Abstract
The role of cannabinoid type 1 (CB1) receptors in tibial and pudendal neuromodulation of bladder overactivity induced by intravesical infusion of 0.5% acetic acid (AA) was determined in α-chloralose anesthetized cats. AA irritation significantly (P < 0.01) reduced bladder capacity to 36.6 ± 4.8% of saline control capacity. Tibial nerve stimulation (TNS) at two or four times threshold (2T or 4T) intensity for inducing toe movement inhibited bladder overactivity and significantly (P < 0.01) increased bladder capacity to 69.2 ± 9.7 and 79.5 ± 7.2% of saline control, respectively. AM 251 (a CB1 receptor antagonist) administered intravenously at 0.03 or 0.1 mg/kg significantly (P < 0.05) reduced the inhibition induced by 2T or 4T TNS, respectively, without changing the prestimulation bladder capacity. However, intrathecal administration of AM 251 (0.03 mg) to L7 spinal segment had no effect on TNS inhibition. Pudendal nerve stimulation (PNS) also inhibited bladder overactivity induced by AA irritation, but AM 251 at 0.01–1 mg/kg iv had no effect on PNS inhibition or the prestimulation bladder capacity. These results indicate that CB1 receptors play an important role in tibial but not pudendal neuromodulation of bladder overactivity and the site of action is not within the lumbar L7 spinal cord. Identification of neurotransmitters involved in TNS or PNS inhibition of bladder overactivity is important for understanding the mechanisms of action underlying clinical application of neuromodulation therapies for bladder disorders.
Keywords: cannabinoid, tibial, pudendal, neuromodulation, bladder
overactive bladder (OAB) symptoms are characterized by urinary urgency, frequency, and nocturia with or without incontinence (1). OAB affects more than 30 million adults in United States (8) with a significant impact on the quality of life (7). Antimuscarinic drugs, which are the first-line pharmacotherapy for OAB, have a limited efficacy with significant adverse effects (3, 4, 6). After pharmacotherapy fails, neuromodulation and intradetrusor botulinum-A toxin injection are the next treatment options for OAB patients (23, 27). Tibial (20, 26) and pudendal (18, 19) neuromodulation are also effective in treating OAB. However, the mechanism underlying bladder neuromodulation is currently uncertain. Understanding the neurotransmitter mechanisms involved in bladder neuromodulation may help in the discovery of molecular targets for developing new therapies for OAB.
Our previous studies in cats have shown that opioid receptors play a critical role in tibial (25, 30) but not pudendal neuromodulation (16) of bladder overactivity induced by acetic acid irritation of the bladder. Application of naloxone (an opioid receptor antagonist) to the surface of pons removed tibial inhibition of irritation-induced bladder overactivity (10) and spinal cord transection at the T9–T10 level eliminated tibial but not pudendal inhibition (29), indicating that tibial neuromodulation probably acts at supraspinal sites. Since the interactions between cannabinoid and opioid mechanisms in the central nervous system are well known (9, 28), it is logical to hypothesize that similar to the opioid receptors the cannabinoid (CB) receptors at a supraspinal site might also be involved in tibial but not pudendal neuromodulation.
CB receptors are G protein-coupled receptors, which include type 1 (CB1) and type 2 (CB2) receptors (17). Previous animal studies have indicated that both CB1 and CB2 receptors are involved in micturition control (2, 12, 15, 22). In this study using α-chloralose anesthetized cats, we focused our attention on the role of CB1 receptors in tibial and pudendal neuromodulation of bladder overactivity. The bladder was irritated with dilute (0.5%) acetic acid to activate the nociceptive bladder C-fiber afferents and induce bladder overactivity. Electrical stimulation was applied to tibial or pudendal nerve to inhibit the bladder overactivity. Then, AM251 (a selective CB1 receptor antagonist) was administrated intravenously to determine the involvement of CB1 receptors in the two types of neuromodulation. AM251 was also administered intrathecally to determine the central site of action.
METHODS AND MATERIALS
The protocol and animal use in this study were approved by the Animal Care and Use Committee at the University of Pittsburgh.
Surgical procedures.
