Abstract
Capsaicin is an active component of chili pepper and a pain relief drug. Capsaicin can activate transient receptor potential vanilloid 1 (TRPV1) channels to increase cytosolic Ca2+ concentration ([Ca2+]cyt). A rise in [Ca2+]cyt in pulmonary artery smooth muscle cells (PASMCs) is an important stimulus for pulmonary vasoconstriction and vascular remodeling. In this study, we observed that a capsaicin-induced increase in [Ca2+]cyt was significantly enhanced in PASMCs from patients with idiopathic pulmonary arterial hypertension (IPAH) compared with normal PASMCs from healthy donors. In addition, the protein expression level of TRPV1 in IPAH PASMCs was greater than in normal PASMCs. Increasing the temperature from 23 to 43°C, or decreasing the extracellular pH value from 7.4 to 5.9 enhanced capsaicin-induced increases in [Ca2+]cyt; the acidity (pH 5.9)- and heat (43°C)-mediated enhancement of capsaicin-induced [Ca2+]cyt increases were greater in IPAH PASMCs than in normal PASMCs. Decreasing the extracellular osmotic pressure from 310 to 200 mOsmol/l also increased [Ca2+]cyt, and the hypo-osmolarity-induced rise in [Ca2+]cyt was greater in IPAH PASMCs than in healthy PASMCs. Inhibition of TRPV1 (with 5′-IRTX or capsazepine) or knockdown of TRPV1 (with short hairpin RNA) attenuated capsaicin-, acidity-, and osmotic stretch-mediated [Ca2+]cyt increases in IPAH PASMCs. Capsaicin induced phosphorylation of CREB by raising [Ca2+]cyt, and capsaicin-induced CREB phosphorylation were significantly enhanced in IPAH PASMCs compared with normal PASMCs. Pharmacological inhibition and knockdown of TRPV1 attenuated IPAH PASMC proliferation. Taken together, the capsaicin-mediated [Ca2+]cyt increase due to upregulated TRPV1 may be a critical pathogenic mechanism that contributes to augmented Ca2+ influx and excessive PASMC proliferation in patients with IPAH.
Keywords: capsaicin, TRPV1, idiopathic pulmonary arterial hypertension, osmotic pressure, mechanosensitive cation channels
idiopathic pulmonary arterial hypertension (IPAH) is a fatal and progressive pulmonary vascular disease that predominantly affects women. In patients with IPAH, the increased pulmonary arterial pressure is caused primarily by increased pulmonary vascular resistance (PVR), whereas cardiac output (CO) is often maintained in the normal range. Regardless of the initial genetic and acquired triggers, elevated PVR is primarily caused by four pathophysiological and pathological changes in the pulmonary vasculature: 1) sustained pulmonary vasoconstriction due to pulmonary arterial smooth muscle cell (PASMC) contraction; 2) concentric pulmonary vascular remodeling due to increased PASMC proliferation, growth, and migration; 3) in situ thrombosis in small pulmonary arteries and asteroids; and 4) enhanced pulmonary vascular wall stiffness and/or myogenic tone due to myofibroblast recruitment, extracellular matrix remodeling, intracellular cytoskeleton disorganization in PASMCs, pulmonary vascular fibroblasts, and endothelial cells.
Intracellular Ca2+ signaling is involved in stimulating cell contraction, migration, and proliferation (6, 16, 31, 37). Enhanced Ca2+ signaling has been observed in PASMCs from patients with IPAH and is in part due to increased Ca2+ influx through upregulated Ca2+-permeable channels in the plasma membrane. An increase in cytosolic free Ca2+ concentration ([Ca2+]cyt) in PASMCs is a major trigger for pulmonary vasoconstriction. A rise in [Ca2+]cyt in PASMCs also activates Ca2+-sensitive signaling cascades via calmodulin and stimulates cell proliferation by promoting quiescent cells to enter the cell cycle and propelling proliferating cells to go through the cell cycle (17, 36). There are several known Ca2+-sensitive signal transduction proteins and transcription factors that are involved in normal and pathological cell proliferation (e.g., CREB, NFAT, MLCK, NF-κB, RhoA-ROCK). Thus, increased Ca2+ signaling in PASMCs contributes to the development and progression of pulmonary arterial hypertension (PAH) by triggering sustained pulmonary vasoconstriction and by inducing pulmonary vascular wall thickening via increasing PASMC proliferation and migration.
In PASMCs, [Ca2+]cyt is increased by Ca2+ release from intracellular stores (e.g., sarcoplasmic reticulum) and Ca2+ influx through Ca2+-permeable channels in the plasma membrane. At least three families of Ca2+-permeable channels are functionally expressed in PASMCs and are responsible for agonist- or ligand-induced Ca2+ influx: 1) voltage-dependent Ca2+ channels that are activated by membrane depolarization and blocked by dihydropyridines (e.g., nifedipine, nicardipine, and verapamil); 2) receptor-operated Ca2+ channels (ROC) that are activated by phospholipase C and diacylglycerol; and 3) store-operated Ca2+ channels (SOC) that are activated by active and passive depletion of Ca2+ from the intracellular stores, such as sarcoplasmic reticulum or endoplasmic reticulum.
Transient receptor potential (TRP) channels are a family of voltage-independent cation channels that contribute to forming ROC and SOC in PASMCs (6, 12). The TRP superfamily is classified into six main subfamilies: TRPC (canonical), TRPV (vanilloid), TRPA (ankyrin), TRPM (melastatin), TRPP (polycystin), and TRPML (mucolipin). Expression of the TRP vanilloid receptor 1 (TRPV1) was observed in human PASMCs and is associated with hypoxia-induced cell proliferation (35). In rat PASMCs, TRPV1 was shown to be involved in hypoxia-induced cell migration, which is due to TRPV1-mediated cytoskeleton reorganization (16, 19). TRPV1 is a nonselective cation channel with a preference for Na+ and Ca2+ and is activated by noxious stimuli, heat (>43°C), protons (or acidic conditions in which pH < 5.9), and various natural products (e.g., capsaicin) (2). TRPV1 is well known for its role in temperature sensing and nociception in sensory neurons (2, 5). However, little is known about the role of capsaicin, temperature, and pH in the activation of TRPV1 in PASMCs. In this report, we investigate the role of TRPV1 in capsaicin-induced Ca2+ signaling in PASMCs from patients with IPAH.
MATERIALS AND METHODS
Cell culture.
Human PASMCs were isolated from explanted lung tissue of healthy subjects [three unsuitable organ donors (n = 3)]: donor 1: age 54, female, Caucasian; donor 2: age 28, female, Caucasian; and donor 3: age 30, male, Caucasian; and six patients with IPAH [diagnosed on the basis of the National Institutes of Health IPAH Registry with an average mean pulmonary arterial pressure of 56 ± 5 mmHg (n = 6)]: patient 1: age 33, female, Caucasian; patient 2: age 32, female, Caucasian; patient 3: age 50, female, Caucasian; no demographic information for patients 4, 5, and 6 is available. PASMCs isolated from healthy subjects (i.e., normal PASMCs) or patients with IPAH were primary cells and were authenticated through positive cell staining for smooth muscle α-actin and negative cell staining for von Willebrand (factor VIII antigen) factor. Approval to use human cells was granted by the University of Arizona Institutional Review Board. Human PASMCs were maintained in a humidified atmosphere at 37°C and 5% CO2 with Medium 199 (Invitrogen, Grand Island, NY) containing 10% FBS (Invitrogen), 25 mg/l of d-valine (Sigma-Aldrich, St. Louis, MO), 100 IU/ml penicillin, 100 μg/ml streptomycin (Sigma-Aldrich), and 20 μg/ml cell growth supplement (BD Biosciences, Franklin Lakes, NJ). Cells at passages 5–8 were used for these studies.
Measurement of [Ca2+]cyt.
Human PASMCs from normal donors and patients with IPAH were grown at 50–60% confluence on 25-mm-diameter circular glass coverslips. Cells were incubated with 4 μM fura-2 acetoxymethyl ester (fura-2/AM; Invitrogen/Molecular Probes, Eugene, OR) in HEPES-buffered solution for 60 min at room temperature (22–24°C). Cells loaded with fura-2/AM were alternatively illuminated at 340 and 380 nM wavelengths by a xenon lamp (Hamamatsu Photonics, Hamamatsu, Japan) connected to an inverted fluorescent microscope (Eclipse Ti-E; Nikon, Tokyo, Japan). The fluorescence emissions (520 nM) were captured with an EM-CC camera (Evolve; Photometrics, Tucson, AZ) and analyzed using NIS Elements 3.2 software (Nikon). [Ca2+]cyt is expressed as 340/380 fluorescence ratio within an area of interest in a cell recorded every 2 s. In some experiments, the 340/380 ratio was used to calculate the [Ca2+]cyt in nanomolar concentration. [Ca2+]cyt was calculated using the following equation: [Ca2+]cyt = Kd × (Sf2/Sf1) × (R−Rmin)/(Rmax−R). Kd (225 nM) is the dissociation constant of the Ca2+-fura-2 complex; and Sf2 and Sf1 and Rmin and Rmax were calculated using a standard protocol (7). The HEPES-buffered solution contained (in mM) 137 NaCl, 5.9 KCl, 1.8 CaCl2, 1.2 MgCl2, 14 glucose, and 10 HEPES (pH was adjusted to 7.4 with 10 N NaOH). The Ca2+-free solution was prepared by replacing 1.8 mM CaCl2 with equimolar MgCl2 and adding 0.1 mM EGTA to chelate residual Ca2+. All experiments for measurement of [Ca2+]cyt were carried out at room temperature (22–24°C).
Western blot.
Human PASMCs from normal donors and patients were lysed in 1× RIPA buffer (Bio-Rad, Hercules, CA) supplemented with protease inhibitor cocktail (Roche, Germany). The lysates were centrifuged at 12,000 revolutions per minute for 15 min at 4°C, and the supernatant was collected and subjected to SDS-polyacrylamide gel electrophoresis. Antibodies specifically recognizing TRPV1 (Novus, Littleton, CO), cAMP response element-binding protein (CREB; Santa Cruz Biotechnology, Dallas, TX), and phosphorylated CREB (p-CREB, Santa Cruz Biotechnology) were used at dilutions of 1:1,000. Signals were detected using a Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific, Waltham, MA). The protein levels of TRPV1, CREB, and p-CREB were normalized to those of β-actin (antibody: 1:1,000; Santa Cruz Biotechnology) and expressed in arbitrary units.
RT-PCR and real-time RT-PCR.
Total RNA was isolated from human PASMCs of donors and patients by the TRIzol method. The extracted RNA was quantified by NanoDrop 1000 (Thermo Scientific). RNA was converted into first-strand cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Semiquantitative RT-PCR was carried out using PCR Nucleotide Mix (Roche, Germany) with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the internal control on a Thermal Cycler T100 (Bio-Rad). The PCR products were electrophoresed through 1.5% agarose gel, and amplified cDNA bands were visualized using Photo Imager (VWR, Radnor, PA). Quantitative RT-PCR was performed on a Bio-Rad CFX96 real-time PCR detection system using an iTaq Universal SYBR Green Supermix (Bio-Rad). The relative expression levels of TRPV1 were normalized to the amount of GAPDH mRNA in the same RNA extract and determined by calculating the ΔΔCt value.
Single-channel current measurement in PASMCs.
Single-channel currents were recorded under the cell-attached configuration to prevent channel rundown. A high-K+ (140 mM) extracellular solution was used to set the cell membrane potential to ~0 mV. Capsaicin was applied via the patch pipette to prevent activation of other channels on the membrane, thereby minimizing the probability of acquiring noise during the experiments (28). Recordings were obtained at room temperature (20–25°C) using an Axopatch 200B amplifier, and Digidata 1440A interface (Molecular Devices, Foster City, CA). Command-voltage protocols and data acquisition were performed using pCLAMP 10.5 software (Molecular Devices). Pipettes were fabricated from borosilicate glass using a P-97 Flaming/Brown Micropipette puller (Sutter Instruments, Novato, CA). Pipette tips were fire-polished with an MF-830 Microforge (Narishige, Japan) before use to improve gigaseal formation. When backfilled with high-Na+ (140 mM) extracellular pipette solution, the pipette resistance was 8–15 MΩ. Cell-attached single-channel recordings were performed using a bath solution containing (in mM) 140 K-gluconate, 2.5 KCl, 1 MgCl2, 5 HEPES, and 1.5 EGTA, adjusted to pH 7.4 with KOH, and a pipette extracellular solution containing (in mM) 140 Na-gluconate, 10 NaCl, 1 MgCl2, 5 HEPES, and 1.5 EGTA, adjusted to pH 7.2 with NaOH. For some experiments, an inhibitor cocktail composed of 1 µM paxilline, 100 nM apamin, 2 mM tetraethylammonium (TEA), and 50 µM niflumic acid (NFA) was added to the internal pipette solution to block large- and small conductance Ca2+-activated K+, voltage-gated K+ and Cl− currents, respectively. Data were collected at a filtering frequency of 2 kHz and sampling frequency of 50 kHz.
Osmotic stretch calculation.
