Abstract
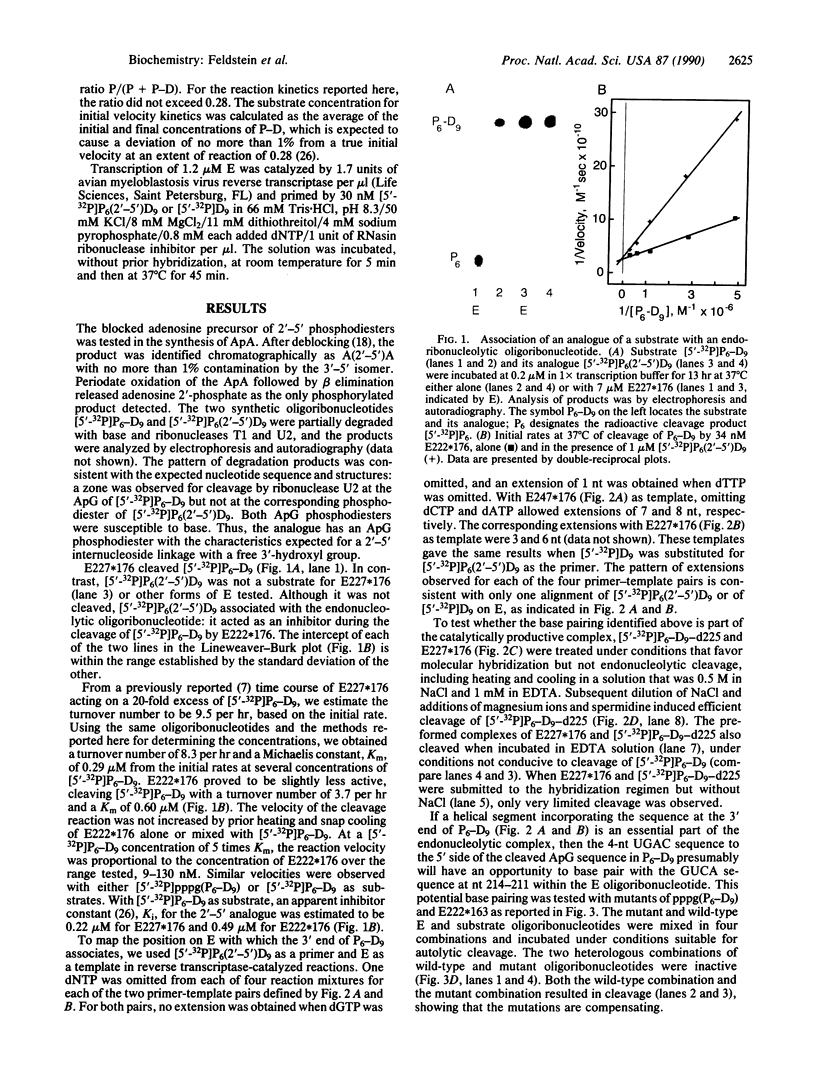
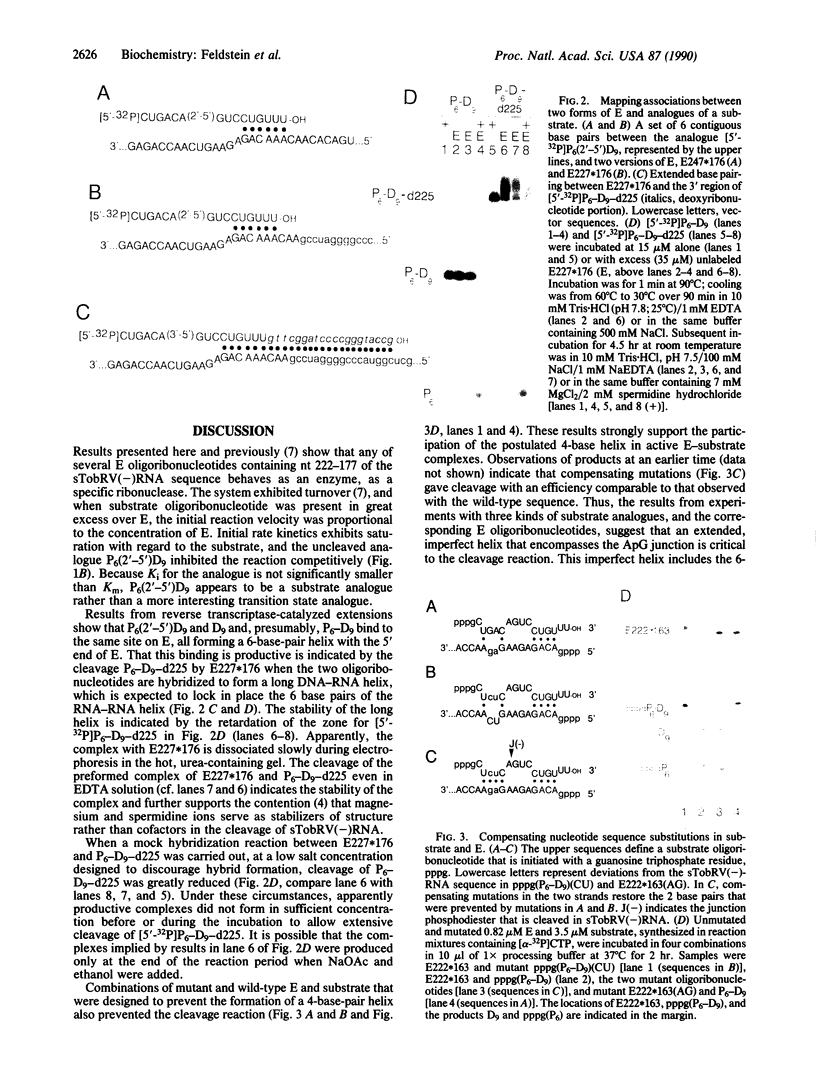
Both polarities of the satellite RNA of tobacco ringspot virus are sources of self-cleaving sequences. RNA of the less abundant, negative polarity, designated sTobRV-(-)RNA, has cleaving activity that was mapped previously to two noncontiguous regions of the polyribonucleotide chain. Endoribonucleolytic oligoribonucleotides (E) corresponding to the larger of the two regions cleaved smaller substrate oligoribonucleotides, at the ApG phosphodiester that is cleaved in sTobRV(-)RNA. An analogue of the substrate, which has a 2'-5' ApG phosphodiester, was not cleaved by E but acted as a competitive inhibitor of the cleavage of substrate. The analogue served as a primer, and E served as template, for reverse transcriptase-catalyzed copying of specific E sequences. The sequences transcribed suggest base pairing between the 5' region of E and a portion of the substrate that is located 3' to, but does not include, the ApG phosphodiester. Results from other experiments indicate this base pairing is a part of the functional cleavage complex. The association of the ends of E and substrate anticipates a second, 4-base-pair association between E and a portion of substrate that is 5' to, but does not include, the ApG phosphodiester. The effects of compensating mutations in E and substrate oligoribonucleotides support the existence of this second association in the active cleavage complex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branch A. D., Benenfeld B. J., Baroudy B. M., Wells F. V., Gerin J. L., Robertson H. D. An ultraviolet-sensitive RNA structural element in a viroid-like domain of the hepatitis delta virus. Science. 1989 Feb 3;243(4891):649–652. doi: 10.1126/science.2492676. [DOI] [PubMed] [Google Scholar]
- Buzayan J. M., Feldstein P. A., Bruening G., Eckstein F. RNA mediated formation of a phosphorothioate diester bond. Biochem Biophys Res Commun. 1988 Oct 14;156(1):340–347. doi: 10.1016/s0006-291x(88)80846-5. [DOI] [PubMed] [Google Scholar]
- Buzayan J. M., Feldstein P. A., Segrelles C., Bruening G. Autolytic processing of a phosphorothioate diester bond. Nucleic Acids Res. 1988 May 11;16(9):4009–4023. doi: 10.1093/nar/16.9.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin J. C., Cramer F. Deoxy oligonucleotide synthesis via the triester method. J Org Chem. 1973 Jan 26;38(2):245–250. doi: 10.1021/jo00942a011. [DOI] [PubMed] [Google Scholar]
