuous
Fingolimod 0.5 mg (n = 783) Switch Placebo–fingolimod 0.5 mg (n = 773) |
MRI scan at screening and at months 6, 12, 24, 36, 48, 60, and 72; PBVC evaluated with SIENA |
| PPMS studies | |||||
| INFORMS NCT00731692 [31] |
Multicenter, phase III, randomized, double-blind, placebo-controlled | 3–5 years | Aged 25–65 years old; PPMS according to 2005 revised McDonald criteria; at least 1 year of disease progression; two or more of the following: positive brain MRI; positive spinal cord MRI; positive cerebrospinal fluid; EDSS score of 3.5–5.6; increase in EDSS score of ≥0.5 points in the past 2 years (N = 970) |
Cohort 1
Fingolimod 1.25 mg (n = 147) Placebo (n = 133) Cohort 2 Fingolimod 0.5 mg (n = 336) Placebo (n = 354) |
MRI scan at screening and every 12 months; PBVC evaluated with SIENA |
AE adverse event, EDSS Expanded Disability Status Scale, EOS end of study, GA glatiramer acetate, IFNβ-1a interferon beta-1a, IM intramuscular, MRI magnetic resonance imaging, MS multiple sclerosis, PBVC percentage brain volume change, PPMS primary progressive multiple sclerosis, RMS relapsing multiple sclerosis, SIENA Structural Image Evaluation, using Normalization, of Atrophy
Fingolimod versus Placebo: FREEDOMS and FREEDOMS II
The FREEDOMS [20] and FREEDOMS II [21] trials were 24-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group studies that compared the efficacy of oral fingolimod (1.25 and 0.5 mg/day) with placebo in patients with RMS (Table 1). In FREEDOMS (N = 1272), both doses of fingolimod significantly reduced the rate of BVL over 2 years, compared with placebo. The reduction in mean rate of BVL was 35% with fingolimod compared with placebo (p < 0.001) after 24 months of treatment. Of note, significant reductions in the rate of BVL compared with placebo were detected as early as 6 months in both treatment groups (Table 2) [20]. Similar effects were seen in the FREEDOMS II study (N = 1083); after 24 months of treatment, the reduction in mean rate of BVL was 33% with fingolimod compared with placebo (p < 0.001), and this effect on BVL was detected as early as 6 months (Table 2) [21].
Table 2.
Percentage change in BV during three pivotal phase III trials of fingolimod in patients with RMS
| Endpoint | Fingolimod 0.5 mg, oral | Placebo | IFNβ-1a IM 30 μg/week |
|---|---|---|---|
| Mean (SD) change in BV from 0 to 6 months, % | |||
| FREEDOMS [20] | −0.22 (0.81); p = 0.006 | −0.34 (0.73) | |
| FREEDOMS II [21] | −0.23 (0.87); p = 0.012 | −0.38 (0.91) | |
| Mean (SD) change in BV from 0 to 12 months, % | |||
| FREEDOMS [20] | −0.50 (1.05); p = 0.03 | −0.65 (1.05) | |
| FREEDOMS II [21] | −0.38 (0.97); p < 0.001 | −0.63 (1.05) | |
| TRANSFORMS [22] | −0.31 (0.65); p < 0.001 | −0.45 (0.73) | |
| Mean (SD) change in BV from 12 to 24 months, % | |||
| FREEDOMS [20] | −0.37 (0.81); p < 0.001 | −0.67 (1.07) | |
| FREEDOMS II [21] | −0.49 (0.90); p = 0.013 | −0.68 (1.10) | |
| Mean (SD) change in BV from 0 to 24 months, % | |||
| FREEDOMS [20] | −0.84 (1.31); p < 0.001 | −1.31 (1.50) | |
| FREEDOMS II [21] | −0.86 (1.22); p < 0.001 | −1.28 (1.50) | |
Measured by SIENA
BV brain volume, IFNβ-1a interferon beta-1a, IM intramuscular, RMS relapsing multiple sclerosis, SD standard deviation, SIENA Structural Image Evaluation, using Normalization, of Atrophy
p Values in FREEDOMS and FREEDOMS II are versus placebo; p values in TRANSFORMS are versus IFNβ-1a IM
Fingolimod versus IFNβ-1a IM: TRANSFORMS
TRANSFORMS (N = 1292) was a 12-month, multicenter, randomized, double-blind, active-controlled, double-dummy, parallel-group study that compared the efficacy of oral fingolimod (1.25 and 0.5 mg/day) with IFNβ-1a IM (30 µg/week) in patients with RMS (Table 1) [22]. In this study, both doses of fingolimod significantly reduced the rate of BVL compared with IFNβ-1a IM; after 12 months of treatment, the reduction in mean rate of BVL was 32% with fingolimod 0.5 mg compared with IFNβ-1a IM (p < 0.001; Table 2) [22].
Post Hoc Analyses of FREEDOMS, FREEDOMS II and TRANSFORMS
An analysis of data pooled from FREEDOMS, FREEDOMS II, TRANSFORMS, and their extensions was conducted to determine the delay in BVL following continuous treatment with fingolimod relative to that following treatment with placebo or IFNβ-1a IM. The analysis calculated the additional time required for fingolimod-treated patients to reach the levels of BVL observed in the control groups at the end of the core study. It showed that patients receiving continuous fingolimod took 56–61% longer than those receiving placebo, and 46% longer than those receiving IFNβ-1a IM, to sustain comparable levels of BVL [32].
Sustained Effects of Fingolimod in Reducing BVL
The long-term effects of fingolimod on BVL have been evaluated in the FREEDOMS, FREEDOMS II, and TRANSFORMS extensions [27–29] (see Table 1 for an overview of extension study designs). Patients who completed each of the three core phase III studies were eligible to enter the respective extension study, in which they either continued to receive fingolimod (continuous groups) or were switched from placebo (FREEDOMS and FREEDOMS II) or from IFNβ-1a IM (TRANSFORMS) to receive fingolimod (switch groups). Patients in the switch groups were randomized 1:1 to receive either fingolimod 1.25 mg or fingolimod 0.5 mg [27–29], although all patients receiving fingolimod were later de-escalated to the 0.5 mg dose. The beneficial effects of fingolimod on BVL that were observed in the FREEDOMS, FREEDOMS II, and TRANSFORMS core studies were maintained among patients who continued to receive fingolimod in the respective extension studies. Furthermore, patients who switched to fingolimod during the extensions (from either placebo or IFNβ-1a IM in the core studies), benefited from reduced rates of BVL relative to the rates experienced during the core studies [27–29].
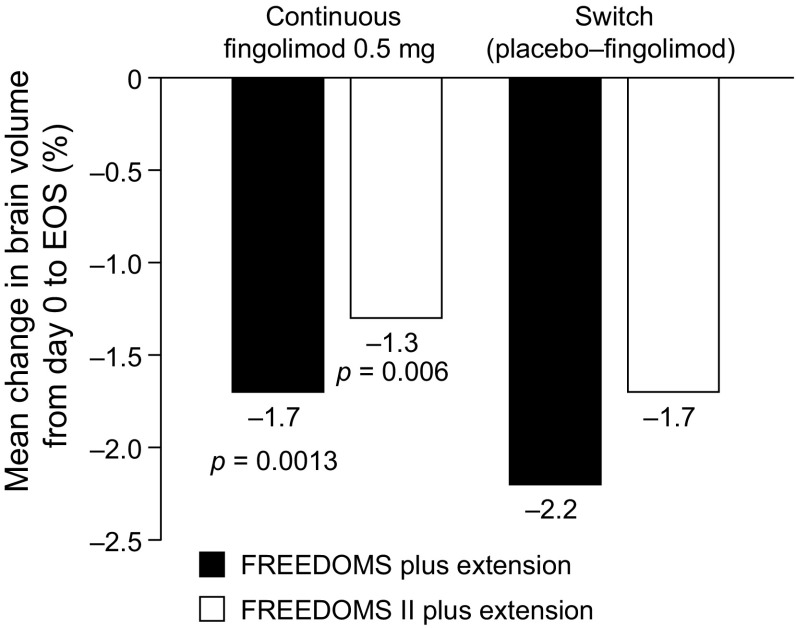
In the FREEDOMS extension (N = 920), patients who received continuous fingolimod experienced less overall BVL at 4 years than patients who were switched from placebo to fingolimod. Mean (standard deviation) exposure to fingolimod was 1394 (208) days in the continuous group and 669 (206) days in the switch group. From day 0 (FREEDOMS baseline) to end of study (EOS) in the FREEDOMS extension, the mean rate of BVL was 23% lower in the continuous fingolimod group (1.7%) than in the switch group (2.2%; p = 0.0013) (Fig. 1). However, mean BVL within the group that switched to fingolimod 0.5 mg was 1.42% during months 0–24 and 0.90% during months 24–48 (p = 0.008). Moreover, during months 24–48, mean rates of BVL in the continuous fingolimod group and in the switch group were similar (0.78 and 0.90%, respectively). The comparably low rates of BVL reported across all groups during the extension study are consistent with the assumption that effects of fingolimod on BVL are not confined to the first 2 years of treatment [27]. Similar effects were seen in the FREEDOMS II extension study (N = 632). From day 0 to EOS, the mean rate of BVL was 25% lower in the continuous fingolimod group than in the switch group (p = 0.006 vs switch group) (Fig. 1). However, from month 24 to EOS, there were no significant between-group differences in BVL [29].
