Abstract
Mammalian glutaredoxin 3 (Grx3) has been shown to be important for regulating cellular redox homeostasis in the cell. Our previous studies indicate that Grx3 is significantly overexpressed in various human cancers including breast cancer and demonstrate that Grx3 controls cancer cell growth and invasion by regulating reactive oxygen species (ROS) and NF-κB signaling pathways. However, it remains to be determined whether Grx3 is required for normal mammary gland development and how it contributes to epithelial cell proliferation and differentiation in vivo. In the present study, we examined Grx3 expression in different cell types within the developing mouse mammary gland (MG) and found enhanced expression of Grx3 at pregnancy and lactation stages. To assess the physiological role of Grx3 in MG, we generated the mutant mice in which Grx3 was deleted specifically in mammary epithelial cells (MECs). Although the reduction of Grx3 expression had only minimal effects on mammary ductal development in virgin mice, it did reduce alveolar density during pregnancy and lactation. The impairment of lobuloalveolar development was associated with high levels of ROS accumulation and reduced expression of milk protein genes. In addition, proliferative gene expression was significantly suppressed with proliferation defects occurring in knockout MECs during alveolar development compared with wild-type controls. Therefore, our findings suggest that Grx3 is a key regulator of ROS in vivo and is involved in pregnancy-dependent mammary gland development and secretory activation through modulating cellular ROS.
Keywords: glutaredoxin, mammary gland, mammary epithelial cells, alveologenesis, breast cancer
Postnatal mammary epithelial cell growth and gland development are controlled by a complex of hormones and signaling pathways (1, 6, 28, 43, 48, 63, 71). During pubertal growth, growth hormone, ovarian estrogen, progesterone, and IGF-I are required to initiate branching morphogenesis to generate a ductal tree that fills fat pads (63, 71). Upon pregnancy, the combined actions of progesterone and prolactin promote the proliferation and the differentiation of specialized structures, i.e., alveoli, where milk is synthesized and secreted during lactation (7, 43, 48). After weaning, the lack of demand for milk triggers the process of involution in which the gland undergoes remodeling to remove the milk-producing epithelial cells and subsequent adipocyte differentiation (43, 70). The different stages of mammary gland development are regulated by numerous signaling pathways (57).
Reactive oxygen species (ROS) are considered by-products of aerobic metabolism in all oxygenic organisms (18). Cells also actively generate ROS as signals through the activation of various oxidases and peroxidases in response to internal developmental cues and external stresses (36). However, when in excess, ROS induce oxidative stress, causing a wide range of damage to macromolecules and eventually leading to apoptotic or necrotic cell death (59, 66, 67), which are often associated with human diseases (22–24, 60, 62, 78).
Intracellular ROS and redox balance have been implicated in mammary epithelial cell growth and differentiation (34, 65). Recent studies indicate that ROS-mediated signaling pathways play an important role in the process of epithelial-to-mesenchymal transition, which is crucial for tumor metastasis (5, 16, 55). Thioredoxin (Trx) and glutaredoxin (Grx) are the two major antioxidant systems that play a vital role in redox homeostasis (2, 14). Grxs are ubiquitous, small heat-stable disulfide oxidoreductases which are conserved in both prokaryotes and eukaryotes (31, 32). Grxs appear to be involved in many cellular processes and play an important role in protecting cells against oxidative stress (39). Grxs can be categorized into dithiol Grxs, which contain two cysteine residues in their active motifs, and monothiol Grxs, which contain a single cysteine residue in their putative motifs (39). There is a growing body of evidence suggesting that monothiol Grxs may have multiple functions in biogenesis of iron-sulfur clusters, iron trafficking and homeostasis, protection of protein oxidation, cell growth, and proliferation (13, 14, 29, 30, 38, 42, 44, 50, 53, 58). In mammalian cells there are two monothiol Grxs, Grx3 and Grx5 (33, 73). Mammalian Grx5 is a mitochondrial Grx that plays a critical role in iron-sulfur cluster biogenesis and heme synthesis in red blood cells (8, 75) and appears to be crucial for protecting osteoblasts from oxidative stress-induced apoptosis (40). Grx3, also termed thioredoxin-like 2 (Txnl2) or PICOT (protein kinase C interacting cousin of thioredoxin), was originally identified through a yeast two-hybrid screen, in which Grx3 physically interacted with the protein kinase C theta isoform (73). Genetic studies also demonstrate that Grx3 is essential for early embryonic growth and development, as the deletion of Grx3 causes embryonic lethality (10, 15).
Our previous work indicated that the knockdown of Grx3 in human breast cancer cells dramatically increased ROS levels and apoptosis, inhibited the proliferation and invasion of breast cancer cells, and diminished mammary tumor growth and metastasis in xenograft models (54). These studies further demonstrated that Grx3 played a critical role in human breast cancer cell growth and metastasis via regulating redox homeostasis and NF-κB signaling (54).
However, the precise function of Grx3 in mammary epithelial cell growth and differentiation and gland development in vivo remains to be fully elucidated. In this study, we investigated the expression of Grx3 during mammary gland developmental stages. We created Grx3 floxed mice by flanking the exon 2 of Grx3 with two loxP sites and further generated Grx3 MEC-specific knockout (KO) mice (Grx3MKO) by intercrossing Grx3 floxed mice with MMTV-Cre transgenic ones. We functionally characterized the Grx3 MKO mice and documented the molecular mechanism underlying Grx3 function in mammary epithelial cell growth and differentiation. Taken together, these findings suggest that the presence of Grx3 in mammary epithelial cells may be critical for lobuloalveolar development during pregnancy and lactation.
MATERIALS AND METHODS
Reagents.
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless stated otherwise. Dulbecco’s modified Eagle medium (DMEM), DMEM/F12, and HyClone Newborn Bovine Calf Serum (CS) were obtained from Thermo Scientific (Waltham, MA). Fetal bovine serum (FBS) was from ATLANTA Biologicals (Lawrence, GA). The 0.25% Trypsin EDTA was from Mediatech (Manassas, VA). Penicillin-Streptomycin Solution was from Global Cell Solutions (Charlottesville, VA). Collagenase type I was purchased from Worthington (Lakewood, NJ). Anti-cyclin D1, anti-PCNA, and anti-cyclin B1 antibodies were purchased from Cell Signaling Technology. Anti-β-actin antibody was bought from Sigma-Aldrich. Anti-GAPDH was bought from Chemicon International. Anti-Keratin 14 polyclonal antibody was from BioLegend (San Diego, CA). Anti-milk specific protein antibody was purchased from Nordic Immunological Laboratory (Tilburg, The Netherlands), and secondary antibodies were from Cell Signaling Technology (Beverly, MA). Grx3 monoclonal antibody was made in-house using full-length human Grx3 recombinant protein. This antibody was validated and used in our previous studies, in which Grx3 only detected a predicted 38-kDa protein in mouse embryos (and embryonic fibroblasts), but not in KO embryos (12). It was also validated when used in normal human and breast cancer patient breast tissues (54).
Generation of a conditional knockout allele for the Grx3 gene in mice.
To generate the targeting vector for Grx3 floxed mice (Fig. 1A), a bacterial artificial chromosome (BAC) clone, RP23–349D22, was obtained, which contained the mouse Grx3 gene from Children’s Hospital Research Center at Oakland (CHRCO, Oakland, CA). Two DNA fragments, 5.0 kb (left arm) and 5.4 kb (right arm) around exon 2 (Fig. 1A), were subcloned from this BAC by recombineering (37) and used for homologous recombination. A 356-bp DNA fragment containing the targeted exon 2 with its immediate 5′- and 3′-introns (partial) was amplified by PCR and inserted in between two loxP sites of the NeoFrtLoxP vector (Fig. 1A) (74). Two TK cassettes were inserted into the 5′-end of the targeting vector. R1 mouse embryonic stem cells (46) were transfected by electroporation with the linearized targeting construct. Positive embryonic stem (ES) cells were selected with G418 (Invitrogen) and ganciclovir. About 200 ES clones were screened by a long-PCR approach and several ES clones were obtained with the targeting construct (Fig. 1B). Two ES clones (no. 2 and no. 6) were expanded and used for the production of chimeras. Blastocyst injection and germline transmission were done by standard techniques. All cloning and genotyping primers are listed in Table 1.
Fig. 1.
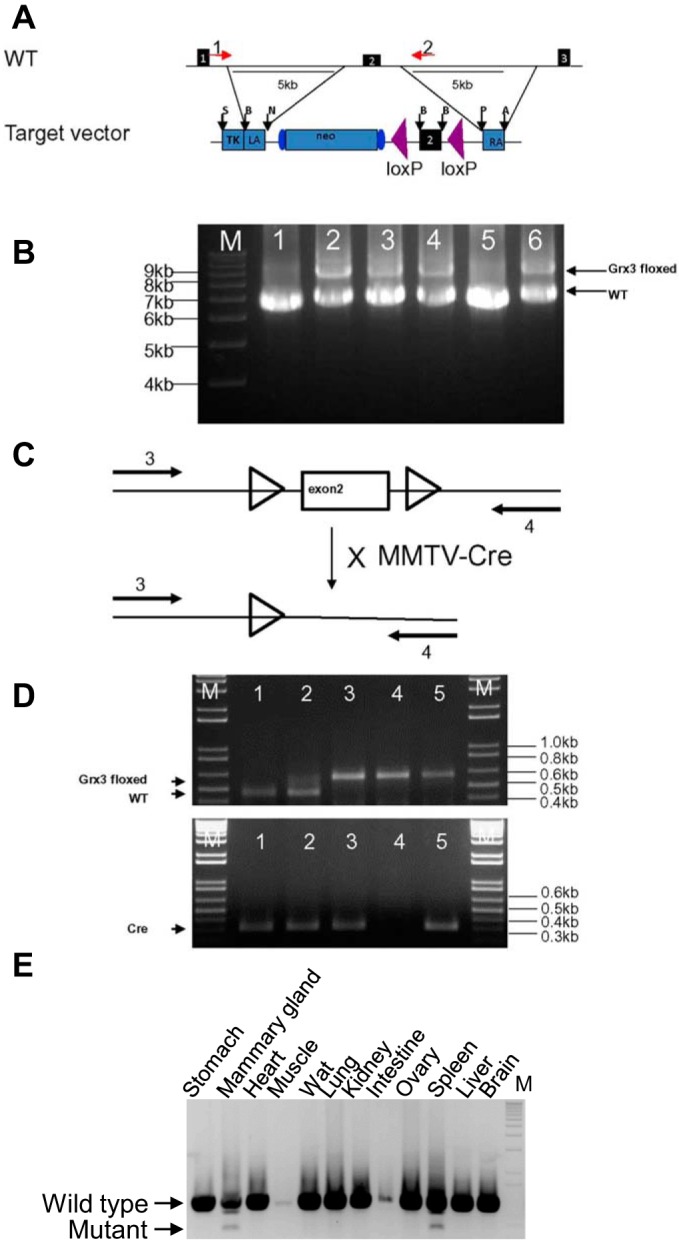
Generation of Grx3 floxed mice. A: diagram of genomic structure (partial) of Grx3 gene and target vector. Purple arrowheads indicate two lox P sites. Red arrows indicate two primers for genotyping PCR. B: genotypic PCR identifies positive ES clones. 1, wild-type ES cells; 2 to 6, Grx3 ES clones; nos. 2, 3, 4, and 6 are positive; no. 5 is negative. C and D: genotyping PCR to identify wt, heterozygous, and homozygous progenes and Cre positive mice. E: deletion of Grx3 genomic DNA was detected in KO MG by genotyping PCR.
