Abstract
Regulated dicarboxylate transport is critical for acid-base homeostasis, prevention of calcium nephrolithiasis, regulation of collecting duct sodium chloride transport, and the regulation of blood pressure. Although luminal dicarboxylate reabsorption via NaDC1 (SLC13A2) is believed to be the primary mechanism regulating renal dicarboxylate transport, the specific localization of NaDC1 in the human kidney is currently unknown. This study’s purpose was to determine NaDC1's expression in normal and neoplastic human kidneys. Immunoblot analysis demonstrated NaDC1 expression with an apparent molecular weight of ~61 kDa. Immunohistochemistry showed apical NaDC1 immunolabel in the proximal tubule of normal human kidney tissue; well-preserved proximal tubule brush border was clearly labeled. Apical NaDC1 expression was evident throughout the entire proximal tubule, including the initial proximal convoluted tubule, as identified by origination from the glomerular tuft, and extending through the terminal of the proximal tubule, the proximal straight tubule in the outer medulla. We confirmed proximal tubule localization by colocalization with the proximal tubule specific protein, NBCe1. NaDC1 immunolabel was not detected other than in the proximal tubule. In addition, NaDC1 immunolabel was not detected in tumors of presumed proximal tubule origin, clear cell and papillary renal cell carcinoma, or in tumors of nonproximal tubule origin, oncocytoma and chromophobe carcinoma. In summary, 1) in the human kidney, apical NaDC1 immunolabel is present throughout the entire proximal tubule, and is not detectable in other renal cells; and 2) NaDC1 immunolabel is not present in renal tumors. These studies provide important information regarding NaDC1’s role in human dicarboxylate metabolism.
Keywords: citrate, human, NaDC1, proximal tubule
dicarboxylate anions have multiple critical roles in renal physiology. Citrate, perhaps the most widely known dicarboxylate, has a critical role in the prevention of nephrolithiasis, in acid-base homeostasis and as an important oxidative energy source for renal proximal tubule cells (2, 39, 57). 2-Oxoglutarate, also known as alpha-ketoglutarate, contributes to acid-base homeostasis through two mechanisms; as a metabolic alkali precursor, its excretion serves as a mechanism of alkali excretion (5, 10), and as a signaling molecule it stimulates pendrin-mediated bicarbonate secretion in distal epithelial cells (56). 2-Oxoglutarate also stimulates distal epithelial NaCl reabsorption through parallel activation of the sodium reabsorbing protein, NDCBE, and the chloride-reabsorbing protein, pendrin (56). A third dicarboxylate is succinate, which through activation of the succinate receptor, GPR91/SUCNR1, can regulate blood pressure (11, 49).
The major mechanism controlling urinary excretion of these dicarboxylate anions appears to be regulated reabsorption in the renal proximal tubule. Studies utilizing brush border membrane vesicles show the presence of a sodium-linked transport activity with broad dicarboxylate specificity (16, 28, 29, 60). Microperfusion proximal tubule studies confirmed proximal tubule dicarboxylate transport, and showed no evidence of transport in more distal epithelial segments (9). Neither changes in serum levels nor changes in glomerular filtration rate appear to be major regulatory mechanisms (16, 17, 43, 57).
The protein, NaDC1, is a sodium-coupled dicarboxylate transporter that is likely to have a critical role in renal dicarboxylate transport. NaDC1 couples movement of three sodium ions with a dicarboxylate, enabling facilitated, secondarily active dicarboxylate apical uptake driven by the low intracellular sodium concentration and by intracellular electronegativity (41, 42, 44, 46, 51). It has specificity for all major renal dicarboxylate ions, including citrate, 2-oxoglutarate, and succinate (41, 42, 44, 46, 51). Although citrate exists in both a tricarboxylate form (citrate3−) and a dicarboxylate form (citrate2−), only the dicarboxylate form appears to be transported by NaDC1. NaDC1 mRNA is expressed in the kidney, and immunohistochemistry in both the rat and mouse has localized NaDC1 to the rodent proximal tubule brush border (3, 40, 51).
To date, NaDC1 expression in the human kidney has not been extensively studied. Northern blot analysis has shown mRNA expression (42). However, neither the presence of protein expression nor the cellular site of NaDC1 protein expression in the human kidney has been identified. Because of the central role of dicarboxylate anions and their appropriate renal transport in multiple aspects of human physiology and pathophysiology, determining the specific expression of NaDC1 in human kidney is of critical importance.
Thus the purpose of the current studies was to identify the pattern of NaDC1 expression in the human kidney. We used immunoblot analysis to confirm protein expression. We then used immunohistochemical analysis to define both the cellular and the subcellular distribution of NaDC1 expression in the human kidney. In addition, because renal tumors can originate from various cell types and because dicarboxylate metabolism is particularly important in oxidative metabolism in proximal tubule cells, we determined the expression of NaDC1 in human renal tumors, of both proximal tubule and intercalated cell origin.
METHODS
Human kidney tissue.
Normal human kidney tissues were obtained from the pathology laboratory of the Gainesville Veterans Affairs (VA) Medical Center. Samples were obtained from unused portions of nephrectomy specimens removed during routine clinical care, most frequently for treatment of renal cell carcinoma. Tissue was fixed in 10% buffered formalin. Tissue from a total of thirteen kidneys was used. These studies were approved by the Institutional Review Board of the Gainesville VA Medical Center and the University of Florida College of Medicine.
Antibodies.
Antibodies to sodium-dependent dicarboxylate transporter 1 (NaDC1/SLC13A2) and antibodies to Na+-coupled bicarbonate cotransporter (NBCe1/SLC4A4) were obtained from Proteintech Group (Rosemont, IL). We previously confirmed the specificity of both the NaDC1 and NBCe1 antibodies using kidneys from NaDC1 and NBCe1 knockout mice (20, 40).
Human whole kidney and cortical protein lysate.
Normal human kidney protein lysates were obtained from Takara Bio USA (Mountain View, CA).
Immunoblot analysis.
Immunoblot analysis was performed as described previously in detail (18, 19). Briefly, renal tissue lysate protein (10 μg/ lane) was electrophoresed on 10% PAGE ReadyGel (Bio-Rad, Hercules, CA). The proteins were then transferred electrophoretically to nitrocellulose membranes, blocked with 5 g/dl nonfat dry milk in Blotto buffer (50 mM Tris, 150 mM NaCl, 5 mM Na2EDTA, and 0.05% Tween 20, pH 7.6), and incubated at 4°C overnight with primary antibody diluted in nonfat dry milk. Loading and transfer equivalence were assessed with Ponceau S staining. After washing, membranes were exposed to secondary antibody, goat anti-rabbit IgG (Cell Signaling Technology, Beverly, MA), conjugated to horseradish peroxidase at a dilution of 1:5,000. Sites of antibody-antigen reaction were visualized by using enhanced chemiluminescence (SuperSignal West Pico Substrate, Pierce) and a Kodak Image Station 440CF digital imaging system.
Immunohistochemistry.
