Abstract
We have examined the pathogenic role of increased complement expression and activation during kidney fibrosis. Here, we show that PDGFRβ-positive pericytes isolated from mice subjected to obstructive or folic acid injury secrete C1q. This was associated with increased production of proinflammatory cytokines, extracellular matrix components, collagens, and increased Wnt3a-mediated activation of Wnt/β-catenin signaling, which are hallmarks of myofibroblast activation. Real-time PCR, immunoblots, immunohistochemistry, and flow cytometry analysis performed in whole kidney tissue confirmed increased expression of C1q, C1r, and C1s as well as complement activation, which is measured as increased synthesis of C3 fragments predominantly in the interstitial compartment. Flow studies localized increased C1q expression to PDGFRβ-positive pericytes as well as to CD45-positive cells. Although deletion of C1qA did not prevent kidney fibrosis, global deletion of C3 reduced macrophage infiltration, reduced synthesis of C3 fragments, and reduced fibrosis. Clodronate mediated depletion of CD11bF4/80 high macrophages in UUO mice also reduced complement gene expression and reduced fibrosis. Our studies demonstrate local synthesis of complement by both PDGFRβ-positive pericytes and CD45-positive cells in kidney fibrosis. Inhibition of complement activation represents a novel therapeutic target to ameliorate fibrosis and progression of chronic kidney disease.
Keywords: pericytes, C1q, C3
the prevalence of chronic kidney disease (CKD) in the US is high and continues to rise in our aging population (6). Independent of the cause of CKD, tubulointerstitial fibrosis and glomerulosclerosis represent major pathways of progression of kidney disease; however, there are no approved drugs to treat kidney fibrosis and/or ameliorate progression of CKD (54). Activated fibroblasts, termed myofibroblasts, are the cells most likely responsible for increased production and deposition of extracellular matrix, including collagens and matrix proteins (28). The source of interstitial myofibroblasts in kidney disease is an area of current controversy (13, 53). Recent studies have identified pericytes as a major source of precursors of scar-producing myofibroblasts during kidney fibrosis (12). When pericytes are activated, they migrate from the pericapillary area to the interstitial space and contribute to vascular instability, an important mechanism for the progression of kidney disease (27, 28, 47). Previous work by several investigators has identified numerous secreted factors that contribute to the persistence of fibrotic response, including transforming growth factor-β (TGFβ), VEGF, connective tissue growth factor, matrix metalloproteinases, WNT ligands, and platelet-derived growth factors (PDGFs) (12, 61, 63, 64). A recent study using Col1A2-Cre and tenascin Cre system to conditionally delete TGFβ type 2 receptor on collagen-producing fibroblasts and medullary interstitial cells in the model of unilateral ureteral obstruction (UUO) demonstrated reduced collagen production but no significant effect on kidney tissue fibrosis, suggesting that signaling pathways other than TGFβ could contribute to the pathogenesis of renal fibrosis (35).
Additional factors that could be involved in the pathogenesis of kidney fibrosis include the activation of the immune response. A component of the innate immune response that has been demonstrated to contribute to renal inflammation is the complement system, which can directly affect cell function and also influence the adaptive immune response (46, 51). The complement system has been characterized primarily as a host defense system that can be activated in the presence of injured cells, accumulating debris, or foreign materials (44). However, recent studies support the notion that an overly active complement system can turn this protective response to a destructive one that drives disease pathogenesis (14, 38, 45). Also, new studies show that complement is regulated not only in the intravascular space but also through local secretion of complement components by injured tissue and infiltrating cells as well as by intracellular complement turnover that contributes to the complement response in various pathological conditions (23, 66). Complement activation occurs through three distinct pathways: the classical pathway (CP) that involves antibody-mediated activation of the C1 complex comprising of C1q, C1r, and C1s, the lectin pathway (LP) triggered by carbohydrate signatures on cell surfaces, and the alternative pathway (AP), which involves direct activation of C3 through surface and properdin (FP) binding of C3b. Within the C1 complex, C1r and C1s are serine proteases responsible for initiating the classical complement pathway. Binding via C1q to immune complexed IgG or to certain pathogens induces a conformational change in C1q that activates the proteases in a stepwise fashion: C1r first autoactivates and then activates C1s. C1s subsequently cleaves substrates C4 and C4 bound C2 to form the C3 convertase (C4b2a), the downstream component of the reaction cascade. Seminal observations have provided a considerable understanding of the important role of AP in kidney disease (32, 36, 37, 55, 58). Upon activation of the complement system, C3 and C5 are among the proteins that are cleaved, thus generating several active downstream products, including anaphylatoxins C3a and C5a. In previous studies using animal models of ischemia reperfusion injury (IRI), complement activation was demonstrated primarily via activation of the alternate and the lectin pathway during acute kidney injury (AKI) (40, 56, 65). Additional studies have characterized the importance of tubulo-interstitial fibrosis in AKI by elucidating the functional role of complement inhibitor Crry expressed in the proximal tubule (57). In addition, recent studies suggest that interstitial fibrosis and macrophage infiltration are significantly reduced in C5-deficient (C5−/−) mice (2), and moreover, C3 deficiency limits renal fibrosis by suppressing EMT processes during degeneration of the nephrotubulus (67). Those above studies had demonstrated complement activation predominantly in renal tubular epithelial cells and proximal tubules (2, 67).
In the present study, we examined whether nonimmune interstitial cells such as PDGF receptor-β (PDGFRβ)-positive pericytes and immune cells isolated from UUO and folic acid-treated mice synthesize and secrete complement components when compared with sham pericytes. In addition, we used mice with deletion of specific complement genes C1qA or C3 to examine the role of complement activation in kidney fibrosis.
METHODS
Animals and reagents.
C1qA−/− mice with targeted disruption of exon 1 of the C1qa gene in C57BL/6 background were obtained from Dr. Andrea Tenner’s laboratory (made by Dr. Marina Botto). C3−/− mice (strain B6.129S4-C3tm1Crr/J) of C57BL/6 genetic background and wild-type (WT) mice (C57BL/6) were obtained from The Jackson Laboratory (Bar Harbor, ME). Antibodies included monoclonal antibody to mouse C1q (Clone JL-1) from Hycult Biotech (Plymouth Meeting, PA), rabbit polyclonal antibody to C1q from Abcam (Cambridge, MA), rat monoclonal antibody to mouse C3b/iC3b/C3c (clone 2/11, which was reported previously) (31), rabbit polyclonal α-SMA from Abcam (Cambridge, MA), mouse monoclonal PCNA from Biolegend (San Diego, CA), rabbit polyclonal F4/80 from Abcam, and mouse monoclonal GAPDH from Santa Cruz Biotechnology (Dallas, TX). For immunofluorescence staining, rabbit monoclonal antibody to C1q was conjugated with Alexa Fluor 488 using the antibody labeling kit (Molecular Probes; Life Technologies, Eugene, OR) according to the manufacturer’s instructions.
Renal fibrosis models.