Table 1.
Primer sequences for cloning and genotyping
| Sequence | |
|---|---|
| Exon2-F | gcc taa gga tcc gca cac tgc agt gga gtt ctt tgt ttt cta gg |
| Exon2-R | gcc taa gga tcc aga gtt caa cac aaa gtt caa g |
| Left arm | |
| Left fra-F | gcc taa gga tcc gcc tgt cta tgt aag ttt tat gtt t |
| Left fra-R | gcc taa gaa ttc gcc cac aca ccc cac cac tga gct |
| Right fra-F | gcc taa gaa ttc gcc agt gac tcc acc gtc agc cca c |
| Right fra-R | gcc taa gcg gcc gcc ttc ctg tct gga taa aca gtc aag |
| Right arm | |
| Left fra-F | gcc taa Gtt taa acc gtt ata ttt gca gca agc tat t |
| Left fra-R | gcc taa gaa ttc gcc gtt tcg caa cat act acc ttc |
| Right fra-F | gcc taa gaa ttc gcc ccc aaa tga gtg aga ccc cct |
| Right fra-R | gcc taa ggc gcg ccc tcc ttc cta aaa caa atc tta c |
| Primer 1 Forward | gca agt tca agg tca gtt tga |
| Primer 2 Reverse | gcc taa gaa ttc gcc gtt tcg caa cat act acc ttc |
| Primer 3 Forward | ctg ggc acc atg ggc tcc ac |
| Primer 4 Reverse | tgt cct ttg ccc att ctg tag c |
| Cre Forward | ctg cat tac cgg tcg atg ca |
| Cre Reverse | cag cat tgc tgt cac ttg gt |
F, forward; R, reverse; Fra, fragment.
Mouse strains.
FVB/NJ mice were purchased from Jackson Laboratory. Grx3 MG knockout mice with mixed background were generated by intercrossing Grx3 floxed mice (B6/129Sc mixed background) with MMTV-Cre mice (Line D-B6129F1). Previous reports indicated that mammary gland lactation in Line D was not affected by expression of MMTV-Cre (56, 76). There was no difference in MG development between Grx3flox/flox and MMTV-cre transgene alone mice. We used mixed background littermates as controls in our studies. All mouse strains were housed in temperature-controlled environment and fed a standard chow diet (LabDiet 5V5R chow; LabDiet, St. Louis, MO) ad libitum unless stated otherwise. The virgin mice used for these studies were housed together before analysis. All animal protocols were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine.
Mammary gland whole mounts.
Mammary gland whole mounts and images were obtained following the procedure as previously described (17). In brief, MMTV-Cre/Grx3flox/flox (Grx3 MKO) and aged-match Grx3flox/flox and MMTV-cre mice as controls at age of 4 wk, 6 wk, 10 wk, pregnancy (P) at day 14–15, and lactation (L) at day 1 were euthanized. Both inguinal mammary gland fat pads (no. 4) were excised, mounted on the Superfrost slides (Thermo Fisher Scientific. Waltham, MA), and fixed for a minimum 2 h in Carnoy’s solution (60% ethanol, 30% chloroform, and 10% glacial acetic acid). The fixed glands were washed in 70% ethanol for 15 min and then rinsed in water for 5 min. The mammary glands were stained overnight at 4°C in carmine alum stain (1 g carmine and 2.5 g aluminum potassium sulfate in 500 ml water). The glands were then dehydrated progressively in 70–95%-100% ethanol, and cleared in xylene for 1 h. Mammary whole mounts were photographed using a MZ6 dissecting microscope (Leica, Deerfield, IL) and an Olympus (Lake Success, NY) Oly 760 video camera. Morphometric analysis of virgin and pregnant mammary ductal development was conducted as previously described (27). After segmentation of the ductal tree, mammary ductal specific end points included ductal area, ductal perimeter, total branch count, and branch density. Morphometric analysis of lobuloalveolar development consisted of determining epithelial area in H&E-stained tissue sections prepared from glands collected at day 1 of lactation.
H&E staining and immunohistochemistry.
Sections of no. 4 mammary glands from Grx3 MKO and wild-type controls were stained with H&E staining (26). Immunohistochemistry was performed using a highly sensitive streptavidin-biotin-peroxidase detection system with an in-house made Grx3 monoclonal antibody (54). Bromodeoxy-uridine labeling was conducted by intraperitoneal administration of a dose of 3 mg/kg body wt. Animals were then euthanized at 2 h after dosing and BrdU staining was conducted as previously reported (11).
Isolation of mammary epithelial cells.
Mammary epithelial cells (MECs) were isolated from control and knockout MGs at P15–18 or L1–2 following the protocol as previously described (72). In brief, MG no. 3 and no. 4 glands (without muscle and lymph nodes) were collected and minced with scissors in 10 cm culture plate. Ten milliliters of digestion buffer (DMEM/F12 medium containing 100 μg/ml gentamycin, 5% FBS, 1X anti-mycotic solution, 2 mg/ml collagenase type A) were added and incubated in 37°C water bath for 2–3 h with gentle shaking until no clumps remained. Digested tissues were transferred to 50-ml tubes and centrifuged at 1,400 rpm for 3 min at 4°C. Supernatant was discarded, and pellets were washed 3 times with 1X PBS at differential speeds (i.e., 1,400, 1,300, 1,200 rpm) until supernatants were clear. One milliliter of 0.25% trypsin was added into each digested tube and incubated for 10 min at 37°C; then 5 ml wash buffer (DMEM/F12 medium containing 5% FBS and 50 μg/ml gentamycin) was added to stop digestion, and centrifuged at 1,400 rpm for 3 min at 4°C. Pellets were then treated with 1 ml of Dispase II (5 mg/ml) for 3 min at 37°C, then washed with 5 ml of wash buffer to stop digestion. Purified MECs were resuspended in 1–2 ml of wash buffer and stored at −80°C until use.
Western blotting analysis.
Mammary tissue homogenates or MEC lysates were run on SDS-PAGE gel and Western blot analysis was conducted following an established procedure (12). Apoptosis was assessed by examining for cleaved caspase 3 in WT and KO MECs extracted from MGs collected at both day 14 of pregnancy and day 1 of lactation.
Quantitative real-time PCR analysis.
Total RNA was extracted from either mammary gland tissues or MECs of wild-type controls and Grx3 MKO mice at 4, 6, 10, and 12 wk of age, pregnancy (14.5 day), and lactation (1 day). Purified RNA samples underwent reverse transcription to yield cDNA. qPCR was performed using the SYBR Green-based system on the Bio-Rad CFX96. Differences in gene expression of control and knockout mice were quantified by the comparative CT method. Keratin 5 was selected to serve as the internal control because its expression level was consistent between WT and KO MECs and relatively stable across different stages compared with keratin 8, whose expression was somewhat affected by Grx3 deletion at different developmental stages.
Detection of ROS by DHE staining.
Dihydroethidium (DHE) staining to measure ROS production in MECs followed published procedures with modification (34, 77). In brief, the wax-embedded MG slides were immersed in xylene twice for 10 min each, then immersed in 100% ethanol twice for 10 min each, in 95% ethanol once for 5 min, in 75% ethanol once for 5 min, in 50% ethanol once for 5 min, and finally in distilled water once for 10 min. Slides were washed 2 times for 10 min each in 1x PBS; placed in ice-cold 4% PFA with PBS for 10 min; washed with PBS 3 × 10 min; incubated for 5 min with 5% Triton X-100 in 1x PBS, and washed twice with PBS for 10 min each. They were then incubated with 5 μM DHE for 1 h at RT, washed 3 x 10 min with PBS, and covered. They were allowed to dry overnight at RT in a cabinet away from light. Vectashield mounting media was placed on the slides and the slides were covered, allowed to dry overnight again at RT away from light, and then imaged under confocal microscope with the same setting.
Statistical analysis.
All results are shown as means ± SE. Student’s t test was used to compare 2 groups. P < 0.05, P < 0.01, and P < 0.001 were used as indicators of the level of significance.
RESULTS
Grx3 expression during mammary gland development.
Immunohistochemical analysis of Grx3 expression in the developing mouse mammary glands (MGs) revealed that Grx3 was expressed in all stages of mammary development (Fig. 2). In the terminal end buds (TEBs) of the peripubertal MG, Grx3 was detected in different cellular subtypes including cap cells, myoepithelial cells, luminal epithelial cells, most of body cells, and stroma cells as well (Fig. 2, A and B). It appears that Grx3 was predominantly localized in the cytoplasm of those cells in TEBs (Fig. 2B). However, more Grx3 proteins were detected in ductal and lobuloalveolar epithelial cells in the mature virgin (Fig. 2C), pregnant (Fig. 2D), and lactating (Fig. 2E) MGs, whereas less Grx3 proteins were detected in involuting MGs (Fig. 2F). It was also evident that Grx3 was present in both cytoplasm and nuclei of luminal cells but was rarely detected in other subtypes of cells (Fig. 2, C–F). In agreement with these observations, double immunolabeling with antibodies against Grx3 and CK14, a marker to label the myoepithelial cells, demonstrated that Grx3 is localized in luminal epithelial cells (Fig. 2, G–I). The quantification of Grx3 expression in the developing MG using q-PCR analysis of Grx3 mRNA levels in isolated mammary epithelial cells (MECs) demonstrated that Grx3 expression was upregulated in MECs of pregnant and lactating MGs compared with those of virgin MGs (Fig. 2J). Taken together, these findings suggest that Grx3 may play an important role in MG development during pregnancy and lactation.
Fig. 2.