Fig. 1.
Mean percentage change in BV (measured with SIENA) from day 0 to EOS in FREEDOMS, FREEDOMS II and their extensions [27, 29]. All p values are versus the respective extension study switch group. BV brain volume, EOS end of study, SIENA Structural Image Evaluation, using Normalization, of Atrophy
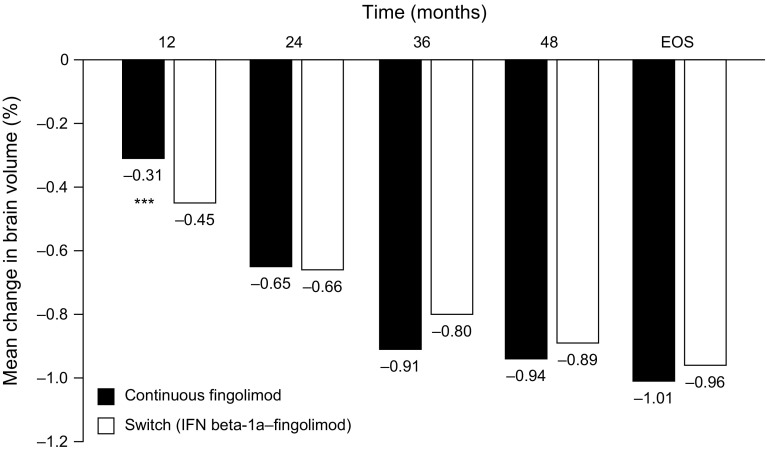
During the TRANSFORMS extension (N = 1027), patients in the fingolimod 0.5 mg switch group experienced pronounced (approximately 50%) and significant reductions in the rate of BVL compared with the reductions sustained with IFNβ-1a IM during the core study. Mean BVL was 0.45% in the 12 months before switching and 0.22% in the 12 months after switching (p = 0.006) [28]. This effect was maintained throughout the TRANSFORMS extension, with patients in the switch groups experiencing a reduced rate of BVL for up to 4.5 years of treatment. By the end of the extension, those in the switch group had ‘caught up’ with those receiving fingolimod continuously, mean BVL from baseline in the two groups being 0.96 and 1.01%, respectively; p = not significant) (Fig. 2) [33].
Fig. 2.
Mean percentage change in BV (measured with SIENA) from core study baseline in the continuous and switched groups of patients participating in TRANSFORMS and its extension. In the switched group, patients were switched from IFN beta-1a IM to fingolimod at month 12. Adapted with permission from Cohen et al. [33]. BV brain volume, EOS end of study, IFN beta-1a interferon beta-1a, IM intramuscular, SIENA Structural Image Evaluation, using Normalization, of Atrophy; ***p < 0.001 versus switch group
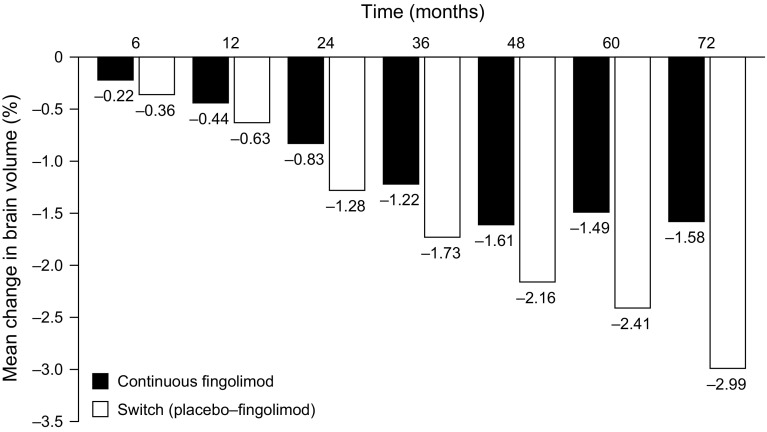
In the ongoing LONGTERMS trial (N = 2355), an open-label, single-arm, long-term extension to various phase II, III and IIIb trials including FREEDOMS, FREEDOMS II, and TRANSFORMS (Table 1), interim data demonstrated that low rates of BVL were sustained for up to 6 years of treatment, although the number of patients at months 60 and 72 was small (analyses to date have included only patients randomized initially to receive fingolimod 0.5 mg in the two FREEDOMS trials). Importantly, in terms of reduced levels of BVL, patients who were randomized to placebo in the core FREEDOMS trials and then switched to fingolimod in the extension studies did not recoup the advantages gained by those who received continuous fingolimod in both the core and extension studies (Fig. 3) [34].
Fig. 3.
Mean percentage change in BV (measured with SIENA) from baseline (core FREEDOMS and FREEDOMS II) in the continuous and switched groups of patients participating in the LONGTERMS extension study [34]. In the switched groups, patients were switched from placebo to fingolimod at month 24. BV brain volume, SIENA Structural Image Evaluation, using Normalization, of Atrophy
Patient History and Disease Characteristics Can Affect BVL, But Do Not Generally Influence the Effect of Fingolimod on BVL
Inflammatory Disease Activity at Baseline: Gadolinium-Enhancing Lesions and Relapses
Patient subgroup analyses were performed in the FREEDOMS population to determine whether BVL, and the effect of fingolimod on BVL, were affected by baseline inflammatory activity, defined as the presence or absence of gadolinium-enhancing (Gd+) lesions at baseline. Overall, BVL was greater in patients with Gd+ lesions at baseline than in those without; however, fingolimod significantly reduced BVL in both subgroups compared with placebo (Fig. 4). In the subgroup of patients with Gd+ lesions at baseline, BVL was similar in the fingolimod 0.5 mg and placebo arms at months 6 and 12, but was significantly lower with fingolimod than with placebo at month 24 (p = 0.01). Among patients with no Gd+ lesions at baseline, a slower rate of BVL was observed with fingolimod than with placebo as early as month 6 (p = 0.01), and this was maintained at month 24 (p = 0.002). During months 12–24, fingolimod resulted in significant reductions in BVL relative to placebo, regardless of Gd+ lesion status at baseline (presence, p < 0.001; absence, p = 0.002). The apparent lack of a treatment effect at months 6 and 12 in the subgroup of patients with Gd+ lesions at baseline may be attributable to accelerated BVL caused by fingolimod reducing inflammatory activity in these patients (i.e., pseudoatrophy). Taken together, the significantly lower rate of BVL at 6 months in patients with no Gd+ lesions on fingolimod (vs on placebo) and the effect of fingolimod from month 12 onwards in patients regardless of their Gd+ lesion status at baseline support the hypothesis that fingolimod has an early and continuous direct beneficial effect on BVL [35].
Fig. 4.
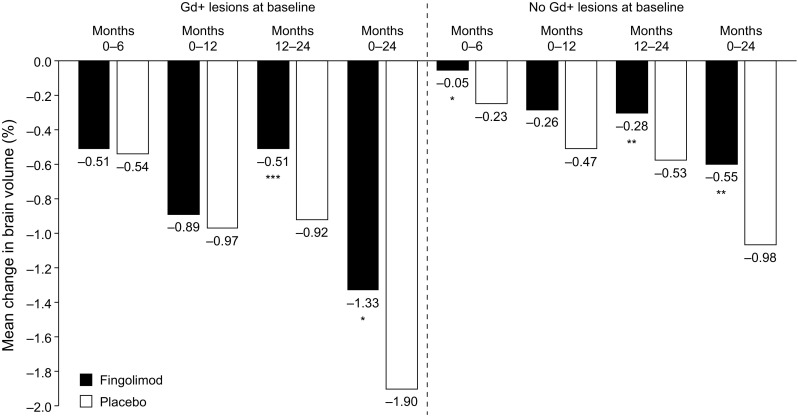
Mean percentage change in BV (measured with SIENA) from baseline in the 2-year FREEDOMS trial, in subgroups of patients with and without gadolinium-enhancing lesions at baseline [35]. BV brain volume, Gd+ gadolinium-enhancing, SIENA Structural Image Evaluation, using Normalization, of Atrophy; *p < 0.05, **p < 0.01, ***p < 0.001 versus placebo
A similar subgroup analysis in the TRANSFORMS population also identified a higher rate of BVL among patients with, than among those without, Gd+ lesions at baseline. Fingolimod reduced BVL relative to IFNβ-1a IM in both subgroups at 12 months, but this between-group difference only reached significance in patients who had Gd+ lesions at baseline [36]. The same analysis of TRANSFORMS also stratified patients by their relapse activity in the 2 years before enrollment. Fingolimod reduced BVL compared with IFNβ-1a IM in all of the subgroups analyzed, with larger effect sizes seen among patients with higher numbers of relapses (two or more relapses during the 2 years before enrollment), and the smallest (non-significant) effect size observed in those with only one relapse in the 2 years before enrollment [36].