All experiments were performed in accordance with the National Institutes of Health’s (NIH) Guide for the Care and Use of Laboratory Animals, and all procedures were approved by the University of Virginia Animal Care and Use Committee. For the UUO model, 8- to 10-wk-old male mice (C57/BL/6 WT, C1qA−/−, or C3−/− mice) were used. The left kidney was exposed through a midline incision under sterile conditions, and the ureter was dissected and securely tied at two places with 6-0 silk sutures. Volume depletion was prevented by administration of 0.1 ml of saline into the peritoneal cavity. The midline incision was closed; the mice were returned to their cages and allowed free access to food and water. As control, sham surgery was performed the same way as UUO without tying the ureter. On days 3 and 10, mice were euthanized and the left kidneys from UUO and sham mice collected for protein, RNA isolation, and histological evaluation. For the folic acid model, 8- to 10-wk-old male mice (C57/BL/6 WT, C1qA−/− or C3−/− mice) received intraperitoneal injection of folic acid (250 µg/g body weight). Mice were euthanized after 2 wk, and kidney tissue was harvested.
Isolation of pericytes/fibroblast from sham, UUO, and folic acid-treated mice.
To isolate PDGFRβ-positive pericytes from sham and UUO kidneys, kidney tissue was minced and digested in Liberase/DNase mix for 30 min at 37°C with vigorous shaking (48). The mixture was filtered with a 40-µm nylon mesh, and single cells were spun down, resuspended in MACS buffer, and incubated with rabbit anti-mouse PDGFRβ antibody (39) for 15 min on ice, followed by incubation with goat anti-rabbit IgG Microbeads (Miltenyl Biotec, San Diego, CA). Cells were washed and applied to a MACS affinity column under a magnetic field. Magnetic bead column-purified cells were cultured in DMEM-F-12 with 10% FBS and insulin/transferrin/selenium on gelatin-coated dishes.
Enrichment of CD45 cells from kidney cell suspension.
Kidney cell suspension was prepared as described previously. The cells were suspended in PEB buffer (PBS + 2 mM EDTA + 0.5% bovine serum albumin) and incubated on ice for 30 min and blocked with Fc block. Then the cells were stained for CD45 and attached to magnetic beads as per the instructions of the manufacturer to positively select the CD45 cells using the Magnisort mouse CD45.2 + ve selection kit (eBiosciences). The positive cells were separated using an AUTOMACS system (Milltenyi Biotec). Positive cells were then washed briefly in phosphate buffer and lysed for RNA preparation, and real-time PCR analysis for various genes was done.
Protein analysis of UUO secretome.
Proteins isolated from supernatants of sham and UUO mice were separated on two- or one-dimensional electrophoresis, and proteins excised from the gel were identified by mass spectrometry. Briefly, proteins isolated from the supernatant of sham or UUO mice (10 days) were suspended in Laemmli buffer containing 2% SDS (wt/vol) and 0.5% (vol/vol) β-mercaptoethanol and heated for 5 min at 95°C. Proteins were separated on SDS acrylamide gradient gels (4–12%) and then stained with SYPRO Ruby (ThermoFisher Scientific and Invitrogen) for visualizing total protein, and 1-mm slices were excised. For two-dimensional separation, protein was suspended in IEF loading buffer (8 M urea, 2% CHAPS, 40 mM DTT, and 0.2% Biolyte) and resolved by 2D gel electrophoresis. Proteins were first separated in ampholytes by isoelectric focusing, pH 3–10, and then electrophoresed on 1% sodium dodecyl-sulfate (SDS) and 4–12% polyacrylamide gradient gels (Invitrogen) and visualized by staining with SYPRO Ruby (Invitrogen). Selective differential proteins were excised. Proteins were digested with trypsin, and peptides were analyzed by high-resolution LC-MS/MS with a Thermo Velos Orbitrap mass spectrometer coupled to a waters nanoACQUITY LC system (4). Proteins were identified by MASCOT (www.matrixscience.com) matching of peptide fragmentation patterns to a database of predicted patterns, as reported previously (4). For clustering, we used DAVID (version 6.7, https://david.abcc.ncifcrf.gov/) analysis to proteins that were either over- or underrepresented in UUO or control samples.
Luminex analysis.
PDGFRβ-positive cells isolated from sham and UUO kidneys were cultured in six-well plates after isolation and grown to confluence in complete media. For measurements of cytokines produced, pericytes were grown for 24 h in serum-free media, and then supernatants were collected and concentrated using an AMICON 3K membrane (UFC800324) and centrifugation at 4,000 g for 40 min. The concentrated samples were used for analysis by Luminex Mag Pix; 25 µl was used for the luminex analysis in duplicate for each sample and the cytokine profile determined using the MAPKIT-31 (Millipex). The standards provided with the kit were used to calibrate the readings and for quality assurance.
Cell proliferation assay.
PDGFRβ-positive cells from P1 passage were grown in 24 wells in complete medium for 7 days at a density of 15,000 cells/well. The cells were then cultured for 16 h in 3% FCS and DMEM-F-12. At the end of this period, complete medium was replaced. Cells were grown for an additional 24 h. Staining for PCNA was carried out using an antibody against PCNA (1:200) and an AF488 conjugated secondary antibody in the wells. DAPI was used to locate and enumerate the nuclei. A Zeiss Axiovision microscope and ×10 magnification were used for microscopic analysis of PCNA-positive cells. From each well at least five spots were recorded, and the percentage of PCNA-positive cells was calculated at each spot. The average value of the percentages for each sample was plotted.
Topflash reporter assays.
Super 8xTOPFlash and 8xFOPFlash were a gift from Randall Moon (Addgene plasmid nos. 12456 and 12457), and recombinant mouse Wnt-3a was from R & D Systems (Minneapolis, MN). Pericytes from sham and day 3 UUO kidneys were plated (75,000 cells/well) in a six-well gelatin-coated plate. After 24 h, cells were transfected with 2.5 µg of either TOPFlash or FOPflash reporter along with a renilla luciferase plasmid using lipofectamine 2000 (Invitrogen). The next day, cells were starved for 3 h before treatment with 10 ng/ml Wnt3a (R & D Systems) and lysed in passive lysis buffer 24 h later. Luciferase activity was measured using the dual luciferase assay system (Promega, Madison, WI) with the GloMax Multi Jr Detection System: Luminometer (Promega). Activity was expressed as relative light units.
