Abstract
T cells have been implicated in the pathogenesis of acute kidney injury (AKI) and its progression to chronic kidney disease (CKD). Previous studies suggest that Th17 cells participate during the AKI-to-CKD transition, and inhibition of T cell activity by mycophenolate mofetil (MMF) or losartan attenuates the development of fibrosis following AKI. We hypothesized that T cell-deficient rats may have reduced levels of IL-17 cytokine leading to decreased fibrosis following AKI. Renal ischemis-reperfusion (I/R) was performed on T cell-deficient athymic rats (Foxn1rnu−/rnu−) and control euthymic rats (Foxn1rnu−/+), and CKD progression was hastened by unilateral nephrectomy at day 33 and subsequent exposure to 4.0% sodium diet. Renal fibrosis developed in euthymic rats and was reduced by MMF treatment. Athymic rats exhibited a similar degree of fibrosis, but this was unaffected by MMF treatment. FACS analysis demonstrated that the number of IL-17+ cells was similar between postischemic athymic vs. euthymic rats. The source of IL-17 production in euthymic rats was predominately from conventional T cells (CD3+/CD161−). In the absence of conventional T cells in athymic rats, a compensatory pathway involving natural killer cells (CD3−/CD161+) was the primary source of IL-17. Blockade of IL-17 activity using IL-17Rc receptor significantly decreased fibrosis and neutrophil recruitment in both euthymic and athymic rats compared with vehicle-treated controls. Taken together, these data suggest that IL-17 secretion participates in the pathogenesis of AKI-induced fibrosis possibly via the recruitment of neutrophils and that the source of IL-17 may be from either conventional T cells or NK cells.
Keywords: lymphocytes, progression
acute kidney injury (AKI), which affects nearly 5% of all hospitalizations, enhances morbidity and mortality and increases the risk of chronic kidney disease (CKD) and end-stage renal disease (ESRD) (3). However, the mechanism for the progression of AKI to CKD/ESRD is not completely defined. Significant evidence suggests that T cells may participate in the pathogenesis of AKI. In animal models of AKI by ischemia-reperfusion (I/R), renal infiltration of T cells is observed as early as 30 min postinjury (8, 35, 39), whereas immunosuppressive drugs or immune deficiency protects against renal injury following I/R (10, 31). Our laboratory and others have identified sustained increases in renal T cells following recovery from AKI (5, 10, 26). In addition, the lymphocyte inhibitor mycophenolate mofetil (MMF) reduced progression of renal fibrosis in post-AKI rats exposed to high-salt diet (31). Given the increased recognition of the contribution of AKI to CKD progression, a better understanding of the mechanisms by which T cell activity may mediate increased fibrosis post-AKI is important in the potential development of further therapies.
CD4+ T cells differentiate into different effector T-helper (Th) cells upon antigen exposure and cytokines in “the postischemic” milieu (22, 42). Initially, it was thought that Th1 cells were activated during kidney injury, whereas Th2 cells contributed to repair function (25). Another T-helper subset, the T-regulatory cell, also promotes recovery following renal I/R injury in an IL-10-dependent pathway (22). However, a recently identified CD4+ T cell subset, referred to as Th17 cells, is characterized by the secretion of the cytokine IL-17 and has been implicated in various diseases, including AKI. IL-17 production is not restricted to T lymphocytes (i.e., Th17 cells) but may also be produced by other cells such as innate immune cells, including lymphoid-tissue inducer (LTi) cells, γδT cells, invariant natural killer T (iNKT) cells, natural killer (NK) cells, neutrophils, and Paneth cells (15). Its activity promotes tissue damage in part by attracting neutrophils (1) and stimulating the development of fibrosis (34). Increased numbers of IL-17+ cells in glomeruli and in the tubulointerstitium have been measured in renal biopsies collected from antineutrophil cytoplasmic antibodies (ACNA)-induced glomerulonephritis patients and correlated to their serum creatinine levels. Interestingly, the number of IL-17+ cells was reduced in renal biopsies of patients receiving immunotherapy (37). Circulating Th17 cells and Th17-associated cytokines are also increased in various experimental models of renal injury such as glomerulonephritis, lupus nephritis, and I/R (24, 30, 33). Furthermore, to demonstrate the role of IL-17 in kidney injury, Chan et al. reported that IL-17a–/– and Rorγt–/– mice as well as in mice treated with anti–IL-17a antibodies were protected from alterations in renal structure and function in response to cisplatin (13).
We recently reported an association of Th17 cells in CKD progression following AKI. Th17 cells were shown to persist in the kidney up to 5 wk following the resolution of the initial I/R injury (5). When injured rats were subsequently placed on high-salt diet for an additional 4 wk to hasten CKD progression, Th17 cells increased by greater than twofold. In addition, inhibition of the angiotensin type 1 receptor (AT1R) with losartan reduced Th17 infiltration and progression of renal fibrosis following recovery from AKI (26). These observations suggested a potential role of Th17 cells in the AKI-to-CKD transition; however, a clear and direct assessment of T cells or specific T cell subsets in this progression has not yet been clearly demonstrated.
As an initial step toward investigating the role of activated T cells, we used athymic rats (Foxn1rnu−/rnu−) in a model of the AKI-to-CKD transition. We hypothesized that athymic rats would manifest impaired activation of T lymphocytes and reduced generation of Th17 cells in response to AKI and subsequent exposure to high-salt diet. We further hypothesized that this impairment would result in a less severe development of renal fibrosis compared with euthymic control rats (Foxn1rnu−/+). Our results indicate that, despite reduced conventional Th17 cell activation in postischemic athymic rats, both athymic and euthymic rats develop fibrosis in an IL17-dependent fashion, but with athymic rats using a compensatory source of IL-17 derived primarily from NK cells during the AKI-to-CKD transition.
MATERIALS AND METHODS
Animals.
Rats were maintained in accordance with the policies of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols were approved by the Institutional Animal Care and Use Committees at Indiana University. Athymic nude and euthymic rats were initially obtained from Harlan (Indianapolis, IN). Male athymic rats (Foxn1rnu−/rnu−) were bred with female euthymic (Foxn1rnu−/+) rats in-house to generate litters of both genotypes. Genotyping of athymic and euthymic rats was based on obvious differences in coat quality. The studies used male rats of both athymic (Foxn1rnu−/rnu−) and euthymic (Foxn1rnu−/+) genotypes with an initial starting weight of 200-250 g.
Study I was designed to evaluate the role of lymphocyte activity following AKI and progression to CKD. Athymic and control euthymic rats were anesthetized with ketamine (100 mg/kg)/xylazine (5 mg/kg), and renal injury was induced by unilateral I/R injury to the left kidney by clamping the renal pedicle for 40 min using a surgical approach described previously (26). The rats were allowed to recover for 33 days on a standard diet (AIN 76A; Dyets) containing 0.4% NaCl. To hasten the development of renal fibrosis, rats were subjected to unilateral nephrectomy (UNx) at day 33 postsurgery. On day 35 all rats were exposed to elevated NaCl diet (AIN76A, 4% NaCl) while rats of both genotypes were randomly treated from day 35 to day 63 with either MMF (30 mg·kg−1·day−1; Accord Healthcare, Durham, NC) or vehicle (sugar-free chocolate pudding at 1 g/kg) one time daily (7). Rats were observed to ensure that the daily doses were ingested completely. Sham-operated rats received similar treatment without clamping on day 0 but with UNx at day 33 (Fig. 1A). On day 63, rats were heavily anesthetized with Fatal Plus, and kidneys were harvested for histological analysis or flow cytometry (see below).
Fig. 1.
