Abstract
Idiopathic pulmonary fibrosis is a well-known age-related disease. However, much less recognized has been the aging associated pathogenesis of this disorder. As we and others previously showed that dysregulation of micro-RNAs (miRNAs) was an important mechanism involved in pulmonary fibrosis, the role of these molecules in this pathology in the aged population has not been investigated (Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F, Thannickal VJ, Cardoso WV, Lü J. Am J Respir Cell Mol Biol 45: 287–294, 2011; Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, Kaminski N, Abraham E. J Exp Med 207: 1589–1597, 2010; Pandit KV, Corcoran D, Yousef H, Yarlagadda M, Tzouvelekis A, Gibson KF, Konishi K, Yousem SA, Singh M, Handley D, Richards T, Selman M, Watkins SC, Pardo A, Ben-Yehudah A, Bouros D, Eickelberg O, Ray P, Benos PV, Kaminski N. Am J Respir Crit Care Med 182: 220–229, 2010). In this study, by using a lung fibrosis model established in old mice, we found that ablation of miR-34a protected aged animals from developing experimental lung fibrosis. miR-34a was upregulated in lung epithelial cells, but not in lung fibroblasts of aged mice, and miR-34a expression was further increased in epithelial cells of the fibrotic lungs of these old animals. We found that miR-34a induced dysfunctions in alveolar epithelial cells (AECs), as evidenced by increased cellular senescence and apoptosis and mitochondrial aberrations. More importantly, these abnormalities were attenuated in AECs of the fibrotic lungs of aged miR-34a−/− mice. We found that miR-34a targeted Sirt1, a master anti-aging regulator, and two key cell cycle modulators, E2F3 and cyclin E2, in lung epithelial cells, and the repression of these targets was relieved in miR-34a-deficient AECs. In summary, our data suggest that elevated AEC miR-34a plays a critical role in the pathogenesis of pulmonary fibrosis in the aged population. Our study also indicates miR-34a to be a more precise miRNA target for treating this disease that overwhelmingly affects people of advanced age.
Keywords: lung fibrosis, micro-RNA, alveolar epithelial cell, aging, apoptosis, senescence
idiopathic pulmonary fibrosis (IPF) is the most common form of lung fibrosis and a dreadful disease that has a life expectancy of 2–6 yr upon diagnosis (29, 42). While it is well recognized that IPF is an age-related disease, mostly because a large majority of IPF patients are 50 yr and older (17, 35, 36, 39), much less known is the mechanism involved in the pathogenesis of this disorder in the aged population (15, 40, 42). Nevertheless, the current lack of insight is about to change, since there has been emerging evidence showing that dysfunctions of lung fibroblasts and alveolar epithelial cells (AECs) related to cellular senescence may play a key role in the development of this disease (5, 18, 32).
micro-RNAs (miRNAs) are 21–22-nucleotide in length, small, noncoding RNAs (1, 2, 6, 14, 47). miRNAs function by downregulating gene expression via binding to the 3′ untranslated region (UTR) of their targets (1, 2, 6, 14, 47). Our laboratory and others had previously demonstrated that dysregulation of miRNAs was a crucial mechanism underlying the pathogenesis of pulmonary fibrosis (10, 12, 25, 30, 31, 43, 45). While these studies significantly advanced our understanding of the role of miRNAs in this disease, almost all of the knowledge was established by experimenting young animals. Given that pulmonary fibrosis predominantly affects the population of advanced age, it is becoming critically important to study this type of molecules in a context that bears closer resemblance to such a demographic feature associated with this pathology.
In recognition of this concept, we established lung fibrosis model in old mice of 20 mo of age. We specifically investigated the role of miR-34a in this model partly because this miRNA had been frequently implicated in cellular senescence and aging. Strikingly, we found that ablation of miR-34a (miR-34a−/−) protected aged mice from developing bleomycin-induced lung fibrosis. To delineate the underlying mechanism, we examined levels of alveolar epithelial miR-34a and found they rose in old mice and were further induced in fibrotic lungs of aged animals. We demonstrated that miR-34a promoted alveolar epithelial senescence, apoptosis, and mitochondrial aberrations, likely by targeting Sirt1, a master anti-aging modulator, and two cell cycle regulators, E2F3 and cyclin E2. Taken together, our findings suggest that elevated AEC miR-34a is an important mechanism underlying the pathogenesis of pulmonary fibrosis in the aged population by promoting profibrotic dysfunctions in these cells.
MATERIALS AND METHODS
Reagents.
Bleomycin was from Besse Medical. miRNA mimics were from Ambion. HiPerFect Transfection Reagent and RNA isolation kit RNeasy Mini were from Qiagen. Type I collagenase, Dispase II, and DNase I were from Worthington. JC-1 dye and MitoSOX Red reagent were from Thermofisher.
Cell lines.
Human lung epithelial cell lines A549 and BEAS-2B were purchased from American Type Culture Collection.
Experimental pulmonary fibrosis model.
C57BL/6 wild-type (WT) and miR-34a−/− mice were purchased from The Jackson Laboratory. Mice were aged to 20 mo old at University of Alabama at Birmingham (UAB) animal facility. Conditional alveolar epithelial type 2 (ATII) miR-34a knockout mice (miR-34aCKO) were generated by crossing floxed miR-34a mice (Mir34atm1.2Aven/J, Jackson Laboratory) with Sftpc-Cre mice [B6.129S-Sftpctm1(cre/ERT2)Blh/J, Jackson Laboratory] at UAB animal facility. ATII ablation of miR-34a was induced by intraperitoneal injection of tamoxifen (75 mg/kg body wt) for 5 consecutive days. The bleomycin-induced lung fibrosis models were previously described (25, 46). The animal protocol was approved by the UAB Institutional Animal Care and Use Committee.
Isolation of primary lung fibroblasts and epithelial cells.
Primary mouse lung fibroblasts and epithelial cells were harvested as previously described (7, 10, 24). Briefly, lungs tissues were minced and digested with tissue digestion buffer containing 0.1% type I collagenase, 0.1% dispase II, and 0.01% DNase I in HBSS for 1 h at 37°C water bath. The digestions were passed through 40-µm cell strainers, and lung cells were pelleted. After red blood cells lysis, the cells were resuspended and incubated with biotin conjugated anti-CD16/32, anti-CD45, and anti-CD31 antibodies (BD Biosciences) for 1 h. Cells were then washed, resuspended, and incubated with streptavidin magnetic beads (Promega) for 30 min. Tubes containing the incubated cells were then applied to a magnet to deplete endothelial cells, lymphocytes, monocyte/macrophages, natural killer cells, neutrophils, and other hematopoietic cells. Clear supernatants were collected and plated into tissue culture plates. After 1-h incubation at 37°C, the suspended lung epithelial cells were harvested for experiments. The adherent lung fibroblasts were cultured in MEM media containing 10% FBS. Fibroblasts at passages 3–5 were used for experiments. To assess the purity of the isolated pulmonary cells, lung epithelial cells were prepared by cytospin and stained with FITC conjugated anti-E-cadherin antibody (eBioscience), and fibroblasts were stained with anti-α-smooth muscle actin (SMA) antibody (Proteintech), followed by incubation with Alexa Fluor 488 conjugated secondary antibody (Invitrogen). Nuclei were counterstained with 4,6-diamidino-2-phenylindole, and cells with green fluorescence were regarded as lung epithelial cells and fibroblasts, respectively. This method has been routinely performed in our laboratory and consistently yields highly purified epithelial cells and lung fibroblasts.
Hydroxyproline content determination.
