Abstract
Hypothalamic inflammation was recently found to mediate obesity-related hypertension, but the responsible upstream mediators remain unexplored. In this study, we show that dietary obesity is associated with extracellular release of mitochondrial DNA (mtDNA) into the cerebrospinal fluid and that central delivery of mtDNA mimics transforming growth factor-β (TGFβ) excess to activate downstream signaling pathways. Physiological study reveals that central administration of mtDNA or TGFβ is sufficient to cause hypertension in mice. Knockout of the TGFβ receptor in proopiomelanocortin neurons counteracts the hypertensive effect of not only TGFβ but also mtDNA excess, while the hypertensive action of central mtDNA can be blocked pharmacologically by a TGFβ receptor antagonist or genetically by TGFβ receptor knockout. Finally, we confirm that obesity-induced hypertension can be reversed through central treatment with TGFβ receptor antagonist. In conclusion, circulating mtDNA in the brain employs neural TGFβ pathway to mediate a central inflammatory mechanism of obesity-related hypertension.
Keywords: mitochondrial DNA, transforming growth factor-β, brain, hypothalamus, hypertension, inflammation
hypertension is among the most prevalent cardiovascular diseases (CVDs) and is a major risk factor for many common causes of morbidity and mortality including congestive heart failure, end-stage renal disease, and stroke (43, 91). Most forms of hypertension are caused by a chronic increase in blood pressure (BP) that arises from the interaction between nonspecific genetic and environmental factors (64). While the etiology remains unknown, it is generally appreciated that several factors contribute to the breakdown of regulatory systems that maintain BP in the normotensive range. Indeed, numerous studies show that these regulatory pathways are under neurological control, involving both the autonomic and central nervous systems (1, 21, 22, 26, 60, 68, 71, 76).
Many forms of hypertension are accompanied by autonomic nervous system abnormalities and/or changes in the central circuitry (36, 49, 59). Increases in BP can be brought on by a dysregulation in the balance between the sympathetic and parasympathetic arms of the autonomic nervous system and frequently as a direct result of an increase in sympathetic drive (1, 20, 22). Alternatively, central alterations at sites that play a role in processing and integrating peripheral stimuli—the circumventricular organs, brain stem, and hypothalamus—can also increase sympathetic tone and BP (1, 20–22, 68, 71, 76). Functional changes in the hypothalamus are particularly relevant (18, 21, 28, 33, 34, 37, 65, 68, 69, 77, 79), considering it contains several nuclei that act as the interface between the nervous and endocrine systems. The paraventricular nucleus, for example, contains preautonomic neurons, which can modulate sympathetic drive and therefore participate in the development of hypertension (18, 28, 33, 34, 79). Another area of interest is the mediobasal hypothalamus, which plays a regulatory role in energy homeostasis by maintaining negative and positive energy balance (72, 75, 97). Several studies suggest that neurons within the mediobasal hypothalamus, and particularly in the arcuate nucleus, are especially important in the development of obesity-induced hypertension (28, 37, 60, 65, 68, 69, 79). Although the precise mechanisms remain unknown, mounting evidence from both experimental and clinical studies points to inflammation in the hypothalamus as an important pathogenic factor in energy imbalance (66, 82) and blood pressure imbalance (36, 77).
Recently, development of obesity-related metabolic syndromes, including prediabetic glucose disorder (89) and hypertension (67), has been causally related to nuclear factor-κB (NF-κB)-driven hypothalamic inflammation. Studies have conclusively demonstrated that proinflammatory NF-κB activity increases with both age and obesity (67, 89, 94, 96), two epidemical risk factors for the development of hypertension. In line with the immunological etiology of hypertension appreciated in the field (45, 50, 62), we recently showed that central TNFα administration activates NF-κB in the mediobasal hypothalamus, resulting in sympathetic upregulation and hypertension (67). However, since TNFα is not only an activator but also a product of NF-κB, it may not be an initial cause of NF-κB-mediated neuroinflammation. Recently, we found that central transforming growth factor-β (TGFβ) overproduction is an early mediator of hypothalamic inflammation in obesity (89). However, although increased levels of TGFβ in the circulation and cardiovascular system are associated with CVDs, including hypertension (4, 48, 55, 93), it is still unknown if the neural action of TGFβ is important for the development of hypertension.
In parallel, mitochondrial dysfunction in endothelial, vascular, and myocardial cells frequently accompanies the development of CVDs including hypertension (9), implicating mitochondria as targets of hypertension-induced inflammation (29). Circulating mitochondrial DNAs (mtDNA) are found in major target organs in hypertension including the heart, kidney, and brain (41, 58); mitochondria are known to retain several properties more reminiscent of organelles in aerobic bacteria than eukaryotic cells including a double membrane and circular DNA (35). Although mtDNA represents a small portion of the DNA in a eukaryotic cell, the mitochondrial genome encodes several essential elements of the electron transport chain that fuels cellular respiration and energy production. It also differs from nuclear DNA in that it is rich in unmethylated CpG motifs that can act as damage-associated molecular patterns to initiate inflammatory response (78). In an elegant set of experiments, Oka et al. demonstrate that mtDNA that escapes from autophagy leads to inflammatory responses in heart muscle cells, inducing abnormalities in cardiac structure/function, heart failure, and increased mortality (41, 58). These findings are in line with previous studies which show that release of mtDNA into the circulation (so-called circulating cell-free mtDNA) can elicit a sepsis-like inflammatory response and widespread organ damage (95). Taken together, these observations implicate mtDNA in the genesis of chronic inflammation that accompanies CVDs. Yet it remains unexplored whether there is a “leak” of mtDNA into the hypothalamic ventricular system in chronic obesity and whether it has an important connection with hypothalamic inflammation-induced hypertension.
In this context, we postulated a role for central circulating mtDNA in the neurogenic hypertension associated with neuroinflammation. Indeed, we show that obesity-induced hypertension is associated with mtDNA excess in the cerebrospinal fluid (CSF). Administration of mtDNA directly into the brain elevated the BP in a physical activity-independent manner. In addition, we found that neurons in the mediobasal hypothalamus were crucial for the hypertensive effects of mtDNA. Loss-of-function studies further showed that inhibition of the TGFß pathway counteracted hypertension induced by mtDNA. These results point to a novel paradigm involving mtDNA-induced neuroinflammation in hypothalamic neurons as a mediator of obesity-associated neurogenic hypertension.
MATERIALS AND METHODS
Experimental animals.
C57BL/6 mice and Tgfbr2flox/flox mice were obtained from The Jackson Laboratory, with Tgfbr2flox/flox being maintained on C57BL/6 background. POMC-Cre mice were used in a previous research study (67) and were also maintained on C57BL/6 background. Tgfbr2flox/flox and POMC-Cre animals were crossed to create mice with TGFβ receptor 2 (TGFβR2) knockout in proopiomelanocortin (POMC) neurons. All mice were housed in a pathogen-free animal facility with a 12:12-h light-dark cycle with ad lib access to normal chow from weaning age to the start of the experiment. Mice with dietary obesity were generated using high-fat diet (HFD) feeding (60% kcal fat; Research Diets) for the time period as indicated. All procedures were approved by the Institutional Animal Care and Use Committee of Albert Einstein College of Medicine.
Circulating cell-free mtDNA in CSF and hypothalamus.
For the CSF collection, an anesthetized mouse was placed onto the stereotactic apparatus with the head forming an angle of ~135° with the body and secured with the head adaptors. A sagittal incision in the neck skin was made inferior to the occiput lateral, and then a capillary tube was used to penetrate into the cisterna magna through the dura mater (89). Then the hypothalamus was dissected from the brain as described previously (96) and immediately frozen. To measure the number of copies of mtDNA, we analyzed the amount of mtDNA vs. a single-copy nuclear gene. NADH dehydrogenase 1 (ND1) was used to represent mtDNA, and β-globin was used as a reference of the single-copy nuclear gene. The following specific primers were used for quantification of mitochondrial DNA and nuclear DNA: ND1, 5′-CTAGCAGAAACAAACCGGGC-3′ and 5′-CCGGCTGCGTATTCTACGTT-3′; β-globin, 5′-GAAGCGATTCTAGGGAGCAG-3′ and 5′-GGAGCAGCGATTCTGAGTAGA-3′.
Tissue harvesting and Western blotting.
The hypothalamus was dissected from the brain as described previously (96). Protein lysates were prepared and dissolved in a lysis buffer, separated by SDS-PAGE, immunoblotted with primary antibodies including rabbit anti-phospho (p)-Smad2, anti-Smad2, anti-p-ERK1/2, anti-ERK1/2, and anti-β-actin (Cell Signaling), and reacted with horseradish peroxidase-conjugated secondary antibody (Pierce). Quantification of Western blots was performed with Image J, and p-Smad2 or p-ERK1/2 levels were normalized according to the protein levels of total Smad2 or total ERK1/2.
Hypothalamic third-ventricle cannulation and injection.
Utilizing an ultraprecise, small animal stereotaxic apparatus (10-µm resolution; David Kopf Instruments), a 26-gauge guide cannula (Plastics One) was implanted into the third ventricle of mice anesthetized with 87.5 mg/kg ketamine and 12.5 mg/kg xylazine before the surgery at the midline coordinates of 1.66 mm posterior to and 5.0 mm below the bregma. We also injected ketoprofen (100 mg/kg) subcutaneously as an analgesic treatment before surgery and postsurgery during 3 consecutive days. The animals were monitored during 7 days, and they did not show any symptom of pain or illness. Following a 1–2-wk recovery period, mice in the experimental group were infused with either mtDNA (60 pg from isolated mitochondria from hypothalamic tissue) or TGFβ1 (4 ng; R&D Systems) dissolved in 0.5-µl artificial cerebrospinal fluid (aCSF). A separate group of mice were administered the same volume of aCSF as vehicle control. Mice were injected with mtDNA or TGFβ1 in the beginning stage of daytime (rest phase) vs. nighttime (active phase) as specified in each experiment. Mice with obesity-induced hypertension were injected with a single dose of 10 µM TGFβ receptor antagonist, SB-431542 (Millipore). All injections were performed through the preimplanted cannula over a 5-min time period using a 33-gauge internal injector (Plastics One) connected to a 5-µl Hamilton syringe.
Telemetric BP probe implantation and recording.