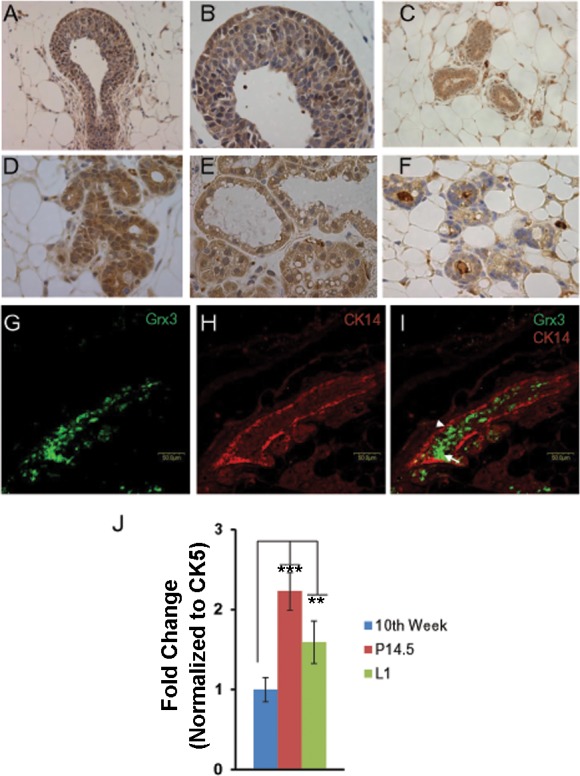
Grx3 expression at different mammary gland developing stages. A and B: terminal end bud of 7-wk-old virgin mice; Grx3 proteins were detected in cap, myoepithelial, luminal epithelial, most of body cells, and stroma cells. Grx3 proteins were examined in myoepithelial cells and luminal epithelial cells in gland ducts at 10th week of age in virgin mice (C), at pregnancy day 14 (D), at lactation day 1 (E), at involution day 7 (F). 200× magnification for A, 400× magnification for B–F. G–I: double immunolabeling of mammary gland tissue sectioned from MG at P14.5 with antibodies against Grx3 (G) and CK14 (H). I: shown is the merged image with Grx3 in luminal epithelial cells (arrow) and CK14 is present in myoepithelial cells (arrowhead). Scale bars, 50 µm. J: q-PCR analysis of Grx3 mRNA levels in mammary epithelial cells (MECs) isolated from glands at 10th week, pregnancy day 14.5, and lactation day 1. Cytokeratin 5 (CK5) was used as an internal control. Student’s t-test; n = 3. **P < 0.01, L1 vs. 10th week; ***P < 0.001, P14.5 vs. 10th week.
Generation of mammary epithelial cell-specific Grx3 knockout mice.
The disruption of Grx3 in germline transgenic mice causes embryonic lethality at 12.5 days postgestation (12). To understand the role of Grx3 in the mature MGs, a conditional knockout mouse line was generated, in which the second exon of Grx3 was deleted through Cre-mediated recombination. The details of the targeting vector and the generation of Grx3 floxed mice are described in materials and methods (Fig. 1). Homozygous mice carrying two floxed Grx3 alleles grew and developed normally; both female and male mice were fertile. When Grx3 floxed mice were intercrossed with MEC specific MMTV-Cre mice (Line D) to generate the MMTV-Cre/ Grx3flox/flox mice, termed as Grx3 MKO mice, the DNA sequence of the second exon of Grx3 was specifically deleted in MECs, which was revealed by PCR analysis of genomic DNA extracted from different tissues (Fig. 1, C–E). Immunohistochemical analysis indicated that Grx3 protein levels were significantly reduced in Grx3 MKO, but remained detected in certain MECs in the ducts of virgin MGs compared with those of control mice (Fig. 3, A and B). In contrast, the reduction of Grx3 expression was profound in the MECs of pregnant and lactating Grx3 MKO MGs (Fig. 3, C, D, and I). In agreement with these findings, q-PCR analysis demonstrated that Grx3 mRNA levels were reduced ~60% in virgin Grx3 MKO MECs (Fig. 3, E and F), whereas more than 95% reduction of Grx3 mRNA levels occurred in pregnant and lactating Grx3 MKO MECs compared with that of control MECs (Fig. 3, G and H), suggesting that MMTV-Cre mediated recombination sufficiently generates Grx3 KO in MECs of pregnant and lactating MGs.
Fig. 3.
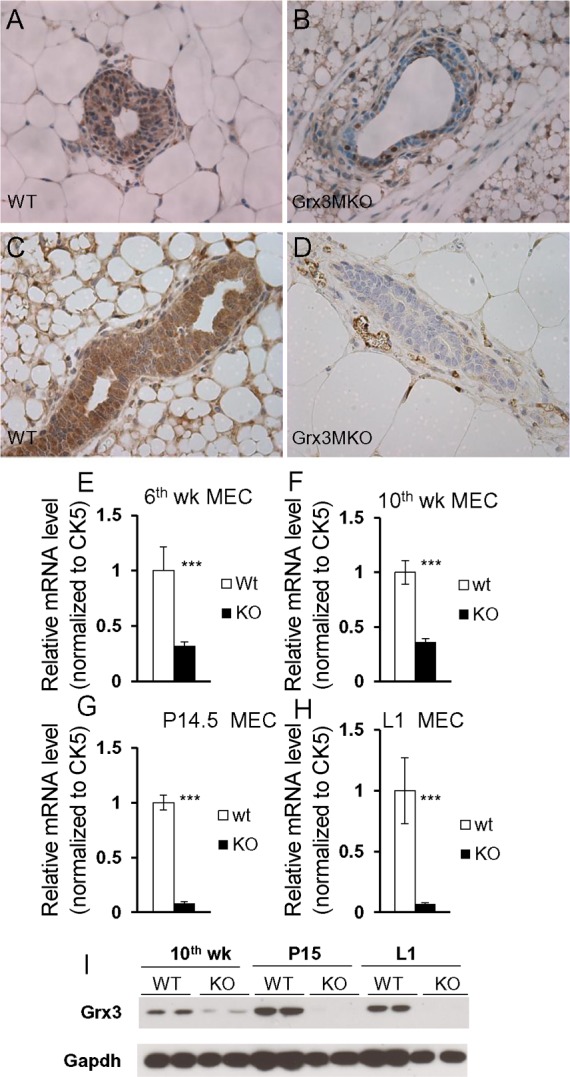
Grx3 deletion in MMTV-Cre/ Grx3flox/flox mammary glands. A–D: immunohistochemical staining at 6th week of age in virgin mice (A and B) and at pregnancy day 14 mice (C and D) shows Grx3 expression in ducts of floxed (A and C) and knockout (B and D) MGs. 400× magnification. E–H: q-PCR analysis of Grx3 expression in MECs from 6th week (E), 10th week (F), pregnancy day 14.5 (G), and lactation day 1 (H). Student’s t-test; n = 3. ***P < 0.001, significant difference between WT and KO. Cytokeratin 5 (CK5) was used as an internal control. I: Western blotting analysis of Grx3 protein levels in WT and KO MECs isolated from 10th week, P15, and L1 of MGs.
Loss of Grx3 impairs pregnancy-dependent lobuloalveolar development.
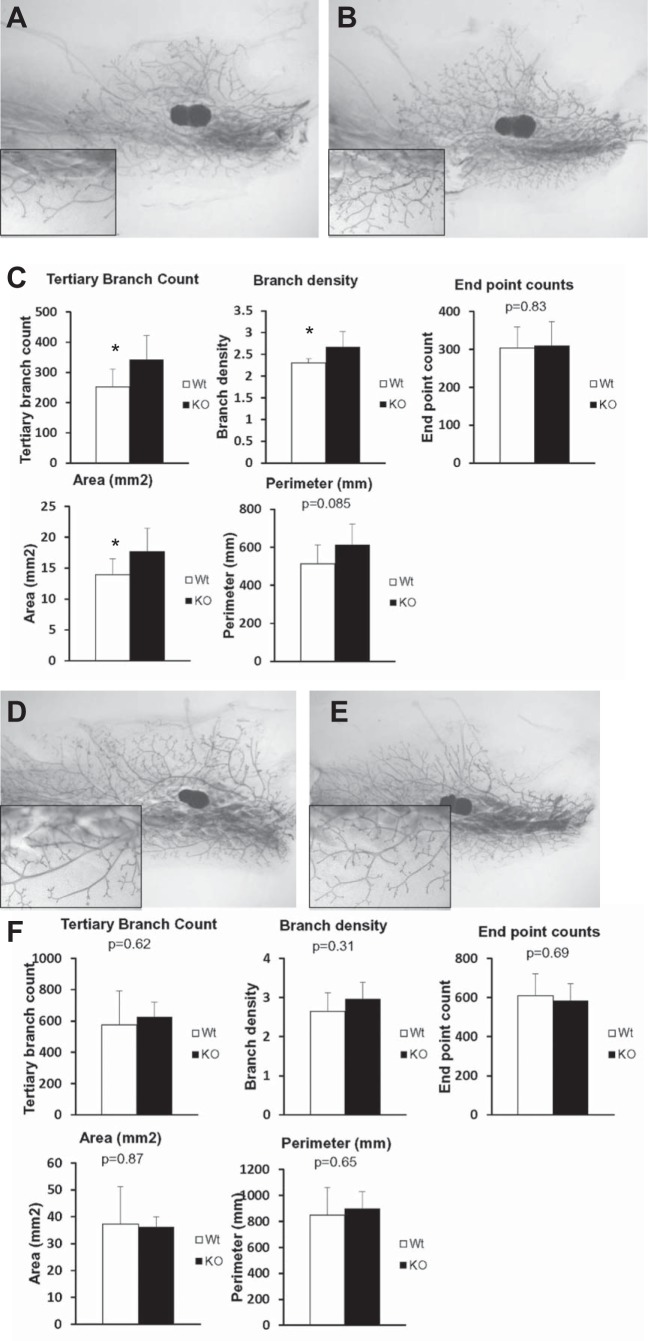
The Grx3 MKO females were viable, fertile, and able to give birth to normal-sized litters and to nurse their youngsters (Fig. 4). Examination of mature Grx3 MKO female mice revealed no dramatic developmental defects. To investigate the specific effects of Grx3 on ductal growth, MG whole mounts of early pubertal (6 wk age) and mature (12 wk age) virgin Grx3 MKO mice were compared with control littermates. Although quantitative morphometric analysis revealed modest developmental differences in ductal outgrowth between Grx3 MKO and control mice at 6 wk of age (Fig. 5, A–C), these differences were not evident at 12 wk of age (Fig. 5, D–F). The results suggest that the loss of Grx3 neither impacts mammary ductal development in the virgin gland nor occurs in a sufficient number of cells to cause consequence.
