Abstract
Recurrent heat stress and dehydration have recently been shown experimentally to cause chronic kidney disease (CKD). One potential mediator may be vasopressin, acting via the type 2 vasopressin receptor (V2 receptor). We tested the hypothesis that desmopressin accelerates CKD in mice subjected to heat stress and recurrent dehydration. Recurrent exposure to heat with limited water availability was performed in male mice over a 5-wk period, with one group receiving desmopressin two times daily and the other group receiving vehicle. Two additional control groups were not exposed to heat or dehydration and received vehicle or desmopressin. The effects of the treatment on renal injury were assessed. Heat stress and recurrent dehydration induced functional changes (albuminuria, elevated urinary neutrophil gelatinase-associated protein), glomerular changes (mesangiolysis, matrix expansion), and tubulointerstitial changes (fibrosis, inflammation). Desmopressin also induced albuminuria, glomerular changes, and tubulointerstitial fibrosis in normal animals and also exacerbated injury in mice with heat stress nephropathy. Both heat stress and/or desmopressin were also associated with activation of the polyol pathway in the renal cortex, likely due to increased interstitial osmolarity. Our studies document both glomerular and tubulointerstitial injury and inflammation in heat stress nephropathy and may be clinically relevant to the pathogenesis of Mesoamerican nephropathy. Our data also suggest that vasopressin may play a role in the pathogenesis of the renal injury of heat stress nephropathy, likely via a V2 receptor-dependent pathway.
Keywords: vasopressin, copeptin, Mesoamerican nephropathy, chronic kidney disease of unknown etiology
historically the development of a prerenal state was thought to be fully reversible without any permanent kidney damage. However, the identification of an epidemic of chronic kidney disease (CKD) of unknown etiology in Central America has renewed interest in this subject. Specifically, the epidemic is occurring in manual workers on the Pacific coast who are at high risk for recurrent dehydration due to the extreme heat and humidity (9, 13). While initial concerns included toxin exposures, the primary risk factor to date has been recurrent heat stress and dehydration (12, 23, 33). Indeed, the observation of similar epidemics in other countries has led to the suggestion that “heat stress nephropathy” might represent a type of CKD that has not been recognized as a major cause of CKD but one that might be increasing due to progressive water shortage and climate changes (14).
To better understand the mechanisms by which heat stress might cause CKD, different animal models have been developed in which animals are exposed repetitively to heat and water restriction. Our group has used a model in which animals are exposed multiple times daily to heat stress and dehydration, and over time the animals develop CKD associated with significant scarring of the tubulointerstitium (30). Studies by the García-Arroyo laboratory have used a model of milder heat stress, and, while CKD does not occur, one can document marked oxidative stress and renal inflammation (11). In these models hydration with water during the heat stress is protective, whereas hydration with sugary beverages makes the injury worse (11, 30).
One of the consequences of heat stress and dehydration is the development of elevated serum osmolarity that activates several mediator systems that may cause renal injury (17). One such system in the polyol-fructokinase pathway. The enzyme aldose reductase is activated by hyperosmolarity (20) and is normally active in the renal medulla where it generates sorbitol from glucose, with the sorbitol acting as an intracellular osmol to protect medullary cells from injury from hypertonicity (6). While sorbitol is the end product of the polyol pathway in the renal medulla, in the outer medulla and renal cortex the sorbitol can be further degraded to fructose by sorbitol dehydrogenase, and the fructose can then become a substrate for the enzyme fructokinase, which is present in the proximal tubule, especially the S3 segment (7, 25). When fructose is metabolized by fructokinase C, there is a fall in ATP levels, adenine nucleotide turnover, and the production of uric acid, oxidants, and chemokines (monocyte chemoattractant protein-1) that can cause local renal injury (7, 25). We recently demonstrated this system is activated with recurrent heat stress and dehydration and that fructokinase knockout mice are protected from the renal damage (30).
The other major mediator system activated by hyperosmolarity is vasopressin. Vasopressin is considered a beneficial hormone, since its primary action is to increase water reabsorption in the collecting duct under conditions of hyperosmolarity or extracellular volume depletion. However, there is a “trade-off” in which chronically elevated vasopressin levels cause renal injury. In fact, most of the supportive data suggest that chronic stimulation of the V2 receptor may lead to kidney damage (2, 8). Specifically, the administration of vasopressin, or the V2 agonist desmopressin, induces glomerular hyperfiltration and albuminuria in humans and laboratory animals (3–5).
In this study we sought to examine the role of vasopressin in our heat stress nephropathy model. Because we were worried that blocking vasopressin might result in reduced survival in our water-restricted mice, we tested the hypothesis that administration of a V2 agonist would worsen the renal injury in heat stress nephropathy.
MATERIALS AND METHODS
Experimental protocol and animals.
Eight-week-old male C57BL/6J mice (Jackson Labs, Bar Harbor ME) (10) were maintained in temperature- and humidity-controlled specific pathogen-free conditions on a 14:10-h dark-light cycle and were fed regular diet ad libitum (no. 2918, containing 58% carbohydrate, 24% protein, and 18% fat and devoid of fructose or sugar; Harlan Teklad).
The experimental protocol was as follows. Mice (n = 7/group) were placed in a heat chamber for 30 min (39.5°C) each hour over an 8-h period each week day (Monday to Friday) with water restriction during that time. One group received desmopressin (Heat + Des) (Sigma-Aldrich, St. Louis, MO) via subcutaneous injection [dose = 32 ng/kg dissolved in physiological saline solution (150 µl)] that was administered 30 min before initiation of heat stress with a second subcutaneous injection 4 h later, in the middle of dehydration time. A second group received a subcutaneous injection of physiological saline solution (150 µl) at the same time (Heat). Two additional control groups included mice not exposed to heat or dehydration given vehicle (Control) or normal mice administered desmopressin (Des). This treatment was continued for a 5-wk period and resulted in no mortality. Mice were killed at 5 wk by anesthesia and cardiac exsanguination with collection of serum, urine from bladder, and kidney tissues for the analysis. All experiments were conducted with adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Colorado.
Biochemical analyses.