Radiotelemetric catheters [model TA11PA-C10; Data Sciences International (DSI)] were preimplanted into the carotid artery, using a previously described procedure (67). Briefly, before anesthesia, animals were injected subcutaneously with ketoprofen (100 mg/kg). Under anesthesia (using the ketamine/xylazine cocktail) a ventral midline skin incision was made for animals, and the left common carotid artery was isolated under a binocular surgical microscope. The proximal end of the artery was ligated right below the carotid bifurcation, and the distal end was occluded with a microclip. A microscissor was used to make a small incision near the proximal end, and then a pressure transmission catheter was guided into the artery, advanced to the aorta, and secured in place with sutures. The transmitter device was placed subcutaneously on the right flank, as close to the hind limb as possible. Finally, the neck incision was closed using 5-0 sutures (Ethicon), and during 3 consecutive days they received analgesia (ketoprofen 100 mg/kg); mice were allowed 1~2 wk of postsurgical recovery under daily monitoring, and they did not show any symptom of pain or illness. Following recovery, BP measurements of each animal under conscious, free-moving conditions were recorded using the computerized DSI software. All animals were adapted to the injection procedure using the vehicle for ~3–4 days, so injection stress was minimal and did not obviously affect blood pressure for the time window of treatment effects. BP data were sampled continuously with a sampling rate of 1,000 Hz for indicated time period. Physical activity of freely-moving animals was simultaneously recorded via radio telemetry and analyzed using DSI software.
Power spectral analysis of heart rate variability.
Power spectra of multiple short-term (5 min) heart rate variability (HRV) was assessed in the frequency domain by means of power spectral density using ART 4.2 software (Data Sciences International). Interbeat intervals were detected by extracting beat-by-beat features from pressure signals and were spline interpolated at a frequency of 50 Hz using cubic order to obtain equidistant data points for spectral analysis. Power spectral density was calculated by an averaged periodogram method using maximum periodogram resolution and an overlapping subseries of 5. As previously established, cutoff frequency ranges for low-frequency and high-frequency HRV were defined as 0.4–1.5 and 1.5–4 Hz, respectively (80).
Statistical analysis.
Data were analyzed using ANOVA followed by appropriate post hoc analyses for studies involving more than two groups for comparisons and two-tailed Student’s t-tests for studies involving only two groups for comparison. Telemetric BP and heart rate over time periods were analyzed using repeated measures ANOVA followed by appropriate post hoc analyses. All data are presented as means ± SE. P values <0.05 were defined as statistically significant.
RESULTS
Obesity-associated central excess in circulating mtDNA activates TGFβ pathway.
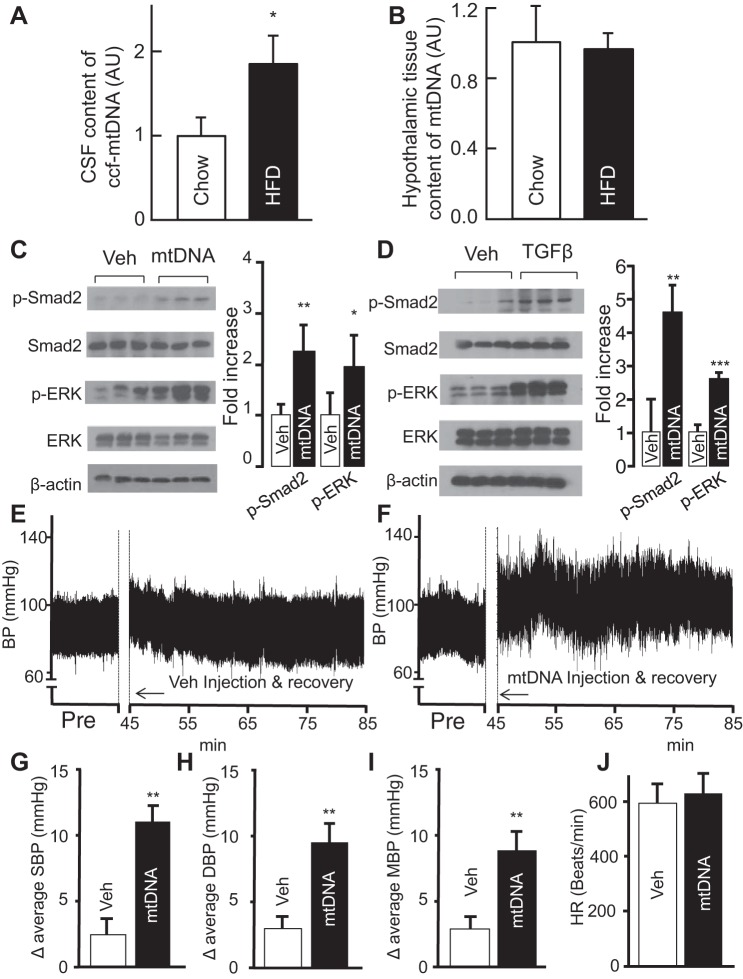
Although mitochondrial dysfunction and oxidative stress are linked to overnutrition-related diseases (5, 11, 16, 23, 51, 86) and hypertension (56, 57), it remains unknown whether obesity can trigger the release of circulating mtDNA in the brain. To address this question, dietary obesity was induced by a 12-wk regimen of high-fat diet (HFD) feeding in C57BL/6 mice, while age-matched control mice were fed normal chow. Both groups were anesthetized, and the CSF samples as well as hypothalamic tissues were collected as described previously (89). Analysis of circulating cell-free mtDNA in the CSF was performed using quantitative PCR. We found a higher amount of mtDNA in HFD-fed mice compared with mice fed with normal chow (Fig. 1A). Changes in CSF were evident even though mtDNA content in the hypothalamic tissues remained comparable between the two groups (Fig. 1B), suggesting that changes in circulating mtDNA are not due to inherent changes in intracellular mtDNA content in the hypothalamus, but rather an increase in extracellular release.
Fig. 1.
Obesity-associated brain excess in circulating mtDNA and the effect on blood pressure (BP). A and B: C57BL/6 mice fed on a HFD vs. normal chow for 12 wk were killed to obtain cerebrospinal fluid and hypothalamic tissues. Data show the fold increases of circulating cell-free (ccf) mtDNA in the cerebrospinal fluid (A) and tissue mtDNA in the hypothalamus (B) of HFD-fed mice compared with chow-fed mice. AU, arbitrary unit. C and D: standard C57BL/6 mice were preimplanted with an injection cannula in the hypothalamic third ventricle. After ~1-wk postsurgery recovery, they received a single injection of mtDNA (60 pg) or TGFβ1 (4 ng) in the third ventricle. Vehicle (Veh)-treated mice were used for comparison in each experiment. Western blots from mtDNA (C) or TGFβ1 (D)-treated mice were used to measure the levels of phospho-Smad2 (p-Smad2) and phospho-ERK (p-ERK) and quantified in graphs at right. E–J: regular C57BL/6 were preimplanted with a telemetric transmitter in the carotid artery and an injection cannula in the hypothalamic third ventricle. All mice were monitored for BP and heart rate following 1-wk postsurgery recovery. E and F: representative preinjection (Pre) period and 40-min telemetric BP tracings during the rest phase of a day. The time of 0–45 min was used for injection and postinjection recovery. G–J: average levels of systolic BP (G), diastolic BP (H), mean BP (I), and heart rate (J). SBP, systolic BP; DBP, diastolic BP; MBP, mean BP; HR, heart rate. *P < 0.05, **P < 0.01, ***P < 0.001; n = 3–6 mice per group (A–D) and n = 6 mice per group (G–J). Error bars reflect means ± SE.
Considering that obesity induces mtDNA excess in CSF, we wanted to examine which molecular pathways are involved. To mimic the rise in central mtDNA that accompanies obesity, we injected a single dose (60 pg) of mtDNA into the third ventricle of normal C57BL/6 mice through a preimplanted cannula. Previous work from our laboratory has highlighted the role of both the hypothalamic NF-κB (67, 89, 94, 96) and TGFβ (89) pathways in metabolic syndrome. Considering that mitochondrial dysfunction (90) and mtDNA (31) are known to trigger activation of the TGFß pathway, we studied levels of downstream signaling molecules including Smad and ERK1/2 via Western blot of hypothalamic tissues. Analysis of hypothalamic proteins revealed that a single hypothalamic infusion of mtDNA increased levels of phosphorylated Smad2 and ERK1/2 (Fig. 1C), suggesting increased activation of the TGFß pathway. Comparable, although not identical, elevations in phosphorylated Smad2 and ERK1/2 were found following TGFβ administration in a similar manner (Fig. 1D). Taken together, the results suggest that central excess of mtDNA mimics TGFβ to activate downstream signaling pathways.
Acute central administration of mtDNA increases BP.
Although excess mtDNA in the periphery is known to be associated with inflammation and CVDs (58), its role in hypothalamic neurogenic hypertension has not been explored. To test whether there might be a cause-effect relationship between excess circulating mtDNA and hypertension, we developed a novel paradigm in which we examined how central administration of mtDNA affects BP. We opted for a single intracerebroventricular infusion of 60-pg mtDNA directly into the hypothalamic third ventricle of healthy, chow-fed mice according to plasma mtDNA levels in humans with diabetes or cardiovascular disease (47). Comprehensive BP measurements including systolic and diastolic BP and heart rate were measured in conscious mice. Readings were done using a telemetry transmitter implanted into the carotid artery of mice; all animals were adapted to the procedure for ~1 wk. The effects of mtDNA on BP and heart rate were measured and statistically compared with the values from vehicle-treated controls, as well as the baseline measures obtained before injection. Compared with vehicle-treated animals (Fig. 1E), we found that even a single administration of mtDNA caused a significant increase in overall BP (Fig. 1F). Quantitative analysis revealed that mtDNA excess induced a much larger increase in systolic BP compared with treatment with vehicle alone (Fig. 1G). Similarly, the change in average diastolic BP was also increased in mtDNA-treated animals (Fig. 1H). Overall, mtDNA excess caused a large elevation in mean BP that was significantly greater than that caused by vehicle solution (Fig. 1I). Conversely, heart rate between the two treatment groups remained comparable (Fig. 1J). The results suggest that excess circulating mtDNA can induce hypertension even under acute, short-term conditions.
Central TGFβ excess mimics circulating mtDNA to induce hypertension.