Disease Burden at Baseline: T2 Lesions and Disability
Patient subgroup analyses also examined whether T2 lesion burden or disability status at baseline influenced the effect of fingolimod. In FREEDOMS, patients with a total baseline T2 lesion volume of >3300 mm3 had a faster rate of BVL than those with a lower T2 lesion burden. Despite this difference in the rate of BVL, fingolimod significantly reduced BVL in both higher and lower lesion-burden subgroups relative to placebo (relative reductions of 34.5% [p < 0.001] and of 37.3% [p = 0.02], respectively) [35]. Dichotomization of patients in TRANSFORMS using the same criteria also identified a higher rate of BVL among patients with higher T2 lesion volumes. Again, fingolimod reduced the rate of BVL in both subgroups relative to IFNβ-1a IM, although the difference between treatments reached significance only among those with a higher T2 lesion burden [36].
To examine whether the effect of fingolimod on BVL was influenced by disability level at baseline, subgroup analyses were conducted based on patients’ baseline Expanded Disability Status Scale (EDSS) scores (EDSS score of 0–3.5 or >3.5). In FREEDOMS, fingolimod reduced BVL substantially relative to placebo at 2 years in both subgroups, but the difference between treatments was not significant among those with more severe disability (relative BVL reduction: EDSS score ≤3.5, 32.7% [p < 0.001]; EDSS score >3.5, 42.2% [p = 0.10]) [35]. Essentially, the same pattern was seen in TRANSFORMS, fingolimod being associated with greater reductions in BVL compared with IFNβ-1a IM, although the difference between treatments was significant in both EDSS subgroups (p = 0.043 and p = 0.008, respectively) [36].
Ongoing Disease Activity: Gd+ Lesions, New or Enlarged T2 Lesions and Disability
To determine whether ongoing inflammatory MRI disease activity influenced the effect of fingolimod on BVL, the following dichotomous subgroups in TRANSFORMS were examined: presence/absence of Gd+ lesions at baseline; presence/absence of new disease activity (Gd+ lesions and new or enlarged T2 lesions) at month 12; and presence/absence of Gd+ lesions at baseline and new disease activity at month 12. There were generally higher rates of BVL among patients with ongoing inflammatory disease activity than among those with none, and fingolimod reduced BVL significantly relative to IFNβ-1a IM over 12 months in most subgroups. For fingolimod and IFNβ-1a IM, BVL was 0.40 and 0.52%, respectively, among patients with new disease activity at month 12 (p = 0.023), and 0.22 and 0.37% among those with no new disease activity at month 12 (p = 0.046). Among patients with Gd+ lesions at baseline and new disease activity at month 12, respective BVL was 0.39 and 0.55% (p = 0.012). However, fingolimod did not exert a significant treatment effect on BVL among patients with no MRI lesion activity at baseline or at month 12 (fingolimod 0.19%; IFNβ-1a IM 0.26%; p = 0.536) [37].
Building on such investigations of disease activity on study, an analysis was undertaken to examine a more comprehensive classification of disease activity than that based on MRI lesion activity alone. Over 4 years in FREEDOMS and its extension, effects on BVL were compared in patients with or without disease activity, based on the following classification of being disease-free: no disability progression (defined as an increase in EDSS score of ≥1.0 confirmed at 3 months), no confirmed relapses, no Gd+ lesions, and no new or enlarged T2 lesions. Throughout the 4-year study period, patients who were disease-free experienced less BVL than those with disease activity (BVL 0–48 months: disease-free 1.36%; not disease-free 2.08%), and this difference was observed as early as 12 months (BVL 0–12 months: disease-free 0.17%; not disease-free 0.60%). Regardless of disease-activity status, however, patients who received fingolimod continuously experienced less BVL than those who were switched from placebo to fingolimod at extension study entry [38].
Treatment History and Response to Previous Treatment
As well as considering whether patients’ disease status and history might influence the effect of fingolimod on BVL, analyses were undertaken to examine whether patients’ treatment history before receiving fingolimod may be important. At month 24 in FREEDOMS, fingolimod reduced BVL by 48.8% in previously treated patients, and by 24.4% in previously untreated patients relative to those receiving placebo (p = 0.002 and p = 0.02, respectively) [35]. At month 12 in TRANSFORMS, fingolimod reduced BVL relative to IFNβ-1a IM in both treatment-naïve (p = 0.01) and previously treated patients, although the between-group effect did not reach significance in the latter subgroup (p = 0.108) [36].
Further analyses then examined patients, either previously treated or treatment-naïve, who had relatively high levels of disease activity at enrollment into the phase III trials, in order to examine BVL outcomes among patients who were apparently non-responsive to other treatments. Patients in FREEDOMS and in TRANSFORMS were stratified into three subgroups, which were categorized based on the European approval criteria for fingolimod at the time. Patients in group 1 had had at least as many relapses in the year before enrollment as in the preceding year, and those in group 2 had had at least one relapse in the year before enrollment, and at least either one Gd+ lesion or nine T2 lesions at baseline; patients in both groups had previously received IFNβ therapy before enrollment. Group 3 comprised treatment-naive patients who had experienced at least two relapses in the year before enrollment and had at least one Gd+ lesion at baseline. In groups 1 and 2 in FREEDOMS, the rate of BVL was reduced with fingolimod by 76–78% relative to placebo over 24 months (p < 0.001, both groups), and in TRANSFORMS, fingolimod reduced BVL over 12 months in both groups by 40% relative to IFNβ-1a (p = 0.006 and p = 0.016, respectively). A similar, non-significant reduction (approximately 38%) was observed relative to IFNβ-1a IM among patients in group 3 in TRANSFORMS, but there was little evidence of a reduction in BVL relative to placebo in group 3 in FREEDOMS [39]. As patients in group 3 were treatment-naïve and had inflammatory disease activity at enrollment, it is likely that BVL measured in those receiving fingolimod was at least in part attributable to pseudoatrophy.
Analysis of groups 1 and 2 (defined as above but among patients receiving any disease-modifying therapy in the year before enrollment) in a large patient population based on data pooled from FREEDOMS and FREEDOMS II identified BVL reductions of 45–50% (p < 0.001) over 24 months with fingolimod relative to placebo [40]. Analysis of patients from this pooled population, who had been previously treated with glatiramer acetate and fulfilled the criteria for categorization in groups 1 and 2, showed that fingolimod reduced median BVL by 56% over 24 months relative to placebo (0.70 and 1.58%, respectively; p = 0.004) [41].
Fingolimod in Primary Progressive MS
Fingolimod Did Not Reduce BVL in the Placebo-Controlled Phase III INFORMS Trial
Approximately 10–15% of patients with MS experience progressive disability from disease onset, with no, or with very infrequent relapses (i.e. PPMS) [42, 43]. The INFORMS trial (N = 970) was a phase III, randomized, double-blind, placebo-controlled, parallel-group study designed to assess the efficacy and safety of fingolimod in patients with PPMS (Table 1) [31]. INFORMS did not meet its primary efficacy endpoint as there was no significant difference in the time to 3-month confirmed disability progression when comparing patients receiving fingolimod 0.5 mg or placebo (hazard ratio 0.95; 95% confidence intervals 0.80–1.12; p = 0.544). However, although overall levels of inflammatory activity in the INFORMS population were low at baseline (13–14% of patients had Gd+ lesions at study entry), fingolimod exerted substantial and significant effects relative to placebo on the number of new or enlarged T2 lesions (73% reduction; p < 0.0001), on the number of Gd+ lesions (78% reduction; p < 0.0001), and on the number of new T1 hypointense lesions (62% reduction; p < 0.0001) [31].