A total of 17 cats (10 males and 7 females, 2.4–4.0 kg; Liberty Research, Waverly, NY) were used in this study. The animals were anesthetized with isoflurane (2–5% in oxygen) during surgery and then switched to α-chloralose anesthesia (initial 65 mg/kg iv and supplemented as needed) during data collection. Left cephalic veins were catheterized for intravenous administration of drugs and fluid. A tracheotomy was performed and a tube was inserted to keep the airway patent. A catheter was inserted into right carotid artery to monitor systemic blood pressure. Heart rate and blood oxygen were monitored by a pulse oximeter (9847V; NONIN Medical, Plymouth, MN) attached to the tongue. Through an abdominal incision, the ureters were isolated, tied, and cut for external drainage. A double lumen catheter was inserted through the urethra into the bladder and secured by a ligature around the urethra. One lumen was connected to a pump to slowly (1–3 ml/min) infuse saline or 0.5% acetic acid (AA). The other lumen was attached to a pressure transducer to measure bladder pressure. The tibial nerve was exposed in the left leg above the ankle, and a tripolar cuff electrode (NC223pt; MicroProbe, Gaithersburg, MD) was implanted for stimulation. The right pudendal nerve was dissected via a 3- to 4-cm incision in the sciatic notch lateral to the tail for implantation of another tripolar cuff electrode. The tibial and pudendal nerve electrodes were connected to different output channels of an electrical stimulator (S88; Grass Medical Instruments, Quincy, MA) via constant voltage stimulus isolators (SIU5; Grass Medical Instruments). In five cats, a small incision was made to remove the L3 spinal process and expose the spinal cord. Then, the spinal dura was pierced and a fine catheter (PE10) was inserted caudally underneath the dura to position the catheter tip at the level of L7 spinal cord for intrathecal administration of AM251, because our recent study in cats has shown that tibial afferent nerves passing through L7 dorsal root play a critical role in tibial inhibition of bladder overactivity (5). The location of the intrathecal catheter was confirmed by a postmortem laminectomy between the L5-S3 spinal processes. After the surgery, the skin and muscle layers were closed by sutures.
Stimulation protocol and drug administration.
Uniphasic rectangular pulses (5-Hz frequency, 0.2-m pulse width) were used to stimulate the tibial or pudendal nerve via the cuff electrode. The intensity threshold (T) for inducing observable toe movement or anal sphincter twitch was determined by gradually increasing the stimulation intensity. Based on our previous studies (25, 30), intensities of 2T or 4T were used in this study to suppress bladder overactivity.
At the beginning of each experiment, multiple cystometrograms (CMGs) were performed by slowly infusing the bladder with saline to determine the bladder capacity that was defined as the bladder volume threshold to induce a bladder contraction of large amplitude (>30 cmH2O) and long duration (>20 s). Then, AA was infused into the bladder to irritate the bladder, activate nociceptive C-fiber afferent nerves, and induce bladder overactivity. Once the control bladder capacity stabilized during repeated AA CMGs, the inhibitory effect of tibial nerve stimulation (TNS) was determined by additional four AA CMGs 1): control CMG without TNS; 2) CMG during 2T TNS; 3); CMG during 4T TNS; and 4); control CMG again to examine any poststimulation effect. Then, the animals were divided into three experimental groups.
In the first group (n = 5 cats), cumulative doses (0.01, 0.03, 0.1, 0.3, and 1.0 mg/kg iv) of AM251 (MedChemExpress) were given. Since AM251 was dissolved in 30% Cremophor (Sigma-Aldrich, St. Louis, MO), in the second group (n = 5 cats) the vehicle solution (30% Cremophor in saline) was administered in increasing volumes corresponding to the volumes of cumulative doses of AM251 (0.01, 0.03, 0.1, 0.3, and 1.0 mg/kg iv). In the third group (n = 5 cats, 3 cats from the vehicle control group), a single dose (0.03 mg it) of AM 251 was given. After administering each dose of drug/vehicle, the four CMGs (control, 2T TNS, 4T TNS, control) were repeated to determine the drug/vehicle effects. A 10-min waiting period after each intravenous dose of AM251/vehicle and a 5-min waiting period after intrathecal AM251 were used for the drug/vehicle to take effect. Waiting period of 2–3 min was also used between CMGs for the bladder reflex to recover.
The same experimental protocol used to test TNS was also used to test the effect of cumulative doses (0.01, 0.03, 0.1, 0.3, and 1.0 mg/kg iv) of AM251 on pudendal nerve stimulation (PNS) in the fourth group of five cats.
Data analysis.
Bladder capacity was measured during each CMG and normalized to the capacity measured during the first control CMG in different experimental groups. Repeated measurements (2–3 CMGs) of control bladder capacity in the same animal under the same conditions (saline or AA) were averaged. The normalized data from different animals are presented as means ± SE. Statistical significance (P < 0.05) was determined by repeated-measures ANOVA followed by Dunnett’s (one-way) or Bonferroni’s (two-way) multiple comparison.
RESULTS
TNS inhibition of bladder overactivity induced by AA irritation.
AA irritation induced bladder overactivity that significantly (P < 0.01) reduced bladder capacity to 36.6 ± 4.8% of saline control capacity (n = 12 cats, Fig. 1). TNS at 2T or 4T intensity inhibited the bladder overactivity and significantly (P < 0.01) increased bladder capacity to 69.2 ± 9.7 and 79.5 ± 7.2% of saline control, respectively (Fig. 1). After the stimulation, AA control capacity returned to prestimulation level, indicating that there was no post-stimulation effect (Fig. 1).
Fig. 1.