We employed external solutions of different osmolarity that were measured with a cryoscopic osmometer (Gonotec, Berlin, Germany). To examine the effect of osmotic stretch of cell membrane on Ca2+ influx through stretch mechano-sensitive cation channel in PASMCs, the isotonic bath solution used in the experiments contained (in mM) 90 NaCl, 5 KCl, 1.3 MgCl2, 2.4 CaCl2, 10 HEPES, and 10 D-glucose, with pH adjusted to 7.4 with NaOH. Mannitol (100 mM) was added to maintain the osmolarity constant at 310 mOsml/kg, whereas the hypo-osmotic solution (200 mOsml/kg) was prepared by omitting mannitol.
Transfection of shRNA and siRNA.
PASMCs were transiently transfected with scramble pGFP-C-shLenti Vector (1 μg, ORIGENE) and human TRPV1 short hairpin RNA (shRNA) constructs in lentiviral green fluorescent protein (GFP) vector (1 μg, ORIGENE) using X-tremeGENE 9 DNA Transfection Reagent (Roche, Germany). [Ca2+]cyt measurement and Western blot using plasmid-transfected cells were performed 48 h after transfection. PASMCs were transiently transfected with scramble silent interfering RNA (siRNA) (80 pM, Santa Cruz Biotechnology) and TRPV1 siRNA (40 and 60 pM, Santa Cruz Biotechnology) using X-tremeGENE siRNA Transfection Reagent (Roche, Germany) for 48 h, after then cell counting and Western blot were performed.
5-Ethynyl-2′-deoxyuridine cell proliferation assay.
PASMCs from donors and patients were grown on 12-mm-diameter coverslips at a density of 4 × 104. Cells were incubated with 10 µM 5-ethynyl-2′-deoxyuridine (EdU) (Life Technologies, Eugene, OR) at 37°C for 24 h either in the presence or absence of the TRPV1 inhibitor capsazepine (CPZ; Tocris Bioscience, Bristol, UK). Cells were fixed in 10% formalin solution (Sigma-Aldrich) and permeabilized with 0.5% Triton-X (Sigma-Aldrich). Detection of EdU incorporation into newly synthesized DNA was performed using a Click-iT EdU Alexa Fluor 594 imaging kit (ThermoFisher Scientific). Hoescht 32333 (1:2,000) was used for staining all nuclear DNA. Stained coverslips were mounted to glass slides using ProLong Diamond Antifade Mountant (Life Technologies). All reagents were prepared in accordance with manufacturers’ instructions. Cells were imaged with a Zeiss AxioObserver.Z1 fluorescence microscope (Carl Zeiss Microscopy, Jena, Germany). Six fields on each coverslip were selected at random for analysis.
Drugs and chemicals.
Capsaicin (Alomone Laboratories, Jerusalem, Israel) was prepared as concentrated stock solution in ethanol. 5′-Iodoresiniferatoxin (5′-IRTX; Tocris), CPZ, BAPTA-AM, and cyclopiazonic acid (CPA) were prepared as stock solutions in DMSO. La3+ and diltiazem were dissolved in distilled water as stock solutions. All stock solutions (in water, ethanol, or DMSO) were aliquoted and kept frozen at −20°C until use. All drugs were from Sigma-Aldrich, unless otherwise indicated.
Statistical analysis.
Data are expressed as means ± SE. Statistical significance between two or among multiple groups was examined using a Student’s t-test or Scheffé test after one-way ANOVA, respectively. Significant difference is expressed in the figures as *P < 0.05, **P < 0.01, and ***P < 0.001; or #P < 0.05, ##P < 0.01, and ###P < 0.001.
RESULTS
Capsaicin-induced increase in [Ca2+]cyt is significantly enhanced in IPAH PASMCs compared with healthy PASMCs.
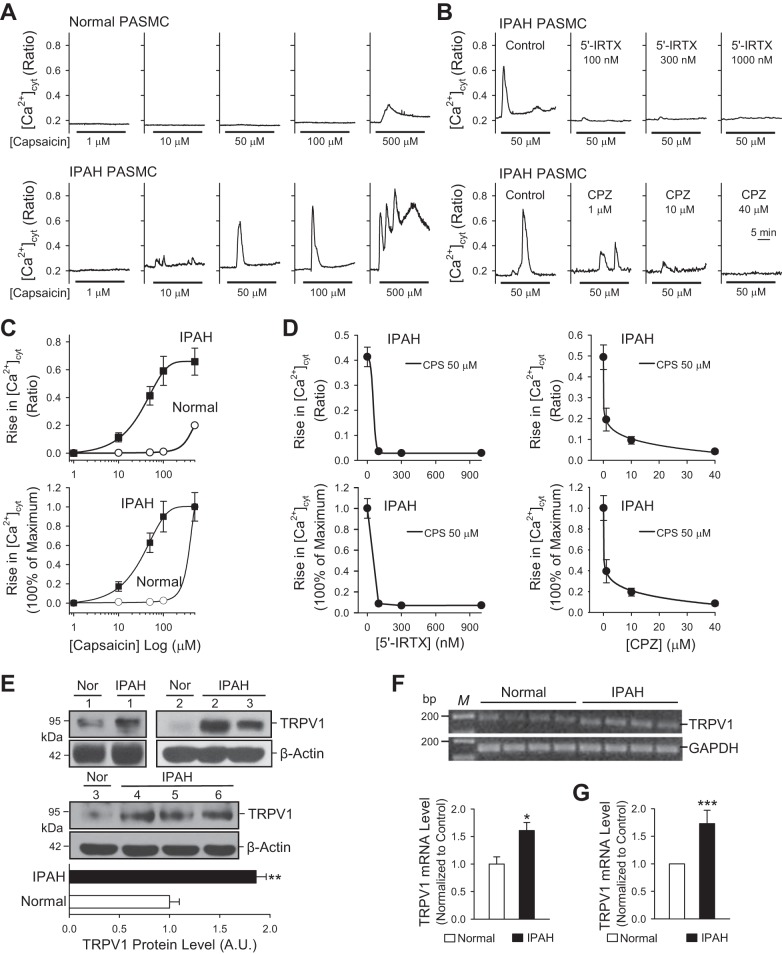
To examine whether a capsaicin-mediated increase in [Ca2+]cyt due to Ca2+ influx through TRPV1 channels is enhanced in PASMCs from patients with IPAH, we compared PASMCs from normal donors and patients with IPAH. PASMCs were cultured for the same amount of time and were used at the same passage number for all experiments. Extracellular application of capsaicin (1, 10, 50, 100, and 500 μM) resulted in a dose-dependent increase in [Ca2+]cyt in PASMCs from donor subjects and patients with IPAH (Fig. 1A). PASMCs from normal donors responded to capsaicin only at a large dose of 500 μM. The capsaicin-induced increase in [Ca2+]cyt was composed of two kinetically distinct components: a transient increase followed by a sustained and/or oscillatory increase (Fig. 1A). The amplitudes of both the transient and sustained increases were significantly higher in PASMCs from patients with IPAH than from normal donors (Fig. 1, A and C). The dose-response curve for PASMCs from patients with IPAH was shifted to the left compared with the curve for normal PASMCs from donors (Fig. 1C, bottom); the estimated EC50 of capsaicin for normal PASMCs was 275 µM, whereas the EC50 for PASMCs from patients with IPAH was ~35 µM (Fig. 1C, bottom). These data indicate that IPAH PASMCs are more sensitive to capsaicin than healthy PASMCs.
Fig. 1.
Capsaicin-induced increase in [Ca2+]cyt is significantly enhanced in pulmonary artery smooth muscle cells (PASMCs) from patients with idiopathic pulmonary arterial hypertension (IPAH). A: representative traces showing changes in [Ca2+]cyt in normal (top) and IPAH (bottom) PASMCs before and during extracellular application of 1, 10, 50, 100, and 500 μM capsaicin. B: representative traces showing changes in [Ca2+]cyt in IPAH PASMCs in response to 50 μM capsaicin in the absence (control) or presence of 100, 300, and 1,000 nM 5′-iodoresiniferatoxin (5′-IRTX) (top) and 1, 10, 40 μM capsazepine (CPZ), the potent antagonists of TRPV1 channels (bottom). C: summarized data (means ± SE) showing the actual (top) and normalized (bottom) dose-response curves of capsaicin-induced increases in [Ca2+]cyt in normal (white circles) and IPAH (black squares) PASMCs (n = 120–150 cells, 3–5 separate experiments). D: summarized data (means ± SE) showing the actual (top) and normalized (bottom) dose-response curves of 5′-IRTX-induced (left) and CPZ-induced (right) inhibition of increases in [Ca2+]cyt in IPAH PASMCs induced by 50 μM capsaicin (n = 90 to 120 cells, 3 separate experiments). E: representative images (top) and summarized data (means ± SE, bottom) showing Western blot analysis of TRPV1 in PASMCs from three normal donor subjects (n = 3) and six patients with IPAH (n = 6). F and G: regular (F) and real-time (G) RT-PCR analyses of TRPV1 in PASMCs from three normal donor subjects (n = 3) and six patients with IPAH (n = 6). *P < 0.05 and ***P < 0.001 compared with normal PASMCs.
Extracellular application of 5′-IRTX, a specific and potent antagonist of the TRPV1 channel, resulted in abolition of capsaicin-mediated increases in [Ca2+]cyt in IPAH PASMCs (Fig. 1, B and D). The inhibitory effect of 5′-IRTX was dose-independent; 100, 300, and 1,000 nM 5′-IRTX all caused 95% inhibition of the capsaicin-induced increase in [Ca2+]cyt in IPAH PASMCs (Fig. 1D). Capsazepine (CPZ), another TRPV1-specific blocker, caused a dose-dependent attenuation of capsaicin-induced increase in [Ca2+]cyt in IPAH PASMCs; 1, 10, and 40 μM CPZ inhibited the increase in [Ca2+]cyt by 61, 81, and 92%, respectively (Fig. 1, B and D). These data indicate that a capsaicin-mediated increase in [Ca2+]cyt in PASMCs is primarily a result of Ca2+ influx through 5′-IRTX- and CPZ-sensitive TRPV1 channels, and that capsaicin-induced Ca2+ entry through TRPV1 channels is greater in IPAH PASMCs than in normal PASMCs. To examine whether mRNA or protein expression levels of TRPV1 were increased in IPAH PASMCs compared with normal PASMCs, we conducted Western blot and regular and real-time RT-PCR experiments. Consistent with the functional experiments shown in Fig. 1, A–D, the Western blot and RT-PCR experiments showed that protein (Fig. 1E) and mRNA (Fig. 1, F and G) expression levels of TRPV1 were significantly higher in IPAH PASMCs (tested in PASMCs isolated from six patients) than in normal PASMCs (tested in PASMCs isolated from three donors). These data suggest that the enhanced capsaicin-induced increase in [Ca2+]cyt in IPAH PASMCs was likely due to upregulated expression of TRPV1.
Characterization of capsaicin-induced TRPV1 currents based on unitary conductance.
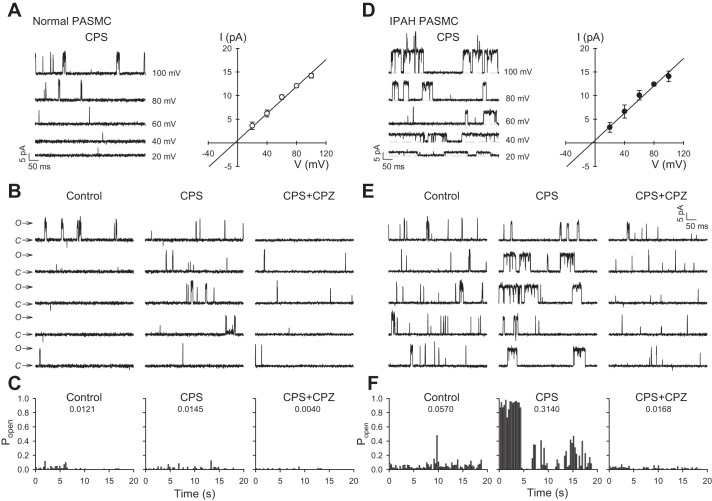
We used the patch clamp technique to measure capsaicin-induced single-channel currents in PASMCs in cell-attached mode. All recordings were carried out in Ca2+-free solutions to avoid Ca2+-induced desensitization (3). Cells were superfused with a high-K+ bath solution to artificially set the membrane potential to ~0 mV. When the pipette extracellular solution contained 1 µM capsaicin, major channel openings were observed in both healthy and IPAH PASMCs, although channel activity in IPAH PASMCs was noticeably enhanced (Fig. 2, A and D, left). The slope conductance of the unitary current was calculated from the I-V relationship curve. The calculated slope conductance for these capsaicin-sensitive currents was 143 and 144 pS, with reversal potential of −3.49 and −3.57 mV for normal and IPAH PASMCs, respectively (Fig. 2, A and D, right). Capsaicin did not appreciably increase the open probability (Popen) of single-channel currents in normal PASMCs compared with cells treated with vehicle or the TRPV1 selective antagonist CPZ (10 µM) (Popen = 0.0121, 0.0145, and 0.0040 for control, CPS, and CPS+CPZ, respectively) (Fig. 2, B and C). In contrast, capsaicin-activated single-channel currents displayed an approximately sixfold increase in Popen in IPAH PASMCs compared with vehicle (Popen = 0.0570 and 0.3140 for control and CPS, respectively) (Fig. 2, E and F). In addition, CPZ markedly decreased Popen of capsaicin-sensitive single-channel currents in IPAH PASMCs (Popen = 0.0168) (Fig. 2, E and F).