The between–treatment-group difference in BVL was non-significant during the study. Mean BVL from baseline to month 36 was 1.49% for fingolimod 0.5 mg and 1.53% for placebo (p = 0.673) [31]. Very little information has been reported on BVL in PPMS. Based on these data, annual BVL over 3 years in INFORMS was approximately 0.5%, a rate that was lower than anticipated. Previously, a comparative study of BVL rates across different MS phenotypes estimated mean annual BVL in untreated PPMS to be 0.56% [2]. Post hoc analyses of INFORMS have indicated that BVL on study correlated with disability worsening in patients with PPMS [44], an association that has also been shown in patients with RMS [45]; and analyses also revealed that BVL on study, and fingolimod’s treatment effect on BVL in INFORMS, were both dependent on the presence of Gd+ lesion activity at baseline [44]. In contrast, the treatment effect of fingolimod on BVL in RMS is independent of baseline Gd+ lesion activity (Sect. 2.3.1). It has been proposed that the nature of neurodegeneration in PPMS and the underlying pathogenesis causing BVL differ in RMS [46–48], in which the underlying pathological mechanisms appear to be influenced by fingolimod in the absence of inflammatory lesions. However, in the presence of inflammation, the treatment effect of fingolimod on BVL in INFORMS was consistent with the effect seen in patients with RMS [44].
Correlates of BV and Its Clinical Relevance
Baseline Correlates of Baseline BV
A post hoc, exploratory analysis of 3635 patients from FREEDOMS, FREEDOMS II, TRANSFORMS, and their extensions, examined whether various demographic, clinical, and MRI parameters correlated with BV and BVL (Table 3). The analyses showed that increasing age, duration of disease, T2 lesion burden, T1-hypointense lesion burden, and disability level (assessed by EDSS and Multiple Sclerosis Functional Composite [MSFC] score) all correlated with reduced normalized BV (NBV) at baseline (all, p < 0.001). Thus, older patients with more advanced disease were likely to have smaller NBV than younger patients with less advanced disease. These associations might be anticipated, given that the measures are indicative of disease severity, but the measures were also found to contribute independently to BVL after adjusting for age, suggesting that BVL reflects not only disease severity, but also disease progression [45]. A separate exploratory analysis of data from TRANSFORMS (N = 1280) also found that several demographic and disease characteristics correlated with baseline NBV. These were baseline T1-hypointense lesion volume (p < 0.001), treatment for MS before enrollment (p = 0.016), baseline T2 lesion volume (p < 0.001), age (p < 0.001), duration of MS (p = 0.012), and the interaction between baseline T1 hypointense lesion volume and T2 lesion volume (p < 0.001) [49].
Table 3.
Summary of methodology used for examining correlates of BV and the associated clinical relevance [45]
| Evaluation | BV parameter | Patient/disease parameters | Analysis methodology |
|---|---|---|---|
| Baseline correlation | Baseline NBV | Baseline parameters: Age Duration of MS since first symptoms Number of relapses during the year before study Number of relapses during the 2 years before study Disability, measured using EDSS score Disability, measured using MSFC score Gd-enhancing lesion number T2 lesion volume T1-hypointense lesion volume |
Pairwise Pearson (or Spearman) correlation: data presented with 95% CIs and p values determined using Fisher z transformation Statistical model selection process to identify the best baseline predictors of NBV: 1. Forward model selection based on multiple regression models and AIC was conducted separately for each study 2. The order of importance was ranked in each study, and ranks were then averaged across studies to weight the studies equally. The best baseline predictors were defined as those with the lowest mean ranks across studies. Only candidate variables that were consistently selected in all studies were considered for inclusion in the final model 3. The analysis was repeated, excluding MSFC as a candidate variable 4. A multiple linear regression model was used to investigate the combined effect of more than one explanatory variable on NBV The final model was fitted to all three studies for parameter estimation |
| Baseline predictors of on-study change | PBVC on-study | Baseline parameters As baseline correlation (above), plus: NBV |
Pairwise Pearson or Spearman correlation as above Statistical model selection process as steps 1–3 above, then: 4. A final multiple regression model with treatment and the two best predictors was then refitted to the data from each of the three studies to quantify PBVC as a function of the best predictors. No adjustment was made for multiplicity |
| Longitudinal (on-study) correlation | PBVC on-study | On-study parameters: Number of confirmed relapses EDSS score MSFC score Cumulative number of Gd-enhancing lesions T2 lesion volume Number of new or enlarged T2 lesions T1-hypointense lesion volume |
Pairwise Pearson or Spearman correlation: data were presented with 95% CIs and p values determined using Fisher z transformation |
AIC Akaike Information Criterion, BV brain volume, CI confidence interval, EDSS Expanded Disability Status Scale, Gd gadolinium, MS multiple sclerosis, MSFC MS Functional Composite, NBV normalized brain volume, PBVC percentage brain volume change
Baseline Predictors of On-Study BVL
In the first of the two analyses discussed in the previous section, demographic variables such as age and sex did not predict on-study BVL, whereas baseline clinical and MRI characteristics such as disability level (MSFC score), disease burden (baseline T2 and T1-hypointense lesion volumes), inflammatory lesion activity (Gd+ lesion count), and NBV all significantly predicted risk of BVL over the 1- to 2-year study period (all p < 0.05). The strongest individual predictors were baseline T2 lesion volume and Gd+ lesion count. Notably, T2 lesion volume was a better predictor of BVL than was T1-hypointense lesion volume, perhaps indicating that T2 lesion volume captures evidence of current lesion activity, and hence is more likely to reflect future lesion activity than is T1-hypointense lesion volume [45]. In the post hoc analysis of the TRANSFORMS data set, baseline Gd+ lesion count, total T2 lesion volume, disease duration, and NBV were all significant predictors of BVL over the 1-year study period (all p < 0.05), and among these, baseline Gd+ lesion count was the strongest predictor [49].
Longitudinal Factors that Correlate with BVL on Study
During FREEDOMS, FREEDOMS II, TRANSFORMS, and their extensions, the greatest levels of BVL correlated with the most pronounced MRI and clinical activity. For example, the number of confirmed relapses, the number of new/enlarged T2 lesions, and the cumulative number of Gd+ lesions all correlated strongly with the observed BVL [45]. After 1 year, there was also a significant correlation between the extent of BVL and the increase in EDSS score from baseline in the combined analysis population, and this relationship between BVL and worsening disability strengthened with time during the 4 years analyzed. As described earlier for this analysis, disability at baseline correlated more strongly with baseline NBV than with BVL on study, even though the strength of correlation between disability worsening and BVL was seen to increase over time. The strength of correlation with NBV at baseline may be indicative of the fact that in these studies, the patients enrolled, on average, 7–11 years after the appearance of first MS symptoms; hence, NBV at baseline effectively reflects BVL from the time of disease onset to enrollment [45].
Clinical Relevance of BVL and Implications for Fingolimod
A recent meta-analysis of 13 placebo-controlled studies that included more than 13,500 patients with RMS (treated with alemtuzumab, dimethyl fumarate, cladribine, fingolimod, glatiramer acetate, IFNβ-1a IM, IFNβ-1b, natalizumab, or teriflunomide) showed that over a 2-year period, treatment effects on BVL and on T2 lesions correlated independently with treatment effects on disability progression. Importantly, treatment effects on BVL and on T2 lesions in combination accounted for a greater proportion of the effect on disability than was accounted for by each parameter in isolation [18]. If T2 lesions are considered to provide a measure of inflammatory disease burden, this result implies that in addition to those changes related directly to inflammatory disease, global changes in BV may capture other aspects of disease progression in MS, such as neurodegeneration.
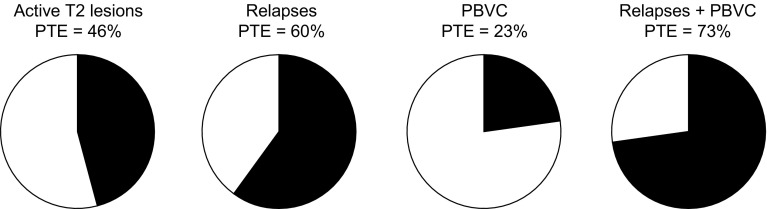
These effects seen at the group level are in accordance with a more recent analysis at the patient level in FREEDOMS, which found the effects of fingolimod on BVL and relapses to be independent of its effect on disability. The analysis set out to examine whether the effect of fingolimod on disability progression was mediated by its effect on lesions, relapses, or BVL. The effect on T2 lesion count and on the number of relapses in year 1 of FREEDOMS accounted, respectively, for 46 and 60% of the overall effect on 6-month confirmed disability progression over 2 years, and the effect on reducing annual BVL accounted for 23% of the overall effect in reducing disability. In a multivariate analysis, relapses and BVL predicted disability progression independently, but T2 lesion count did not, and the effect of fingolimod on relapses and BVL in combination accounted for 73% of the effect on disability (Fig. 5). Taken together, these findings suggest that, in RMS cohorts, fingolimod may reduce BVL via a mechanism that is at least in part independent of any reduction directly attributable to its anti-inflammatory activity [50].
