Abstract
Acute respiratory distress syndrome (ARDS) is a devastating critical illness disproportionately affecting the elderly population, with both higher incidence and mortality. The integrity of the lung endothelial cell (EC) monolayer is critical for preservation of lung function. However, mechanisms mediating EC barrier regulation in the context of aging remain unclear. We assessed the severity of acute lung injury (ALI) in young (2 mo) and aged (18 mo) mice using a two-hit preclinical model. Compared with young cohorts, aged mice exhibited increased ALI severity, with greater vascular permeability characterized by elevated albumin influx and levels of bronchoalveolar lavage (BAL) cells (neutrophils) and protein. Aged/injured mice also demonstrated elevated levels of reactive oxygen species (ROS) in the BAL, which was associated with upregulation of the ROS–generating enzyme, Nox4. We evaluated the role of aging in human lung EC barrier regulation utilizing a cellular model of replicative senescence. Senescent EC populations were defined by increases in β-galactosidase activity and p16 levels. In response to lipopolysaccharide (LPS) challenge, senescent ECs demonstrate exacerbated permeability responses compared with control “young” ECs. LPS challenge led to a rapid induction of Nox4 expression in both control and senescent ECs, which was posttranslationally mediated via the proteasome/ubiquitin system. However, senescent ECs demonstrated deficient Nox4 ubiquitination, resulting in sustained expression of Nox4 and alterations in cellular redox homeostasis. Pharmacological inhibition of Nox4 in senescent ECs reduced LPS-induced alterations in permeability. These studies provide insight into the roles of Nox4/senescence in EC barrier responses and offer a mechanistic link to the increased incidence and mortality of ARDS associated with aging.
Keywords: senescence, oxidative stress, Nox4, reactive oxygen species
acute respiratory distress syndrome (ARDS) is an inflammatory lung condition affecting ~200,000 U.S patients/year (36). A key pathological feature of the inflammatory response in ARDS is the disruption of lung endothelial barrier integrity with paracellular gap formation (13). The endothelium is a monolayer that lines the luminal surface of the entire vascular system and provides a semipermeable barrier between circulating blood and underlying tissues. The functional integrity of the endothelial cell (EC) monolayer is essential to prevent vascular leakage. However, during the pathobiology of inflammatory lung injury, pulmonary vascular permeability to circulating fluids, macromolecules, and leukocytes is dramatically increased, resulting in alveolar flooding and severe oxygenation impairment, which can lead to life-threatening multiorgan failure. There are no FDA-approved drug treatments for ARDS, and present management strategies only target underlying etiologies, aggressive fluid removal, and supportive mechanical ventilation. ARDS patients pose a major challenge to intensive care unit mortality, with ARDS mortality remaining 30–50% (44).
Aging is a major risk factor for ARDS. The incidence of ARDS increases with age; in young individuals (15–19 yr of age), 16 cases appear per 100,000 person-years vs. elderly individuals (75–84 yr of age) with 306 cases per 100,000 person-years (36). Likewise, the mortality from ARDS increases with advancing age, ranging from 24% in adolescents (15–19 yr of age) to 60% in elderly patients (>85 yr of age) (36). Although preexisting comorbidities in elderly patients likely contribute to the higher mortality rate observed in advanced age, the mortality risk in middle-aged adults is also significantly higher compared with adolescents (36). Despite the strong association between aging and ARDS, few studies have investigated the molecular mechanisms that account for this age-associated predilection.
Aging and ARDS are both associated with cumulative oxidant burden. A substantial body of evidence has implicated redox imbalance as a major contributor to the pathogenesis of ARDS (5, 24, 28, 34, 46). The “oxidative stress theory” posits that the progressive and irreversible accumulation of damage caused by reactive oxygen species (ROS) impacts critical aspects of the aging process by contributing to impaired physiological function, increased incidence of disease, and reduced life span. Oxidative stress can lead to extensive modifications and damage to macromolecules including DNA, lipids, and proteins and increased production of cytokines. The lungs are particularly prone to ROS-mediated injury, given their direct exposure to the environment and inspired air (30). Environmental insults to the lung may serve as a second hit, which may accelerate the aging process by promoting persistently elevated oxidative stress levels, leading to an increased susceptibility to disease (40). ARDS has a myriad of etiologies although excessive oxidative stress may represent a core and common pathway in the disease pathogenesis. Despite the well-recognized role of oxidative stress in ARDS, the specific contribution of ROS generated by nonphagocytic cells to the development or perpetuation of disease pathogenesis is not well understood. Elucidating the mechanisms that mediate redox imbalance of ECs in the context of aging may be critical to the development of more effective therapeutic strategies for ARDS.
In the present study, reparative responses to lung injury in young and aged mice were evaluated using a two-hit (LPS and mechanical ventilation) preclinical model of acute lung injury (ALI). The results demonstrate that aged mice exhibit significantly increased severity of ALI associated with impaired vascular barrier-regulatory responses. Senescent ECs demonstrate exacerbated permeability responses mediated by sustained expression of the ROS-generating enzyme NADPH oxidase-4 (Nox4). We demonstrate that Nox4 expression is rapidly increased by posttranslational regulation via the proteasome-ubiquitin pathway, and dysregulation of Nox4 ubiquitination leads to sustained expression of Nox4 in senescent ECs. Pharmacological targeting of Nox4 in senescent ECs led to protection from LPS-induced alterations in permeability. Together, these studies provide novel insight into the role of Nox4/senescence in regulating EC barrier responses and offer a mechanistic link to the increased incidence and mortality of ARDS associated with aging.
METHODS
Reagents.
We purchased protease inhibitor cocktail set III and MG132 from EMD Chemicals (San Diego, CA). We purchased the following antibodies: GAPDH from Cell Signaling (Danvers, MA), Nox4 and β-actin from Abcam (Cambridge, MA), and p16 from BD Biosciences (San Jose, CA). Secondary horseradish peroxidase-conjugated anti-mouse and anti-rabbit antibodies were purchased from Bio-Rad (Hercules, CA). All other chemicals/reagents were purchased from Sigma, unless otherwise stated.
Cell culture.
Human pulmonary artery ECs and EGM-2 bullet kit culture media were purchased from Lonza (Walkersville, MD).
Cells were cultured at 37°C in 5% CO2, 95% air. For senescence studies, ECs at low population doublings were utilized as control cells; these cells were also expanded in culture to achieve high population doublings and induction of replicative senescence.
Murine model of ALI.
Young (2 mo) and aged (18 mo) male C57BL/6 mice (The Jackson Laboratory or National Institute on Aging) were used for in vivo studies. We employed a previously described two-hit preclinical model (Fig. 1) (15, 27, 35). Mice were anesthetized using intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). Lipopolysaccharide (LPS) diluted in PBS (0.2 mg/kg; 50 μl total volume) was administered intratracheally to induce lung injury. Following recovery, mice were returned to their cages. At 20 h post-LPS administration, mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and intubated for mechanical ventilation; additional doses of anesthesia were given as needed during the ventilation-induced lung injury (VILI) protocol to ensure adequate anesthetic depth and body temperature was maintained with a heating pad. A 20-gauge catheter was orally inserted into the trachea, and mice were connected to a Harvard Apparatus Inspira rodent ventilator (Harvard Apparatus, Cambridge, MA) and subjected to high tidal volume ventilation (room air, 20 ml/kg tidal volume, 85 breaths/min) for 4 h under continuous anesthesia. For control mice, we used intact (uninjured) 18-mo-old female mice that were not subjected to LPS/vent. While under deep anesthesia, mice subjected to LPS/VILI (postinjury) or uninjured (control) mice were euthanized by exsanguination and bilateral thoracotomy, and lung tissue/sample harvest was performed. All experiments and procedures involving animals were conducted in accordance with the approval of the Institutional Animal Care and Use Committee (IACUC) under guidelines at the University of Arizona.