Mouse right lungs were homogenized in 2 ml H2O. Homogenates (100 µl) were mixed with 100 µl 12 N HCl, and the samples were incubated at 120°C for 3 h. Hydroxyproline contents were then determined using Hydroxyproline Assay Kit (BioVision), according to the manufacturer’s instructions.
Collagen content determination.
The collagen contents in right lungs were determined by Sircol collagen assay, as previously described (10).
Immunohistochemistry and Masson's trichrome staining.
Immunohistochemistry for α-SMA and Masson's trichrome staining for collagen fibers was performed as described previously (45).
Real-time PCR.
miR-34a levels were determined by TaqMan MicroRNA Assay, and small nucleolar RNAs sno135 was used as an internal reference (Applied Biosystems). RNA levels of protein coding genes were determined by real-time PCR using SYBR Green Master Mix Kit (Roche). Primer sequences were as below: human tubulin-β1, sense 5′ TGGACTCTGTTCGCTCAGGTCCTT 3′, antisense 5′ AGTGGCCTTTGGCCCAGTTGTTAC 3′; mouse tubulin-α1, sense 5′ GGATGCTGCCAATAACTATGCTCGT 3′, antisense 5′ GCCAAAGCTGTGGAAAACCAAGAAG 3′; mouse pri-miR-34a, sense 5′ GCAGCCTCTCCATCTTCCTGTGACT 3′, antisense 5′ CTGACCTCTGACCTTTTCCTTCTCG 3′; mouse fibronectin, sense 5′ TCTGGGAAATGGAAAAGGGGAATGG 3′, antisense 5′ CACTGAAGCAGGTTTCCTCGGTTGT 3′; mouse Col1A1, sense 5′ GGAGGGCGAGTGCTGTGCTTT 3′, antisense 5′ GGGACCAGGAGGACCAGGAAGT 3′; human p21, sense 5′ TTCCTCATCCACCCCATCCCTC 3′, antisense 5′ CCTGTCCATAGCCTCTACTGCC 3′; mouse p21, sense 5′ ATAGCACTTTGGAAAAATGAGTAG 3′, antisense 5′ GAGCAATGTCAAGAGTCGGGAT 3′; human PAI-1, sense 5′ CCACTGGAAAGGCAACATGACCAGG 3′, antisense 5′ GCCATGCGGGCTGAGACTATGACAG 3′; mouse PAI-1, sense 5′ CTCATCAGACAATGGAAGGGCAACA 3′, antisense 5′ ATCGGTCTATAACCATCTCCGTGGG 3′; human Sirt1, sense 5′ AGATTAGTAGGCGGCTTGATGGT 3′, antisense 5′ ATGGGTTCTTCTAAACTTGGACTC 3′; mouse Sirt1, sense 5′ GTTGCAGAAACAGTGAGAAAATG 3′, antisense 5′ CACAGGAACTAGAGGACAAGACG 3′; human cyclin E2, sense 5′ TGGAGGCATTATGACACCACCGAAG 3′, antisense 5′ GGGCAATCAATCACAGCACTACTTTC 3′; mouse cyclin E2, sense 5′ GGATGGTGCCTTTTGTTAGTGTTGTAA 3′, antisense 5′ AATTTGTGTGTGTCTGGATATTGTGTC 3′; human E2F3, sense 5′ GTCCAAAAACTCCAAAATCTCCCTC 3′, antisense 5′ CTGCTGCCTTGTTCAAATCCAATAC 3′; mouse E2F3, sense 5′ TGTTGTCCCTTCCTACCTTCTTCCTC 3′, antisense 5′ CACCTGATTGCACATCTTCTCACTTG 3′. To calculate fold change in the expression of these genes, ΔCt values were first obtained: ΔCt = Ct of tubulin − Ct of individual genes. ΔΔCt values were then obtained: ΔΔCt = ΔCt of treated groups − ΔCt of untreated control groups. Fold change was calculated as 2ΔΔCt, with control groups as onefold.
Western blotting.
Western blotting was performed as previously described (26). Mouse anti-α-tubulin and anti-β-actin antibodies were from Sigma. Rabbit anti-p21 was from Proteintech. Rabbit anti-PAI-1 antibody was from Molecular Innovations. Rabbit anti-SFTPC (pro-surfactant protein C) antibody was from Seven Hills Bioreagents.
Terminal deoxynucleotidyl transferase dUTP nick-end labeling staining.
Apoptotic cells were detected by ApopTag Plus Fluorescein In Situ Apoptosis Detection Kit (EMD Millipore), according to the manufacturer’s instructions.
Apoptosis assay.
Cells were collected and stained with FITC-annexin V and propidium iodide using FITC Annexin V Apoptosis Detection Kit (BD Biosciences), according to the manufacturer’s instructions. Annexin V and propidium iodide positive cells were determined by flow cytometry.
Cellular senescence assay.
Cellular senescence was evaluated by determining senescence-associated β-galactosidase activity with a flow cytometry-based assay, as previously reported (13). Briefly, cells were pretreated with 100 nM bafilomycin A1 to induce lysosomal alkalinization. Cells were then incubated with 50 nM β-galactosidase substrate fluorescein di-β-d-galactopyranoside for an additional 2 h at 37°C. Cells were washed and harvested for flow cytometric analysis with excitation at 488 nm and emission at 530 ± 30 nm.
Determination of mitochondrial reactive oxygen species assay.
Mitochondrial superoxide was determined after MitoSOX Red incubation, followed by flow cytometric analysis. Briefly, cells were incubated with 2.5 µM MitoSOX Red for 10 min at 37°C. Cells were then fixed with 2% paraformaldehyde and analyzed by flow cytometry.
Mitochondrial membrane potential assay.
Mitochondrial membrane potential was measured by incubating cells with JC-1 dye, followed by flow cytometric analysis. The mean fluorescence intensity ratio of red to green was used as an index of mitochondrial membrane potential.
Cell proliferation assay.
Cell proliferative activity was determined with BrdU Cell Proliferation Assay Kit (Cell Signaling Technology), according to the manufacturer's instructions.
Lentivirus production and cell transduction.
The lentiviral constructs that express human Sirt1 were generated by cloning the full-length Sirt1 open reading frame into lentiviral vector pCDH-EF1-MCS (CD502A-1) (System Biosciences). Primer sequences for Sirt1 open reading frame amplification were as follows: sense, 5′ TCTAGAGCCGCCATGGCGGACGAGGCGGCCCTCGCCC 3′; antisense, 5′ GGATCCCTATGATTTGTTTGATGGATAGTTC 3′. Lentiviruses were produced in human embryonic kidney-293T cells, as previously described (10), and used to infect cells in the presence of polybrene (8 µg/ml).
Statistical analysis.
One-way ANOVA followed by the Bonferroni test was used for multiple group comparisons. The Student's t-test was used for comparison between two groups. P < 0.05 was considered statistically significant.