Fig. 5.
Relationships between individual outcomes and disability progression in the FREEDOMS trial. Reproduced from Sormani et al. [50]. Pies show the PTE of fingolimod on disability progression at 2 years accounted for by its effect on active T2 lesions at 1 year, on relapses at 1 year, on PBVC at 2 years, and on relapses and PBVC combined, both of which were determined to be independent predictors of disability progression. PBVC percentage brain volume change, PTE proportion of treatment effect
This hypothesis is supported by a post hoc analysis of the pooled FREEDOMS and FREEDOMS II populations, in which the effects of fingolimod on BVL were examined with and without adjustment for relapses and active MRI lesions on study. At month 24 in the unadjusted analysis, there was an absolute difference in BVL of 0.51% favoring fingolimod over placebo, compared with an absolute difference of 0.27% in the analysis adjusted for relapses and lesion activity. The ratio of these effect sizes implies that about half of the reduction in BVL associated with fingolimod treatment occurs independently of its effect on inflammatory disease in MS. This analysis was complemented by an analysis which found that fingolimod reduced BVL relative to placebo by 48.2% (p = 0.004) at month 24 in the subgroup of patients in the pooled FREEDOMS and FREEDOMS II population who had no Gd+ lesions at baseline and who had experienced neither relapses nor new lesion activity on study. A treatment effect on BVL in this group of patients implies that fingolimod is acting on pathologies affecting non-lesional tissues in the brain or possibly on lesional tissue that is clinically silent or radiologically inapparent [51].
Disease Activity and BVL
The correlation between BVL and disability progression, the association between treatment effects to reduce BVL and the slowing of disability progression, and the evidence that such treatment effects may influence both inflammatory and neurodegenerative pathologies in the brain in MS lend weight to the notion that measurement of BVL might become a useful part of routine patient follow-up. For example, it could provide neurologists with information about treatment response and subclinical disease progression between relapses, before worsening disability manifests clinically. The effect of including BVL in a combination assessment of disease activity has been investigated in a population of patients pooled from the two FREEDOMS trials [52]. A four-parameter measure of ‘no evident disease activity’ (NEDA-4) assessed the presence of relapses, T2 lesion activity, 6-month confirmed disability progression, and annual BVL, and adopted a threshold rate of 0.4% [52], below which annual BVL may be considered to be in the normal range [8]. At 2 years, 19.7% of patients receiving fingolimod attained NEDA-4 status, compared with 5.3% of patients on placebo (odds ratio 4.41; p < 0.0001). Excluding assessment of BVL found that 31.0% of patients on fingolimod and 9.9% of patients on placebo (odds ratio 4.07; p < 0.0001) had no evidence of disease activity, so inclusion of BVL certainly appeared to improve the stringency of the combination measure. The ultimate aim of applying such a combination measure in clinical practice would be to establish that remission from disease progression is complete; such an aspiration for NEDA-4 will require validation in long-term studies [52].
While likely to provide information about rates of disease progression, assessment of BVL relies on waiting for changes in BV (and therefore neurodegenerative damage) to manifest. An alternative, cross-sectional approach has been reported in which patients were categorized based on whether their actual NBV was lower than, similar to, or greater than a value estimated for them based on various demographic and disease characteristics. Regression analysis of patient data from the two FREEDOMS trials that accounted for age, disease duration, sex, disability status, and T2 lesion volume was used to generate a formula to estimate NBV on an individual basis. Patients categorized as having low NBV had the highest risk of 3-month confirmed disability progression over 2 years (hazard ratio 1.75, relative to the high NBV group; p = 0.001) [53]. Based on this example from the two FREEDOMS trial populations, the approach could be extended using data from a broad range of patients with MS to produce a generally applicable regression equation. This in turn could be used to inform treatment decisions based on a single NBV measurement.
Monitoring BVL in Clinical Practice
Guideline Recommendations
Despite the key role that MRI plays in the diagnosis and monitoring of disease progression in MS, and in the assessment of treatment efficacy, BVL assessment is excluded from almost all national guidelines for the management of MS, and few guidelines even refer to it.
European Guideline Recommendations
In 2015, the second part of the Magnetic Resonance Imaging in MS (MAGNIMS) consensus guidelines on the use of MRI in MS was published. The guidelines make a number of important recommendations for the use of MRI in disease prognosis and monitoring, but only discuss measurement of BV or BVL briefly and cautiously, noting the problems of confounding factors and pseudoatrophy affecting measurements [54].
Canadian Guideline Recommendations
In 2013, the Canadian Multiple Sclerosis Working Group (CMSWG) published a set of recommendations for treatment optimization in MS. Technical obstacles to assessment of BV were noted, as was the lack of available evidence that BVL signals a suboptimal response to treatment that warrants treatment review [55]. However, there is evidence that this position may be changing. In 2015, the Canadian Expert Panel Recommendations for MRI Use in MS Diagnosis and Monitoring guidelines made the recommendation “[to] include three-dimensional T1 pre-contrast, diffusion-weighted imaging … in anticipation of future automatic volumetric analysis for brain atrophy” [56].
Subsequent to the CMSWG guidelines, the Canadian MRI Working Group published recommendations on the evolving role of MRI assessments in MS treatment optimization, focusing specifically on the standardization of MRI use, acquisition, and reporting in patients with RMS who are already on treatment. Qualitative reporting of BV changes was recommended as part of routine MRI assessment, and the guidelines also stated that “In comparing scans, the radiologist … should comment on whether there is evidence of significant brain atrophy. The reporting of brain atrophy will usually be confined to two scans obtained at least one year apart” [57].
Although assessment of BVL is not mandated, advocating that MRI assessments of BV are conducted regularly for retrospective assessment is an important development in MS clinical practice. Adopting these techniques may become more widespread in routine clinical care as our understanding of the value of BVL assessment increases. For example, a recent study found that non-responders to IFNβ could be identified with greater sensitivity if measures of BVL were added to known predictive factors [58].
Challenges to the Adoption of Routine BV Assessment in Clinical Practice
Longitudinal assessment of BVL is not performed routinely in clinical practice, and a number of challenges will need to be overcome before estimation of BV can become routine (Table 4). One example of such challenges is the possibility that physiological variables may confound analysis (e.g., level of hydration and inflammation-related volume changes) [57], and another is the variation between different centers in the methods used for advanced quantitative imaging [54, 57].
Table 4.
Challenges to the adoption of BV assessment in routine clinical practice
| Category | Topic |
|---|---|
| Physiological factors [11, 57, 64, 66, 70, 74, 75] | Age Body mass index Diurnal variation Genotype (ApoE expression) Hydration state Menstrual cycle |
| Disease-related factors [11, 57, 64, 74–77] | Fluid-level changes attributable to inflammation (edema) Fluid-level changes attributable to resolution of inflammation (treatment-related pseudoatrophy) |
| Habits and comorbidities [11, 64, 66, 74] | Alcohol consumption Cardiovascular hypertension Diabetes mellitus Obesity Smoking |
| MRI-related factors [11, 57, 64, 65, 74, 76] | Changes in acquisition protocols Changes in scanner type and scanner upgrades Factors influencing scan quality (head motion, distortions, inhomogeneity artifacts) Measurement error Non-standardized quantification methods Patient repositioning in the scanner |
| Logistical factors [11, 57, 64, 65, 76] | Reimbursement Complexity of use (e.g. PACS integration) Lack of normative data Methods dependent on real-time data Poor integration of some image formats |
ApoE apolipoprotein E gene, BV brain volume, MRI magnetic resonance imaging, PACS Picture Archiving and Communication System
Issues with Algorithms Used for Estimating BV and BVL
Cross-sectional algorithms such as SIENAX, NeuroQuant®, and MSmetrix™ are used to estimate BV in patients with MS [6, 14, 59, 60]. SIENAX is an automated method used by several expert reading centers that measures BV from a single MRI scan normalized to a standard skull [6]. NeuroQuant is US Food and Drug Administration (FDA)-approved software that automatically segments and measures volumes of brain structures using MRI images obtained in routine clinical practice or in clinical trials [59, 61]. Finally, MSmetrix is also an FDA-approved automated technique that measures global and local brain atrophy, as well as lesion load [60, 62]. Despite automation, none of these techniques provides accurate assessments every time they are used, and each presents different challenges when correcting measurement errors. A recent comparative analysis of SIENAX with NeuroQuant and MSMetrix in patients with RMS (n = 61) or clinically isolated syndrome (n = 2) found that both NeuroQuant and MSMetrix quantified whole BV with an accuracy comparable with that yielded with SIENAX, suggesting that either algorithm could be used for fully automated routine cross-sectional BV estimation [62].