Immunolocalization was accomplished using standard immunoperoxidase procedures in routine use in our laboratory (18, 19, 32, 33, 40). Briefly, sections were deparaffinized in xylene, rehydrated, and then rinsed in PBS. Antigen retrieval was accomplished by immersing slides in Trilogy (Cell Marque, Rocklin, CA) at 96°C for 1 h. Endogenous peroxidase activity was blocked by incubation of the sections in 3% H2O2 in methanol for 45 min. After washing in PBS, the sections were treated with 0.5% Triton X-100 in PBS for 15 min. The sections then underwent several washes in PBS containing 1% bovine serum albumin (BSA), 0.05% saponin, and 0.2% gelatin, followed by incubation with Serum-Free Protein Block (Dako Cytomation) for 15 min, and then incubation at 4°C overnight with primary antibody. After several washes in PBS containing 0.1% BSA, 0.05% saponin, and 0.2% gelatin, the sections were washed in PBS and incubated for 1 h with polymer-linked, peroxidase-conjugated goat anti-rabbit IgG (MACH2, Biocare Medical, Concord, CA), again washed with PBS, and then exposed to diaminobenzidine (DAB) for 5 min. The sections were washed in distilled water, dehydrated with xylene, mounted, and observed by light microscopy. Sections were examined on a Nikon E600 microscope equipped with DIC optics and photographed using a DMX1200F digital camera and ACT-1 software (Nikon). Color correction was performed using Adobe Photoshop software (Adobe Systems, San Jose, CA).
Double-immunolabeling procedure.
Double immunolabeling was accomplished using sequential immunoperoxidase procedures described in detail previously (18, 19, 32, 33, 40). Briefly, tissue sections were labeled with the first primary antibody using Vector SG (Vector Laboratories, Burlingame, CA) as the chromogen to produce a blue label, as described above. After the Vector SG reaction, sections were washed in PBS and then blocked using 3% H2O2 in methanol. The above procedure was repeated with the substitution of a second primary antibody and the substitution of DAB for Vector SG. This label was easily distinguishable from the blue label produced by the Vector SG. Sections were then washed with glass distilled water, dehydrated with xylene, mounted with Permount, and observed by light microscopy.
RESULTS
Immunoblot analysis.
Immunoblot analysis of human whole kidney and cortical protein revealed expression of a 61-kDa protein in NaDC1 antibodies (Fig. 1). No protein was detected when either the primary antibody or the secondary antibody was omitted from the immunoblot procedure. These studies confirmed the presence of NaDC1 protein in human kidney tissues.
Fig. 1.
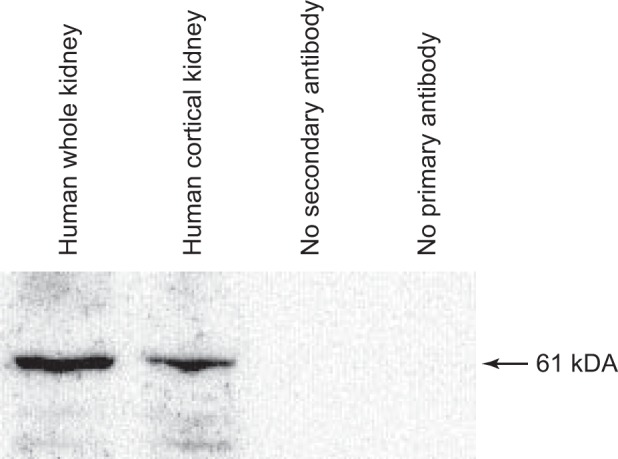
Immunoblot of normal human kidney protein. Immunoblot analysis of human whole kidney and human cortical kidney revealed expression of a 61-kDa protein in NaDC1 antibodies. No protein was detected when either the primary antibody or the secondary antibody was omitted from the immunoblot procedure.
NaDC1 immunolocalization.
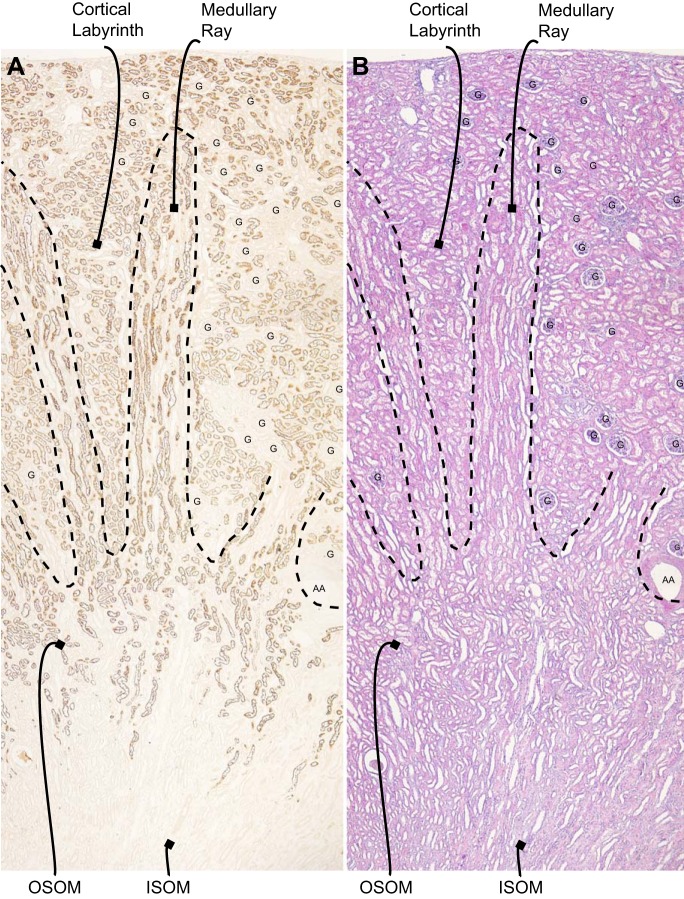
We next determined NaDC1’s cell-specific expression in the human kidney using immunohistochemistry. Low-power microscopy showed NaDC1 immunolabeling was present in a subset of cells in the cortex in the labyrinth, in the medullary ray in the cortex and in the outer stripe of the outer medulla. There was no significant detectable expression in the inner stripe of the outer medulla (Fig. 2).
Fig. 2.
Distribution of NaDC1 immunolabel in the human kidney. A: low-power magnification of NaDC1 immunolabel in the human kidney. Immunoreactivity is present in a subset of cells in the cortex in the labyrinth, in the medullary ray in the cortex, and in the outer stripe of the outer medulla (OSOM). No expression is observed in the inner stripe of the outer medulla (ISOM). B: hematoxylin and eosin (H&E) staining of a serial section of the same kidney. G, glomerulus; AA, arcuate artery.