- Davanloo P., Rosenberg A. H., Dunn J. J., Studier F. W. Cloning and expression of the gene for bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2035–2039. doi: 10.1073/pnas.81.7.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Bruce A. G., Uhlenbeck O. C. Specific labeling of 3' termini of RNA with T4 RNA ligase. Methods Enzymol. 1980;65(1):65–74. doi: 10.1016/s0076-6879(80)65011-3. [DOI] [PubMed] [Google Scholar]
- Feldstein P. A., Buzayan J. M., Bruening G. Two sequences participating in the autolytic processing of satellite tobacco ringspot virus complementary RNA. Gene. 1989 Oct 15;82(1):53–61. doi: 10.1016/0378-1119(89)90029-2. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of plus and minus RNAs of a virusoid and a structural model for the active sites. Cell. 1987 Apr 24;49(2):211–220. doi: 10.1016/0092-8674(87)90562-9. [DOI] [PubMed] [Google Scholar]
- Gough G. R., Collier K. J., Weith H. L., Gilham P. T. The use of barium salts of protected deoxyribonucleoside-3' p-chlorophenyl phosphates for construction of oligonucleotides by the phosphotriester method: high-yield synthesis of dinucleotide blocks. Nucleic Acids Res. 1979 Dec 11;7(7):1955–1964. doi: 10.1093/nar/7.7.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel A., Tritz R. RNA catalytic properties of the minimum (-)sTRSV sequence. Biochemistry. 1989 Jun 13;28(12):4929–4933. doi: 10.1021/bi00438a002. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Sequences required for self-catalysed cleavage of the satellite RNA of tobacco ringspot virus. Gene. 1989 Oct 15;82(1):43–52. doi: 10.1016/0378-1119(89)90028-0. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988 Aug 18;334(6183):585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- Hutchins C. J., Rathjen P. D., Forster A. C., Symons R. H. Self-cleavage of plus and minus RNA transcripts of avocado sunblotch viroid. Nucleic Acids Res. 1986 May 12;14(9):3627–3640. doi: 10.1093/nar/14.9.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. M., Tousignant M. E., Steger G. Nucleotide sequence predicts circularity and self-cleavage of 300-ribonucleotide satellite of arabis mosaic virus. Biochem Biophys Res Commun. 1988 Jul 15;154(1):318–325. doi: 10.1016/0006-291x(88)90687-0. [DOI] [PubMed] [Google Scholar]