Urine was collected at the end of the study from the bladder and analyzed for urine osmolality using Freezing-Point Osmometry (Advance Instruments Micro Osmometer, Norwood, MA), urinary albumin was measured by the Albuwell M ELISA kit (Exocell, Philadelphia, PA), and urine neutrophil gelatinase-associated protein (NGAL) was measured using the Mouse Lipocalin-2/NGAL Quantikine ELISA Kit (R&D Systems, Minneapolis, MN). Serum osmolality was measured by Freezing-Point Osmometry, and serum and urine creatinine were analyzed by the high-performance liquid chromatography–tandem mass spectrometry method (32). Urinary urea was measured using a Colorimetric QuantiChrom Urea Assay Kit from BioAssay Systems (Hayward, CA). Urine ammonia was measured using the Ammonia Assay Kit ab83360 (Abcam, Cambridge, MA).
Histology.
Tissues were fixed in 10% formalin or methyl Carnoy’s and embedded in paraffin. Sections (3 μm) were stained with periodic acid-Schiff reagent (PAS). On coronal sections of the kidney, all glomeruli (50–100 glomeruli) were examined for evaluation of glomerular hypertrophy. Glomerular hypertrophy was determined by outlining the glomerular tuft in all glomeruli and measuring the area using Aperio software (Aperio Technologies, Vista, CA). Mesangial matrix expansion was determined by measuring the area of type IV collagen deposition in glomeruli using Aperio software on tissue sections stained for type IV collagen using rabbit anti-type IV collagen antibody (Chemicon, Temecula, CA) as described elsewhere (22). Specifically, the relative mesangial area (proportion of type IV collagen-positive area/glomerular tuft area) was calculated using Aperio Software.
Renal fibrosis was determined by staining for type III collagen with a goat anti-type III collagen antibody (Southern Biotech, Birmingham, AL), and infiltrating macrophages were detected using the mouse F4/80 monoclonal antibody (Serotec, Oxford, UK). Immunostaining for angiotensin-converting enzyme (ACE) was also performed to assess proximal tubular brush border (16, 22) using rabbit anti-ACE antibody (Chemicon). Staining of renal tissues for C3 was performed using anti-C3 antibody ab11862 (Abcam) to look for evidence for intrarenal complement expression. Briefly, after deparaffinization, the sections were treated with 3% H2O2 for 10 min to inactivate endogenous peroxidase activity. After incubation with a background sniper (Biocare Medical, Concord, CA) for 15 min, sections were incubated with primary antibodies overnight at 4°C. The sections were also incubated with secondary antibodies for 60 min before 3,3-diaminobenzidine (DAB) staining (Vector Laboratories, Burlingame, CA). The percent positive area was then determined as the DAB-positive pixel values per negative pixel values in each section using an Aperio scanner (Aperio Technologies). The software allows color recognition of immunostained tissues as percent positive color saturation at 20 magnification and was evaluated in a blinded manner using at least 15 fields for each biopsy sample.
Western blot and renal monocyte chemoattractant protein-1 measurement.
Renal cortical monocyte chemoattractant protein-1 (MCP-1) levels were measured in kidney tissue homogenates using the MCP-1 Elisa Kit Invitrogen (Life Technologies). Briefly, tissues (~50 mg) were homogenized in 500 μl of mitogen-activated protein kinase lysis buffer (21) containing 0.5% Triton X-100, 2 mM MgCl2, 1 mM EGTA, and 1 mM dithiothreitol supplemented with protease and phosphatase inhibitors (Roche). Samples were then incubated on ice for 30 min with occasional vortex and spun at 13,000 revolutions/min (rpm) for 15 min at 4oC, and the assay for MCP-1 was performed with correction for total protein using the BCA protein assay kit (Pierce, Rockford, IL).
For Western blotting, kidney lysates using the same mitogen-activated protein kinase lysis buffer (21) were prepared from mouse cortical tissue (~50 mg) and were incubated on ice for 30 min with occasional vortex and then spun at 13,000 rpm for 15 min at 4oC. The supernatant was collected, content was determined by the BCA protein assay (Pierce), and then 50 μg protein were loaded per lane for SDS–PAGE (10% wt/vol) analysis and transferred to polyvinylidene difluoride membranes. Membranes were incubated with primary antibodies (all at a 1:1,000 dilution {fructokinase (HPA007040; Sigma), aldose reductase [rabbit anti-mouse aldose reductase (AKR1B1) antibody (Novus Biologicals, Littleton, CO)], β-actin (4967S; Cell Signaling), KHK (HPA007040; Sigma)} and were visualized using a horseradish peroxidase secondary antibody (HRP, 1:2,000) and the HRP Supersignal West Pico Chemiluminescent Substrate (ThermoFisher Scientific). Chemiluminescence was recorded with an Image Station 440CF, and results were analyzed with 1D Image Software (Kodak Digital Science, Rochester, NY). Kidney homogenized supernatant cortex was collected for uric acid measurement using the QuantiChrom Uric Acid assay kit and urea with the Quantichrom Urea Assay Kit (both from BioAssay Systems).
Statistical analysis.
Statistical analysis was performed using one-way analysis Bonferroni of variance by rank. A two-sided value of P < 0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism version 6 (GraphPad Software, La Jolla, CA). All data are presented as means ± SE.
RESULTS
Hydration status and effect of desmopressin.
The model of heat stress nephropathy we used is one in which animals are recurrently exposed to heat over an 8-h period with complete water restriction during that time (30). It results in relatively severe acute dehydration, with ~14% weight loss at the end of the heating period. However, animals fully rehydrate at night, there is no mortality, and body weights remain stable over the 5-wk period (Table 1) (30). Additionally, by the end of the dehydration/heat stress period, there is a marked increase in serum osmolality that occurs in association with an increase in serum copeptin [a more stable peptide derived from the same precursor molecule as vasopressin (19)] and a rise in urinary osmolality and urine creatinine, consistent with urinary concentration.
Table 1.