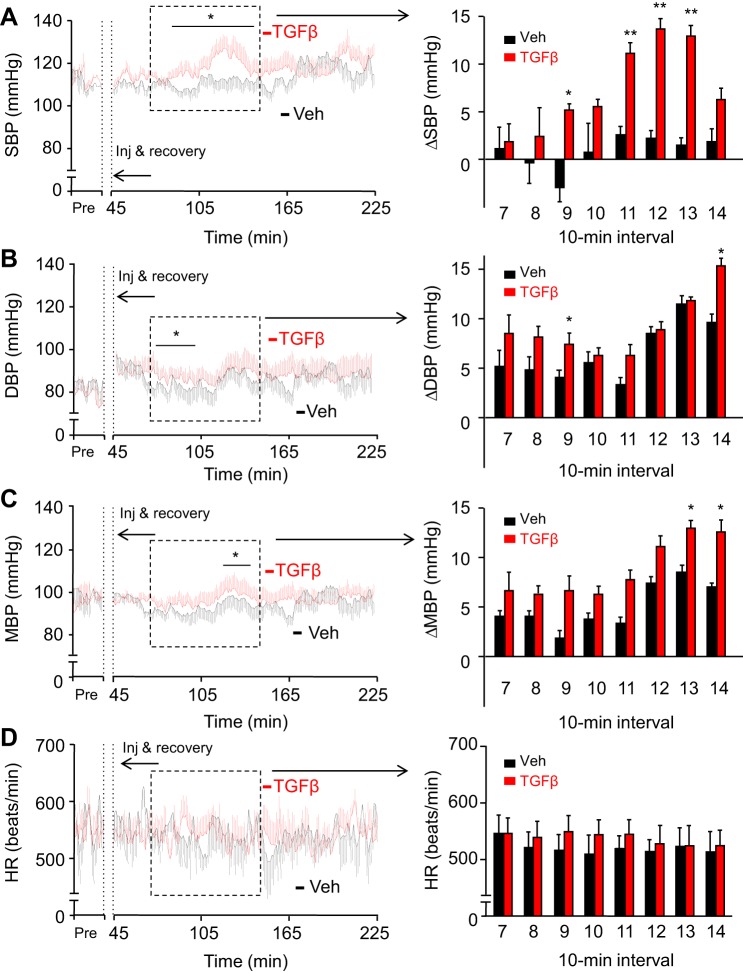
To test the hypothesis that central TGFβ is able to induce an increase in blood pressure like that previously observed with central administration of mtDNA, we designed a study to examine the central administration of TGFβ during rest. To study this effect, we injected a single dose (4 ng) of TGFβ1, a prototypical ligand in TGFβ signaling, into the third ventricle of normal C57BL/6 mice through a preimplanted cannula, at the beginning of either the rest or active phase. Dosage and expected pharmacokinetics were evaluated in our previous study (89) and demonstrated the physiological relevance of this approach. Systolic and diastolic BP, as well as heart rate, were measured via a telemetry transmitter implanted into the carotid artery; all animals were adapted to the procedure for at least 3 days. Subsequently, the effects of TGFβ administration on BP and heart rate were measured, which were compared with the values from vehicle-treated animals as well as baseline measures of TGFβ-treated mice obtained before injection.
Compared with controls, animals that received an injection of TGFβ showed an increase in BP (Fig. 2, A–C), which began ~1.5-h postinjection. This increase in systolic BP peaked ~2-h postinjection at ~15% higher than at baseline. Systolic BP in TGFβ-treated mice returned to control levels roughly 2.5 h or longer postinjection (Fig. 2A). By grouping systolic BP measurements into 10-min intervals, we observed a significant increase in TGFβ-treated mice compared with vehicle-treated controls from 90- to 130-min postinjection (Fig. 2A). In contrast, diastolic BP levels in these animals were not affected by TGFβ treatment (Fig. 2B). Mean BP values of these mice, calculated as two-thirds diastolic BP plus one-third systolic BP, did not reach significance for most of the follow-up duration, though there was a general increase in mean BP in TGFβ-treated animals (Fig. 2C). TGFβ-injected mice generally had higher heart rates compared with controls (Fig. 2D), potentially reflecting a sympathetic input to the heart in addition to the vascular system.
Fig. 2.
BP-raising effect of a single TGFβ injection via the hypothalamic ventricle. Standard C57BL/6 mice (chow-fed males, ~4 mo old) were preimplanted with a telemetric transmitter in the carotid artery and an injection cannula in the hypothalamic third ventricle. All mice were monitored for BP and heart rate during 1-wk postsurgery recovery. For the experiment, these mice were injected with a single dose of TGFβ1 (4 ng) vs. vehicle (Veh) in the hypothalamic third ventricle during the rest phase, and systolic BP, diastolic BP, mean BP, and heart rate preinjection and postinjection were continuously recorded. Curves at left present the minute-by-minute average systolic (A), diastolic (B), and mean (C) BPs and heart rate (D) over a representative preinjection (Pre) period and 3-h postinjection period. The time period of 0–45 min was used for injection (inj) and postinjection recovery. Bar graphs on the right present changes (Δ) in systolic (A), diastolic (B), and mean (C) BP compared with the baseline, as well as heart rate (D) over duration segment 7–14 of the 10-min intervals for the time period outlined on the curves. SBP, systolic BP; DBP, diastolic BP; MBP, mean BP; HR, heart rate. *P < 0.05, **P < 0.01, compared with time-matched Veh-treated group; n = 9 mice per group. Error bars reflect means ± SE.
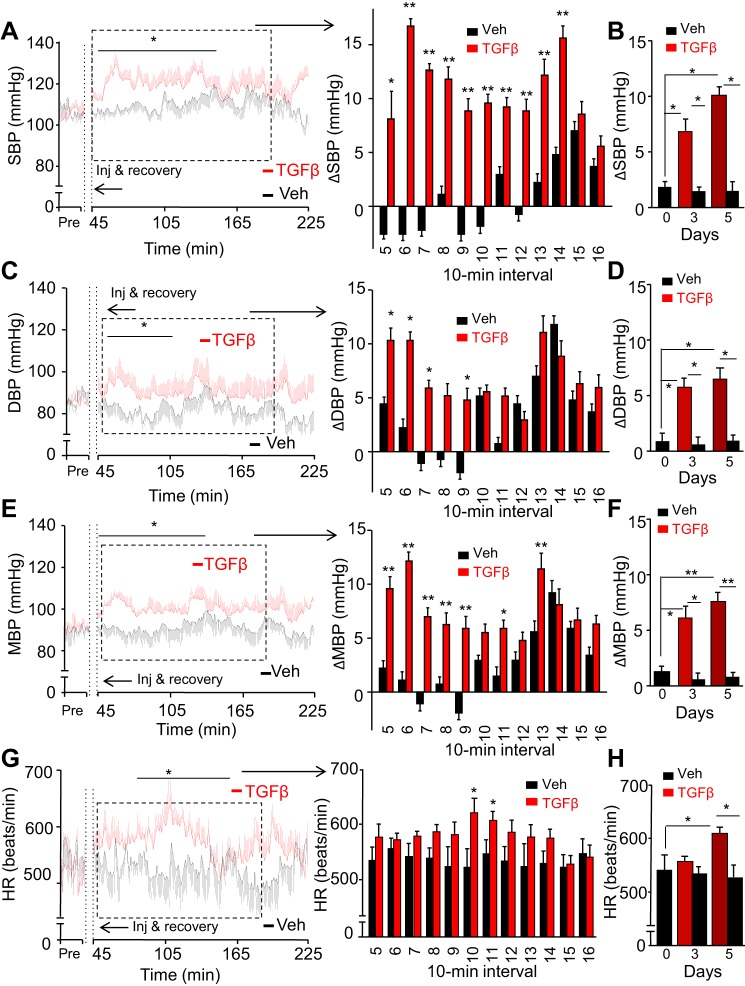
TGFβ content is known to chronically increase in the hypothalamus with aging or obesity development (8, 88, 89). To study the effect of a pathological, chronic excess of this cytokine, we used an identical experimental design as detailed above. Compared with vehicle-injected controls, mice that chronically received TGFβ injections displayed increased systolic BP values in a time course-dependent manner (Fig. 3, A and B). On day 5, this systolic BP increase occurred almost immediately following the postinjection recovery (~45 min after injection). On day 5, the highest systolic BP levels in some TGFβ-treated mice reached 130–150 mmHg, much higher than the normal range of systolic BP (105–115 mmHg) in vehicle-treated mice. Furthermore, the elevations in systolic BP of TGFβ-injected mice remained high for 2 h (Fig. 3A), more than twice as long compared with the effects from a single TGFβ injection (Fig. 2A). Simultaneously, diastolic BP also clearly increased (Fig. 3, C and D); although these increases were more pronounced than those after a single administration (Fig. 2B), they were less substantial compared with the magnitude in systolic BP. The highest diastolic BP levels in some TGFβ-treated mice reached 95–110 mmHg, higher than the normal range of diastolic BP (70–85 mmHg) in vehicle-treated mice. In the 10-min average measurements, mean BP increased up to 12 mmHg following 5-day TGFβ treatment (Fig. 3E), and this effect was already noticeable by day 3 during the treatment (Fig. 3F). Compared with chronic infusion, acute administration led to smaller and less frequent differences in mean BP (Fig. 2C). While acute TGFβ treatment did not increase heart rate (Fig. 2D), chronic infusion displayed an increasing trend during this 5-day treatment as presented in Fig. 3D, and on day 5, this increase was more obvious and statistically significant (Fig. 3, G and H), suggesting that TGFβ excess in the brain, especially with chronic infusion has a stimulatory impact on the heart which might also contribute to BP increase.
Fig. 3.
Effects of chronic central TGFβ excess on BP and heart rate in mice. Standard C57BL/6 mice followed the same procedure as described in Fig. 2. After postsurgery recovery, mice were injected with TGFβ1 (4 ng) vs. vehicle (Veh) in the third ventricle every 12 h at the beginning of light and dark phases consecutively for 5 days, and SBP (A and B), DBP (C and D), MBP (E and F), and HR (G and H) were recorded before injection and postinjection. A, C, E, and G: curves at left present the minute-by-minute average SBP (A), DBP (C), MBP (E), and HR (G) on day 5 over a representative preinjection (Pre) period and a 3-h period after the injection in the light phase. Bars at right present changes (Δ) of postinjection SBP (A), DBP (C), and MBP (E) compared with the preinjection baseline and heart rate (G) over duration segment 5–16 of 10-min intervals for the time period outlined on the curves. B, D, F, and H: bars show 40-min peak changes (Δ) of SBP (B), DBP (D), and MBP (F) compared with the preinjection baseline and heart rate (H) in TGFβ- vs. Veh-injected mice in the light phase of days 0, 3, and 5. *P < 0.05, **P < 0.01, compared with time-matched Veh-treated group (A, C, E, and G) and between groups as indicated (B, D, F, and H); n = 6 mice per group. Error bars reflect means ± SE.
Central mtDNA and TGFβ excess induces hypertension independent of physical activity.
