Abstract
Individuals with alcohol (ethanol)-use disorders are at increased risk for lung infections, in part, due to defective mucociliary clearance driven by motile cilia in the airways. We recently reported that isolated, demembranated bovine cilia (axonemes) are capable of producing nitric oxide (∙NO) when exposed to biologically relevant concentrations of alcohol. This increased presence of ∙NO can lead to protein S-nitrosylation, a posttranslational modification signaling mechanism involving reversible adduction of nitrosonium cations or ∙NO to thiolate or thiyl radicals, respectively, of proteins forming S-nitrosothiols (SNOs). We quantified and compared SNO content between isolated, demembranated axonemes extracted from bovine tracheae, with or without in situ alcohol exposure (100 mM × 24 h). We demonstrate that relevant concentrations of alcohol exposure shift the S-nitrosylation status of key cilia regulatory proteins, including 20-fold increases in S-nitrosylation of proteins that include protein phosphatase 1 (PP1). With the use of an ATP-reactivated axoneme motility system, we demonstrate that alcohol-driven S-nitrosylation of PP1 is associated with PP1 activation and dysfunction of axoneme motility. These new data demonstrate that alcohol can shift the S-nitrothiol balance at the level of the cilia organelle and highlight S-nitrosylation as a novel signaling mechanism to regulate PP1 and cilia motility.
Keywords: S-nitrosylation, alcohol, cilia, redox regulation
alcohol drinking is associated with an increase in pneumonia related to inhaled pathogens (19). Individuals with a history of excessive alcohol [ethanol (EtOH)] use demonstrate a decrease in exhaled nitric oxide (∙NO) levels (1), suggesting diminished ∙NO production or a fate alternative to excretion. ∙NO plays key roles in airway physiology, including regulation of bronchial smooth muscle and pulmonary vascular tone, bactericidal activity, and regulation of mucociliary clearance by stimulation of ciliary beat frequency (CBF) (24). A likely contributor to lung disease with excessive alcohol use is the impairment of mucociliary clearance as an innate host-defense mechanism.
In the context of ciliated airway epithelium lining the small airways and trachea, consumed alcohol that has diffused into bronchial air condenses and recycles during exhalation, resulting in high and prolonged concentrations of alcohol to motile cilia (15). Bovine-ciliated airway epithelial cells exposed to alcohol generate a rapid increase in ∙NO production (33). Moreover, alcohol rapidly and transiently stimulates CBF at the organelle, cellular, and tissue level, and this stimulation is ∙NO dependent through the stimulation of soluble guanylyl cyclase to activate protein kinase G (PKG) (36); concurrently, alcohol activates PKA. Sequential activation of PKG and then PKA is needed for maximal stimulation of CBF by alcohol (32). In contrast, prolonged alcohol exposure blocks cilia stimulation by β-agonists. Prolonged alcohol exposure activates axoneme-localized protein phosphatase 1 (PP1), causing desensitization of PKA and preventing an increase in CBF (27). The mechanism of PP1 activation by alcohol exposure is unknown.
Chronic alcohol drinking shifts the lung reduction/oxidation (redox) balance by the following: 1) altering the NAD+/NADH ratio necessary to mediate coupled electron transfer controlling oxidant formation and 2) depleting the levels of the antioxidant (reduced GSH) within the alveolar space by as much as 80–90% (2, 17). These redox imbalances lead to generation of reactive oxygen species, such as superoxide, and increase lung susceptibility to aberrant oxidant-mediated signaling (17, 26). The reversible oxidation of thiol residues by nitrogen oxides to generate S-nitrosothiol (SNO), or S-nitrosylation (14), is gaining increasing appreciation as a posttranslational protein modification in the airway.
The combination of alcohol and peroxynitrite (the product of the reaction of superoxide + ∙NO) results in the in vivo formation of ethyl nitrite (6), which drives SNO formation of endogenous proteins at levels formed with biologically relevant concentrations of alcohol (25). Therefore, we hypothesized that alcohol shifts the airway cilia proteome to an S-nitrosylated phenotype. To test this hypothesis, we characterized changes in S-nitrosylation in isolated axonemes extracted from bovine tracheae exposed to alcohol. We used a previously established, highly sensitive, quantitative proteomics approach: site-specific, high-throughput identification of protein S-nitrosylation (23) to identify that 24 h in situ alcohol exposure alters the axoneme S-nitrosoproteome. We found that alcohol robustly promotes S-nitrosylation of key cilia regulatory proteins, such as PP1. We demonstrate that in situ alcohol exposure-driven S-nitrosylation of PP1 is associated with PP1 activation and desensitization of isolated, demembranated axonemes to cAMP-mediated stimulation of CBF.
MATERIALS AND METHODS
Chemicals and reagents.
Ammonium bicarbonate, CaCl2 standard solution (1 M), NaCl, urea, HEPES, Nonidet P-40 (NP-40), EDTA, PMSF, thiopropyl-sepharose 6B resin, sodium ascorbate, DTT, and iodoacetamide were purchased from Sigma-Aldrich (St. Louis, MO). Thiourea was from Acros Organics (Morris Plains, NJ). Sequence-grade trypsin solution was from Promega (Madison, WI). Acetonitrile was purchased from Honeywell (Burdick & Jackson; Muskegon, MI). PBS, DMEM, and FBS were purchased from MediaTech, Corning Life Sciences (Tewksbury, MA). Tris buffer, penicillin/streptomycin, and neocuproine (1,10-phenanthroline) were from Thermo Fisher Scientific (Waltham, MA). Coomassie protein assay reagent was purchased from Thermo Fisher Scientific.