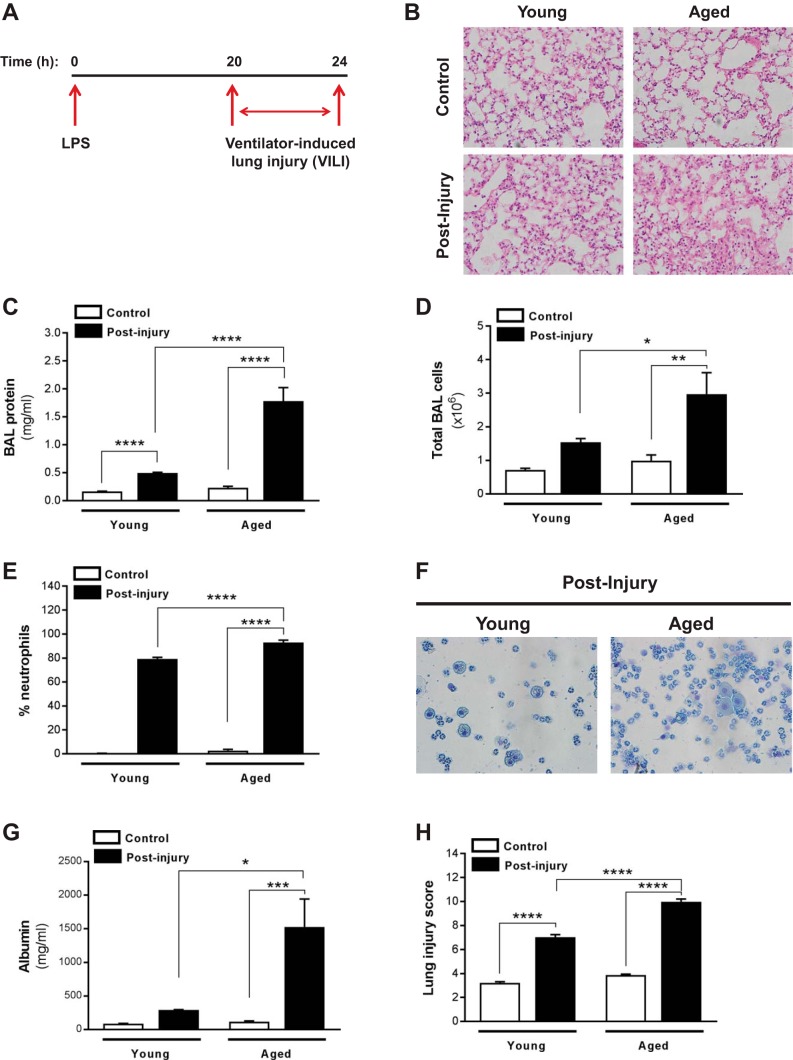
Fig. 1.
Aged mice exhibit increased severity of acute lung injury (ALI). Young (2 mo old) and aged (18 mo old) C57BL/6 mice were subjected to lung injury by airway instillation of intratracheal LPS (0.2 mg/kg) for 20 h followed by ventilator-induced lung injury (VILI) for 4 h; postinjury refers to mice subjected to this LPS/VILI injury protocol, and control refers to uninjured mice. A: schematic diagram illustrating the time course for the in vivo 2-hit injury model of ALI. B–H: bronchoalveolar lavage (BAL) fluid was collected, and lung tissue was harvested from both young and aged control (uninjured) mice and from young and aged mice postinjury (subjected to LPS/VILI). Severity of ALI was assessed by histopathology (B), total BAL protein (C), total BAL cells (D), and neutrophil influx (E and F); cytospin was performed, and BAL neutrophil counts were quantified (E). Representative cytospin image (F), albumin assay (G), and lung injury score (H) are shown. Values represent means ± SE; n = 5–9 mice/group; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 using 2-way ANOVA with Bonferroni’s multiple-comparison test.
Bronchoalveolar lavage.
Bronchoalveolar lavage (BAL) fluid was recovered by flushing the lungs with 1 ml of HBSS through the tracheal cannula. BAL samples were processed for multiple assays, including evaluation of total BAL protein, total number of cells, and determination of BAL cell differentials, as previously described (33, 37). Albumin assay kit was purchased from Bethyl Laboratories (Montgomery, TX), and samples were evaluated according to the manufacturer’s instructions. BAL H2O2 levels were determined using the Amplex red assay kit.
Senescence assays.
We used a high-sensitivity substrate (fluorescein di-β-D-galactosidase, βgal) for quantitative assessment of cellular senescence (Marker Gene Technologies, Eugene, OR), according to the manufacturer’s instructions. Cell number was normalized by DAPI (Fluorescent Cell Count Normalization Kit; Marker Gene Technologies). We also used a senescence detection kit designed to histochemically detect βgal activity in cultured cells (Abcam).
Ubiquitin kit.
Cell lysates were prepared in RIPA buffer, and ubiquitinated Nox4 was evaluated using the UBI-QAPTURE-Q kit from Enzo Life Science (Farmingdale, NY), according to the manufacturer’s instructions. The kit facilitates pull-down of both mono- and polyubiquitinylated proteins (independent of lysine residue chain linkage) from cell extracts through use of a high-binding-affinity matrix. Briefly, cell lysates were incubated with the affinity matrix to capture any ubiqutinylated proteins. After a thorough wash, the proteins were eluted in SDS-PAGE gel loading buffer and subjected to Western blot analysis using the Nox4 antibody utilized for all other studies (Abcam).
Lung histology.
Lung tissue was fixed in 10% formalin. Tissues were then processed, paraffin embedded, sectioned, and stained with hematoxylin and eosin (H and E) at NDBbio (Baltimore, MD).
Lung injury score.
Histological sections stained with H and E were utilized to evaluate lung injury, as previously described (15). Briefly, severity of ALI was blindly evaluated using a scoring system to determine inflammation, alveolar septal thickening, and edema. Inflammation was detected by the total number of inflammatory cells/×100 field; 5–7 random fields were counted/slide (n = 4–6 animals/group). Edema was characterized as follows: absent (score = 0), mild (<10% alveoli involved; score = 1), moderate (10–50% alveoli involved; score = 2), or severe (>50% alveoli involved; score = 3). Alveolar septal thickening was characterized as follows: normal (15–20 µm; score = 1), mild (2-fold of the normal thickening; score = 2), moderate (3-fold of the normal thickening; score = 3), or severe (4-fold of the normal thickening; score = 4). Inflammation, edema, and septal thickening scores for each treatment group were averaged, and the final score represents the sum the scores from each of the three injury parameters.
Measurement of transendothelial electrical resistance.