Fig. 2.
Effects of capsaicin on single-channel activity in membrane patches of human PASMCs. A and D: representative current records for normal PASMCs and IPAH PASMCs in a cell-attached membrane patch at various test potentials ranging from +20 to +100 mV in 20-mV steps (A and D, left). Composite current-voltage relationship (I-V curve) indicates the slope conductance of this capsaicin-sensitive channel is 143 pS for normal PASMCs and 144 pS for IPAH PASMCs (A and D, right). B and E: representative unitary currents in a cell-attached membrane patch elicited by a sustained depolarization at +80 mV for normal PASMCs (B) and IPAH PASMCs (E) exposed to vehicle (control), 1 µM capsaicin (CPS), or 1 µM capsaicin plus 10 µM capsazepine (CPS+CPZ). C and F: bar graphs showing the steady-state open probability [P(o)] of normal PASMCs (C) and IPAH PASMCs (F) as a function of time for the sample recordings in B and E, respectively. n = 4–6 cells per group.
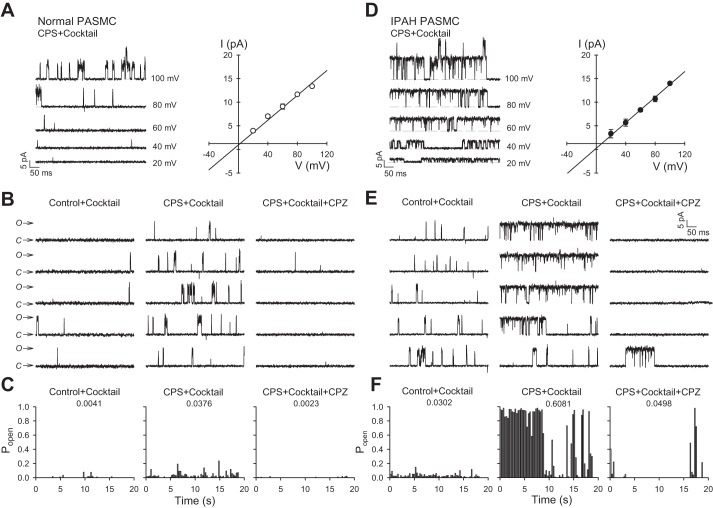
To verify the capsaicin-sensitive single-channel currents were not influenced by Ca2+-activated K+ current, voltage-gated K+ current, or Cl− currents, we repeated these series of experiments by adding to the pipette an inhibitor cocktail containing 1 µM paxilline, 100 nM apamin, 2 mM TEA, and 50 µM NFA. Our data show that even in the presence of the inhibitors of K+ channel (paxilline and apamin) and Cl− channel (tetraethylammonium and niflumic acid), capsaicin (1 µM) was capable of evoking single-channel currents in both normal and IPAH PASMCs (Fig. 3, A and D, left). The calculated slope conductance of the capsaicin-sensitive currents was 131 and 135 pS, with reversal potential of −7.15 and −1.77 mV for normal and IPAH PASMCs, respectively (Fig. 3, A and D, right). In normal PASMCs, Popen of capsaicin-treated cells was considerably increased compared with basal condition or the CPZ-treated group (Popen = 0.0041, 0.0376, and 0.0023 for Control+Cocktail, CPS+Cocktail, and CPS+Cocktail+CPZ, respectively) (Fig. 3, B and C). For IPAH PASMCs, capsaicin-activated single-channel currents were significantly enhanced as indicated by the elevated steady-state open probability compared with vehicle (Popen = 0.0302, 0.6081 for control and CPS, respectively) (Fig. 3, E and F). In addition, capsazepine markedly decreased Popen for capsaicin-sensitive single-channel currents in IPAH PASMCs (Popen = 0.0498) (Fig. 3, E and F). Although Popen for inhibitor cocktail-treated IPAH PASMCS was considerably small (Popen = 0.1254) compared with IPAH PASMCs from Fig. 2, E and F (Popen = 0.3055), there was still an approximately sixfold increase in single-channel activity than its respective group. Something of note is that normal PASMCs displayed increased sensitivity to capsaicin when the inhibitor cocktail was applied to the pipette solution, as indicated by the sixfold increase in channel activation. The mechanisms of action for these ion channel inhibitors are poorly understood. However, there is evidence to suggest that TEA can activate protein kinase C (PKC). Ramakers et al. (23) found that TEA not only induces long-term potentiation in rat hippocampus, but it also increases phosphorylation of pre- and postsynaptic PKC substrate. Therefore, it is possible that TEA can activate PKC, thereby leading to inhibition of Ca2+-activated and voltage-dependent K+ channels. This also could explain the increased sensitivity observed in PASMCs when the inhibitor cocktail was applied (e.g., phosphorylation of Ser502 and Ser801 by PKC has been shown to potentiate the activation of TRPV1 single-channel current) (28). Overall, these data suggest that upregulated expression of TRPV1 channels is associated with the increased Popen of capsaicin-activated single-channel currents in IPAH PASMCs compared with normal PASMCs.
Fig. 3.
K+ and Cl- channels do not contribute to the capsaicin-mediated activation of single-channel currents in membrane patches of human PASMCs. Inhibitor cocktail (IC) comprised of 1 µM paxilline, 100 nM apamin, 2 mM tetraethylammonium (TEA), and 50 µM niflumic acid (NFA) were added to the pipette A: representative current records for normal PASMCs and IPAH PASMCs in a cell-attached membrane patch at various test potentials ranging from +20 to +100 mV in 20 mV steps (A and D, left). Composite current-voltage relationship (I-V curve) indicates the slope conductance of this capsaicin-sensitive channel is 131 pS for normal PASMC and 135 pS for IPAH PASMCs (A and D, right). B and E: representative unitary currents in a cell attached membrane patch elicited by a sustained depolarization at +80 mV for normal PASMCs (B) and IPAH PASMCs (E) exposed to vehicle (control+cocktail), 1 µM capsaicin (CPS+cocktail) or 1 µM capsaicin plus 10 µM capsazepine (CPZ) plus inhibitor cocktail (CPS+cocktail+CPZ). C and F: bar graphs showing the steady-state open probability [P(o)] of normal PASMCs (C) and IPAH PASMCs (F) as a function of time for the sample recordings in B and E, respectively. n = 3–4 cells per group.
Capsaicin-induced [Ca2+]cyt increase is dependent on extracellular Ca2+ and regulated by extracellular pH and temperature.
Removal of extracellular Ca2+ abolished the capsaicin-induced increase in [Ca2+]cyt in both normal and IPAH PASMCs. As shown in Fig. 4, extracellular application of a large dose of capsaicin (500 µM) in the presence of 1.8 mM extracellular Ca2+ increased [Ca2+]cyt in normal PASMCs that were tested; however, 500 µM capsaicin had no effect on [Ca2+]cyt in the absence of extracellular Ca2+ (0 mM Ca) (Fig. 4, A, C, and E). In IPAH PASMCs, a lower dose of capsaicin (100 µM) resulted in significant increases in [Ca2+]cyt in the presence of 1.8 mM extracellular Ca2+; the amplitude of 100 µM capsaicin-induced rises in [Ca2+]cyt in IPAH PASMCs was much greater than the amplitude of 500 µM capsaicin-induced increases in normal PASMCs (Fig. 4, B, D, and F). Removal of extracellular Ca2+ (0 mM Ca) also abolished capsaicin-induced increases in [Ca2+]cyt in IPAH PASMCs (Fig. 4, B, D, and F). Taken together with the inhibitory effect of 5′-IRTX on the capsaicin-induced rise in [Ca2+]cyt, our data support the conclusion that capsaicin-induced [Ca2+]cyt increases are due to Ca2+ influx through TRPV1 channels in PASMCs.
Fig. 4.
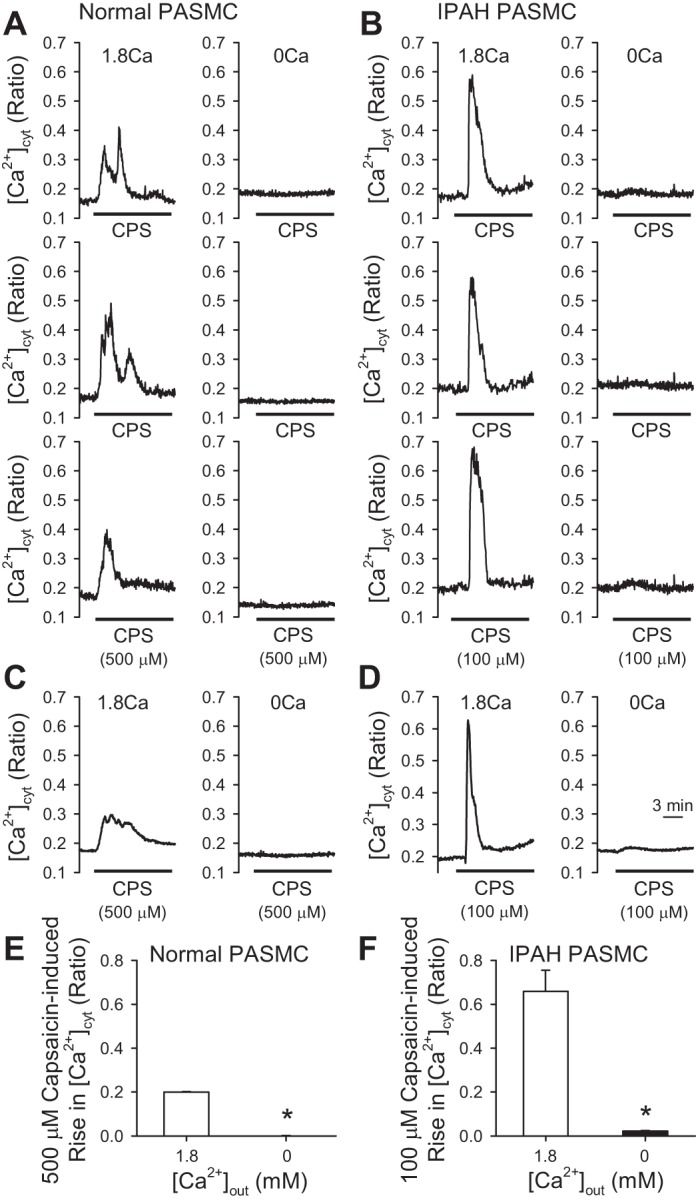
Removal of extracellular Ca2+ abolishes the capsaicin-induced increase in [Ca2+]cyt in PASMCs. A and B: representative individual traces showing changes in [Ca2+]cyt in normal and IPAH PASMCs before and during extracellular application of 500 μM (normal) and 100 μM (IPAH) capsaicin when cells were superfused with 1.8 mM Ca2+-containing bath solution or in Ca2+-free bath solution. C and D: representative averaged traces showing changes in [Ca2+]cyt in normal and IPAH PASMCs before and during extracellular application of 500 μM (normal) and 100 μM (IPAH) capsaicin when cells were superfused with 1.8 mM Ca2+-containing bath solution or in Ca2+-free bath solution. E and F: summarized data (means ± SE) showing the amplitudes of 500 μM capsaicin-induced increases in [Ca2+]cyt in normal and 100 μM capsaicin-induced increases in [Ca2+]cyt in IPAH in the absence and presence of 1.8 mM extracellular Ca2+ (n = 60–90 cells, 3 separate experiments). *P < 0.05 compared with 1.8 mM Ca2+.