In situ treatment of bovine tracheae and airway axoneme extraction.
No live animals were used in the course of this research. Excess tissue from a local abattoir was received under agreement and approval from the U.S. Department of Agriculture for use in this study. Bovine tracheae obtained from a local abattoir were treated with or without 100 mM alcohol and axonemes, prepared as described previously (27, 35). To preserve SNO bonds, axoneme preparation samples were homogenized in a 1:15-vol ice-cold homogenization buffer consisting of 8 M urea, 25 mM HEPES, 50 mM NaCl, 1 mM EDTA, 0.1 mM neocuproine, 1% NP-40, and 0.5 mM PMSF plus protease inhibitor cocktail at pH 7.4.
Light restriction.
To the extent possible, all steps up through the reduction of the SNO bond with ascorbate were performed in the dark to minimize photodegradation of the SNO bond.
SNO content.
Total SNO content was assessed by modifications of the Griess and Saville assays (11). In brief, axonemes isolated from alcohol or control trachea were normalized to 0.25 μg/ml protein and then incubated for 10 min, with or without 20 mM HgCl2. Total nitrite was then measured using the Nitrate/Nitrite Colorimetric Assay Kit (Cayman Chemical, Ann Arbor, MI), according to the manufacturer’s instructions. The difference between nitrite concentrations, with and without addition of HgCl2, was taken as the concentration of SNO.
Mass spectrometry sample preparation.
Proteomic analysis was performed on axonemes extracted from a single bovine trachea that was cut in half, and each half was treated with media plus 100 mM alcohol for 24 h or media alone and axonemes isolated, as described above. In brief, following airway axoneme isolation for proteomics analysis, axoneme samples were homogenized in a 1:15-vol ice-cold homogenization buffer consisting of 8 M urea, 25 mM HEPES, 50 mM NaCl, 1 mM EDTA, 0.1 mM neocuproine, 1% NP-40, and 0.5 mM PMSF plus protease inhibitor cocktail (Sigma-Aldrich) at pH 7.4. S-Nitrosylated peptide enrichment was performed as described previously (23), and the liquid chromatography (LC)-mass spectrometry (MS)/MS experiment was performed on an in-house-built nanoLC system (38) and an LTQ-Orbitrap mass spectrometer, equipped with a nanoelectrospray ion source (Thermo Fisher Scientific). The nanoLC columns were prepared as described previously (23). Exponential gradient elution was performed by two mobile phases (A and B) by increasing mobile phase B from 0 to 60% over 100 min. To identify the eluting peptides, the mass spectrometer was operated in the data-dependent mode to switch automatically between a full MS scan, followed by 10 MS2 scans. Survey full-scan MS spectra (from mass-to-charge ratio 300 to 2,000) were acquired in the LTQ-Orbitrap with resolution of 6,000. The 10 most intense ions (depending on signal intensity) were sequentially isolated for fragmentation in the linear ion trap by collision-induced dissociation. The capillary was maintained at 200°C; the spray voltage was kept at 2.3 kV.
Raw MS spectra were processed with DeconMSn to generate a .mgf file (5). The MS2 spectra were searched with the Ontario Municipal Social Services Association search engine (version 2.1.9; National Center for Biotechnology Information, Bethesda, MD) (9). Ontario Municipal Social Services Association matched the MS2 fragmentation spectra with sequences from the composite bovine International Protein Index (IPI) (18) protein sequence database (version 3.58) containing normal IPI bovine proteins, commonly observed contaminants, and reverse sequences of all proteins. In the database searching, oxidation of methionine, carbamidomethylation, and propionamidation of cysteine were set as the variable modifications. The precursor tolerance was set as 0.2 Da, and MS2 tolerance was 0.5 Da. E-value cutoff was set at 0.1. The false discovery rate was set at 1% at peptide level by filtering on E-value of all forward and reversed peptide identifications.
Biotin switch and NeutrAvidin pulldown.
Following axoneme isolation, a modification of the biotin switch, as described by Jaffrey and Snyder (16), using the S-Nitrosylated Protein Detection Kit (Cayman Chemical) to label and detect SNO bonds, as per the manufacturer’s instructions, with and without preexposure to UV light (366 nm), was performed using a handheld Mineralight lamp as a control for specificity of SNO reduction (11). After biotinylation, protein concentrations were determined and then normalized with cold wash buffer. Sample (50 μl) before NeutrAvidin (Thermo Fisher Scientific) pulldown was saved as input protein. The remaining sample (130 μl) was added to 1.5 ml tubes and biotinylated proteins isolated by NeutrAvidin, according to the manufacturer’s protocol. To elute the biotinylated sample, the resin was resuspended in 5 resin-bed vol Laemmli buffer (Bio-Rad Laboratories, Hercules, CA) containing 5% β-mercaptoethanol and boiled at 100°C for 10 min.
SDS-PAGE and Western blot.