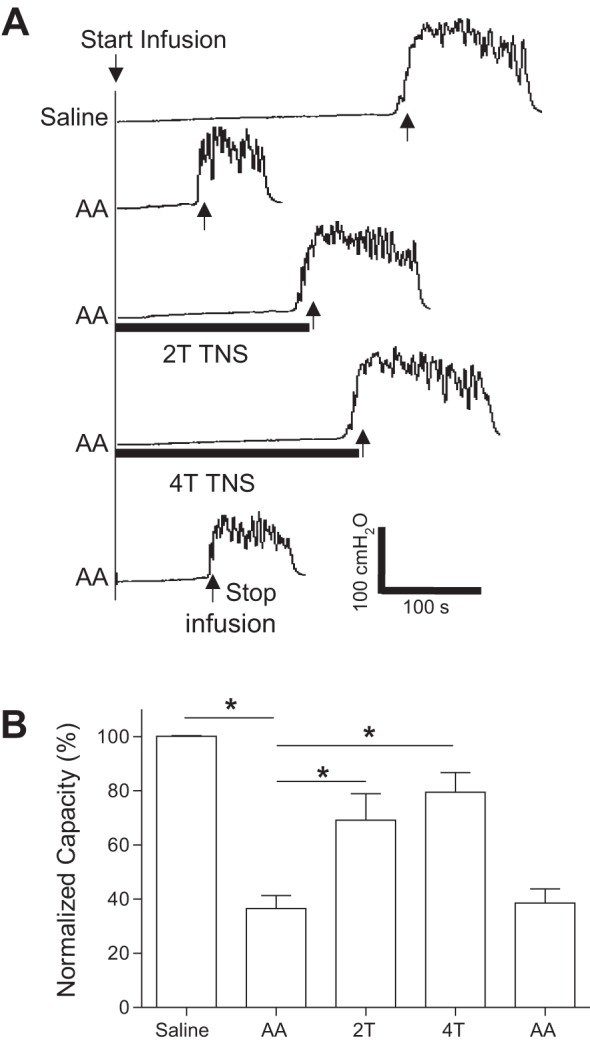
Tibial nerve stimulation (TNS) inhibits bladder overactivity induced by 0.5% acetic acid (AA) irritation. A: repeated cystometrograms (CMGs) during saline or AA infusion with or without TNS. T, threshold TNS intensity for inducing toe twitching. Black bars under the bladder pressure traces indicate the duration of TNS (5 Hz, 0.2 ms, T = 2.2 V). Infusion rate = 2 ml/min. B: summarized results from 12 cats. The bladder capacity is normalized to the measurement during saline infusion. *Significantly (P < 0.01) different from the AA control (one-way ANOVA).
Effect of AM251 on TNS or PNS inhibition of bladder overactivity.
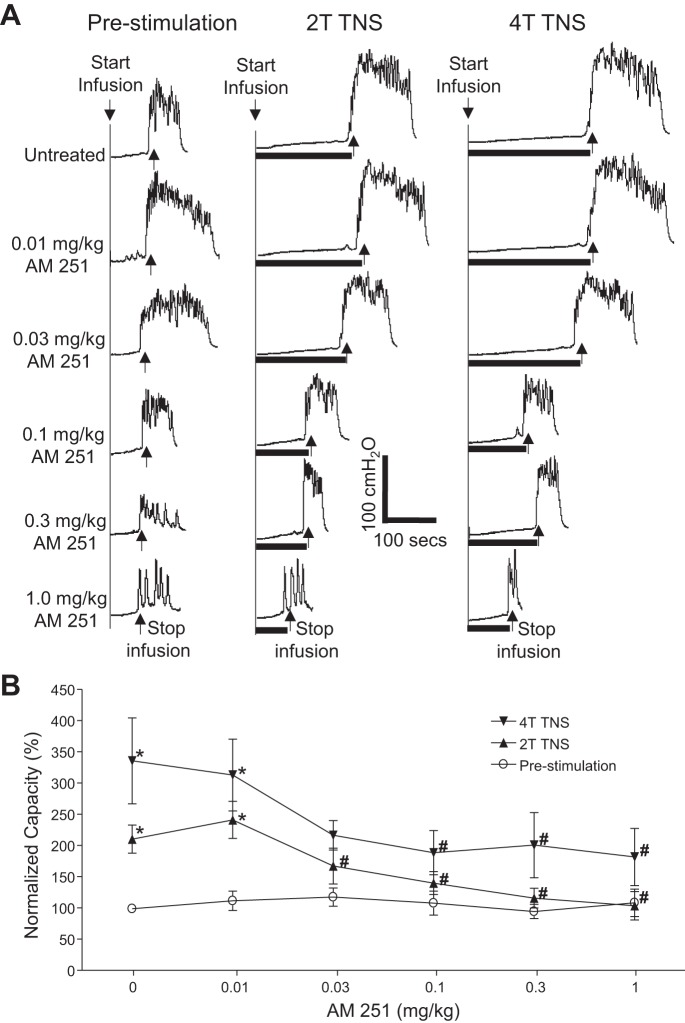
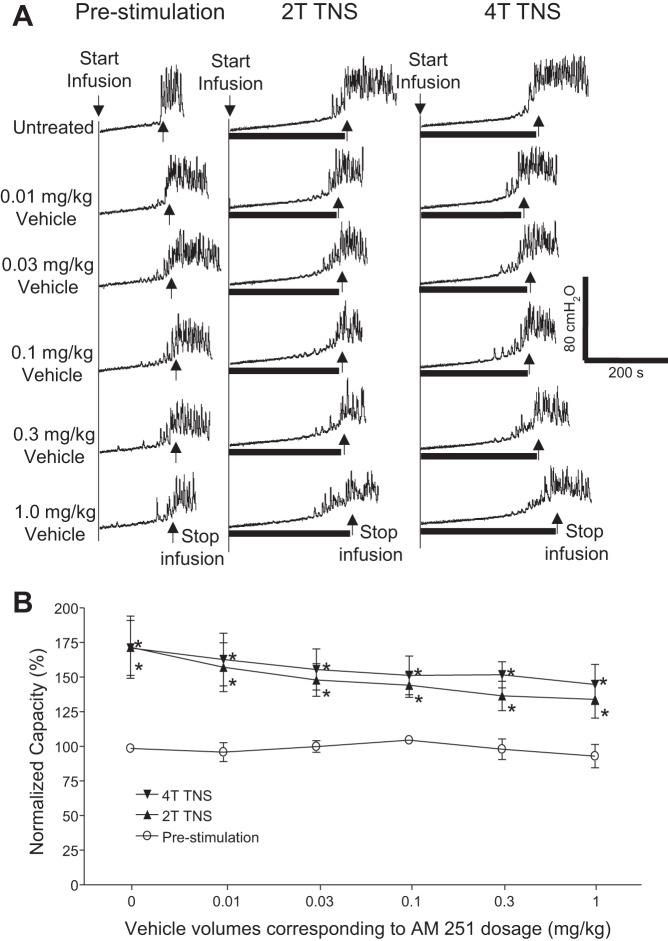
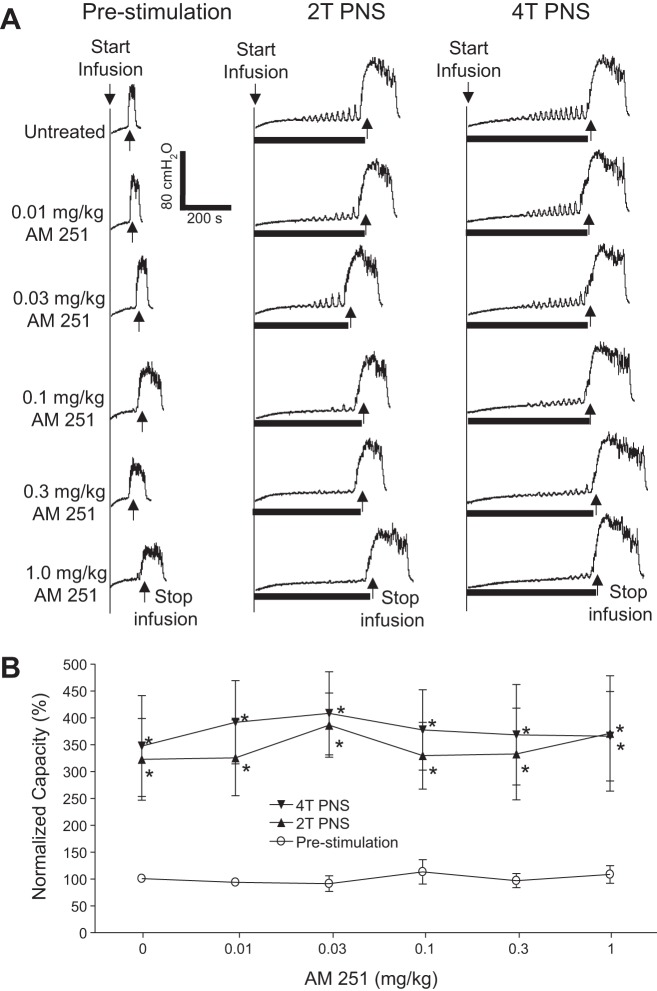
AM251 (intravenous) dose dependently reduced TNS inhibition of bladder overactivity without changing the prestimulation bladder capacity during repeated AA CMGs (Fig. 2A). The increase in bladder capacity induced by TNS was significantly (P < 0.05) reduced by AM251 at 0.03 and 0.1 mg/kg for 2T TNS and 4T TNS, respectively (Fig. 2B). The vehicle (30% Cremophor in saline) at volumes corresponding to those used to administer different intravenous doses of AM215 did not have a significant effect on TNS inhibition or prestimulation bladder capacity during repeated AA CMGs (Fig. 3). AM251 (0.03 mg it) had no effect on TNS inhibition or prestimulation bladder capacity during AA CMGs (Fig. 4), indicating that the site of action of the intravenous doses of drug is not within the lumbar L7 spinal cord. AM251 at 0.01–1 mg/kg iv had no effect on PNS inhibition of bladder overactivity or prestimulation bladder capacity during repeated AA CMGs (Fig. 5). No poststimulation effect was observed after any dose of drug/vehicle treatment (data not shown).
Fig. 2.