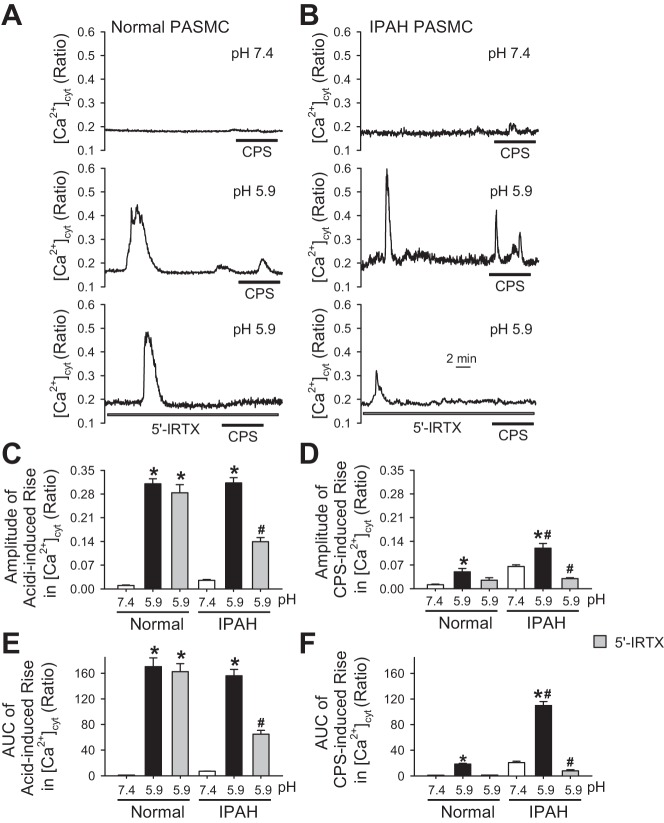
It has been reported that TRPV1 channels are sensitive to changes in extracellular pH and temperature (2). Studies have shown that acidic by-products of inflammation can lower the pH value of the affected tissue to as low as 5.9 (21, 33). Chronic inflammation has been identified as a characteristic feature and has been shown to be involved in the pathology of PAH (20, 22). Indeed, increasing the extracellular acidity or decreasing the extracellular pH from 7.4 to 5.9 resulted in increased [Ca2+]cyt (Fig. 5, A and B); however, the amplitude of acidity-induced increase in [Ca2+]cyt showed no significant difference between normal and IPAH PASMCs (Fig. 5C). In addition to the increase in [Ca2+]cyt induced by extracellular acidity, the capsaicin-induced increase in [Ca2+]cyt was also significantly enhanced by changing the extracellular pH from 7.4 to 5.9 in both normal and IPAH PASMCs, whereas the amplitude of capsaicin-induced increase in [Ca2+]cyt in acidic condition was greater in IPAH PASMCs than in normal PASMCs (Fig. 5, A, B, and D). Furthermore, the area under the curve (AUC) of the increase in [Ca2+]cyt, which represents the total amount of Ca2+ that enters the cell induced by capsaicin under acidic conditions (pH = 5.9) but not by changing pH alone, was significantly greater in IPAH PASMCs than in normal PASMCs (Fig. 5, E and F). Extracellular application of 5′-IRTX markedly attenuated the acidity-induced increase in [Ca2+]cyt (both amplitude and AUC) in IPAH PASMCs but not in normal PASMCs (Fig. 5, A–F), whereas 5′-IRTX inhibited the capsaicin-induced increase in [Ca2+]cyt (both amplitude and AUC) under acidic condition in both normal and IPAH PASMCs (Fig. 5, A–F). Acidity induced not only similar amplitude but also longer spike duration of [Ca2+]cyt increase in normal PASMCs compared with IPAH PASMCs. However, 5′-IRTX negligibly affected the acidity-induced [Ca2+]cyt increase in normal PASMCs, suggesting that other acidity-sensitive receptors/channels rather than TRPV1, such as acid-sensing ion channels (ASICs), might be primarily responsible for sensing acidity (low pH) in normal PASMCs.
Fig. 5.
Decreasing the extracellular pH from 7.4 to 5.9 raises [Ca2+]cyt and significantly enhances capsaicin-induced increases in [Ca2+]cyt in IPAH PASMCs. A: representative traces showing different patterns of [Ca2+]cyt changes in normal PASMCs before and during application of 10 μM capsaicin (CPS) when the pH values in the extracellular solution were at 7.4 or 5.9, and the different patterns of [Ca2+]cyt changes in normal PASMCs before and during application of 10 μM CPS in the presence of 5′-IRTX when the pH values in the extracellular solution was at 5.9 (bottom). B: representative traces showing different patterns of [Ca2+]cyt changes IPAH PASMCs before and during application of 10 μM CPS when the pH values in the extracellular solution were at 7.4 or 5.9, and the different patterns of [Ca2+]cyt changes in IPAH PASMCs before and during application of 10 μM CPS in the presence of 5′-IRTX when the pH values in the extracellular solution was at 5.9 (bottom). C: summarized data (means ± SE) showing the amplitude of increases in [Ca2+]cyt at pH = 7.4 or 5.9 with or without 300 nM 5′-IRTX in normal and IPAH PASMCs (n = 45–60 cells, 3 separate experiments). *P < 0.05 vs. pH 7.4, #P < 0.05 vs. pH = 7.4 and pH = 5.9. D: summarized data (means ± SE) showing the amplitude of 10 μM CPS-induced increases in [Ca2+]cyt at pH = 7.4 or 5.9 with 10 μΜ CPS and with or without 300 nM 5′-IRTX in normal and IPAH PASMCs (n = 45–60 cells, 3 separated experiments). *P < 0.05 vs. pH 7.4, #P < 0.05 vs. pH = 7.4 and pH = 5.9. E: summarized data (means ± SE) showing the area under the curve (AUC) of increases in [Ca2+]cyt at pH = 7.4 or 5.9 with or without 300 nM 5′-IRTX in normal and IPAH PASMCs (n = 45–60 cells, 3 separate experiments). *P < 0.05 vs. pH = 7.4, #P < 0.05 vs. pH = 7.4 and pH = 5.9. F: summarized data (means ± SE) showing the AUC of 10 μM CPS-induced increases in [Ca2+]cyt at pH = 7.4 or 5.9 with or without 300 nM 5′-IRTX in normal and IPAH PASMCs (n = 45–60 cells, 3 separate experiments). *P < 0.05 vs. pH = 7.4, #P < 0.05 vs. pH = 7.4 and pH = 5.9.
In addition to the effect of changing extracellular pH, we also examined the effect of changing the extracellular temperature on the capsaicin-induced increase in [Ca2+]cyt. As shown in Fig. 6, increasing the extracellular temperature from 23°C to 43°C alone had a negligible effect on [Ca2+]cyt in both normal and IPAH PASMCs, but it further enhanced the amplitude and AUC of the capsaicin-induced transient increase in [Ca2+]cyt; both amplitude and AUC were greater in IPAH PASMCs than in normal PASMCs (Fig. 6, A–D). Collectively, these results suggest that 1) the pH-sensitive and temperature-sensitive TRPV1 is the upregulated channel that contributes to the enhanced capsaicin-induced Ca2+ influx in IPAH PASMCs compared with normal PASMCs, and 2) lowering the extracellular pH (or increasing the extracellular acidity) and increasing the extracellular temperature not only directly activates (low pH value) TRPV1 to increase [Ca2+]cyt in IPAH PASMCs, but it also enhances capsaicin-induced increases in [Ca2+]cyt.
Fig. 6.
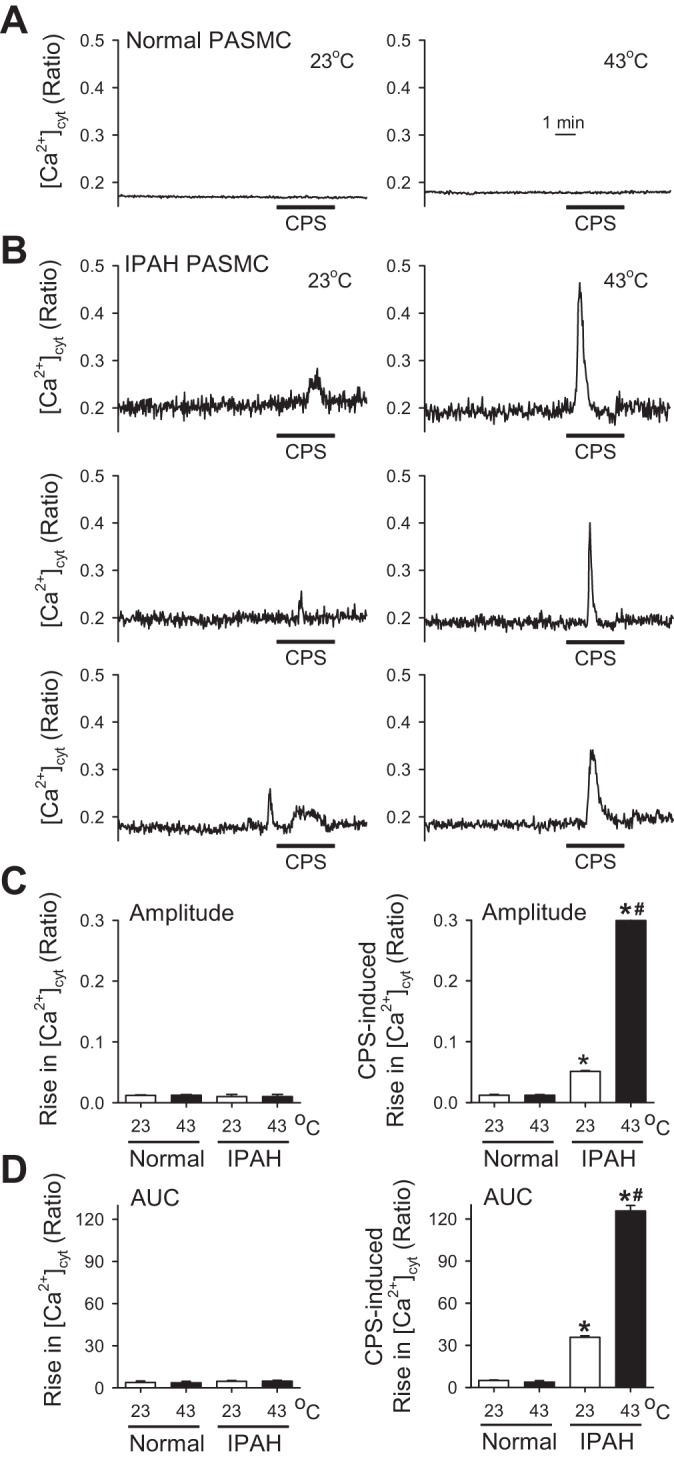
Increasing the extracellular temperature from 23 to 43°C raises [Ca2+]cyt and significantly enhances capsaicin-induced increases in [Ca2+]cyt in IPAH PASMCs. A: representative records showing different patterns of [Ca2+]cyt changes in normal PASMCs before and during application of 10 μM capsaicin (CPS) when the temperature in the extracellular solution was at 23°C (left) or 43°C (right). B: representative individual records showing different patterns of [Ca2+]cyt changes in IPAH PASMCs before and during application of 10 μM CPS when the temperature in the extracellular solution was at 23°C (left) or 43°C (right). C: summarized data (means ± SE) showing the amplitude of increases in [Ca2+]cyt at 23 or 43°C without (left) and with (right) 10 μM CPS in normal (white bars) and IPAH (black bars) PASMCs (n = 60–100 cells, 3–5 separated experiments). *P < 0.05 vs. normal PASMCs, #P < 0.05 vs temperature of 23°C. D: summarized data (means ± SE) showing the AUC of increases in [Ca2+]cyt at 23 or 43°C without (left) and with (right) 10 μM CPS in normal (white bars) and IPAH (black bars) PASMCs (n = 60–100 cells, 3–5 separate experiments). *P < 0.05 vs. normal PASMCs, #P < 0.05 vs. 23°C.
Osmotic stretch induces a significant increase in [Ca2+]cyt in IPAH PASMCs and this [Ca2+]cyt increase is partially mediated by Ca2+ influx through 5′-IRTX-sensitive TRPV1 channels.
Osmotic stretch is a common mechanical stimulus in the pulmonary circulation system, especially during hypertension and inflammation, during which the hydrostatic condition or permeability are increased. Among the mammalian TRP channels that bear mechanosensitivity (e.g., TRPV1, 2, 4; TRPC1, 5, 6; TRPM3, 7; TRPA1; TRPP2), TRPV1 is an important mechanosensitive cation channel (8, 11). Therefore, we examined and compared the effect of hypo-osmolarity, which leads to cell membrane stretch, on [Ca2+]cyt in normal and IPAH PASMCs. As shown in Fig. 7A, osmotic stretch induced by superfusion of hypo-osmotic solution (200 mOsml/kg) resulted in a very small increase in [Ca2+]cyt in normal PASMCs, but a significant increase in [Ca2+]cyt in IPAH PASMCs. The summarized data indicate that the osmotic-mediated transient increase in [Ca2+]cyt is significantly enhanced in IPAH PASMCs compared with normal PASMCs. We also examined the effect of hyperosmolarity on [Ca2+]cyt in normal and IPAH PASMCs. As shown in Fig. 7B, application of hyperosmotic solution (400 mOsml/kg) induced only a minor increase in [Ca2+]cyt in IPAH PASMCs, but not in normal PASMCs. These data indicate that PASMCs from patients with IPAH have greater sensitivity and responses to mechanical stimuli, including flow shear stress and osmotic stretch.
Fig. 7.