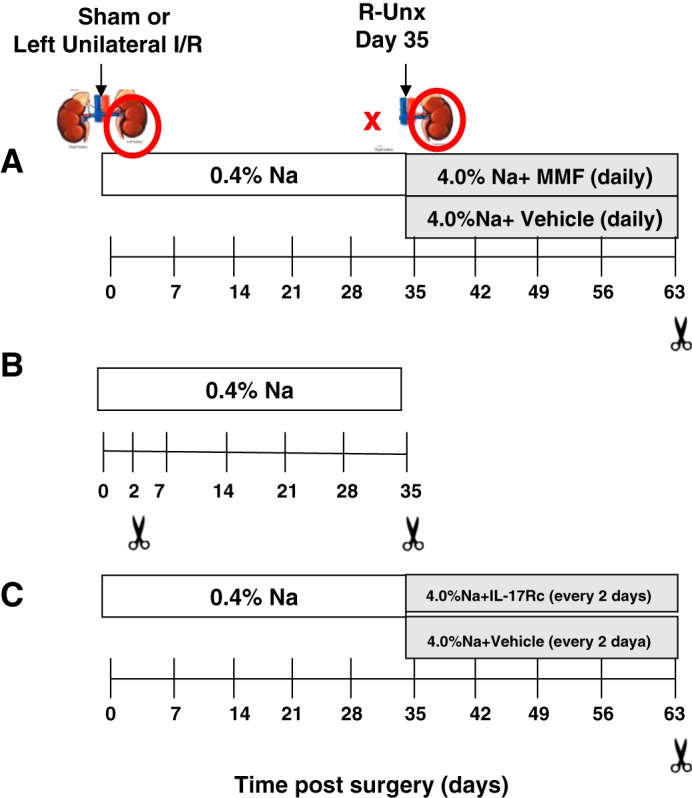
Experimental schema of studies carried out to investigate the role of T cells in chronic kidney disease (CKD) progression following acute kidney injury (AKI). Renal injury was induced by unilateral ischemia-reperfusion (I/R) injury to the left kidney by clamping the renal pedicle for 40 min (red circle). The rats were allowed to recover for 33 days on a standard diet containing 0.4% NaCl. To hasten CKD, rats were subjected to unilateral nephrectomy (UNx) at day 33 postsurgery (red X) and subsequently placed on high-salt diet (4% NaCl). Sham-operated rats received similar treatment without clamping on day 0 but also with UNx at day 33. A: study I investigated the effect of T cell inhibition in the AKI-CKD transition. Rats from both genotypes were treated from day 35 to day 63 with either mycophenolate mofetil (MMF, 30 mg·kg−1·day−1) or vehicle (sugar-free chocolate pudding at 1 g/kg) daily. B: study II was designed to identify the source of I/R-induced IL-17 production, with cohorts sacrificed 2 or 35 days postsurgery. C: study III was designed to investigate the effect of IL-17 antagonism by administration of IL-17Rc along with high-salt diet.
Study II was designed to study the specific cell types contributing to IL-17 production in the postischemic kidney. In these studies, unilateral I/R was performed on both athymic and euthymic rats as described in study 1; however, the rats were sacrificed at either 2 or 35 days postsurgery (Fig. 1B).
Study III was designed to study the effect of IL-17 antagonism on the development of renal fibrosis following recovery from I/R. This study was performed in both athymic and euthymic rats using a timeline similar to that described in study I. To inhibit the effects of IL17, mouse recombinant IL-17Rc soluble receptor (2270-ml-050: 150 ng/day ip; R&D Systems) was administered to rats every other day beginning at the time of exposure to high-salt diet (from day 35 to day 63) (Fig. 1C) (14).
Measurements of renal functions.
To measure creatinine, blood from rats was collected via tail clipping in heparin-containing tubes and spun to collect plasma. Plasma creatinine was measured using a Pointe Scientific Analyzer and Creatinine Assay reagents using methods outlined by the manufacturer (Pointe Scientific, Canton, MI).
Renal histology and immunohistochemistry.
At the time of tissue harvest, kidneys were bisected, one-half was fixed by immersion in 10% formalin and embedded in paraffin, and 5-µm sections were stained with picosirus red to assess fibrosis. For quantitative analysis, five random images of renal outer medulla were obtained using a Leica DMLB (Scientific Instruments, Columbus, OH) microscope with a ×20 objective. The percent area of picrosirus red stain was obtained using Image J (NIH) using procedures described previously (26).
Neutrophil staining was conducted on deparaffinized sections following antigen retrieval by incubating slides in sodium citrate buffer, pH 6.0, at a temperature of 121oC and a pressure of 15 psi for 6 min. After blockade using a buffer consisting of 10% horse serum, the tissues were incubated overnight in primary antibody (rabbit anti-rat neutrophil, no. ABIN2586050; online.com antibodies) at a concentration of 2 µg/ml. After being washed, the signals were detected following incubation with horse anti-rabbit HRP (Vector Laboratories, Burlingame, CA) and subsequent development with Di-amino-benzidine according to the manufacturer’s instructions (Sigma, St. Louis, MO). Images were obtained with a Leica DMLB (Scientific Instruments) microscope using a ×20 objective. Scoring of neutrophil content was quantified by overlaying an arbitrary array of gridlines to a density of 520 boxes/visual field with the aid of ImageJ software. The number of boxes containing neutrophil staining were counted, and data were expressed as the percent area of neutrophil-positive grids per visual field.
FACS analysis.
Harvested kidneys were minced and digested in liberase (2 μg/ml; Roche, Indianapolis IN) for 15 min at 37°C with the help of Gentle MACs (Miltenyli, San Diego, CA). The digested tissue was filtered through a 100-μm filter mesh and washed with DMEM containing 10% fetal bovine serum (Cell Applications, San Diego, CA). The mononuclear cells were separated by Percoll (Sigma) and counted by hemocytometer. To evaluate T lymphocytes, the cells were stained with antibodies against rat CD4 (PE-Cy7; BD Biolgend, San Diego, CA), CD8a (Alexa 647; BD Biolgend), CD25 (FITC; BD Biolgend), CD3 (PE; BD Biolegend), and CD161(PerCp; eBiosciences, San Diego, CA). To evaluate the cytokines secreted by T cells, the cells were stained for the CD4 surface marker, permeabilized using 0.1% saponin, and stained with antibodies against rat IFN-γ (FITC; BD Biolgend) or IL-17 (FITC; BD Biolgend). Cells were scanned using flow cytometry (FACSCalibur; BD Biosciences), and scans were analyzed using Flowjo software (Tree Star, Ashland, OR). The gating strategy used for these analyses was exactly as we have shown in our previous publication (26). The total numbers of the different T cell populations in the harvested kidney were calculated using the percentage of each cell type and the total cell number measured per gram of kidney. The data are expressed as total number of each T cell population per gram of kidney.
RNA analysis.
Total RNA was obtained from kidney using Trizol and the Zymogen RNA extraction kit (Zymo Research, Irvine, CA), and cDNA was prepared using Moloney murine leukemia virus enzyme. Quantitative real-time PCR using gene-specific primers (Life Technologies) was performed using a 7500 ABI biosystem machine. Absolute mRNA numbers were calculated using the comparative threshold value.
Statistical analysis.
All data are expressed as means ± SE. Differences in means were established by Student’s t-test or one-way ANOVA as indicated the legends for Figs. 1–8.
Fig. 8.
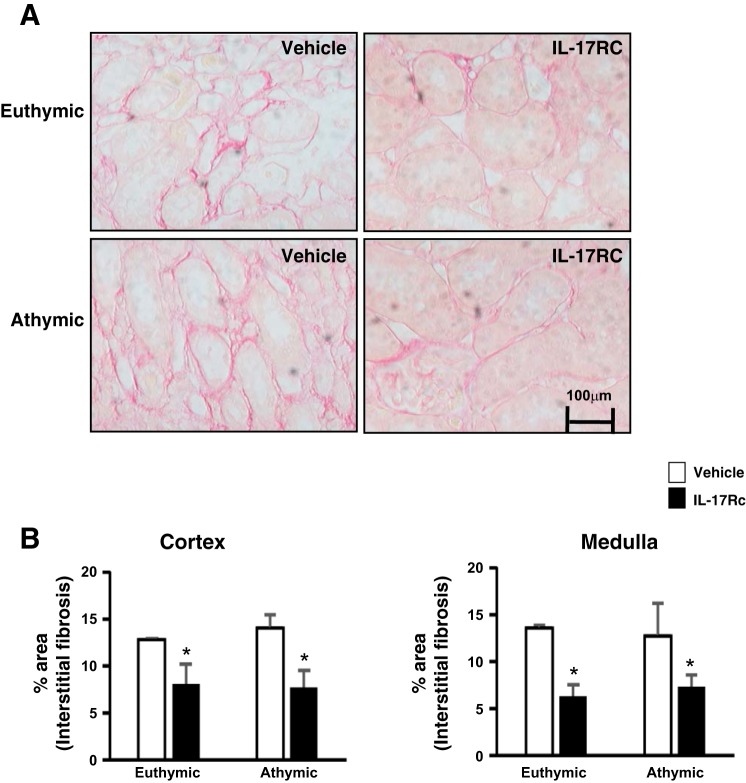
Effect of systemic IL-17 blockade on AKI-induced renal fibrosis. A: representative images through the renal outer medulla are shown for picosirius red-stained sections of kidneys from vehicle-treated rats and IL-17Rc-treated rats. Increase in interstitial fibrosis is apparent in vehicle-treated rats post-I/R, which was reduced by IL-17Rc treatment. Magnification is shown. B: quantification of interstitial fibrosis area based on picosirius red staining. Data are means ± SE. *P < 0.05, IL-17Rc vs. vehicle using Student’s t-test (n = 3-5 animals/group).