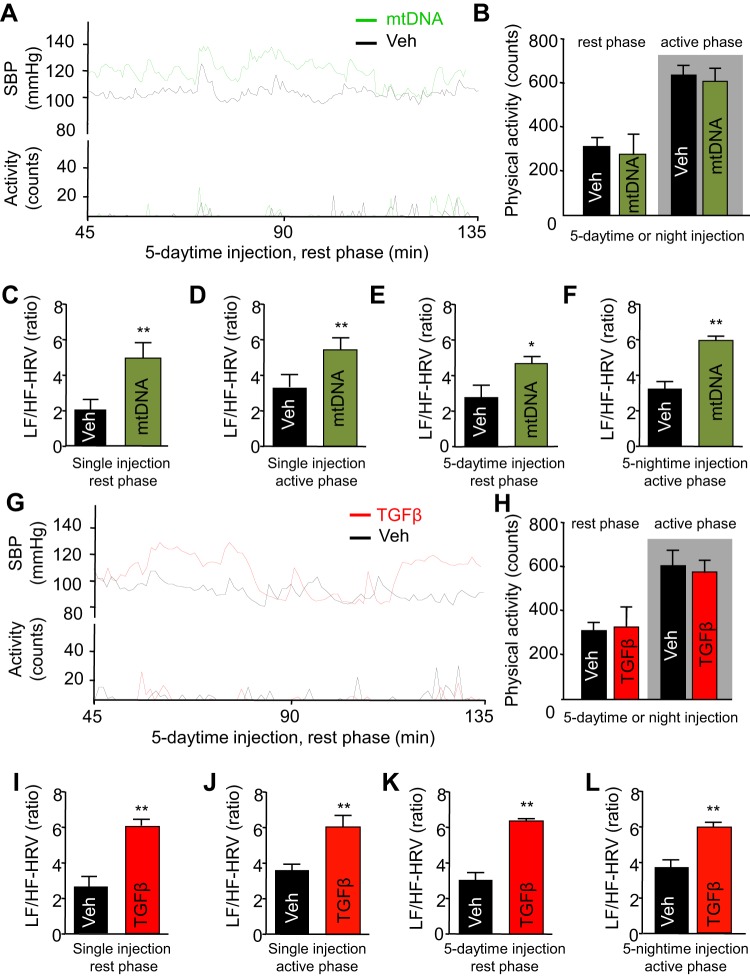
Exercise is known to raise systolic BP to a greater degree than diastolic BP (46, 52, 92). Since acute central mtDNA (data not shown) and TGFβ excess (Fig. 2) affected systolic BP more than diastolic BP, a relevant question is, could the BP changes we observed be a result of increased physical activity? To answer this question, we examined if mtDNA and TGFβ administration could affect physical activity through simultaneously collecting data on both physical activity and BP under telemetric recordings. Focusing on a 90-min time window in which mtDNA and TGFβ excess strongly increased systolic BP, we observed that 5-day repeated injections of mtDNA (Fig. 4, A and B) or TGFβ (Fig. 4, G and H) had no influence on physical activity compared with vehicle-treated animals. We noted similar findings in single-injection response (data not shown). These observations are demonstrated through representative tracings of systolic BP following the last injection after chronic treatment (Fig. 4, A and G) or average physical activity assessed automatically via telemetric recordings (Fig. 4, B and H). Analysis revealed that mice receiving mtDNA (Fig. 4B) and TGFβ (Fig. 4H) injections display physical activity similar to that of control animals treated with vehicle during both active and rest phases. Hence the BP increases observed under central mtDNA and TGFβ excess were not caused by a change in physical activity, though the same mechanisms involved in the exercise-induced BP rise might also be used by central mtDNA and TGFβ to affect rest-phase systolic BP.
Fig. 4.
Physical activity-independent sympathetic activation by TGFβ and mtDNA excess. Physical activity and sympathetic hemodynamics were calculated from mice treated with a single injection or five daily injections of mtDNA or TGFβ1 in the hypothalamic third ventricle. A: tracing of BP and physical activity of mice recorded in the rest phase (daytime) after five daytime injections of mtDNA (60 pg per injection). B: average rest-phase and active-phase physical activity of mice, respectively, following five daytime or nighttime injections of mtDNA (60 pg per injection). C–F: LH/HF-HRV over 30-min peak changes in mice recorded following a single daytime or nighttime injection (C and D) or five daytime or nighttime injections (E and F) of mtDNA (60 pg per injection). G: tracing of BP and physical activity of mice recorded in the rest phase after five daytime injections of TGFβ1 (4 ng per injection). H: average rest-phase and active-phase physical activity of mice, respectively, following five daytime and five nighttime injections of TGFβ1 (4 ng per injection). I–L: LH/HF-HRV over 30-min peak changes in mice following a single daytime or nighttime injection (I and J) or five daytime and nighttime injections (K and L) of TGFβ1 (4 ng per injection). *P < 0.05, **P < 0.01, compared with Veh-treated groups; n = 6 mice per group. Error bars reflect means ± SE.
Sympathetic activation underlies the hypertensive effect of mtDNA and TGFβ excess.
Sympathetic regulation is an essential contributor to BP increase (38, 87), and several forms of hypertension, including obesity-related hypertension, are believed to involve an upregulation in sympathetic activation (65, 68). Therefore understanding how sympathetic activity could be changing with mtDNA or TGFβ infusion can provide further etiological evidence for the observed increases in BP. Methodologically, sympathetic control of hemodynamics can be reported using the low-frequency to high-frequency heart rate variability (LF/HF-HRV), a parameter which is consistent with the level of sympathetic outflow in regulating hemodynamics (67). Using this approach, we calculated the LF/HF-HRV values during peak BP elevations following acute and chronic treatment with mtDNA (Fig. 4, C–F) and TGFβ (Fig. 4, I–L). We found that central mtDNA significantly increased LF/HF-HRV in mice following a single injection (Fig. 4, C and D) or 5-day injection regimen (Fig. 4, E and F), and these effects were seen for both the rest phase (Fig. 4, C and E) and active phase (Fig. 4, D and F). In line with other findings in this paper supporting overlapping pathways, TGFβ induced similar changes in sympathetic outflow. In particular, mice with excess central TGFβ displayed increased LF/HF-HRV in all conditions studied including rest phase following acute injection (Fig. 4I), active phase after a single dose (Fig. 4J), rest phase after chronic TGFβ administration (Fig. 4K), and, finally, the active phase after 5-day injections (Fig. 4L). Taken together, these findings suggest that for both rest and active phases and under acute or chronic conditions, increased sympathetic outflow to the cardiovascular system represents the overall physiological basis for central mtDNA and TGFβ-induced hypertension.
Activation of POMC neurons mediates the hypertensive effect of mtDNA and TGFβ excess.
The hypothalamus is the central regulator of energy homeostasis because it senses metabolic cues and, in turn, modulates neurohormonal and neurotransmitter systems via endocrine signaling, inflammatory signaling, and neuronal plasticity (12, 74). Proopiomelanocortin (POMC) and agouti-related peptide (AGRP) neurons in the mediobasal hypothalamus reciprocally regulate energy balance via anorexigenic and orexigenic effects, respectively (72, 75, 97). Previous work from our laboratory revealed that TNFα stimulation of the NF-κB pathway in POMC neurons leads to an elevation in blood pressure through sympathetic nervous system activation (67). Here, we asked whether activation of this cell subpopulation in the mediobasal hypothalamus could also be important for the hypertensive effects elicited by mtDNA and TGFβ excess. To study this possibility, we tested whether central mtDNA/TGFβ-induced hypertension could be affected by genetic inhibition of TGFβR2, the TGFβ receptor subunit that is required for inducing TGFβ signaling and actions (30). We carried out this study using POMC neuron-specific TGFβR2 knockout (POMC/TGFβRlox/lox) mice generated by breeding POMC-Cre mice with TGFβR2lox/lox mice as described in detail in our recent work (89). POMC/TGFβRlox/lox mice and littermate wild-type controls with matched genetic background (TGFβRlox/lox mice) were injected with a single dose (60 pg) of mtDNA or TGFβ via a preimplanted hypothalamic third-ventricle cannula.
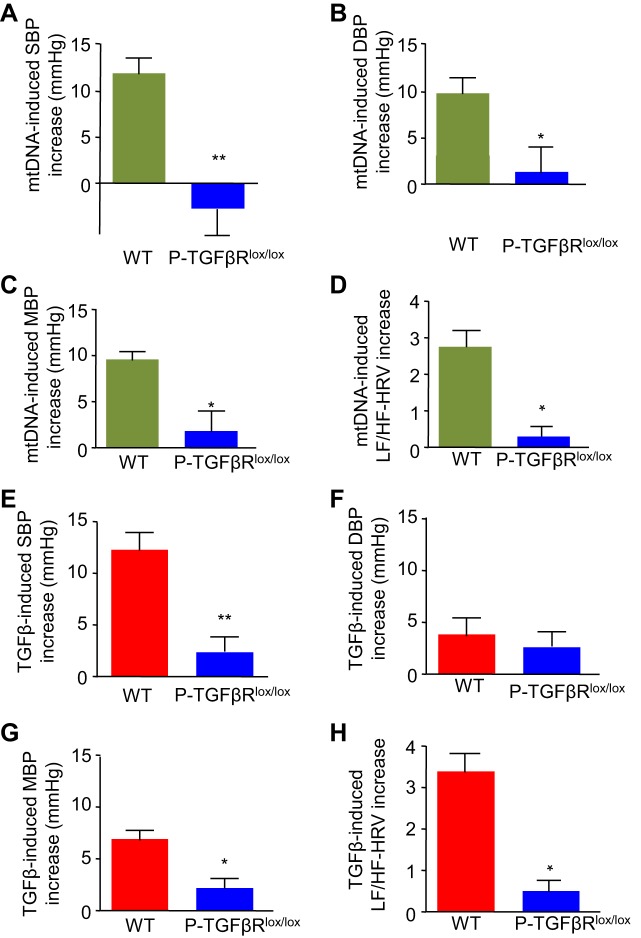
We studied whether POMC/TGFβRlox/lox mice are protected from the hypertensive effects of mtDNA and TGFβ excess compared with wild-type TGFβRlox/lox controls. Compared with wild-type mice, which showed a great increase in systolic BP in response to mtDNA injection, POMC/TGFβRlox/lox mice were clearly protected against this increase (Fig. 5A). Diastolic BP in POMC/TGFβRlox/lox mice was also significantly lower than in wild-type controls (Fig. 5B). Reductions in both systolic and diastolic BP resulted in significantly lower mean BP values in POMC/TGFβRlox/lox mice (Fig. 5C). The observation that mtDNA did not increase sympathetic hemodynamics in the knockout mice (Fig. 5D) further supports the involvement of sympathetic upregulation in mtDNA-induced hypertension. In response to TGFβ injection, POMC/TGFβRlox/lox failed to develop the increase in systolic BP that was present in wild-type controls (Fig. 5E). Diastolic BP was not significantly affected (Fig. 5F). Compared with wild-type controls, mean BP values in POMC/TGFβRlox/lox mice were less affected by TGFβ injection, due to the counteraction against systolic BP rise in these mice (Fig. 5G). Similar to the response to mtDNA excess, TGFβ also failed to increase sympathetic hemodynamics in knockout mice (Fig. 5H). These findings indicate that the pathogenesis of mtDNA-mediated hypertension critically engages the TGFβ receptor in POMC neurons. This may be explained by the fact that TGFβ is believed to directly modulate POMC mRNA expression in the hypothalamus (14) and that the major peptide product produced by hypothalamic POMC mRNA is α-melanocyte-stimulating hormone, which is known to act on melanocortin-4 receptors to stimulate sympathetic outflow and affect metabolic and cardiovascular outcomes (24, 28).