SDS-PAGE was based on the method previously described using precast 4–20% Tris-HCl gels (Bio-Rad Laboratories) (31) with 5 μg input ciliary protein or 50 μl NeutrAvidin elute. After SDS-PAGE, proteins were transferred to nitrocellulose, incubated with Ponceau S reagent (Amresco, Solon, OH) for 10 min, rinsed twice with deionized water, and scanned. After two subsequent rinse steps, the membranes were blocked with wash buffer (0.005 M Tris, 0.15 M NaCl, 0.005% Tween-20, pH 7.5), with 3% BSA, and then incubated with the following primary antibodies: PP1α (anti-mouse; 7482; Santa Cruz Biotechnology, Dallas, TX), sorcin (SR1; anti-rabbit; NBP2-24479SS; Novus Biologicals, Littleton, CO), or sperm-associated antigen 6 (SPAG6; anti-rabbit; NBP2-20462; Novus Biologicals), diluted (1:1000) in wash buffer overnight at 4°C. The membranes were then washed three times with wash buffer, followed by incubation with horseradish peroxidase-conjugated secondary antibodies (goat anti-rabbit or rabbit anti-mouse; Sigma-Aldrich), diluted (1:10,000) in wash buffer with 3% BSA for 1 h at room temperature with shaking. Proteins were identified using SuperSignal West Femto substrate (Thermo Fisher Scientific) and exposing the membranes to autoradiography film or a C-DiGit blot scanner (LI-COR, Lincoln, NE).
Protein phosphatase activity.
Phosphatase activity was determined, as previously described, using the Ser/Thr Phosphatase Assay Kit 1 (KR-pT-IRR; EMD Millipore, Billerica, MA) (27). Isolated axonemes from naive or EtOH-treated tracheae were incubated with 30 μM ascorbate for 10 min or were preincubated with 2.0 nM inhibitor-2 (I-2) before adding the KR-pT-IRR peptide. Once the peptide was added, the reaction was incubated for 10 min at room temperature and stopped with Malachite green.
Ciliary beat frequency.
CBF of isolated axonemes was assessed, as described previously, and readings reported after 5 min initiation of motility (35). Ascorbate was prepared by first diluting to 1 M in ddH2O and then diluting to five times the indicated final concentration in cilia resuspension buffer. CBF data are represented as the mean frequency (hertz) ± SE at 5 min postaddition of cilia activation buffer.
Statistics.
One-way ANOVA with post hoc Tukey’s test statistics was performed in GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA). Statistical significance was set at P ≤ 0.05.
RESULTS
Alcohol shifts the S-nitrosoproteome of airway axoneme proteins.
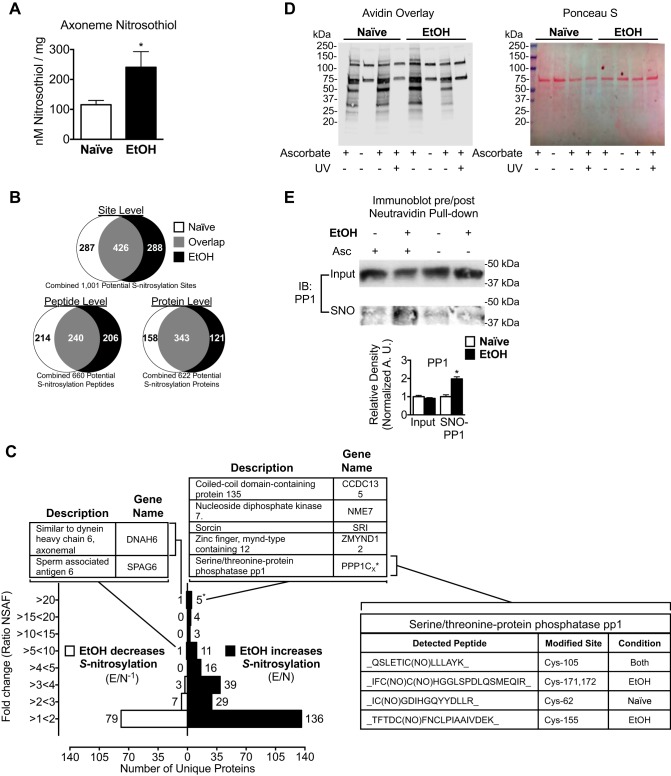
We hypothesized that alcohol exposure increases axoneme S-nitrosylation. To test this hypothesis, we quantified and compared SNO content between isolated, demembranated axonemes extracted from alcohol-naive or 24 h alcohol (100 mM)-exposed bovine tracheae (Fig. 1A). Total SNO content was increased in axonemes extracted from alcohol-exposed tracheae compared with naive (240.6 ± 52.36 vs. 115.5 ± 14.36 nM/mg, P = 0.0502, n = 5). We then sought to determine which specific axoneme proteins showed differential S-nitrosylation with alcohol compared with control. We identified 1,001 potential S-nitrosylated sites on 622 S-nitrosylated proteins (Fig. 1B). There are 426 shared S-nitrosylated sites, 240 shared S-nitrosylated peptides, and 343 shared S-nitrosylated proteins between the control and alcohol-treated samples (Fig. 1B). Unique S-nitrosylation sites were observed on similar peptides for different proteins in both groups. These data suggest that alcohol exposure shifts the S-nitrosoproteome of airway axonemes.
Fig. 1.