RESULTS
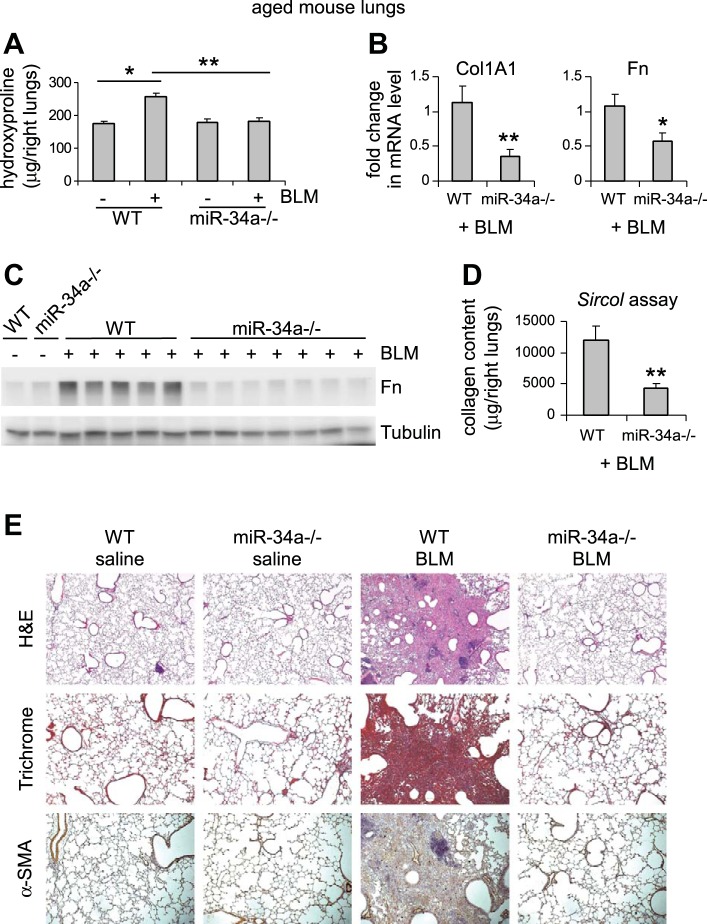
Aged miR-34a−/− mice are protected from experimental lung fibrosis.
To determine the role of miRNAs in the pathogenesis of pulmonary fibrosis in the aged population, we initially focused on miR-34a primarily due to its well-established role in cellular senescence and aging (4, 9, 38, 44). We aged WT and miR-34a−/− mice to 20 mo and administered intratracheally saline or bleomycin to these mice. Aged miR-34a−/− mice had similar weight as aged WT animals [average body weight: 31.1 g (miR-34a−/−) vs. 30.9 g (WT)]. We found that, while basal levels of collagen in the lungs were comparable, aged miR-34a−/− mice were protected from bleomycin-induced pulmonary fibrosis, compared with aged WT animals (Fig. 1A). The remarkable reduction in collagen content in the lungs of bleomycin-treated aged miR-34a−/− mice was also consistent with significant decrease in the mRNA and protein levels of collagen I and fibronectin in the lungs of these mice (Fig. 1, B–D). Additionally, histological examination of lung sections, trichrome staining of collagen deposition, and in situ immunostaining of α-SMA underscored strikingly diminished pulmonary fibrosis in the lungs of bleomycin-treated aged miR-34a−/− mice (Fig. 1E). Together, these data suggest that miR-34a promotes lung fibrosis in the aged population.
Fig. 1.
Aged miR-34a−/− mice are protected from experimental lung fibrosis. Aged wild-type (WT) or miR-34a−/− mice (20 mo old) were intratracheally instilled with saline or bleomycin (BLM; 1.5 U/kg body wt in 50 μl saline). Three weeks after BLM instillation, mice were killed and lungs collected. A: hydroxyproline levels in right lungs were determined. Values are means ± SE; n = 3, 10, 3, and 7 WT mice without and with BLM, and miR-34a−/− mice without and with BLM, respectively. *P < 0.05. **P < 0.01. B: mRNA levels of Col1A1 and fibronectin (Fn) in BLM-treated lungs were determined by real-time PCR. C: protein levels of Fn in the lungs were determined by Western blotting. D: acid- and pepsin-soluble collagens in BLM-treated lungs were determined by Sircol collagen assays. B–D: values are means ± SE; n = 5 and 7 WT and miR-34a−/− BLM-treated mice, respectively. *P < 0.05 and **P < 0.01 compared with BLM WT group. E: lung sections were prepared from the above experiments. Hematoxylin and eosin (H&E) staining (original magnification, × 5), Masson's trichrome staining, and immunohistochemistry assay for α-SMA were performed (original magnification, × 10).
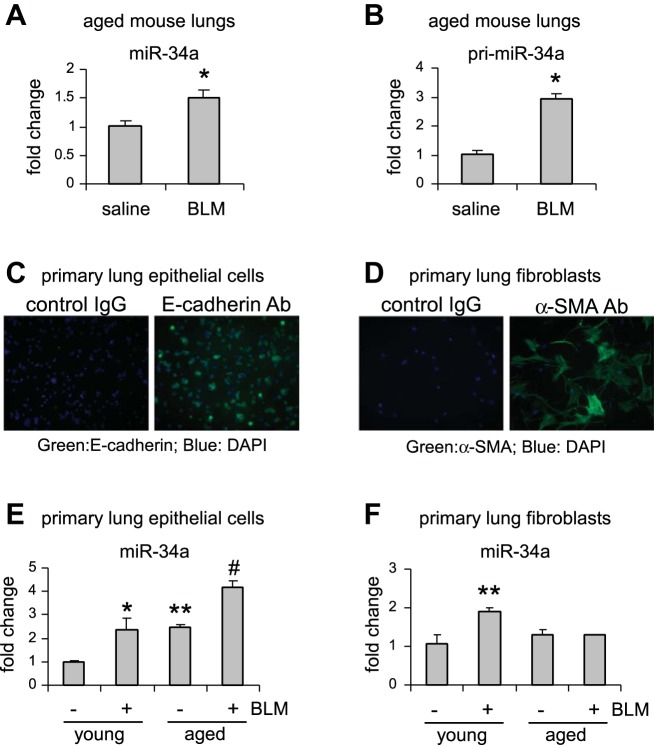
miR-34a is upregulated in AECs, but not in lung fibroblasts, of aged mice, and AEC miR-34a is further increased in aged fibrotic lungs.
To determine the mechanism underlying the profibrotic effect of miR-34a in the lungs of aged mice, we characterized its expression in the whole lungs and in respective pulmonary cells that are directly affected in this disease. First, we found that miR-34a was significantly upregulated in the lungs of aged mice that were treated with bleomycin (Fig. 2A). Additionally, the primary transcripts of miR-34a (pri-miR-34a) were also induced in these fibrotic lungs (Fig. 2B), suggesting an enhanced de novo transcription of miR-34a in the aged lungs after bleomycin treatment.
Fig. 2.
miR-34a is upregulated in AECs, but not in lung fibroblasts, of aged mice, and AEC miR-34a is further increased in aged fibrotic lungs. A and B: aged mice (20 mo old) were intratracheally instilled with bleomycin (BLM) (1.5 U/kg body wt in 50 μl saline). Three weeks after BLM instillation, lungs were collected, and levels of mature miR-34a (A) and primary miR-34a (B) in the lungs were determined by real-time PCR. Values are means ± SE; n = 3 and 6 saline and BLM mice, respectively. *P < 0.05 compared with saline group. C–F: young (10 wk old) and aged mice (20 mo old) were intratracheally treated with saline or BLM. At day 21 after BLM treatment, mice were killed and lungs collected. Primary lung epithelial cells and lung fibroblasts were isolated as described in materials and methods. Purity of lung epithelial cells and lung fibroblasts was determined by staining of epithelial marker E-cadherin (C) and fibroblast marker α-SMA (D), respectively. Levels of miR-34a in primary lung epithelial cells (E) and lung fibroblasts (F) were determined by real-time PCR. Values are means ± SE; n = 3, 4, 3, and 3 (B), and n = 3, 8, 3, and 4 (C) young mice without and with BLM and aged mice without and with BLM, respectively. *P < 0.05 and **P < 0.01 compared with young saline group. #P < 0.05 compared with young BLM group. Ab, antibody; DAPI, 4,6-diamidino-2-phenylindole.