Gene expression analysis by quantitative real-time PCR:
Total RNA from pericytes and kidney tissue was isolated using a Spinsmart RNA Mini purification kit from Denville Scientific (Metuchen, NJ). Real-time PCR was carried out using the StepOnePlus real-time PCR system (Invitrogen) with iTaq SYBR Green Supermix with Rox (Bio-Rad, Hercules, CA). Specificity of the amplified product was confirmed by melting curve. For relative quantification, a standard curve was generated from a six-step cDNA dilution series. The primer sequences in the RT-PCR were as follows: for C1qa, 5′-aggactgaagggcgtgaaag-3′ (forward) and 5′-caagcgtcattgggttctgc-3′ (reverse); for C1qb, 5′-aagcatcacaga acaccagga-3′ (forward) and 5′-accccactgtgtcttcatcag-3′ (reverse); for C1qc, 5′-ctgtctgggagaacagg acg-3′ (forward) and 5′-actgggtccaacgaccatc-3′ (reverse); for C1s, 5′-tgaaggaagagggaaagacaag-3′ (forward) and 5′-gattttggaggtaaagggcagt-3′(reverse); for C1r, 5′-acttccgctacatcaccacaa-3′ (forward) and 5′-ctctccttcc tcttcattcttcc-3′ (reverse); for C3, 5′-acaaactcacacagagcaaga-3′ (forward) and 5′-atccatgaaga caccagcatag-3′ (reverse); for C5, 5′-cagcaaggaggagtcaacat-3′(forward) and 5′-tccacaagagccc gtaaatc-3′ (reverse); for C4, 5′-accccctaaataacctgg-3′ (forward) and 5′-cctcatgtatcctttttgga-3′ (reverse); for C3aR, 5′-ccccaagacattgcctccat-3′ (forward) and 5′-gactgtgttcacggtcgtct-3′ (reverse); for C5aR, 5′-taccacagaacccaggagga-3′ (forward) and 5′-gccatccgcaggtatgttag-3′ (reverse); for α-SMA, 5′-ctgacag aggcaccactgaa-3′ (forward) and 5′-catctccaga gtccagcaca-3′ (reverse); for fibronectin, 5′-tccacagccattcctgcgcc (forward) and 5′-gttcacccg cacccggtagc-3′ (reverse); for collagen 1A1, 5′-gccccaagggtccttccggt-3′ (forward) and 5′-aggacc agggctgccaggac-3′ (reverse); for TGFβ1, 5′-cgaggcggtgctcgctttgt-3′ (forward) and 5′-catagatggcgttg ttgcggtcca-3′ (reverse); for VCAM-1, 5′-acaagtctacatctctcccaggaatac-3′ (forward) and 5′-cacagca ccaccctcttgaa-3′ (reverse); for ICAM-1, 5′-atggg aatgtcaccaggaatg-3′ (forward) and 5′-tcacgaggcccacaatga-3′ (reverse); and for 18s, 5′-ggagtgggcctgcggctta-3′ (forward) and 5′-aacggccatgcaccaccacc-3′ (reverse).
Protein analysis and Western blotting.
Pericytes from sham UUO and folic acid-treated kidneys were grown to confluence in six-well dishes and serum starved for 24 h. Cells were lysed in RIPA lysis buffer with protease inhibitor cocktail (Roche, Mannheim, Germany). Culture supernatants were concentrated using Amicon Ultra (EMD Millipore, Billerica, MA). Kidney tissue lysates were prepared by homogenizing in ice-cold RIPA buffer for 30 s using an ultrasonic processor (Cole-Parmer). Lysates were clarified by centrifugation, and protein concentration was determined by the BCA protein assay (Thermo Fisher Scientific, Rockford, IL). Proteins were separated on 10% SDS-PAGE and transferred to PVDF membranes (EMD Millipore). The membranes were blocked with Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) for 1 h at room temperature and then incubated with primary antibodies rabbit polyclonal anti-C1q (1:1,000), mouse monoclonal anti-C1q (1:50), rabbit polyclonal α-smooth muscle actin (1:1,000), rat monoclonal anti-C3b/iC3b/C3c (1:1,000), and mouse GAPDH (1:200) overnight at 4°C and then with goat anti-rabbit IRDye 680RD-, goat anti-mouse IRDye 800CW-, or goat anti-rat IRDye 680LT- labeled secondary antibody (1:15,000) (LI-COR Biosciences) in Odyssey blocking buffer with 0.1% Tween for 1 h at room temperature. Membranes were imaged, and densitometric analysis of bands was performed using the LI-COR Odyssey Infrared Imaging System with Image Studio Lite 4.0 analytical software.
Native gel electrophoresis for C3 fragments.
Native gels of 8%, cast in Tris·HCl (pH 8.0), were run with Tris-glycine buffer to analyze kidney lysates; 120 µg of the protein quantified by the BCA method was run in each lane. Samples were run under nonreducing conditions, blotted, and probed using the procedure or the method of Mastellos et al. (31). Densitometry analysis of the signals was done, and the average values for sham or UUO were plotted.
Immunofluorescence microscopy.
Kidneys were fixed in 2% PLP (1% paraformaldehyde, 1.4% dl-lysine, and 0.2% sodium periodate in 0.1 M phosphate buffer, pH 7.4) overnight at 4°C, incubated in 30% sucrose for 24 h at 4°C, and embedded in OCT compound (Fischer Scientific, Houston, TX). Frozen kidney sections (6 µm) were permeabilized with 0.3% Triton X-100 and blocked with 15% horse serum and 15% chicken serum for 1 h at room temperature, followed by blocking with anti-mouse CD16/32 (clone 2.4G2; StemCell Technologies, Vancouver, BC, Canada) with 0.02% sodium azide overnight at room temperature. Sections were labeled with FITC-labeled anti-C1q, mouse monoclonal anti-actin smooth muscle-Cy3 (Sigma-Aldrich, St. Louis, MO), and PE-conjugated anti-F4/80 (Clone BM8; Biolegend, San Diego, CA) all at 1:25 PM at room temperature for 2 h. Sections were mounted with ProLong Gold Antifade reagent with DAPI (Molecular Probes, Life Technologies, Eugene, OR). Images were acquired using the Carl Zeiss Axiovert 200 microscopy system with ApoTome imaging and Axiovision software (Carl Zeiss Microscopy, Thornwood, NY).
Immunohistochemistry and picrosirius red staining.
Kidneys embedded in paraffin were sectioned (5 µm). Sections were deparaffinzed, fixed in 4% paraformaldehyde, rinsed in PBS, and stained with picrosirius red solution (Polysciences, Warrington, PA) for 1 h at room temperature. Slides were washed in acid water, dehydrated, and mounted with cytoseal and viewed by light microscopy (Carl Zeiss Axioskop). Photographs were taken with an SPOT RT camera (software version 3.3; Diagnostic Instruments, Sterling Heights, MI). The extent of picrosirius red-positive area was quantitated using the grid method (266 squares) applied to images in Photoshop (Adobe Systems, San Jose, CA) that were obtained at either ×200 or ×400 magnification. Fibrosis was expressed as a percentage of the total area. A total of four to five images were evaluated per kidney, and the mean values were calculated. For immunohistochemistry, antigen retrieval was performed using the antigen unmasking solution from Vector Laboratories (Burlingame, CA). Sections were blocked with 5% goat serum containing 10% Triton X-100 for 1 h at room temperature and incubated with rat monoclonal anti-C3b/iC3b/C3c or rabbit polyclonal F4/80 antibodies both at 1:100 at 4°C overnight, followed by incubation with biotinylated secondary antibodies (1:250; Vector Laboratories) for 1 h at room temperature. Color was developed using diaminobenzidine (Vector Laboratories), and sections were counterstained with hematoxylin, rinsed, air-dried, and mounted using cytoseal. Quantitation of F4/80 positive areas in four to five images per kidney obtained at ×400 magnification was done using the grid method and expressed as percentage of F4/80-positive areas.