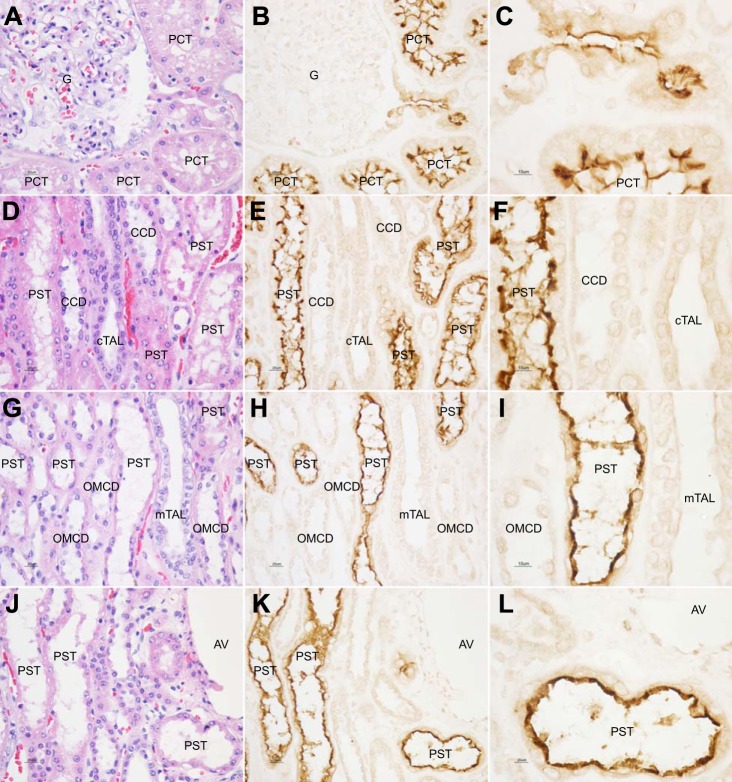
NaDC1 positive cells in the cortical labyrinth had an apical brush border, which showed strong NaDC1 immunolabeling (Fig. 3, A–C). Apical and brush-border NaDC1 immunolabeling was present in tubule segments that originated from the glomerular tuft, that is, the initial proximal convoluted tubule. All cells with an apical brush border had brush-border NaDC1 immunolabeling.
Fig. 3.
High-power micrographs of NaDC1 immunolabel in human kidney. A and B show H&E staining and NaDC1 expression in the renal cortical labyrinth. Proximal tubule segment originating from the glomerular tuft, the initial proximal convoluted tubule (PCT), shows strong apical immunolabel. No expression is observed in the glomerulus. In addition, well-preserved proximal tubule brush-border membrane is clearly labeled (C, high-power micrograph). All cells with an apical brush border had brush-border NaDC1 immunolabeling. D and E show H&E staining and NaDC1 expression in the medullary ray in the cortex. In the medullary ray in the cortex, which consists of proximal straight tubule (PST), cortical thick ascending limb (cTAL) of the loop of Henle and cortical collecting duct (CCD) segments, NaDC1 immunolabeling is located over the abundant apical brush border of PST cells (F, high-power micrograph). No NaDC1 immunolabeling is present in the cTAL or CCD. G and H show H&E staining and NaDC1 expression in the outer stripe of the outer medulla. As in the medullary ray in the cortex, NaDC1 immunolabeling is located over the abundant apical brush border of PST cells (I, high-power micrograph). No NaDC1 immunolabeling is present in the mTAL and outer medullary collecting duct (OMCD). J and K show H&E staining and NaDC1 expression in the transition from cortical to outer medullary region. There is no evident intensity variation in the transition from the PST in the cortical labyrinth to the PST in the outer stripe of outer medulla (L, high-power micrograph). AV, arcuate vein.
In the medullary ray in the cortex, which consists of proximal straight tubule, cortical thick ascending limb of the loop of Henle and collecting duct segments, apical NaDC1 immunolabeling was present in a subpopulation of tubular epithelial segments (Fig. 3, D–F). The apical NaDC1 immunolabel was exclusively in tubules with an abundant apical brush border, thereby identifying them as proximal straight tubules. No NaDC1 immunolabeling was present in tubular epithelial segments with cells with morphological characteristics of either cortical thick ascending limb of the loop of Henle or cortical collecting duct principal or intercalated cells.
In the outer stripe of the outer medulla, a subpopulation of tubular profiles showed apical NaDC1 immunolabeling (Fig. 3, G–I). Similar to the medullary ray in the cortex, strong NaDC1 immunolabel was present in the abundant apical brush-border of proximal tubules. No NaDC1 immunolabeling was present in cells with morphological characteristics of thick ascending limb or collecting duct cells. In many cases, NaDC1-positive proximal tubule segments in the medullary ray in the cortex extended into the outer stripe of the outer medulla. Apical NaDC1 immunolabeling was continuous and did not show evident intensity variations from the transition from cortical to outer medullary regions (Fig. 3, J–L).
There was no evidence of NaDC1 immunolabeling in glomerular tuft cells, in vascular tissues, or in interstitial cells.
Overall, these findings show evidence of NaDC1 apical immunolabeling throughout the entire proximal tubule of the human kidney, from the initial proximal convoluted tubule through the terminal portion of the proximal straight tubule in the outer stripe of the outer medulla. NaDC1 immunolabeling was present only in the proximal tubule, and there was no detectable expression in non-proximal tubule cells.
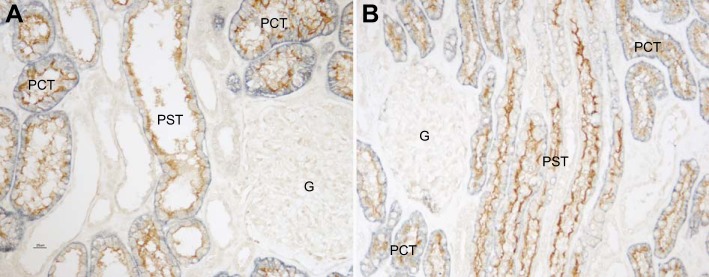
Colocalization of NaDC1 with NBCe1.
To confirm that NaDC1 expression was specific to the proximal tubule, we performed colocalization of NaDC1 with the proximal tubule-specific protein NBCe1 (SLC4A4). NBCe1 is the sodium-coupled, electrogenic bicarbonate cotransporter present in the basolateral plasma membrane throughout the entire proximal tubule (38, 48, 50). NBCe1 plays a key role in proximal tubule bicarbonate reabsorption (1, 8, 25) and is necessary for normal proximal tubule ammonia and organic anion metabolism (20, 40). Figure 4 shows representative findings. All renal epithelial cells with apical NaDC1 immunolabel showed strong basolateral NBCe1 immunolabel. These colocalization findings confirm that NaDC1 is present throughout the entire proximal tubule of the human kidney.
Fig. 4.
Colocalization of NaDC1 with proximal tubule specific protein, NBCe1. A: cortical labyrinth. B: medullary ray. In both the cortical labyrinth and medullary ray, all cells with apical NaDC1 (brown) immunolabel exhibit basolateral NBCe1 (blue) immunolabel, and vice versa. No cells exhibit only NaDC1 or only NBCe1 immunolabel. These findings indicate that NaDC1 is expressed solely in renal proximal tubule cells.