Next, we assessed the expression of miR-34a in AECs and lung fibroblasts isolated from both young and aged animals that were treated with saline or bleomycin. We first evaluated the purity of the isolated cells and found that there was homogeneous positive staining for E-cadherin in the isolated AECs, and almost all of the isolated fibroblasts were positive for α-SMA (Fig. 2, C and D). We found that there was increased expression of miR-34a in lung epithelial cells of aged mice compared with that in lung epithelial cells of young animals (Fig. 2E). More importantly, miR-34a expression was further increased in lung epithelial cells of aged mice that were treated with bleomycin (Fig. 2E). In contrast, we found that miR-34a was only upregulated in lung fibroblasts of bleomycin-treated young, but not aged, mice (Fig. 2F). Of note, we found that there was no evident increase of miR-34a expression in AECs of 8-mo-old mice compared with those of young animals (data not shown), suggesting that miR-34a upregulation in AECs may occur in the late stage of life. Together, these data suggest that the phenotype of lung epithelial cells, but not lung fibroblasts, in aged mice could be impacted by miR-34a elevation during pathological fibrogenesis.
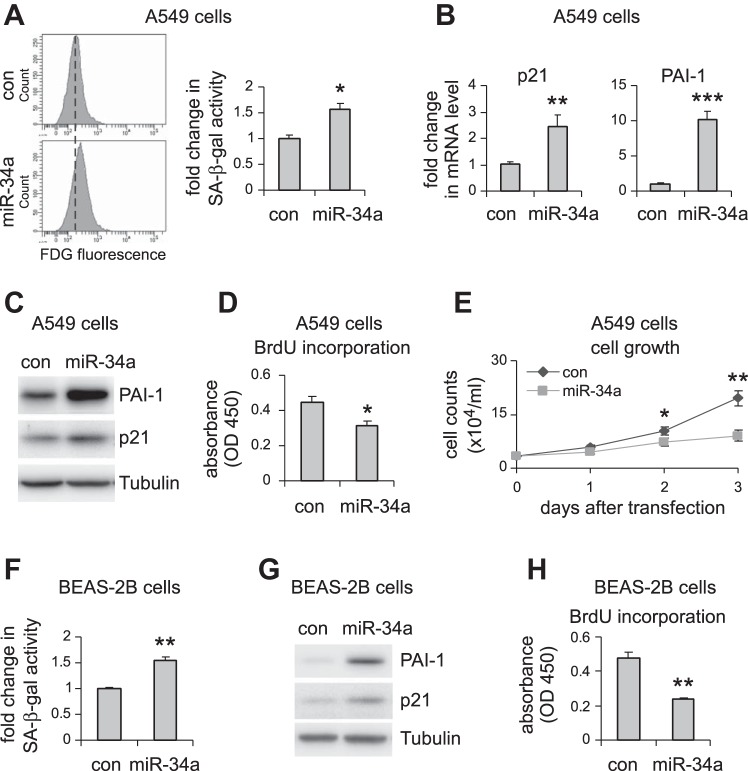
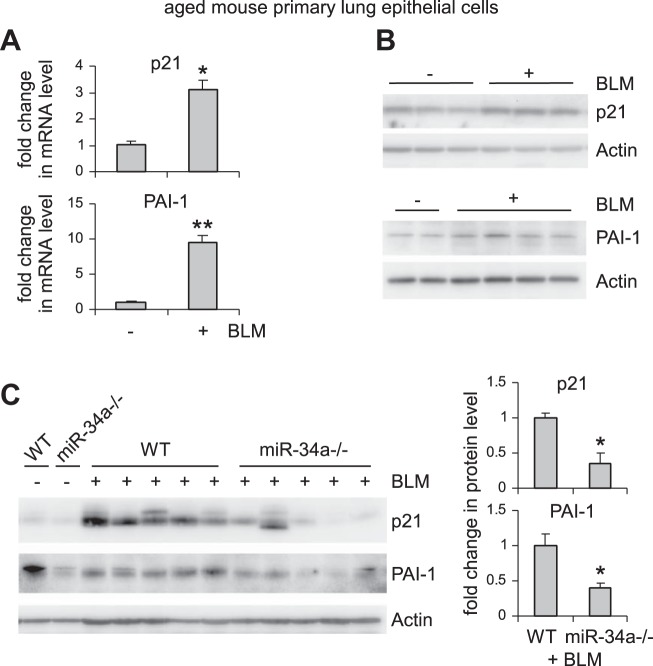
miR-34a induces lung epithelial cell senescence, and this senescent phenotype is attenuated in the fibrotic lungs of aged miR-34a−/− mice.
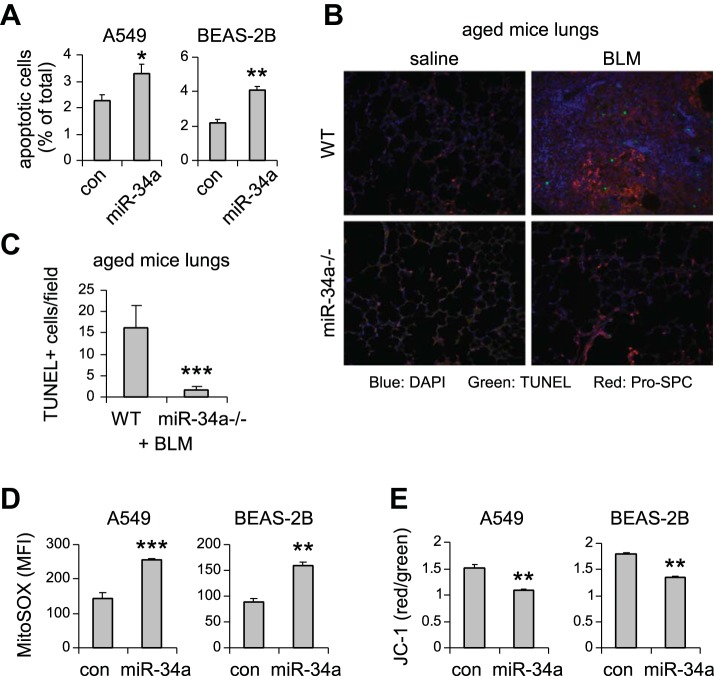
miR-34a is known to regulate senescence in cancer cells (9, 38, 44). Given that alveolar epithelial senescence has been recently shown to be a characteristic feature in IPF (8, 27), we next investigated if the elevated miR-34a in AECs had similar activity and thereby promoted fibrosis in the lungs of aged mice. As shown in Fig. 3, A–C, miR-34a was potent in inducing senescence of human lung epithelial A549 cells, as indicated by elevated senescence-associated β-galactosidase activity and increased expression of senescence markers p21 and PAI-1 in miR-34a transfected lung epithelial cells. Consistent with this senescent phenotype, miR-34a transfected lung epithelial cells also demonstrated decreased proliferation (Fig. 3, D and E). The prosenescence activity of miR-34a was also confirmed in human lung epithelial cell line BEAS-2B (Fig. 3, F–H). In ex vivo experiments, we found that lung epithelial cells of bleomycin-treated WT aged mice demonstrated increased senescent phenotype, as evidenced by greater expression of p21 and PAI-1 in these cells (Fig. 4, A and B). More importantly, this senescent phenotype was diminished in lung epithelial cells of bleomycin-treated aged miR-34a−/− mice (Fig. 4C). Together, these data suggest that miR-34a-induced lung epithelial cell senescence promotes lung fibrosis in aged mice, and deletion of miR-34a in these cells protects aged animals from developing this pathology.