Fig. 4.
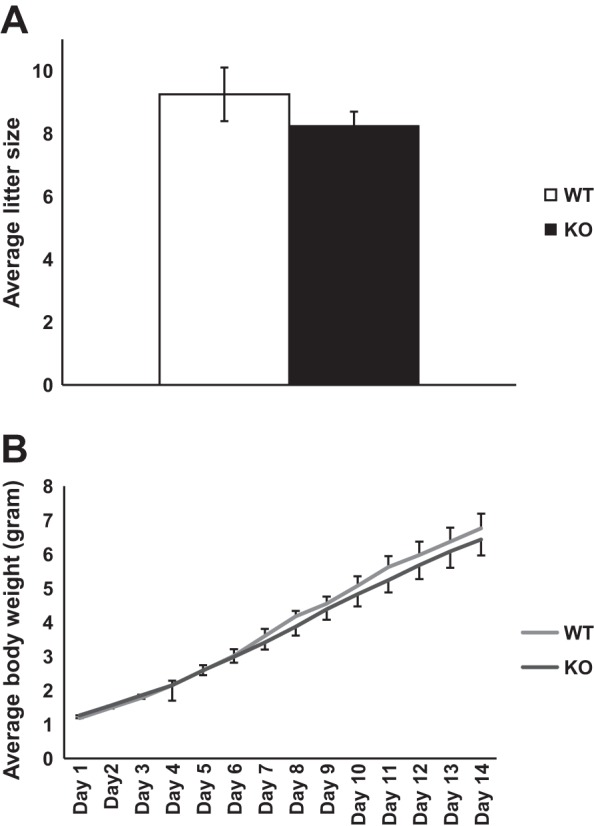
Grx3 MKO female mice have normal litter size and body weight of pups. A: average litter size of MKO female mice (n = 5) and controls (n = 4) was recorded and there is no significant difference between MKO and control mice. B: average body weight of each individual pup of total 42 and 37 pups from MKO mice and their littermate controls, respectively, was monitored for 14 days after birth. There is no significant difference between MKO and control mice.
Fig. 5.
Effects of Grx3 on ductal-branching in virgin mice. A–C: compared with controls (A), Grx3 MKO mice (B) displayed more tertiary branching and branch density at 6th week of age. C: quantification of parameters measured. Student’s t test; *P < 0.05, significance between controls (n = 9) and Grx3 MKO (n = 9) MGs. D–F: compared with controls (D), Grx3 MKO mice (E) displayed no difference in ductal tertiary branching and branch density at 12th week. F: quantification of parameters measured. Controls (n = 4) and Grx3 MKO (n = 6) MGs. 1.1× magnification; 40× magnification for insets.
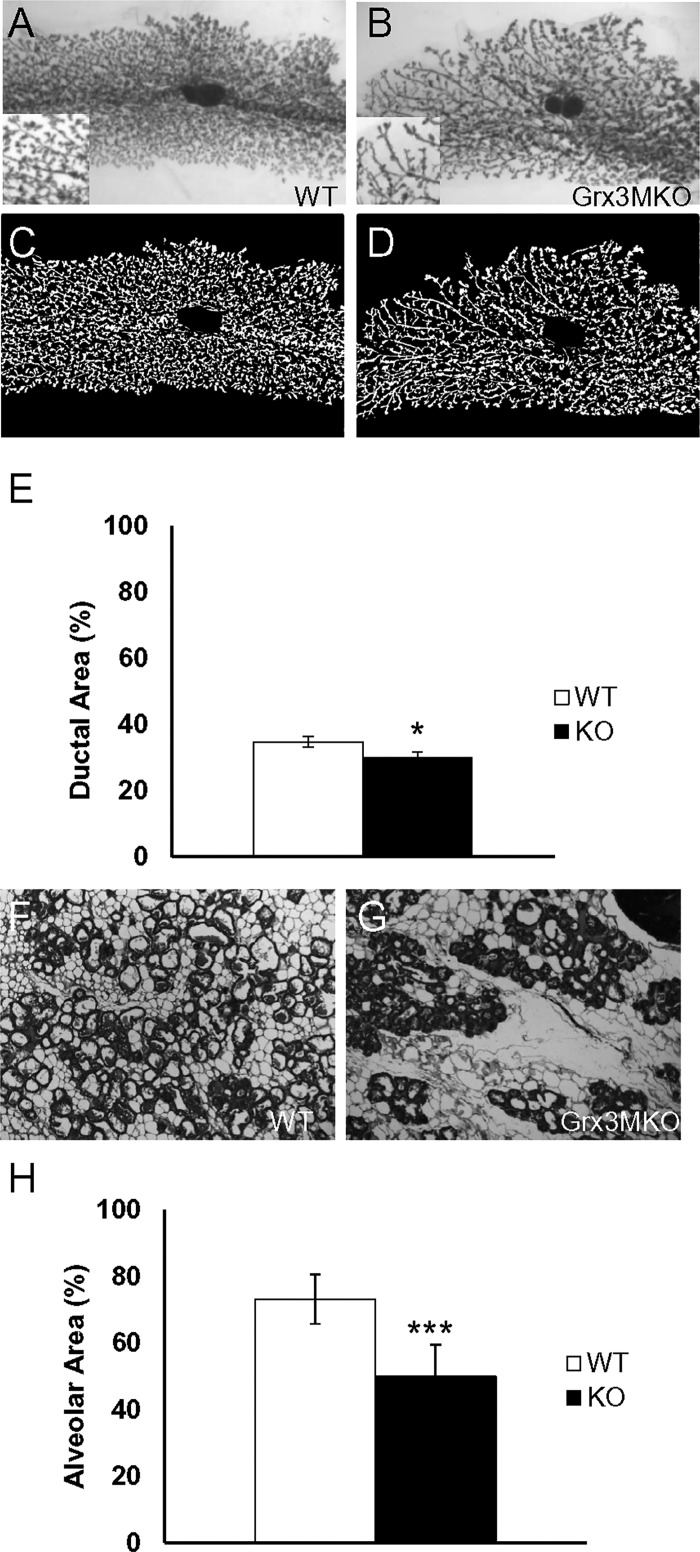
Because the morphogenesis of the ductal tree in mature Grx3 MKO mice was similar to that in the control littermates and because the number of cells undergoing Cre-dependent deletion of Grx3 during pregnancy was much more extensive than that in virgin mice, lobuloalveolar development was evaluated at mid pregnancy and at day 1 postpartum. The visual comparison of whole-mount MGs from Grx3 MKO mice at midpregnancy indicated that although pregnancy-dependent alveolar budding was present, the expansion of this budding was less profound than that of control littermates (Fig. 6, A–D). Quantitative measurement of epithelial area further revealed a lower density of lobuloalveolar units in those MGs derived from Grx3 MKO mice (Fig. 6E; P < 0.05, one-tailed). On day 1 of lactation, H&E staining showed that in control littermates, the extent of lobuloalveolar development completely filled the mammary fat pad with dilated ducts, indicating active milk secretion (Fig. 6F). In contrast, the mammary glands of lactating Grx3 MKO mice appeared to have normally differentiated alveoli, but at significantly reduced density (Fig. 6, G and H; P < 0.01) supporting the conclusion that alveolar development during pregnancy is compromised in the Grx3 MKO females.
Fig. 6.
Lobuloalveolar growth impairment as a result of Grx3 loss. Whole mount staining of MGs at pregnancy day 14.5 reveals reduced lobuloalveolar density in Grx3 MKO mice compared with control MGs. MGs were examined by whole-mount staining (A and B). 1.1× magnification; 40× magnification for insets. C and D: background subtracted images of the ductal tree from A and B). 1.1× magnification. E: quantification of parameters measured. Student’s t-test, *P < 0.05, one tailed, indicates the significance between controls (n = 6) and Grx3 MKO (n = 7) MGs. F–H: reduced lobuloalveolar growth in Grx3 KO MGs at lactation. H&E staining of lactating MGs from wild-type control (F) and Grx3 KO mice (G). 100× magnification. H: quantification of parameters measured. Student’s t-test; ***P < 0.001, significance between controls (n = 3) and Grx3 MKO (n = 3) MGs.
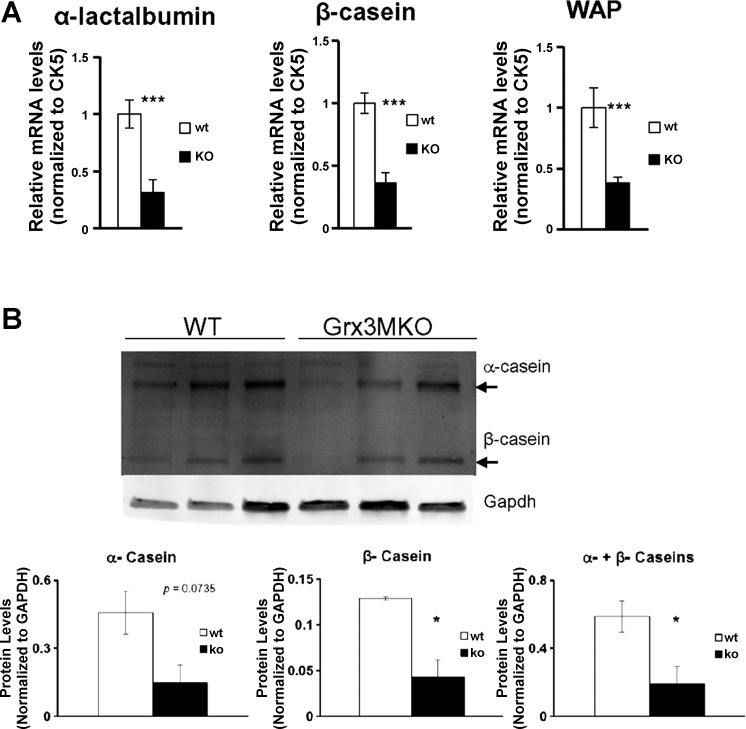
In addition to the morphological defects, the mRNA levels of lactogenic genes in Grx3 MKO mammary epithelial cells were significantly reduced in comparison to that of control MECs (Fig. 7A). Furthermore, the production of major milk proteins, such as caseins, was also decreased in Grx3 MKO MECs (Fig. 7B). Taken together, these results suggest that not only does Grx3 play a supporting role in normal lobuloalveolar development, but it may also be required for complete differentiation of the mammary epithelium during secretory activation.
Fig. 7.