Dose-dependent effect of AM 251 (intravenous) on the inhibition of bladder overactivity induced by tibial nerve stimulation (TNS). A: repeated CMGs at increasing cumulative doses of AM 251 were performed during acetic acid (AA) infusion with or without TNS. T, threshold TNS intensity for inducing toe twitching. Black bars under the pressure trace indicate the duration of TNS (5 Hz, 0.2 ms, T = 2.2 V). Infusion rate = 2 ml/min. B: summarized results from 5 cats. The bladder capacity is normalized to the untreated condition before TNS. *Significantly (P < 0.05) different from prestimulation at each dosage (two-way ANOVA). #Significantly (P < 0.05) different from the bladder capacity measured during TNS before AM 251 treatment (one-way ANOVA).
Fig. 3.
Vehicle (30% Cremophor in saline, intravenous) has no significant effect on the inhibition of bladder overactivity induced by tibial nerve stimulation (TNS). A: repeated CMGs were performed during acetic acid (AA) infusion with or without TNS after administering different vehicle volumes corresponding to those cumulative doses of AM251. T, threshold TNS intensity for inducing toe twitching. Black bars under the pressure trace indicate the duration of TNS (5 Hz, 0.2 ms, T = 0.7 V). Infusion rate = 3 ml/min. B: summarized results from 5 cats. The bladder capacity is normalized to the untreated condition before TNS. *Significantly (P < 0.05) different from prestimulation at each dosage (two-way ANOVA).
Fig. 4.
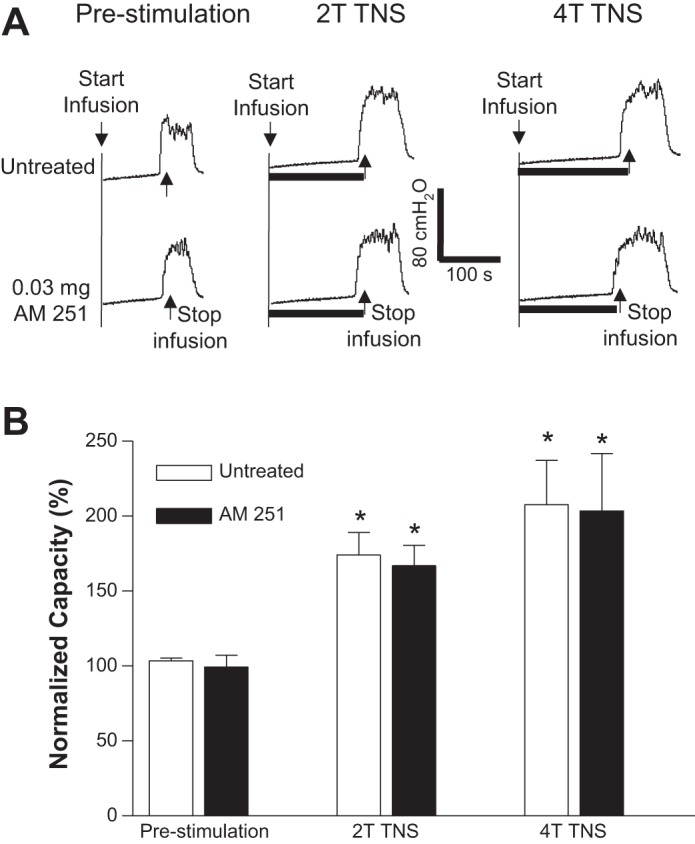
Intrathecal administration of AM 251 does not alter the inhibition of bladder overactivity induced by tibial nerve stimulation (TNS). A: repeated CMGs were performed during acetic acid (AA) infusion with or without TNS. T, threshold TNS intensity for inducing toe twitching. Black bars under the pressure trace indicate the duration of TNS (5 Hz, 0.2 ms, T = 0.8 V). Infusion rate = 3 ml/min. B: summarized results from 5 cats. The bladder capacity is normalized to the untreated condition before TNS. *Significantly (P < 0.05) different from prestimulation in untreated or AM 251 treated conditions (one-way ANOVA).
Fig. 5.
AM 251 (intravenous) does not alter the inhibition of bladder overactivity induced by pudendal nerve stimulation (PNS). A: repeated CMGs at increasing cumulative doses of AM 251 were performed during acetic acid (AA) infusion with or without PNS. T, threshold PNS intensity for inducing anal sphincter twitching. Black bars under the pressure trace indicate the duration of PNS (5 Hz, 0.2 ms, T = 2.4 V). Infusion rate = 2 ml/min. B: summarized results from 5 cats. The bladder capacity is normalized to the untreated condition before PNS. *Significantly (P < 0.05) different from prestimulation at each dosage (two-way ANOVA).
DISCUSSION
This study in anesthetized cats revealed that CB1 receptors play a critical role in TNS inhibition (Figs. 2 and 3) but not in PNS inhibition (Fig. 5) of bladder overactivity induced by AA irritation. Furthermore, the fact that the intravenous administration of the CB1 antagonist reduced the TNS inhibition but intrathecal administration to lumbar L7 spinal cord was ineffective (Fig. 4) suggests that the inhibition occurs at a central site distant from L7 spinal cord or at a peripheral site in the bladder. The data also indicate that during AA irritation CB1 receptor inhibitory mechanisms are inactive except during TNS because the antagonist did not alter baseline bladder capacity before TNS (Figs. 2–5).