Fig. 3.
miR-34a induces cellular senescence and inhibits cell proliferation in pulmonary epithelial cells in vitro. A–D: human pulmonary epithelial cells A549 were transfected with 50 nM control (con) mimics or miR-34a mimics. A: 3 days after transfection, cells were collected, and senescence-associated β-galactosidase (SA-β-gal) activities in the cells were assessed by flow cytometry. mRNA and protein levels of p21 and PAI-1 were determined by real-time PCR (B) and Western blotting (C). D: cell proliferation was assessed by BrdU incorporation assay. E: A549 cells were transfected with con mimics or miR-34a mimics. Cells were trypsinized and counted at the indicated time points after transfection. A, B, D, and E: values are means ± SD; n = 3. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with con miR group. F–H: human lung epithelial cells BEAS-2B were transfected with con mimics or miR-34a mimics as in A–D. Three days after transfection, cells were collected, and SA-β-gal activities in the cells were assessed by flow cytometry (F), PAI-1 and p21 levels by Western blotting (G), and cell proliferation by BrdU incorporation assay (H). F and H: values are means ± SD, n = 3. **P < 0.01 compared with con miR group. FDG, fluorescein di-β-d-galactopyranoside; OD, optical density.
Fig. 4.
Bleomycin (BLM) induces lung epithelial cell senescence in aged mice, and this senescent phenotype is attenuated in the fibrotic lungs of aged miR-34a−/− mice. A and B: aged WT mice (20 mo old) were intratracheally instilled with BLM. Three weeks after treatment, mice were killed, and lung epithelial cells isolated. mRNA and protein levels of p21 and PAI-1 were determined by real-time PCR (A) and Western blotting (B). A: values are means ± SE; n = 3. *P < 0.05 and **P < 0.01 compared with saline group. C: aged WT and miR-34a−/− mice were intratracheally instilled with BLM. Three weeks after treatment, mice were killed, and lung epithelial cells isolated. Levels of p21 and PAI-1 were determined by Western blotting and densitometric analyses performed using ImageJ (National Institutes of Health). *P < 0.05 compared with BLM WT group.
miR-34a promotes lung epithelial cell apoptosis and mitochondrial dysfunction.
To further characterize the functional significance of miR-34a-induced lung epithelial cell senescence, we examined additional phenotypic alterations associated with lung epithelial cells that are critical to pathological lung fibrogenesis. As shown in Fig. 5A, we found that upregulating miR-34a in lung epithelial A549 and BEAS-2B cells subjected these cells to apoptosis. More importantly, the number of terminal deoxynucleotidyl transferase dUTP nick-end labeling positive apoptotic cells in areas of lung epithelial cells in bleomycin-treated aged miR-34a−/− mice was diminished compared with that in bleomycin-treated WT aged animals (Fig. 5, B and C). Additionally, miR-34a upregulation also increased mitochondrial reactive oxygen species stress and impaired mitochondrial membrane potential (Fig. 5, D and E), two well-recognized profibrotic phenotypic abnormalities, in lung epithelial cells (5). Again, these data suggest that miR-34a promotes lung fibrosis in aged animals by inducing lung epithelial cell senescence, apoptosis, and mitochondrial dysfunction.
Fig. 5.
miR-34a promotes lung epithelial cell apoptosis and mitochondrial dysfunction. A: human lung epithelial cells A549 and BEAS-2B were transfected with 50 nM control (con) mimics or miR-34a mimics. Three days after transfection, cells were collected, and apoptosis determined by FITC-annexin V/propidium iodide assay. Values are means ± SD; n = 3. *P < 0.05 and **P < 0.01 compared with con miR group. B and C: aged WT and miR-34a−/− mice were intratracheally treated with BLM. Three weeks after instillation, mice were killed, and lung sections prepared. Apoptotic cells were assessed by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay (green fluorescence). B: slides were costained with pro-surfactant protein C (SPC; red). C: the number of TUNEL+ cells was counted (original magnification, × 20). ***P < 0.001 compared with WT+ group. D: A549 and BEAS-2B cells were transfected with 50 nM con mimics or miR-34a mimics. Three days after transfection, mitochondrial ROS were determined using MitoSOX Red followed by flow cytometry. E: A549 and BEAS-2B cells were transfected as in D. Three days after transfection, cells were collected, and mitochondrial membrane potential was determined by JC-1 assay. D and E: values are means ± SD; n = 3. **P < 0.01 and ***P < 0.001 compared with con miR group. MFI, mean fluorescence intensity.
Alveolar epithelial ablation of miR-34a protects mice from experimental lung fibrosis.
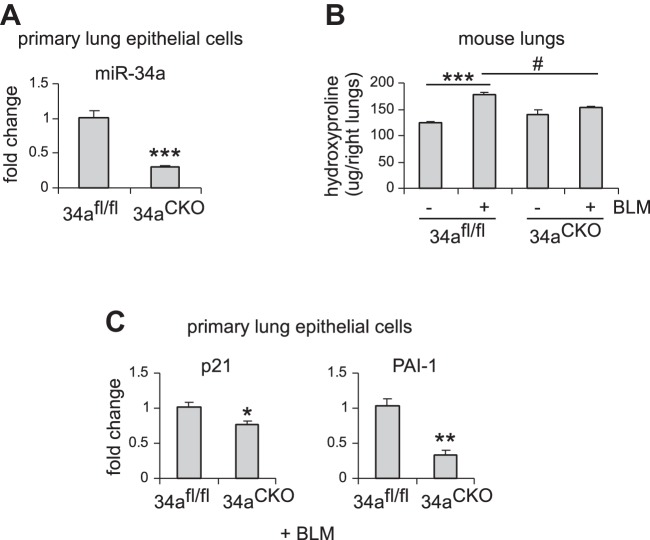
We have found that miR-34a was specifically upregulated in AECs, but not in fibroblasts, in the fibrotic lungs of aged mice. We also showed that aged miR-34a−/− mice were protected from bleomycin-induced lung fibrosis. To further characterize the role of alveolar epithelial miR-34a and also directly demonstrate the profibrotic activity of AEC miR-34a, we generated mice with conditional knockout of miR-34a in ATII cells by breeding floxed miR-34a mice and mice that inducibly express Cre recombinase in ATII cells. We first confirmed effective knockdown of miR-34a in AECs of these mice after tamoxifen induction (Fig. 6A). We then treated these mice intratracheally with saline or bleomycin and found that ablation of miR-34a in ATII cells dramatically diminished bleomycin-induced lung fibrosis (Fig. 6B). Consistently, levels of senescence markers, such as p21 and PAI-1, were also reduced in AECs from the fibrotic lungs of these conditional miR-34−/− mice (Fig. 6C).
Fig. 6.
Alveolar epithelial ablation of miR-34a protects mice from experimental lung fibrosis. A: alveolar epithelial type 2 (ATII) conditional knockout mice (miR-34aCKO) and control miR-34afl/fl mice were administered tamoxifen (75 mg/kg body wt in 100 μl corn oil) intraperitoneally for 5 consecutive days. Three weeks after the first injection, mice were killed, and lung epithelial cells isolated. miR-34a levels were determined by real-time PCR. Values are means ± SE; n = 5. ***P < 0.001 compared with control mice. B and C: ATII conditional knockout mice (miR-34aCKO) and control miR-34afl/fl mice were treated with tamoxifen as in A. At day 7 after the first injection, mice were intratracheally treated with saline or bleomycin (BLM) (1.5 U/kg body wt in 50 μl saline). Two weeks after BLM instillation, mice were killed, and lungs collected. Hydroxyproline levels in right lungs were determined. B: values are means ± SE; n = 3, 5, 3, and 5 miR-34afl/fl mice without and with BLM and miR-34aCKO mice without and with BLM, respectively. ***P < 0.001 compared with saline-injected miR-34afl/fl mice group. #P < 0.05 compared with BLM-injected miR-34afl/fl mice group. C: mRNA levels of p21 and PAI-1 were determined by real-time PCR. Values are means ± SE; n = 5. *P < 0.05 and **P < 0.01 compared with BLM-injected miR-34afl/fl mice group.
miR-34a targets key regulators of cellular senescence, proliferation, and mitochondrial function.