RESULTS
Compensatory role of NK cells in IL-17 production in the absence of conventional T cells in postischemic athymic rats.
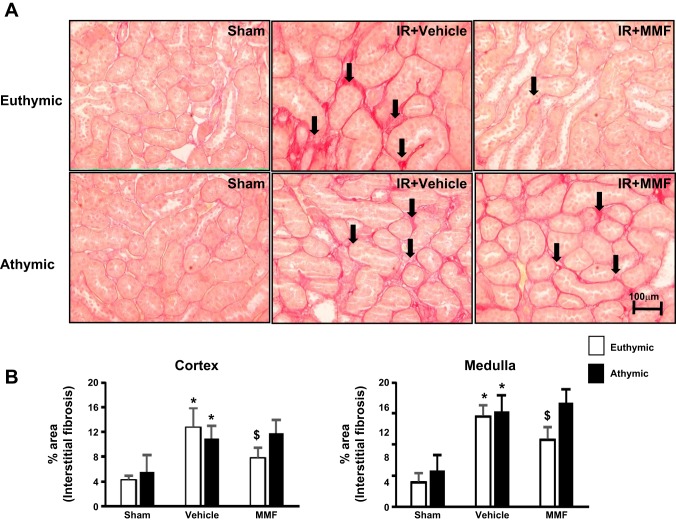
Previous work has shown that the immunosuppressive agent MMF decreased infiltrating cells, reduced damage, and normalized blood pressure in post-AKI rats fed a high-salt diet, suggesting that immune cells may contribute to long-term progression of AKI (31). We also showed that Th17 cells are increased when post-AKI rats were placed on high-salt diet to a much greater extent than either Th1 or Th2 subsets (26). To further elucidate the role of T cells in progression following AKI, we subjected either athymic or euthymic rats to I/R injury. We first investigated the degree of AKI in response to bilateral I/R and showed that the loss of renal function in both athymic and euthymic rats was similar as determined by the level of serum creatinine at 24 h post-I/R (3.1±0.03 vs. 2.7±0.98 mg/dl, not significant). This suggested that impaired T cell activation in athymic rats does not significantly reduce the initial degree of AKI in response to ischemia. To determine the long-term effect of T cell deficiency, we used the unilateral model of AKI-CKD transition as outlined in Fig. 1A. In euthymic rats, there was an increase in the degree of interstitial fibrosis following I/R compared with sham-operated control rats that was significantly reduced by MMF treatment (Fig. 2, A and B). This effect of MMF is similar to results reported previously using Sprague-Dawley rats (31). Unexpectedly, athymic rats also manifested significant renal fibrosis following AKI to a level that was not different from euthymic rats. However, in contrast to euthymic rats, MMF treatment had no effect on the level of renal fibrosis in athymic rats following I/R injury (Fig. 2, A and B).
Fig. 2.
Effect of MMF on renal structure in postischemic and sham-operated rats on high-salt diet. A: representative picosirius red-stained sections showing fibrosis (black arrows) through the renal outer medulla are shown from sham-operated (left), I/R + vehicle (middle), and I/R + MMF (right) athymic and euthymic rats. Magnification is shown. B: quantification of interstitial fibrosis (%area) in both cortex and medulla based on the scoring of picosirius red staining. Data are means ± SE. P < 0.05, injury vs. sham group (*) and MMF vs. vehicle ($) using ANOVA and Student-Neuman-Keuls post hoc test (n = 5–8 animals/group).
Similar to previous results using Sprague-Dawley rats (31), serum creatinine was not elevated in either postischemic athymic or euthymic rats 63 days following I/R. To elucidate further the degree of renal damage, we measured the mRNA expression of the renal injury marker neutrophil gelatinase-associated lipocalin (NGAL) and the expression of IL-6 in whole kidney. As expected, there was a significant increase in these markers in both postischemic euthymic and postischemic athymic rats compared with sham-operated rats (Table 1). Similar to the effects on fibrosis, MMF treatment significantly reduced both NGAL and IL-6 expression in euthymic rats but not in athymic rats (Table 1). Furthermore, we evaluated the mRNA levels of the profibrotic factor TGF-β. TGF-β expression tended to be elevated in postischemic rats relative to sham, and appeared to be higher in athymic vs. euthymic rats, but the values were highly variable and were not statistically significant. Moreover, MMF did not appear to affect TGF-β levels in either group (Table 1).
Table 1.
Message levels of different renal injury markers and cytokines measured in the kidney of postischemic rats treated with MMF
| Euthymic Sham | Euthymic Vehicle | Euthymic MMF | Athymic Sham | Athymic Vehicle | Athymic MMF | |
|---|---|---|---|---|---|---|
| NGAL | ND | 28 ± 8* | 15 ± 2.6# | ND | 30 ± 9* | 23 ± 3 |
| IL-6 | ND | 1.3 ± 0.5* | 0.7 ± 0.01# | ND | 1.1 ± 0.002* | 0.7 ± 0.1 |
| TGF-β | 0.03 ± 0.001 | 0.067 ± 0.05 | 0.048 ± 0.04 | 0.01 ± 0.003 | 0.55 ± 0.1 | 0.59 ± 0.006 |
| Foxp3 | 0.69 ± 0.6 | 0.74 ± 0.9 | 0.39 ± 1.2 | 0.78 ± 0.8 | 0.67 ± 0.9 | 0.9 ± 1 |
Data are means ± SE; n = 5–6 animals/group. MMF, mycophenolate mofetil; NGAL, neutrophil gelatinase-associated lipocalin; ND, not determined. mRNA levels were measured in the kidney isolated from injured athymic and euthymic rats fed high-salt diet along with/without MMF. The values are expressed as absolute numbers (2−Ct target gene − Ct Endo gene).
P < 0.05, injury vs. sham group.
P < 0.05, MMF vs. vehicle using ANOVA and Student-Neuman-Keuls post hoc test.
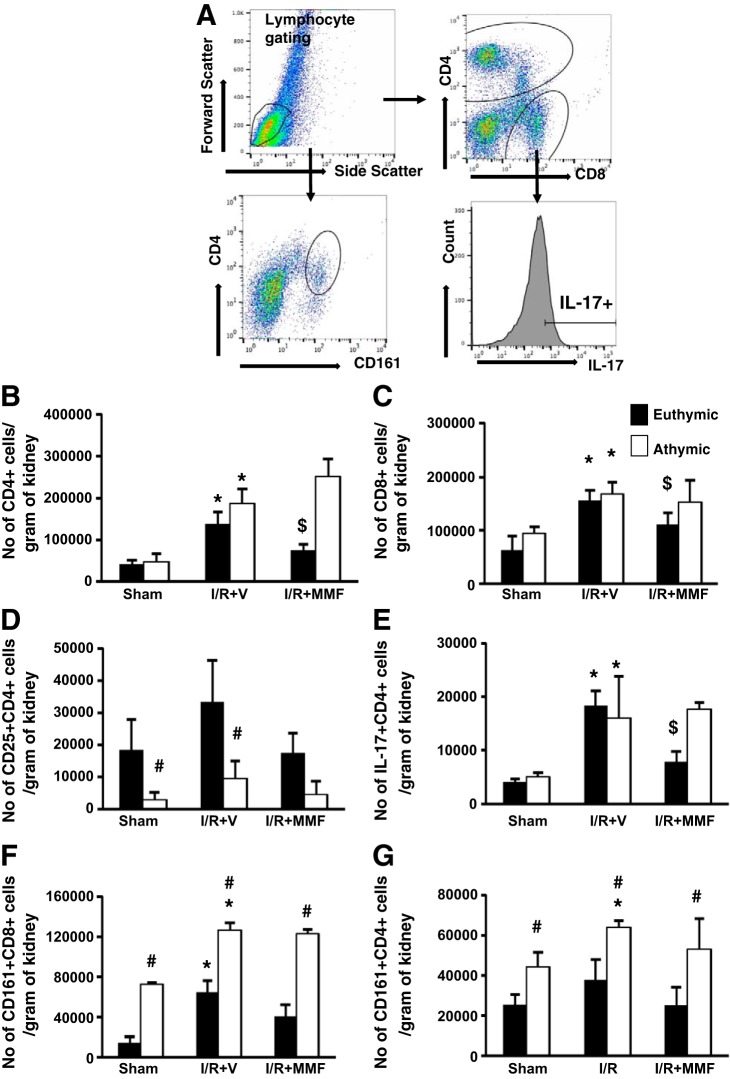
Because MMF is thought to inhibit lymphocyte activity, these data suggest that athymic rats use an alternative MMF-insensitive pathway to induce renal fibrosis following AKI. To evaluate changes in lymphocyte activity in athymic rats vs. euthymic rats, FACS analysis to quantify specific lymphocyte subpopulations as a function of AKI and inhibition with MMF was conducted. The gating strategy used was similar to our previous study and has been diagrammed in Fig. 3A. As expected, both renal CD4+ and CD8+ cells were elevated in postischemic euthymic rats compared with sham-operated rats, and these levels were significantly reduced by MMF treatment (Fig. 3, B and C). There was no difference in the number of infiltrating CD4+ cells and CD8+ cells in postischemic athymic rats compared with euthymic rats. In addition, MMF treatment did not affect infiltrating CD4+ and CD8+ cells in athymic rats (Fig. 3, B and C). Despite similar levels of CD4+ cells, activated CD4+ T cells, i.e., CD4+CD25+, were drastically reduced in postischemic athymic vs. euthymic rats to levels similar to sham (Fig. 3D). As expected, MMF reduced the number infiltrating CD25+ T cells in euthymic rats (Fig. 3D). Because the number of activated cells was so few in post-schemic athymic rats, we determined if this reduction was associated with reduced cytokine expression. Similar to our previous data, there was a significant increase in CD4+IL-17-expressing cells in postischemic rats fed high-salt diet compared with sham-operated rats (Fig. 3E). In euthymic rats, MMF treatment significantly reduced the number of IL-17-producing cells in postischemic kidney. Surprisingly, the number of CD4+IL-17+ was similar in athymic rats compared with euthymic rats and was unaffected by MMF treatment (Fig. 3E).