Effects of Grx3 on MG lactogenesis. A: significant decreases in lactogenic gene expression in MECs of Grx3 MKO mice compared with control mice. Student’s t-test, n = 3; ***P < 0.001, significant difference between WT and KO. Cytokeratin 5 (CK5) was used as an internal control. B: major milk protein productions, such as caseins, were decreased in Grx3 MKO MECs. Student’s t-test, n = 3; *P < 0.05, significant difference between WT and KO.
Increased ROS levels in Grx3 MKO MECs.
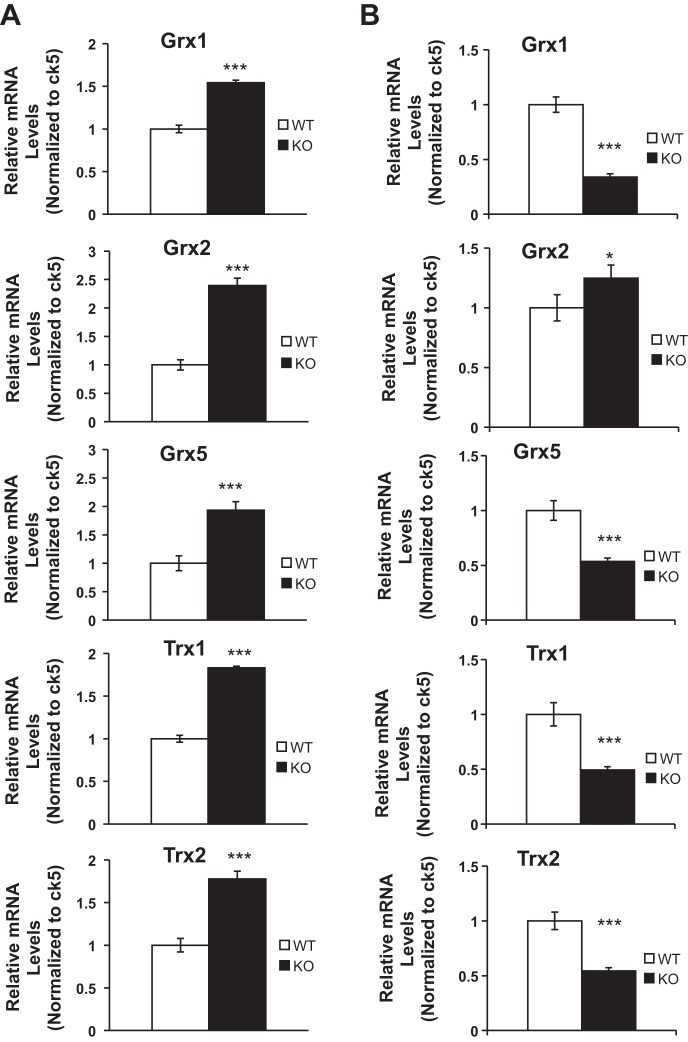
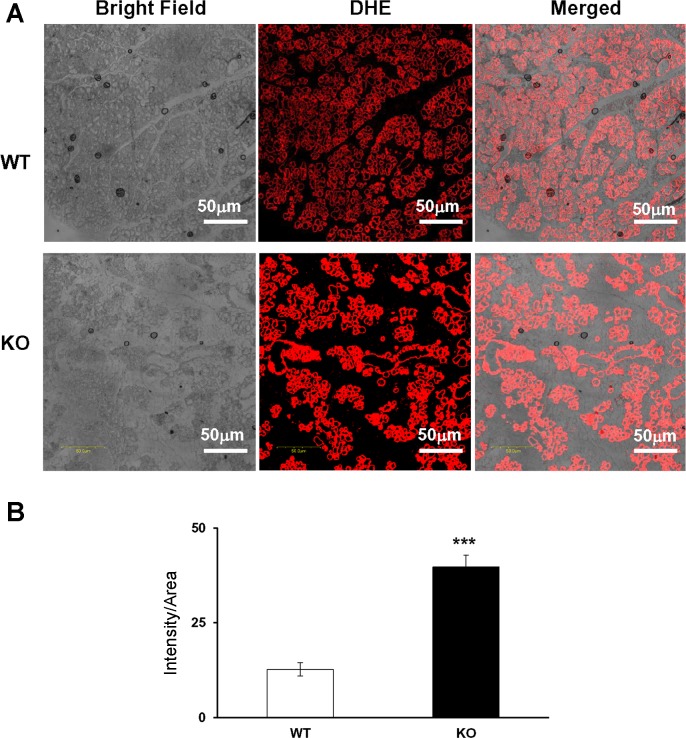
Our previous studies indicated that the knockdown of Grx3 in human cancer cells resulted in ROS accumulation, inhibition of cell proliferation, and eventually cell death (52, 54). Our studies further demonstrated that deletion of Grx3 in mouse embryonic fibroblasts (MEFs) inhibited cell proliferation through controlling redox states and cell cycle progression at G2/M phase (12, 52). Given that a group of Grx and thioredoxin (Trx) genes exist in the genome, it will be interesting to determine if there is a compensatory increase in the expression of those genes in the Grx3 MKO mouse MECs. Our q-PCR analysis indicated that both Grx and Trx gene expression was enhanced during pregnancy (Fig. 8A). In contrast, only Grx2 was induced in Grx3 MKO mouse MECs, whereas other Grx and Trx gene expression was, in fact, decreased at lactation (Fig. 8B). To further determine whether the deletion of Grx3 could affect cellular redox homeostasis that contributes to defective cell proliferation in MECs, ROS (superoxide) levels in WT and KO MECs were measured using dihydroethidium (DHE) staining. As shown in Fig. 9A, the signals from lactating KO MECs were stronger and brighter compared with those of wild-type controls, indicating a significant increase of ROS production in the absence of Grx3 (Fig. 9B). This finding suggests that Grx3 is critical for maintaining redox states in MECs during mammary development.
Fig. 8.
Effects of Grx3 deletion on redox gene expression. q-PCR analysis of redox gene mRNA levels in mammary epithelial cells (MECs) isolated from glands at pregnancy day 14.5 (A) and lactation day 1 (B). Cytokeratin 5 (CK5) was used as an internal control. Student’s t-test, n = 3; *P < 0.05, ***P < 0.001.
Fig. 9.
ROS levels were increased in Grx3 MKO alveoli. A: representative images showed strong DHE-staining signals as indicators of ROS levels in the lactating MGs of Grx3 MKO mice compared with wild-type controls. B: quantification of signal intensity per area indicated that ROS levels was increased ~3-fold in Grx3 MKO compared with wild-type controls. Student’s t-test, n = 3; ***P < 0.001, significance between controls and Grx3 MKO MGs.
Altered expression of proliferating genes in Grx3 MKO MECs.
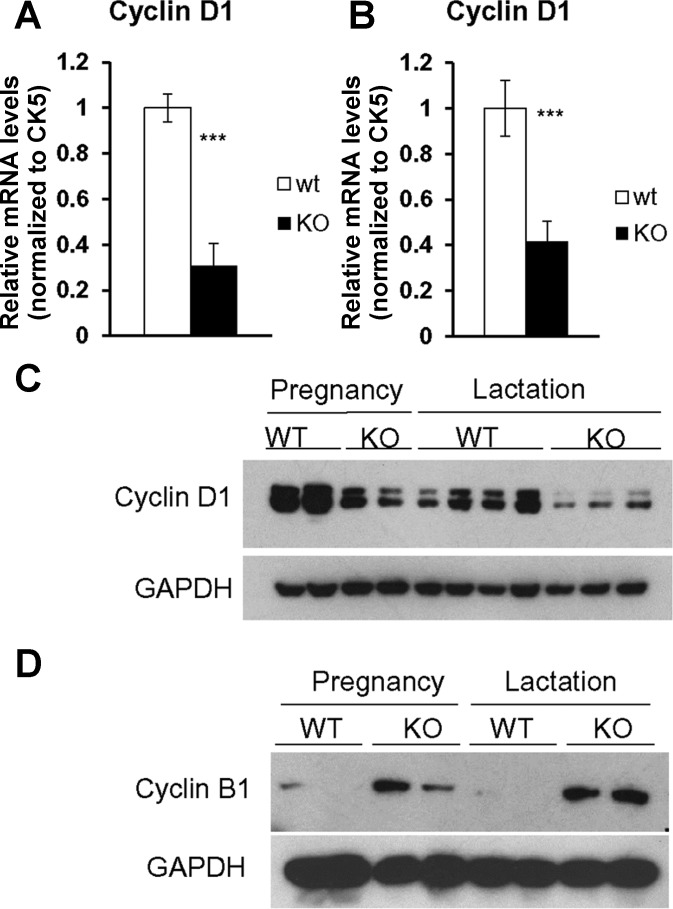
The decreased alveolar development in Grx3 MKO mice could be due to defects in the pregnancy-dependent proliferation of MECs. In support of this notion, levels of Cyclin D1, a key regulator in cell proliferation of mammary gland development (19, 61), were significantly reduced in Grx3 MKO MECs compared with those in control MECs in both pregnant and lactating MGs as shown by q-PCR analysis (Fig. 10, A and B). Furthermore, Cyclin D1 protein levels were also decreased in Grx3 MKO MECs as revealed by Western blot analysis (Fig. 10C). Interestingly, cyclin B1 protein levels were significantly increased in Grx3 MKO MECs in comparison to those of control MECs (Fig. 10D), suggesting that Grx3 MKO MECs may have defects in cell cycle progression at G2/M phase.
Fig. 10.
Expression of proliferation genes in Grx3 MKO MECs. q-PCR analysis of Cyclin D1 mRNA levels in Grx3 MKO MECs compared with controls. A: pregnancy day 14.5. B: lactation day 1. Student’s t-test, n = 3; ***P < 0.001, significant difference between WT and KO. Cytokeratin 5 (CK5) was used as an internal control. C–D: Western blot analysis of Cyclin D1 (C) and cyclin B1 (D) protein levels in Grx3 MKO MECs at pregnancy and lactation compared with controls.
Pgr-RANKL-RANK pathway is attenuated in Grx3 MKO MECs.