Alcohol exposure drives S-nitrosylation of key motility regulatory enzymes in bovine airway axonemes. A: axonemes extracted from bovine tracheae exposed to 100 mM alcohol (EtOH) contain more S-nitrosothiol (SNO) content compared with axonemes from alcohol-naive (Naive) bovine tracheae (240.6 ± 52.36 vs. 115.5 ± 14.36 nM/mg, n = 5). B: a total of 1,001 putative S-nitrosylated sites (cysteine residues) on 660 peptides corresponding to 622 SNO proteins were identified [Naive sample and EtOH (100 mM × 24 h)-treated sample]. There are 426 shared SNO sites, 240 shared SNO peptides, and 343 shared SNO proteins between the Naive and EtOH-treated samples. C: fold change increase or decrease in SNO in EtOH compared with Naive-identified peptides quantified by normalized spectral abundance factor (NSAF). The fold change in the S-nitrosylation increase was calculated by dividing EtOH by the Naive sample NSAF (E/N). The fold-change SNO decreased was calculated by performing the inverse (E/N−1) of values <1. Inset tables list the proteins (with gene names) identified with the indicated NSAF ratio, specific peptides detected, and cysteine residues modified from the bovine International Protein Index (IPI). *No peptides observed for protein phosphatase 1 (PP1) were isoform unique, and therefore, all peptides for PP1 were counted as 1 protein. Proteomic analyses are representative of replicate analyses performed on axonemes extracted from a single bovine tracheae that was cut in half, and each half was treated with media plus 100 mM alcohol for 24 h or media alone. D, left: axoneme proteins extracted from Naive (lanes 1–4) or EtOH (lanes 5–8)-treated bovine tracheae, labeled for total S-nitrosylation by the biotin switch technique (BST) in the presence (lanes 1, 3, 4, 5, 7, and 8) or absence (lanes 2 and 6) of ascorbate or prephotolyzed with UV light (lanes 4 and 8) as a control and assessed by avidin-horseradish peroxidase overlay. Right: Ponceau S staining as a loading control for the biotin switch assay. E: total and S-nitrosylated PP1 were then determined by NeutrAvidin pulldown and immunoblot (IB), demonstrating increased S-nitrosylation of PP1. Quantification of S-nitrosylation by densitometry is represented as the average ± SE of 3 independent experiments, 1 bovine trachea split into 2 parts for each experiment. Asc, ascorbate. *P ≤ 0.05.
To quantify the relative abundance of S-nitrosylation between the alcohol-exposed and alcohol-naive samples on individual proteins, we compared the normalized spectral abundance factor (NSAF) between the groups. The highest relative changes in S-nitrosylation with alcohol exposure are among proteins that demonstrate increased S-nitrosylation (Fig. 1C). A disproportionately high number of proteins demonstrated fivefold or greater increases in NSAF with alcohol compared with control. Strikingly, nine proteins in the alcohol-exposed axonemes demonstrated >20-fold NSAF increases compared with control axonemes (Fig. 1C). Interestingly, only one protein—dynein heavy chain 6—had spectral counts >20-fold higher in the control samples compared with the alcohol-exposed axonemes (Fig. 1C).
In addition to those demonstrating a >20-fold increase in NSAF, 18 additional proteins identified from the alcohol group had NSAF counts greater than fivefold higher than control (Fig. 1C). Unlike increases in S-nitrosylation, only one other protein (SPAG6; decreased 7.28-fold) demonstrated a greater than fivefold change with alcohol exposure compared with control. The remaining proteins demonstrated a less-than fivefold decrease in S-nitrosylation with alcohol exposure. The biotin switch technique (BST), performed in the absence of ascorbate or on samples prephotolyzed with UV light to control for artifactual labeling (Fig. 1D), was used to validate S-nitrosylation of select proteins, including PP1 (Fig. 1E), sorcin (SR1), and SPAG6 (data not shown).
Alcohol-induced ciliary motility dysfunction persists at the level of the axoneme and is PP1 and oxidation dependent.
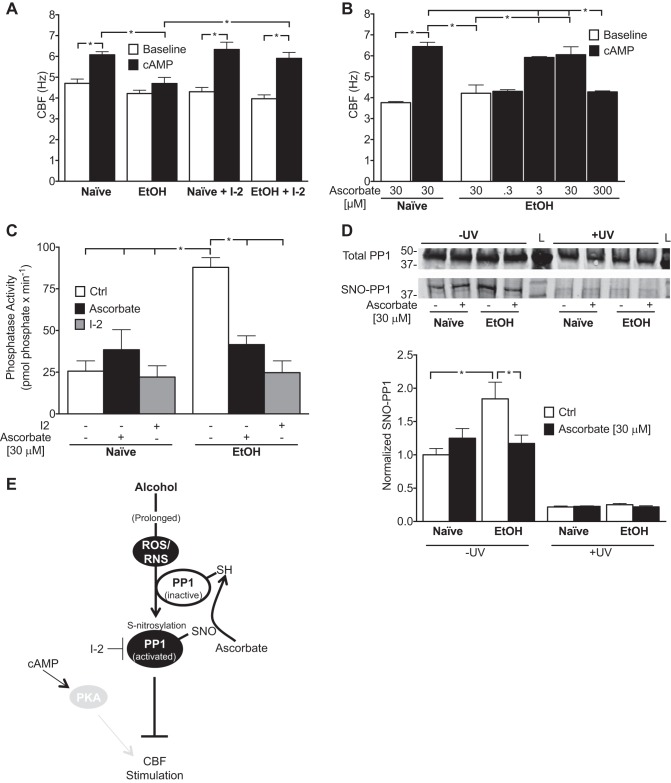
Previous studies indicate that alcohol-induced ciliary motility dysfunction (AICD) is PP1 dependent at the cell and tissue level and that key ciliary kinases are inactivated on the axoneme. We hypothesized that AICD is persistent at the organelle level. To test this hypothesis, we reactivated naive and alcohol-exposed axonemes with ATP and then stimulated with cAMP and measured CBF, with or without the PP1-specific inhibitor, recombinant I-2. Baseline ATP-reactivated CBF was not statistically different between alcohol-exposed and alcohol-naive axonemes (4.22 ± 0.16 and 4.70 ± 0.20 Hz, respectively), and PP1 inhibition did not alter baseline CBF (EtOH, 3.97 ± 0.19 Hz; naive, 4.30 ± 0.21 Hz) with either condition (Fig. 2A). Alcohol exposure blunted stimulated CBF compared with naive (4.70 ± 0.30 and 6.08 ± 0.15 Hz, respectively, P < 0.05, n = 3; Fig. 2A). I-2 restored CBF responsiveness to cAMP in alcohol-exposed axonemes to levels similar to control (EtOH + I-2, 5.90 ± 0.29 Hz, compared with naive + I-2, 6.33 ± 0.35 Hz, P < 0.05, n = 3; Fig. 2A).