Transendothelial electrical resistance (TER) measurements were performed as previously described (14). Briefly, ECs were plated in a confluent monolayer in electric cell-substrate impedance sensing (ECIS) array plates coated with 0.1% gelatin. After 24 h, cells were simultaneously treated with LPS (200 ng/ml) and GKT137831 (15 μM), a first-in-class small-molecule inhibitor of Nox1/4 (Genkyotex, Geneva, Switzerland), or vehicle (DMSO) for the duration of the experiment (10 h). TER measurements were performed using an electrical cell substrate impedance sensing system (Applied Biophysics, Troy, NY), and relative TER values were calculated by dividing actual TER values at each time point by the initial TER values (1,300–1,500 Ohms). The relative values were plotted as the means ± SE.
Western immunoblotting.
We prepared cell lysates in RIPA buffer, subjected them to SDS-PAGE under reducing conditions, and performed Western immunoblotting. Lysates were quantitated using a Micro BCA Protein assay kit (Pierce, Waltham, MA) according to instructions. We used enhanced chemiluminescence Western blotting substrate (Pierce) and My ECL Imager (Pierce) to detect specific immunoreactive signals.
RNA isolation and real-time PCR.
Total RNA was extracted from ECs using RNeasy mini kits from Qiagen (Germantown, MD), and cDNA was synthesized using High-Capacity cDNA Reverse Transcription kit from Invitrogen (Carlsbad, CA). PCR was carried out using KAPA SYBR FAST Master Mix (Wilmington, MA), and relative quantification was calculated with the 2-ΔΔCT method. The primer sequences used for PCR are as follows: GAPDH: Fwd- CTCTCTGCTCCTCCTGTTCG and Rev- TTAAAAGCAGCCCTGGTGAC; Nox4: Fwd- TGTCCCAGTGTATCTGCATTAG and Rev- GACCTCATAGTTCAGTGATTCCTC.
Hydrogen peroxide (H2O2) detection.
H2O2 levels in BAL and H2O2 generated by ECs were determined by Amplex red assay kit (Molecular Probes/Life Technologies, Eugene, OR). The fluorescence intensity was measured at 525 nm for excitation and emission in the range of 580–640 nm using the Glomax multi detection system (Promega, Madison, WI).
Statistical analysis.
Graphs were made and statistical analyses were performed with GraphPad Prism (GraphPad Software, San Diego, CA). Data are expressed as means ± SE. Differences among more than two groups were assessed using two-way ANOVA with a Bonferroni’s multiple-comparisons test and between two groups using Student’s two-tailed t-test; statistical significance is denoted by *P < 0.05, **P < 0.01, ***P < 0.001, or ****P < 0.0001.
RESULTS
Aged mice demonstrate increased susceptibility to ALI.
We first evaluated the responses to lung injury in young and aged mice subjected to a two-hit preclinical model of lung injury, combining exposure to both endotoxin and VILI to more accurately mimic human ARDS. Specifically, mice were subjected to intratracheal administration of LPS (0.2 mg/kg; 50 μl total volume) for 20 h, followed by high tidal volume ventilation (20 ml/kg) for 4 h (Fig. 1A; schematic diagram illustrating time course of injury model). Compared with young cohorts, aged mice exhibited increased severity of lung injury, as demonstrated by greater diffuse alveolar damage and alveolar wall thickening (Fig. 1B, histopathology), and increased alveolar and vascular permeability characterized by elevated BAL protein and cellularity (Fig. 1C, total BAL protein; Fig. 1D, total BAL cells). Specifically, BAL fluid from aged/injured mice showed greater neutrophil influx (Fig. 1, E and F, quantification of percent neutrophil influx and representative cytological images, respectively), as well as elevated albumin influx (Fig. 1G). Finally, aged mice subjected to LPS/VILI demonstrated a significantly elevated lung injury score compared with young cohorts (Fig. 1H). Taken together, these data indicate that barrier-regulatory responses may be impaired in aged mice, ultimately contributing to the increased severity of ALI.
Increased ALI severity in aged mice is associated with elevated Nox4 expression and ROS.
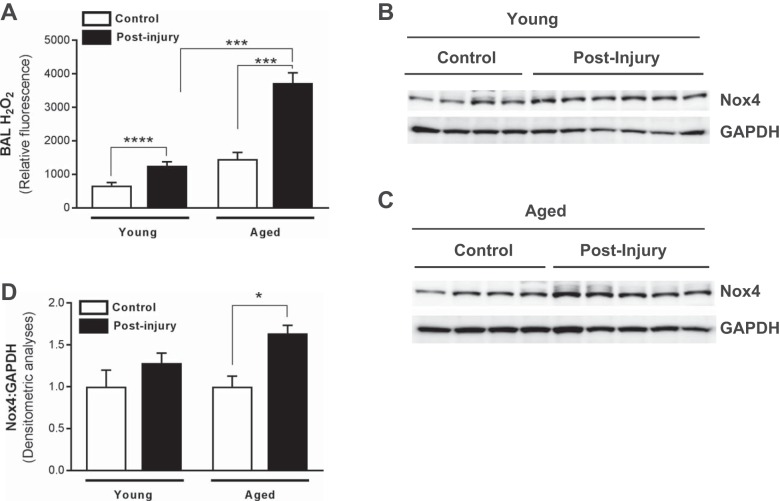
Numerous studies demonstrate a strong association with oxidative stress and ARDS (5, 24, 28, 34, 46). We therefore sought to determine whether increased severity of ALI in aged mice was also associated with elevated ROS. Although both young and aged mice exhibited increased H2O2 in the BAL following injury (Fig. 2A), H2O2 levels were significantly higher in aged/injured mice (Fig. 2A). Furthermore, significantly elevated BAL H2O2 levels in aged/injured mice corresponded with elevated expression of the oxidant-generating enzyme, Nox4 (Fig. 2, C and D), whereas Nox4 expression was not elevated in young mice postinjury (Fig. 2, B and D).
Fig. 2.
Age-dependent increased severity of ALI is associated with elevated NADPH oxidase-4 (Nox4) expression and reactive oxygen species (ROS) generation. Whole lung tissue and BAL fluid were collected from young and aged control (uninjured) mice and mice postinjury (subjected to LPS/VILI; 24 h). A–C: Nox4 expression in whole lung tissue was assessed in young (A) and aged (B) mice by Western immunoblotting; densitometric analyses are shown (C). D: BAL fluid was assessed for the presence of H2O2 by Amplex red assay. Values represent means ± SE; n = 4–6 mice/group; *P < 0.05, ***P < 0.001, ****P < 0.0001 using 2-way ANOVA with Bonferroni’s multiple-comparison test.
Defective barrier-regulatory responses in senescent ECs are associated with Nox4-mediated redox imbalance.