Our previous studies in cats showed that opioid receptors also play a critical role in TNS inhibition of bladder overactivity (25, 30). Thus in future experiments it will be important to investigate the potential interaction between opioid and CB1 receptors in tibial neuromodulation because it is well known that these two types of receptor mechanisms interact at other sites in the central nervous system (9, 28). In addition the similar role of the two mechanisms in TNS inhibition of bladder overactivity raises the question of whether this similarity extends to TNS inhibition of normal bladder reflexes during saline CMGs. Saline distention of bladder activates nonnociceptive Aδ afferent fibers that trigger a supraspinal micturition reflex while the nociceptive C-fiber afferents are silent (14). During AA irritation the nociceptive C-fiber afferents are activated, which triggers a spinal micturition reflex and produces bladder overactivity (11, 14). Since our previous study in cats revealed that opioid receptors do not play a role in TNS inhibition of normal bladder activity induced by saline distention (25), it is possible that CB1 receptors might also not be involved in TNS inhibition of normal bladder activity particularly if the interaction between CB1 and opioid receptors is required for the expression of the CB1 receptor-mediated inhibition.
CB1 receptors are not involved in PNS inhibition of bladder overactivity induced by AA irritation (Fig. 5), which also mirrors very well our previous study showing that opioid receptor mechanisms do not play a role in PNS inhibition of bladder overactivity (16) and have only a minor role in PNS inhibition of normal bladder activity during saline CMGs. Thus it is possible that CB1 receptor might also play a minor role in PNS inhibition of normal bladder activity. The different results between PNS and TNS may also reflect the fact that PNS and TNS activate afferent inputs to different spinal segments. Our recent study in cats has shown that tibial afferent input to lumbar L7 spinal cord plays a critical role in TNS inhibition of bladder overactivity, while pudendal afferent inputs to both sacral S1 and S2 spinal cord are important for PNS inhibition (5).
The probability that supraspinal CB1 receptors contribute to TNS inhibition of bladder overactivity (Fig. 4) is similar to the site of action of the opioid receptors in TNS inhibition of bladder overactivity (10, 29). Our previous studies in cats showed that TNS did not inhibit the AA irritation-induced spinal bladder reflex after acute T9/T10 spinal cord transection (29) but application of naloxone (an opioid receptor antagonist) to the surface of the pons blocked TNS inhibition of AA irritation-induced bladder overactivity (10). Therefore, it is possible that CB1 and opioid receptors might interact synergistically in the pons to produce TNS inhibition of bladder overactivity.
Although it is known that both CB1 and CB2 receptors are involved in micturition control in rats (2, 15, 22), previous studies focused primarily on the role of these receptors in the peripheral nervous system and bladder muscle (2, 15, 22) and the functional roles of both spinal and supraspinal CB receptors in micturition control are not fully understood (2). A recent study in awake rats showed that intrathecal AM251, a selective CB1 receptor antagonist, reduced the bladder overactivity induced by chronic urethral outlet obstruction (12), indicating tonic activation of CB1 receptors elicits an excitatory effect on spinal micturition circuitry. However, our results in anesthetized cats show that neither central nor peripheral CB1 receptors tonically regulate the bladder overactivity induced by AA irritation (Figs. 2–5). This difference could be due to the different species (rat and cat) or anesthesia or that chronic urethral outlet obstruction (14 days) is needed for a tonic activation of CB1 receptors in the spinal cord (12) while acute AA irritation (<8 h) in our study was insufficient to upregulate CB1 receptor mechanism. Nevertheless, TNS was able to activate CB1 receptors and inhibit bladder overactivity (Figs. 2–4).
Similarly, our previous studies in cats (16, 25, 30) also show that opioid receptors do not tonically regulate the bladder overactivity induced by AA irritation. However, normal bladder activity induced by saline distention in cats is tonically inhibited by a central opioid receptor mechanism (21). Therefore, it is possible that CB1 receptors in cats might also tonically inhibit the normal micturition reflex. This possibility is supported by a recent study in rats with normal bladders (12) showing that intrathecal administration of a CB1 antagonist induced bladder overactivity.
AM251 has a 300-fold selectivity for the CB1 receptor over the CB2 receptor (13, 24). Since the effect of AM251 on TNS inhibition was observed at a relatively low dose (0.03 mg/kg, Fig. 2B), it is highly likely due to the antagonism of CB1 receptors rather than CB2 receptors. A recent study (24) has shown that AM251 can also bind to μ-opioid receptors with a similar affinity to CB2 receptors and at a high dose (10 mg/kg ip) of AM251 can antagonize the analgesic effect of morphine (a μ-opioid receptor agonist). Our previous studies (25, 30) have also shown that a μ-opioid receptor antagonist can significantly reduce TNS inhibition of bladder overactivity. However, the effective intraperitoneal dose (10 mg/kg) for AM251 to antagonize μ-opioid receptors is more than 300 times higher than the effective intravenous dose (0.03 mg/kg) for AM251 to reduce TNS inhibition. Therefore, it is reasonable to believe that the AM251 effect observed in this study (Fig. 2) is due to blocking CB1 receptors and not due to an effect on μ-opioid receptors. Meanwhile, it can also be expected that a AM251 dose much higher than 0.03 mg/kg iv will be needed to see a possible effect of CB2 receptor antagonism on TNS inhibition because AM251 has a similar low affinity for both CB2 and μ-opioid receptors (24).