General characteristics of experimental groups
| Body Wt | Control (n = 7) | Desmopressin (n = 7) | Heat (n = 7) | Heat + Desmopressin (n = 7) | P Values |
|---|---|---|---|---|---|
| Basal, g | 22.4 ± 1.4 | 23.5 ± 1.2 | 23.5 ± 1.1 | 23.5 ± 1.8 | NS |
| After 5 wk, g | 24.2 ± 1.2 | 24.4 ± 1.7 | 23.0 ± 0.6 | 23.0 ± 1.4 | NS |
| Loss after dehydration, % | 14.1 ± 1.1 | 14.5 ± 3.0 | NS |
Values are means ± SE; n, no. of mice. NS, not significant.
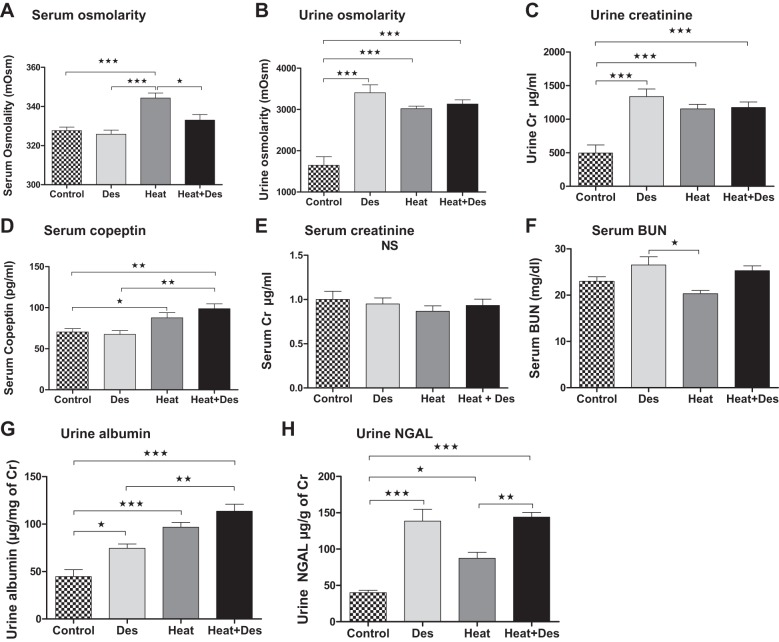
In this study, the administration of desmopressin to healthy hydrated mice resulted in urinary concentration as noted by a rise in urine osmolality and urinary creatinine associated with a slight and nonsignificant decrease in serum osmolality (Fig. 1). Mice exposed to heat stress and dehydration developed a significant rise in serum osmolality and also showed signs of urinary concentration (Fig. 1). The administration of desmopressin to mice with heat stress ameliorated the rise in serum osmolarity with heat stress but did not further change urine osmolality (Fig. 1). Interestingly, the rise in urine osmolality was greatest in control mice receiving desmopressin, and the tendency for lower urine osmolality in mice exposed to heat stress might reflect the fact that these animals were developing chronic tubulointerstitial disease that might partially impair their urinary concentration ability (see below).
Fig. 1.
Effects of desmopressin (Des) on hydration status and renal function. Serum osmolality (A) increased in the heat stress control group and tended to be lower in the heat group that received desmopressin. Urine osmolality (B) and urine creatinine (Cr, C) increased with heat stress but also in both groups receiving desmopressin. Serum copeptin (D) increased in the two groups of mice exposed to heat stress and dehydration. No differences in serum creatinine (E) was observed, whereas an increase in serum blood urea nitrogen (BUN) (F) tended to increase in the desmopressin groups. Urine albumin (G) stepwise increased with desmopressin alone, followed by heat stress/dehydration, and then heat stress plus desmopressin. Urinary neutrophil gelatinase-associated protein (NGAL) excretion (H) was highest in groups receiving desmopressin. NS, not significant. *P < 0.05, **P < 0.01, and ***P < 0.001.
Effect of heat stress and desmopressin on renal function.
Serum creatinine (measured by HPLC) was no different among groups. Serum BUN levels tended to be elevated in mice receiving desmopressin with or without heat stress, but this was not significant. However, albuminuria increased stepwise with desmopressin alone, followed by heat stress, and then by heat stress plus desmopressin (Fig. 1). Urine NGAL was also significantly elevated in both desmopressin groups and to a lesser extent in the heat stress alone group compared with normal healthy controls (Fig. 1). Whether this reflects renal injury or a prerenal state remains unclear because urinary NGAL has also been reported to be elevated in prerenal conditions (27).
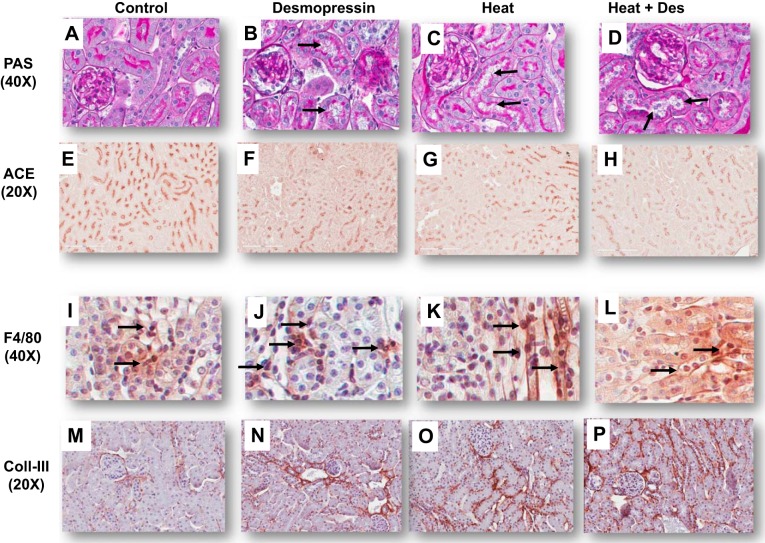
Effect of heat stress and desmopressin on renal histology.