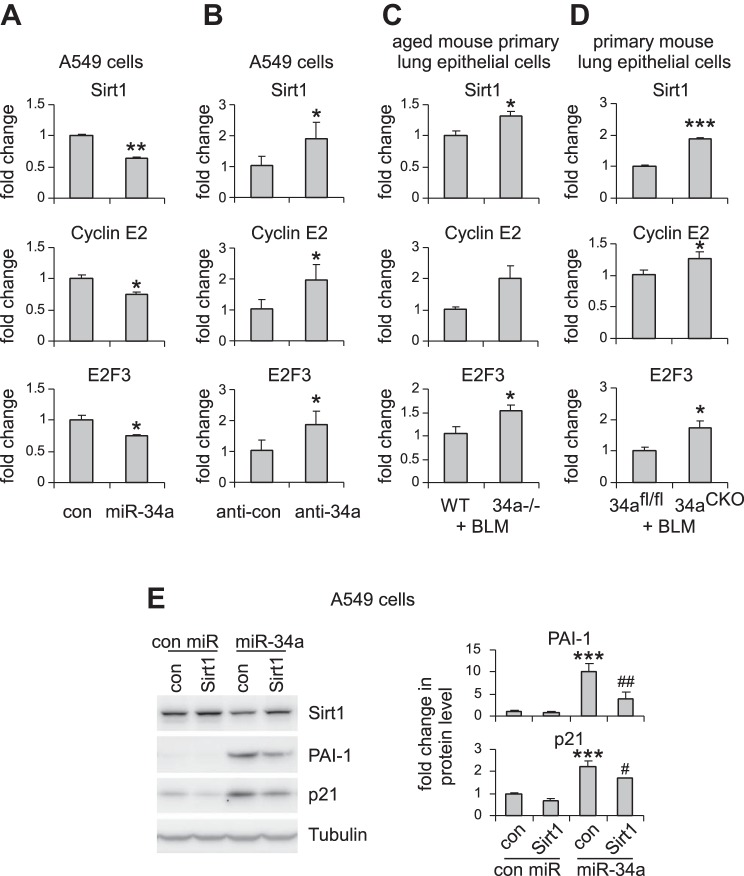
We have found that miR-34a promoted senescence of lung epithelial cells. We next determined to identify miR-34a target(s) that potentially mediated these effects. Among those predicted target genes, Sirt1 stood out as a primary candidate because it had been previously shown to be a pivotal modulator of cellular senescence (19, 21). We confirmed that Sirt1 expression was decreased in lung epithelial cells that were transfected with miR-34a (Fig. 7A). We also found that predicted targets that directly regulate cell cycle, such as cyclin E2 and E2F3, were also downregulated by miR-34a in lung epithelial cells (Fig. 7A). In contrast, miR-34a knockdown significantly increased the expression of these targets in lung epithelial cells (Fig. 7B). More importantly, the expression of these targets was enhanced in AECs of bleomycin-treated aged miR-34a−/− mice compared with those in their respective WT counterparts (Fig. 7C). Consistently, the expression of Sirt1, cyclin E2, and E2F3 was also increased in AECs of bleomycin-treated mice with specific ablation of ATII miR-34a (Fig. 7D). In addition, we generated a construct that contained full-length Sirt1 cDNA but without the Sirt1 3′-UTR. Lack of 3′-UTR deprives miR-34a of its regulation of the exogenous expression of Sirt1 in A549 cells. We found that Sirt1 overexpression partially rescued miR-34a-induced cellular senescence in A549 cells, as evidenced by reduced expression of PAI-1 and p21 in these cells (Fig. 7E). Together, these data suggest that Sirt1 mediates miR-34a-induced lung epithelial senescence and pulmonary fibrosis in aged mice.
Fig. 7.
miR-34a targets key regulators of cellular senescence, proliferation, and mitochondrial function. A: A549 cells were transfected with 50 nM control (con) or miR-34a mimics. Two days after transfection, levels of Sirt1, cyclin E2, and E2F3 were determined by real-time PCR. Values are means ± SD; n = 4. *P < 0.05 and **P < 0.01 compared with con miR group. B: A549 cells were transfected with 50 nM con inhibitors or inhibitors against miR-34a. Two days after transfection, levels of Sirt1, cyclin E2, and E2F3 were determined by real-time PCR. Values are means ± SD; n = 4. *P < 0.05 compared with the con inhibitor group. C: aged WT and miR-34a−/− mice were intratracheally instilled with bleomycin (BLM). Three weeks after treatment, lung epithelial cells were harvested. Levels of Sirt1, cyclin E2, and E2F3 were determined by real-time PCR. Values are means ± SE; n = 5 and 6 WT and miR-34a−/− mice, respectively. *P < 0.05 compared with BLM WT con. D: ATII conditional knockout mice (miR-34aCKO) and con miR-34afl/fl mice were given tamoxifen (75 mg/kg body wt in 100 μl corn oil) by intraperitoneal injection, followed by intratracheal BLM treatment. Two weeks after BLM treatment, mice were killed, and lung epithelial cells isolated. Levels of Sirt1, cyclin E2, and E2F3 were determined by real-time PCR. Values are means ± SE; n = 5. *P < 0.05 and ***P < 0.001 compared with BLM-injected miR-34afl/fl mice group. E: A549 cells were transduced with con lentiviruses or lentiviruses that expressed human Sirt1. One day after transduction, cells were transfected with 50 nM con mimics or miR-34a mimics for another 48 h. Levels of Sirt1, p21, and PAI-1 in the cells were determined by Western blotting, and densitometric analyses were performed using ImageJ (National Institutes of Health). ***P < 0.001 compared with the con virus, con miR group. #P < 0.05 and ##P < 0.01 compared with the con virus, miR-34a group.
DISCUSSION
While we demonstrated here that miR-34a deletion protected aged mice from bleomycin-induced pulmonary fibrosis, in our parallel study, we found paradoxically that miR-34a−/− young animals developed more severe fibrosis than WT young counterparts in response to bleomycin lung injury (11). The unexpected disparate effect of miR-34a deficiency on lung fibrosis between young and old animals leads to our hypothesis that miR-34a, probably like those well-recognized examples of regulators involved in tumorigenesis (3, 34, 41), plays an antagonistically pleiotropic role in that it is beneficial early in life, but deleterious later in life, in response to profibrotic lung injury. We found that the divergent role of miR-34a in pulmonary fibrosis in young and old mice could be attributed to its distinct expression patterns in different types of lung cells in these two age groups.
We found that miR-34a was upregulated in AECs, but not fibroblasts of the fibrotic lungs of aged mice. These data suggest that functions of AECs, but not lung fibroblasts in aged animals, are the ones to be likely impacted by the elevated miR-34a in response to profibrotic lung injury. However, miR-34a expression was increased in both AECs and fibroblasts in the fibrotic lungs of young animals. Although we found that ablation of miR-34a in AECs also protected young mice from developing lung fibrosis, young mice with global miR-34a knockout demonstrated more severe lung fibrosis compared with their WT counterparts (11). These findings suggest that the activity of elevated miR-34a in lung fibroblasts and likely other lung cell populations, but not that in AECs, dominates the pathological outcome after profibrotic lung injury in young animals. These disparities between young and old subjects particularly highlight an imperative that age-related diseases, including pulmonary fibrosis, need to be studied precisely in more appropriate contexts.