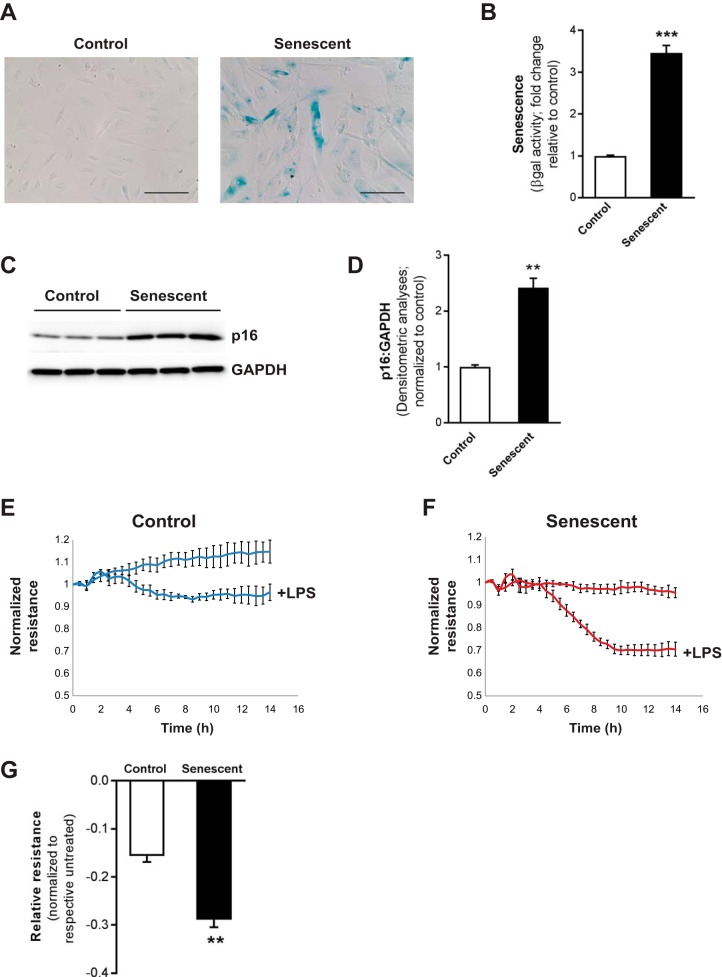
To evaluate the role of aging in vascular barrier-regulatory responses, a cellular model of replicative senescence was employed, human lung ECs at low and high population doublings (control and senescent ECs, respectively). In vitro replicative senescence models permit the study of the mechanisms of changes that occur while the cells undergo a predictable and reproducible deterioration in a controlled environment (10). This has been a valuable model for explaining the basis of mechanisms underlying aging changes observed in situ and for testing hypotheses that investigate common mechanisms underlying cell deterioration and loss of integrative functions, including the regulation and effects of ROS in cellular aging (10). Here, senescent ECs were defined by having at least three- to fourfold increased βgal activity (Fig. 3A, cellular staining for βgal; Fig. 3B, quantitative fluorescence assay for βgal activity) and elevated levels of the senescence marker, p16 (Fig. 3C, Western immunoblotting; Fig. 3D, densitometric analyses) compared with young control cells. Using this model, we first sought to evaluate whether senescence of ECs led to altered permeability responses. We utilized ECIS, an impedance-based cell-monitoring technology method, to evaluate TER as a measure of permeability responses to LPS. As expected, LPS challenge led to increased permeability in control ECs (Fig. 3, E and G). However, alterations in the LPS-induced permeability response were significantly exacerbated in senescent ECs, reflecting an exaggerated loss of barrier integrity in senescent ECs (Fig. 3, F and G).
Fig. 3.
Senescent endothelial cells (ECs) demonstrate exacerbated permeability responses. Control and senescent ECs (at low and high population doublings, respectively) were utilized and cultured ex vivo. Senescence was evaluated by β-galactosidase (βgal) staining (A); quantitative measurement of βgal activity, fluorescence assay, normalized to total number of cells (B); expression of p16 by Western immunoblotting (C); and densitometric analyses (D). E–G: control (E) and senescent (F) ECs were challenged with LPS, and transendothelial electrical resistance (TER) was evaluated. G: bar graph represents peak differences in resistance of control and senescent ECs, normalized to respective untreated group. Values represent means ± SE; n = 3; **P < 0.01, ***P < 0.001 using Student’s 2-tailed t-test.
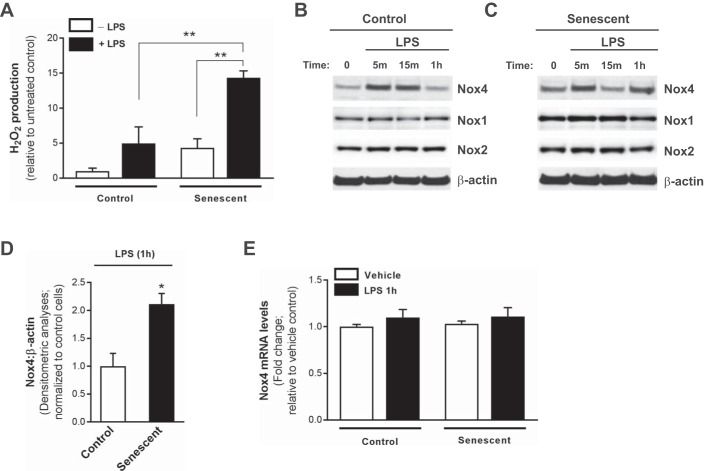
ROS has been increasingly recognized as one of the key substances modulating endothelial dysfunction and disruption responsible for the principle clinical manifestations of the syndrome (7), and we demonstrate that ROS is elevated in the lungs of aged mice, which exhibit increased severity of lung injury. We therefore evaluated LPS-dependent ROS production in control and senescent ECs. Following LPS challenge, senescent ECs demonstrated significantly elevated H2O2 production compared with control cells (Fig. 4A). Previous studies have demonstrated that Nox4, an oxidant-generating enzyme, is induced in ECs following LPS challenge (31); however, Nox4-dependent responses to LPS have not been characterized in senescent ECs. In response to challenge with LPS, Nox4 expression was rapidly induced in control ECs and also in senescent ECs (5–15 min) (Fig. 4, B and C, Western immunoblotting). Although Nox4 induction was transient in control cells (Fig. 4B), senescent ECs demonstrated sustained elevated levels of Nox4 expression at 1 h (Fig. 4, C and D; Western immunoblotting and densitometric analyses). Interestingly, Nox1 and Nox2 expressions levels were not affected by LPS at these early time points (Fig. 4, B and C).These data indicate that impaired permeability responses in senescent ECs are associated with a rapid and sustained upregulation of Nox4 and persistently elevated ROS levels.
Fig. 4.
Increased permeability in senescent ECs is associated with Nox4-mediated redox imbalance. Control and senescent ECs were utilized and cultured ex vivo. A: H2O2 production was evaluated by Amplex red assay at 1 h post-LPS (100 ng/ml). Data was normalized to total number of cells and expressed as fold change relative to untreated control cells. **P < 0.01, using 2-way ANOVA with Bonferroni’s multiple-comparison test. B–D: Nox4, Nox1, Nox2, and β-actin expressions were detected by Western immunoblotting in control (B) and senescent (C) ECs following LPS challenge. D: densitometric analyses of Nox4:β-actin following LPS treatment; *P < 0.05 using Student’s 2-tailed t-test. All data comparing expression in control vs. senescent cells (including representative images shown in B and C) were run on the same gel and were subjected to identical exposure times. E: real-time PCR of Nox4 levels in control and senescent ECs. Values represent means ± SE; n = 3–5.
Aberrant posttranslational regulation of Nox4 in senescent ECs.