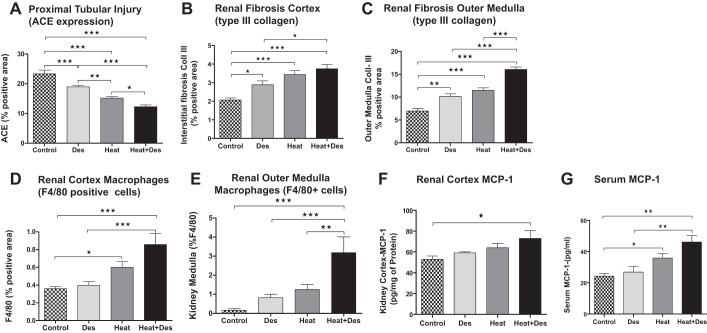
Light microscopy (PAS staining) was associated with a stepwise increase in proximal tubular injury (with loss of brush border as noted by both PAS and immunostaining for ACE) (22) in control mice, control mice receiving desmopressin, followed by heat stress/dehydration, and then combined desmopressin and heat stress/dehydration (Figs. 2 and 3). Similar findings were shown for interstitial fibrosis (noted by type III collagen deposition) and interstitial macrophage inflammation (noted by F4/80 positive staining) in both the renal cortex and outer medulla (Figs. 2 and 3). Desmopressin significantly worsened proximal tubular injury, as well as renal fibrosis and inflammation, in the outer medulla in mice exposed to heat stress compared with heat stress alone. There was also a stepwise increase in serum MCP-1 and in renal cortical MCP-1 levels (Fig. 3).
Fig. 2.
Renal cortex histology. Proximal tubular injury [loss of brush border with tubular dilation (black arrows)] was observed in desmopressin, Heat, and Heat + Des groups [periodic acid-Schiff reagent (PAS), A–D] and was associated with a progressive loss of brush border, as noted by staining for angiotensin-converting enzyme (ACE, E–H). Macrophage infiltration (F4/80, I–L) was increased in Des, Heat, and Heat + Des groups compared with the control group (black arrows). Interstitial collagen III deposition was minimal in normal controls (M) but was increased in the Des, Heat, and Heat + Des groups (N–P).
Fig. 3.
Semiquantitative histological changes. Tissues were stained for ACE as a marker of the proximal tubular brush border (which for this marker included both cortical and outer medullary proximal tubules). A progressive loss of ACE was associated with the addition of desmopressin, heat, and heat plus desmopressin (A). Interstitial collagen III deposition in the renal cortex and outer medulla increased in parallel with decreasing ACE expression (B and C). Similarly, infiltrating macrophages showed a similar pattern both in the renal cortex and outer medulla (noted by F4/80 scoring; D and E) and were significantly worsened by desmopressin in the outer medulla of heat stress-exposed mice. Renal cortical MCP-1 protein levels were also elevated in the heat stress plus desmopressin group with a tendency to increase in the heat group (F). Serum monocyte chemoattractant protein-1 (MCP-1) represents a biomarker of inflammation; it was increased in the animals exposed to heat and tended to worsen with desmopressin (G). Statistical analysis used Bonferroni one-way analysis of variance by ranks. A two-sided value of P < 0.05 was considered statistically significant. *P < 0.05, **P < 0.01, and ***P < 0.001.
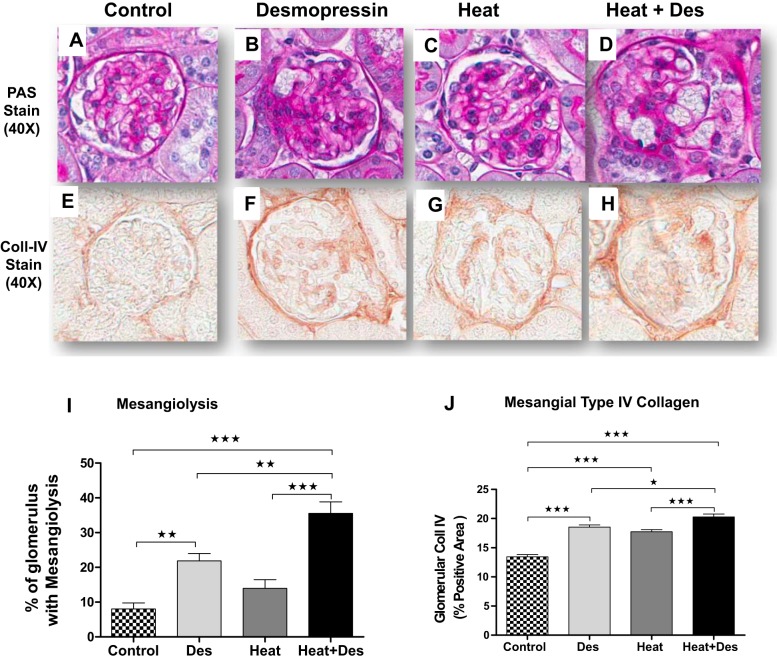
There were also glomerular changes observed. Interestingly, desmopressin was found to induce mesangiolysis in both normal mice and heat stress-exposed mice. However, heat stress alone did not result in mesangiolysis (Fig. 4). Mesangial matrix expansion, as noted by type IV collagen deposition, was induced by desmopressin in normal mice, as well as in heat stress-exposed mice, and was significantly enhanced in heat stress-exposed mice that also received desmopressin (Fig. 4).
Fig. 4.
Focal glomerular mesangiolysis. Heat stress was associated with focal mesangiolysis as were control mice administered desmopressin compared with healthy control mice (A–D, with quantification of mean percent glomeruli involved in I). This was accompanied by parallel changes in mesangial matrix expansion [as noted by type IV collagen immunostaining (E–H), with quantification in J]. The effects of desmopressin to induce mesangiolysis and mesangial expansion of type IV collagen were significant. *P < 0.05, **P < 0.01, and ***P < 0.001.
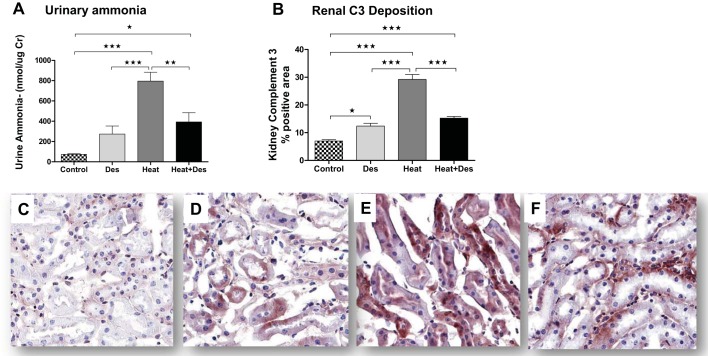
Effect of desmopressin on ammonia production and complement activation.