- Kuo M. Y., Goldberg J., Coates L., Mason W., Gerin J., Taylor J. Molecular cloning of hepatitis delta virus RNA from an infected woodchuck liver: sequence, structure, and applications. J Virol. 1988 Jun;62(6):1855–1861. doi: 10.1128/jvi.62.6.1855-1861.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M. Y., Sharmeen L., Dinter-Gottlieb G., Taylor J. Characterization of self-cleaving RNA sequences on the genome and antigenome of human hepatitis delta virus. J Virol. 1988 Dec;62(12):4439–4444. doi: 10.1128/jvi.62.12.4439-4444.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T. G., Regnier F. E., Weith H. L. Separation of synthetic oligonucleotides on columns of microparticulate silica coated with crosslinked polyethylene imine. Anal Biochem. 1983 Aug;133(1):85–93. doi: 10.1016/0003-2697(83)90225-7. [DOI] [PubMed] [Google Scholar]
- Prody G. A., Bakos J. T., Buzayan J. M., Schneider I. R., Bruening G. Autolytic processing of dimeric plant virus satellite RNA. Science. 1986 Mar 28;231(4745):1577–1580. doi: 10.1126/science.231.4745.1577. [DOI] [PubMed] [Google Scholar]
- Sharmeen L., Kuo M. Y., Dinter-Gottlieb G., Taylor J. Antigenomic RNA of human hepatitis delta virus can undergo self-cleavage. J Virol. 1988 Aug;62(8):2674–2679. doi: 10.1128/jvi.62.8.2674-2679.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson N., Gurevitz M., Ford J., Apirion D. Self cleavage of a precursor RNA from bacteriophage T4. J Mol Biol. 1984 Jan 25;172(3):301–323. doi: 10.1016/s0022-2836(84)80028-5. [DOI] [PubMed] [Google Scholar]
- Wu H. N., Lai M. M. Reversible cleavage and ligation of hepatitis delta virus RNA. Science. 1989 Feb 3;243(4891):652–654. doi: 10.1126/science.2492677. [DOI] [PubMed] [Google Scholar]
- Wu H. N., Lin Y. J., Lin F. P., Makino S., Chang M. F., Lai M. M. Human hepatitis delta virus RNA subfragments contain an autocleavage activity. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1831–1835. doi: 10.1073/pnas.86.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tol H., Gross H. J., Beier H. Non-enzymatic excision of pre-tRNA introns? EMBO J. 1989 Jan;8(1):293–300. doi: 10.1002/j.1460-2075.1989.tb03376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]