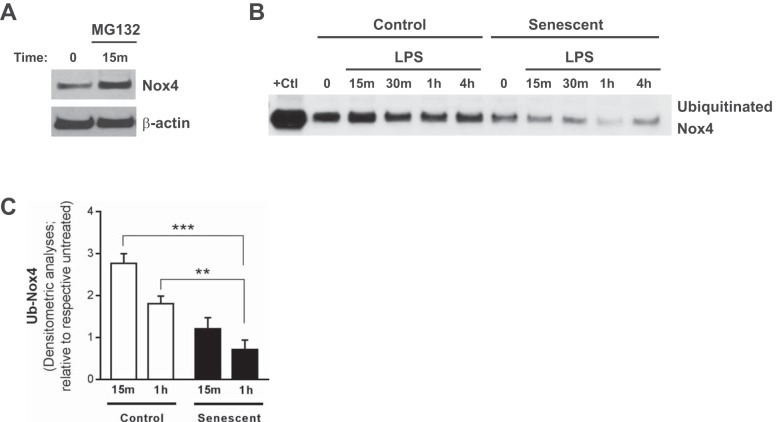
We next investigated mechanism(s) by which Nox4 is rapidly induced in control and senescent ECs. Although Nox4 is primarily regulated at the transcriptional level (16, 17), the rapid induction of Nox4 expression (as early as 5 min post-LPS challenge) in both control and senescent ECs (Fig. 4, B–D) suggested that it may be posttranslationally regulated. Nox4 transcript levels were similar in both control and senescent ECs and were not altered by LPS at this early time point, confirming that the elevated expression of Nox4 1 h post-LPS was not due to transcriptional regulation (Fig. 4E). Nox4 can be controlled by the ubiquitin-proteasomal system in fibroblast cells (11); however, posttranslational regulation of Nox4 has not been previously reported in ECs. To gain further insight into the potential mechanism(s) by which Nox4 is posttranslationally regulated in ECs, we examined Nox4 protein levels in the presence of the ubiquitin-proteasomal inhibitor carbobenzoxy-Leu-Leu-leucinal (MG132). Treatment with MG132 (25 μM, 15 min) led to a rapid increase in Nox4 protein levels (Fig. 5A), indicating that Nox4 may be regulated by the ubiquitin-proteasome system. We next evaluated the levels of ubiquitinated Nox4 in control vs. senescent ECs following LPS challenge. Control cells demonstrated significantly elevated levels of ubiquitinated Nox4 following LPS challenge (2–3-fold increase; Fig. 5, B and C) in contrast to lower levels of ubiquitinated Nox4 in senescent ECs (Fig. 5, B and C; Western immunoblotting and densitometric analyses). Together, these data indicate that senescent ECs exhibit a defect in ubiquitin-proteasome-mediated regulation of Nox4. This mechanism may in part explain the persistent expression of Nox4 in response to LPS challenge in senescent ECs.
Fig. 5.
Posttranslational regulation of Nox4 by the ubiquitin-proteasome pathway is defective in senescent ECs. A: ECs were cultured ex vivo and treated with MG132 (25 μM, 15 min), and Nox4 expression was evaluated by Western immunoblotting. B: control and senescent ECs were treated with LPS (100 ng/ml) for the time points indicated. Ubiquitinated Nox4 in control and senescent ECs following LPS treatment was detected by Western immunoblotting and quantified by densitometric analyses (C). Values represent means ± SE; n = 3; **P < 0.01, ***P < 0.001 using 2-way ANOVA with Bonferroni’s multiple-comparison test.
Pharmacological targeting of Nox4 reduces LPS-induced permeability responses in senescent ECs.
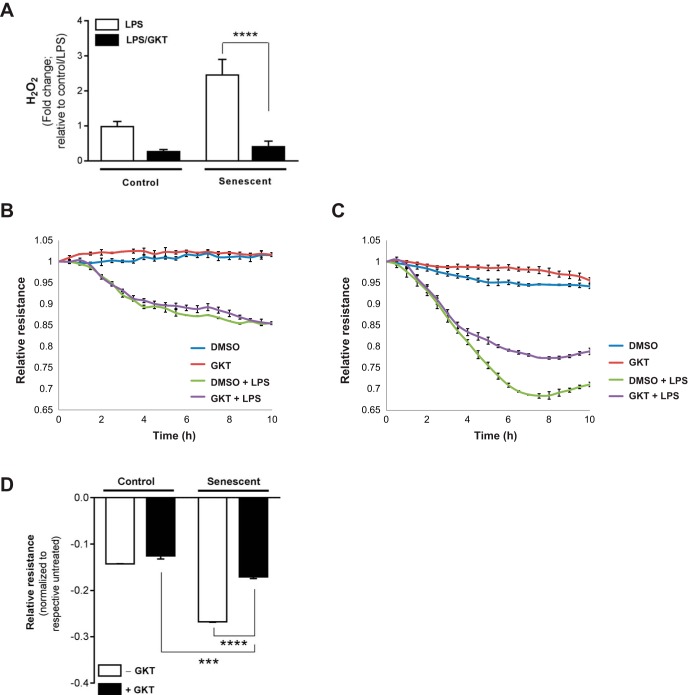
A first-in-class small-molecule inhibitor of Nox1/4 (GKT137831), developed by Genkyotex, was utilized to evaluate the role of Nox4 in mediating endothelial barrier-regulatory responses. Control and senescent cells were treated with both GKT137831 (15 μM) and with or without LPS (200 ng/ml) simultaneously, and we first confirmed that GKT137831 led to inhibition of H2O2 production (Fig. 6A; Amplex red assay). Next, EC barrier-regulatory responses were evaluated by TER for 10 h after GKT137831/LPS treatment. Although pharmacological targeting of Nox4 did not appear to significantly alter permeability responses of control cells (Fig. 6, B and D), Nox4 inhibition by GKT137831 led to significant protection from LPS-induced alterations in permeability in senescent ECs (Fig. 6, C and D).
Fig. 6.
Pharmacological targeting of Nox4 is protective in LPS-induced alterations in EC permeability. Control and senescent ECs were plated in a confluent monolayer in electric cell-substrate impedance sensing (ECIS) array plates coated with 0.1% gelatin. After 24 h, cells were simultaneously treated with GKT137831 (selective Nox1/4 inhibitor; 15 μM) with or without LPS (200 ng/ml). A: H2O2 was evaluated 3 h post-LPS/GKT by Amplex red assay. B–D: TER measurements for control (B) and senescent (C) ECs were obtained for 10 h post-LPS/GKT treatment, and values at each time point were normalized to the initial pretreatment values and plotted as the means ± SE. D: bar graph represents peak differences in resistance of control and senescent ECs, normalized to respective untreated (without LPS) group. ***P < 0.001, ****P < 0.0001 using 2-way ANOVA with Bonferroni’s multiple-comparison test.
DISCUSSION
As the average life expectancy continues to increase, the elderly population is rapidly growing. Between 2015 and 2050, the proportion of the world's population over 60 yr is expected to nearly double (from 12% to 22%). With this shift in the elderly demographic, it has become increasingly important to understand the contribution of aging to disease pathogenesis, risk, and severity. ARDS has many etiologies; however, aging is a uniform and well-established ARDS risk factor (36). Adolescents have lower incidence and mortality from ARDS compared with middle aged and elderly adults, and ARDS mortality directly increases with advancing age (36). However, investigating the mechanistic basis for these epidemiological observations in human ARDS has proven challenging because of the heterogeneity of causes and the paucity of biological samples (biopsies, autopsies, BAL).