Desmopressin has been reported to increase urinary ammonia levels in vasopressin-deficient mice (1) and in turn could theoretically lead to local activation of complement due to its ability to activate the alternative complement pathways that could lead to tubulointerstitial injury (26). In our mice, urinary ammonia levels tended to increase in the control mice but were markedly increased in mice exposed to heat stress (Fig. 5). Interestingly, the combination of desmopressin and heat stress resulted in less ammonia excretion than observed with heat stress alone. The same pattern was observed with C3 localization in the kidney. Here there was minimal C3 expression in normal mouse kidney, but with desmopressin one could identify focal staining, especially in the proximal tubules. This was markedly enhanced in mice exposed to heat stress, but, similar to the pattern of ammonia excretion, there was a reduction in C3 staining with combined desmopressin and heat stress associated with reduced proximal tubular staining (Fig. 5).
Fig. 5.
Urine ammonia and C3 expression. Desmopressin has been reported to increase urine ammonia, which has been shown to activate the alternative complement pathway. Consistent with this hypothesis, we found a tendency for an increase in urinary ammonia and renal C3 expression that was primarily identified in proximal tubules [control mice (C) and desmopressin-injected control mice (D)]. Heat stress-exposed mice showed the highest urinary ammonia excretion and renal tubular C3 expression (A, B, and E), whereas heat stress-exposed mice administered desmopressin paradoxically showed a reduction in urinary ammonia and renal C3 deposition (F). *P < 0.05, **P < 0.01, and ***P < 0.001.
Effect of desmopressin and heat on the aldose reductase-fructokinase pathway.
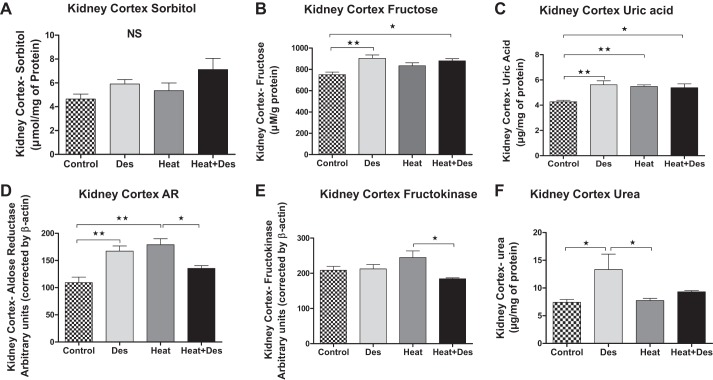
Evidence for increased activity of the polyol-fructokinase pathway with heat stress was suggested by the significant increase in both cortical aldose reductase protein and uric acid concentrations in heat stress-exposed mice and a tendency for higher fructose, fructokinase, and sorbitol levels. Treatment with desmopressin in normal mice also led to higher aldose reductase, fructose, and uric acid levels. Similarly, the combination of desmopressin with heat stress also resulted in higher fructose and uric acid levels compared with control mice (Fig. 6). However, an interesting finding was that the expression of aldose reductase and fructokinase was lower in heat-exposed mice receiving desmopressin compared with mice receiving heat stress alone (Fig. 6).
Fig. 6.
Effect of desmopressin on the polyol-fructokinase pathway in the kidney cortex. Renal cortical homogenates tended to show higher levels of sorbitol (A) with heat stress and/or desmopressin, but this was not significant. In contrast, renal cortical fructose (B) and uric acid (C) were higher with desmopressin independent of whether animals were exposed to heat stress, and uric acid was also elevated in animals with heat stress alone compared with healthy controls. Western blot documented increased aldose reductase protein in renal cortex of desmopressin controls and mice exposed to heat, with a tendency for higher expression in animals exposed to heat and desmopressin (D). Fructokinase protein tended to be higher in the heat-exposed mice compared with controls, but this was not significant (E). Cortical urea concentration (F) was observed in control animals receiving desmopressin. *P < 0.05 and **P < 0.01.
Vasopressin is known to increase the reabsorption of urea, which helps in urinary concentration (36). An increase in interstitial urea might extend into the renal cortex where it might facilitate water absorption by the cortical collecting duct. Consistent with this finding, we found that desmopressin increased cortical urea concentration in normal mice (Fig. 6). However, these effects were not observed in mice with heat-induced injury.
DISCUSSION
In this study, we explored the role of vasopressin, and particularly the V2 receptor, in a model of heat stress nephropathy. Our hypothesis was that stimulation of the V2 receptor would accelerate the renal injury associated with heat stress nephropathy. Our primary finding was that the administration of desmopressin, a V2 agonist, accelerated renal injury with greater glomerular and tubulointerstitial damage. In animals exposed to heat stress, the addition of desmopressin worsened proximal tubular injury, as noted by immunostaining for ACE, which marks the brush border (16, 22), and this was associated with a tendency for greater inflammation and fibrosis. In the outer medulla, the addition of desmopressin resulted in a significantly greater infiltration of F4/80-positive macrophages and greater interstitial fibrosis (type III collagen immunostaining) compared with heat stress alone. The administration of desmopressin to either control animals or animals exposed to heat stress also resulted in mesangiolysis that was not observed in control animals or animals exposed to heat stress alone. Another result was significantly amplified mesangial type IV collagen deposition compared with mice exposed to heat stress alone. We also found evidence that stimulation of the V2 receptor could activate the polyol-fructokinase pathway independent of heat stress-induced injury, as noted by its effects on renal fructose and uric acid accumulation. These data therefore suggest that vasopressin is likely a mediator of renal injury under conditions of recurrent heat stress and dehydration and again emphasize the importance of maintaining good hydration and normal serum osmolarity to promote renal health.