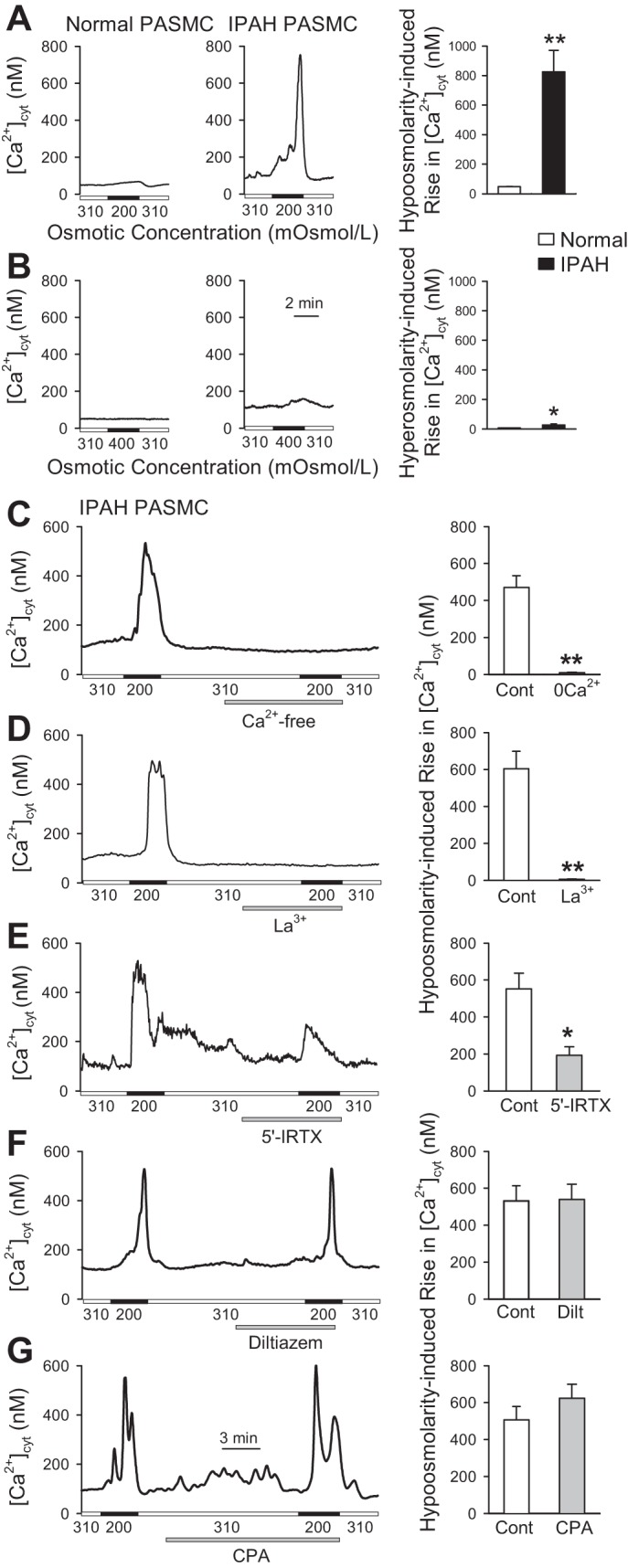
Osmotic stretch-induced increase in [Ca2+]cyt is increased in IPAH PASMCs partially through 5′-IRTX-sensitive TRPV1 channels. A: representative traces showing the changes in [Ca2+]cyt before, during (solid horizontal bars), and after application of hypotonic (200 mOsml/kg) solution in normal and IPAH PASMCs. Summarized data (means ± SE, right) showing the amplitude of hypo-osmolality (200 mOsml/kg)-mediated increase in [Ca2+]cyt in normal and IPAH PASMCs (n = 3 separate experiments). **P < 0.01 vs. normal. B: representative trace showing changes in [Ca2+]cyt before, during (solid horizontal bars) and after application of hypertonic (400 mOsml/kg) solution in normal and IPAH PASMCs. Summarized data (means ± SE, right) showing the amplitude of hyperosmolality (400 mOsml/kg)-mediated increase in [Ca2+]cyt in normal and IPAH PASMCs (n = 3 separate experiments). *P < 0.05 vs. normal. C: representative trace (left) showing the changes in [Ca2+]cyt in IPAH PASMCs before, during, and after application of hypotonic (200 mOsml/kg) solution in the presence or absence of extracellular Ca2+. Summarized data (means ± SE, right) showing the amplitude of hypo-osmolality-mediated increases in [Ca2+]cyt in IPAH PASMCs superfused with control solution containing 1.8 mM Ca2+ or Ca2+-free solution (n = 3 separate experiments). **P < 0.01 vs. control. D–G: representative traces showing the changes in [Ca2+]cyt in IPAH PASMCs before, during, and after application of hypotonic (200 mOsml/kg) solution in the absence or presence of 100 µM La3+ (D), 300 nM 5′-IRTX (E), 10 µM diltiazem (F), or 10 µM cyclopiazonic acid (CPA) (G). Summarized data (means ± SE) on right showing the amplitude of hypo-osmolarity-mediated increases in [Ca2+]cyt in IPAH PASMCs before (Cont), during, and after application of hypotonic (200 mOsml/kg) solution without or with the treatment of La3+ (D), 5′-IRTX (E), diltiazem (F), or CPA (G) (n = 3 separate experiments for each). *P < 0.05 and **P < 0.01 vs. control.
To test whether TRPV1 is involved in the osmotic stretch-mediated [Ca2+]cyt increase in IPAH PASMCs, we examined the effect of specific TRPV1 channel antagonist 5′-IRTX on the osmotic stretch-mediated [Ca2+]cyt increase in IPAH PASMCs. As shown in Fig. 7C, removal of extracellular Ca2+ abolished the osmotic stretch-mediated rise in [Ca2+]cyt in IPAH PASMCs, indicating that the stretch-mediated increase in [Ca2+]cyt is mainly due to Ca2+ influx through Ca2+-permeable channels in the plasma membrane. Extracellular application of 100 µM La3+, a nonselective TRP channel blocker, abolished the osmotic stretch-mediated [Ca2+]cyt increase in IPAH PASMCs (Fig. 7D), whereas the TRPV1-specific antagonist 5′-IRTX inhibited 50% of osmotic stretch-mediated [Ca2+]cyt increase in IPAH PASMCs (Fig. 7E). However, blockage of voltage-gated Ca2+ channels (VGCC) with diltiazem (10 µM) had little effect on the osmotic stretch-mediated [Ca2+]cyt increase in IPAH PASMCs (Fig. 7F). Furthermore, depletion of intracellular Ca2+ stores using 10 µM CPA had no effect on the osmotic stretch-mediated [Ca2+]cyt increase in IPAH PASMCs (Fig. 7G). These data suggest that 1) osmotic stretch-induced increase in [Ca2+]cyt in IPAH PASMCs is due to Ca2+ influx through La3+-sensitive TRP channels, and 2) TRPV1 channels are partially (50%) responsible for sensing osmotic stretch and mediating Ca2+ influx in IPAH PASMCs.
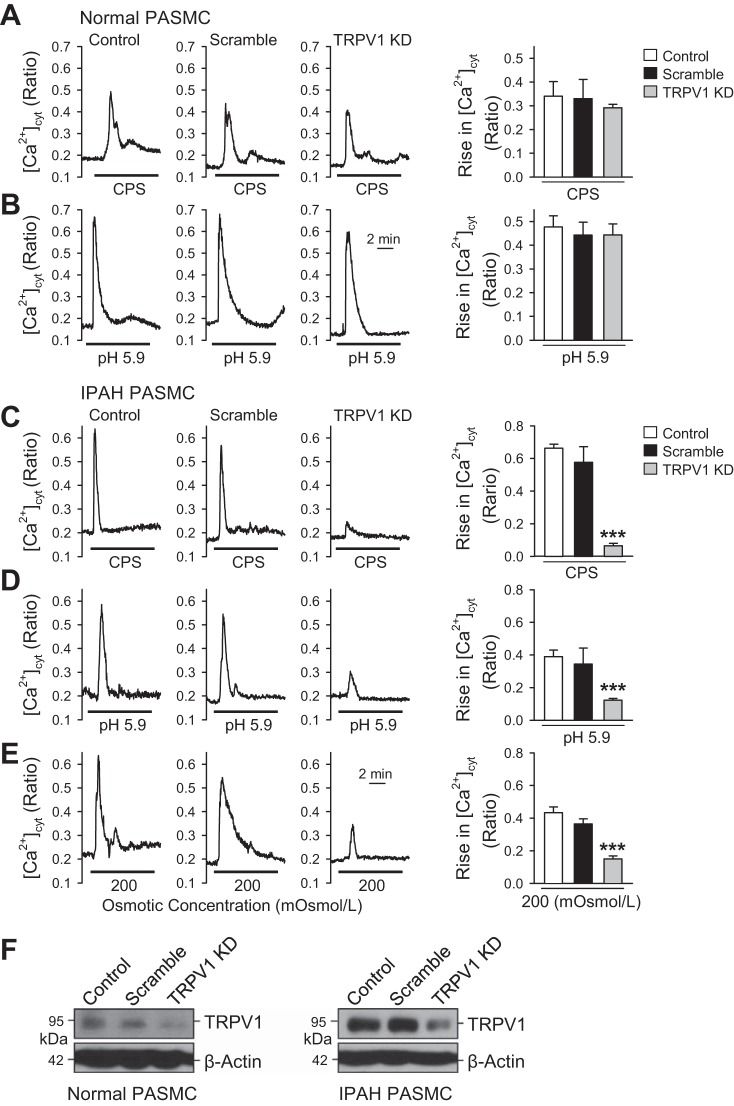
Knockdown of TRPV1 attenuates capsaicin-, acidity-, and osmotic stretch-induced increase in [Ca2+]cyt in IPAH PASMCs.
Although 5′-IRTX is a potent, high-affinity, and selective antagonist for TRPV1, it is possible that it interacts nonspecifically with other vanilloid receptor isoforms. To confirm the conclusion that overexpressed TRPV1 is responsible for capsaicin-, acidity-, and osmotic stretch-induced increase in [Ca2+]cyt in IPAH PASMCs, knockdown experiments were undertaken using specific TRPV1 shRNA. Knockdown of TRPV1 was achieved by expressing four unique TRPV1-specific shRNAs expressed in a lentivirus GFP vector. Single cell calcium imaging was performed only in cells that showed green fluorescence after 48 h of transfection with the shRNA-containing vector. In normal PASMCs, knockdown of TRPV1 negligibly affected the capsaicin- and acidity (pH 5.9)-induced increase in [Ca2+]cyt (Fig. 8, A and B). Capsaicin at 500 μM was chosen here based on the results from Fig. 1, which showed that only a large dose of capsaicin can evoke normal PASMCs. In IPAH PASMCs, knockdown of TRPV1 significantly attenuated capsaicin (50 μM)-, acidity-, and osmotic stretch-induced increase in [Ca2+]cyt in IPAH PASMCs (Fig. 8, C–E). To evaluate the silencing effect of shRNA, the protein expression level of TRPV1 was examined after 48 h of transfection. Western blot data show that the protein expression level of TRPV1 was remarkably reduced in IPAH PASMCs after transfection with specific shRNA, whereas the effect was not detectable in normal PASMCs due to the already low expression level of TRPV1 (Fig. 8F). These results suggest and confirm that 1) 500 μM capsaicin-induced increase in [Ca2+]cyt is a nonspecific response from other TRPV channels due to low protein expression level of TRPV1 in normal PASMCs, 2) TRPV1 channels are not major channels responsible for sensing acidity (pH) in normal PASMCs, and 3) upregulation of TRPV1 contributes to capsaicin-, acidity-, and osmotic stretch-induced increase in [Ca2+]cyt in IPAH PASMCs.
Fig. 8.
Knockdown of TRPV1 attenuates capsaicin-, acidity-, and osmotic stretch-induced increase in [Ca2+]cyt in IPAH PASMCs. A: representative traces showing the changes in [Ca2+]cyt RNA (shRNA), and TRPV1 shRNA constructs in transfected normal PASMCs. Summarized data (means ± SE, left) showing the amplitude of capsaicin-induced increases in [Ca2+]cyt in control normal PASMCs and PASMCs transfected with scramble shRNA or TRPV1 shRNA (n = 9–12 cells, 3 separate experiments for each). B: representative traces showing the changes in [Ca2+]cyt before and during the process of decreasing the extracellular solution pH to 5.9 in control, scramble shRNA, and TRPV1 shRNA transfected normal PASMCs. Summarized data (means ± SE, left) showing the amplitude of acidity-induced (pH = 5.9) increases in [Ca2+]cyt in control normal PASMCs and PASMCs transfected with scramble shRNA and TRPV1 shRNA (n = 9–12 cells, 3 separate experiments for each). C–E: representative traces showing the changes in [Ca2+]cyt before and during application of 50 μΜ capsaicin (C), acidic extracellular solution (pH = 5.9) (D), and hypotonic (200 mOsml/kg) solution (E) in control, scramble shRNA, and TRPV1 shRNA transfected IPAH PASMCs. Summarized data (means ± SE) showing that the amplitude of increase in [Ca2+]cyt in control IPAH PASMCs and PASMCs transfected with scramble shRNA and TRPV1 shRNA activated by capsaicin (C, left), acidity (pH = 5.9) (D, left), and hypo-osmolality (E, left) (n = 9–12 cells, 3 separate experiments for each). ***P < 0.001 vs. control and scramble. F: representative Western blot images showing the protein expression level of TRPV1 in control PASMCs (left) and IPAH PASMCs (right) transfected with scramble shRNA and TRPV1 shRNA (n = 3).