In summary, this study revealed that CB1 receptors at supraspinal sites play a critical role in TNS but not PNS inhibition of bladder overactivity induced by AA irritation of the bladder in cats. Revealing the neurotransmitter mechanisms involved in neuromodulation of bladder reflexes is important for understanding the possible mechanisms underlying clinical application of neuromodulation therapies for OAB.
GRANTS
This study was supported by the National Institutes of Diabetes, Digestive and Kidney Diseases Grants DK-094905, DK-102427, and DK-091253.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
X.J., M.Y., J.U., T.F., C.J., B.S., J.W., J.R.R., W.d.G., and C.T. conceived and designed research; X.J., M.Y., J.U., T.F., C.J., B.S., J.W., J.R.R., W.d.G., and C.T. performed experiments; X.J., M.Y., J.U., T.F., C.J., B.S., J.W., J.R.R., W.d.G., and C.T. analyzed data; X.J., M.Y., J.U., T.F., C.J., B.S., J.W., J.R.R., W.d.G., and C.T. interpreted results of experiments; X.J., M.Y., J.U., T.F., C.J., B.S., J.W., J.R.R., W.d.G., and C.T. prepared figures; X.J., M.Y., J.U., T.F., C.J., B.S., J.W., J.R.R., W.d.G., and C.T. drafted manuscript; X.J., M.Y., J.U., T.F., C.J., B.S., J.W., J.R.R., W.d.G., and C.T. edited and revised manuscript; X.J., M.Y., J.U., T.F., C.J., B.S., J.W., J.R.R., W.d.G., and C.T. approved final version of manuscript.
REFERENCES
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol 187: 116–126, 2002. doi: 10.1067/mob.2002.125704. [DOI] [PubMed] [Google Scholar]
- 2.Andersson KE. Potential future pharmacological treatment of bladder dysfunction. Basic Clin Pharmacol Toxicol 119, Suppl 3: 75–85, 2016. doi: 10.1111/bcpt.12577. [DOI] [PubMed] [Google Scholar]
- 3.Andersson KE, Pehrson R. CNS involvement in overactive bladder: pathophysiology and opportunities for pharmacological intervention. Drugs 63: 2595–2611, 2003. doi: 10.2165/00003495-200363230-00003. [DOI] [PubMed] [Google Scholar]
- 4.Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev 56: 581–631, 2004. doi: 10.1124/pr.56.4.4. [DOI] [PubMed] [Google Scholar]
- 5.Bansal U, Fuller TW, Jiang X, Bandari J, Zhang Z, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Lumbosacral spinal segmental contributions to tibial and pudendal neuromodulation of bladder overactivity in cats. Neurourol Urodyn 24 October 2016 [Epub ahead of print]. doi: 10.1002/nau.23159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D. The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol 54: 543–562, 2008. doi: 10.1016/j.eururo.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 7.Coyne KS, Sexton CC, Irwin DE, Kopp ZS, Kelleher CJ, Milsom I. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int 101: 1388–1395, 2008. doi: 10.1111/j.1464-410X.2008.07601.x. [DOI] [PubMed] [Google Scholar]
- 8.Coyne KS, Sexton CC, Vats V, Thompson C, Kopp ZS, Milsom I. National community prevalence of overactive bladder in the United States stratified by sex and age. Urology 77: 1081–1087, 2011. doi: 10.1016/j.urology.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 9.Fattore L, Deiana S, Spano SM, Cossu G, Fadda P, Scherma M, Fratta W. Endocannabinoid system and opioid addiction: behavioural aspects. Pharmacol Biochem Behav 81: 343–359, 2005. doi: 10.1016/j.pbb.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 10.Ferroni MC, Slater RC, Shen B, Xiao Z, Wang J, Lee A, Roppolo JR, de Groat WC, Tai C. Role of the brain stem in tibial inhibition of the micturition reflex in cats. Am J Physiol Renal Physiol 309: F242–F250, 2015. doi: 10.1152/ajprenal.00135.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 9: 453–466, 2008. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Füllhase C, Schreiber A, Giese A, Schmidt M, Montorsi F, Gratzke C, La Croce G, Castiglione F, Stief C, Hedlund P. Spinal neuronal cannabinoid receptors mediate urodynamic effects of systemic fatty acid amide hydrolase (FAAH) inhibition in rats. Neurourol Urodyn 35: 464–470, 2016. doi: 10.1002/nau.22753. [DOI] [PubMed] [Google Scholar]
- 13.Gatley SJ, Lan R, Pyatt B, Gifford AN, Volkow ND, Makriyannis A. Binding of the non-classical cannabinoid CP 55,940, and the diarylpyrazole AM251 to rodent brain cannabinoid receptors. Life Sci 61: PL191–PL197, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Häbler HJ, Jänig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol 425: 545–562, 1990. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedlund P, Gratzke C. The endocannabinoid system–a target for the treatment of LUTS? Nat Rev Urol 13: 463–470, 2016. doi: 10.1038/nrurol.2016.110. [DOI] [PubMed] [Google Scholar]