An important distinction in this study was the observation that desmopressin could induce mesangiolysis in both normal mice and mice exposed to heat stress, whereas heat stress alone was not associated with mesangiolysis. This might suggest that the pathogenic mechanisms by which desmopressin induces renal injury may be separate from that associated with heat stress and thereby challenge the hypothesis that desmopressin might simply be a mechanism for amplifying the stimulation of V2 receptors that occurs in this model. However, we believe desmopressin does reflect an amplification of the underlying mechanisms of injury rather than a different mechanism, since vasopressin (copeptin) levels were high in this model. Hence, some activation of V2 receptors is highly likely. Indeed, in renal biopsies of subjects with Mesoamerican nephropathy, there is evidence for glomerulosclerosis in addition to the well-recognized tubulointerstitial fibrosis, and the authors of the original publication have argued that the glomerulosclerosis is likely a primary process rather than developing secondary to chronic kidney damage (34). Desmopressin has been recognized in other studies to cause glomerular hyperfiltration and albuminuria in both experimental animals and in humans (3–5), which might predispose to the development of glomerulosclerosis. Interestingly, because V2 receptors are not present in glomeruli, the mechanisms for how vasopressin induces these changes are not completely understood, although it has been posited to be by altering tubuloglomerular feedback (5). Alternatively, studies by Grgic et al. have shown that isolated injury to the proximal tubule may also cause glomerular injury (15). Whether this may represent a similar process is unknown.
The mechanism by which desmopressin may induce proximal tubular injury was not shown in this model, but one potential mechanism could be by increasing ammonia production and excretion that might activate complement in the urine, leading to local tubular injury (26). Indeed, we documented an increase in ammonia with heat stress and to a lesser extent with desmopressin alone, but, interestingly, there was less urinary ammonia excretion in mice exposed to heat stress and desmopressin. We also found increased C3 present in the proximal tubules following heat stress, but again tubular expression of C3 also decreased in mice exposed to heat stress and desmopressin. We hypothesize that this might be due to the fact that combined desmopressin and heat stress resulted in greater proximal tubular injury as noted by immunostaining for ACE. Because the proximal tubule is the site of ammoniagenesis and was also the site for C3 expression, it could potentially explain this finding.
The renal injury mediated by desmopressin could also involve a cross talk between the vasopressin and fructokinase systems. Vasopressin, by increasing urea and sodium reabsorption, will increase interstitial osmolarity. Aldose reductase has an osmolarity-sensitive promoter, and activity increases in parallel with increasing osmolarity (31). We observed a significant increment in cortical urea with desmopressin; however, this effect was not significant in mice exposed to either heat and/or heat stress with desmopressin (Fig. 6). Nevertheless, renal cortical fructose and uric acid were elevated both with desmopressin alone and with heat stress with desmopressin. Again, this apparent paradox might be due to the greater proximal tubular injury observed in mice exposed to heat stress that received desmopressin, since the proximal tubule is the site where fructokinase C is expressed (25) and where aldose reductase can be induced (22). Greater tubular injury might also alter the ability of the renal medullary tubules to maintain high interstitial osmolarity. This could explain why cortical urea was not as high in mice with heat-associated injury compared with desmopressin alone.
The generation of fructose in the renal cortex is associated with fructokinase-dependent tubular injury both in response to heat stress and in diabetes (22, 30). Fructose induces tubular injury due to the generation of uric acid, oxidative stress, and the production of chemokines such as MCP-1 (22, 28, 30). In turn, we have shown in a model of diabetic nephropathy that fructokinase-mediated tubular injury is also associated with worse albuminuria and glomerular injury (22), suggesting this might represent the link between vasopressin and glomerular injury. Consistent with this cross talk, we have recently reported evidence for tubular injury in patients with chronic hyponatremia, and in these patients urinary fructose levels are elevated (24). In addition, fructose also stimulates vasopressin release (11, 35), thereby leading to a positive feedback system (18).
In summary, these studies provide evidence that both the aldose reductase-fructokinase system (30) and vasopressin may modulate heat stress nephropathy. Rehydration with sugary beverages also accelerates this pathway not only by providing fructose substrate but also by stimulating vasopressin (11). Our studies also suggest uric acid may be involved because of its known ability to stimulate inflammatory pathways and also because of its potential crystal-dependent and -independent effects (28, 29). Thus, we encourage further studies to investigate whether these processes may be involved in the current epidemics of CKD occurring under conditions of documented heat stress (14).
GRANTS
This study was supported by a grant from the Department of Defense (PR130106). M. Kuwabara was the recipient of a grant for studying abroad from the Federation of National Public Service Personnel Mutual Aid Association in Japan. T. Jensen and P. Bjornstad were supported by National Institute of Diabetes and Digestive and Kidney Diseases T32 Training Grants 5T32-DK-007446-34 (T. Jensen) and T32-DK-063687 (P. Bjornstad).
DISCLOSURES
The authors disclose no conflicts of interest related to the manuscript. CRJ, MAL, LGL and RJJ are members of Colorado Research Partners, LLC that is developing inhibitors of fructose metabolism for the treatment of metabolic syndrome and kidney disease. Dr. Johnson is also on the Scientific Board of Amway. This paper is considered a contribution by the University of Colorado Climate Change and Health consortium.
AUTHOR CONTRIBUTIONS
C.A.R.-J., T.M., A.A.-H., T.J., Z.S., and L.-G.S.-L. performed experiments; C.A.R.-J., T.M., A.A.-H., M.K., T.J., L.-G.S.-L., M.A.L., and R.J.J. analyzed data; C.A.R.-J., T.M., M.K., L.-G.S.-L., M.A.L., and R.J.J. interpreted results of experiments; C.A.R.-J. and A.A.-H. prepared figures; C.A.R.-J., M.K., and T.J. drafted manuscript; C.A.R.-J., P.B., G.E.G., Y.S., L.-G.S.-L., M.A.L., and R.J.J. edited and revised manuscript; C.A.R.-J. and R.J.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Lise Bankir for suggestions in the experimental design of the study.