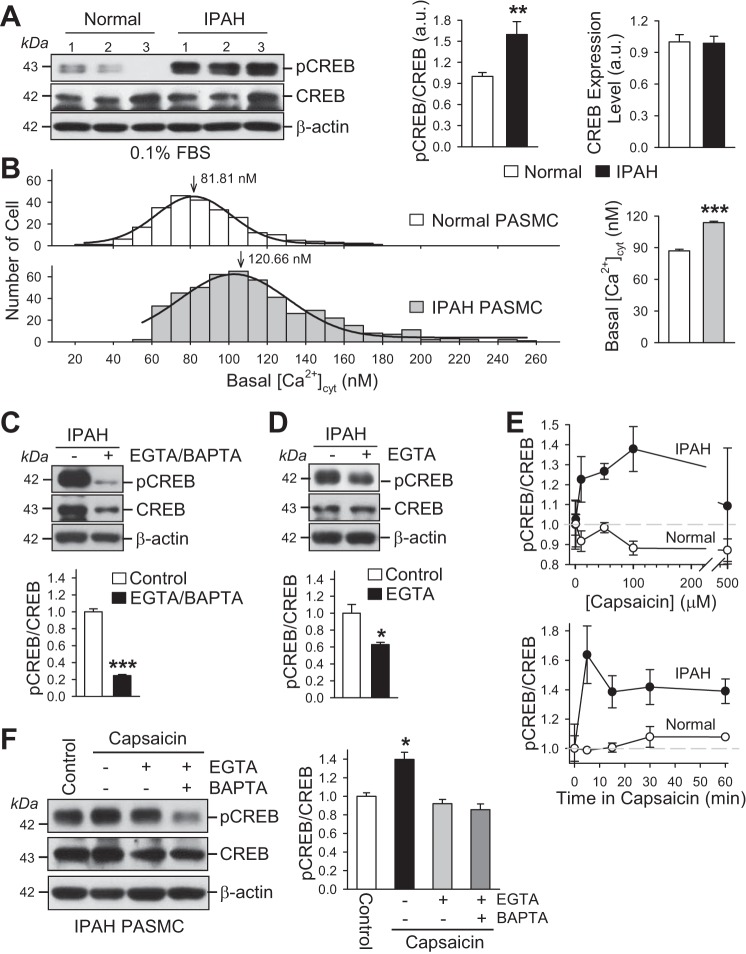
Increased basal [Ca2+]cyt in IPAH PASMCs is associated with an increased phosphorylation of CREB.
In vascular smooth muscle cells, including PASMCs, there are several known Ca2+/calmodulin-sensitive signaling proteins and transcription factors. The cAMP response element-binding protein (CREB) is a transcription factor involved in growth factor-dependent cell proliferation and cell survival (14, 39). Phosphorylation of CREB is an important step in promoting cellular gene expression and cell proliferation/survival in response to growth factor signals and mitogenic second messengers (e.g., cAMP). Compared with normal PASMCs, the phosphorylated CREB (pCREB) was significantly increased in IPAH PASMCs (Fig. 9A); the increased pCREB was associated with an increased basal [Ca2+]cyt (Fig. 9B). In these experiments, normal and IPAH PASMCs were growth arrested in 0.1% FBS media (for 48 h) before the experiments were performed.
Fig. 9.
Increased phosphorylation of CREB is associated with high basal [Ca2+]cyt and a capsaicin-induced increase in [Ca2+]cyt in IPAH PASMCs. A: Western blot analysis of phosphorylated CREB (pCREB) and total CREB (CREB) in normal PASMCs and IPAH PASMCs cultured in low serum (0.1% FBS) media (left). Summarized data (means ± SE) showing the ratio of pCREB to total CREB (pCREB/CREB) (middle) and protein level of total CREB (CREB) (right) in normal and IPAH PASMCs (n = 3). **P < 0.01 vs. normal PASMCs. B: histogram showing the distribution of basal [Ca2+]cyt in normal PASMCs (open bars) and IPAH PASMCs (dark gray bars). The peak values of basal [Ca2+] are 81.94 and 120.66 nM in normal and IPAH PASMCs, respectively. Summarized data (means ± SE) showing the average basal [Ca2+]cyt in normal (n = 300 cells) and IPAH (n = 600 cells) PASMCs (right). ***P < 0.001 vs. normal PASMCs. C: Western blot analysis of pCREB and CREB in IPAH PASMCs treated with (+EGTA/BAPTA) or without (−EGTA/BAPTA) 2 mM EGTA and 30 µM BAPTA-AM for 24 h (top). Summarized data (means ± SE, bottom) showing the averaged pCREB/CREB ratios in control IPAH PASMCs and IPAH PASMCs treated with EGTA and BAPTA-AM (+EGTA/BAPTA) (n = 3). ***P < 0.001 vs. control IPAH PASMCs. D: Western blot analysis of pCREB and CREB in IPAH PASMCs treated with (+EGTA) or without (−EGTA) 2 mM EGTA for 24 h (top). Summarized data (means ± SE, bottom) showing the averaged pCREB/CREB ratios in control IPAH PASMCs and IPAH PASMCs treated with EGTA (n = 3). *P < 0.05 vs. control IPAH PASMCs. E: dose-response curves (top) and the time-course curves (bottom) for capsaicin-induced changes in pCREB/CREB ratio in normal and IPAH PASMCs (n = 3). Data are presented as means ± SE. The time for each dose of capsaicin was 15 min for the dose-response experiment, and the dose for each time of capsaicin treatment was 50 µM for the time-course experiment (n = 3). Data are presented as means ± SE. F: Western blot analysis of pCREB and CREB in IPAH PASMCs treated with 50 µM capsaicin in the absence (−) or presence (+) of 2 mM EGTA or EGTA plus 30 µM BAPTA-AM for 15 min (left). Summarized data (means ± SE, right) showing the averaged pCREB/CREB ratios in control IPAH PASMCs and IPAH PASMCs treated with capsaicin in the absence or presence of EGTA or EGTA plus BAPTA-AM (n = 3). *P < 0.05 vs. control IPAH PASMCs and IPAH PASMCs treated with EGTA/BAPTA or EGTA.
To examine whether increased pCREB in IPAH PASMCs was due to increased [Ca2+]cyt, we pretreated the IPAH PASMCs with a medium containing 2 mM EGTA, a Ca2+ chelator to reduce the free [Ca2+] in the culture medium to 500 nM (30); and 30 µM BAPTA-AM, a membrane-permeable Ca2+ chelator that can be accumulated and chelate free Ca2+ in the cytosol, the intracellular organelles (e.g., sarcoplasmic reticulum, mitochondria) and the nucleus (26). As shown in Fig. 9C, chelation of extracellular and intracellular free Ca2+ with EGTA and BAPTA-AM significantly decreased the phosphorylation of CREB in IPAH PASMCs. The protein expression level of total CREB was also decreased in IPAH PASMCs when extracellular and intracellular Ca2+ was chelated with EGTA and BAPTA. Interestingly, the ratio of pCREB to total CREB was significantly decreased by Ca2+ chelation. Given that BAPTA-AM chelates intracellular Ca2+, which can be the summation of both Ca2+ release and Ca2+ influx, we pretreated IPAH PASMCs with medium containing 2 mM EGTA only, to examine the contribution of extracellular Ca2+ (Ca2+ influx-induced increased basal [Ca2+]cyt) to the increased pCREB in IPAH PASMCs. As shown in Fig. 9D, extracellular chelation of free Ca2+ with EGTA decreased pCREB in IPAH PASMCs without also resulting in significant changes in total CREB protein level. These data indicate that the significantly higher basal [Ca2+]cyt in IPAH PASMCs, compared with normal PASMCs, is sufficient to cause a significant increase in pCREB (i.e., pCREB/CREB ratio). Removal or chelation of extracellular and/or intracellular free Ca2+ significantly inhibits the phosphorylation of CREB in IPAH PASMCs.
In normal PASMCs, extracellular application of capsaicin (1 to 100 µM) had no effect on [Ca2+]cyt until the dose of capsaicin was increased to 500 µM, resulting in a slight increase in [Ca2+]cyt (Fig. 1, A and C). The dose-response curve of the effect of capsaicin on the ratio of pCREB/CREB showed no increase in pCREB/CREB in normal PASMCs (Fig. 9E, top), which was consistent with its effect on [Ca2+]cyt in normal PASMCs (Fig. 1, A and C). In IPAH PASMCs, however, capsaicin resulted in a significant increase in the ratio of pCREB/CREB in a dose-dependent manner (Fig. 9E, top); the time course of the capsaicin-induced increase in pCREB indicated that the augmenting effect of capsaicin on pCREB/CREB ratio rapidly occurred within 5 min (Fig. 9E, bottom). Extracellular chelation of free Ca2+ with EGTA or intracellular chelation of free Ca2+ with BAPTA-AM significantly inhibited the capsaicin-induced increase in pCREB in IPAH PASMCs (Fig. 9F). These data indicate that 1) the basal increase in the ratio of pCREB to CREB in IPAH PASMCs is likely due to the rise in basal [Ca2+]cyt associated, at least in part, with upregulated TRPV1 channels and enhanced Ca2+ influx through these upregulated receptor- and/or store-operated Ca2+ channels in the presence of extracellular agonists and stimulators; and 2) capsaicin, as a ligand of the upregulated TRPV1 in IPAH PASMCs, increases [Ca2+]cyt, induces CREB phosphorylation, and may cause PASMC contraction, migration, and proliferation.
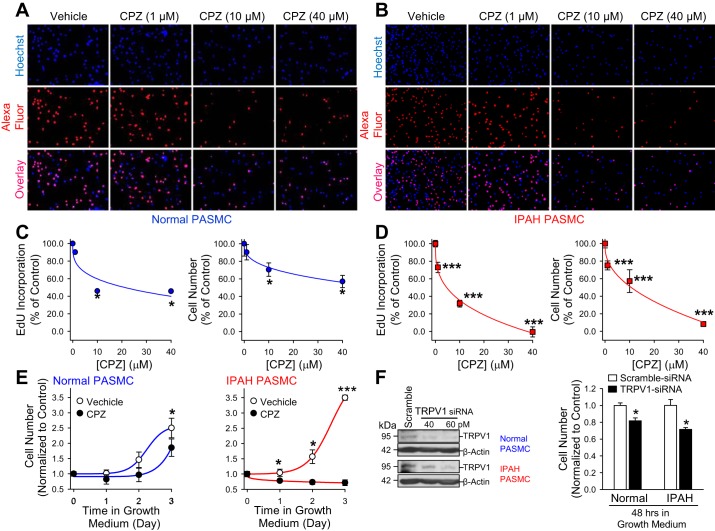
Inhibition of TRPV1 attenuates IPAH PASMC proliferation.
To investigate whether blockage of TRPV1 inhibits cell proliferation, we incubated normal and IPAH PASMCs in medium with CPZ for 48 h. We then determined the number of cells that were positively stained with Alexa fluor, a dye that tells how much EdU was incorporated into the newly synthesized DNA. For future in vivo study on the role of TRPV1 in the development of PAH, CPZ but not 5′-IRTX, was used for the cell proliferation experiments due to the robust hypothermia effect of 5′-IRTX on the animal body (4, 25). Cell proliferation that was measured before treatment was used as a basal reference value to evaluate the toxicity of the drug (data not shown). CPZ caused a dose-dependent attenuation of cell proliferation in both normal and IPAH PASMCs. However, cell proliferation was significantly decreased in PASMCs from patients with IPAH compared with cells from healthy donors. The number of EdU-positive cells was reduced by 10, 54, and 54% in normal PASMCs, whereas the number of EdU-positive cells was reduced by 30, 70, and 105% in IPAH PASMCs, respectively (treated with 1, 10, and 40 μM CPZ; Fig. 10, A–D). A high concentration of CPZ (40 μM) that caused a 105% reduction in cell number in IPAH PASMCs was cytotoxic to cells, thus accounting for the value higher than 100%. To determine whether CPZ caused inhibition of EdU incorporation, we counted the total cell number in addition to EdU measurements. As shown in Fig. 10, C and D, incubation of cells with CPZ resulted in dose-dependent decrease in total cell number. The decreased cell number is significantly higher in IPAH PASMCs compared with normal PASMCs. To further evaluate the effect of TRPV1 inhibition on cell proliferation, cell proliferation rate was evaluated by treating cells with 10 μM CPZ for 72 h. Compared with vehicle control cells, CPZ treatment resulted in a significant decrease in cell proliferation rate in both normal and IPAH PASMCs (Fig. 10E). Cells from healthy donor subjects exhibited an increased trend toward cell proliferation even under CPZ conditions; however, the cell growth of IPAH PASMCs was arrested during 72 h of treatment with CPZ. Knockdown of TRPV1 also reduced cell proliferation by 30% in IPAH PASMCs at the maximum dose of siRNA, compared with a decrease of 19% in normal PASMCs (Fig. 10F). Knockdown of TRPV1 resulted in residual expression of TRPV1, which may explain why pharmacological inhibition of TRPV1 results in a more pronounced decrease in cell proliferation. These data indicate that 1) upregulation of TRPV1 is associated with increased proliferation of PASMCs from patients with IPAH, and 2) the TRPV1 channel is an important target in developing therapeutic approaches for IPAH.
Fig. 10.