Fig. 2.
Activation and S-nitrosylation of PP1 are associated in isolated cilia-motility dysfunction. A and B: axonemes isolated from Naive or EtOH-treated bovine tracheae were centrifuged onto a 96-well plate and reactivated with ATP (2.5 mM; Baseline) and then stimulated with cAMP (10 μM) in the presence or absence of the PP1 inhibitor, inhibitor-2 (I-2; 2 nM; A). Alternatively, isolated axonemes were preincubated with ascorbate (0.3, 3, 30, and 300 μM) and reactivated with ATP in the presence or absence of cAMP (B). Ciliary beat frequency (CBF; in hertz) was obtained by capturing axoneme bending with high-speed video microscopy at 30× and analyzed by Sisson Ammons Video Analysis software after 5 min incubation with all reagents present in each condition. Data are represented as the average ± SE for CBF at t = 5 min from 3 bovine tracheae with 3 technical replicates each. *P < 0.05. C: phosphatase activity of Naive and EtOH-isolated axonemes in the presence or absence of additional DTT (ascorbate; 30 μM for 10 min) or I-2 (2 nM), determined by colorimetric change of Malachite green after incubation with the serine/threonine phosphatase-specific peptide, KR-pT-IRR. Data are represented as the average ± SE for phosphatase activity assays performed in duplicate from 5 cow tracheae. *P < 0.05. D: S-nitrosylation of PP1 determined by the biotin switch technique (BST). Isolated axoneme proteins were incubated as in B and then subjected to the BST, with or without ascorbate as a control. Densitometry data are represented as the average ± SE of immunoblots performed in duplicate for 3 bovine tracheae. *P < 0.05. E: proposed model of PP1 activation by alcohol. Prolonged alcohol exposure drives increased reactive oxygen and reactive nitrogen species (ROS/RNS), resulting in S-nitrosylation and activation of PP1 and blunting of CBF stimulation by cAMP via PKA. SH, reduced thiol.
To understand the functional role of S-nitrosylation as an oxidative, posttranslational modification, we performed isolated axoneme motility assays with the addition of the reducing agent sodium ascorbate (ascorbate; 0.3–3,000 μM). Ascorbate is an antioxidant known to reduce SNOs selectively (16). Ascorbate dose dependently restored CBF responsiveness to cAMP in axonemes that had been exposed to alcohol (0.3 μM, 4.303 ± 0.081 Hz; 3 μM, 5.92 ± 0.04 Hz; and 30 μM, 6.05 ± 0.37 Hz, n = 3; Fig. 2B). Interestingly, high doses of ascorbate (3 mM) completely stopped ciliary beating in both alcohol-treated and alcohol-naive axonemes (data not shown), likely due to the pro-oxidant H2O2-promoting capacity of ascorbate at elevated concentrations (3). Ascorbate at lower doses did not significantly affect baseline CBF in alcohol-exposed (4.21 ± 0.40 Hz) or alcohol-naive (3.76 ± 0.06 Hz) axonemes and did not affect CBF in naive-stimulated axonemes (6.44 ± 0.20 Hz; Fig. 2B).
Alcohol-induced PP1 activation is associated with S-nitrosylation.
We next sought to understand the relationship of PP1 S-nitrosylation with its activation by prolonged alcohol exposure. To explore this relationship, we measured phosphatase activity from alcohol-treated or alcohol-naive isolated axonemes after in vitro treatment with ascorbate (relatively specific for SNO bonds) or with the PP1-specific inhibitor, I-2, in parallel. Alcohol stimulated PP1 activity and increased S-nitrosylation compared with the alcohol-naive control (Fig. 2, C and D). Ascorbate and I-2 independently attenuated phosphatase activity in alcohol-treated compared with alcohol-naive isolated axonemes (Fig. 2C). No change in PP1 activity was observed in alcohol-naive axonemes treated with ascorbate (Fig. 2C). S-Nitrosylation of PP1 was attenuated by ascorbate, which was extinguished with UV prephotolysis, associating PP1 activation with S-nitrosylation status (Fig. 2D).
DISCUSSION
Our data demonstrate that 24 h, 100 mM alcohol exposure alters the S-nitrosylation status of key proteins within our isolated, demembranated axoneme + basal-body preparation. Indeed, alcohol exposure stimulated S-nitrosylation on a total of 121 proteins, including PP1. S-Nitrosylation of PP1 is correlative with its activation and subsequent desensitization of cilia-motility responsiveness. These data corroborate our previous finding that alcohol stimulates axoneme-localized PP1 activity and highlight the novel role of alcohol to drive oxidative signaling within the confinement of the axoneme and the ciliary metabolon.