Fig. 5.
Role of POMC neurons in mtDNA-induced hypertension. POMC/TGFβR2lox/lox mice (P-TGFβRlox/lox) and littermate wild-type controls (WT) were maintained in standard feeding and housing conditions. At an adult age, they were preimplanted with a telemetric transmitter in the carotid artery and/or an injection cannula in the hypothalamic third ventricle. All the experiments were done after 1 wk of postsurgery recovery. For BP and sympathetic activity, one group of mice was injected with a single dose of mtDNA (60 pg) vs. vehicle (Veh) in the third ventricle during the rest phase (daytime), and systolic BP, diastolic BP, mean BP, and heart rate were continuously recorded before injection and postinjection. Data show mtDNA injection-induced peak increases in SBP (A), DBP (B), MBP (C), and LF/HF-HRV (D) over a 30-min postinjection period compared with the preinjection baseline levels in each genotype. Another group of POMC/TGFβR2lox/lox and WT mice were injected with a single dose of TGFβ1 (4 ng) vs. Veh in the third ventricle. Data show TGFβ injection-induced peak increases in SBP (E), DBP (F), MBP (G), and LF/HF-HRV (H). *P < 0.05, **P < 0.01, compared with WT group; n = 6 mice per group. Error bars reflect means ± SE.
Protection against mtDNA-induced increase in BP with TGFβ receptor antagonist.
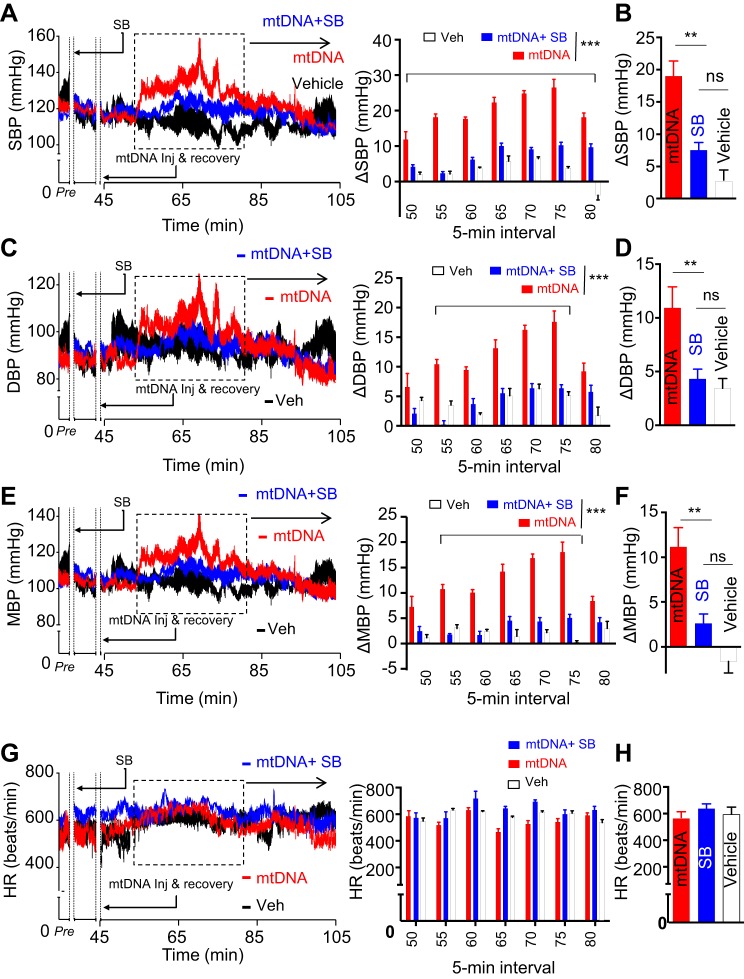
Although the findings above suggest that central delivery of mtDNA mimics TGFβ excess to activate downstream signaling pathways and promote hypertension, they did not functionally examine this potential relationship. One way to address this concern is to block TGFβ receptor in the context of mtDNA excess and examine the influence on mtDNA-induced hypertension. If mtDNA indeed mediates its effects through the TGFβ receptor, then inhibiting the receptor should abolish, or at least attenuate, the hypertensive response to mtDNA excess. Alternatively, if inhibition of the TGFβ receptor fails to protect against mtDNA-induced hypertension, that would suggest that other mechanisms may be at play. To dissect this relationship further, we pretreated animals with an intrahypothalamic third-ventricle injection of the TGFβ receptor antagonist SB-431542 at 30 min before induction of excess mtDNA. We chose a low dose (0.5 μl, 10 μM SB-431542), which theoretically leads to roughly 1–2 μM of this drug in the CSF of mice right after injection. Control animals received a vehicle solution 30 min before mtDNA administration. Systolic and diastolic BPs were recorded via radio telemetry as described above. Compared with vehicle-treated mtDNA excess animals and wild-type controls, mice treated with SB-431542 showed a significant reduction in systolic BP, most pronounced between 50 and 80 min after mtDNA injection (Fig. 6, A and B). Analysis of diastolic BP similarly revealed significantly lower BP following SB-431542 administration compared with both vehicle-treated and wild-type animals (Fig. 6, C and D). Mean BP showed similar trends (Fig. 6, E and F), suggesting that pharmacological inhibition of the TGFβ receptor protects against mtDNA-induced hypertension in mice. Conversely, pretreatment with SB-431542 had no noticeable effect on heart rate (Fig. 6, G and H). Overall, the finding that TGFβ receptor antagonism normalizes, not merely attenuates, BP points to TGFβ receptor-mediated signaling as the primary pathway involved in hypertension produced by excess circulating mtDNA.
Fig. 6.
Centrally antagonizing TGFβ receptor suppresses mtDNA-induced hypertension. Standard C57BL/6 mice followed the same procedure as described in Fig. 2. After 1-wk postsurgery recovery, these mice were injected with a single dose of TGFβ receptor antagonist, SB-431542 (SB; 5 pmol) or/and mtDNA (60 pg) vs. vehicle (Veh) in the third ventricle, and systolic BP, diastolic BP, mean BP, and heart rate preinjection and postinjection were continuously recorded. The time period of 0–45 min was used for injection (inj) and postinjection recovery from the last injection (mtDNA injection). SBP (A and B), DBP (C and D), MBP (E and F), and HR (G and H) were recorded before injection and postinjection. A, C, E, and G: curves at left present the every-10-s average SBP (A), DBP (C), MBP (E), and HR (G) over a representative preinjection (Pre) period and a 1-h period after the injection in the rest phase. Bars at right present changes of postinjection in SBP (A), DBP (C), and MBP (E) compared with the preinjection baseline and heart rate (G) over duration segment of 5-min intervals for the time period outlined on the curves. B, D, F, and H: bars show 30-min peak changes (Δ) of SBP (B), DBP (D), and MBP (F) compared with the preinjection baseline and heart rate (H) in indicated groups. SBP, systolic BP; DBP, diastolic BP; MBP, mean BP; HR, heart rate; ns, nonsignificant, *P < 0.05, **P < 0.01, ***P < 0.001, compared between indicated groups; n = 6 mice per group. Error bars reflect means ± SE.
Treatment of obesity-related hypertension by central administration of a TGFβ antagonist.
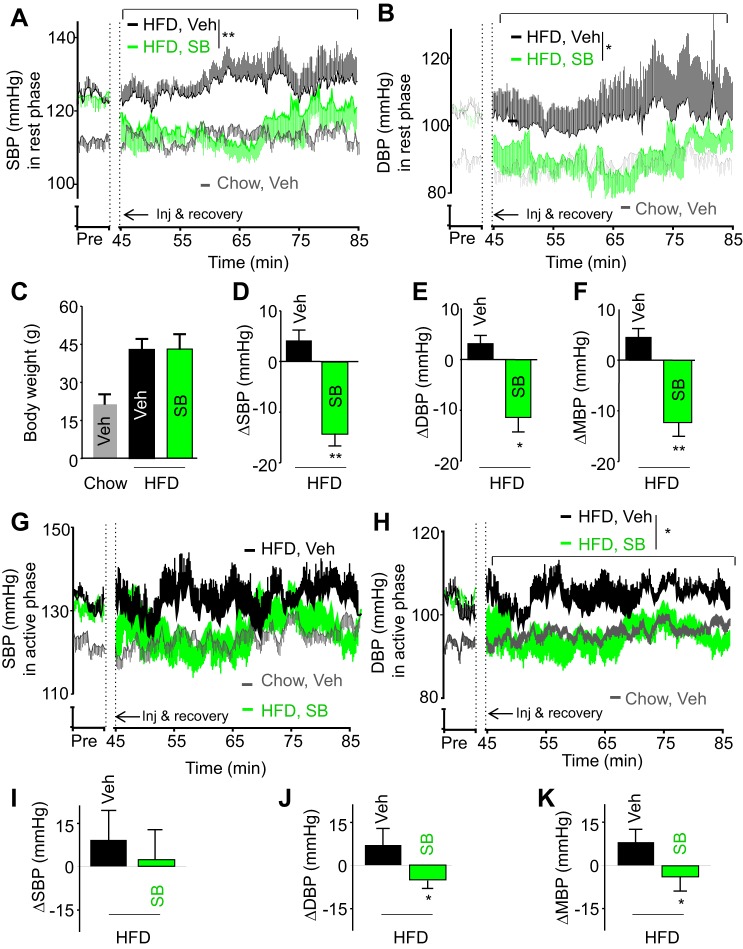
Finally, perhaps one of the most important questions in this study was, could central TGFβ be pharmacologically targeted to treat hypertension? Because hypothalamic TGFβ excess is closely associated with obesity (89), we decided to use obesity-related hypertension as a model to assess this possibility. To do so, adult C57BL/6 mice were maintained on a HFD for 5 mo, during which obesity-induced hypertension developed. Subsequently, these mice received a hypothalamic third-ventricle injection of either vehicle or TGFβ receptor antagonist SB-431542, in the beginning of the rest phase or active phase. Systolic and diastolic BPs were recorded via radio telemetry. As shown in Fig. 7, A and B, despite a low-dose and single injection, administration of SB-431542 during the rest phase resulted in a strong reversal of hypertension, using a mouse model of HFD-induced obesity (Fig. 7C). Comparing the BP differences preinjection and postinjection (Fig. 7, D–F), treatment with SB-431542 led to greatly decreased in systolic BP (Fig. 7D), diastolic BP (Fig. 7E), and mean BP (Fig. 7F). The magnitude of systolic BP drop appeared to be larger than that of diastolic BP, agreeing with the hypothesis that during the rest phase, central TGFβ excess affects systolic BP more than diastolic BP.