Capsazepine (CPZ) attenuates IPAH PASMC proliferation. A and B: representative images showing nuclear staining (top, Hoechst, blue), EdU staining (middle, AlexaFluor, red), and overlay images (bottom, overlay, purple) of normal and IPAH PASMCs incubated without (vehicle) or with 1, 10, and 40 μM CPZ for 48 h. C and D: summarized data (means ± SE) showing the percentage of cells with EdU incorporation (left) and the percentage of total cell number (right) in vehicle- and CPZ-treated normal and IPAH PASMCs (n = 3 separate experiments for normal and n = 9 separate experiments for IPAH). *P < 0.05 and ***P < 0.001 compared with control normal and IPAH PASMCs. E: summarized data (means ± SE) showing the percentage of total cell number in vehicle- and 10 μM CPZ-treated normal (left) and IPAH (right) PASMCs for 72 h (n = 3 separate experiments for normal and IPAH PASMCs, respectively). *P < 0.05 and ***P < 0.001 compared with control normal and IPAH PASMCs. F: representative Western blot images (left) showing the protein expression level of TRPV1 in normal PASMCs (top) and IPAH PASMCs (bottom) transfected with 80 pM scramble siRNA and 40 pM and 60 pM TRPV1 siRNA, respectively (n = 3). Summarized data (means ± SE) (right) showing the percentage of total cell number in 80 pM scramble siRNA- and 80 pM TRPV1 siRNA-treated normal and IPAH PASMCs for 48 h (n = 3 separate experiments for normal and IPAH PASMCs, respectively). *P < 0.05 and #P < 0.05 compared with scramble siRNA-treated normal and IPAH PASMCs.
DISCUSSION
Regardless of the initial genetic, environmental, and acquired causes for the disease, the increased pulmonary arterial pressure in patients with IPAH is mainly caused by increased pulmonary vascular resistance due to sustained pulmonary vasoconstriction, concentric pulmonary vascular remodeling, in situ thrombosis, and/or increased pulmonary arterial wall stiffness. An increase in cytosolic free Ca2+ concentration ([Ca2+]cyt) in PASMCs is a major trigger for pulmonary vasoconstriction and an important stimulus for PASMC migration and proliferation, which contributes to concentric pulmonary arterial wall thickening. The increased pulmonary vascular tone and thickened pulmonary vascular wall also contribute to increased vascular wall stiffness, which limits the recruitment and extension of the pulmonary arteriole and capillary.
In this study, we found the following: 1) the capsaicin-induced increase in [Ca2+]cyt correlated with increased protein expression of TRPV1 in IPAH PASMCs; 2) increasing the extracellular proton concentration and temperature significantly enhanced the capsaicin-induced increase in [Ca2+]cyt in IPAH PASMCs compared with normal PASMCs and hypo-osmotic pressure induced a transient increase in [Ca2+]cyt only in PASMCs from patients with IPAH but not in PASMCs isolated from normal subjects; 3) the steady-state open probability of capsaicin-sensitive TRPV1 single-channel currents was significantly enhanced in IPAH PASMCs compared with normal PASMCs; 4) inhibition or downregulation of TRPV1 inhibited capsaicin-, acidity-, and hypo-osmolarity-induced increases in [Ca2+]cyt in IPAH PASMCs; 5) the increased basal pCREB and capsaicin-induced enhanced pCREB (determined by the ratio of pCREB to total CREB) in IPAH PASMCs were associated with a significant increased basal [Ca2+]cyt and capsaicin-mediated enhanced [Ca2+]cyt increase compared with normal PASMCs; and 6) inhibition of TRPV1 markedly attenuated IPAH PASMC proliferation. Collectively, the observations of this study indicate that 1) increased Ca2+ influx through upregulated TRPV1 channels in PASMCs contributes, at least partly, to the augmented Ca2+ signaling and inappropriate cell proliferation in patients with IPAH; and 2) inhibition of the upregulated TRPV1 channels with special antagonists may represent a novel therapeutic approach for pulmonary arterial hypertension.
Capsaicin is an ingredient that can be extracted from chili pepper and is included in the daily diet for many people in the world. Capsaicin and several related compounds are called capsaicinoids, and are produced as secondary metabolites by chili peppers. Capsaicinoids and capsacinoids have also been used for weight control or weight management and pain control (15). When TRPV1 is upregulated in the pulmonary vasculature or PASMCs, the possibility that TRPV1 is activated by capsaicin is increased, which may result in increased Ca2+ influx and may induce activation or phosphorylation of CREB, a transcription factor that is involved in cell proliferation, cell survival, and cell apoptosis (Fig. 7) (14, 39). In the airways, capsaicinoids cause an inflammatory effect as well as airway epithelial cell death through activation of TRPV1 channels (24). In the pulmonary vasculature, the data from this study showing capsaicin-mediated enhanced increase in [Ca2+]cyt and CREB phosphorylation in IPAH PASMCs, suggests a potential pathogenic mechanism to induce sustained pulmonary vasoconstriction and excessive pulmonary vascular remodeling in patients with IPAH. In addition to capsaicin sensitivity, TRPV1 is also sensitive to acid and heat (>42°C). Both normal and IPAH PASMCs actively responded to a low pH (pH = 5.9) in a similar pattern, whereas acid significantly enhanced the sensitivity of TRPV1 to capsaicin in IPAH PASMCs compared with that in normal PASMCs. As for heat, neither cells from subjects nor those from patients with IPAH patients showed a response to high temperature (43°C), however, the sensitivity of TRPV1 to capsaicin is significantly enhanced under high temperature. Therefore, although the individual stimulus intensity (capsaicin, acid, and heat) studied here is hard to reach under pathophysiological conditions, the combined effect of these stimuli may significantly enhance the sensitivity of TRPV1 in vivo. Thus, upregulated TRPV1 in PASMCs may serve as an important target for inhibiting Ca2+ signaling and Ca2+-mediated cell contraction, migration, and proliferation in the pulmonary vasculature in response to capsaicin, fever, and acidic conditions. Accordingly, blockade of TRPV1 (by capsazepine, for example) may be a strategy for developing novel therapies for patients with PAH, especially for patients who have a high intake of spicy food, and those with inflammation and infection (which results in extracellular high temperature and acidic stimuli for TRPV1 channels).
TRPV1 is a major heat sensor in a subset of peripheral sensor neurons. Studies of TRPV1 in sensory neurons or in heterologously expressed HEK293 cells have shown that 42°C is the activation threshold of TRPV1 (29). In this study, we stimulated TRPV1 at 43°C. but did not observe activation of TRPV1. Some studies have reported that TRPV1 lacking the C-terminal 88 amino acids could not be activated by heat in normal calcium solution (13). In other studies, a truncated C-terminal variant of TRPV1 lacking the terminal 62 amino acids found in T ganglia has a reduced thermal threshold (34). Therefore, we speculate that the TRPV1 expressed in PASMCs may have mutations in C-terminal that cause the reduced sensitivity to heat activation. Further studies are needed to test this hypothesis.
The canonical transient receptor potential channels (TRPC1/6) have been reported to form functional receptor-and store-operated Ca2+ channels in many cell types, including PASMCs (1, 6, 9, 37); downregulation of TRPC1/6 inhibited receptor-mediated and store-operated Ca2+ influx and efficiently attenuated PASMC proliferation (6, 31, 37). It has been demonstrated that the protein expression levels of TRPC3 and TRPC6 channels are greater in PASMCs from patients with IPAH than from healthy subjects (36). Our previous study also shows that the mechanical stress-sensitive TRPM7 and TRPV4 channels are both upregulated in IPAH PASMCs, which are involved in the enhanced shear stress-induced increase in [Ca2+]cyt compared with normal PASMCs (27). Recently, a study has shown that TRPC6 functionally interacts with calcium-sensing receptor (CaSR) to regulate [Ca2+]cyt in PASMCs (32). Taken together, these data suggest that transient receptor potential (TRP) channel genes are prone to being overexpressed in IPAH PASMCs, and most importantly, the overexpressed TRP channels affect intracellular Ca2+ signaling to regulate cell proliferation and growth. Interestingly, the data from this study show that enhanced basal phosphorylated CREB in IPAH PASMCs is associated with increased resting [Ca2+]cyt, which is due, at least in part, to Ca2+ influx from the extracellular space (EGTA chelation) (Fig. 7, A–D). Given that the ability to sense mechanical stress and to form the receptor-operated and store-operated Ca2+ channels, it is fair to speculate that the upregulated TRPV1 channels, together with other TRP channels (TRPC3, TRPC6, TRPV4 and TRPM7), may contribute to the increased resting [Ca2+]cyt and enhanced Ca2+ signaling in PASMCs from patients with IPAH. Taken together, this mechanism may in part explain the excessive proliferation of PASMCs leading to pulmonary vascular remodeling, and ultimately, increased pulmonary vascular resistance and pulmonary arterial pressure.
In PASMCs from healthy donor subjects, the amplitude of acidity (pH = 5.9)-induced [Ca2+]cyt increase was similar to that of PASMCs from patients with IPAH, but the spike duration (data not shown) was longer than in PASMCs from patients with IPAH, whereas using TRPV1 potent blocker 5′-IRTX or knockdown of TRPV1 negligibly affected the acidity (pH = 5.9)-induced [Ca2+]cyt increase in normal PASMCs. It must be emphasized that TRPV1 channels are not the only proton-sensing (H+) channels in PASMCs. Previous studies have shown that acid-sensing ion channel1 (ASIC1) is expressed in PASMCs and was involved in hypoxia-induced augmented store-operated Ca2+ entry (SOCE); hypoxia can promote ASIC1 function through enhanced ASIC1 plasma membrane localization without altering total ASIC1 protein level in human and murine PASMCs (10, 18). Here, we observed that acidity induces a significant increase in [Ca2+]cyt in normal PASMCs without being affected by blockage or downregulation of the TRPV1 channel, indicating that ASIC1 might be the major acidity-sensing channel in normal PASMCs, whereas, in IPAH PASMCs, TRPV1 plays an important role in proton (H+) sensing. Furthermore, it is speculated that the ability of ASIC1 to sense H+ protons is decreased in IPAH PASMCs, either functionally or through downregulated protein expression. Future studies will investigate whether ASIC1 also participates in the augmented SOCE in PASMCs from patients with IPAH.
In this study, we used cultured PASMCs, which undergo major dedifferentiation with phenotype changes from a contractile phenotype to proliferative phenotype. In vivo, PASMCs in patients with IPAH characterized by excessive proliferation and PASMCs in normal subjects are more contractive with less proliferation. Therefore, the data collected from cultured normal PASMCs may not reflect the in vivo biological characterizations. Additionally, the samples we used are from limited numbers of subjects (3 healthy donors, 6 patients with IPAH). Future studies will focus on understanding the universal significance of our findings by examining additional PASMC samples and by modeling our findings in a murine model.
It should be noted that the calculated EC50 (~35 μM in IPAH PASMC and 275 μM in normal PASMC) from the dose-response curve obtained from the Ca2+ imaging experiments (Fig. 1, C and D, bottom) indicated lower sensitivity of TRPV1 to capsaicin in normal and IPAH PASMCs compared with reported studies in other literature. One possible explanation for this discrepancy could be the overall resolution of the Ca2+ imaging technique. In other words, the Ca2+ fluorescence indicator fura-2 used in the digital imaging fluorescence microscopy experiments requires cytosolic free [Ca2+] to increase or decrease by a certain amount to produce a detectable fluorescence signal; thus, even if TRPV1 is activated by submicromolar concentrations of capsaicin, because of the low expression level (or low channel density in the plasma membrane) in PASMCs, the amount of increased free [Ca2+] may not be sufficient to cause significant fluorescence changes of fura-2. This may also explain why cultured rat PASMCs do not always respond to 10 µM capsaicin (<20%) as reported by Martin et al. (16). It is likely that the EC50 derived from Ca2+ imaging data does not reflect the actual potency of capsaicin or sensitivity of TRPV1 measured patch clamp techniques, but rather it indicates that the expression level of TRPV1 is too low to sufficiently detect. Another consideration is that the single-channel patch clamp technique provides higher resolution, making it best used to characterize the biophysical properties of TRPV1 channels, including open probability and gating, because the high resistance from a gigaseal formation is effective in significantly reducing background noise of the recording. In essence, the TRPV1 current is easily resolved at lower concentrations of capsaicin.
In this study, 10 μM and 50 μM capsaicin were used in all the experiments besides the dose-response curve and patch clamp experiments. Knockdown of TRPV1 results in ~90% attenuation of the capsaicin (50 μM)-induced increase in [Ca2+]cyt in IPAH PASMCs, suggesting the existence of nonspecific responses from other TRPV channels that are sensitive to capsaicin. Therefore, the data collected from using a high concentration of capsaicin may not completely indicate the effect of activation of TRPV1 on cell function. Because of the aim of this study is to characterize and compare TRPV1-mediated Ca2+ signaling in normal and IPAH PASMCs, digital imaging fluorescence microscopy is thus the preference for this purpose. Future studies will place more effort on seeking more sensitive Ca2+ sensors or improving Ca2+ imaging experimental condition to minimize the nonspecific response caused by the relatively high concentration of agonists, or to use a more sensitive patch clamp technique to characterize the biophysical and pharmacological properties of the upregulated TRPV1 channels in PASMCs from patients.