Tight regulation of S-nitrosylation is necessary to maintain homeostatic SNO balance within the cell. This balance is achieved by catalytic degradation of SNOs by enzymes, such as alcohol dehydrogenase 5 (class III; also known as GSH SNO reductase), thioredoxin reductase, and thioredoxin (8). Moreover, thiol-containing antioxidant proteins, such as GSH, are important sinks for SNOs (10). Chronic alcohol drinking shifts the redox balance within the lung by the following: 1) altering the NAD+/NADH ratio necessary to mediate coupled electron transfer controlling the formation of free radicals and 2) depleting the antioxidant (reduced GSH) within the alveolar space by as much as 80–90% (2, 17). In addition, brief alcohol exposure is known to stimulate robust ∙NO synthase-dependent ∙NO production in isolated bovine axonemes (32). The production of ∙NO in the presence of depleted, reducing capacity is a likely mechanism through which alcohol drives S-nitrosylation of airway axonemes. Despite this, more work is needed to elucidate the precise role of alcohol to alter SNO production and/or metabolism.
Prolonged alcohol exposure activates PP1, desensitizing PKA and rendering cilia unresponsive to external stimuli, such as β-agonists (27). Interestingly, PP1 was among the most heavily S-nitrosylated proteins by alcohol exposure compared with control. Previous reports have identified potential roles of oxidative signaling to regulate PP1 activity (7, 20, 21, 29).
PP1 contains several elements that might render it under regulation of oxidative mechanisms. First, metals (likely Fe2+ and Zn2+) are in its native active site (13). Indeed, H2O2, generated by NADPH oxidase 4, has been demonstrated to inhibit recombinant PP1 by one electron loss within a dinuclear metal center (29). Recombinant PP1 is reversibly inactivated by H2O2, which is coincident with oxidation of residues Cys62 and Cys105 to sulfenic or sulfinic or irreversibly, to sulfonic acid (20). These results are yet to be validated with native PP1, as the metals contained in recombinant PP1 are likely different and subject to different oxidation energies (13). Interestingly, we observed nitrosylation of Cys62 only in control samples, suggesting that axoneme-localized PP1 is inactivated under normal conditions if nitrosylation also serves to inactivate PP1. Cys105 is nitrosylated in both control and alcohol conditions, suggesting that this residue is not key to the regulation of PP1 activity in the context of alcohol exposure (Fig. 1D). Second, PP1 contains a putative oxidoreductase active site (7). Interestingly, one of the unique residues (Cys155) that we identified as S-nitrosylated in response to alcohol on PP1 is within the putative oxidoreductase site (Fig. 1D). In this context, the mechanism by which S-nitrosylation stimulates PP1 activity can only be speculated. S-Nitrosylation of the oxidoreductase site could directly alter PP1 catalytic activity or alter binding with any of the >250 identified binding partners of PP1 (4). Whereas it is clear that ascorbate coincidentally decreases PP1 activity and S-nitrosylation, as detected by the biotin switch, restoring CBF responsiveness, S-nitrosylation may not be the primary redox modification responsible for PP1 activation. The specific reduction of S-nitrosylation in our phosphatase activity and CBF experiments depends on selectivity of ascorbate. Whereas ascorbate does indeed preferentially cleave SNO bonds, ascorbate has also been shown to reduce disulfides. In addition to ascorbate reversing PP1 activity, DTT, a less-specific reducing agent, more selective for disulfide bonds, also reverses alcohol-stimulated PP1 activity and CBF desensitization. In addition, DTT reversed PP1 S-nitrosylation, giving no further information about the specificity of the redox modification of axoneme PP1 in the context of alcohol exposure (data not shown). Despite this, our data indeed suggest oxidation and possibly S-nitrosylation of PP1 as novel mechanisms of activation in bovine-isolated airway axonemes.
Consistent with these findings, previous work in a mouse model of alcohol feeding suggests that AICD is an oxidant-mediated mechanism. Mice that were fed 6 wk, 20% wt/vol alcohol, with concomitant antioxidants (either procysteine or N-acetyl-l-cysteine), were protected from ciliary dysfunction, whereas CBF from tracheae, extracted from those that received alcohol alone, was desensitized to stimulation by β-agonists (30). Moreover, CBF responsiveness was restored within 2 wk of alcohol abstinence following the 6-wk alcohol feeding.
We chose 100 mM alcohol, based on previous studies in our laboratory, indicating that ad libitum, in vivo-prolonged drinking of alcohol by mice or rats results in ciliary dysfunction (30, 37). Whereas the legal blood-alcohol concentration to drive in the United States is ~17 mM, 100 mM is consistent with blood-alcohol concentrations of patients with chronic alcohol-use disorders (22, 34). Moreover, the condensation and recycling of alcohol as it cools, when exhaled from bronchi, further increase and prolong the exposure of the ciliated airway epithelium. The characterization of alcohol-mediated S-nitrosylation in our axoneme preparation is limited to ciliary axoneme proteins. The means by which we prepare axonemes excludes the ciliary phospholipid bilayer (12). Furthermore, SNO bonds are notoriously evanescent and subject to both false-positive and -negative detection by the inadvertent labeling of sulfenic or sulfinic acids or presence of oxidizing metals or sunlight (photolysis) (28). The site-specific, high-throughput identification of the protein SNO method that we have chosen for detection uses a dual blocking strategy and photolysis (data not shown) to control for most of the potential artifact in identifying S-nitrosylation (23). However, alternative methods of validation, such as demonstrated with the BST (Fig. 2D), and mutagenesis studies are needed to understand the precise relationship of S-nitrosylation to regulation of PP1 activity in the context of alcohol exposure.
In summary, we have identified that alcohol exposure drives S-nitrosylation in airway ciliary axonemes. Alcohol-driven S-nitrosylation is enriched in several key cilia regulatory proteins, including PP1, which we have recently identified as key to the mechanism of alcohol-induced ciliary dysfunction. Importantly, S-nitrosylation may be a key regulatory mechanism for ciliary motility. Alcohol drinking may be an important driving factor of S-nitrosylation to alter the physiology of innate defense via mucociliary clearance.