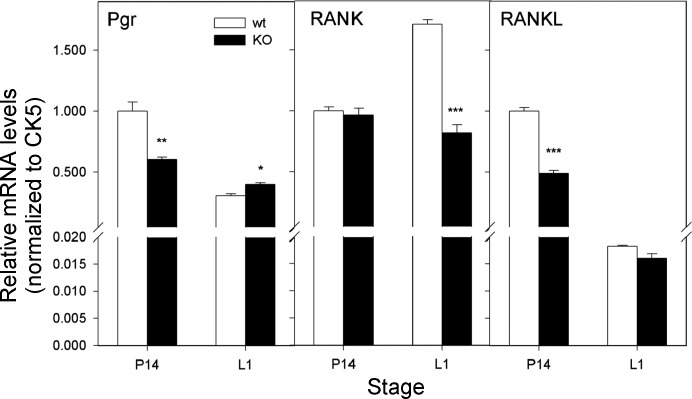
Receptor activator of nuclear factor κB (RANK) and its ligand (RANKL) are key osteoclast differentiation/activation factors for bone metabolism (47). Signaling by this pathway is also essential for mammary gland development (9, 20, 21, 25), through mediating progesterone-driven epithelial cell proliferation (4, 45, 49, 64). Measurement of mRNA levels of progesterone receptor (Pgr) indicated a significant decrease in Grx3 MKO MECs from pregnant mice compared with wild-type controls (Fig. 11), whereas Pgr levels were increased in lactating Grx3 MKO MECs (Fig. 11). RANKL levels were decreased during pregnancy but remained unchanged during lactation (Fig. 11). In contrast, RANK levels did not change during pregnancy but were significantly decreased during lactation in Grx3 MKO MECs compared with those of wild-type controls (Fig. 11). Together, with the significant decrease of Cyclin D1 levels in Grx3 MKO MECs during both pregnancy and lactation (Fig. 10), these results indicate that Grx3 may play an important role in the Pgr-RANKL-RANK pathway regulating epithelial cell proliferation and lobuloalveolar development.
Fig. 11.
Expression of progesterone receptor (Pgr) and NF-κB pathways in Grx3 MKO MECs. q-PCR analysis of Pgr, RANK, RANKL mRNA levels in Grx3 MKO MECs compared with controls. A: pregnancy day 14.5. B: lactation day 1. Student’s t-test, n = 3; *P < 0.05, **P < 0.01, ***P < 0.001, significant difference between WT and KO. Cytokeratin 5 (CK5) was used as an internal control.
DISCUSSION
In this study, we generated a mammary gland-specific Grx3 KO mouse model and demonstrated that Grx3 may play an important role in mammary gland development through modulating the RANKL-RANK-NF-κB signaling pathway to control epithelial cell proliferation.
Our previous studies indicated that Grx3 is ubiquitously present in multiple tissues and organs in mice (12). The current work showed that Grx3 is expressed in many cell types of MGs during mammary gland development (Fig. 2). The temporal and spatial expression of Grx3 in MGs is dependent on the developmental stages, in which Grx3 expression is significantly increased in the pregnant and lactating MGs (Fig. 2J). This suggests that the expression of Grx3 in MGs is regulated by the same developmental cues that control lobuloalveolar development and lactogenesis. This result is consistent with the finding that Grx3 expression in human breast cancer cells is induced by growth hormone, IGF-I (54). Also, the Grx3 proteins appear to localize in both the cytoplasm and the nuclei during developmental stages (Fig. 2, A–F). Interestingly, our recent report demonstrated that the nuclear accumulation of Grx3 induced by oxidative stress was partially inhibited by a synthetic anti-progestogen, mifepristone (RU-486) (52). Whether and how the subcellular location of Grx3 in MECs during MG development is modulated by hormones, such as progesterone, during mammary gland development remains to be further investigated.
By crossing with the MMTV-Cre transgenic line, Grx3 gene (exon 2) was efficiently deleted (Fig. 1) and the expression of Grx3 was specifically reduced in MECs (Fig. 3). There are multiple MMTV-cre lines available, such as Lines A, D, and F (69), and several reports indicated that MG growth and development were compromised in lines A and F (56, 76). There are no reports indicating the defective effect of MMTV-Cre transgenic line D, which was used to generate Grx3 MKO. The MMTV-cre transgene was also found to express in the spleen, which is consistent with the previous report (35). However, how Grx3 expression in spleen is affected and whether this impacts mammary gland development have not been evaluated in the present study. Importantly, the efficacy of Grx3 deletion in the mammary ductal tree was found to vary depending on developmental stage. In the virgin gland, the deletion of Grx3 was incomplete resulting in a significant level of residual expression. This residual expression could very well be the reason that developmental effects in the virgin mammary gland were minimal in comparison to those observed during pregnancy, where the loss of expression is almost complete in the epithelial compartment.
In contrast to virgin MECs, pregnant and lactating MECs showed significantly enhanced Grx3 expression (Fig. 2J), suggesting an important function that Grx3 may play during those developmental stages. In support of this notion, the deletion of Grx3 in pregnant and lactating MECs in Grx3 MKO mice displayed an impairment of lobuloalveolar development (Fig. 6), indicating the requirement of Grx3 in this process. Furthermore, Grx3 appeared to be required for complete differentiation of secretory epithelial cells since the expression of milk protein genes was significantly decreased in the Grx3 MKO (Fig. 7A). These results suggest that Grx3 may affect prolactin (PRL)/prolactin receptor (PRLR)-mediated JAK2-STAT5 signaling in regulating epithelial proliferation and differentiation. However, in contrast to Jak2 and Stat5 mammary gland-specific deletion mice that are unable to nurse their youngsters because of no/reduced milk production (41, 68), the effect of Grx3 on milk production seems more modest (Fig. 7B). In this regard, although Grx3 MKO mice were able to nurse their litters, the possibility that milk production is compromised even more modestly in these animals has not been definitively evaluated under more stringent conditions when comparing the growth of size- and weight-matched cross-foster litters. It is unlikely that Grx3 effect on lobuloalveolar development is mediated by the JAK2-STAT5 pathway because there was no difference in phosphorylation of STAT5 observed in isolated MECs of Grx3 MKO MGs and cultured MECs stimulated with PRL compared with controls (data not shown).
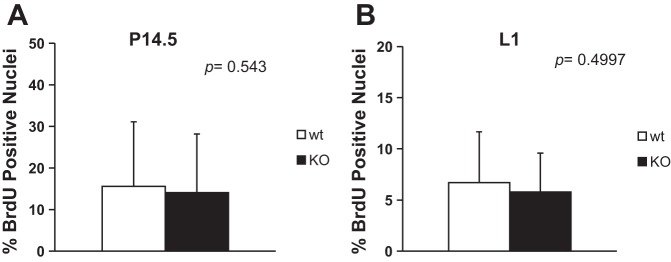
In Grx3 KO MECs, both cyclin D1 mRNA and protein levels were significantly decreased (Fig. 10, A–C), suggesting that Grx3 may regulate cell cycle progression in MECs and is an important regulator in epithelial cell proliferation during pregnancy and lactation. Surprisingly, the results from BrdU labeling assays were not consistent with cyclin D1 gene expression analyses (Fig. 10; Fig. 12, A and B). Our previous studies with MEFs also indicated that there were no differences in the BrdU labeling of Grx3 KO MEFs and wild-type controls; instead, Grx3 KO MEFs had higher levels of cyclin B1 and increased numbers of cells with double nuclei that accounted for G2/M arrest of MEFs during proliferation (12). Interestingly, in Grx3 KO MECs, more cyclin B1 accumulated compared with controls (Fig. 10D), suggesting that Grx3 may play a similar role in MECs.
Fig. 12.
BrdU labeling to measure proliferating cells in pregnant and lactating MGs. BrdU positive cells for controls and Grx3 MKOMGs were counted and expressed as a ratio of positive nuclei to the total number of nuclei counted in the percentage. A: ducts in pregnancy day 14.5; 18–28 ducts were counted. B: ducts in lactation day 1; 18–24 ducts were counted.
The RANKL-RANK signaling pathway is important for mammary gland development. Although the overexpression of either RANKL or RANK gene in mammary glands promotes proliferation of MECs, ductal outgrowth, and alveologenesis (21, 25), the deletion of either one significantly impairs epithelial cell proliferation and gland development (20). It is also evident that RANKL-RANK signaling activates the NF-κB pathway to regulate cyclin D1 expression responsible for cell proliferation in the gland (9). Here, we found that the RANKL/RANK expression was downregulated in Grx3 MECs compared with controls (Fig. 11). Given the RANKL-RANK-axis functions in the same regulatory pathway during mammary gland development, one would expect that the regulation of RANKL and RANK expression mediated by Grx3 could be simultaneous. Surprisingly, in our study, we observed that RANK and RANKL expression are affected at different stages in the MKO mice. One plausible explanation is that RANKL and RANK expressions are regulated by multiple hormones and factors. Also, previous studies have found that RANKL and RANK show differential expression, with inhibitory effects on each other, during mammary gland development (21, 25). Furthermore, Grx3 effects on RANKL and RANK expression could be distinct in different types of luminal epithelial cells, like ER+/PR+ cells vs. ER-PR− cells. Therefore, the molecular mechanism by which Grx3 regulates expression of RANKL and RANK and the downstream signaling pathway remain to be explored in future studies.
When Grx3 was knocked down in human breast cancer cells, neither Grx1 nor Trx1 protein levels were changed (54). In Grx3 MKO mouse MECs, however, both Grx and Trx gene expression was enhanced during pregnancy (Fig. 8A), suggesting that a compensatory effect occurs. This could explain why the impact of Grx3 deletion was less profound at pregnancy (Fig. 6, A–E). In contrast, other Grx and Trx gene expression was decreased in Grx3 MKO mouse MECs during lactation (Fig. 8B). This is consistent with the result that more ROS were significantly accumulated compared with wild-type controls during lactation (Fig. 9). In our current study, we hypothesize that the RANKL-RANK-NF-κB signaling pathway is involved in controlling ROS in MECs and that the dysregulation of this pathway could cause defective cell cycle progression in MECs. The direct link between RANKL/RANK signaling and ROS scavenging in mammary epithelial cell proliferation remains to be explored. However, a recent study reported that RANKL/RANK signaling promoted FoxO-regulated antioxidant pathways to attenuate ROS production during osteoclastogenesis (3). Furthermore, dysregulated redox states in mammary epithelial cells can cause developmental arrest in mammary glands as seen in mice with loss of mitochondrial superoxide dismutase (51). Given that Grx3 has been shown to be essential for cancer cell proliferation and survival (12, 54), and reduced Grx3 in human breast cancer cells attenuates NF-κB activation under oxidative stress (54), our findings suggest that a potential redox-dependent RANKL-RANK-NF-κB signaling pathway, mediated by Grx3, may play a unique role in regulating mammary epithelial growth and gland development.