Fig. 7.
Central injection of TGFβ receptor antagonist-treated obesity-related hypertension. C57BL/6 mice were fed a HFD for 5 mo to establish obesity-related hypertension compared with age-matched chow-fed mice. These mice were implanted with a telemetric transmitter in the carotid artery and an injection cannula in the hypothalamic third ventricle. BP and heart rate were recorded during 1-wk postsurgery recovery. Mice were injected with TGFβ receptor antagonist, SB-431542 (SB; 5 pmol) vs. the vehicle in the third ventricle during the rest phase (A–F) or during the active phase (G–K). A, B, G, and H: average SBP (A and G) and DBP (B and H) every 10 s over a representative preinjection (Pre) period during the basal condition, and 40-min postinjection period. C: body weight of mice at the end of experiment. D–F and I–K: changes (Δ) of postinjection SBP (D and I), DBP (E and J), and MBP (F and K) averaged from 30-min peak effects compared with the preinjection baseline. *P < 0.05, **P < 0.01, compared with Veh-treated HFD-fed mice; n = 6 mice per group. Error bars reflect means ± SE.
Also, driven by curiosity regarding whether TGFβ antagonism would affect BP during the active phase (nighttime), we performed TGFβ receptor antagonist treatment during the nighttime, using a different cohort of HFD-fed mice. A single injection of SB-431542 in the hypothalamic third ventricle decreased diastolic BP to a greater degree than systolic BP (Fig. 7, G and H). This therapeutic effect reinforces our findings that TGFβ excess has a stronger influence on diastolic BP during the active phase. Regardless of the dynamics by which the TGFβ receptor antagonist lowers BP, the body of these results supports the conclusion that suppression of central TGFβ bears a therapeutic potential for hypertension treatment. This understanding might be clinically important for hypertension, considering that clinical trials using TGFβ antagonists have been ongoing for patients with pulmonary fibrosis (3), scleroderma (25) and cancers (2); thus adding a new clinical implication for treating hypertension and related cardiovascular diseases is both innovative and feasible.
DISCUSSION
For the first time, we revealed that excess circulating mtDNA induces the development of hypertension through the central nervous system, and particularly the hypothalamus. Our detailed analyses demonstrated the following: First, obesity induces an increase in circulating mtDNA in the cerebrospinal fluid. Second, we observed that mtDNA could induce a strong activation of the TGFβ pathway. Third, when mtDNA and TGFβ are chronically in excess, it leads to elevations in systolic and diastolic BPs, mimicking the pathogenesis of hypertension. Fourth, in mechanistic studies, we found that the hypertensive effect of central mtDNA excess is mediated by the TGFβ receptor in POMC neurons in the mediobasal hypothalamus. Finally, and therapeutically, the central administration of a TGFβ receptor antagonist was found to alleviate obesity-related hypertension, signaling an interventional target and mechanism for treating this disease.
Under physiological conditions, mitochondria transform energetic substrates (e.g., lipids and glucose) and oxygen into energy. These membrane-bound organelles also modulate cellular survival and apoptosis, generation of reactive oxygen species, and intracellular calcium homeostasis. Since mtDNA encodes several essential elements required to maintain critical cellular functions, it comes as no surprise that damage to mtDNA is a common feature in pathogenic conditions characterized by premature aging (42, 84), neurodegeneration (27, 39, 85), malignancy (15, 32, 39), and metabolic oversupply (53, 61, 81). Accumulation of mutated mtDNA increases the production of reactive oxygen species, and oxidative stress is tightly associated with brain inflammation in overnutrition-related diseases (5, 11, 16, 23, 51, 86) and hypertension (56, 57). Damaged mtDNA is degraded by autophagy (40, 73), and it is notable that under obesogenic conditions, decline of hypothalamic autophagy leads to neuroinflammatory reaction driven by NF-κB (54, 83); thus escaped mtDNA from obesity-impaired autophagy may contribute to the formation of an obesity-associated neural inflammatory response (41, 58, 95). In light of the potential mechanisms by which mtDNA stimulates TGFβ signaling, mtDNA contains inflammatogenic unmethylated CpG motifs (19, 63) that could drive the overproduction of inflammatory cytokines including TGFβ and then likely employ an autocrine approach to increase TGFβ signaling as similarly observed in other experimental conditions (13). Also, as documented in the literature (6), mtDNA can activate toll-like receptor 9 (TLR9), and this action on the cell surface might have an interaction with TGFβ signaling leading to enhanced TGFβ production and signaling, which has been reported before with several TLRs (10, 44). Further research is warranted to examine whether TLR might represent a link between mtDNA and activation of TGFβ signaling in the hypothalamus under conditions of obesity.
Furthermore, hypothalamic inflammatory mechanisms of hypertension have recently been suggested to act through a process mediated by the proinflammatory NF-κB pathway which produces cytokines such as TNFα to increase BP (67).Therefore an outstanding question that remains to be answered is how hypothalamic NF-κB is activated under relevant etiological conditions leading to hypertension. Previous work from our laboratory has revealed that TGFβ is capable of activating NF-κB by reducing IκBα mRNA stability (89), thus circumventing the need for IKK signaling. This new knowledge led us to predict that the central action of TGFβ is potentially important for hypertension, in agreement with previous studies (45). Then, we observed that both mtDNA and TGFβ are able to activate the TGFβ pathway. Thus, while beyond the scope of this study, which focused on hypertension, it can be speculated that hypothalamic cell-free mtDNA and TGFβ signaling contributes to the hypertension associated with obesity. In fact, brain and hypothalamic TGFβ contents chronically increase in aging and obesity, and this pathophysiological priming of the sympathetic upregulation of BP can, at least in part, explain the greater prevalence of hypertension in aged and obese populations.
From a loss-of-function perspective, we showed that transgenic mice not expressing the TGFβ receptor in POMC neurons did not experience mtDNA and TGFβ-mediated increase in either sympathetic hemodynamics or BP, probably because we did not observe an increase on POMC activation after mtDNA or TGFβ injection, suggesting that mtDNA activates POMC through the TGFβ receptor. This is supported by our data showing that mtDNA is able to activate TGFβ signaling (Fig. 1). On the other hand, if we apply TGFβ receptor antagonist prior to mtDNA injection, we observe an inhibition of the increase in BP, indicating that the activity is through the TGFβ pathway. Indeed, similar to TGFβ, hypothalamic NF-κB activity also increases in obesity, together delineating the role of the hypothalamic mtDNA → TGFβ → NF-κB → TNFα axis in the central control of hypertension and related CVD in these etiological conditions. Here, we observed an increase in cell-free mtDNA levels in obesity; however, in aging, it remains unknown, and it could be interesting to see if there is any relationship.
In parallel, we used a mouse model of obesity-induced hypertension to show that central suppression of the TGFβ pathway was sufficient to reduce the extent of hypertension. Altogether, these findings strongly indicate that the TGFβ pathway in the brain is a valuable target for the treatment of hypertension. As there are not any available treatments targeting cell-free mtDNA, we decided to focus on TGFβ. TGFβ has been implicated in the pathogenesis of a large number of health problems ranging from cancers to many chronic diseases, and clinical trials looking at TGFβ suppression medications are actively ongoing (2, 3, 25). To date, there has been a favorable outcome on issues such as pharmacokinetics, pharmacodynamics, and toxicology, causing TGFβ inhibitors to be generally regarded as safe (2, 70). This clinical and pharmacological background on TGFβ inhibitors is an advantage compared with the strategy of developing NF-κB or TNFα inhibitors—which have pitfalls such as immune compromise (7). Indeed, as the number of individuals with hypertension and obesity is expected to substantially increase in the future, and with some forms of hypertension such as obesity-related hypertension responding poorly to currently available medications (17), fully understanding how mtDNA/TGFβ causes hypertension and whether TGFβ inhibitors can treat this disease is meaningful for combating this health epidemic.
In conclusion, central mtDNA excess dynamically mediates hypertension development through the mediobasal hypothalamus. This conjecture expands the literature by demonstrating that the central nervous system and particularly the hypothalamus are crucial for mediating the pathogenesis of hypertension. While additional studies are required to fully elucidate the detailed neurological events in the process of mtDNA-induced hypertension, targeting the central action of TGFβ provides a novel pharmacological and physiological basis for future hypertension therapeutics.
GRANTS
This study was supported by National Institutes of Health Grants R01-DK-078750, R01-AG-031774, R01-HL-113180, and R01-DK-099136 (all to D. Cai).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.A., Y.Z., and C.H. performed experiments; A.A., Y.Z., and C.H. analyzed data; A.A., Y.Z., C.H., and D.C. interpreted results of experiments; A.A., Y.Z., and C.H. prepared figures; A.A. and D.C. drafted manuscript; A.A., Y.Z., C.H., and D.C. edited and revised manuscript; A.A., Y.Z., C.H., and D.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We sincerely thank D. Cai’s laboratory members for technical assistance.