NaDC1 expression in renal tumors.
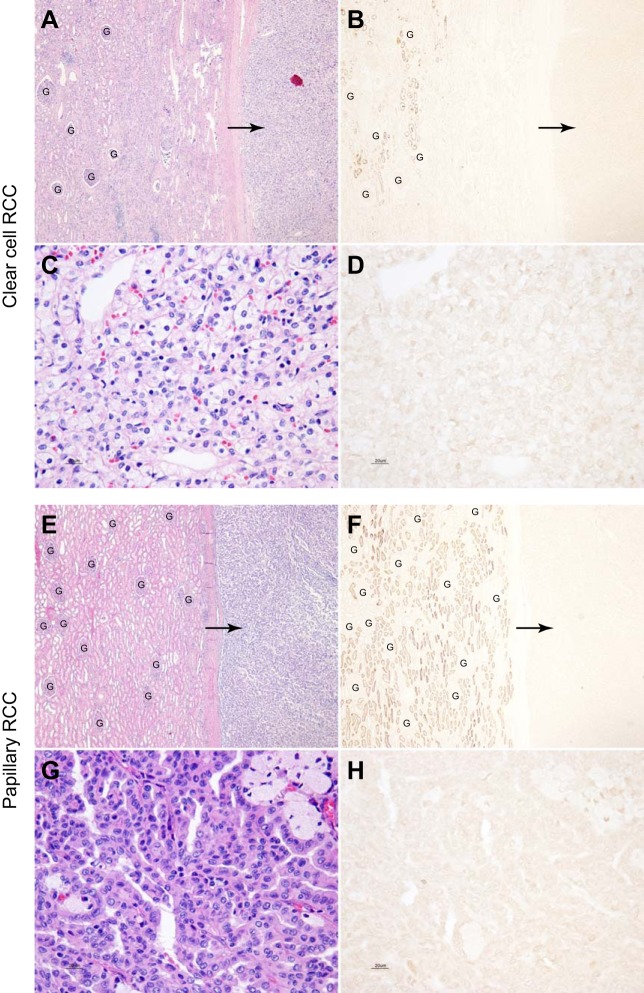
Because of the high level of NaDC1 expression in the kidney and because of the possible role of citrate as an organic anion substrate for energy metabolism, we examined whether renal tumors, particularly those of proximal tubule origin, would express significant levels of NaDC1 protein. Figure 5 shows representative findings. Most evidence indicates that both clear cell and papillary renal cell carcinoma are of proximal tubule origin. However, no significant NaDC1 immunolabel was detected in either clear cell (n = 7) or papillary renal cell carcinoma (n = 6). Adjacent, nonneoplastic kidney tissue in each specimen was examined, and showed normal levels of NaDC1 apical immunolabel. Thus the absence of NaDC1 immunolabel in neoplastic tissue was not due to nonspecific effects associated with tissue preservation, handling, or the immunohistochemical procedure.
Fig. 5.
NaDC1 expression in the clear cell and papillary renal cell carcinoma. A and B show low-power magnification of hematoxylin and eosin staining of a clear cell renal cell carcinoma (RCC) and adjacent normal renal cortical tissue and NaDC1 immunolabel in a serial section of the same human kidney tissue. No NaDC1 immunolabel is evident in the clear cell carcinoma (arrows). Normal NaDC1 apical immunolabel is present in proximal tubule cells in uninvolved adjacent renal tissue. C and D show high-power magnification of hematoxylin and eosin staining of a clear cell RCC and NaDC1 immunolabel in a serial section of the same clear cell RCC. No detectable NaDC1 immunoreactivity is observed in clear cell RCC. E and F show low-power micrographs of hematoxylin and eosin staining of a papillary RCC and adjacent normal renal cortical tissue and NaDC1 immunolabel in a serial section of the same human kidney tissue. No NaDC1 immunolabel is evident in the papillary RCC (arrows). Normal NaDC1 apical immunolabel is present in proximal tubule cells in uninvolved adjacent renal tissue. G and H show high-power magnification of hematoxylin and eosin staining of a papillary RCC and NaDC1 immunolabel in a serial section of the same a papillary RCC. No detectable NaDC1 immunoreactivity is observed in the papillary RCC. These findings indicate that NaDC1 expression is not detectable in either clear cell or papillary RCC. Glomeruli (“G”) are present in A, B, E, and F. No detectable NaDC1 immunolabel is present in glomeruli.
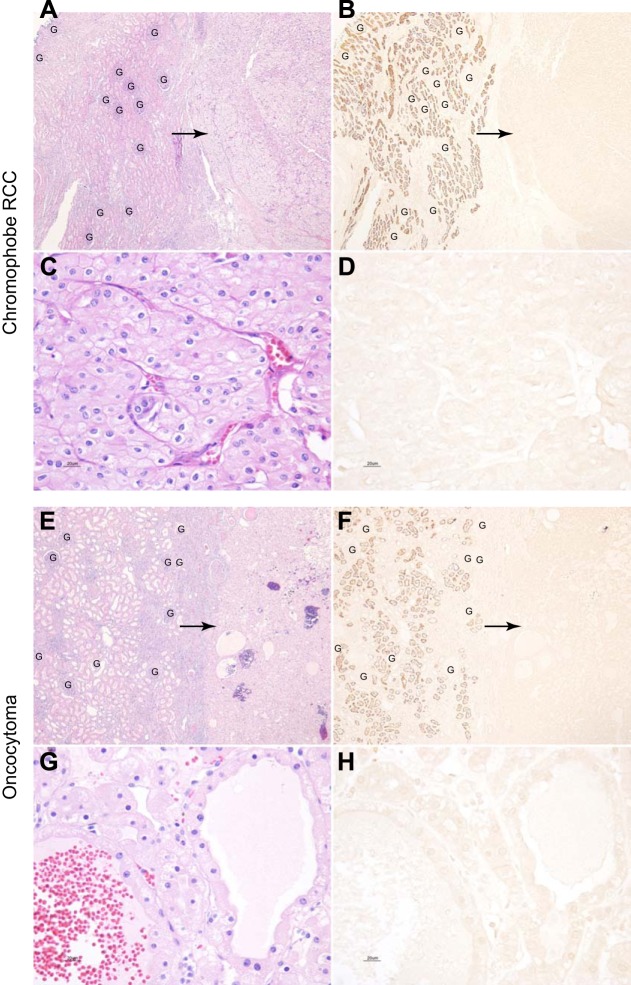
Two other forms of renal tumors are chromophobe renal cell carcinoma and oncocytoma. Most evidence suggests that these are of intercalated cell origin. Consistent with this cellular origin, there was no evidence of NaDC1 immunolabel in either chromophobe renal cell carcinoma (n = 3) or oncocytoma tissues (n = 5, Fig. 6). Again, adjacent, non-neoplastic kidney tissue in each specimen showed normal levels of apical NaDC1 immunolabel in proximal tubule segments.
Fig. 6.