REFERENCES
- 1.Amlal H, Sheriff S, Faroqui S, Ma L, Barone S, Petrovic S, Soleimani M. Regulation of acid-base transporters by vasopressin in the kidney collecting duct of Brattleboro rat. Am J Nephrol 26: 194–205, 2006. doi: 10.1159/000093305. [DOI] [PubMed] [Google Scholar]
- 2.Bankir L, Bouby N, Ritz E. Vasopressin: a novel target for the prevention and retardation of kidney disease? Nat Rev Nephrol 9: 223–239, 2013. doi: 10.1038/nrneph.2013.22. [DOI] [PubMed] [Google Scholar]
- 3.Bardoux P, Bichet DG, Martin H, Gallois Y, Marre M, Arthus MF, Lonergan M, Ruel N, Bouby N, Bankir L. Vasopressin increases urinary albumin excretion in rats and humans: involvement of V2 receptors and the renin-angiotensin system. Nephrol Dial Transplant 18: 497–506, 2003. doi: 10.1093/ndt/18.3.497. [DOI] [PubMed] [Google Scholar]
- 4.Bardoux P, Martin H, Ahloulay M, Schmitt F, Bouby N, Trinh-Trang-Tan MM, Bankir L. Vasopressin contributes to hyperfiltration, albuminuria, and renal hypertrophy in diabetes mellitus: study in vasopressin-deficient Brattleboro rats. Proc Natl Acad Sci U S A 96: 10397–10402, 1999. doi: 10.1073/pnas.96.18.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouby N, Ahloulay M, Nsegbe E, Déchaux M, Schmitt F, Bankir L. Vasopressin increases glomerular filtration rate in conscious rats through its antidiuretic action. J Am Soc Nephrol 7: 842–851, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Burg MB. Molecular basis of osmotic regulation. Am J Physiol Renal Fluid Electrolyte Physiol 268: F983–F996, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Cirillo P, Gersch MS, Mu W, Scherer PM, Kim KM, Gesualdo L, Henderson GN, Johnson RJ, Sautin YY. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J Am Soc Nephrol 20: 545–553, 2009. doi: 10.1681/ASN.2008060576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark WF, Sontrop JM, Huang SH, Moist L, Bouby N, Bankir L. Hydration and Chronic Kidney Disease Progression: A Critical Review of the Evidence. Am J Nephrol 43: 281–292, 2016. doi: 10.1159/000445959. [DOI] [PubMed] [Google Scholar]
- 9.Correa-Rotter R, Wesseling C, Johnson RJ. CKD of unknown origin in Central America: the case for a Mesoamerican nephropathy. Am J Kidney Dis 63: 506–520, 2014. doi: 10.1053/j.ajkd.2013.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diggle CP, Shires M, McRae C, Crellin D, Fisher J, Carr IM, Markham AF, Hayward BE, Asipu A, Bonthron DT. Both isoforms of ketohexokinase are dispensable for normal growth and development. Physiol Genomics 42A: 235–243, 2010. doi: 10.1152/physiolgenomics.00128.2010. [DOI] [PubMed] [Google Scholar]
- 11.García-Arroyo FE, Cristóbal M, Arellano-Buendía AS, Osorio H, Tapia E, Soto V, Madero M, Lanaspa MA, Roncal-Jiménez C, Bankir L, Johnson RJ, Sánchez-Lozada LG. Rehydration with soft drink-like beverages exacerbates dehydration and worsens dehydration-associated renal injury. Am J Physiol Regul Integr Comp Physiol 311: R57–R65, 2016. doi: 10.1152/ajpregu.00354.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.García-Trabanino R, Jarquín E, Wesseling C, Johnson RJ, González-Quiroz M, Weiss I, Glaser J, José Vindell J, Stockfelt L, Roncal C, Harra T, Barregard L. Heat stress, dehydration, and kidney function in sugarcane cutters in El Salvador–A cross-shift study of workers at risk of Mesoamerican nephropathy. Environ Res 142: 746–755, 2015. doi: 10.1016/j.envres.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Garcia Trabiano R, Aguilar R, Silva CR, Mercado MO, Merino RL. [End-stage renal disease among patients in a referral hospital in El Salvador] Rev Panam Salud Publica. 12: 202–206, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Glaser J, Lemery J, Rajagopalan B, Diaz HF, Garcia-Trabanino R, Taduri G, Madero M, Amarasinghe MD, Abraham G, Anutrakulchai S, Jha V, Stenvinkel P, Roncal-Jiménez C, Lanaspa M, Correa-Rotter R, Sheik-Hamad D, Burdmann EA, Andres-Hernando A, MIlagres T, Weiss I, Kanbay M, Wesseling C, Sanchez-Lozada LG, Johnson RJ. Climate Change and the Emergent Epidemic of Chronic Kidney Disease from Heat Stress in Rural Communities: The Case for Heat Stress Nephropathy. Clin J Am Soc Nephrol 11: 1472–1483, 2016. doi: 10.2215/CJN.13841215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grgic I, Campanholle G, Bijol V, Wang C, Sabbisetti VS, Ichimura T, Humphreys BD, Bonventre JV. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int 82: 172–183, 2012. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikemoto F, Song GB, Tominaga M, Kanayama Y, Yamamoto K. Angiotensin-converting enzyme in the rat kidney. Activity, distribution, and response to angiotensin-converting enzyme inhibitors. Nephron 55, Suppl 1: 3–9, 1990. doi: 10.1159/000186027. [DOI] [PubMed] [Google Scholar]
- 17.Johnson RJ, Rodriguez-Iturbe B, Roncal-Jimenez C, Lanaspa MA, Ishimoto T, Nakagawa T, Correa-Rotter R, Wesseling C, Bankir L, Sanchez-Lozada LG. Hyperosmolarity drives hypertension and CKD--water and salt revisited. Nat Rev Nephrol 10: 415–420, 2014. doi: 10.1038/nrneph.2014.76. [DOI] [PubMed] [Google Scholar]
- 18.Johnson RJ, Stenvinkel P, Jensen T, Lanaspa MA, Roncal C, Song Z, Bankir L, Sánchez-Lozada LG. Metabolic and kidney diseases in the setting of climate change, water shortage, and survival factors. J Am Soc Nephrol 27: 2247–2256, 2016. doi: 10.1681/ASN.2015121314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan SQ, Dhillon OS, O’Brien RJ, Struck J, Quinn PA, Morgenthaler NG, Squire IB, Davies JE, Bergmann A, Ng LL. C-terminal provasopressin (copeptin) as a novel and prognostic marker in acute myocardial infarction: Leicester Acute Myocardial Infarction Peptide (LAMP) study. Circulation 115: 2103–2110, 2007. doi: 10.1161/CIRCULATIONAHA.106.685503. [DOI] [PubMed] [Google Scholar]