The SIENA algorithm was designed specifically for longitudinal analyses and has an error rate of 0.15% when determining longitudinal changes in BV [6]. In addition to changes in BV, MSmetrix can also measure longitudinal changes in white matter and lesion volume, which is useful for monitoring disease progression [63]. Regional longitudinal assessment of changes in ventricular cerebrospinal fluid volumes can be made using NeuroSTREAM® (Neurological Software Tool for REliable Atrophy Measurement), a tool under development that compares individual patient data with a normative database adjusted for age, sex, disease duration, disability level, and MS disease course [64].
Other recommendations that begin to address the challenges of conducting longitudinal MRI measurements of BV include (i) acquisition of images using isotropic three-dimensional pulse sequences with multiple image contrasts, to allow for more comprehensive analyses of lesion load and atrophy across time points; (ii) use of robust automated methods that have demonstrated precise BV assessment in scan–rescan data; and (iii) creation of a standardized dataset of benchmark results, to facilitate the development, calibration and objective evaluation of the image analysis methods that are used in MS [65].
Biological Factors that can Confound BV and BVL Measurement
As mentioned earlier, it is known that hydration levels and inflammation-related volume changes can affect BV, but atrophy rates can also be affected by various other factors. For example, significantly higher atrophy rates have been associated with older age; higher glycated hemoglobin A levels, body mass index, and alcohol intake; severe white matter hyperintensity; and being a carrier of the APOE epsilon4 gene [66]. They can also be confounded by factors such as treatment-induced pseudoatrophy, smoking status, and hydration level [67]. Treatment-induced pseudoatrophy merits particular attention. Anti-inflammatory drugs used to treat MS have often been associated with a paradoxical acceleration of BVL following therapy initiation; this is termed pseudoatrophy [64, 68]. It may be attributable to resolution of inflammatory edema, or to other mechanisms that cause changes in the water content of tissues, but to allow for this potentially confounding effect, some studies have excluded the first 6 months of BV measurement from analyses of BVL [11, 69]. Finally, a recent analysis of over 3000 serial scans from 755 patients with MS established that diurnal variations in BV occur, with BV generally being greater in the morning than at other times of day [70].
BVL as a Biomarker for Regulatory Approval of MS Therapies
In RMS, relapses, disability worsening, and MRI lesion activity are the measures generally used to determine the therapeutic efficacy of an MS drug in pivotal interventional trials. However, these measures can overlook clinically silent neurodegenerative disease progression. In contrast, BVL measurement captures global changes in the brain, so potentially provides a more sensitive assessment of disease progression than these other measures. There are several aspects to consider before adopting BVL as a biomarker for regulatory approval of MS therapies. Some of these aspects present obstacles but others have been addressed successfully.
In terms of obstacles, there is currently little precedent for the use of BVL as a primary study endpoint in RMS. It was the primary endpoint in a phase II placebo-controlled trial of riluzole and IFNβ-1a IM in early MS [71], but to date, BVL has not been the primary endpoint in a phase III interventional trial. Also, as discussed earlier, there is a lack of methodological standardization both for BVL measurement and for data processing that allows for confounding factors. One further obstacle to overcome is clinical adoption. If BVL was the primary measure of therapeutic efficacy for MS therapies, more widespread measurement of volumetric MRI within routine clinical follow-up would be needed.
What is encouraging is that the clinical relevance and the clinical definition of BVL as a biomarker in MS have been investigated and established. The correlation between BVL and disability in MS [45, 49–51], and the association between treatment effects on BVL and on disability [18], have been demonstrated in post hoc analyses of several large study populations. Furthermore, in terms of a clinical definition, analyses at the patient level have defined a threshold rate of BVL that distinguishes normal from pathological BVL with good specificity and sensitivity [8].
Conclusions
There is comprehensive evidence from several large phase III studies that fingolimod significantly reduces the extent and rate of BVL in patients with RMS. In these studies, fingolimod reduced annual BVL by approximately one-third relative to that observed in patients receiving placebo or IFNβ-1a IM, and this reduced rate was sustained in individuals treated continuously with fingolimod in the study extensions. Subgroup analyses have shown that the ability of fingolimod to reduce BVL is independent of treatment history, baseline disability level, and inflammatory lesion activity both at baseline and on study. Data from post hoc analyses of fingolimod phase III studies have identified a number of predictors of baseline NBV and of changes in BV during treatment, including age, disability level, disease burden, and inflammatory lesion activity; baseline T2 lesion and Gd+ lesion burdens at baseline were particularly strong predictors of BVL in the subsequent 2 years. Understanding changes in BV that occur during the disease course and in response to existing disease-modifying therapies may help to provide neurologists with new insights into their patients’ prognoses, and to guide them when reviewing treatment. Several obstacles to routine MRI monitoring of BV could be overcome in the near future, but its increased adoption in the wider neurological community is almost certainly reliant on raising awareness of the potential benefits afforded to patients by routine BV assessment.
Acknowledgments
The authors acknowledge Jeremy Bright of Oxford PharmaGenesis, Oxford, UK, for providing medical writing support (including preparing a manuscript outline based on teleconferences and correspondence with the authors, collating the authors’ comments during manuscript development, and providing assistance with data checking, figure production, and submission).
Compliance with Ethical Standards
Funding
Editorial and medical writing support for the development of this manuscript was funded by Novartis Pharma AG. Journal open-access costs were also met by Novartis Pharma AG.
Conflicts of Interest
NDS has received honoraria from Biogen-Idec, Genzyme, Merck Serono, Novartis, Roche, and Teva for consulting services, speaking, and travel support. He serves on advisory boards for Merck Serono, Novartis, Biogen-Idec, Roche, and Genzyme, and he has received research grant support from the Italian MS Society. DS is an employee of Novartis Pharma AG. MHB has received institutional support from Biogen, Genzyme, Merck, Novartis, and Teva, and is a research consultant for Medical Safety Systems.