NaDC1 expression in the chromophobe renal cell carcinoma and oncocytoma. A and B show low-power magnification of hematoxylin and eosin staining of a renal chromophobe RCC and adjacent normal renal cortical tissue and NaDC1 immunolabel in a serial section of the same human kidney tissue. No NaDC1 immunolabel is evident in the chromophobe RCC (arrows). Normal NaDC1 apical immunolabel is present in proximal tubule cells in uninvolved adjacent renal tissue. C and D show high-power magnification of hematoxylin and eosin staining of a chromophobe RCC and NaDC1 immunolabel in a serial section of the same chromophobe RCC. No detectable NaDC1 immunoreactivity is observed in chromophobe RCC. E and F show low-power micrographs of hematoxylin and eosin staining of a oncocytoma and adjacent normal renal cortical tissue and NaDC1 immunolabel in a serial section of the same human kidney tissue. No NaDC1 immunolabel is evident in the oncocytoma (arrows). Normal NaDC1 apical immunolabel is present in proximal tubule cells in uninvolved adjacent renal tissue. G and H show high-power magnification of hematoxylin and eosin staining of a oncocytoma and NaDC1 immunolabel in a serial section of the same a oncocytoma. No detectable NaDC1 immunoreactivity is observed in the oncocytoma. These findings indicate that NaDC1 expression is not detectable in either chromophobe RCC or oncocytoma. Glomeruli (“G”) are present in panels A, B, E, and F. No detectable NaDC1 immunolabel is present in glomeruli.
DISCUSSION
These studies are the first report of the cell-specific expression of NaDC1 in the normal and neoplastic human kidney. Immunoblot analysis of human whole kidney and kidney cortex protein isolates demonstrated expression of a protein of ~61 kDa. Immunohistochemistry showed apical NaDC1 immunolabel in the proximal tubule of normal human kidney tissue. NaDC1 was present throughout the entire proximal tubule, from the initial proximal convoluted tubule through the terminal portion of the proximal straight tubule in the outer stripe of the outer medulla. Expression was apical and appeared to involve the brush border. Proximal tubule-specific localization was confirmed by classic morphological characteristics of the proximal tubule, by demonstration of expression in proximal tubule segments arising from the glomerular capillary tuft, and by colocalization with the proximal tubule-specific protein, NBCe1. Finally, there was no detectable NaDC1 expression in a variety of renal tumors, including tumors of presumed proximal tubule origin, clear cell and papillary renal cell carcinoma, or in renal tumors of intercalated cells origin, chromophobe renal cell carcinoma and oncocytoma. These findings provide an important advance in our understanding of human renal dicarboxylate transport.
NaDC1 is a member of a protein family (SLC13) that facilitates sodium-coupled movement of Krebs cycle intermediates or sulfates across plasma membranes. SLC13A1 and SLC13A4 are sodium-coupled sulfate transporters, also known as NaSi-1 and NaSi-2, respectively. They do not have known roles in transport of dicarboxylate, in general, or of citrate, specifically (47). SLC13A3, also known as NaDC3, is a high-affinity, sodium-coupled dicarboxylate cotransporter with broad tissue expression. In the kidney, SLC13A3 appears to be expressed specifically in proximal tubule basolateral membranes (4). Its most important physiological substrates appear to be glutarate and alpha-ketoglutarate (2-oxoglutarate), and it may have an important role in drug and xenobiotic excretion related to its interactions with organic anion transporters (47). SLC13A5, also known as NaCT, is found predominantly in the liver and brain and appears to be involved in multiple critical metabolic processes (26, 27, 47). Deletion of the SLC13A5 gene in mice causes substantial changes in signaling pathways regulating energy balance; the mice are smaller in size, are protected against nutritionally induced obesity, are protected against high-fat diet-induced hepatic steatosis and insulin resistance, have increased mitochondrial metabolism that involves AMPK activation, and are protected against aging-related adiposity and insulin resistance (7).
Multiple lines of evidence indicate that NaDC1 has a critical role in renal dicarboxylate transport. It is present in the apical brush border of proximal tubules in the human (current study), rat (3, 21, 51), and mouse (40) kidney. Functional expression studies show that it has transport characteristics of sodium-coupled, electrogenic dicarboxylate transport with broad dicarboxylate affinity, including citrate, 2-oxoglutarate, and succinate (41, 42, 44, 51). Citrate exists in both a tricarboxylate form (citrate3−) and a dicarboxylate form (citrate2−), with pKa of ~6.4, and current evidence indicates that NaDC1 transports citrate exclusively in the dicarboxylate form (41, 42, 51, 61). Experimental studies in rodents indicate that NaDC1 expression is increased by metabolic acidosis (3, 40) through a mechanism that may involve the endothelin B receptor (36). Each of these characteristics is essentially identical to findings examining proximal tubule dicarboxylate transport, whether studied using perfused proximal tubule segments or brush-border membrane vesicles (9, 13, 16, 28, 52, 60).
Urinary dicarboxylate excretion is determined by the difference between glomerular filtration and tubular reabsorption. Plasma dicarboxylates are small-molecular-weight molecules that are not protein bound, and are essentially completely filtered at the glomerulus. Although significant components of plasma citrate can complex with calcium, magnesium, or sodium (58, 59), both ionized and complexed citrate are small-molecular-weight molecules that are essentially completely filtered at the glomerulus. In general, changes in plasma dicarboxylate levels are not considered an important regulatory mechanism affecting their urinary excretion. Current evidence indicates that essentially all tubular reabsorption occurs in the proximal tubule (6, 9). Limited proximal tubule 2-oxoglutarate secretion can occur in response to metabolic alkalosis (37), which may involve apical OAT10-mediated transport (14). Although an early study suggested the presence of distal tubular citrate uptake (12), subsequent evidence localized citrate transport exclusively to the proximal tubule (9, 15, 31). Citrate transported into proximal tubule cells is completely metabolized via the Krebs cycle, enabling citrate to serve as a significant component of renal oxidative metabolism (16).
The current study suggests several important differences exist between the axial distribution of protein expression in the human kidney and reports of expression and/or citrate transport activity in the rat and rabbit kidney. In particular, the current study found no axial variation in NaDC1 immunolabel intensity between the early proximal tubule and the proximal straight tubule. This contrasts with findings in the rat in which NaDC1 immunolabel was found exclusively in S2 and S3 proximal tubule segments (51). However, another study in the rat identified NaDC1 expression throughout the S1, S2, and S3 proximal tubule (3). Our present study in human kidney also contrasts with citrate transport measurements in isolated perfused rabbit proximal tubule segments, where the mean rate of transport in rabbit proximal convoluted tubule segments was ~14 times greater than in proximal straight tubule segments (9). These differences likely reflect aspects of species-dependent differences in cellular expression, regulation of transport activity other than solely through NaDC1 expression, and contributions of proteins other than NaDC1 to proximal tubule citrate reabsorption.