Although we found that miR-34a expression was differentially regulated in lung fibroblasts and epithelial cells in young and aged mice, which likely led to the disparate effects of this miRNA on lung fibrosis in young and aged animals, the unsolved issue is how the differential expression of miR-34a takes place. This actually invites an even bigger question with regard to how cellular physiology of different lung cell types evolves with aging, and it probably involves the states of many layers of regulatory networks, including at the genetic, epigenetic, and posttranslational levels, in lung cells of young and aged individuals. With improved knowledge of the aging process of lung cells, we will probably have better understanding of the precise role of many genes that were implicated in pathological lung fibrogenesis in different stages of both human and animal lives.
This study, as well as our laboratory's parallel one, provided some invaluable evidence to help settling the debate on whether cellular senescence is pro- or anti-fibrotic (22, 28). Our findings suggest that the precise role of cellular senescence in tissue fibrosis is essentially hinged on which cell population is primarily impacted by this cellular process. It is also noteworthy that age itself is likely another important factor that decides the effects of this cellular process, as we have previously shown that senescence of lung fibroblasts led to failed resolution of lung fibrosis in aged animals (18).
We showed compelling evidence that Sirt1 is a target of miR-34a in AECs, not only because Sirt1 was downregulated by miR-34a in AECs in vitro, but also the repression of Sirt1 expression was partially relieved in miR-34a−/− AECs. Sirt1 is a key regulator of cellular senescence and mitochondrial functions (19, 20, 33). This led us to the conclusion that miR-34a promotes AEC senescence and mitochondrial dysfunctions likely by targeting Sirt1 in aged lungs. Despite the important functions of Sirt1 in anti-senescence and maintenance of mitochondrial homeostasis, little is known of the role of this master regulator in IPF. Given that highly specific Sirt1 activators have already been in clinical trials for treating several age-related diseases (20), it is becoming particularly appealing to elevate our effort in pursuit of this lead in IPF.
The role of AEC dysfunctions has long been recognized in the pathogenesis of pulmonary fibrosis (29, 37). Early investigations identified AECs as a source of profibrotic myofibroblasts in fibrotic lungs through a process called epithelial-mesenchymal transition (23). More recent studies found prominent senescent phenotype in AECs in IPF (8, 27). This has apparent mechanistic implications in the pathology, because ATII cell senescence may compromise the regenerative capacity of this type of cells. ATII regeneration is well known to be a crucial step for replenishment of ATI cells in the event of lung injury (37). Additionally, senescent AECs may secrete a number of soluble molecules, many of which likely have nonautonomous profibrotic activity in the lung, such as transforming growth factor-β1 (16). Taken together, AEC dysfunctions caused by elevated miR-34a may constitute one of the key mechanisms underlying the pathogenesis of pulmonary fibrosis in the aged population.
Although we have identified Sirt1 and two other key cell cycle regulators, cyclin E2 and E2F3, as miR-34a targets that presumably mediate its activities of prosenescence and promitochondrial dysfunction in aged AECs, there are most likely additional mediators that are also involved in this process, as this has been true for many other miRNAs. This body of evidence indicates that the functions of specific miRNAs are often executed by collective actions of multiple targets. These findings also suggest miRNA be at the very top in the mechanistic network associated with complex diseases like IPF, for which they become superior therapeutic targets.
In summary, we found that miR-34a upregulation in AECs of aged animals promoted dysfunctions of these cells by inducing cellular senescence, mitochondrial aberration, and cell death and thus leading to pulmonary fibrosis in the aged population. Moreover, our finding that miR-34a had antagonistic roles in lung fibrosis in young and aged mice carries additional implications to the field, because almost all studies used young animals to identify and test therapeutic targets for treating lung fibrosis. Therefore, we may need to use extra caution when translating this body of knowledge into developing new therapies for human IPF, a disease that overwhelmingly affects people of advanced age.
GRANTS
This work was supported by US National Heart, Lung, and Blood Institute Grants HL-105473 and HL-126737 (to G. Liu) and HL-114470 (to V. J. Thannickal.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.C., J.G., N.X., S.B., Y.Z., R.-M.L. and G.L. performed experiments; H.C., J.G., N.X., S.B., Y.Z., R.-M.L., and G.L. analyzed data; H.C., J.G., N.X., S.B., Y.Z., R.-M.L., V.J.T., and G.L. interpreted results of experiments; H.C. and G.L. prepared figures; H.C., V.J.T., and G.L. drafted manuscript; H.C., V.J.T., and G.L. edited and revised manuscript; H.C., J.G., N.X., S.B., Y.Z., R.-M.L., V.J.T., and G.L. approved final version of manuscript.
REFERENCES
- 1.Ambros V. microRNAs: tiny regulators with great potential. Cell 107: 823–826, 2001. doi: 10.1016/S0092-8674(01)00616-X. [DOI] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297, 2004. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 3.Blagosklonny MV. Revisiting the antagonistic pleiotropy theory of aging: TOR-driven program and quasi-program. Cell Cycle 9: 3171–3176, 2010. doi: 10.4161/cc.9.16.13120. [DOI] [PubMed] [Google Scholar]
- 4.Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, Heydt S, Kaluza D, Tréguer K, Carmona G, Bonauer A, Horrevoets AJ, Didier N, Girmatsion Z, Biliczki P, Ehrlich JR, Katus HA, Müller OJ, Potente M, Zeiher AM, Hermeking H, Dimmeler S. MicroRNA-34a regulates cardiac ageing and function. Nature 495: 107–110, 2013. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- 5.Bueno M, Lai YC, Romero Y, Brands J, St. Croix CM, Kamga C, Corey C, Herazo-Maya JD, Sembrat J, Lee JS, Duncan SR, Rojas M, Shiva S, Chu CT, Mora AL. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest 125: 521–538, 2015. doi: 10.1172/JCI74942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol 23: 175–205, 2007. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 7.Cakarova L, Marsh LM, Wilhelm J, Mayer K, Grimminger F, Seeger W, Lohmeyer J, Herold S. Macrophage tumor necrosis factor-alpha induces epithelial expression of granulocyte-macrophage colony-stimulating factor: impact on alveolar epithelial repair. Am J Respir Crit Care Med 180: 521–532, 2009. doi: 10.1164/rccm.200812-1837OC. [DOI] [PubMed] [Google Scholar]
- 8.Chilosi M, Carloni A, Rossi A, Poletti V. Premature lung aging and cellular senescence in the pathogenesis of idiopathic pulmonary fibrosis and COPD/emphysema. Transl Res 162: 156–173, 2013. doi: 10.1016/j.trsl.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Christoffersen NR, Shalgi R, Frankel LB, Leucci E, Lees M, Klausen M, Pilpel Y, Nielsen FC, Oren M, Lund AH. p53-independent upregulation of miR-34a during oncogene-induced senescence represses MYC. Cell Death Differ 17: 236–245, 2010. doi: 10.1038/cdd.2009.109. [DOI] [PubMed] [Google Scholar]