GRANTS
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (F30 AA024676, to M. E. Price; R01 AA008769, to J. H. Sisson; and R01 AA017993, to T. A. Wyatt), Department of Veterans Affairs (I01 BX003635; to T. A. Wyatt), and Pulmonary, Critical Care, Sleep & Allergy Division, Department of Internal Medicine, at the University of Nebraska Medical Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.E.P., S-J.D., and J.H.S. conceived and designed research; M.E.P., J.A.P., and M.L. performed experiments; M.E.P., J.A.P., and S-J.D. analyzed data; M.E.P., S-J.D., T.A.W., and J.H.S. interpreted results of experiments; M.E.P. and J.A.P. prepared figures; M.E.P. and J.H.S. drafted manuscript; M.E.P., J.A.P., M.L., S-J.D., T.A.W., and J.H.S. edited and revised manuscript; M.E.P., J.A.P., M.L., S-J.D., T.A.W., and J.H.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge Lisa Chudomelka for editorial manuscript assistance.
REFERENCES
- 1.Afshar M, Poole JA, Cao G, Durazo R, Cooper RC, Kovacs EJ, Sisson JH. Exhaled nitric oxide levels among adults with excessive alcohol consumption. Chest 150: 196–209, 2016. doi: 10.1016/j.chest.2016.02.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boé DM, Vandivier RW, Burnham EL, Moss M. Alcohol abuse and pulmonary disease. J Leukoc Biol 86: 1097–1104, 2009. doi: 10.1189/jlb.0209087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E, Choyke PL, Pooput C, Kirk KL, Buettner GR, Levine M. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci USA 104: 8749–8754, 2007. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen PT. Protein phosphatase 1—targeted in many directions. J Cell Sci 115: 241–256, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 26: 1367–1372, 2008. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 6.Deng XS, Bludeau P, Deitrich RA. Formation of ethyl nitrite in vivo after ethanol administration. Alcohol 34: 217–223, 2004. doi: 10.1016/j.alcohol.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Fetrow JS, Siew N, Skolnick J. Structure-based functional motif identifies a potential disulfide oxidoreductase active site in the serine/threonine protein phosphatase-1 subfamily. FASEB J 13: 1866–1874, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Gaston BM, Carver J, Doctor A, Palmer LA. S-Nitrosylation signaling in cell biology. Mol Interv 3: 253–263, 2003. doi: 10.1124/mi.3.5.253. [DOI] [PubMed] [Google Scholar]
- 9.Geer LY, Markey SP, Kowalak JA, Wagner L, Xu M, Maynard DM, Yang X, Shi W, Bryant SH. Open mass spectrometry search algorithm. J Proteome Res 3: 958–964, 2004. doi: 10.1021/pr0499491. [DOI] [PubMed] [Google Scholar]
- 10.Gould N, Doulias PT, Tenopoulou M, Raju K, Ischiropoulos H. Regulation of protein function and signaling by reversible cysteine S-nitrosylation. J Biol Chem 288: 26473–26479, 2013. doi: 10.1074/jbc.R113.460261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gow A, Doctor A, Mannick J, Gaston B. S-Nitrosothiol measurements in biological systems. J Chromatogr B Analyt Technol Biomed Life Sci 851: 140–151, 2007. doi: 10.1016/j.jchromb.2007.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hastie AT, Dicker DT, Hingley ST, Kueppers F, Higgins ML, Weinbaum G. Isolation of cilia from porcine tracheal epithelium and extraction of dynein arms. Cell Motil Cytoskeleton 6: 25–34, 1986. doi: 10.1002/cm.970060105. [DOI] [PubMed] [Google Scholar]
- 13.Heroes E, Rip J, Beullens M, Van Meervelt L, De Gendt S, Bollen M. Metals in the active site of native protein phosphatase-1. J Inorg Biochem 149: 1–5, 2015. doi: 10.1016/j.jinorgbio.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6: 150–166, 2005. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 15.Hlastala MP, Anderson JC. Alcohol breath test: gas exchange issues. J Appl Physiol (1985) 121: 367–375, 2016. doi: 10.1152/japplphysiol.00548.2015. [DOI] [PubMed] [Google Scholar]
- 16.Jaffrey SR, Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE 2001: pl1, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Kaphalia L, Calhoun WJ. Alcoholic lung injury: metabolic, biochemical and immunological aspects. Toxicol Lett 222: 171–179, 2013. doi: 10.1016/j.toxlet.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kersey PJ, Duarte J, Williams A, Karavidopoulou Y, Birney E, Apweiler R. The International Protein Index: an integrated database for proteomics experiments. Proteomics 4: 1985–1988, 2004. doi: 10.1002/pmic.200300721. [DOI] [PubMed] [Google Scholar]
- 19.Kershaw CD, Guidot DM. Alcoholic lung disease. Alcohol Res Health 31: 66–75, 2008. [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HS, Song MC, Kwak IH, Park TJ, Lim IK. Constitutive induction of p-Erk1/2 accompanied by reduced activities of protein phosphatases 1 and 2A and MKP3 due to reactive oxygen species during cellular senescence. J Biol Chem 278: 37497–37510, 2003. doi: 10.1074/jbc.M211739200. [DOI] [PubMed] [Google Scholar]