Fig. 3.
Phenotypic analysis of infiltrating mononuclear cells in immune-deficient athymic rats and immune-competent euthymic rats following I/R and exposure to high-salt diet. Lymphocytes were obtained from postischemic rats fed high-salt diet (4.0% NaCl) with or without MMF as labeled. A: gating strategy for FACS analysis. Lymphocytes were gated based on forward and side scatter plot. These were further gated based on the surface expression of CD4, CD8, and CD161. With the use of a histogram, CD4+ cells were further analyzed for expression of IL-17. B and C: no. of CD4+ (B) and CD8+ (C) cells isolated from kidneys. D: no. of activated T cells (CD4+CD25+) isolated from post-I/R or sham-operated rats fed high-salt diet with or without MMF. E–G: no. of CD4+IL-17+ (E), CD8+ natural killer T (NKT) cells (CD8+CD161+) (F), and CD4+NKT (CD4+CD161+) (G) cells isolated from athymic and euthymic rats treated with or without MMF. Data are means ± SE. P < 0.05, injury vs. sham group (*), MMF vs. vehicle ($), and athymic vs. euthymic (#) using ANOVA and Student-Neuman-Keuls post hoc test (n = 5–8 animals/group).
A similar relationship was observed with CD4+IFN-γ+ cells, i.e., postischemic euthymic and athymic rats had a similar number of double-positive cells, and MMF treatment reduced CD4+IFN-γ+ cells only in the euthymic rats but not in athymic rats (data not shown). However, the total number of CD4+IFN-γ+ cells was dramatically less than CD4+IL17+ cells in postischemic rats (~800 ± 150 CD4+IFN-γ+ vs. ~18,000 ± 2,300 CD4+IL-17+ cells). To understand whether T cell deficiency might influence T-regulatory cells, we measured the mRNA expression of Foxp3 and showed no difference in postischemic athymic vs. euthymic rats (Table 1). Given the low number of activated T cells in athymic rats, these data suggested that the source of IL-17 may not be from conventional CD4+ cells but rather another subtype that is insensitive to MMF treatment.
NK cells are innate immune cells that are characterized by the presence of the killer cell lectin-like receptor subfamily B, member 1, also known as NK1.1, KLRB1, NKR-P1A, or CD161, and secrete large amounts of cytokines upon stimulation. A previous report suggests that basal levels of NK (CD161+) cells are increased in athymic rats relative to euthymic rats (18). Therefore, we sought to verify whether NK cells were increased in response to renal I/R. Interestingly, both sham-operated and postischemic athymic rats had significantly higher levels of both CD4+ and CD8+ NK cells compared with euthymic rats (Fig. 3, F and G). Furthermore, CD4+ and CD8+ NK cell infiltration was insensitive to MMF in both genotypes (Fig. 3, F and G), suggesting that NK cells may play a compensatory role in cytokine production in postischemic kidney of athymic rats.
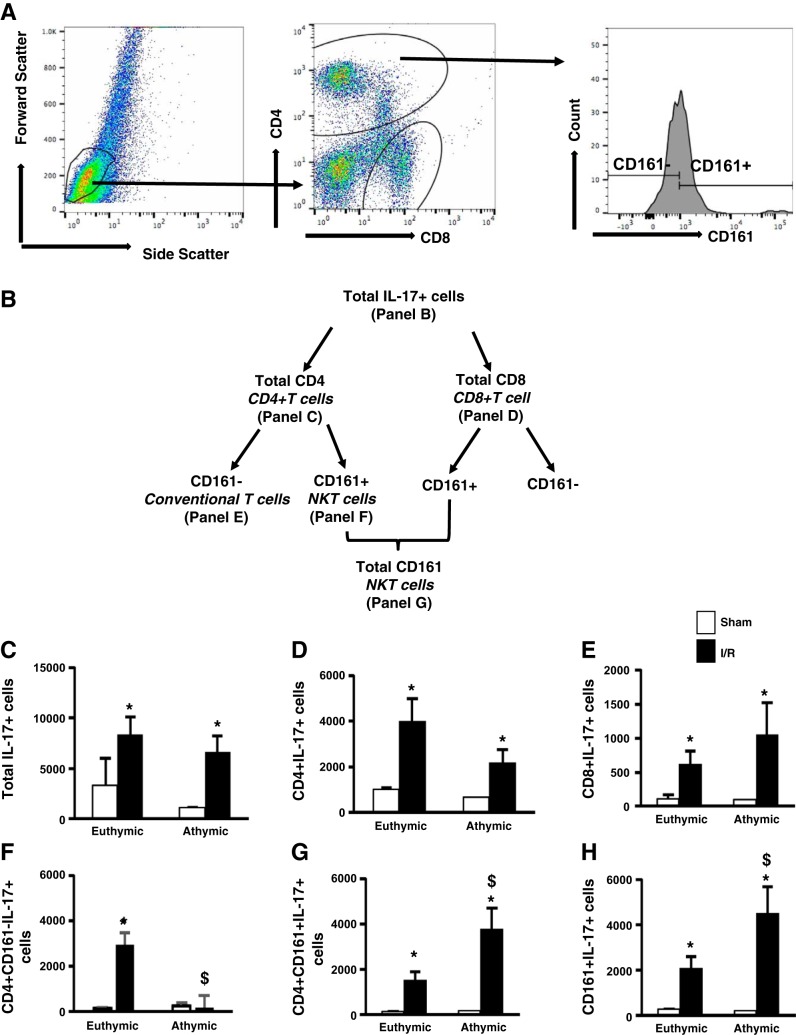
To further elucidate the source of IL-17 in athymic rats, we costained renal lymphocytes with markers against different immune cells. The gating strategy and flow chart showing the different sources of IL-17-secreting cells are depicted in Fig. 4, A and B. Initial studies focused on tissues 2 days following renal I/R, since previous studies demonstrated that CD4+IL-17+ cells were markedly increased at this time point (26). As expected, there was a similar increase in total IL-17+ cells in both athymic and euthymic rats compared with sham-operated rats (Fig. 4C). In addition, the numbers of total CD4+IL-17+ and CD8+IL-17+ cells were significantly elevated postischemia in both athymic and euthymic rats (Fig. 4, D and E). We further analyzed total CD4+IL-17+ cells depending on the expression of CD161, i.e., NK cells (CD4+CD161+) and conventional T cells (CD4+CD161−), and observed that IL-17 production in athymic rats postischemia was predominantly from NK cells, since the number of triple-positive CD4+CD161+IL-17+ cells was two- to threefold higher compared with euthymic rats (Fig. 4G). Conversely, in the postischemic euthymic rats, the increased IL-17 production relative to sham derived primarily from conventional T cells (~66%; CD4+CD161−IL-17+) and a smaller proportion from NK (~33%; CD4+CD161+) (Fig. 4, F and G). In addition, the number of total CD161+IL-17+ (comprising both CD4+ and CD8+ NK cells) was significantly increased in postischemic athymic rats compared with euthymic rats (Fig. 4H). Similar results were obtained following 5 wk of recovery from I/R (Table 2).
Fig. 4.