REFERENCES
- 1.Abboud FM, Harwani SC, Chapleau MW. Autonomic neural regulation of the immune system: implications for hypertension and cardiovascular disease. Hypertension 59: 755–762, 2012. doi: 10.1161/HYPERTENSIONAHA.111.186833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov 11: 790–811, 2012. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azuma A. Pirfenidone treatment of idiopathic pulmonary fibrosis. Ther Adv Respir Dis 6: 107–114, 2012. doi: 10.1177/1753465812436663. [DOI] [PubMed] [Google Scholar]
- 4.Badri KR, Yue M, Carretero OA, Aramgam SL, Cao J, Sharkady S, Kim GH, Taylor GA, Byron KL, Schuger L. Blood pressure homeostasis is maintained by a P311-TGF-β axis. J Clin Invest 123: 4502–4512, 2013. doi: 10.1172/JCI69884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell 120: 483–495, 2005. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Bao W, Xia H, Liang Y, Ye Y, Lu Y, Xu X, Duan A, He J, Chen Z, Wu Y, Wang X, Zheng C, Liu Z, Shi S. Toll-like receptor 9 can be activated by endogenous mitochondrial DNA to induce podocyte apoptosis. Sci Rep 6: 22579, 2016. doi: 10.1038/srep22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov 8: 33–40, 2009. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beggs ML, Nagarajan R, Taylor-Jones JM, Nolen G, Macnicol M, Peterson CA. Alterations in the TGFbeta signaling pathway in myogenic progenitors with age. Aging Cell 3: 353–361, 2004. doi: 10.1111/j.1474-9728.2004.00135.x. [DOI] [PubMed] [Google Scholar]
- 9.Bernal-Mizrachi C, Gates AC, Weng S, Imamura T, Knutsen RH, DeSantis P, Coleman T, Townsend RR, Muglia LJ, Semenkovich CF. Vascular respiratory uncoupling increases blood pressure and atherosclerosis. Nature 435: 502–506, 2005. doi: 10.1038/nature03527. [DOI] [PubMed] [Google Scholar]
- 10.Bhattacharyya S, Kelley K, Melichian DS, Tamaki Z, Fang F, Su Y, Feng G, Pope RM, Budinger GR, Mutlu GM, Lafyatis R, Radstake T, Feghali-Bostwick C, Varga J. Toll-like receptor 4 signaling augments transforming growth factor-β responses: a novel mechanism for maintaining and amplifying fibrosis in scleroderma. Am J Pathol 182: 192–205, 2013. doi: 10.1016/j.ajpath.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature 464: 529–535, 2010. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blouet C, Schwartz GJ. Hypothalamic nutrient sensing in the control of energy homeostasis. Behav Brain Res 209: 1–12, 2010. doi: 10.1016/j.bbr.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 13.Bouché M, Canipari R, Melchionna R, Willems D, Senni MI, Molinaro M. TGF-beta autocrine loop regulates cell growth and myogenic differentiation in human rhabdomyosarcoma cells. FASEB J 14: 1147–1158, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Bouret S, Chuoi-Mariot MT, Prevot V, Croix D, Takumi T, Jegou S, Vaudry H, Beauvillain JC, Mitchell V. Evidence that TGF beta may directly modulate POMC mRNA expression in the female rat arcuate nucleus. Endocrinology 142: 4055–4065, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Brandon M, Baldi P, Wallace DC. Mitochondrial mutations in cancer. Oncogene 25: 4647–4662, 2006. doi: 10.1038/sj.onc.1209607. [DOI] [PubMed] [Google Scholar]
- 16.Cai D. Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends Endocrinol Metab 24: 40–47, 2013. doi: 10.1016/j.tem.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 51: 1403–1419, 2008. doi: 10.1161/HYPERTENSIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 18.Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin II-induced hypertension is modulated by nuclear factor-κBin the paraventricular nucleus. Hypertension 59: 113–121, 2012. doi: 10.1161/HYPERTENSIONAHA.111.182154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardon LR, Burge C, Clayton DA, Karlin S. Pervasive CpG suppression in animal mitochondrial genomes. Proc Natl Acad Sci U S A 91: 3799–3803, 1994. doi: 10.1073/pnas.91.9.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlson SH, Wyss JM. Neurohormonal regulation of the sympathetic nervous system: new insights into central mechanisms of action. Curr Hypertens Rep 10: 233–240, 2008. doi: 10.1007/s11906-008-0044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carmichael CY, Wainford RD. Hypothalamic signaling mechanisms in hypertension. Curr Hypertens Rep 17: 39, 2015. doi: 10.1007/s11906-015-0550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carthy ER. Autonomic dysfunction in essential hypertension: a systematic review. Ann Med Surg (Lond) 3: 2–7, 2013. doi: 10.1016/j.amsu.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 262: 689–695, 1993. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 24.da Silva AA, do Carmo J, Dubinion J, Hall JE. The role of the sympathetic nervous system in obesity-related hypertension. Curr Hypertens Rep 11: 206–211, 2009. doi: 10.1007/s11906-009-0036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denton CP, Merkel PA, Furst DE, Khanna D, Emery P, Hsu VM, Silliman N, Streisand J, Powell J, Akesson A, Coppock J, Hoogen F, Herrick A, Mayes MD, Veale D, Haas J, Ledbetter S, Korn JH, Black CM, Seibold JR; Cat-192 Study Group; Scleroderma Clinical Trials Consortium . Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum 56: 323–333, 2007. doi: 10.1002/art.22289. [DOI] [PubMed] [Google Scholar]
- 26.DiBona GF. Sympathetic nervous system and hypertension. Hypertension 61: 556–560, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00633. [DOI] [PubMed] [Google Scholar]
- 27.DiMauro S, Schon EA. Mitochondrial disorders in the nervous system. Annu Rev Neurosci 31: 91–123, 2008. doi: 10.1146/annurev.neuro.30.051606.094302. [DOI] [PubMed] [Google Scholar]
- 28.do Carmo JM, da Silva AA, Rushing JS, Pace B, Hall JE. Differential control of metabolic and cardiovascular functions by melanocortin-4 receptors in proopiomelanocortin neurons. Am J Physiol Regul Integr Comp Physiol 305: R359–R368, 2013. doi: 10.1152/ajpregu.00518.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eirin A, Lerman A, Lerman LO. Mitochondrial injury and dysfunction in hypertension-induced cardiac damage. Eur Heart J 35: 3258–3266, 2014. doi: 10.1093/eurheartj/ehu436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falk S, Wurdak H, Ittner LM, Ille F, Sumara G, Schmid MT, Draganova K, Lang KS, Paratore C, Leveen P, Suter U, Karlsson S, Born W, Ricci R, Götz M, Sommer L. Brain area-specific effect of TGF-beta signaling on Wnt-dependent neural stem cell expansion. Cell Stem Cell 2: 472–483, 2008. doi: 10.1016/j.stem.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Fang F, Goncalves Marangoni R, Zhou X, Yang Y, Ye B, Shangguang A, Qin W, Wang W, Bhattacharyya S, Wei J, Tourtellotte WG, Varga J. Toll-like receptor 9 signaling is augmented in systemic sclerosis and elicits transforming growth factor β-dependent fibroblast activation. Arthritis Rheumatol 68: 1989–2002, 2016. doi: 10.1002/art.39655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fliss MS, Usadel H, Caballero OL, Wu L, Buta MR, Eleff SM, Jen J, Sidransky D. Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science 287: 2017–2019, 2000. doi: 10.1126/science.287.5460.2017. [DOI] [PubMed] [Google Scholar]
- 33.Glass MJ, Wang G, Coleman CG, Chan J, Ogorodnik E, Van Kempen TA, Milner TA, Butler SD, Young CN, Davisson RL, Iadecola C, Pickel VM. NMDA receptor plasticity in the hypothalamic paraventricular nucleus contributes to the elevated blood pressure produced by angiotensin II. J Neurosci 35: 9558–9567, 2015. doi: 10.1523/JNEUROSCI.2301-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goncharuk VD, Van Heerikhuize J, Swaab DF, Buijs RM. Paraventricular nucleus of the human hypothalamus in primary hypertension: activation of corticotropin-releasing hormone neurons. J Comp Neurol 443: 321–331, 2002. doi: 10.1002/cne.10124. [DOI] [PubMed] [Google Scholar]
- 35.Gray MW, Burger G, Lang BF. Mitochondrial evolution. Science 283: 1476–1481, 1999. doi: 10.1126/science.283.5407.1476. [DOI] [PubMed] [Google Scholar]
- 36.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 37.Harlan SM, Guo DF, Morgan DA, Fernandes-Santos C, Rahmouni K. Hypothalamic mTORC1 signaling controls sympathetic nerve activity and arterial pressure and mediates leptin effects. Cell Metab 17: 599–606, 2013. doi: 10.1016/j.cmet.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirooka Y, Kishi T, Ito K, Sunagawa K. Potential clinical application of recently discovered brain mechanisms involved in hypertension. Hypertension 62: 995–1002, 2013. doi: 10.1161/HYPERTENSIONAHA.113.00801. [DOI] [PubMed] [Google Scholar]
- 39.Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y, Hayashi J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320: 661–664, 2008. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 40.Kamat PK, Kalani A, Kyles P, Tyagi SC, Tyagi N. Autophagy of mitochondria: a promising therapeutic target for neurodegenerative disease. Cell Biochem Biophys 70: 707–719, 2014. doi: 10.1007/s12013-014-0006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konstantinidis K, Kitsis RN. Cardiovascular biology: escaped DNA inflames the heart. Nature 485: 179–180, 2012. doi: 10.1038/485179a. [DOI] [PubMed] [Google Scholar]
- 42.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309: 481–484, 2005. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 43.Lackland DT, Weber MA. Global burden of cardiovascular disease and stroke: hypertension at the core. Can J Cardiol 31: 569–571, 2015. doi: 10.1016/j.cjca.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Lafyatis R, Farina A. New insights into the mechanisms of innate immune receptor signalling in fibrosis. Open Rheumatol J 6: 72–79, 2012. doi: 10.2174/1874312901206010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lanz TV, Ding Z, Ho PP, Luo J, Agrawal AN, Srinagesh H, Axtell R, Zhang H, Platten M, Wyss-Coray T, Steinman L. Angiotensin II sustains brain inflammation in mice via TGF-beta. J Clin Invest 120: 2782–2794, 2010. doi: 10.1172/JCI41709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levy AM, Tabakin BS, Hanson JS. Hemodynamic responses to graded treadmill exercise in young untreated labile hypertensive patients. Circulation 35: 1063–1072, 1967. doi: 10.1161/01.CIR.35.6.1063. [DOI] [PubMed] [Google Scholar]
- 47.Liu J, Cai X, Xie L, Tang Y, Cheng J, Wang J, Wang L, Gong J. Circulating cell free mitochondrial DNA is a biomarker in the development of coronary heart disease in the patients with type 2 diabetes. Clin Lab 61: 661–667, 2015. [DOI] [PubMed] [Google Scholar]