References
- 1.Bermel RA, Bakshi R. The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol. 2006;5:158–170. doi: 10.1016/S1474-4422(06)70349-0. [DOI] [PubMed] [Google Scholar]
- 2.De Stefano N, Giorgio A, Battaglini M, et al. Assessing brain atrophy rates in a large population of untreated multiple sclerosis subtypes. Neurology. 2010;74:1868–1876. doi: 10.1212/WNL.0b013e3181e24136. [DOI] [PubMed] [Google Scholar]
- 3.Rudick RA, Fisher E, Lee JC, Simon J, Jacobs L. Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing–remitting MS. Multiple Sclerosis Collaborative Research Group. Neurology. 1999;53:1698–1704. doi: 10.1212/WNL.53.8.1698. [DOI] [PubMed] [Google Scholar]
- 4.Anderson VM, Fox NC, Miller DH. Magnetic resonance imaging measures of brain atrophy in multiple sclerosis. J Magn Reson Imaging. 2006;23:605–618. doi: 10.1002/jmri.20550. [DOI] [PubMed] [Google Scholar]
- 5.Barkhof F, Calabresi PA, Miller DH, Reingold SC. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol. 2009;5:256–266. doi: 10.1038/nrneurol.2009.41. [DOI] [PubMed] [Google Scholar]
- 6.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 7.Fox NC, Jenkins R, Leary SM, et al. Progressive cerebral atrophy in MS: a serial study using registered, volumetric MRI. Neurology. 2000;54:807–812. doi: 10.1212/WNL.54.4.807. [DOI] [PubMed] [Google Scholar]
- 8.De Stefano N, Stromillo ML, Giorgio A, et al. Establishing pathological cut-offs of brain atrophy rates in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87:93–99. doi: 10.1136/jnnp-2016-314597.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takao H, Hayashi N, Ohtomo K. A longitudinal study of brain volume changes in normal aging. Eur J Radiol. 2012;81:2801–2804. doi: 10.1016/j.ejrad.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Hedman AM, van Haren NE, Schnack HG, Kahn RS, Hulshoff Pol HE. Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Hum Brain Mapp. 2012;33:1987–2002. doi: 10.1002/hbm.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Stefano N, Airas L, Grigoriadis N, et al. Clinical relevance of brain volume measures in multiple sclerosis. CNS Drugs. 2014;28:147–156. doi: 10.1007/s40263-014-0140-z. [DOI] [PubMed] [Google Scholar]
- 12.Fisher E, Rudick RA, Simon JH, et al. Eight-year follow-up study of brain atrophy in patients with MS. Neurology. 2002;59:1412–1420. doi: 10.1212/01.WNL.0000036271.49066.06. [DOI] [PubMed] [Google Scholar]
- 13.Jacobsen C, Hagemeier J, Myhr KM, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. 2014;85:1109–1115. doi: 10.1136/jnnp-2013-306906. [DOI] [PubMed] [Google Scholar]
- 14.Miller DH, Barkhof F, Frank JA, Parker GJ, Thompson AJ. Measurement of atrophy in multiple sclerosis: pathological basis, methodological aspects and clinical relevance. Brain. 2002;125:1676–1695. doi: 10.1093/brain/awf177. [DOI] [PubMed] [Google Scholar]
- 15.Zivadinov R, Bakshi R. Central nervous system atrophy and clinical status in multiple sclerosis. J Neuroimaging. 2004;14:27S–35S. doi: 10.1111/j.1552-6569.2004.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 16.Filippi M, Rocca MA, Barkhof F, et al. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol. 2012;11:349–360. doi: 10.1016/S1474-4422(12)70003-0. [DOI] [PubMed] [Google Scholar]
- 17.Rudick RA, Fisher E. Preventing brain atrophy should be the gold standard of effective therapy in MS (after the first year of treatment): Yes. Mult Scler. 2013;19:1003–1004. doi: 10.1177/1352458513482385. [DOI] [PubMed] [Google Scholar]
- 18.Sormani MP, Arnold DL, De Stefano N. Treatment effect on brain atrophy correlates with treatment effect on disability in multiple sclerosis. Ann Neurol. 2014;75:43–49. doi: 10.1002/ana.24018. [DOI] [PubMed] [Google Scholar]
- 19.Novartis. Gilenya® (fingolimod) summary of product characteristics. 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002202/WC500104528.pdf. Accessed 02 Nov 2016.
- 20.Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 21.Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing–remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13:545–556. doi: 10.1016/S1474-4422(14)70049-3. [DOI] [PubMed] [Google Scholar]
- 22.Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 23.Khan O, Bao F, Shah M, et al. Effect of disease-modifying therapies on brain volume in relapsing–remitting multiple sclerosis: results of a five-year brain MRI study. J Neurol Sci. 2012;312:7–12. doi: 10.1016/j.jns.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 24.Sastre-Garriga J, Tur C, Pareto D, et al. Brain atrophy in natalizumab-treated patients: a 3-year follow-up. Mult Scler. 2015;21:749–756. doi: 10.1177/1352458514556300. [DOI] [PubMed] [Google Scholar]
- 25.Arnold DL, Fisher E, Brinar VV, et al. Superior MRI outcomes with alemtuzumab compared with subcutaneous interferon beta-1a in MS. Neurology. 2016;87:1464–1472. doi: 10.1212/WNL.0000000000003169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanasescu R, Constantinescu CS. Pharmacokinetic evaluation of fingolimod for the treatment of multiple sclerosis. Expert Opin Drug Metab Toxicol. 2014;10:621–630. doi: 10.1517/17425255.2014.894019. [DOI] [PubMed] [Google Scholar]
- 27.Kappos L, O’Connor P, Radue EW, et al. Long-term effects of fingolimod in multiple sclerosis: the randomized FREEDOMS extension trial. Neurology. 2015;84:1582–1591. doi: 10.1212/WNL.0000000000001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khatri B, Barkhof F, Comi G, et al. Comparison of fingolimod with interferon beta-1a in relapsing–remitting multiple sclerosis: a randomised extension of the TRANSFORMS study. Lancet Neurol. 2011;10:520–529. doi: 10.1016/S1474-4422(11)70099-0. [DOI] [PubMed] [Google Scholar]
- 29.Reder AT, Jeffery D, Goodin D, et al. Long-term efficacy of fingolimod in patients with relapsing remitting multiple sclerosis: results from the phase 3 FREEDOMS II extension study. Mult Scler. 2013;19(Suppl. 11):510–511. [Google Scholar]
- 30.Kappos L, Cohen JA, Barkhof F, Cappiello L, Zhang Y, Von Rosenstiel P. Relapse rates and disability remain consistently low with long-term fingolimod therapy: five-year interim results of the LONGTERMS extension study. Mult Scler. 2013;19(Suppl. 11):486–487. [Google Scholar]
- 31.Lublin F, Miller DH, Freedman MS, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387:1075–1084. doi: 10.1016/S0140-6736(15)01314-8. [DOI] [PubMed] [Google Scholar]
- 32.Häring D, Tomic D, Piani Meier D, Sfikas N, Chin P, Francis G. Time to brain atrophy is prolonged in continuously fingolimod-treated MS patients vs placebo or interferon beta 1-a in phase 3 studies of fingolimod. Mult Scler. 2014;20(Suppl. 1):103–104. [Google Scholar]
- 33.Cohen JA, Khatri B, Barkhof F, et al. Long-term (up to 4.5 years) treatment with fingolimod in multiple sclerosis: results from the extension of the randomised TRANSFORMS study. J Neurol Neurosurg Psychiatry. 2016;87:468–475. doi: 10.1136/jnnp-2015-310597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radue EW, Barkhof F, Cohen J, et al. Sustained low rate of brain volume loss under long-term fingolimod treatment in relapsing multiple sclerosis: results from the LONGTERMS study. Mult Scler. 2014;20(Suppl. 1):261–262. [Google Scholar]
- 35.Radue EW, O’Connor P, Polman CH, et al. Impact of fingolimod therapy on magnetic resonance imaging outcomes in patients with multiple sclerosis. Arch Neurol. 2012;69:1259–1269. doi: 10.1001/archneurol.2012.1051. [DOI] [PubMed] [Google Scholar]
- 36.Cohen JA, Barkhof F, Comi G, et al. Fingolimod versus intramuscular interferon in patient subgroups from TRANSFORMS. J Neurol. 2013;260:2023–2032. doi: 10.1007/s00415-013-6932-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barkhof F, Cohen J, Montalbán X, et al. Fingolimod (FTY720) reduces brain volume loss over 12 months compared with intramuscular interferon beta-1a: subgroup analyses of TRANSFORMS data based on inflammatory disease activity. Mult Scler. 2011;17(Suppl. 10):S406. [Google Scholar]
- 38.Radue E, de Stefano N, O’Connor P, et al. Brain atrophy and disease-free status over four years: analyses of the FREEDOMS core and extension trial data. Mult Scler. 2013;19(Suppl. 11):481. [Google Scholar]
- 39.Radue EW, Barkhof F, Cohen J, Holdbrook F, Francis G, Kappos L. MRI analyses in RRMS patients with highly active disease: results from FREEDOMS and TRANSFORMS phase 3 studies. Neurology. 2012;78(Suppl. 1):P01.134.
- 40.Bergvall N, Sfikas N, Chin P, Tomic D, Von Rosenstiel P, Kappos L. Efficacy of fingolimod in pre-treated patients with disease activity: pooled analyses of FREEDOMS and FREEDOMS II. Neurology. 2014;82(Suppl. 10):P3.174.
- 41.Jeffrey D, Radue EW, Karlsson G, Zheng H, Von Rosenstiel P, Kappos L. Efficacy benefits of fingolimod 0.5 mg once daily in patients previously treated with glatiramer acetate: pooled analysis of the phase 3, placebo-controlled FREEDOMS and FREEDOMS II studies in relapsing multiple sclerosis. Neurology. 2014;82(Suppl. 10):PS.193.
- 42.Antel J, Antel S, Caramanos Z, Arnold DL, Kuhlmann T. Primary progressive multiple sclerosis: part of the MS disease spectrum or separate disease entity? Acta Neuropathol. 2012;123:627–638. doi: 10.1007/s00401-012-0953-0. [DOI] [PubMed] [Google Scholar]
- 43.Koch M, Kingwell E, Rieckmann P, Tremlett H. The natural history of primary progressive multiple sclerosis. Neurology. 2009;73:1996–2002. doi: 10.1212/WNL.0b013e3181c5b47f. [DOI] [PubMed] [Google Scholar]
- 44.Wolinsky JS, Häring DA, Sormani MP, et al. Brain volume as a predictor of disability in primary progressive multiple sclerosis: evidence from the INFORMS trial. Neurology. 2016;86(Suppl. 16):S51.007.