Compensatory role of NKT cells in IL-17 production in T cell-deficient postischemic athymic rats fed high-salt diet. Lymphocytes were isolated from postischemic euthymic and athymic rats 2 days post-I/R. The FACS gating strategy is shown in A. In brief, lymphocytes are gated based of their forward and side scatter. These cells are then further subdivided into CD4+ and CD8+ fractions. CD4+ cells were then separated into CD161+ and CD161− cells using a histogram plot. B: schematic diagram for different IL-17-secreting cells by flow cytometry. C: no. of total IL-17+ cells isolated from sham-operated and postischemic kidneys. D and E: total no. of CD4+IL-17+ cells (D) and CD8+IL-17+ cells (E) isolated from postischemic and sham-operated rats. F and G: portion of CD4+IL-17+ cells that are either CD161− (F) or CD161+ (G). H: no. of total IL-17-secreting NKT cells (CD161+) that comprise both CD4+NKT and CD8+NKT. Data are means ± SE. P < 0.05, injury vs. sham group (*) and athymic vs. euthymic ($) using ANOVA and Student-Neuman-Keuls post hoc test (n = 5–6 animals/group).
Table 2.
Number of IL-17+ cells from different sources isolated postischemic rats
| Euthymic Sham | Euthymic I/R | Athymic Sham | Athymic I/R | |
|---|---|---|---|---|
| Total IL-17 | 223 ± 747 | 8,279 ± 1,766 | 1,011 ± 689 | 6,514 ± 1,696 |
| CD4+IL-17+ | 988 ± 77 | 3,980 ± 1,006 | 606 ± 59 | 2,154 ± 586 |
| CD8+IL-17+ | 1,668 ± 981 | 3,138 ± 1,583 | 1,166 ± 1,092 | 3,808 ± 2,573 |
| CD161+IL-17+ | 249 ± 21 | 2,057 ± 556 | 173 ± 51 | 4,493 ± 1,206* |
| CD4+CD161+ | 80 ± 63 | 1,492 ± 389 | 117 ± 28 | 3,752 ± 958* |
| CD4+CD161− | 205 ± 193 | 2,252 ± 451 | 181 ± 69 | 807 ± 147* |
Data are means ± SE; n = 5–6 animals/group. I/R, ischemia-reperfusion. The table depicts the number of IL-17+ cells/g of kidney from different cell subtypes isolated from 35 days post-I/R.
P < 0.05, injury vs. sham group using ANOVA and Student-Neuman-Keuls post hoc test.
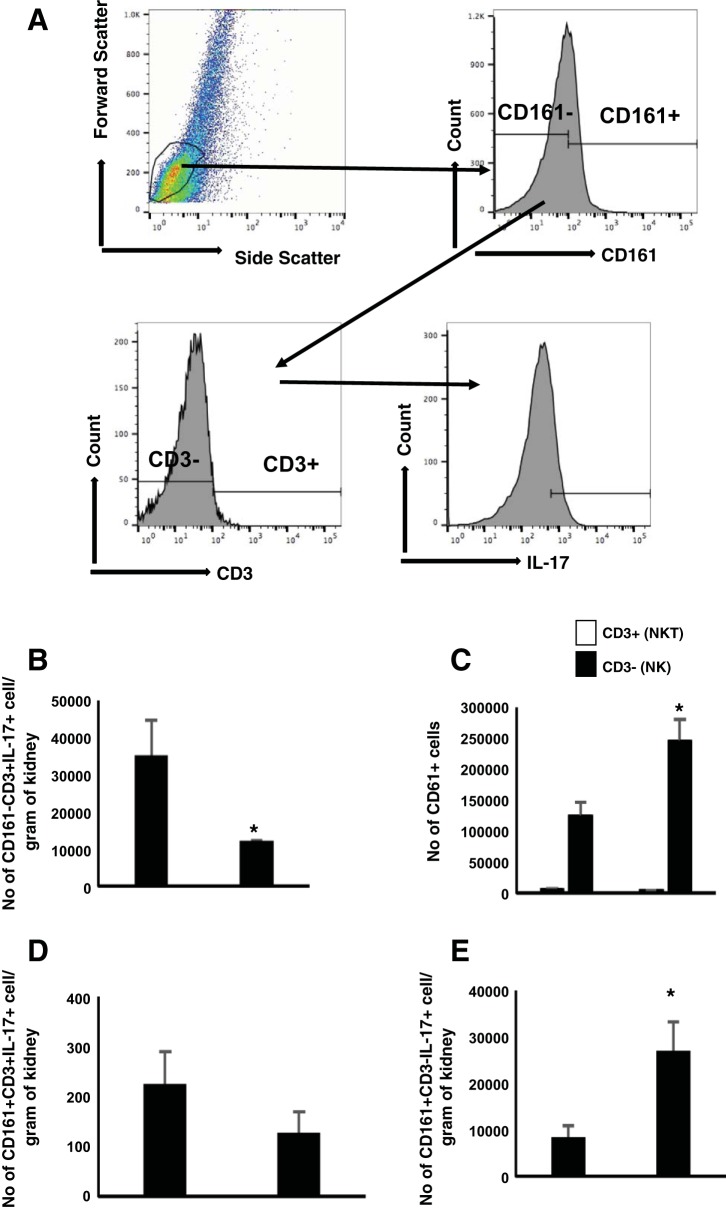
These data suggest that conventional CD4+ T cells are the major source of IL-17 secretion in euthymic rats, whereas NK cells are the major producers in athymic rats. However, because NK cells cannot be distinguished from natural killer T cells (NKT) on the basis of CD4 or CD8 expression alone (6, 12), we further characterized the source of CD161+/IL-17+ as either CD3− (NK cells) or CD3+ (NKT cells) (Fig. 5A). As expected, the number of IL-17+ cells in postischemic euthymic rats was predominantly conventional T cells, defined by CD3+/CD161− (Fig. 5B). In athymic rats, CD161+ expression was higher relative to euthymic rats, and ~98% of these cells were CD3−, indicative of NK cells, whereas ~2% were CD3+, indicative of NKT cells (Fig. 5C). As expected, the predominant source of IL-17 in CD161+ cells was NK cells relative to NKT (Fig. 5D), and these were significantly greater in athymic vs. euthymic rats (Fig. 5E). Taken together, these data suggest that, in the absence of conventional T cells, NK cells play a compensatory role in the secretion of IL-17 in athymic rats post-I/R injury.
Fig. 5.
Identification of source of IL-17-producing cells in postischemic athymic rats fed a high-salt diet. A: lymphocytes were gated based on the forward and side scatter plot. These gated cells were further subdivided based on their CD161 expression. CD161+ cells were further gated for CD3 expression. Each population was analyzed for the expression of IL-17. B: no. of IL-17+ cells in the conventional T cells (CD3+CD161−). C: no. of CD3+ (NKT) and CD3− (NK) cells gated on CD161+ fraction. D and E: no. of IL-17+ cells in the NKT and NK populations. Data are means ± SE. *P < 0.05, athymic vs. euthymic by Student’s t-test (n = 3 animals/group).
IL-17 inhibition reduces renal fibrosis in both athymic and euthymic rats following AKI.