Single-nucleotide polymorphisms in human NaDC1 are known to alter transport activity and protein expression. For example, several common variants, including M45L, A310P, V117I, and F254L, cause decreased protein expression and transport activity (45). Other variants, A310P, P385S, and V477M, change substrate selectivity (45). The V477M variant showed stimulation by lithium, suggesting involvement of the high-affinity binding site (45). The I550V variant has an increased sensitivity to inhibition by lithium, but no significant effect on protein abundance (45). Thus, not only is human NaDC1 well-positioned, through its expression in the apical membrane of the proximal tubule, to play a critical role in renal citrate excretion, common polymorphisms are likely to cause clinically important variations in renal transport and excretion of critical citric acid cycle intermediates.
Studies in mice with genetic deletion of NaDC1 have provided important information regarding its role in metabolism of renal citrate and other dicarboxylates. Homozygous NaDC1 deletion increased urinary excretion of citrate, as well as succinate, 2-oxoglutarate, fumarate, and malate, dicarboxylate compounds that are transported by NaDC1 (24, 55). Although heterozygous deletion did not alter excretion of any of these (24), heterozygous deletion of many renal transport proteins often has minimal to no physiological effect. This may involve either adaptive changes in expression and/or regulation in response to a 50% decrease in allele presence, hormonal changes, or adaptive changes in transcription efficiency, mRNA stability, translation efficiency, or protein stability. Studies examining NaDC1 gene deletion have shown evidence of significant residual citrate reabsorption (24, 55), suggesting the presence of additional apical proximal tubule citrate transport mechanisms. One likely candidate is a calcium-sensitive, acidosis-stimulated dicarboxylate transport activity that has been detected, although the specific protein mediating this activity has not been identified (22, 23).
NaDC1 expression was not identified in renal tumors, of either proximal tubule or non-proximal tubule origin. The lack of expression in tumors of presumed proximal tubule origin, i.e., clear cell and papillary renal cell carcinoma, is consistent with a shift of metabolic pathways away from oxidative pathways and towards glycolytic pathways (34, 35, 54, 62). Under normal conditions, citrate metabolism, primarily related to apical citrate uptake mediated by NaDC1, provides ~15% of proximal tubule oxidative metabolism (16, 30). Lack of expression in oncocytoma and chromophobe renal cell carcinoma tumors is consistent with the cell origin of these tumors being intercalated cells (18, 53), a cell type which had no evidence of NaDC1 expression in the current study.
In summary, NaDC1 has a critical role in proximal tubule dicarboxylate transport, most evident in its role in citrate transport, and thereby is critical both for prevention of nephrolithiasis and for acid-base homeostasis. The current studies demonstrate for the first time that apical NaDC1 protein is present throughout the human proximal tubule. Importantly, this demonstrates that NaDC1 distribution in the human kidney is identical to that observed in laboratory animals used for examining the physiological role and regulation of NaDC1 in ion transport. Accordingly, these current studies indicate that the role of NaDC1 in the human kidney is likely to be very similar to that observed in rodent and rabbit kidneys.
GRANTS
The studies were supported by funds from the National Institute of Diabetes and Digestive and Kidney Diseases (Grants R01-DK-045788 and R01-DK-107798) and from the Department of Veterans Affairs (1I01BX000818).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.-W.L., M.E.H., W.L.C., and D.N.W. performed experiments; H.-W.L., J.W.V., and I.D.W. analyzed data; H.-W.L., M.E.H., J.W.V., and I.D.W. interpreted results of experiments; H.-W.L. prepared figures; H.-W.L. and I.D.W. drafted manuscript; H.-W.L., M.E.H., G.O., W.L.C., D.N.W., J.W.V., and I.D.W. edited and revised manuscript; H.-W.L., M.E.H., G.O., W.L.C., J.W.V., and I.D.W. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank G. Cowsert for secretarial assistance.
REFERENCES
- 1.Aalkjaer C, Frische S, Leipziger J, Nielsen S, Praetorius J. Sodium coupled bicarbonate transporters in the kidney, an update. Acta Physiol Scand 181: 505–512, 2004. doi: 10.1111/j.1365-201X.2004.01324.x. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal KP, Narula S, Kakkar M, Tandon C. Nephrolithiasis: molecular mechanism of renal stone formation and the critical role played by modulators. Biomed Res Int 2013: 292953, 2013. doi: 10.1155/2013/292953. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Aruga S, Wehrli S, Kaissling B, Moe OW, Preisig PA, Pajor AM, Alpern RJ. Chronic metabolic acidosis increases NaDC-1 mRNA and protein abundance in rat kidney. Kidney Int 58: 206–215, 2000. doi: 10.1046/j.1523-1755.2000.00155.x. [DOI] [PubMed] [Google Scholar]
- 4.Bai X, Chen X, Feng Z, Hou K, Zhang P, Fu B, Shi S. Identification of basolateral membrane targeting signal of human sodium-dependent dicarboxylate transporter 3. J Cell Physiol 206: 821–830, 2006. doi: 10.1002/jcp.20553. [DOI] [PubMed] [Google Scholar]
- 5.Balagura S, Pitts RF. Renal handling of α-ketoglutarate by the dog. Am J Physiol 207: 483–494, 1964. [DOI] [PubMed] [Google Scholar]
- 6.Baruch SB, Burich RL, Eun CK, King VF. Renal metabolism of citrate. Med Clin North Am 59: 569–582, 1975. doi: 10.1016/S0025-7125(16)32009-0. [DOI] [PubMed] [Google Scholar]
- 7.Birkenfeld AL, Lee HY, Guebre-Egziabher F, Alves TC, Jurczak MJ, Jornayvaz FR, Zhang D, Hsiao JJ, Martin-Montalvo A, Fischer-Rosinsky A, Spranger J, Pfeiffer AF, Jordan J, Fromm MF, König J, Lieske S, Carmean CM, Frederick DW, Weismann D, Knauf F, Irusta PM, De Cabo R, Helfand SL, Samuel VT, Shulman GI. Deletion of the mammalian INDY homolog mimics aspects of dietary restriction and protects against adiposity and insulin resistance in mice. Cell Metab 14: 184–195, 2011. doi: 10.1016/j.cmet.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boron WF. Acid-base transport by the renal proximal tubule. J Am Soc Nephrol 17: 2368–2382, 2006. doi: 10.1681/ASN.2006060620. [DOI] [PubMed] [Google Scholar]