- 20.Ko BC, Ruepp B, Bohren KM, Gabbay KH, Chung SS. Identification and characterization of multiple osmotic response sequences in the human aldose reductase gene. J Biol Chem 272: 16431–16437, 1997. doi: 10.1074/jbc.272.26.16431. [DOI] [PubMed] [Google Scholar]
- 21.Lanaspa MA, Almeida NE, Andres-Hernando A, Rivard CJ, Capasso JM, Berl T. The tight junction protein, MUPP1, is up-regulated by hypertonicity and is important in the osmotic stress response in kidney cells. Proc Natl Acad Sci U S A 104: 13672–13677, 2007. doi: 10.1073/pnas.0702752104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanaspa MA, Ishimoto T, Cicerchi C, Tamura Y, Roncal-Jimenez CA, Chen W, Tanabe K, Andres-Hernando A, Orlicky DJ, Finol E, Inaba S, Li N, Rivard CJ, Kosugi T, Sanchez-Lozada LG, Petrash JM, Sautin YY, Ejaz AA, Kitagawa W, Garcia GE, Bonthron DT, Asipu A, Diggle CP, Rodriguez-Iturbe B, Nakagawa T, Johnson RJ. Endogenous fructose production and fructokinase activation mediate renal injury in diabetic nephropathy. J Am Soc Nephrol 25: 2526–2538, 2014. doi: 10.1681/ASN.2013080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laws RL, Brooks DR, Amador JJ, Weiner DE, Kaufman JS, Ramírez-Rubio O, Riefkohl A, Scammell MK, López-Pilarte D, Sánchez JM, Parikh CR, McClean MD. Changes in kidney function among Nicaraguan sugarcane workers. Int J Occup Environ Health 21: 241–250, 2015. doi: 10.1179/2049396714Y.0000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SK, Lanaspa MA, Sánchez-Lozada LG, Johnson RJ. Hyponatremia with Persistent Elevated Urinary Fractional Uric Acid Excretion: Evidence for Proximal Tubular Injury? Kidney Blood Press Res 41: 535–544, 2016. doi: 10.1159/000447928. [DOI] [PubMed] [Google Scholar]
- 25.Nakayama T, Kosugi T, Gersch M, Connor T, Sanchez-Lozada LG, Lanaspa MA, Roncal C, Perez-Pozo SE, Johnson RJ, Nakagawa T. Dietary fructose causes tubulointerstitial injury in the normal rat kidney. Am J Physiol Renal Physiol 298: F712–F720, 2010. doi: 10.1152/ajprenal.00433.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nath KA, Hostetter MK, Hostetter TH. Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest 76: 667–675, 1985. doi: 10.1172/JCI112020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nejat M, Pickering JW, Devarajan P, Bonventre JV, Edelstein CL, Walker RJ, Endre ZH. Some biomarkers of acute kidney injury are increased in pre-renal acute injury. Kidney Int 81: 1254–1262, 2012. doi: 10.1038/ki.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roncal CA, Mu W, Croker B, Reungjui S, Ouyang X, Tabah-Fisch I, Johnson RJ, Ejaz AA. Effect of elevated serum uric acid on cisplatin-induced acute renal failure. Am J Physiol Renal Physiol 292: F116–F122, 2007. doi: 10.1152/ajprenal.00160.2006. [DOI] [PubMed] [Google Scholar]
- 29.Roncal-Jimenez C, García-Trabanino R, Barregard L, Lanaspa MA, Wesseling C, Harra T, Aragón A, Grases F, Jarquin ER, González MA, Weiss I, Glaser J, Sánchez-Lozada LG, Johnson RJ. Heat Stress Nephropathy From Exercise-Induced Uric Acid Crystalluria: A Perspective on Mesoamerican Nephropathy. Am J Kidney Dis 67: 20–30, 2016. doi: 10.1053/j.ajkd.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 30.Roncal Jimenez CA, Ishimoto T, Lanaspa MA, Rivard CJ, Nakagawa T, Ejaz AA, Cicerchi C, Inaba S, Le M, Miyazaki M, Glaser J, Correa-Rotter R, González MA, Aragón A, Wesseling C, Sánchez-Lozada LG, Johnson RJ. Fructokinase activity mediates dehydration-induced renal injury. Kidney Int 86: 294–302, 2014. doi: 10.1038/ki.2013.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruepp B, Bohren KM, Gabbay KH. Characterization of the osmotic response element of the human aldose reductase gene promoter. Proc Natl Acad Sci U S A 93: 8624–8629, 1996. doi: 10.1073/pnas.93.16.8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi N, Boysen G, Li F, Li Y, Swenberg JA. Tandem mass spectrometry measurements of creatinine in mouse plasma and urine for determining glomerular filtration rate. Kidney Int 71: 266–271, 2007. doi: 10.1038/sj.ki.5002033. [DOI] [PubMed] [Google Scholar]
- 33.Wesseling C, Aragón A, González M, Weiss I, Glaser J, Bobadilla NA, Roncal-Jiménez C, Correa-Rotter R, Johnson RJ, Barregard L. Kidney function in sugarcane cutters in Nicaragua--A longitudinal study of workers at risk of Mesoamerican nephropathy. Environ Res 147: 125–132, 2016. doi: 10.1016/j.envres.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Wijkström J, Leiva R, Elinder CG, Leiva S, Trujillo Z, Trujillo L, Söderberg M, Hultenby K, Wernerson A. Clinical and pathological characterization of Mesoamerican nephropathy: a new kidney disease in Central America. Am J Kidney Dis 62: 908–918, 2013. doi: 10.1053/j.ajkd.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 35.Wolf JP, Nguyen NU, Dumoulin G, Berthelay S. Influence of hypertonic monosaccharide infusions on the release of plasma arginine vasopressin in normal humans. Horm Metab Res 24: 379–383, 1992. doi: 10.1055/s-2007-1003340. [DOI] [PubMed] [Google Scholar]
- 36.Yang B, Bankir L. Urea and urine concentrating ability: new insights from studies in mice. Am J Physiol Renal Physiol 288: F881–F896, 2005. doi: 10.1152/ajprenal.00367.2004. [DOI] [PubMed] [Google Scholar]