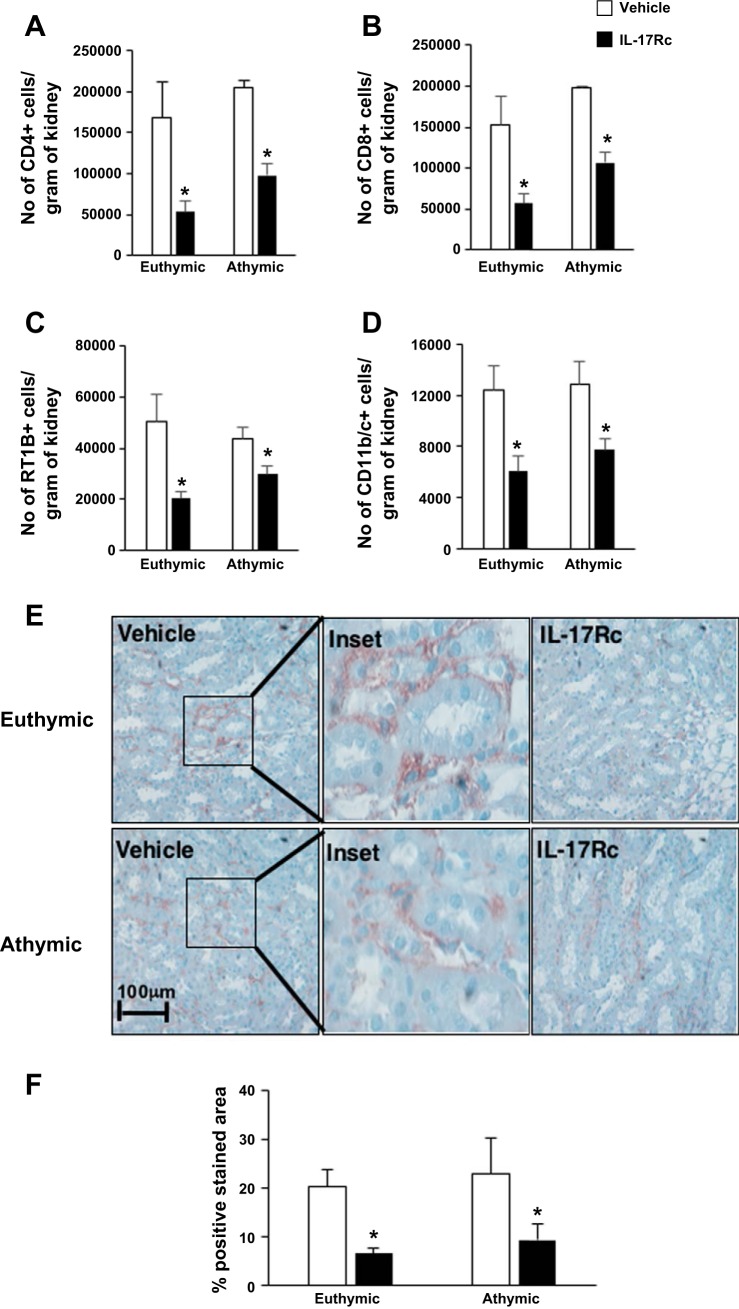
To directly address the role of IL-17 in the development of renal fibrosis following recovery from I/R, both postischemic euthymic and athymic rats were injected with mouse recombinant IL-17Rc soluble receptor at the time of exposure to high-salt diet (Fig. 1). This approach was previously used to attenuate the development of hypertension in the rat model of preeclampsia (14). Multiple studies suggest that IL-17 acts as a chemoattractant for immune cells and promotes fibrosis via the recruitment of neutrophils (17, 28, 34). Systemic IL-17Rc soluble receptor treatment significantly reduced the number of CD4+ and CD8+ infiltration by ~66% in euthymic rats and ~52% in athymic rats (Fig. 6, A and B) compared with vehicle. Furthermore, IL-17 inhibition also reduced the total number of B (RT1B+) cells and dendritic/macrophage cells (CD11b/c+) by 63 and 30%, respectively (Fig. 6, C and D). We then tested whether AKI-induced IL-17 also influenced the recruitment of neutrophils. Neutrophil infiltration was observed primarily in the interstitial area of renal medulla following AKI (Fig. 6E), although some neutrophils were also observed in the glomerulus (data not shown). Neutrophil content in both athymic and euthymic postischemic rats was significantly attenuated by treatment with IL-17Rc (Fig. 6, E and F).
Fig. 6.
Effect of systemic IL-17 blockade on infiltrating immune cells in postischemic rats fed high-salt diet. A–D: no. of total CD4 (A), CD8 (B), B cells (Rt1B+) (C), and DC/Mac (CD11b/c+) (D) isolated from post-AKI kidney of rats treated with IL-17Rc antagonist or vehicle. E: representative images of neutrophil staining in kidneys from vehicle or IL-17Rc-treated rats. Magnification is shown. F: quantification of neutrophil infiltration (%positive area) in renal medulla. Data are means ±SE. *P < 0.05, IL-17Rc vs. vehicle using Student’s t-test (n = 3-5 animals/group).
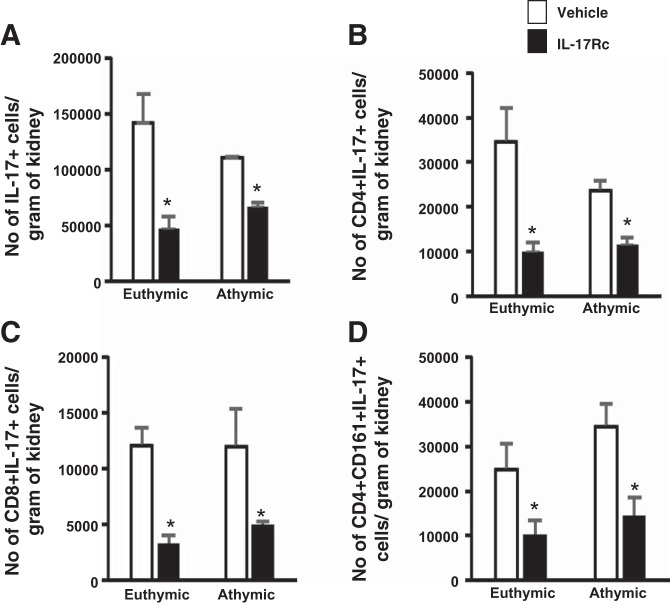
Treatment with IL-17Rc also reduced the expression of total IL-17-producing cells by ~50 and 40% in euthymic rats and athymic rats, respectively (Fig. 7A). This reduction in IL-17 expression was observed from multiple different sources such as CD4+, CD8+, and CD161+ populations (Fig. 7, B, C, and D). Taken together, these data highlight the efficacy of IL-17Rc systemic treatment in postischemic rats and suggest that IL-17 secretion, regardless of the source, augments the activation and infiltration of other immune cells.
Fig. 7.
Quantification of IL-17+ cells in response to IL-17 antagonism. Lymphocytes were obtained from postischemic euthymic and athymic rats fed high-salt diet (4.0% NaCl) and treated with vehicle or IL-17Rc antagonist. A: no. of total IL-17-producing cells isolated from rats treated with and without IL-17Rc. B–D: different sources of IL-17-producing cells: CD4+IL-17+ (B), CD8+IL-17+ (C), and CD4+CD161+IL-17+ (D). Data are means ±SE. *P < 0.05, IL-17Rc vs. vehicle using Student’s t-test (n = 3-5 animals/group).
Kidney sections were stained with picosirus red to investigate the development of renal fibrosis following recovery from I/R. A similar degree of fibrosis was observed in euthymic and athymic rats postischemia (Fig. 8, A and B), similar to results in Fig. 2. IL-17Rc inhibition significantly reduced renal fibrosis by ~50% in both athymic and euthymic rats (Fig. 8, A and B), suggesting an association between IL-17 activity and the development of fibrosis following recovery from AKI. However, IL-17Rc did not significantly affect the expression of NGAL, IL-6, or TGF-β mRNA in either athymic or euthymic rats (data not shown).
DISCUSSION
The pathogenesis of CKD following AKI is a complex interplay involving alterations in renal tubular, vascular, and interstitial remodeling and is also influenced by inflammatory processes (3). Multiple studies in mice have suggested that T cells are involved in mediating early AKI. For example, T cell-deficient (nu/nu) mice were protected from postischemic renal injury; however, when the knockout mice were reconstituted with wild-type T cells, injury levels were similar to that of wild-type mice (11). Furthermore, CD4+ cell-deficient mice showed more efficient recovery compared with CD8+-deficient mice (9). Modulating and inhibiting T cell activity using a soluble P- and L-selectin ligand has been reported to be protective against ischemic reperfusion injury-induced alterations in rodents (35). The association of T cells and I/R is not limited to kidney, since T cell-deficient mice are also protected from I/R in liver and gut (19, 43).
The role of T cells in CKD progression following AKI has received some attention in recent years. Studies from Burne-Taney et al. demonstrated that CD4 lymphocytes persisted in the kidney up to 6 wk following recovery from AKI in mice (11) and further demonstrated that adoptive transfer of splenocytes from AKI mice resulted in proteinuria in naïve recipient mice (10). In rats, CKD is significantly hastened post-AKI following exposure to increased dietary sodium (4), and treatment with the lymphocyte inhibitor MMF attenuates this progression (31). However, the specific lymphocytes responsible for this activity have not yet been identified.
Th17 cells have been recognized as important mediators of renal injury associated with autoimmune-mediated nephritis induced by injection of sheep serum in mice (30), which was blunted in IL-17 knockout mice (30). Similarly, Th17 cells were also shown to influence autoimmune renal disease in a model of systemic lupus erythematosus (21). IL17 secreted from Th17 and (γδ) T cells promotes renal fibrosis by regulating T cell-expressing markers and immune cell infiltration. Recently, we demonstrated that Th17 cells are the predominant T-helper subtype present in the early post-I/R period and are further increased during the in AKI-CKD progression induced by high-salt diet (26). Losartan, an AT1R blocker, significantly reduced Th17 cell activity and the development of fibrosis in post-AKI rats fed-high salt (26). However, a direct role of Th17 cells in progression of CKD following AKI is unclear.