Flow cytometry.
Phosphate-buffered saline-perfused kidneys were obtained from sham and 10-day UUO mice and single-cell suspensions prepared by digesting the tissue with Liberase (Roche Diagnostics) and DNase for 30 min at 37°C, with constant shaking and vortexing every 5 min for 30 s. The suspension was filtered and centrifuged, and RBC lysis was done on the pellet, followed by neutralization and filtration though a 40-μm filter. The filtrate was centrifuged and the cell pellet washed in PBS and resuspended in PBS for live dead staining using the Live Dead Kit (Life Technologies). The cell suspension was then blocked with Fc Block (BD PharMingen). The cells were stained for surface markers, followed by fixation and permeabilization. The permeabilized cell suspension was stained with AF488-conjugated anti-mouse C1q or an isotype control. Data acquisition was done using a Cytek upgraded FACS Calibur, and data were analyzed using the Flowjo software. Single color controls were prepared from spleen cell suspension for the various fluorochromes used. The following antibodies were used: CD 45.2 (clone 104) from eBiosciences; Ly6c (clone AL-21) and Fc block CD16/32, both from BD Biosciences; F4/80 (clone BM8), CD11c (clone418), CD8a (clone 53-6.7), CD4 (clone GK1.5), MRC1 (clone C068C2), PDGFRβ (clone APB5), Ly6G (clone IA8), CD11b (clone M/170), and CD31 (clone 390), all from Biolegend; and Collectrin rabbit polyclonal antibody from Dr. Thu Le, Department of Medicine, University of Virginia.
Macrophage depletion.
Clophosome-neutral liposomal clodronate and control liposome (7 mg/ml) obtained from FormuMAX Scientific (Palo Alto, CA) were injected intraperitoneally in 8- to 10-wk-old male mice 2 days before UUO surgery. Following surgery, mice were injected with 75 µl of liposomes on days 1, 3, and 5. On day 7, left kidneys from UUO and sham mice were collected for flow cytometry, RNA isolation, and histological evaluation.
Statistics.
All experiments were performed at least three times. All values are expressed as means ± SD. Statistical analysis was performed using an unpaired Student’s t-test. A P value of <0.05 was considered to be statistically significant.
RESULTS
Increased secretion of extracellular matrix components and complement in secretome of UUO pericytes.
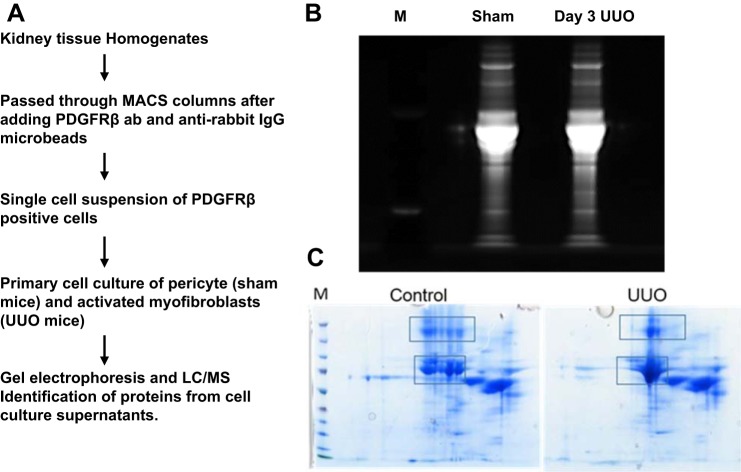
Previous studies have shown that platelet-derived growth factor receptor-β (PDGFRβ) is expressed on all renal myofibroblasts (18). To investigate the cellular pathways involved in UUO-mediated pericyte to myofibroblast transition, we isolated PDGFRβ-positive cells from sham and UUO mice and established primary cell cultures as described (48). The scheme of isolation of these cells is shown in Fig. 1A. These cell cultures have been well characterized in earlier studies by Dr. Jeremy Duffield, who has shown that these PDGFRβ-positive pericytes lack epithelial, endothelial, leukocyte, and podocyte markers (48). We collected supernatants of these cells grown in serum-free media, concentrated them, and performed a proteomic analysis on selected protein spots that were different when comparing supernatants from UUO and control samples (Fig. 1, B and C). Proteins identified by mass spectrometry from differential spots from 2D gels were serotransferrin, fibronectin, thrombospondin, vinculin, and myosin 1, 6, 7, and 8 isoforms. The entire secretome identified a total of 750 proteins, and from these a total of 214 proteins showed differences between levels of expression when comparing cells isolated from sham and UUO secretome. Supplemental Table S1 (all Supplemental Material for this article is available online at the AJP-Renal Physiology website) shows that PDGFRβ-positive pericytes isolated from UUO mice secrete increased amounts of collagens, including α1, α2, (I and XII), fibrillin-1, proteoglycans like fibronectin, cathepsins B and D, fibulin-2, osteopontin, thrombospondins 1 and 2, and several complement components. Among the proteins that had increased phosphorylation in UUO secretome compared with sham are osteopontin, vimentin, latent TGF-β, insulin-like growth factor-binding protein 3 and 5, vinculin, and several others, as shown in Supplemental Table S2. The most significantly enriched categories from DAVID gene ontology (GO) (https://david.ncifcrf.gov/) terms or pathways are listed in Table 1. Complement C1q was one of the enriched GO term glycoproteins, with a 6.6-fold increase in the UUO secretome.
Fig. 1.
Analysis of the secretome of platelet-derived growth factor receptor-β (PDGFRβ)-positive pericytes isolated from sham and unilateral ureteral obstruction (UUO) mice. A: schematic representation of the procedure used for isolation of cells. B and C: SDS-PAGE separation (B) and 2D gel separation (C) of proteins isolated from pericyte supernatants from sham and day 3 UUO. Regions sliced for protein mass spectrometry analysis are marked in rectangular boxes. M, molecular weight marker.
Table 1.