- 16.Mally AD, Matsuta Y, Zhang F, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Role of opioid and metabotropic glutamate 5 receptors in pudendal inhibition of bladder overactivity in cats. J Urol 189: 1574–1579, 2013. doi: 10.1016/j.juro.2012.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pertwee RG, Ross RA. Cannabinoid receptors and their ligands. Prostaglandins Leukot Essent Fatty Acids 66: 101–121, 2002. doi: 10.1054/plef.2001.0341. [DOI] [PubMed] [Google Scholar]
- 18.Peters KM, Feber KM, Bennett RC. Sacral versus pudendal nerve stimulation for voiding dysfunction: a prospective, single-blinded, randomized, crossover trial. Neurourol Urodyn 24: 643–647, 2005. doi: 10.1002/nau.20174. [DOI] [PubMed] [Google Scholar]
- 19.Peters KM, Killinger KA, Boguslawski BM, Boura JA. Chronic pudendal neuromodulation: expanding available treatment options for refractory urologic symptoms. Neurourol Urodyn 29: 1267–1271, 2010. doi: 10.1002/nau.20823. [DOI] [PubMed] [Google Scholar]
- 20.Peters KM, Macdiarmid SA, Wooldridge LS, Leong FC, Shobeiri SA, Rovner ES, Siegel SW, Tate SB, Jarnagin BK, Rosenblatt PL, Feagins BA. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol 182: 1055–1061, 2009. doi: 10.1016/j.juro.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 21.Roppolo JR, Booth AM, De Groat WC. The effects of naloxone on the neural control of the urinary bladder of the cat. Brain Res 264: 355–358, 1983. doi: 10.1016/0006-8993(83)90841-7. [DOI] [PubMed] [Google Scholar]
- 22.Ruggieri MR., Sr Cannabinoids: potential targets for bladder dysfunction. Handb Exp Pharmacol 202: 425–451, 2011. doi: 10.1007/978-3-642-16499-6_20. [DOI] [PubMed] [Google Scholar]
- 23.Schulte-Baukloh H, Weiss C, Stolze T, Herholz J, Stürzebecher B, Miller K, Knispel HH. Botulinum-A toxin detrusor and sphincter injection in treatment of overactive bladder syndrome: objective outcome and patient satisfaction. Eur Urol 48: 984–990, 2005. doi: 10.1016/j.eururo.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Seely KA, Brents LK, Franks LN, Rajasekaran M, Zimmerman SM, Fantegrossi WE, Prather PL. AM-251 and rimonabant act as direct antagonists at mu-opioid receptors: implications for opioid/cannabinoid interaction studies. Neuropharmacology 63: 905–915, 2012. doi: 10.1016/j.neuropharm.2012.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tai C, Larson JA, Ogagan PD, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC. Differential role of opioid receptors in tibial nerve inhibition of nociceptive and nonnociceptive bladder reflexes in cats. Am J Physiol Renal Physiol 302: F1090–F1097, 2012. doi: 10.1152/ajprenal.00609.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vandoninck V, van Balken MR, Finazzi Agrò E, Petta F, Micali F, Heesakkers JP, Debruyne FM, Kiemeney LA, Bemelmans BL. Percutaneous tibial nerve stimulation in the treatment of overactive bladder: urodynamic data. Neurourol Urodyn 22: 227–232, 2003. doi: 10.1002/nau.10111. [DOI] [PubMed] [Google Scholar]
- 27.van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, Lycklama á Nijholt AA, Siegel S, Jonas U, Fowler CJ, Fall M, Gajewski JB, Hassouna MM, Cappellano F, Elhilali MM, Milam DF, Das AK, Dijkema HE, van den Hombergh U. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol 178: 2029–2034, 2007. doi: 10.1016/j.juro.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 28.Viganò D, Rubino T, Parolaro D. Molecular and cellular basis of cannabinoid and opioid interactions. Pharmacol Biochem Behav 81: 360–368, 2005. doi: 10.1016/j.pbb.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 29.Xiao Z, Rogers MJ, Shen B, Wang J, Schwen Z, Roppolo JR, de Groat WC, Tai C. Somatic modulation of spinal reflex bladder activity mediated by nociceptive bladder afferent nerve fibers in cats. Am J Physiol Renal Physiol 307: F673–F679, 2014. doi: 10.1152/ajprenal.00308.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Z, Slater RC, Ferroni MC, Kadow BT, Lyon TD, Shen B, Xiao Z, Wang J, Kang A, Roppolo JR, de Groat WC, Tai C. Role of µ, κ, and δ opioid receptors in tibial inhibition of bladder overactivity in cats. J Pharmacol Exp Ther 355: 228–234, 2015. doi: 10.1124/jpet.115.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]