In the current study, we sought to explore the role of activated lymphocytes in the AKI-to-CKD transition in rats because rats manifest salt sensitivity to progression, which is blocked by MMF. By using a genetic model in which the development of the thymus is impaired, we reasoned that T cell activation would be reduced relative to their heterozygous immunocompetent littermates and show attenuated CKD progression. We further anticipated that adoptive transfer studies would then allow investigation of specific subpopulations and evaluate their role in progression post-AKI. Surprisingly, T cell-deficient athymic rats had similar levels of renal fibrosis postischemia as did control euthymic rats. This was an unexpected finding given the lack of T cell activation in athymic rats. However, studies using genetic mutant animals should be interpreted, keeping in mind potential compensatory effects from other immune cells and their influence on the contribution to disease. Indeed, the number of IL-17-expressing cells was similar in both athymic and euthymic rats, representing a possible explanation for the similar degree of fibrosis in both genotypes. Further analysis of athymic rats suggested that NK cells were playing a compensatory role in the production of IR-induced IL-17 and that these cells appeared insensitive to treatment with MMF (Fig. 5).
NK cells are critical to the innate immune system and have the ability to recognize injured cells in the absence of major histocompatibility complex (38). NK cell infiltration has been implicated in tubular epithelial cell death following renal I/R injury (41). NK cell activity has also been identified as mediating fibrosis in models of liver injury (16), but its role in kidney fibrosis is less clear. Recent studies using a chronic allograft nephropathy model in which parent-to-F1 kidney transplants were conducted in Rag1-deficient mice lacking T and B cells demonstrated an increase in NK cell activity and a similar degree of nephropathy relative to wild-type recipients replete with T and B cells, suggesting that NK cells may mediate allograft nephropathy in the absence of conventional lymphocytes (40). It has been suggested that persistent development of allograft nephropathy may ensue despite improvements in T cell-specific immunosuppression, which may be the result of NK activity (40). In this regard, it is interesting that, in our current study, MMF did not suppress IL-17 secretion from the NK population or renal fibrosis in immunocompromised rats. In contrast, Vincent et al. demonstrated that immunoneutralization of NK cells attenuated the fibrotic response in immunocompetent mice following renal I/R (37a). Therefore, the potential role of NK cells in the AKI-to-CKD transition is not yet clear.
NK cells, in response to various stimuli, produce large amounts of IFN-γ and IL-17 (2, 29) and therefore may represent an alternate or additional source for these cytokines when conventional T cell activation is impaired. Regardless of the source of IL-17, the current study demonstrated that blockade of IL-17 using the recombinant IL-17Rc reduced fibrosis and neutrophil infiltration in both euthymic and athymic rats.
Activated Th17 cells secrete IL-17A, IL-17F, IL-21, IL-22, IL-23, and TNF-α, which then promote tissue inflammation by induction of other proinflammatory mediators and recruitment of leukocytes, including neutrophils, to the site of inflammation (27). IL-17 can induce the expression of neutrophil-attracting chemokines, like CXCL1, CXCL2, or CXCL8, in various cell types, among them a variety of epithelial and endothelial cell types, but IL-17 itself can also act to mobilize and activate neutrophils (27). Laan et al. have elegantly shown that treatment with human IL-17 increases the release of the major neutrophil chemoattractant, the C-X-C chemokine IL-8, in human bronchial epithelial and venous endothelial cells in vitro, thereby suggesting a direct effect of IL-17 on neutrophil migration (23). The association of IL-17 with fibrosis has also been observed in humans. Cystic fibrosis patients have higher levels of IL-17 protein and IL-23 mRNA in their sputum compared with healthy controls (36). Even though multiple studies have suggested a direct association between IL-17 on either neutrophil migration or fibrosis, these activities have not been demonstrated in renal injury. In our study, systemic blockade of IL-17 by IL-17Rc effectively reduced both fibrosis and immune cell infiltration, including neutrophils.
Taken together, to our knowledge this is the first study demonstrating a potential role of IL-17 in fibrosis following recovery from AKI. Interestingly, in immunocompetent animals, conventional Th-17 cells represent the primary source of IL-17, whereas NK cells play a compensatory role in the IL-17 production in immunocompromised athymic rats. We propose that IL-17, regardless of the source, promotes the AKI-to CKD transition possibly via the recruitment of neutrophils and that modulation of IL-17 activity represents a potential therapeutic target to mitigate the development of CKD following AKI.
GRANTS
This work is supported by National Institutes of Health (NIH) Grant DK-063114 (D. P. Basile) and the Ralph W. and Grace M. Showalter Research Trust Fund (P. Mehrota). Support for J. A. Collett was provided by NIH Grant T32-HL-07995.
AUTHOR CONTRIBUTIONS
P.M., J.A.C., S.M., J.S., and C.M.I. performed experiments; P.M. and J.A.C. analyzed data; P.M., J.A.C., and D.P.B. interpreted results of experiments; P.M. prepared figures; P.M. drafted manuscript; P.M. and D.P.B. edited and revised manuscript; P.M. and D.P.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Robert Bacallao for important discussions and reading of this manuscript and Dr. Jesus Dominguez and Danhui Xie for guidance on renal neutrophil staining.
Portions of this work were presented at the 2015 meeting of the American Society of Nephrology.
REFERENCES
- 1.Allen JE, Sutherland TE, Rückerl D. IL-17 and neutrophils: unexpected players in the type 2 immune response. Curr Opin Immunol 34: 99–106, 2015. doi: 10.1016/j.coi.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Arase H, Arase N, Saito T. Interferon gamma production by natural killer (NK) cells and NK1.1+ T cells upon NKR-P1 cross-linking. J Exp Med 183: 2391–2396, 1996. doi: 10.1084/jem.183.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol 2: 1303–1353, 2012. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basile DP, Leonard EC, Beal AG, Schleuter D, Friedrich J. Persistent oxidative stress following renal ischemia-reperfusion injury increases ANG II hemodynamic and fibrotic activity. Am J Physiol Renal Physiol 302: F1494–F1502, 2012. doi: 10.1152/ajprenal.00691.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basile DP, Leonard EC, Tonade D, Friedrich JL, Goenka S. Distinct effects on long-term function of injured and contralateral kidneys following unilateral renal ischemia-reperfusion. Am J Physiol Renal Physiol 302: F625–F635, 2012. doi: 10.1152/ajprenal.00562.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein HB, Wang G, Plasterer MC, Zack JA, Ramasastry P, Mumenthaler SM, Kitchen CMR. CD4+ NK cells can be productively infected with HIV, leading to downregulation of CD4 expression and changes in function. J Immunol 177: 3669–3676, 2006. doi: 10.4049/jimmunol.177.6.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boesen EI, Williams DL, Pollock JS, Pollock DM. Immunosuppression with mycophenolate mofetil attenuates the development of hypertension and albuminuria in deoxycorticosterone acetate-salt hypertensive rats. Clin Exp Pharmacol Physiol 37: 1016–1022, 2010. doi: 10.1111/j.1440-1681.2010.05428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burne MJ, Daniels F, El Ghandour A, Mauiyyedi S, Colvin RB, O’Donnell MP, Rabb H. Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest 108: 1283–1290, 2001. doi: 10.1172/JCI200112080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burne MJ, Daniels F, El Ghandour A, Mauiyyedi S, Colvin RB, O’Donnell MP, Rabb H. Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest 108: 1283–1290, 2001. doi: 10.1172/JCI200112080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burne-Taney MJ, Liu M, Ascon D, Molls RR, Racusen L, Rabb H. Transfer of lymphocytes from mice with renal ischemia can induce albuminuria in naive mice: a possible mechanism linking early injury and progressive renal disease? Am J Physiol Renal Physiol 291: F981–F986, 2006. doi: 10.1152/ajprenal.00229.2005. [DOI] [PubMed] [Google Scholar]