Preclinical animal models that can more accurately reproduce the clinical features of ARDS are fundamentally critical to continued investigation of the pathogenesis and to the future development of therapeutic interventions. Several preclinical animal models have been used to investigate the mechanisms of ALI, most of which are based on reproducing a single risk factor, such as pneumonia or sepsis (37), mechanical ventilation (18, 29), or lipid pulmonary embolism secondary to bone fracture (47). However, none of these models fully recapitulate the features of human ARDS, as ARDS is associated with complex interactions between multiple risk factors. Bacterial pneumonia and sepsis are the most common predisposing clinical conditions (25), and LPS, the major cell wall endotoxin of Gram-negative bacteria, is an important mediator of ALI. Thus intratracheal administration of LPS has become a widely used model of ALI (26). However, LPS challenge alone does not cause the severe endothelial and epithelial injury observed in human ARDS (45). Mechanical ventilation often is the only life-saving intervention for critically ill patients with respiratory failure; however, mechanical ventilation is now well known to also contribute directly to lung inflammation and injury, a process known as VILI (1, 12, 26). Combined exposure to LPS and mechanical ventilation challenge synergistically increase neutrophilic alveolitis and alveolar permeability (4, 15, 27). However, a limitation of many present LPS/VILI models is the short duration of these studies (typically killed 4–8 h postinjury) (4, 8, 49).
Another key limitation of many present LPS/VILI models is the overwhelming utilization of young animals. One study demonstrated that adult mice (4 mo) exhibited increased severity of ALI compared with juvenile mice (21 days) (38); however, the synergistic effects of LPS/VILI have not been evaluated in old animals. To understand the contribution of aging to the development of ARDS in a clinically relevant animal model, we utilized an established two-hit preclinical model of ARDS (15, 27) in young (2 mo) and aged (18 mo) mice. This aging mouse model of ARDS has several strengths in addition to being a demographically relevant model. An age-relevant model is in line with patients at risk for ARDS, exploiting the early developmental period of disease, and provides for the ability to assess the role of VILI after intervention with mechanical ventilation, mimicking clinical practice, as the severity of the initial insult often does not equate to immediate respiratory failure requiring mechanical support. Our studies demonstrate that aged mice exhibit increased severity of lung injury that is accompanied by significantly elevated ROS levels in lung BAL; these findings support the concept that synergistic lung inflammatory and injury responses are acquired with advanced age. This model may have important implications for clinical translation of drug candidates, allowing adequate duration to assess therapeutic efficacy and therapeutic targeting of age-associated pathological mechanisms.
Although the complex mechanisms involved in ARDS pathogenesis remain unclear, loss of vascular integrity attributable to EC activation or injury is an essential pathological feature of the inflammatory response in ARDS and key to the profound physiological perturbations that ensue. Increased lung vascular permeability attributable to EC paracellular gap formation is intimately involved with the development of multisystem organ dysfunction involving distal organs, including the kidney, liver, and intestine. Although senescent ECs have been identified in vivo, little is known about the consequences of EC senescence on the barrier-regulatory function of the endothelial monolayer and/or whether EC senescence contributes to the pathogenesis of ARDS. The increased occurrence of senescent cells at the sites of age-associated pathologies, such as atherosclerosis (43), supports a role for senescence in the pathogenesis. The fact that many stimuli produce the senescent phenotype suggests that this phenotype may be a final, common pathway for replicating cells in which signaling or metabolic imbalances occur (9). Previous studies indicate that replicative senescence in vitro is an attractive model of vascular aging in vivo as cells exhibit some features of senescence that are maintained in a controlled environment (10). The presence of replicative senescent human umbilical vein ECs in a nonsenescent monolayer led to disruption of tight junction morphology of surrounding young cells and increased permeability of the monolayer (21). Our studies demonstrate that senescent ECs exhibit impaired barrier-regulatory properties and responses and may provide insight into the mechanisms responsible for increased ARDS severity in vivo. Additionally, the in vitro model of EC senescence described here may provide a valuable tool for high-throughput screening approaches of drug candidates aimed to treat age-associated vascular barrier dysfunction.
There is significant evidence that increased ROS generation in lung ECs during ARDS contributes to loss of barrier integrity (39) with ARDS-relevant stimuli such as TNF and LPS inducing ROS production in ECs. ROS participate in the modulation of cell-signaling pathways that activate key transcription factors, such as NF-κB, that mediate proinflammatory responses (20). LPS directly increases ROS generation in ECs through Nox-dependent modulation (3). Endothelial targeting of a nonselective Nox inhibitor led to significant protection from LPS-induced ALI, whereas nontargeted delivery was less effective (19). These studies implicate endothelial-specific Nox-dependent ROS production in the pathogenesis of ARDS. Mounting evidence also suggests that the Nox4 homolog, rather than Nox2, plays a critical role in ARDS pathogenesis. Nox4 is the most abundantly expressed Nox homolog in ECs, with significantly lower expression levels of Nox2 (2). Nox4 mRNA levels are expressed at 100-fold higher levels than Nox2 in human ECs (42) and are increased in response to hyperoxia (32). Finally, it has been suggested that therapeutic strategies targeting Nox2 would not be favorable for ARDS, as Nox2 inhibition may lead to increased susceptibility to infection (6). Thus we sought to further assess Nox4-dependent mechanisms that mediate barrier-regulatory responses, particularly in the context of EC senescence. Our data indicate that LPS challenge rapidly induces increased Nox4 expression in ECs, with highly divergent responses in control vs. senescent ECs, whereas Nox1 and Nox2 expression levels were not affected by LPS in control or senescent ECs at the same time points. Nox4 is aberrantly regulated in senescent ECs, with persistently elevated expression resulting in redox imbalance and exacerbated permeability responses. The rapid induction of Nox4 levels in both control and senescent ECs further validates findings of its posttranslational regulation by the ubiquitin-proteasomal system. In agreement with previous reports of the regulation of Nox4 by ubiquitination in fibroblasts (11), the present data show an acute increase in ubiquitinated Nox4 after LPS challenge in control ECs. Importantly, ubiquitinated Nox4 levels were lower in senescent vs. control cells following LPS challenge. This finding offers an attractive and novel pathway for therapeutic modulation. Our data indicate that Nox4 is elevated in the lungs of aged/injured mice, and our proof-of-concept studies demonstrate that pharmacological inhibition of Nox4 led to protection from LPS-induced permeability responses in senescent ECs. Nox4 may also contribute to other aspects of the ARDS-associated inflammatory response; Nox4 is known to be involved in LPS-induced NF-κB-dependent IL-8, MCP-1, and ICAM-1 gene expression in human aortic ECs (31). Future studies are warranted to determine whether these signaling pathways are altered in pulmonary EC aging/senescence, when Nox4 is persistently expressed.