In conclusion, upregulated TRPV1, together with other upregulated TRP channels (e.g., TRPC1/3/6, TRPV4, TRPM7) (27, 36–38), plays an important role in mediating increases in [Ca2+]cyt required for causing PASMC contraction, migration, proliferation, and growth in patients with IPAH. The upregulated TRPV1 channels in PASMCs may make patients more sensitive to capsaicin or capsaicinoids (e.g., a high level of intake of chili powder for pain relief and/or weight loss), high temperature (e.g., fever), acidic microenvironment (e.g., increased inflammation or metabolic abnormalities that decrease extracellular pH), and mechanical stimulation (e.g., ventilator use) and more likely to develop sustained pulmonary vasoconstriction and concentric pulmonary vascular wall thickening, and ultimately, pulmonary hypertension. Thus, developing pharmacological interventions specially aimed at decreasing the gene expression of TRPV1 or inhibiting the function of TRPV1 may greatly help in the development of new therapeutic strategies for patients with idiopathic, heritable and associated PAH.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants HL-115014, HL-066012, and HL-125208.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.S. and J.X.-J.Y. conceived and designed research; S.S., R.J.A., A.Y., H.Y., S.D., A.B., H.T., X.S., A.G.C., Z.I.K., S.M.B., A.A.D., F.R., K.M.M., J.G.G., and A.M. performed experiments; S.S., R.J.A., A.Y., H.Y., S.D., A.B., H.T., X.S., A.G.C., Z.I.K., S.M.B., A.A.D., F.R., K.M.M., J.G.G., A.M., and J.X.-J.Y. analyzed data; S.S., R.J.A., A.Y., H.Y., S.D., A.B., H.T., X.S., A.G.C., Z.I.K., S.M.B., A.A.D., F.R., K.M.M., J.G.G., A.M., and J.X.-J.Y. interpreted results of experiments; S.S., R.J.A., A.Y., H.Y., S.D., A.B., H.T., X.S., A.G.C., Z.I.K., S.M.B., A.A.D., F.R., K.M.M., J.G.G., A.M., and J.X.-J.Y. prepared figures; S.S. drafted manuscript; S.S., R.J.A., A.Y., H.Y., S.D., A.B., H.T., X.S., A.G.C., Z.I.K., S.M.B., A.A.D., F.R., K.M.M., J.G.G., A.M., and J.X.-J.Y. edited and revised manuscript; S.S., R.J.A., A.Y., H.Y., S.D., A.B., H.T., X.S., A.G.C., Z.I.K., S.M.B., A.A.D., F.R., K.M.M., J.G.G., A.M., and J.X.-J.Y. approved final version of manuscript.
REFERENCES
- 1.Bergdahl A, Gomez MF, Dreja K, Xu SZ, Adner M, Beech DJ, Broman J, Hellstrand P, Swärd K. Cholesterol depletion impairs vascular reactivity to endothelin-1 by reducing store-operated Ca2+ entry dependent on TRPC1. Circ Res 93: 839–847, 2003. doi: 10.1161/01.RES.0000100367.45446.A3. [DOI] [PubMed] [Google Scholar]
- 2.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288: 306–313, 2000. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 3.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398: 436–441, 1999. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 4.Dogan MD, Patel S, Rudaya AY, Steiner AA, Székely M, Romanovsky AA. Lipopolysaccharide fever is initiated via a capsaicin-sensitive mechanism independent of the subtype-1 vanilloid receptor. Br J Pharmacol 143: 1023–1032, 2004. doi: 10.1038/sj.bjp.0705977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynn R, Chapman K, Iftinca M, Aboushousha R, Varela D, Altier C. Targeting the transient receptor potential vanilloid type 1 (TRPV1) assembly domain attenuates inflammation-induced hypersensitivity. J Biol Chem 289: 16675–16687, 2014. doi: 10.1074/jbc.M114.558668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol 280: H746–H755, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985. [PubMed] [Google Scholar]
- 8.Hill-Eubanks DC, Gonzales AL, Sonkusare SK, Nelson MT. Vascular TRP channels: performing under pressure and going with the flow. Physiology (Bethesda) 29: 343–360, 2014. doi: 10.1152/physiol.00009.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular α1-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res 88: 325–332, 2001. doi: 10.1161/01.RES.88.3.325. [DOI] [PubMed] [Google Scholar]
- 10.Jernigan NL. Smooth muscle acid-sensing ion channel 1: pathophysiological implication in hypoxic pulmonary hypertension. Exp Physiol 100: 111–120, 2015. doi: 10.1113/expphysiol.2014.081612. [DOI] [PubMed] [Google Scholar]
- 11.Liedtke WB. TRPV channels’ function in osmo- and mechanotransduction. In TRP Ion Channel Function In Sensory Transduction and Cellular Signaling Cascades, edited by Liedtke WB, Heller S. Boca Raton, FL: CRC Press, 2007, p. 303–318. [PubMed] [Google Scholar]
- 12.Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 95: 496–505, 2004. doi: 10.1161/01.RES.0000138952.16382.ad. [DOI] [PubMed] [Google Scholar]
- 13.Liu B, Ma W, Ryu S, Qin F. Inhibitory modulation of distal C-terminal on protein kinase C-dependent phospho-regulation of rat TRPV1 receptors. J Physiol 560: 627–638, 2004. doi: 10.1113/jphysiol.2004.069054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Q, Patel B, Harrington EO, Rounds S. Transforming growth factor-β1 causes pulmonary microvascular endothelial cell apoptosis via ALK5. Am J Physiol Lung Cell Mol Physiol 296: L825–L838, 2009. doi: 10.1152/ajplung.90307.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo XJ, Peng J, Li YJ. Recent advances in the study on capsaicinoids and capsinoids. Eur J Pharmacol 650: 1–7, 2011. doi: 10.1016/j.ejphar.2010.09.074. [DOI] [PubMed] [Google Scholar]
- 16.Martin E, Dahan D, Cardouat G, Gillibert-Duplantier J, Marthan R, Savineau JP, Ducret T. Involvement of TRPV1 and TRPV4 channels in migration of rat pulmonary arterial smooth muscle cells. Pflugers Arch 464: 261–272, 2012. doi: 10.1007/s00424-012-1136-5. [DOI] [PubMed] [Google Scholar]
- 17.Means AR. Calcium, calmodulin and cell cycle regulation. FEBS Lett 347: 1–4, 1994. doi: 10.1016/0014-5793(94)00492-7. [DOI] [PubMed] [Google Scholar]
- 18.Nitta CH, Osmond DA, Herbert LM, Beasley BF, Resta TC, Walker BR, Jernigan NL. Role of ASIC1 in the development of chronic hypoxia-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 306: H41–H52, 2014. doi: 10.1152/ajpheart.00269.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parpaite T, Cardouat G, Mauroux M, Gillibert-Duplantier J, Robillard P, Quignard JF, Marthan R, Savineau JP, Ducret T. Effect of hypoxia on TRPV1 and TRPV4 channels in rat pulmonary arterial smooth muscle cells. Pflugers Arch 468: 111–130, 2016. doi: 10.1007/s00424-015-1704-6. [DOI] [PubMed] [Google Scholar]
- 20.Perros F, Dorfmüller P, Souza R, Durand-Gasselin I, Mussot S, Mazmanian M, Hervé P, Emilie D, Simonneau G, Humbert M. Dendritic cell recruitment in lesions of human and experimental pulmonary hypertension. Eur Respir J 29: 462–468, 2007. doi: 10.1183/09031936.00094706. [DOI] [PubMed] [Google Scholar]
- 21.Punnia-Moorthy A. Evaluation of pH changes in inflammation of the subcutaneous air pouch lining in the rat, induced by carrageenan, dextran and Staphylococcus aureus. J Oral Pathol 16: 36–44, 1987. doi: 10.1111/j.1600-0714.1987.tb00674.x. [DOI] [PubMed] [Google Scholar]
- 22.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res 115: 165–175, 2014. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramakers GM, Pasinelli P, van Beest M, van der Slot A, Gispen WH, De Graan PN. Activation of pre- and postsynaptic protein kinase C during tetraethylammonium-induced long-term potentiation in the CA1 field of the hippocampus. Neurosci Lett 286: 53–56, 2000. doi: 10.1016/S0304-3940(00)01081-8. [DOI] [PubMed] [Google Scholar]
- 24.Reilly CA, Taylor JL, Lanza DL, Carr BA, Crouch DJ, Yost GS. Capsaicinoids cause inflammation and epithelial cell death through activation of vanilloid receptors. Toxicol Sci 73: 170–181, 2003. doi: 10.1093/toxsci/kfg044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimizu I, Iida T, Horiuchi N, Caterina MJ. 5-Iodoresiniferatoxin evokes hypothermia in mice and is a partial transient receptor potential vanilloid 1 agonist in vitro. J Pharmacol Exp Ther 314: 1378–1385, 2005. doi: 10.1124/jpet.105.084277. [DOI] [PubMed] [Google Scholar]
- 26.Song S, Jacobson KN, McDermott KM, Reddy SP, Cress AE, Tang H, Dudek SM, Black SM, Garcia JG, Makino A, Yuan JX. ATP promotes cell survival via regulation of cytosolic [Ca2+] and Bcl-2/Bax ratio in lung cancer cells. Am J Physiol Cell Physiol 310: C99–C114, 2016. doi: 10.1152/ajpcell.00092.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song S, Yamamura A, Yamamura H, Ayon RJ, Smith KA, Tang H, Makino A, Yuan JX. Flow shear stress enhances intracellular Ca2+ signaling in pulmonary artery smooth muscle cells from patients with pulmonary arterial hypertension. Am J Physiol Cell Physiol 307: C373–C383, 2014. doi: 10.1152/ajpcell.00115.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Studer M, McNaughton PA. Modulation of single-channel properties of TRPV1 by phosphorylation. J Physiol 588: 3743–3756, 2010. doi: 10.1113/jphysiol.2010.190611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun X, Zakharian E. Regulation of the temperature-dependent activation of transient receptor potential vanilloid 1 (TRPV1) by phospholipids in planar lipid bilayers. J Biol Chem 290: 4741–4747, 2015. doi: 10.1074/jbc.M114.611459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sweeney M, McDaniel SS, Platoshyn O, Zhang S, Yu Y, Lapp BR, Zhao Y, Thistlethwaite PA, Yuan JX. Role of capacitative Ca2+ entry in bronchial contraction and remodeling. J Appl Physiol 92: 1594–1602, 2002. doi: 10.1152/japplphysiol.00722.2001. [DOI] [PubMed] [Google Scholar]
- 31.Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol 283: L144–L155, 2002. doi: 10.1152/ajplung.00412.2001. [DOI] [PubMed] [Google Scholar]
- 32.Tang H, Yamamura A, Yamamura H, Song S, Fraidenburg DR, Chen J, Gu Y, Pohl NM, Zhou T, Jiménez-Pérez L, Ayon RJ, Desai AA, Goltzman D, Rischard F, Khalpey Z, Black SM, Garcia JG, Makino A, Yuan JX. Pathogenic role of calcium-sensing receptors in the development and progression of pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 310: L846–L859, 2016. doi: 10.1152/ajplung.00050.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuchiya H, Mizogami M, Ueno T, Takakura K. Interaction of local anaesthetics with lipid membranes under inflammatory acidic conditions. Inflammopharmacology 15: 164–170, 2007. doi: 10.1007/s10787-007-1601-5. [DOI] [PubMed] [Google Scholar]
- 34.Valente P, García-Sanz N, Gomis A, Fernández-Carvajal A, Fernández-Ballester G, Viana F, Belmonte C, Ferrer-Montiel A. Identification of molecular determinants of channel gating in the transient receptor potential box of vanilloid receptor I. FASEB J 22: 3298–3309, 2008. doi: 10.1096/fj.08-107425. [DOI] [PubMed] [Google Scholar]
- 35.Wang YX, Wang J, Wang C, Liu J, Shi LP, Xu M, Wang C. Functional expression of transient receptor potential vanilloid-related channels in chronically hypoxic human pulmonary arterial smooth muscle cells. J Membr Biol 223: 151–159, 2008. doi: 10.1007/s00232-008-9121-9. [DOI] [PubMed] [Google Scholar]
- 36.Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci USA 101: 13861–13866, 2004. doi: 10.1073/pnas.0405908101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, Yuan JX. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol 284: C316–C330, 2003. doi: 10.1152/ajpcell.00125.2002. [DOI] [PubMed] [Google Scholar]
- 38.Zhang S, Patel HH, Murray F, Remillard CV, Schach C, Thistlethwaite PA, Insel PA, Yuan JX. Pulmonary artery smooth muscle cells from normal subjects and IPAH patients show divergent cAMP-mediated effects on TRPC expression and capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 292: L1202–L1210, 2007. doi: 10.1152/ajplung.00214.2006. [DOI] [PubMed] [Google Scholar]
- 39.Zhang S, Remillard CV, Fantozzi I, Yuan JX. ATP-induced mitogenesis is mediated by cyclic AMP response element-binding protein-enhanced TRPC4 expression and activity in human pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol 287: C1192–C1201, 2004. doi: 10.1152/ajpcell.00158.2004. [DOI] [PubMed] [Google Scholar]