- 11.Burne-Taney MJ, Yokota-Ikeda N, Rabb H. Effects of combined T- and B-cell deficiency on murine ischemia reperfusion injury. Am J Transplant 5: 1186–1193, 2005. doi: 10.1111/j.1600-6143.2005.00815.x. [DOI] [PubMed] [Google Scholar]
- 12.Campbell JP, Guy K, Cosgrove C, Florida-James GD, Simpson RJ. Total lymphocyte CD8 expression is not a reliable marker of cytotoxic T-cell populations in human peripheral blood following an acute bout of high-intensity exercise. Brain Behav Immun 22: 375–380, 2008. doi: 10.1016/j.bbi.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Chan AJ, Alikhan MA, Odobasic D, Gan PY, Khouri MB, Steinmetz OM, Mansell AS, Kitching AR, Holdsworth SR, Summers SA. Innate IL-17A-producing leukocytes promote acute kidney injury via inflammasome and Toll-like receptor activation. Am J Pathol 184: 1411–1418, 2014. doi: 10.1016/j.ajpath.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 14.Cornelius DC, Hogg JP, Scott J, Wallace K, Herse F, Moseley J, Wallukat G, Dechend R, LaMarca B. Administration of interleukin-17 soluble receptor C suppresses TH17 cells, oxidative stress, and hypertension in response to placental ischemia during pregnancy. Hypertension 62: 1068–1073, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol 10: 479–489, 2010. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 16.Fasbender F, Widera A, Hengstler JG, Watzl C. Natural Killer Cells and Liver Fibrosis. Front Immunol 7: 19, 2016. doi: 10.3389/fimmu.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.François A, Gombault A, Villeret B, Alsaleh G, Fanny M, Gasse P, Adam SM, Crestani B, Sibilia J, Schneider P, Bahram S, Quesniaux V, Ryffel B, Wachsmann D, Gottenberg JE, Couillin I. B cell activating factor is central to bleomycin- and IL-17-mediated experimental pulmonary fibrosis. J Autoimmun 56: 1–11, 2015. doi: 10.1016/j.jaut.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Grzelak I, Olszewski WL, Fossum S, Engeset A. Natural killer (NK) cell cytotoxicity in athymic (nude) rats. Arch Immunol Ther Exp (Warsz) 32: 549–556, 1984. [PubMed] [Google Scholar]
- 19.Horie Y, Wolf R, Chervenak RP, Jennings SR, Granger DN. T-lymphocytes contribute to hepatic leukostasis and hypoxic stress induced by gut ischemia-reperfusion. Microcirculation 6: 267–280, 1999. doi: 10.1111/j.1549-8719.1999.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 21.Jacob N, Yang H, Pricop L, Liu Y, Gao X, Zheng SG, Wang J, Gao HX, Putterman C, Koss MN, Stohl W, Jacob CO. Accelerated Pathological and Clinical Nephritis in Systemic Lupus Erythematosus-Prone New Zealand Mixed 2328 Mice Doubly Deficient in TNF Receptor 1 and TNF Receptor 2 via a Th17-Associated Pathway. J Immunol 182: 2532–2541, 2009. doi: 10.4049/jimmunol.0802948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, Ju ST, Okusa MD. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol 20: 1744–1753, 2009. doi: 10.1681/ASN.2008111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, Skoogh BE, Linden A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol 162: 2347–2352, 1999. [PubMed] [Google Scholar]
- 24.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55: 500–507, 2010. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marques VP, Gonçalves GM, Feitoza CQ, Cenedeze MA, Fernandes Bertocchi AP, Damião MJ, Pinheiro HS, Antunes Teixeira VP, dos Reis MA, Pacheco-Silva A, Saraiva Câmara NO. Influence of TH1/TH2 switched immune response on renal ischemia-reperfusion injury. Nephron Exp Nephrol 104: e48–e56, 2006. doi: 10.1159/000093676. [DOI] [PubMed] [Google Scholar]
- 26.Mehrotra P, Patel JB, Ivancic CM, Collett JA, Basile DP. Th-17 cell activation in response to high salt following acute kidney injury is associated with progressive fibrosis and attenuated by AT-1R antagonism. Kidney Int 88: 776–784, 2015. doi: 10.1038/ki.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mi S, Li Z, Yang HZ, Liu H, Wang JP, Ma YG, Wang XX, Liu HZ, Sun W, Hu ZW. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-beta1-dependent and -independent mechanisms. J Immunol 187: 3003–3014, 2011. doi: 10.4049/jimmunol.1004081. [Erratum in J Immunol. 193: 5345–5346, 2014. doi:] [DOI] [PubMed] [Google Scholar]
- 28.Mohamed R, Jayakumar C, Chen F, Fulton D, Stepp D, Gansevoort RT, Ramesh G. Low-Dose IL-17 Therapy Prevents and Reverses Diabetic Nephropathy, Metabolic Syndrome, and Associated Organ Fibrosis. J Am Soc Nephrol 27: 745–765, 2016. doi: 10.1681/ASN.2014111136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Passos ST, Silver JS, O’Hara AC, Sehy D, Stumhofer JS, Hunter CA. IL-6 promotes NK cell production of IL-17 during toxoplasmosis. J Immunol 184: 1776–1783, 2010. doi: 10.4049/jimmunol.0901843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paust HJ, Turner JE, Steinmetz OM, Peters A, Heymann F, Hölscher C, Wolf G, Kurts C, Mittrücker HW, Stahl RA, Panzer U. The IL-23/Th17 axis contributes to renal injury in experimental glomerulonephritis. J Am Soc Nephrol 20: 969–979, 2009. doi: 10.1681/ASN.2008050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pechman KR, Basile DP, Lund H, Mattson DL. Immune suppression blocks sodium-sensitive hypertension following recovery from ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol 294: R1234–R1239, 2008. doi: 10.1152/ajpregu.00821.2007. [DOI] [PubMed] [Google Scholar]
- 32.Peng X, Xiao Z, Zhang J, Li Y, Dong Y, Du J. IL-17A produced by both γδ T and Th17 cells promotes renal fibrosis via RANTES-mediated leukocyte infiltration after renal obstruction. J Pathol 235: 79–89, 2015. doi: 10.1002/path.4430. [DOI] [PubMed] [Google Scholar]
- 33.Pisitkun P, Ha HL, Wang H, Claudio E, Tivy CC, Zhou H, Mayadas TN, Illei GG, Siebenlist U. Interleukin-17 cytokines are critical in development of fatal lupus glomerulonephritis. Immunity 37: 1104–1115, 2012. doi: 10.1016/j.immuni.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray S, De Salvo C, Pizarro TT. Central role of IL-17/Th17 immune responses and the gut microbiota in the pathogenesis of intestinal fibrosis. Curr Opin Gastroenterol 30: 531–538, 2014. doi: 10.1097/MOG.0000000000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takada M, Nadeau KC, Shaw GD, Marquette KA, Tilney NL. The cytokine-adhesion molecule cascade in ischemia/reperfusion injury of the rat kidney. Inhibition by a soluble P-selectin ligand. J Clin Invest 99: 2682–2690, 1997. doi: 10.1172/JCI119457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor PR, Bonfield TL, Chmiel JF, Pearlman E. Neutrophils from F508del cystic fibrosis patients produce IL-17A and express IL-23 - dependent IL-17RC. Clin Immunol 170: 53–60, 2016. doi: 10.1016/j.clim.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 37.Velden J, Paust HJ, Hoxha E, Turner JE, Steinmetz OM, Wolf G, Jabs WJ, Özcan F, Beige J, Heering PJ, Schröder S, Kneißler U, Disteldorf E, Mittrücker HW, Stahl RA, Helmchen U, Panzer U. Renal IL-17 expression in human ANCA-associated glomerulonephritis. Am J Physiol Renal Physiol 302: F1663–F1673, 2012. doi: 10.1152/ajprenal.00683.2011. [DOI] [PubMed] [Google Scholar]
- 37a.Vincent I, Moscalu S, Chhoun C, Ye H, Huang L, Sung SS, Okusa M. Natural killer (NK) cells but not NK T cells attenuate renal fibrosis after acute kidney injury (AKI) (IRC8P.480).(Abstract) J Immunol 192, Suppl: 190.8, 2014. [Google Scholar]
- 38.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol 9: 503–510, 2008. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 39.Ysebaert DK, De Greef KE, De Beuf A, Van Rompay AR, Vercauteren S, Persy VP, De Broe ME. T cells as mediators in renal ischemia/reperfusion injury. Kidney Int 66: 491–496, 2004. doi: 10.1111/j.1523-1755.2004.761_4.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhang ZX, Huang X, Jiang J, Lau A, Yin Z, Liu W, Haig A, Jevnikar AM. Natural Killer Cells Mediate Long-term Kidney Allograft Injury. Transplantation 99: 916–924, 2015. doi: 10.1097/TP.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 41.Zhang ZX, Wang S, Huang X, Min WP, Sun H, Liu W, Garcia B, Jevnikar AM. NK cells induce apoptosis in tubular epithelial cells and contribute to renal ischemia-reperfusion injury. J Immunol 181: 7489–7498, 2008. doi: 10.4049/jimmunol.181.11.7489. [DOI] [PubMed] [Google Scholar]
- 42.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 28: 445–489, 2010. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zwacka RM, Zhang Y, Halldorson J, Schlossberg H, Dudus L, Engelhardt JF. CD4(+) T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest 100: 279–289, 1997. doi: 10.1172/JCI119533. [DOI] [PMC free article] [PubMed] [Google Scholar]