GO analysis: enrichment of functional annotation terms for proteins differentially represented in UUO compared with control secretome
| Term | Count | Fold Δ | FDR |
|---|---|---|---|
| GO terms down in UUO secretome (44 proteins) | |||
| Signal* | 19 | 4.8 | 1E–8 |
| Glycoprotein* | 16 | 3.3 | 2.7E–4 |
| Glycosylation site: N-linked (GlcNAc) | 16 | 3.2 | 8.6E–4 |
| Extracellular region* | 10 | 6.7 | 3.1E–4 |
| EGF-like* | 6 | 2.2 | 4.1E–4 |
| GO terms up in UUO secretome (74 proteins) | |||
| Signal peptide* | 45 | 3.7 | 7.7E–15 |
| Disulfide bond | 32 | 3.1 | 3.6E–7 |
| Glycoprotein* | 33 | 2.2 | 6.9E–4 |
| Lysosome | 13 | 2.2 | 1.0E–10 |
| Glycosidase | 8 | 2.6 | 3.7E–6 |
| EGF-like calcium binding* | 5 | 1.4 | 1.5E–2 |
| Extracellular region* | 17 | 4.2 | 1.4E–4 |
| Complement subcomponent C1q chain A | 3 | 6.6 | 3.8E–3 |
GO, gene ontology; UUO, unilateral ureteral obstruction; EGF, epidermal growth factor; GlcNAc, N-acetyl-d-glucosamine; FDR, false discovery rate. GO terms are listed along with Bonferroni-corrected FDR and corresponding fold changes in differential proteins in UUO.
These proteins are in both the “up” and “down” lists; i.e., separate subsets of proteins in these GO categories increased and decreased with UUO.
UUO pericytes are activated myofibroblasts.
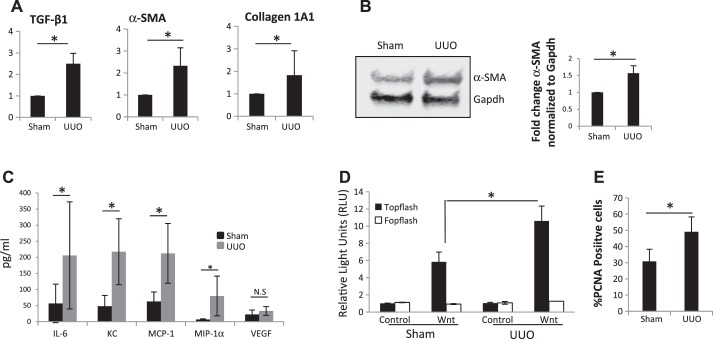
Previous studies have established that myofibroblasts are inflammatory cells that can generate cytokines and chemokines and promote injury (12, 43). Pericyte activation, detachment, and transition to myofibroblasts are driven primarily by Wnt/β-catenin signaling in vivo in response to kidney injury (29). Whereas transient Wnt activation in epithelial cells is associated with repair mechanisms, Wnt activation in pericytes/myofibroblasts leads to fibrotic changes (30). To establish that the cultures we obtained from UUO kidneys are indeed activated myofibroblasts, we characterized our cell populations for the positivity of all of the hallmarks of myofibroblasts. Pericytes isolated from UUO kidneys showed elevated mRNA expression of α-smooth muscle actin (α-SMA), collagen 1A1, and TGFβ (Fig. 2A) and increased α-SMA protein (Fig. 2B). Luminex analysis of cell culture supernatants from UUO pericytes shows increased secretion of proinflammatory chemokines IL-6, KC, MCP-1, and MIP-1α when compared with pericytes isolated from sham mice (Fig. 2C). The Wnt/β catenin signaling pathway activity measured using a TOPflash reporter assay in the presence of Wnt3a showed increased Wnt signaling in UUO pericytes (Fig. 2D). UUO pericytes had enhanced cell proliferation when compared with sham (Fig. 2E). Altogether, these results suggest that PDGFRβ-positive pericytes isolated from UUO mice have several features of activated myofibroblasts.
Fig. 2.
Characterization of PDGFRβ-positive pericytes isolated from sham and UUO mice. A: real-time PCR analysis of fibrotic gene expression in sham and day 10 UUO pericytes (n = 3). B: Western blot for α-smooth muscle actin (α-SMA) in cell lysates from sham and day 10 UUO pericytes (n = 4). C: luminex analysis for chemokines and cytokines in culture supernatants from sham and day 10 UUO pericytes (n = 5). D: Topflash reporter assay for Wnt signaling in sham and day 10 UUO pericytes (n = 3). E: PCNA proliferation assay showing %proliferating cells in sham and day 10 UUO pericytes (n = 3). *P < 0.05. TGFβ1, transforming growth factor-β1; NS, not significant.
C1q expression is increased in whole kidney tissue and PDGFRβ-positive pericytes isolated from UUO and folic acid-treated kidneys.
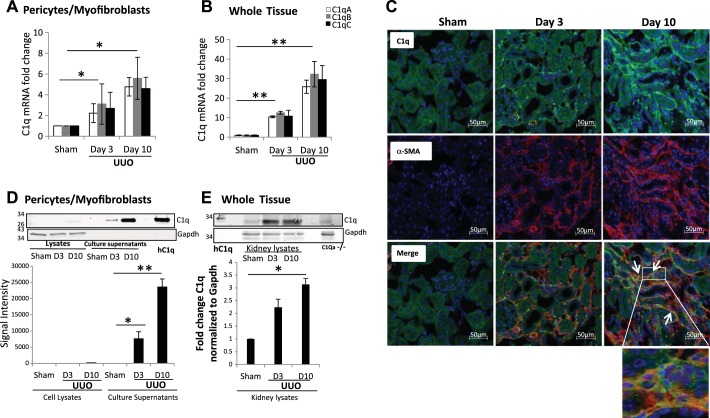
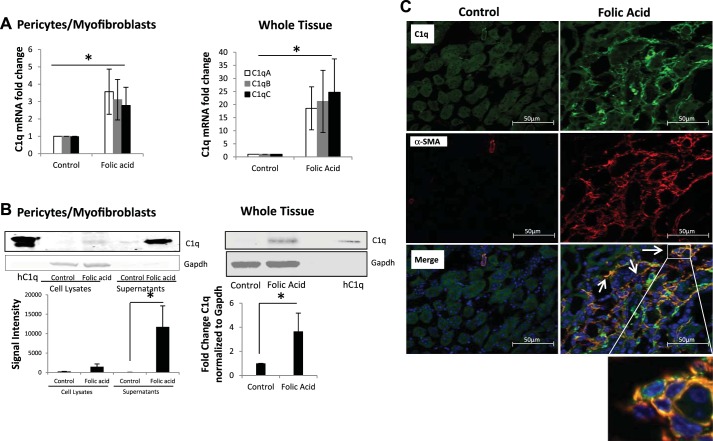
We observed by mass spectrometry analysis of the secretome that UUO pericytes have increased C1q production. To confirm these findings, we analyzed cell lysates of PDGFRβ-positive pericytes isolated from sham and UUO mice. The mRNA levels of C1qA, -B, and -C subunits were elevated in UUO pericytes (Fig. 3A) as well as in whole kidney tissue lysates obtained from mice subjected to UUO for 3 and 10 days (Fig. 3B). Immunostaining localized C1q expression to the interstitium, with no significant staining in the tubular epithelium of UUO mice, whereas sham kidneys showed little or no staining (Fig. 3C). Kidney tissue sections were also stained with anti-α-SMA antibodies, and the majority of the staining was detected in the interstitium, with significant costaining with C1q shown as double-positive cells (Fig. 3C). We confirmed increased C1q protein expression and secretion in UUO pericytes by Western blotting of cell lysates and culture supernatants (Fig. 3D). In whole kidney lysates, C1q protein was elevated in UUO mice on day 3 and day 10 (Fig. 3E). To determine whether increased synthesis of C1q was also present in other models of kidney fibrosis, we performed similar measurements of C1q in whole kidney tissue as well as in PDGFRβ-positive pericytes isolated from folic acid-treated mice. After 2 wk of folic acid given intraperitoneally, there is significant proteinuria (26), tubulointerstitial fibrosis with reduced fatty acid oxidation (19), and reduced mitochondrial biogenesis (52), which are metabolic signatures of progressive kidney disease. The mRNA levels of C1qA, -B, and -C were also elevated in kidney tissue of folic acid-treated mice and in PDGFRβ-positive pericytes (Fig. 4A). Western blotting showed an increased amount of C1q protein in culture supernatants from pericytes isolated from folic acid-injected mice and in whole kidney tissues (Fig. 4B). Similar to the UUO model, in the folic acid mice, staining for C1q was localized to the interstitium with significant colocalization with α-SMA staining (Fig. 4C). These observations confirm that the increased complement C1q expression occurs predominantly in the interstitial space in two different models of kidney fibrosis.