- 48.Lu A, Zuo C, He Y, Chen G, Piao L, Zhang J, Xiao B, Shen Y, Tang J, Kong D, Alberti S, Chen D, Zuo S, Zhang Q, Yan S, Fei X, Yuan F, Zhou B, Duan S, Yu Y, Lazarus M, Su Y, Breyer RM, Funk CD, Yu Y. EP3 receptor deficiency attenuates pulmonary hypertension through suppression of Rho/TGF-β1 signaling. J Clin Invest 125: 1228–1242, 2015. doi: 10.1172/JCI77656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev 90: 513–557, 2010. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 50.Marvar PJ, Lob H, Vinh A, Zarreen F, Harrison DG. The central nervous system and inflammation in hypertension. Curr Opin Pharmacol 11: 156–161, 2011. doi: 10.1016/j.coph.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci 7: 278–294, 2006. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McHam SA, Marwick TH, Pashkow FJ, Lauer MS. Delayed systolic blood pressure recovery after graded exercise: an independent correlate of angiographic coronary disease. J Am Coll Cardiol 34: 754–759, 1999. doi: 10.1016/S0735-1097(99)00269-7. [DOI] [PubMed] [Google Scholar]
- 53.Medikayala S, Piteo B, Zhao X, Edwards JG. Chronically elevated glucose compromises myocardial mitochondrial DNA integrity by alteration of mitochondrial topoisomerase function. Am J Physiol Cell Physiol 300: C338–C348, 2011. doi: 10.1152/ajpcell.00248.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meng Q, Cai D. Defective hypothalamic autophagy directs the central pathogenesis of obesity via the IkappaB kinase beta (IKKbeta)/NF-kappaB pathway. J Biol Chem 286: 32324–32332, 2011. doi: 10.1074/jbc.M111.254417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meyer A, Wang W, Qu J, Croft L, Degen JL, Coller BS, Ahamed J. Platelet TGF-β1 contributions to plasma TGF-β1, cardiac fibrosis, and systolic dysfunction in a mouse model of pressure overload. Blood 119: 1064–1074, 2012. doi: 10.1182/blood-2011-09-377648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in obesity-induced hypertension. Circulation 119: 978–986, 2009. doi: 10.1161/CIRCULATIONAHA.108.824730. [DOI] [PubMed] [Google Scholar]
- 57.Nishihara M, Hirooka Y, Kishi T, Sunagawa K. Different role of oxidative stress in paraventricular nucleus and rostral ventrolateral medulla in cardiovascular regulation in awake spontaneously hypertensive rats. J Hypertens 30: 1758–1765, 2012. doi: 10.1097/HJH.0b013e32835613d7. [DOI] [PubMed] [Google Scholar]
- 58.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 485: 251–255, 2012. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J 33: 1058–1066, 2012. doi: 10.1093/eurheartj/ehs041. [DOI] [PubMed] [Google Scholar]
- 60.Peterson JR, Sharma RV, Davisson RL. Reactive oxygen species in the neuropathogenesis of hypertension. Curr Hypertens Rep 8: 232–241, 2006. doi: 10.1007/s11906-006-0056-1. [DOI] [PubMed] [Google Scholar]
- 61.Picard M, Turnbull DM. Linking the metabolic state and mitochondrial DNA in chronic disease, health, and aging. Diabetes 62: 672–678, 2013. doi: 10.2337/db12-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pober JS. Is hypertension an autoimmune disease? J Clin Invest 124: 4234–4236, 2014. doi: 10.1172/JCI77766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pollack Y, Kasir J, Shemer R, Metzger S, Szyf M. Methylation pattern of mouse mitochondrial DNA. Nucleic Acids Res 12: 4811–4824, 1984. doi: 10.1093/nar/12.12.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet 386: 801–812, 2015. doi: 10.1016/S0140-6736(14)61468-9. [DOI] [PubMed] [Google Scholar]
- 65.Prior LJ, Davern PJ, Burke SL, Lim K, Armitage JA, Head GA. Exposure to a high-fat diet during development alters leptin and ghrelin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension 63: 338–345, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02498. [DOI] [PubMed] [Google Scholar]
- 66.Purkayastha S, Cai D. Neuroinflammatory basis of metabolic syndrome. Mol Metab 2: 356–363, 2013. doi: 10.1016/j.molmet.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Purkayastha S, Zhang G, Cai D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-β and NF-κB. Nat Med 17: 883–887, 2011. doi: 10.1038/nm.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension 45: 9–14, 2005. doi: 10.1161/01.HYP.0000151325.83008.b4. [DOI] [PubMed] [Google Scholar]
- 69.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes 54: 2012–2018, 2005. doi: 10.2337/diabetes.54.7.2012. [DOI] [PubMed] [Google Scholar]
- 70.Rodon J, Carducci MA, Sepulveda-Sánchez JM, Azaro A, Calvo E, Seoane J, Braña I, Sicart E, Gueorguieva I, Cleverly AL, Pillay NS, Desaiah D, Estrem ST, Paz-Ares L, Holdhoff M, Blakeley J, Lahn MM, Baselga J. First-in-human dose study of the novel transforming growth factor-β receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin Cancer Res 21: 553–560, 2015. doi: 10.1158/1078-0432.CCR-14-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sakai K, Sigmund CD. Molecular evidence of tissue renin-angiotensin systems: a focus on the brain. Curr Hypertens Rep 7: 135–140, 2005. doi: 10.1007/s11906-005-0088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron 36: 199–211, 2002. doi: 10.1016/S0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 73.Sarparanta J, García-Macia M, Singh R. Autophagy and mitochondria in obesity and type 2 diabetes. Curr Diabetes Rev 12: 1–17, 2016. doi: 10.2174/1573399812666160217122530. [DOI] [PubMed] [Google Scholar]
- 74.Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000. [DOI] [PubMed] [Google Scholar]
- 75.Schwartz MW, Woods SC, Seeley RJ, Barsh GS, Baskin DG, Leibel RL. Is the energy homeostasis system inherently biased toward weight gain? Diabetes 52: 232–238, 2003. doi: 10.2337/diabetes.52.2.232. [DOI] [PubMed] [Google Scholar]
- 76.Shi P, Raizada MK, Sumners C. Brain cytokines as neuromodulators in cardiovascular control. Clin Exp Pharmacol Physiol 37: e52–e57, 2010. doi: 10.1111/j.1440-1681.2009.05234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi Z, Li B, Brooks VL. Role of the paraventricular nucleus of the hypothalamus in the sympathoexcitatory effects of leptin. Hypertension 66: 1034–1041, 2015. doi: 10.1161/HYPERTENSIONAHA.115.06017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, Arditi M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36: 401–414, 2012. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Simonds SE, Pryor JT, Ravussin E, Greenway FL, Dileone R, Allen AM, Bassi J, Elmquist JK, Keogh JM, Henning E, Myers MG Jr, Licinio J, Brown RD, Enriori PJ, O’Rahilly S, Sternson SM, Grove KL, Spanswick DC, Farooqi IS, Cowley MA. Leptin mediates the increase in blood pressure associated with obesity. Cell 159: 1404–1416, 2014. doi: 10.1016/j.cell.2014.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stauss HM. Identification of blood pressure control mechanisms by power spectral analysis. Clin Exp Pharmacol Physiol 34: 362–368, 2007. doi: 10.1111/j.1440-1681.2007.04588.x. [DOI] [PubMed] [Google Scholar]
- 81.Suzuki S, Hinokio Y, Komatu K, Ohtomo M, Onoda M, Hirai S, Hirai M, Hirai A, Chiba M, Kasuga S, Akai H, Toyota T. Oxidative damage to mitochondrial DNA and its relationship to diabetic complications. Diabetes Res Clin Pract 45: 161–168, 1999. doi: 10.1016/S0168-8227(99)00046-7. [DOI] [PubMed] [Google Scholar]
- 82.Tang Y, Purkayastha S, Cai D. Hypothalamic microinflammation: a common basis of metabolic syndrome and aging. Trends Neurosci 38: 36–44, 2015. doi: 10.1016/j.tins.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122: 153–162, 2012. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly-Y M, Gidlöf S, Oldfors A, Wibom R, Törnell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429: 417–423, 2004. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 85.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39: 359–407, 2005. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu KL, Chan SH, Chan JY. Neuroinflammation and oxidative stress in rostral ventrolateral medulla contribute to neurogenic hypertension induced by systemic inflammation. J Neuroinflammation 9: 212, 2012. doi: 10.1186/1742-2094-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wyss JM. The role of the sympathetic nervous system in hypertension. Curr Opin Nephrol Hypertens 2: 265–273, 1993. doi: 10.1097/00041552-199303000-00014. [DOI] [PubMed] [Google Scholar]
- 88.Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, Zerfas P, Zhigang D, Wright EC, Stuelten C, Sun P, Lonning S, Skarulis M, Sumner AE, Finkel T, Rane SG. Protection from obesity and diabetes by blockade of TGF-β/Smad3 signaling. Cell Metab 14: 67–79, 2011. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yan J, Zhang H, Yin Y, Li J, Tang Y, Purkayastha S, Li L, Cai D. Obesity- and aging-induced excess of central transforming growth factor-β potentiates diabetic development via an RNA stress response. Nat Med 20: 1001–1008, 2014. doi: 10.1038/nm.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yi E-Y, Park S-Y, Jung S-Y, Jang W-J, Kim Y-J. Mitochondrial dysfunction induces EMT through the TGF-β/Smad/Snail signaling pathway in Hep3B hepatocellular carcinoma cells. Int J Oncol 47: 1845–1853, 2015. doi: 10.3892/ijo.2015.3154. [DOI] [PubMed] [Google Scholar]
- 91.Yoon SS, Gu Q, Nwankwo T, Wright JD, Hong Y, Burt V. Trends in blood pressure among adults with hypertension: United States, 2003 to 2012. Hypertension 65: 54–61, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zabetakis PM. Profiling the hypertensive patient in sports. Clin Sports Med 3: 137–152, 1984. [PubMed] [Google Scholar]
- 93.Zacchigna L, Vecchione C, Notte A, Cordenonsi M, Dupont S, Maretto S, Cifelli G, Ferrari A, Maffei A, Fabbro C, Braghetta P, Marino G, Selvetella G, Aretini A, Colonnese C, Bettarini U, Russo G, Soligo S, Adorno M, Bonaldo P, Volpin D, Piccolo S, Lembo G, Bressan GM. Emilin1 links TGF-beta maturation to blood pressure homeostasis. Cell 124: 929–942, 2006. doi: 10.1016/j.cell.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 94.Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 497: 211–216, 2013. doi: 10.1038/nature12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464: 104–107, 2010. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 135: 61–73, 2008. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zigman JM, Elmquist JK. Minireview: From anorexia to obesity--the yin and yang of body weight control. Endocrinology 144: 3749–3756, 2003. doi: 10.1210/en.2003-0241. [DOI] [PubMed] [Google Scholar]