Loss of proteostasis is considered to be one of the major hallmarks of aging (23). A number of age-related disorders are associated with inefficiencies in proteasome-ubiquitin signaling (22), and there are remarkable examples of genetic manipulations that improve proteostasis and delay aging in mammals (48). Ubiquitination is a posttranslational modification and a fundamental mechanism of proteostasis that regulates a myriad of cellular functions, with specificity and diversity of ubiquitination outcomes dictated by a hierarchical cascade of ubiquitin conjugation. Dysregulated ubiquitination is involved in the pathogenesis of respiratory lung diseases, including COPD and asthma (41); however, the role of ubiquitination in ARDS remains poorly understood. These studies indicate that senescent ECs exhibit deficient ubiquitination of Nox4, leading to persistent expression and ultimately ROS-mediated barrier-regulatory dysfunction. These studies prompt more intense future evaluation to determine the specific ubiquitin enzymes involved in Nox4 regulation, in both physiological and pathogenic settings. Novel therapeutic modalities could be developed by selectively targeting specific elements of the ubiquitin pathway that modulate Nox4 expression.
The present study indicates that therapeutic strategies aimed at targeting redox imbalance, specifically selective targeting of Nox4 in the pulmonary endothelium, hold promise for the development of ARDS treatments, which corresponds with significantly elevated Nox4 expression levels. There are presently no selective Nox4 inhibitors that are clinically available. The identification of novel drug candidates that selectively target Nox4 and/or the development of transgenic/conditional mice that selectively modulate endothelial Nox4 expression will be instrumental to an improved understanding of the role of Nox4 in ARDS.
GRANTS
This work was supported by the Veterans Administration Health System grant 1IK2BX001477 (L. Hecker) and National Institutes of Health (NIH) grant P01HL126609-01A1 (J. Garcia). Salary support for M. Mohamed was provided by NIH/NHLBI grant no. R25HL108837, “Short-Term Training to Increase the Diversity Pipeline in Heart/Lung/Blood Research.”
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.P., Y.-J.S., H.Q., M.M., S.S., and L.H. performed experiments; S.P., Y.-J.S., K.A., A.A.D., S.S., T.W., J.G.G., and L.H. analyzed data; S.P., Y.-J.S., K.A., A.A.D., B.A.C., T.W., J.G.G., and L.H. interpreted results of experiments; S.P., Y.-J.S., A.K., and L.H. prepared figures; S.P., K.A., A.A.D., and L.H. drafted manuscript; S.P., Y.-J.S., K.A., A.A.D., A.K., B.A.C., T.W., J.G.G., and L.H. edited and revised manuscript; S.P., Y.-J.S., K.A., A.A.D., H.Q., M.M., A.K., B.A.C., T.W., J.G.G., and L.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Genkyotex (Geneva, Switzerland) for providing us with the first-in-class small-molecule inhibitor of Nox1/4, GKT137831 (GKT).
REFERENCES
- 1.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342: 1301–1308, 2000. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 2.Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation 109: 227–233, 2004. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 3.Al Ghouleh I, Magder S. Nicotinamide adenine dinucleotide phosphate (reduced form) oxidase is important for LPS-induced endothelial cell activation. Shock 29: 553–559, 2008. doi: 10.1097/SHK.0b013e318157ebc8. [DOI] [PubMed] [Google Scholar]
- 4.Altemeier WA, Matute-Bello G, Frevert CW, Kawata Y, Kajikawa O, Martin TR, Glenny RW. Mechanical ventilation with moderate tidal volumes synergistically increases lung cytokine response to systemic endotoxin. Am J Physiol Lung Cell Mol Physiol 287: L533–L542, 2004. doi: 10.1152/ajplung.00004.2004. [DOI] [PubMed] [Google Scholar]
- 5.Bunnell E, Pacht ER. Oxidized glutathione is increased in the alveolar fluid of patients with the adult respiratory distress syndrome. Am Rev Respir Dis 148: 1174–1178, 1993. doi: 10.1164/ajrccm/148.5.1174. [DOI] [PubMed] [Google Scholar]
- 6.Carnesecchi S, Pache JC, Barazzone-Argiroffo C. NOX enzymes: Potential target for the treatment of acute lung injury. Cell Mol Life Sci 69: 2373–2385, 2012. doi: 10.1007/s00018-012-1013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chabot F, Mitchell JA, Gutteridge JM, Evans TW. Reactive oxygen species in acute lung injury. Eur Respir J 11: 745–757, 1998. [PubMed] [Google Scholar]
- 8.Cherpanath TG, Smeding L, Hirsch A, Lagrand WK, Schultz MJ, Groeneveld AB. Low tidal volume ventilation ameliorates left ventricular dysfunction in mechanically ventilated rats following LPS-induced lung injury. BMC Anesthesiol 15: 140, 2015. doi: 10.1186/s12871-015-0123-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cristofalo VJ, Beck J, Allen RG. Cell senescence: An evaluation of replicative senescence in culture as a model for cell aging in situ. J Gerontol A Biol Sci Med Sci 58: B776–B779, 2003. doi: 10.1093/gerona/58.9.B776. [DOI] [PubMed] [Google Scholar]
- 10.Cristofalo VJ, Lorenzini A, Allen RG, Torres C, Tresini M. Replicative senescence: A critical review. Mech Ageing Dev 125: 827–848, 2004. doi: 10.1016/j.mad.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Desai LP, Zhou Y, Estrada AV, Ding Q, Cheng G, Collawn JF, Thannickal VJ. Negative regulation of NADPH oxidase 4 by hydrogen peroxide-inducible clone 5 (Hic-5) protein. J Biol Chem 289: 18270–18278, 2014. doi: 10.1074/jbc.M114.562249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dreyfuss D, Saumon G. Ventilator-induced lung injury: Lessons from experimental studies. Am J Respir Crit Care Med 157: 294–323, 1998. doi: 10.1164/ajrccm.157.1.9604014. [DOI] [PubMed] [Google Scholar]
- 13.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol 91: 1487–1500, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 108: 689–701, 2001. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldman JL, Sammani S, Kempf C, Saadat L, Letsiou E, Wang T, Moreno-Vinasco L, Rizzo AN, Fortman JD, Garcia JG. Pleiotropic effects of interleukin-6 in a “two-hit” murine model of acute respiratory distress syndrome. Pulm Circ 4: 280–288, 2014. doi: 10.1086/675991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, Meldrum E, Sanders YY, Thannickal VJ. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med 6: 231ra47, 2014. doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15: 1077–1081, 2009. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong SB, Huang Y, Moreno-Vinasco L, Sammani S, Moitra J, Barnard JW, Ma SF, Mirzapoiazova T, Evenoski C, Reeves RR, Chiang ET, Lang GD, Husain AN, Dudek SM, Jacobson JR, Ye SQ, Lussier YA, Garcia JG. Essential role of pre-B-cell colony enhancing factor in ventilator-induced lung injury. Am J Respir Crit Care Med 178: 605–617, 2008. doi: 10.1164/rccm.200712-1822OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hood ED, Greineder CF, Dodia C, Han J, Mesaros C, Shuvaev VV, Blair IA, Fisher AB, Muzykantov VR. Antioxidant protection by PECAM-targeted delivery of a novel NADPH-oxidase inhibitor to the endothelium in vitro and in vivo. J Control Release 163: 161–169, 2012. doi: 10.1016/j.jconrel.