Fig. 3.
C1q expression in PDGFRβ-positive pericytes and whole kidney tissue from sham and UUO mice. A: C1q mRNA expression increases in pericytes cultured from UUO kidneys (n = 3). B: C1q expression is increased in the UUO mouse model of kidney injury (n = 5). C1qA (open bars), C1qB (gray bars), and C1qC (black bars) C: immunofluorescence staining showing increased C1q (green) expression in UUO kidneys in the interstitium on day 3 (D3) and day 10 (D10) and colocalization (white arrows) with α-SMA (red; original magnification ×200). Inset shows enlarged magnification of coexpression of C1q and α-SMA in the same interstitial cell. D: Western blot showing that C1q is secreted into the culture medium in pericytes from UUO kidneys but not in pericytes from sham kidneys, hC1q, human C1q protein used as positive control (n = 4). E: Western blot showing increased C1q protein in UUO kidneys on D3 and D10. *P < 0.05; **P < 0.001.
Fig. 4.
C1q expression increases in folic acid-treated mice. A: real-time PCR analysis for expression of C1qA, -B, and -C in pericyte cultures from vehicle- and folic acid-treated mice and in total kidneys of folic acid-treated mice 2 wk postinjection (n = 3). B: Western blot showing increased C1q protein in supernatants of PDGFRβ-positive pericytes cultured from vehicle and folic acid-treated mice and in total kidneys of folic acid mice. hC1q, human C1q protein used as positive control. C. immunofluorescence staining showing increased C1q (green) expression in folic acid-treated mice in the interstitium 2 wk postinjury and colocalization (white arrows) with α-SMA (red; original magnification, ×400). Inset shows enlarged magnification of coexpression of C1q and α-SMA in the same interstitial cell. *P < 0.05.
Flow cytometry study shows increased synthesis of C1q by PDGFRβ-positive pericytes and immune cells during UUO.
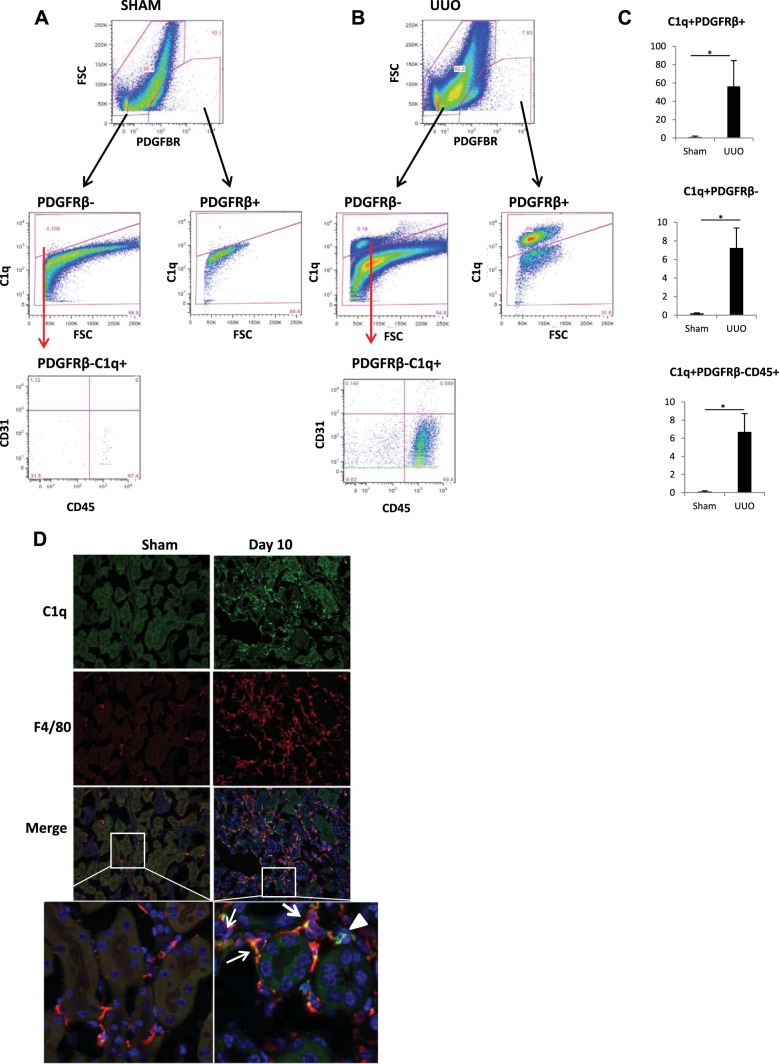
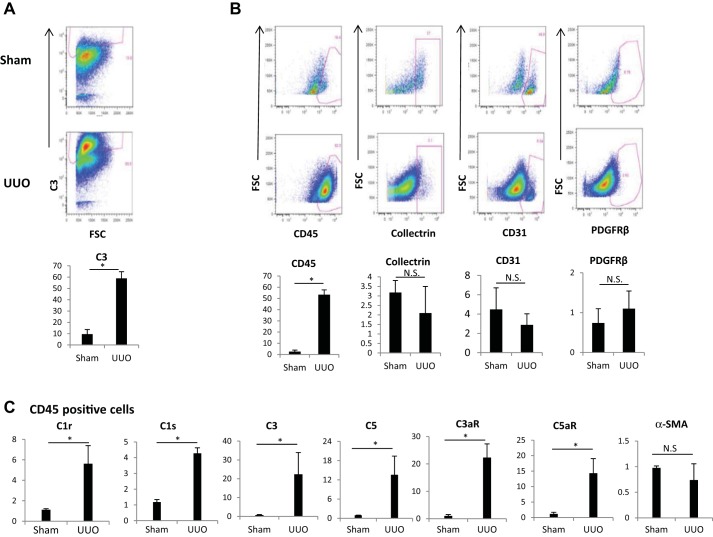
To define the cell types that synthesize C1q in kidney tissue during fibrosis, we performed flow cytometry experiments only in UUO mice. Since the majority of C1q protein synthesized in UUO pericytes was secreted into the supernatant, and no surface staining could be obtained, suspended single-kidney cells were permeabilized to detect C1q by flow cytometry. Figure 5, A and B, top, shows a population of cells that stained positive for PDGFRβ in both sham and UUO mice. During UUO, a significant proportion of PDGFRβ-positive pericytes (56.92 ± 3.8%) stained for C1q compared with 1.09 ± 0.9% in sham mice (Fig. 5C). In addition to the increased expression of C1q in pericytes, we also observed that a vast majority of PDGFRβ-negative cells were C1q positive and stained positive for CD45 (Fig. 5, A and B) and Lower panels marked with red arrows). These populations also increase significantly during UUO (Fig. 5C, bottom). Since our flow cytometry studies suggested that, in addition to PDGFRβ-positive pericytes, there was increased synthesis of C1q protein by macrophages, we next performed double immunostain on kidney sections of sham and UUO mice using antibodies for C1q and F4/80. As shown in Fig. 5D, there was an increased positive staining for both C1q and F4/80 in kidney sections of UUO mice, which was predominant in the interstitial compartment.