GRANTS
This work was supported by the United States Department of Agriculture/Agricultural Research Service under Cooperation Agreement 6250–51000–054 (to N. Cheng); National Institutes of Health (NIH) Grant R01-GM115622 and Cancer Prevention and Research Institute of Texas Grant CPRIT R1104 (to J. Wang); NIH Grant CA151610, the Avon Foundation Grant 02–2014–063, David Salomon Translational Breast Cancer Research Fund Grant, the Fashion Footwear Charitable Foundation of New York, Inc. Grant, and the Margie and Robert E. Petersen Foundation Grant (to X. Cui and Y. Qu); and National Heart, Lung, and Blood Institute Grants R01-HL-089598, R01-HL-091947, R01-HL-117641, R41-HL-129570 and American Heart Association (AHA) Established Investigator Grant 13EIA14560061(to X. H. Wehrens).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.P., J.D., X.J., Y.Q., and H.Y. performed experiments; K.P., J.D., X.J., Y.Q., H.Y., W.O., J.C.M., J.W., X.H.W., X.C., Y.L., D.L.H., and N.C. analyzed data; K.P., J.D., Y.Y., Y.L., D.L.H., and N.C. interpreted results of experiments; K.P., X.J., Y.Q., H.Y., and N.C. prepared figures; K.P. and N.C. drafted manuscript; K.P., J.D., X.J., Y.Q., H.Y., Y.Y., W.O., J.C.M., L.C., J.W., X.H.W., X.C., Y.L., D.L.H., and N.C. approved final version of manuscript; J.C.M., L.C., X.H.W., X.C., Y.L., D.L.H., and N.C. edited and revised manuscript.
REFERENCES
- 1.Anderson SM, Rudolph MC, McManaman JL, Neville MC. Key stages in mammary gland development. Secretory activation in the mammary gland: it’s not just about milk protein synthesis! Breast Cancer Res 9: 204, 2007. doi: 10.1186/bcr1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnér ES, Holmgren A. The thioredoxin system in cancer. Semin Cancer Biol 16: 420–426, 2006. doi: 10.1016/j.semcancer.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Bartell SM, Kim HN, Ambrogini E, Han L, Iyer S, Serra Ucer S, Rabinovitch P, Jilka RL, Weinstein RS, Zhao H, O’Brien CA, Manolagas SC, Almeida M. FoxO proteins restrain osteoclastogenesis and bone resorption by attenuating H2O2 accumulation. Nat Commun 5: 3773, 2014. doi: 10.1038/ncomms4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beleut M, Rajaram RD, Caikovski M, Ayyanan A, Germano D, Choi Y, Schneider P, Brisken C. Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc Natl Acad Sci USA 107: 2989–2994, 2010. doi: 10.1073/pnas.0915148107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boudreau HE, Casterline BW, Rada B, Korzeniowska A, Leto TL. Nox4 involvement in TGF-beta and SMAD3-driven induction of the epithelial-to-mesenchymal transition and migration of breast epithelial cells. Free Radic Biol Med 53: 1489–1499, 2012. doi: 10.1016/j.freeradbiomed.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brisken C, O’Malley B. Hormone action in the mammary gland. Cold Spring Harb Perspect Biol 2: a003178, 2010. doi: 10.1101/cshperspect.a003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brisken C, Rajaram RD. Alveolar and lactogenic differentiation. J Mammary Gland Biol Neoplasia 11: 239–248, 2006. doi: 10.1007/s10911-006-9026-0. [DOI] [PubMed] [Google Scholar]
- 8.Camaschella C, Campanella A, De Falco L, Boschetto L, Merlini R, Silvestri L, Levi S, Iolascon A. The human counterpart of zebrafish shiraz shows sideroblastic-like microcytic anemia and iron overload. Blood 110: 1353–1358, 2007. doi: 10.1182/blood-2007-02-072520. [DOI] [PubMed] [Google Scholar]
- 9.Cao Y, Bonizzi G, Seagroves TN, Greten FR, Johnson R, Schmidt EV, Karin M. IKKalpha provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell 107: 763–775, 2001. doi: 10.1016/S0092-8674(01)00599-2. [DOI] [PubMed] [Google Scholar]
- 10.Cha H, Kim JM, Oh JG, Jeong MH, Park CS, Park J, Jeong HJ, Park BK, Lee YH, Jeong D, Yang DK, Bernecker OY, Kim DH, Hajjar RJ, Park WJ. PICOT is a critical regulator of cardiac hypertrophy and cardiomyocyte contractility. J Mol Cell Cardiol 45: 796–803, 2008. doi: 10.1016/j.yjmcc.2008.09.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakravarty G, Hadsell D, Buitrago W, Settleman J, Rosen JM. p190-B RhoGAP regulates mammary ductal morphogenesis. Mol Endocrinol 17: 1054–1065, 2003. doi: 10.1210/me.2002-0428. [DOI] [PubMed] [Google Scholar]
- 12.Cheng N-H, Zhang W, Chen W-Q, Jin J, Cui X, Butte NF, Chan L, Hirschi KD. A mammalian monothiol glutaredoxin, Grx3, is critical for cell cycle progression during embryogenesis. FEBS J 278: 2525–2539, 2011. doi: 10.1111/j.1742-4658.2011.08178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng NH, Hirschi KD. Cloning and characterization of CXIP1, a novel PICOT domain-containing Arabidopsis protein that associates with CAX1. J Biol Chem 278: 6503–6509, 2003. doi: 10.1074/jbc.M210883200. [DOI] [PubMed] [Google Scholar]
- 14.Cheng NH, Liu JZ, Brock A, Nelson RS, Hirschi KD. AtGRXcp, an Arabidopsis chloroplastic glutaredoxin, is critical for protection against protein oxidative damage. J Biol Chem 281: 26280–26288, 2006. doi: 10.1074/jbc.M601354200. [DOI] [PubMed] [Google Scholar]
- 15.Cheng NH, Zhang W, Chen WQ, Jin J, Cui X, Butte NF, Chan L, Hirschi KD. A mammalian monothiol glutaredoxin, Grx3, is critical for cell cycle progression during embryogenesis. FEBS J 278: 2525–2539, 2011. doi: 10.1111/j.1742-4658.2011.08178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cichon MA, Radisky DC. ROS-induced epithelial-mesenchymal transition in mammary epithelial cells is mediated by NF-kB-dependent activation of Snail. Oncotarget 5: 2827–2838, 2014. doi: 10.18632/oncotarget.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong J, Huang S, Caikovski M, Ji S, McGrath A, Custorio MG, Creighton CJ, Maliakkal P, Bogoslovskaia E, Du Z, Zhang X, Lewis MT, Sablitzky F, Brisken C, Li Y. ID4 regulates mammary gland development by suppressing p38MAPK activity. Development 138: 5247–5256, 2011. doi: 10.1242/dev.069203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 19.Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev 9: 2364–2372, 1995. doi: 10.1101/gad.9.19.2364. [DOI] [PubMed] [Google Scholar]
- 20.Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey DL, Boyle WJ, Khokha R, Penninger JM. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103: 41–50, 2000. doi: 10.1016/S0092-8674(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Valdivia R, Mukherjee A, Ying Y, Li J, Paquet M, DeMayo FJ, Lydon JP. The RANKL signaling axis is sufficient to elicit ductal side-branching and alveologenesis in the mammary gland of the virgin mouse. Dev Biol 328: 127–139, 2009. doi: 10.1016/j.ydbio.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 408: 239–247, 2000. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 23.Franco R, Schoneveld O, Georgakilas AG, Panayiotidis MI. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett 266: 6–11, 2008. doi: 10.1016/j.canlet.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 24.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest 115: 500–508, 2005. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez-Suarez E, Branstetter D, Armstrong A, Dinh H, Blumberg H, Dougall WC. RANK overexpression in transgenic mice with mouse mammary tumor virus promoter-controlled RANK increases proliferation and impairs alveolar differentiation in the mammary epithelia and disrupts lumen formation in cultured epithelial acini. Mol Cell Biol 27: 1442–1454, 2007. doi: 10.1128/MCB.01298-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hadsell DL, Bonnette S, George J, Torres D, Klimentidis Y, Gao S, Haney PM, Summy-Long J, Soloff MS, Parlow AF, Sirito M, Sawadogo M. Diminished milk synthesis in upstream stimulatory factor 2 null mice is associated with decreased circulating oxytocin and decreased mammary gland expression of eukaryotic initiation factors 4E and 4G. Mol Endocrinol 17: 2251–2267, 2003. doi: 10.1210/me.2002-0031. [DOI] [PubMed] [Google Scholar]
- 27.Hadsell DL, Hadsell LA, Olea W, Rijnkels M, Creighton CJ, Smyth I, Short KM, Cox LL, Cox TC. In-silico QTL mapping of postpubertal mammary ductal development in the mouse uncovers potential human breast cancer risk loci. Mamm Genome 26: 57–79, 2015. doi: 10.1007/s00335-014-9551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hennighausen L, Robinson GW. Signaling pathways in mammary gland development. Dev Cell 1: 467–475, 2001. doi: 10.1016/S1534-5807(01)00064-8. [DOI] [PubMed] [Google Scholar]
- 29.Herrero E, de la Torre-Ruiz MA. Monothiol glutaredoxins: a common domain for multiple functions. Cell Mol Life Sci 64: 1518–1530, 2007. doi: 10.1007/s00018-007-6554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann B, Uzarska MA, Berndt C, Godoy JR, Haunhorst P, Lillig CH, Lill R, Mühlenhoff U. The multidomain thioredoxin-monothiol glutaredoxins represent a distinct functional group. Antioxid Redox Signal 15: 19–30, 2011. doi: 10.1089/ars.2010.3811. [DOI] [PubMed] [Google Scholar]
- 31.Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem 264: 13963–13966, 1989. [PubMed] [Google Scholar]
- 32.Holmgren A, Aslund F. Glutaredoxin. Methods Enzymol 252: 283–292, 1995. doi: 10.1016/0076-6879(95)52031-7. [DOI] [PubMed] [Google Scholar]
- 33.Isakov N, Witte S, Altman A. PICOT-HD: a highly conserved protein domain that is often associated with thioredoxin and glutaredoxin modules. Trends Biochem Sci 25: 537–539, 2000. doi: 10.1016/S0968-0004(00)01685-6. [DOI] [PubMed] [Google Scholar]
- 34.Kannan N, Nguyen LV, Makarem M, Dong Y, Shih K, Eirew P, Raouf A, Emerman JT, Eaves CJ. Glutathione-dependent and -independent oxidative stress-control mechanisms distinguish normal human mammary epithelial cell subsets. Proc Natl Acad Sci USA 111: 7789–7794, 2014. doi: 10.1073/pnas.1403813111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumaraswamy E, Carlson BA, Morgan F, Miyoshi K, Robinson GW, Su D, Wang S, Southon E, Tessarollo L, Lee BJ, Gladyshev VN, Hennighausen L, Hatfield DL. Selective removal of the selenocysteine tRNA [Ser]Sec gene (Trsp) in mouse mammary epithelium. Mol Cell Biol 23: 1477–1488, 2003. doi: 10.1128/MCB.23.5.1477-1488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lambeth JD, Neish AS. Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu Rev Pathol 9: 119–145, 2014. doi: 10.1146/annurev-pathol-012513-104651. [DOI] [PubMed] [Google Scholar]