- 10.Cui H, Banerjee S, Xie N, Ge J, Liu RM, Matalon S, Thannickal VJ, Liu G. MicroRNA-27a-3p is a negative regulator of lung fibrosis by targeting myofibroblast differentiation. Am J Respir Cell Mol Biol 54: 843–852, 2016. doi: 10.1165/rcmb.2015-0205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui H, Ge J, Xie N, Banerjee S, Zhou Y, Antony VB, Thannickal VJ, Liu G. miR-34a Inhibits Lung Fibrosis by Inducing Lung Fibroblast Senescence. Am J Respir Cell Mol Biol 56: 168–178, 2017. doi: 10.1165/rcmb.2016-0163OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F, Thannickal VJ, Cardoso WV, Lü J. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol 45: 287–294, 2011. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc 4: 1798–1806, 2009. doi: 10.1038/nprot.2009.191. [DOI] [PubMed] [Google Scholar]
- 14.Engels BM, Hutvagner G. Principles and effects of microRNA-mediated post-transcriptional gene regulation. Oncogene 25: 6163–6169, 2006. doi: 10.1038/sj.onc.1209909. [DOI] [PubMed] [Google Scholar]
- 15.Faner R, Rojas M, Macnee W, Agustí A. Abnormal lung aging in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 186: 306–313, 2012. doi: 10.1164/rccm.201202-0282PP. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez IE, Eickelberg O. The impact of TGF-β on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc 9: 111–116, 2012. doi: 10.1513/pats.201203-023AW. [DOI] [PubMed] [Google Scholar]
- 17.Hagood JS. Beyond the genome: epigenetic mechanisms in lung remodeling. Physiology (Bethesda) 29: 177–185, 2014. doi: 10.1152/physiol.00048.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, Meldrum E, Sanders YY, Thannickal VJ. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med 6: 231ra47, 2014. doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 13: 225–238, 2012. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hubbard BP, Sinclair DA. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol Sci 35: 146–154, 2014. doi: 10.1016/j.tips.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang ES. Senescence suppressors: their practical importance in replicative lifespan extension in stem cells. Cell Mol Life Sci 71: 4207–4219, 2014. doi: 10.1007/s00018-014-1685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jun JI, Lau LF. Cellular senescence controls fibrosis in wound healing. Aging (Albany NY) 2: 627–631, 2010. doi: 10.18632/aging.100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA 103: 13180–13185, 2006. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Königshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, Rose F, Fink L, Seeger W, Schaefer L, Günther A, Eickelberg O. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest 119: 772–787, 2009. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, Kaminski N, Abraham E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med 207: 1589–1597, 2010. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu G, Park YJ, Abraham E. Interleukin-1 receptor-associated kinase (IRAK)-1-mediated NF-kappaB activation requires cytosolic and nuclear activity. FASEB J 22: 2285–2296, 2008. doi: 10.1096/fj.07-101816. [DOI] [PubMed] [Google Scholar]
- 27.Minagawa S, Araya J, Numata T, Nojiri S, Hara H, Yumino Y, Kawaishi M, Odaka M, Morikawa T, Nishimura SL, Nakayama K, Kuwano K. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-β-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 300: L391–L401, 2011. doi: 10.1152/ajplung.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 15: 482–496, 2014. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 29.Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest 122: 2756–2762, 2012. doi: 10.1172/JCI60323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pandit KV, Corcoran D, Yousef H, Yarlagadda M, Tzouvelekis A, Gibson KF, Konishi K, Yousem SA, Singh M, Handley D, Richards T, Selman M, Watkins SC, Pardo A, Ben-Yehudah A, Bouros D, Eickelberg O, Ray P, Benos PV, Kaminski N. Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 182: 220–229, 2010. doi: 10.1164/rccm.200911-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parker MW, Rossi D, Peterson M, Smith K, Sikström K, White ES, Connett JE, Henke CA, Larsson O, Bitterman PB. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest 124: 1622–1635, 2014. doi: 10.1172/JCI71386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel AS, Song JW, Chu SG, Mizumura K, Osorio JC, Shi Y, El-Chemaly S, Lee CG, Rosas IO, Elias JA, Choi AM, Morse D. Epithelial cell mitochondrial dysfunction and PINK1 are induced by transforming growth factor-beta1 in pulmonary fibrosis. PLoS One 10: e0121246, 2015. doi: 10.1371/journal.pone.0121246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riera CE, Dillin A. Can aging be ‘drugged’? Nat Med 21: 1400–1405, 2015. doi: 10.1038/nm.4005. [DOI] [PubMed] [Google Scholar]
- 34.Rodier F, Campisi J, Bhaumik D. Two faces of p53: aging and tumor suppression. Nucleic Acids Res 35: 7475–7484, 2007. doi: 10.1093/nar/gkm744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rojas M, Mora AL, Kapetanaki M, Weathington N, Gladwin M, Eickelberg O; Clinical Impact and Cellular and Molecular Pathways . Aging and lung disease. Ann Am Thorac Soc 12: S222–S227, 2015. doi: 10.1513/AnnalsATS.201508-484PL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selman M, Pardo A. Revealing the pathogenic and aging-related mechanisms of the enigmatic idiopathic pulmonary fibrosis. an integral model. Am J Respir Crit Care Med 189: 1161–1172, 2014. doi: 10.1164/rccm.201312-2221PP. [DOI] [PubMed] [Google Scholar]
- 37.Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc 3: 364–372, 2006. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- 38.Tazawa H, Tsuchiya N, Izumiya M, Nakagama H. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc Natl Acad Sci USA 104: 15472–15477, 2007. doi: 10.1073/pnas.0707351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thannickal VJ, Henke CA, Horowitz JC, Noble PW, Roman J, Sime PJ, Zhou Y, Wells RG, White ES, Tschumperlin DJ. Matrix biology of idiopathic pulmonary fibrosis: a workshop report of the national heart, lung, and blood institute. Am J Pathol 184: 1643–1651, 2014. doi: 10.1016/j.ajpath.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thannickal VJ, Murthy M, Balch WE, Chandel NS, Meiners S, Eickelberg O, Selman M, Pardo A, White ES, Levy BD, Busse PJ, Tuder RM, Antony VB, Sznajder JI, Budinger GR. Blue journal conference. Aging and susceptibility to lung disease. Am J Respir Crit Care Med 191: 261–269, 2015. doi: 10.1164/rccm.201410-1876PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ungewitter E, Scrable H. Antagonistic pleiotropy and p53. Mech Ageing Dev 130: 10–17, 2009. doi: 10.1016/j.mad.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol 9: 157–179, 2014. doi: 10.1146/annurev-pathol-012513-104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie N, Liu G. ncRNA-regulated immune response and its role in inflammatory lung diseases. Am J Physiol Lung Cell Mol Physiol 309: L1076–L1087, 2015. doi: 10.1152/ajplung.00286.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu X, Chen W, Miao R, Zhou Y, Wang Z, Zhang L, Wan Y, Dong Y, Qu K, Liu C. miR-34a induces cellular senescence via modulation of telomerase activity in human hepatocellular carcinoma by targeting FoxM1/c-Myc pathway. Oncotarget 6: 3988–4004, 2015. doi: 10.18632/oncotarget.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang S, Banerjee S, de Freitas A, Sanders YY, Ding Q, Matalon S, Thannickal VJ, Abraham E, Liu G. Participation of miR-200 in pulmonary fibrosis. Am J Pathol 180: 484–493, 2012. doi: 10.1016/j.ajpath.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang S, Cui H, Xie N, Icyuz M, Banerjee S, Antony VB, Abraham E, Thannickal VJ, Liu G. miR-145 regulates myofibroblast differentiation and lung fibrosis. FASEB J 27: 2382–2391, 2013. doi: 10.1096/fj.12-219493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng Y. Principles of micro-RNA production and maturation. Oncogene 25: 6156–6162, 2006. doi: 10.1038/sj.onc.1209908. [DOI] [PubMed] [Google Scholar]