- 45.Radue EW, Barkhof F, Kappos L, et al. Correlation between brain volume loss and clinical and MRI outcomes in multiple sclerosis. Neurology. 2015;84:784–793. doi: 10.1212/WNL.0000000000001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kutzelnigg A, Lassmann H. Pathology of multiple sclerosis and related inflammatory demyelinating diseases. Handb Clin Neurol. 2014;122:15–58. doi: 10.1016/B978-0-444-52001-2.00002-9. [DOI] [PubMed] [Google Scholar]
- 47.Steenwijk MD, Daams M, Pouwels PJ, et al. What explains gray matter atrophy in long-standing multiple sclerosis? Radiology. 2014;272:832–842. doi: 10.1148/radiol.14132708. [DOI] [PubMed] [Google Scholar]
- 48.Dickens AM, Larkin JR, Griffin JL, Cavey A, Matthews L, Turner MR, et al. A type 2 biomarker separates relapsing–remitting from secondary progressive multiple sclerosis. Neurology. 2014;83:1492–1499. doi: 10.1212/WNL.0000000000000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barkhof F, de Jong R, Sfikas N, de Vera A, Francis G, Cohen J. The influence of patient demographics, disease characteristics and treatment on brain volume loss in Trial Assessing Injectable Interferon vs FTY720 Oral in Relapsing-Remitting Multiple Sclerosis (TRANSFORMS), a phase 3 study of fingolimod in multiple sclerosis. Mult Scler. 2014;20:1704–1713. doi: 10.1177/1352458514532317. [DOI] [PubMed] [Google Scholar]
- 50.Sormani MP, De Stefano N, Francis G, et al. Fingolimod effect on brain volume loss independently contributes to its effect on disability. Mult Scler. 2015;21:916–924. doi: 10.1177/1352458515569099. [DOI] [PubMed] [Google Scholar]
- 51.De Stefano N, Tomic D, Radue EW, et al. Effect of fingolimod on diffuse brain tissue damage in relapsing–remitting multiple sclerosis patients. Mult Scler Relat Disord. 2016;7:98–101. doi: 10.1016/j.msard.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 52.Kappos L, De Stefano N, Freedman MS, et al. Inclusion of brain volume loss in a revised measure of ‘no evidence of disease activity’ (NEDA-4) in relapsing–remitting multiple sclerosis. Mult Scler. 2016;22:1297–1305. doi: 10.1177/1352458515616701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sormani MP, Kappos L, Radue EW, et al. Defining brain volume cutoffs to identify clinically relevant atrophy in RRMS. Mult Scler. 2016. pii:1352458516659550. [DOI] [PubMed]
- 54.Wattjes MP, Rovira A, Miller D, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis—establishing disease prognosis and monitoring patients. Nat Rev Neurol. 2015;11:597–606. doi: 10.1038/nrneurol.2015.157. [DOI] [PubMed] [Google Scholar]
- 55.Freedman MS, Selchen D, Arnold DL, et al. Treatment optimization in MS: Canadian MS Working Group updated recommendations. Can J Neurol Sci. 2013;40:307–323. doi: 10.1017/S0317167100014244. [DOI] [PubMed] [Google Scholar]
- 56.Traboulsee A, Letourneau-Guillon L, Freedman MS, et al. Canadian expert panel recommendations for MRI use in MS diagnosis and monitoring. Can J Neurol Sci. 2015;42:159–167. doi: 10.1017/cjn.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arnold DL, Li D, Hohol M, et al. Evolving role of MRI in optimizing the treatment of multiple sclerosis: Canadian Consensus recommendations. Mult Scler J Exp Transl Clin. 2015;1:1–9. doi: 10.1177/2055217315589775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rojas JI, Patrucco L, Miguez J, Besada C, Cristiano E. Brain atrophy as a non-response predictor to interferon-beta in relapsing–remitting multiple sclerosis. Neurol Res. 2014;36:615–618. doi: 10.1179/1743132813Y.0000000304. [DOI] [PubMed] [Google Scholar]
- 59.Cortech Labs. Discover NeuroQuant. 2016. http://www.cortechslabs.com/neuroquant/. Accessed 02 Nov 2016.
- 60.Jain S, Sima DM, Ribbens A, et al. Automatic segmentation and volumetry of multiple sclerosis brain lesions from MR images. Neuroimage Clin. 2015;8:367–375. doi: 10.1016/j.nicl.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nielsen AS, Kita M, Baker J, Haug J, Siegal J. Predictor status of disease modifying therapy time exposure to brain atrophy using NeuroQuant. Mult Scler. 2014;20(Suppl. 1):S302. [Google Scholar]
- 62.Wang C, Beadnall HN, Hatton SN, et al. Automated brain volumetrics in multiple sclerosis: a step closer to clinical application. J Neurol Neurosurg Psychiatry. 2016;87:754–757. doi: 10.1136/jnnp-2015-312304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jain S, Sima DM, Maertens A, Van Huffel S, Maes F, Smeets D. MSmetrix: accurate untrained method for longitudinal lesion segmentation. Mult Scler. 2015;23(11 Suppl):193–4.P450.
- 64.Zivadinov R, Jakimovski D, Gandhi S, et al. Clinical relevance of brain atrophy assessment in multiple sclerosis. Implications for its use in a clinical routine. Expert Rev Neurother. 2016;16:777–793. doi: 10.1080/14737175.2016.1181543. [DOI] [PubMed] [Google Scholar]
- 65.Vrenken H, Jenkinson M, Horsfield MA, et al. Recommendations to improve imaging and analysis of brain lesion load and atrophy in longitudinal studies of multiple sclerosis. J Neurol. 2013;260:2458–2471. doi: 10.1007/s00415-012-6762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Enzinger C, Fazekas F, Matthews PM, et al. Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology. 2005;64:1704–1711. doi: 10.1212/01.WNL.0000161871.83614.BB. [DOI] [PubMed] [Google Scholar]
- 67.Rovira A, Wattjes MP, Tintore M, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis—clinical implementation in the diagnostic process. Nat Rev Neurol. 2015;11:471–482. doi: 10.1038/nrneurol.2015.106. [DOI] [PubMed] [Google Scholar]
- 68.De Stefano N, Arnold DL. Towards a better understanding of pseudoatrophy in the brain of multiple sclerosis patients. Mult Scler. 2015;21:675–676. doi: 10.1177/1352458514564494. [DOI] [PubMed] [Google Scholar]
- 69.Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- 70.Nakamura K, Brown RA, Narayanan S, Collins DL, Arnold DL. Diurnal fluctuations in brain volume: statistical analyses of MRI from large populations. Neuroimage. 2015;118:126–132. doi: 10.1016/j.neuroimage.2015.05.077. [DOI] [PubMed] [Google Scholar]
- 71.Waubant E, Maghzi AH, Revirajan N, Spain R, Julian L, Mowry EM, et al. A randomized controlled phase II trial of riluzole in early multiple sclerosis. Ann Clin Transl Neurol. 2014;1:340–347. doi: 10.1002/acn3.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vollmer T, Jeffery D, Goodin D, et al. Long-term safety of fingolimod in patients with relapsing–remitting multiple sclerosis: results from phase 3 FREEDOMS II extension study. Neurology. 2013;80(7 Suppl.):P01.165.
- 73.Cohen J, Kappos L, Selmaj K, et al. Long-term safety and effectiveness of fingolimod: 7 year data from the LONGTERMS study. Mult Scler. 2015;21(11 Suppl.):280.P591.
- 74.Giorgio A, Battaglini M, Smith SM, De Stefano N. Brain atrophy assessment in multiple sclerosis: importance and limitations. Neuroimaging Clin N Am. 2008;18:675–686. doi: 10.1016/j.nic.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 75.Nakamura K, Brown RA, Araujo D, Narayanan S, Arnold DL. Correlation between brain volume change and T2 relaxation time induced by dehydration and rehydration: implications for monitoring atrophy in clinical studies. Neuroimage Clin. 2014;6:166–170. doi: 10.1016/j.nicl.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Giorgio A, De Stefano N. Clinical use of brain volumetry. J Magn Reson Imaging. 2013;37:1–14. doi: 10.1002/jmri.23671. [DOI] [PubMed] [Google Scholar]
- 77.Zivadinov R, Reder AT, Filippi M, et al. Mechanisms of action of disease-modifying agents and brain volume changes in multiple sclerosis. Neurology. 2008;71:136–144. doi: 10.1212/01.wnl.0000316810.01120.05. [DOI] [PubMed] [Google Scholar]