- 9.Brennan TS, Klahr S, Hamm LL. Citrate transport in rabbit nephron. Am J Physiol Renal Fluid Electrolyte Physiol 251: F683–F689, 1986. [DOI] [PubMed] [Google Scholar]
- 10.Cheema-Dhadli S, Lin SH, Halperin ML. Mechanisms used to dispose of progressively increasing alkali load in rats. Am J Physiol Renal Physiol 282: F1049–F1055, 2002. doi: 10.1152/ajprenal.00006.2001. [DOI] [PubMed] [Google Scholar]
- 11.Deen PMT, Robben JH. Succinate receptors in the kidney. J Am Soc Nephrol 22: 1416–1422, 2011. doi: 10.1681/ASN.2010050481. [DOI] [PubMed] [Google Scholar]
- 12.Edwards KDG, Mody NJ, Crawford MA. Citrate utilization by the renal tubules in primates. S Afr J Med Sci 27: 45–50, 1962. [Google Scholar]
- 13.Edwards RM, Stack E, Trizna W. alpha-Ketoglutarate transport in rat renal brush-border and basolateral membrane vesicles. J Pharmacol Exp Ther 281: 1059–1064, 1997. [PubMed] [Google Scholar]
- 14.Grimm PR, Lazo-Fernandez Y, Delpire E, Wall SM, Dorsey SG, Weinman EJ, Coleman R, Wade JB, Welling PA. Integrated compensatory network is activated in the absence of NCC phosphorylation. J Clin Invest 125: 2136–2150, 2015. doi: 10.1172/JCI78558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grollman AP, Walker WG, Harrison HC, Harrison HE. Site of reabsorption of citrate and calcium in the renal tubule of the dog. Am J Physiol 205: 697–701, 1963. [DOI] [PubMed] [Google Scholar]
- 16.Hamm LL. Renal handling of citrate. Kidney Int 38: 728–735, 1990. doi: 10.1038/ki.1990.265. [DOI] [PubMed] [Google Scholar]
- 17.Hamm LL, Simon EE. Roles and mechanisms of urinary buffer excretion. Am J Physiol Renal Fluid Electrolyte Physiol 253: F595–F605, 1987. [DOI] [PubMed] [Google Scholar]
- 18.Han KH, Croker BP, Clapp WL, Werner D, Sahni M, Kim J, Kim HY, Handlogten ME, Weiner ID. Expression of the ammonia transporter, rh C glycoprotein, in normal and neoplastic human kidney. J Am Soc Nephrol 17: 2670–2679, 2006. doi: 10.1681/ASN.2006020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han K-H, Lee HW, Handlogten ME, Whitehill F, Osis G, Croker BP, Clapp WL, Verlander JW, Weiner ID. Expression of the ammonia transporter family member, Rh B Glycoprotein, in the human kidney. Am J Physiol Renal Physiol 304: F972–F981, 2013. doi: 10.1152/ajprenal.00550.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Handlogten ME, Osis G, Lee HW, Romero MF, Verlander JW, Weiner ID. NBCe1 expression is required for normal renal ammonia metabolism. Am J Physiol Renal Physiol 309: F658–F666, 2015. doi: 10.1152/ajprenal.00219.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He Y, Chen X, Yu Z, Wu D, Lv Y, Shi S, Zhu H. Sodium dicarboxylate cotransporter-1 expression in renal tissues and its role in rat experimental nephrolithiasis. J Nephrol 17: 34–42, 2004. [PubMed] [Google Scholar]
- 22.Hering-Smith KS, Mao W, Schiro FR, Coleman-Barnett J, Pajor AM, Hamm LL. Localization of the calcium-regulated citrate transport process in proximal tubule cells. Urolithiasis 42: 209–219, 2014. doi: 10.1007/s00240-014-0653-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hering-Smith KS, Schiro FR, Pajor AM, Hamm LL. Calcium sensitivity of dicarboxylate transport in cultured proximal tubule cells. Am J Physiol Renal Physiol 300: F425–F432, 2011. doi: 10.1152/ajprenal.00036.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho HTB, Ko BCB, Cheung AKH, Lam AKM, Tam S, Chung SK, Chung SSM. Generation and characterization of sodium-dicarboxylate cotransporter-deficient mice. Kidney Int 72: 63–71, 2007. doi: 10.1038/sj.ki.5002258. [DOI] [PubMed] [Google Scholar]
- 25.Igarashi T, Sekine T, Inatomi J, Seki G. Unraveling the molecular pathogenesis of isolated proximal renal tubular acidosis. J Am Soc Nephrol 13: 2171–2177, 2002. doi: 10.1097/01.ASN.0000025281.70901.30. [DOI] [PubMed] [Google Scholar]
- 26.Inoue K, Zhuang L, Ganapathy V. Human Na+ -coupled citrate transporter: primary structure, genomic organization, and transport function. Biochem Biophys Res Commun 299: 465–471, 2002. doi: 10.1016/S0006-291X(02)02669-4. [DOI] [PubMed] [Google Scholar]
- 27.Inoue K, Zhuang L, Maddox DM, Smith SB, Ganapathy V. Structure, function, and expression pattern of a novel sodium-coupled citrate transporter (NaCT) cloned from mammalian brain. J Biol Chem 277: 39469–39476, 2002. doi: 10.1074/jbc.M207072200. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins AD, Dousa TP, Smith LH. Transport of citrate across renal brush border membrane: effects of dietary acid and alkali loading. Am J Physiol Renal Fluid Electrolyte Physiol 249: F590–F595, 1985. [DOI] [PubMed] [Google Scholar]
- 29.Kippen I, Hirayama B, Klinenberg JR, Wright EM. Transport of tricarboxylic acid cycle intermediates by membrane vesicles from renal brush border. Proc Natl Acad Sci USA 76: 3397–3400, 1979. doi: 10.1073/pnas.76.7.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein KL, Wang MS, Torikai S, Davidson WD, Kurokawa K. Substrate oxidation by isolated single nephron segments of the rat. Kidney Int 20: 29–35, 1981. doi: 10.1038/ki.1981.100. [DOI] [PubMed] [Google Scholar]
- 31.Kook YJ, Lotspeich WD. Citrate excretion during intrarenal arterial precursor infusion in the alkalotic dog. Am J Physiol 215: 282–288, 1968. [DOI] [PubMed] [Google Scholar]
- 32.Lee HW, Osis G, Handlogten ME, Lamers WH, Chaudhry FA, Verlander JW, Weiner ID. Proximal tubule-specific glutamine synthetase deletion alters basal and acidosis-stimulated ammonia metabolism. Am J Physiol Renal Physiol 310: F1229–F1242, 2016. doi: 10.1152/ajprenal.00547.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee HW, Verlander JW, Handlogten ME, Han K-H, Weiner ID. Effect of collecting duct-specific deletion of both Rh B Glycoprotein (Rhbg) and Rh C Glycoprotein (Rhcg) on renal response to metabolic acidosis. Am J Physiol Renal Physiol 306: F389–F400, 2014. doi: 10.1152/ajprenal.00176.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linehan WM, Ricketts CJ. The metabolic basis of kidney cancer. Semin Cancer Biol 23: 46–55, 2013. doi: 10.1016/j.semcancer.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linehan WM, Srinivasan R, Schmidt LS. The genetic basis of kidney cancer: a metabolic disease. Nat Rev Urol 7: 277–285, 2010. doi: 10.1038/nrurol.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L, Zacchia M, Tian X, Wan L, Sakamoto A, Yanagisawa M, Alpern RJ, Preisig PA. Acid regulation of NaDC-1 requires a functional endothelin B receptor. Kidney Int 78: 895–904, 2010. doi: 10.1038/ki.2010.264. [DOI] [PubMed] [Google Scholar]