Fig. 5.
Flow cytometry analysis of kidney tissue from sham and UUO mice. A: flow analysis of kidney cell suspension from sham mice. B: flow analysis of kidney cell suspension from day 10 UUO mice showing PDGFRβ-positive and PDGFRβ-negative cells based on C1q staining. C: quantitation of cells as a fraction of live singlet cells (n = 3). *P < 0.05. C1q gating is based on an isotype control and a fluorescence minus one control to exclude any nonspecific signal. After dead cells were excluded using a live dead stain, followed by a forward scatter (FSC) vs. forward scatter width (FSCW) gating to include only single cells, the total %C1q-positive cells was quantified in the sham and UUO mice. The same gates were applied to UUO and sham samples. D: immunofluorescence staining showing increased C1q (green) expression in UUO kidneys in the interstitium on day 10 and colocalization with F4/80 (red; ×20 magnification). Inset is enlargement of dual-stained interstitial cells marked with white arrows. Arrowhead shows cells positive for C1q alone.
C1qA deficiency does not prevent UUO-mediated kidney fibrosis.
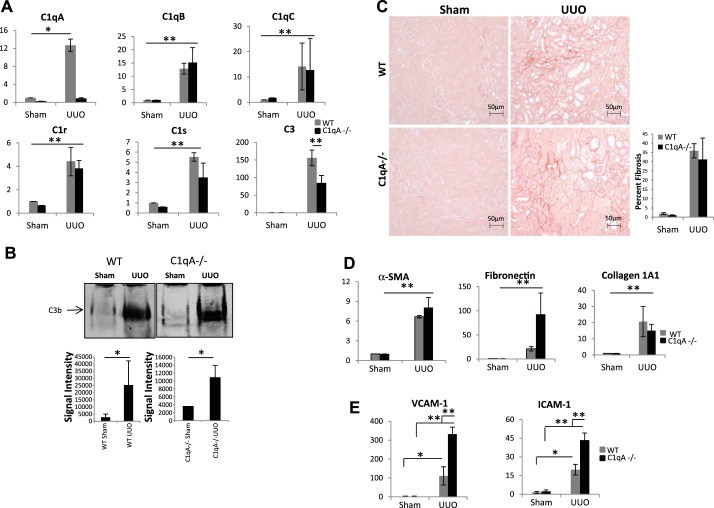
C1qA mRNA levels after UUO were increased only in wild-type but not in C1qA−/− mice; however, C1qB, C1qC, C1r, C1s, and C3 levels were persistently elevated in kidney tissue of both wild-type and C1qA-null mice after UUO (Fig. 6A). Previous studies characterizing complement activation in kidney tissue have used complement C3 antibodies that do not recognize complement C3 fragments generated upon complement activation. To address the issue of whether complement activation occurs in kidney tissue after obstructive injury in wild-type and C1q-null mice, we used in our studies rat monoclonal antibodies (clone 2/11) that preferentially react with the cleaved C3 fragments (31). These antibodies have been well characterized in previous studies (31). Western blot analysis using this antibody showed that protein levels for C3 fragments were increased to the same extent after UUO in both wild-type and C1qA−/− mice (Fig. 6B). C1qA−/− mice also showed similar levels of fibrosis as WT mice when assessed by picrosirius red staining (Fig. 6C). Fibrogenic gene expression, including α-SMA, fibronectin, and collagen 1A1, was also significantly increased after UUO in both wild-type and C1qA−/− mice, as shown in Fig. 6D. A recent study demonstrated that targeted deletion of C1q in mice alters pulmonary vascular homeostasis by augmenting the expression of endothelial adhesion markers associated with enhanced susceptibility of the lung endothelium to injury (49). To examine the potential mechanism by which fibrosis was still observed in C1qA−/− mice, we measured mRNA levels of two adhesion molecules involved in endothelial cell injury, ICAM-1 and VCAM-1. As shown in Fig. 6E, we found that transcript levels for ICAM-1 and VCAM-1 were significantly enhanced after UUO, suggesting increased endothelial damage in kidney tissue from C1qA−/− mice. Our results support the presence of complement C3 activation in kidney tissue during obstructive injury leading to kidney fibrosis, an effect that cannot be prevented solely by the use of C1q-deficient mice. Similar to the model of UUO injury, we also found lack of protection against kidney fibrosis in C1q-null mice subjected to folic acid injury (results not shown). Our results showing that increased expression of endothelial cell adhesion markers occurs in C1q-null mice during obstructive injury points toward a potential protective role of C1q on vascular endothelium. This role of C1q has been suggested to be independent from its role in classical complement activation (24).
Fig. 6.
C1qA-deficient mice are not protected from fibrosis. A: real time PCR analysis for expression of complement genes in wild-type (WT; gray bars) and C1qA−/− (black bars) mouse kidneys (sham and day 7 UUO; n = 3). B: native gel electrophoresis for C3b expression in kidney lysates from sham and day 7 UUO mice (n = 3). C: comparison of picrosirius red staining for collagen deposition between WT and C1qA−/− day 7 UUO mice and quantitation of fibrotic areas (original magnification ×200). D: quantification of mRNA levels for fibrotic genes α-SMA, fibronectin, and collagen 1A1 in WT (gray bars) and C1qA−/− (black bars) mice subjected to UUO. (n = 3) E: quantification of endothelial cell adhesion marker expression (n = 4). *P < 0.05; **P < 0.001. NS, not significant.
C3 deficiency reduces infiltration of inflammatory cells and fibrosis in obstructive and folic acid injury.