- 37.Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics 73: 56–65, 2001. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- 38.Li H, Mapolelo DT, Dingra NN, Keller G, Riggs-Gelasco PJ, Winge DR, Johnson MK, Outten CE. Histidine 103 in Fra2 is an iron-sulfur cluster ligand in the [2Fe-2S] Fra2-Grx3 complex and is required for in vivo iron signaling in yeast. J Biol Chem 286: 867–876, 2011. doi: 10.1074/jbc.M110.184176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lillig CH, Berndt C, Holmgren A. Glutaredoxin systems. Biochim Biophys Acta 1780: 1304–1317, 2008. doi: 10.1016/j.bbagen.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Linares GR, Xing W, Govoni KE, Chen ST, Mohan S. Glutaredoxin 5 regulates osteoblast apoptosis by protecting against oxidative stress. Bone 44: 795–804, 2009. doi: 10.1016/j.bone.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev 11: 179–186, 1997. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- 42.Lopreiato R, Facchin S, Sartori G, Arrigoni G, Casonato S, Ruzzene M, Pinna LA, Carignani G. Analysis of the interaction between piD261/Bud32, an evolutionarily conserved protein kinase of Saccharomyces cerevisiae, and the Grx4 glutaredoxin. Biochem J 377: 395–405, 2004. doi: 10.1042/bj20030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macias H, Hinck L. Mammary gland development. Wiley Interdiscip Rev Dev Biol 1: 533–557, 2012. doi: 10.1002/wdev.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mühlenhoff U, Molik S, Godoy JR, Uzarska MA, Richter N, Seubert A, Zhang Y, Stubbe J, Pierrel F, Herrero E, Lillig CH, Lill R. Cytosolic monothiol glutaredoxins function in intracellular iron sensing and trafficking via their bound iron-sulfur cluster. Cell Metab 12: 373–385, 2010. doi: 10.1016/j.cmet.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA 100: 9744–9749, 2003. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA 90: 8424–8428, 1993. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagy V, Penninger JM. The RANKL-RANK Story. Gerontology 61: 534–542, 2015. doi: 10.1159/000371845. [DOI] [PubMed] [Google Scholar]
- 48.Oakes SR, Hilton HN, Ormandy CJ. The alveolar switch: coordinating the proliferative cues and cell fate decisions that drive the formation of lobuloalveoli from ductal epithelium. Breast Cancer Res 8: 207, 2006. doi: 10.1186/bcr1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Obr AE, Edwards DP. The biology of progesterone receptor in the normal mammary gland and in breast cancer. Mol Cell Endocrinol 357: 4–17, 2012. doi: 10.1016/j.mce.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ojeda L, Keller G, Muhlenhoff U, Rutherford JC, Lill R, Winge DR. Role of glutaredoxin-3 and glutaredoxin-4 in the iron regulation of the Aft1 transcriptional activator in Saccharomyces cerevisiae. J Biol Chem 281: 17661–17669, 2006. doi: 10.1074/jbc.M602165200. [DOI] [PubMed] [Google Scholar]
- 51.Parmar H, Melov S, Samper E, Ljung BM, Cunha GR, Benz CC. Hyperplasia, reduced E-cadherin expression, and developmental arrest in mammary glands oxidatively stressed by loss of mitochondrial superoxide dismutase. Breast 14: 256–263, 2005. doi: 10.1016/j.breast.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Pham K, Pal R, Qu Y, Liu X, Yu H, Shiao SL, Wang X, O’Brian Smith E, Cui X, Rodney GG, Cheng N. Nuclear glutaredoxin 3 is critical for protection against oxidative stress-induced cell death. Free Radic Biol Med 85: 197–206, 2015. doi: 10.1016/j.freeradbiomed.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pujol-Carrion N, Belli G, Herrero E, Nogues A, de la Torre-Ruiz MA. Glutaredoxins Grx3 and Grx4 regulate nuclear localisation of Aft1 and the oxidative stress response in Saccharomyces cerevisiae. J Cell Sci 119: 4554–4564, 2006. doi: 10.1242/jcs.03229. [DOI] [PubMed] [Google Scholar]
- 54.Qu Y, Wang J, Ray PS, Guo H, Huang J, Shin-Sim M, Bukoye BA, Liu B, Lee AV, Lin X, Huang P, Martens JW, Giuliano AE, Zhang N, Cheng NH, Cui X. Thioredoxin-like 2 regulates human cancer cell growth and metastasis via redox homeostasis and NF-κB signaling. J Clin Invest 121: 212–225, 2011. doi: 10.1172/JCI43144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z, Bissell MJ. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436: 123–127, 2005. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robinson GW, Hennighausen L. MMTV-Cre transgenes can adversely affect lactation: considerations for conditional gene deletion in mammary tissue. Anal Biochem 412: 92–95, 2011. doi: 10.1016/j.ab.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosen JM, Roarty K. Paracrine signaling in mammary gland development: what can we learn about intratumoral heterogeneity? Breast Cancer Res 16: 202, 2014. doi: 10.1186/bcr3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rouhier N, Couturier J, Johnson MK, Jacquot JP. Glutaredoxins: roles in iron homeostasis. Trends Biochem Sci 35: 43–52, 2010. doi: 10.1016/j.tibs.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal 9: 49–89, 2007. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- 60.Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer 14: 709–721, 2014. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sicinski P, Donaher JL, Parker SB, Li T, Fazeli A, Gardner H, Haslam SZ, Bronson RT, Elledge SJ, Weinberg RA. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82: 621–630, 1995. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 62.Singh DK, Winocour P, Farrington K. Oxidative stress in early diabetic nephropathy: fueling the fire. Nat Rev Endocrinol 7: 176–184, 2011. doi: 10.1038/nrendo.2010.212. [DOI] [PubMed] [Google Scholar]
- 63.Sternlicht MD. Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res 8: 201, 2006. doi: 10.1186/bcr1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanos T, Sflomos G, Echeverria PC, Ayyanan A, Gutierrez M, Delaloye J-F, Raffoul W, Fiche M, Dougall W, Schneider P, Yalcin-Ozuysal O, Brisken C. Progesterone/RANKL is a major regulatory axis in the human breast. Sci Transl Med 5: 182ra55, 2013. doi: 10.1126/scitranslmed.3005654. [DOI] [PubMed] [Google Scholar]
- 65.Thomas E, Zeps N, Rigby P, Hartmann P. Reactive oxygen species initiate luminal but not basal cell death in cultured human mammary alveolar structures: a potential regulator of involution. Cell Death Dis 2: e189, 2011. doi: 10.1038/cddis.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trachootham D, Lu W, Ogasawara MA, Rivera-Del Valle N, Huang P. Redox regulation of cell survival. Antioxid Redox Signal 10: 1343–1374, 2008. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ueda S, Masutani H, Nakamura H, Tanaka T, Ueno M, Yodoi J. Redox control of cell death. Antioxid Redox Signal 4: 405–414, 2002. doi: 10.1089/15230860260196209. [DOI] [PubMed] [Google Scholar]
- 68.Wagner KU, Krempler A, Triplett AA, Qi Y, George NM, Zhu J, Rui H. Impaired alveologenesis and maintenance of secretory mammary epithelial cells in Jak2 conditional knockout mice. Mol Cell Biol 24: 5510–5520, 2004. doi: 10.1128/MCB.24.12.5510-5520.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wagner KU, Wall RJ, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L, Li M, Furth PA, Hennighausen L. Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res 25: 4323–4330, 1997. doi: 10.1093/nar/25.21.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Watson CJ. Key stages in mammary gland development–Involution: apoptosis and tissue remodelling that convert the mammary gland from milk factory to a quiescent organ. Breast Cancer Res 8: 203, 2006. doi: 10.1186/bcr1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Watson CJ, Khaled WT. Mammary development in the embryo and adult: a journey of morphogenesis and commitment. Development 135: 995–1003, 2008. doi: 10.1242/dev.005439. [DOI] [PubMed] [Google Scholar]
- 72.Welm BE, Dijkgraaf GJP, Bledau AS, Welm AL, Werb Z. Lentiviral transduction of mammary stem cells for analysis of gene function during development and cancer. Cell Stem Cell 2: 90–102, 2008. doi: 10.1016/j.stem.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Witte S, Villalba M, Bi K, Liu Y, Isakov N, Altman A. Inhibition of the c-Jun N-terminal kinase/AP-1 and NF-kappaB pathways by PICOT, a novel protein kinase C-interacting protein with a thioredoxin homology domain. J Biol Chem 275: 1902–1909, 2000. doi: 10.1074/jbc.275.3.1902. [DOI] [PubMed] [Google Scholar]
- 74.Yang Y, Chang BH, Yechoor V, Chen W, Li L, Tsai MJ, Chan L. The Krüppel-like zinc finger protein GLIS3 transactivates neurogenin 3 for proper fetal pancreatic islet differentiation in mice. Diabetologia 54: 2595–2605, 2011. doi: 10.1007/s00125-011-2255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ye H, Jeong SY, Ghosh MC, Kovtunovych G, Silvestri L, Ortillo D, Uchida N, Tisdale J, Camaschella C, Rouault TA. Glutaredoxin 5 deficiency causes sideroblastic anemia by specifically impairing heme biosynthesis and depleting cytosolic iron in human erythroblasts. J Clin Invest 120: 1749–1761, 2010. doi: 10.1172/JCI40372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuan T, Wang Y, Pao L, Anderson SM, Gu H. Lactation defect in a widely used MMTV-Cre transgenic line of mice. PLoS One 6: e19233, 2011. doi: 10.1371/journal.pone.0019233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zeng H, Vaka VR, He X, Booz GW, Chen J-X. High-fat diet induces cardiac remodelling and dysfunction: assessment of the role played by SIRT3 loss. J Cell Mol Med 19: 1847–1856, 2015. doi: 10.1111/jcmm.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao Y, Zhao B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid Med Cell Longev 2013: 316523, 2013. doi: 10.1155/2013/316523. [DOI] [PMC free article] [PubMed] [Google Scholar]