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koay MA, Christman JW, Segal BH, Venkatakrishnan A, Blackwell TR, Holland SM, Blackwell TS. Impaired pulmonary NF-kappaB activation in response to lipopolysaccharide in NADPH oxidase-deficient mice. Infect Immun 69: 5991–5996, 2001. doi: 10.1128/IAI.69.10.5991-5996.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krouwer VJ, Hekking LH, Langelaar-Makkinje M, Regan-Klapisz E, Post JA. Endothelial cell senescence is associated with disrupted cell-cell junctions and increased monolayer permeability. Vasc Cell 4: 12, 2012. doi: 10.1186/2045-824X-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwak MK, Wakabayashi N, Greenlaw JL, Yamamoto M, Kensler TW. Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Mol Cell Biol 23: 8786–8794, 2003. doi: 10.1128/MCB.23.23.8786-8794.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 153: 1194–1217, 2013. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthay MA, Geiser T, Matalon S, Ischiropoulos H. Oxidant-mediated lung injury in the acute respiratory distress syndrome. Crit Care Med 27: 2028–2030, 1999. doi: 10.1097/00003246-199909000-00055. [DOI] [PubMed] [Google Scholar]
- 25.Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA Jr, Hoffman E, Hubmayr RD, Leppert M, Matalon S, Munford R, Parsons P, Slutsky AS, Tracey KJ, Ward P, Gail DB, Harabin AL. Future research directions in acute lung injury: Summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 167: 1027–1035, 2003. doi: 10.1164/rccm.200208-966WS. [DOI] [PubMed] [Google Scholar]
- 26.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L379–L399, 2008. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mekontso Dessap A, Voiriot G, Zhou T, Marcos E, Dudek SM, Jacobson JR, Machado R, Adnot S, Brochard L, Maitre B, Garcia JG. Conflicting physiological and genomic cardiopulmonary effects of recruitment maneuvers in murine acute lung injury. Am J Respir Cell Mol Biol 46: 541–550, 2012. doi: 10.1165/rcmb.2011-0306OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metnitz PG, Bartens C, Fischer M, Fridrich P, Steltzer H, Druml W. Antioxidant status in patients with acute respiratory distress syndrome. Intensive Care Med 25: 180–185, 1999. doi: 10.1007/s001340050813. [DOI] [PubMed] [Google Scholar]
- 29.Moitra J, Evenoski C, Sammani S, Wadgaonkar R, Turner JR, Ma SF, Garcia JG. A transgenic mouse with vascular endothelial over-expression of the non-muscle myosin light chain kinase-2 isoform is susceptible to inflammatory lung injury: Role of sexual dimorphism and age. Transl Res 151: 141–153, 2008. doi: 10.1016/j.trsl.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nanthakumar CB, Hatley RJ, Lemma S, Gauldie J, Marshall RP, Macdonald SJ. Dissecting fibrosis: Therapeutic insights from the small-molecule toolbox. Nat Rev Drug Discov 14: 693–720, 2015. doi: 10.1038/nrd4592. [DOI] [PubMed] [Google Scholar]
- 31.Park HS, Chun JN, Jung HY, Choi C, Bae YS. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc Res 72: 447–455, 2006. doi: 10.1016/j.cardiores.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Pendyala S, Gorshkova IA, Usatyuk PV, He D, Pennathur A, Lambeth JD, Thannickal VJ, Natarajan V. Role of Nox4 and Nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid Redox Signal 11: 747–764, 2009. doi: 10.1089/ars.2008.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med 169: 1245–1251, 2004. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 34.Reddy SP, Hassoun PM, Brower R. Redox imbalance and ventilator-induced lung injury. Antioxid Redox Signal 9: 2003–2012, 2007. doi: 10.1089/ars.2007.1770. [DOI] [PubMed] [Google Scholar]
- 35.Rizzo AN, Sammani S, Esquinca AE, Jacobson JR, Garcia JG, Letsiou E, Dudek SM. Imatinib attenuates inflammation and vascular leak in a clinically relevant two-hit model of acute lung injury. Am J Physiol Lung Cell Mol Physiol 309: L1294–L1304, 2015. doi: 10.1152/ajplung.00031.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693, 2005. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 37.Sammani S, Moreno-Vinasco L, Mirzapoiazova T, Singleton PA, Chiang ET, Evenoski CL, Wang T, Mathew B, Husain A, Moitra J, Sun X, Nunez L, Jacobson JR, Dudek SM, Natarajan V, Garcia JG. Differential effects of sphingosine 1-phosphate receptors on airway and vascular barrier function in the murine lung. Am J Respir Cell Mol Biol 43: 394–402, 2010. doi: 10.1165/rcmb.2009-0223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith LS, Gharib SA, Frevert CW, Martin TR. Effects of age on the synergistic interactions between lipopolysaccharide and mechanical ventilation in mice. Am J Respir Cell Mol Biol 43: 475–486, 2010. doi: 10.1165/rcmb.2009-0039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tasaka S, Amaya F, Hashimoto S, Ishizaka A. Roles of oxidants and redox signaling in the pathogenesis of acute respiratory distress syndrome. Antioxid Redox Signal 10: 739–753, 2008. doi: 10.1089/ars.2007.1940. [DOI] [PubMed] [Google Scholar]
- 40.Thannickal VJ, Zhou Y, Gaggar A, Duncan SR. Fibrosis: Ultimate and proximate causes. J Clin Invest 124: 4673–4677, 2014. doi: 10.1172/JCI74368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vadász I, Weiss CH, Sznajder JI. Ubiquitination and proteolysis in acute lung injury. Chest 141: 763–771, 2012. doi: 10.1378/chest.11-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal 7: 308–317, 2005. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 43.Vasile E, Tomita Y, Brown LF, Kocher O, Dvorak HF. Differential expression of thymosin β-10 by early passage and senescent vascular endothelium is modulated by VPF/VEGF: Evidence for senescent endothelial cells in vivo at sites of atherosclerosis. FASEB J 15: 458–466, 2001. doi: 10.1096/fj.00-0051com. [DOI] [PubMed] [Google Scholar]
- 44.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 45.Wiener-Kronish JP, Albertine KH, Matthay MA. Differential responses of the endothelial and epithelial barriers of the lung in sheep to Escherichia coli endotoxin. J Clin Invest 88: 864–875, 1991. doi: 10.1172/JCI115388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson JN, Pierce JD, Clancy RL. Reactive oxygen species in acute respiratory distress syndrome. Heart Lung 30: 370–375, 2001. doi: 10.1067/mhl.2001.118298. [DOI] [PubMed] [Google Scholar]
- 47.Xiang L, Lu S, Mittwede PN, Clemmer JS, Hester RL. Inhibition of NADPH oxidase prevents acute lung injury in obese rats following severe trauma. Am J Physiol Heart Circ Physiol 306: H684–H689, 2014. doi: 10.1152/ajpheart.00868.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang C, Cuervo AM. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med 14: 959–965, 2008. doi: 10.1038/nm.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Gao J, Wang CJ, Zhou LJ, Fang XZ, Yang LQ. Low tidal volume ventilation preconditioning ameliorates lipopolysaccharide-induced acute lung injury in rats. Acta Anaesthesiol Scand 60: 780–789, 2016. doi: 10.1111/aas.12691. [DOI] [PubMed] [Google Scholar]