- 21.Kohr MJ, Davis JP, Ziolo MT. Peroxynitrite Increases protein phosphatase activity and promotes the interaction of phospholamban with protein phosphatase 2a in the myocardium. Nitric Oxide 20: 217–221, 2009. doi: 10.1016/j.niox.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labianca DA, Simpson G. Medicolegal alcohol determination: variability of the blood- to breath-alcohol ratio and its effect on reported breath-alcohol concentrations. Eur J Clin Chem Clin Biochem 33: 919–925, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Liu M, Hou J, Huang L, Huang X, Heibeck TH, Zhao R, Pasa-Tolic L, Smith RD, Li Y, Fu K, Zhang Z, Hinrichs SH, Ding SJ. Site-specific proteomics approach for study protein S-nitrosylation. Anal Chem 82: 7160–7168, 2010. doi: 10.1021/ac100569d. [DOI] [PubMed] [Google Scholar]
- 24.Marozkina NV, Gaston B. Nitrogen chemistry and lung physiology. Annu Rev Physiol 77: 431–452, 2015. doi: 10.1146/annurev-physiol-021113-170352. [DOI] [PubMed] [Google Scholar]
- 25.Moya MP, Gow AJ, McMahon TJ, Toone EJ, Cheifetz IM, Goldberg RN, Stamler JS. S-Nitrosothiol repletion by an inhaled gas regulates pulmonary function. Proc Natl Acad Sci USA 98: 5792–5797, 2001. doi: 10.1073/pnas.091109498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polikandriotis JA, Rupnow HL, Elms SC, Clempus RE, Campbell DJ, Sutliff RL, Brown LA, Guidot DM, Hart CM. Chronic ethanol ingestion increases superoxide production and NADPH oxidase expression in the lung. Am J Respir Cell Mol Biol 34: 314–319, 2006. doi: 10.1165/rcmb.2005-0320OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price ME, Pavlik JA, Sisson JH, Wyatt TA. Inhibition of protein phosphatase 1 reverses alcohol-induced ciliary dysfunction. Am J Physiol Lung Cell Mol Physiol 308: L577–L585, 2015. doi: 10.1152/ajplung.00336.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin Y, Dey A, Daaka Y. Protein S-nitrosylation measurement. Methods Enzymol 522: 409–425, 2013. doi: 10.1016/B978-0-12-407865-9.00019-4. [DOI] [PubMed] [Google Scholar]
- 29.Santos CX, Hafstad AD, Beretta M, Zhang M, Molenaar C, Kopec J, Fotinou D, Murray TV, Cobb AM, Martin D, Zeh Silva M, Anilkumar N, Schröder K, Shanahan CM, Brewer AC, Brandes RP, Blanc E, Parsons M, Belousov V, Cammack R, Hider RC, Steiner RA, Shah AM. Targeted redox inhibition of protein phosphatase 1 by Nox4 regulates eIF2α-mediated stress signaling. EMBO J 35: 319–334, 2016. doi: 10.15252/embj.201592394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simet SM, Pavlik JA, Sisson JH. Dietary antioxidants prevent alcohol-induced ciliary dysfunction. Alcohol 47: 629–635, 2013. doi: 10.1016/j.alcohol.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simet SM, Pavlik JA, Sisson JH. Proteomic analysis of bovine axonemes exposed to acute alcohol: role of endothelial nitric oxide synthase and heat shock protein 90 in cilia stimulation. Alcohol Clin Exp Res 37: 609–615, 2013. doi: 10.1111/acer.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sisson JH, Pavlik JA, Wyatt TA. Alcohol stimulates ciliary motility of isolated airway axonemes through a nitric oxide, cyclase, and cyclic nucleotide-dependent kinase mechanism. Alcohol Clin Exp Res 33: 610–616, 2009. doi: 10.1111/j.1530-0277.2008.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stout SL, Wyatt TA, Adams JJ, Sisson JH. Nitric oxide-dependent cilia regulatory enzyme localization in bovine bronchial epithelial cells. J Histochem Cytochem 55: 433–442, 2007. doi: 10.1369/jhc.6A7089.2007. [DOI] [PubMed] [Google Scholar]
- 34.Teplin LA, Abram KM, Michaels SK. Blood alcohol level among emergency room patients: a multivariate analysis. J Stud Alcohol 50: 441–447, 1989. doi: 10.15288/jsa.1989.50.441. [DOI] [PubMed] [Google Scholar]
- 35.Wyatt TA, Forgèt MA, Adams JM, Sisson JH. Both cAMP and cGMP are required for maximal ciliary beat stimulation in a cell-free model of bovine ciliary axonemes. Am J Physiol Lung Cell Mol Physiol 288: L546–L551, 2005. doi: 10.1152/ajplung.00107.2004. [DOI] [PubMed] [Google Scholar]
- 36.Wyatt TA, Forgèt MA, Sisson JH. Ethanol stimulates ciliary beating by dual cyclic nucleotide kinase activation in bovine bronchial epithelial cells. Am J Pathol 163: 1157–1166, 2003. doi: 10.1016/S0002-9440(10)63475-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wyatt TA, Gentry-Nielsen MJ, Pavlik JA, Sisson JH. Desensitization of PKA-stimulated ciliary beat frequency in an ethanol-fed rat model of cigarette smoke exposure. Alcohol Clin Exp Res 28: 998–1004, 2004. doi: 10.1097/01.ALC.0000130805.75641.F4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao R, Ding SJ, Shen Y, Camp DG II, Livesay EA, Udseth H, Smith RD. Automated metal-free multiple-column nanoLC for improved phosphopeptide analysis sensitivity and throughput. J Chromatogr B Analyt Technol Biomed Life Sci 877: 663–670, 2009. doi: 10.1016/j.jchromb.2008.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]