- 37.Martin M, Ferrier B, Baverel G. Transport and utilization of alpha-ketoglutarate by the rat kidney in vivo. Pflugers Arch 413: 217–224, 1989. doi: 10.1007/BF00583533. [DOI] [PubMed] [Google Scholar]
- 38.Maunsbach AB, Vorum H, Kwon TH, Nielsen S, Simonsen B, Choi I, Schmitt BM, Boron WF, Aalkjaer C. Immunoelectron microscopic localization of the electrogenic Na/HCO(3) cotransporter in rat and ambystoma kidney. J Am Soc Nephrol 11: 2179–2189, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Moe OW, Preisig PA. Dual role of citrate in mammalian urine. Curr Opin Nephrol Hypertens 15: 419–424, 2006. doi: 10.1097/01.mnh.0000232882.35469.72. [DOI] [PubMed] [Google Scholar]
- 40.Osis G, Handlogten ME, Lee H-W, Hering-Smith KS, Huang W, Romero MF, Verlander JW, Weiner ID. Effect of NBCe1 deletion on renal citrate and 2-oxoglutarate handling. Physiol Rep 4: e12778, 2016. doi: 10.14814/phy2.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pajor AM. Sequence and functional characterization of a renal sodium/dicarboxylate cotransporter. J Biol Chem 270: 5779–5785, 1995. [DOI] [PubMed] [Google Scholar]
- 42.Pajor AM. Molecular cloning and functional expression of a sodium-dicarboxylate cotransporter from human kidney. Am J Physiol 270: F642–F648, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Pajor AM. Citrate transport by the kidney and intestine. Semin Nephrol 19: 195–200, 1999. [PubMed] [Google Scholar]
- 44.Pajor AM, Sun NN. Molecular cloning, chromosomal organization, and functional characterization of a sodium-dicarboxylate cotransporter from mouse kidney. Am J Physiol Renal Physiol 279: F482–F490, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Pajor AM, Sun NN. Single nucleotide polymorphisms in the human Na+-dicarboxylate cotransporter affect transport activity and protein expression. Am J Physiol Renal Physiol 299: F704–F711, 2010. doi: 10.1152/ajprenal.00213.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pajor AM, Valmonte HG. Expression of the renal Na+/dicarboxylate cotransporter, NaDC-1, in COS-7 cells. Pflugers Arch 431: 645–651, 1996. doi: 10.1007/BF02191915. [DOI] [PubMed] [Google Scholar]
- 47.Pajor AM. Sodium-coupled dicarboxylate and citrate transporters from the SLC13 family. Pflugers Arch 466: 119–130, 2014. doi: 10.1007/s00424-013-1369-y. [DOI] [PubMed] [Google Scholar]
- 48.Romero MF, Hediger MA, Boulpaep EL, Boron WF. Expression cloning and characterization of a renal electrogenic Na+/HCO3- cotransporter. Nature 387: 409–413, 1997. doi: 10.1038/387409a0. [DOI] [PubMed] [Google Scholar]
- 49.Sadagopan N, Li W, Roberds SL, Major T, Preston GM, Yu Y, Tones MA. Circulating succinate is elevated in rodent models of hypertension and metabolic disease. Am J Hypertens 20: 1209–1215, 2007. doi: 10.1016/j.amjhyper.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 50.Schmitt BM, Biemesderfer D, Romero MF, Boulpaep EL, Boron WF. Immunolocalization of the electrogenic Na+-HCO-3 cotransporter in mammalian and amphibian kidney. Am J Physiol Renal Physiol 276: F27–F38, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Sekine T, Cha SH, Hosoyamada M, Kanai Y, Watanabe N, Furuta Y, Fukuda K, Igarashi T, Endou H. Cloning, functional characterization, and localization of a rat renal Na+-dicarboxylate transporter. Am J Physiol 275: F298–F305, 1998. [DOI] [PubMed] [Google Scholar]
- 52.Sheridan E, Rumrich G, Ullrich KJ. Reabsorption of dicarboxylic acids from the proximal convolution of rat kidney. Pflugers Arch 399: 18–28, 1983. doi: 10.1007/BF00652517. [DOI] [PubMed] [Google Scholar]
- 53.Störkel S, Steart PV, Drenckhahn D, Thoenes W. The human chromophobe cell renal carcinoma: its probable relation to intercalated cells of the collecting duct. Virchows Arch B Cell Pathol Incl Mol Pathol 56: 237–245, 1989. doi: 10.1007/BF02890022. [DOI] [PubMed] [Google Scholar]
- 54.Sudarshan S, Karam JA, Brugarolas J, Thompson RH, Uzzo R, Rini B, Margulis V, Patard JJ, Escudier B, Linehan WM. Metabolism of kidney cancer: from the lab to clinical practice. Eur Urol 63: 244–251, 2013. doi: 10.1016/j.eururo.2012.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teran FJ, Huang W, Hamm LL, Hering-Smith KS. NaDC1 knockout: effects on blood pressure and urine pH (Abstract) J Am Soc Nephrol 26: 382A, 2015. [Google Scholar]
- 56.Tokonami N, Morla L, Centeno G, Mordasini D, Ramakrishnan SK, Nikolaeva S, Wagner CA, Bonny O, Houillier P, Doucet A, Firsov D. α-Ketoglutarate regulates acid-base balance through an intrarenal paracrine mechanism. J Clin Invest 123: 3166–3171, 2013. doi: 10.1172/JCI67562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Unwin RJ, Capasso G, Shirley DG. An overview of divalent cation and citrate handling by the kidney. Nephron Physiol 98: 15–20, 2004. doi: 10.1159/000080259. [DOI] [PubMed] [Google Scholar]
- 58.Walser M. Divalent cations: physiochemical state in glomerular filtrate and urine and renal excretion. In: Handbook of Physiology. Renal Physiology. Washington, DC: Am. Physiol. Soc., 1973, sect. 8, chapt. 18, p. 555–586. [Google Scholar]
- 59.Walser M. Ion association. VI. Interactions between calcium, magnesium, inorganic phosphate, citrate and protein in normal human plasma. J Clin Invest 40: 723–730, 1961. doi: 10.1172/JCI104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wright SH, Wunz TM. Succinate and citrate transport in renal basolateral and brush-border membranes. Am J Physiol Renal Fluid Electrolyte Physiol 253: F432–F439, 1987. [DOI] [PubMed] [Google Scholar]
- 61.Wright SH, Kippen I, Wright EM. Effect of pH on the transport of Krebs cycle intermediates in renal brush border membranes. Biochim Biophys Acta 684: 287–290, 1982. doi: 10.1016/0005-2736(82)90019-0. [DOI] [PubMed] [Google Scholar]
- 62.Yang OC, Maxwell PH, Pollard PJ. Renal cell carcinoma: translational aspects of metabolism and therapeutic consequences. Kidney Int 84: 667–681, 2013. doi: 10.1038/ki.2013.245. [DOI] [PubMed] [Google Scholar]