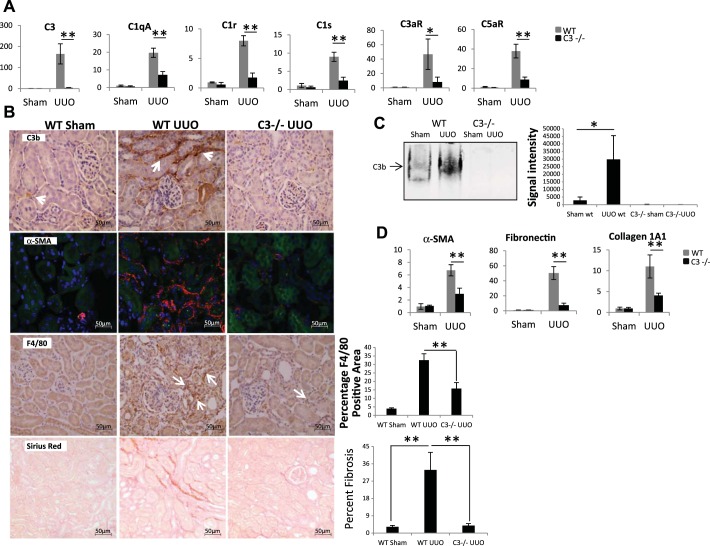
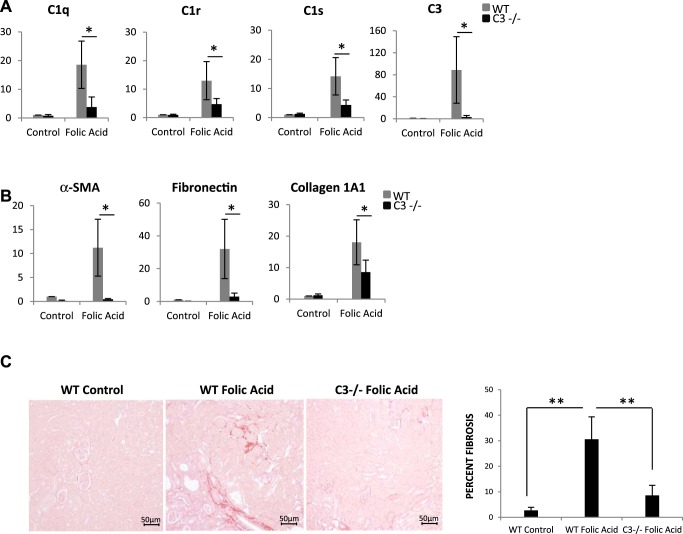
Compared with wild-type mice, C3−/− mice had reduced expression of C1qA, C1r, and C1s as well as C3a and C5a receptors (Fig. 7A). Immunohistochemistry analysis of kidney sections from wild-type and C3−/− UUO mice indicated that complement activation measured by increased C3 fragments after UUO in wild-type mice was localized predominantly in the interstitium, with no significant staining in the tubular epithelium (Fig. 7B). No staining for C3 fragments was seen in C3−/− mice subjected to UUO. Staining for α-SMA was also reduced in C3−/− UUO kidneys. Of importance is the observation that C3−/− mice had marked reduction in macrophage infiltration when compared with wild-type mice after UUO (Fig. 7B). Consistent with these observations is that reduced fibrosis was observed in UUO kidneys of C3−/− mice (Fig. 7B, bottom). Western blotting for C3 fragments showed increased C3 protein in WT UUO kidneys, which was absent in C3−/− mice (Fig. 7C). Fibrogenic gene expression after UUO in C3−/− mice was reduced compared with wild-type mice (Fig. 7D). We also examined the effect of C3 deficiency on kidney fibrosis in the folic acid injury model, and again we observed significant protection against fibrosis by C3−/− mice. There was reduced synthesis of the complement genes C1q, C1r, and C1s in C3-deficient mice (Fig. 8A) and reduced expression of fibrogenic genes, including α-SMA, fibronectin, and collagen1A1 (Fig. 8B), as well as collagen staining by picrosirius red (Fig. 8C). Our study is the first one to demonstrate increased complement activation in kidney tissue after obstructive injury using antibodies that recognize complement C3 fragments generated upon complement activation and significant protection from fibrosis in two different animal models of fibrosis when using C3-deficient mice.
Fig. 7.
C3 deficiency reduces complement expression, macrophage infiltration, and fibrosis during UUO. A: real-time PCR analysis for expression of complement genes in WT (gray bars) and C3−/− (black bars; sham and day 7 UUO) mice (n = 3). B: images showing increased staining for C3 fragments in the interstitium (white arrows) on day 7 UUO by immunohistochemistry; increased staining for α-SMA on day 7 UUO on frozen sections and increased infiltration of F4/80 cells in WT day 7 UUO mice (white arrows) when compared with WT sham and C3−/− UUO mice (histogram shows quantitation of F4/80-positive areas). Bottom: picrosirius red staining for collagen deposition in WT day 7 UUO mice (histogram shows quantitation of fibrotic areas; original magnification ×400). C: native gel electrophoresis for C3b expression in kidney lysates from sham and day 7 UUO mice (n = 3). D: real-time PCR analysis for expression of fibrotic genes in WT (gray bars) and C3−/− (black bars) mice (n = 4). *P < 0.05; **P < 0.001.
Fig. 8.
C3-deficient mice are protected from fibrosis in folic acid injury model. A: real-time PCR analysis for expression of complement genes in WT (gray bars) and C3−/− (black bars; control and folic acid-injected) mice 2 wk postinjection (n = 3). B: real-time PCR analysis for expression of fibrotic genes in WT (gray bars) and C3−/− (black bars) mice. (n = 4). C: images showing picrosirius red staining for collagen deposition in folic acid-treated mice, comparing WT control and C3−/− folic acid-treated mice (histogram shows quantitation of fibrotic areas; original magnification, ×200). *P < 0.05; **P < 0.001.
Flow cytometry shows C3 complement activation in inflammatory cells during UUO.
Our immunohistochemistry studies showed increased staining for C3 fragments in the interstitium of UUO kidneys. To understand which cell surfaces in the kidney predominantly bind active forms of C3 protein (C3b, iC3b, and C3c) we performed flow analysis of kidney cell suspension using markers for PDGFRβ, collectrin, CD45, and CD31 to identify pericytes/myofibroblasts, proximal tubular cells, immune cells, and endothelial cells, respectively. Figure 9A, top and middle, shows the plot of the positive population gated out from the live singlet population. The histograms depicting increased staining of C3 fragments in kidney cell suspension from UUO mice with clone 2–11 when compared with sham are presented in Figure 9A, bottom. We observed increased C3 fragment staining from 2.6 ± 1.3 to 53.3 ± 4.3%, which was detected predominantly in the CD45-positive cells during UUO (expressed as %live single cells). There were no significant differences in the staining of collectrin- or CD31-expressing cells (mainly endothelial cells) between sham and UUO when expressed as a fraction of live singlet cells (Fig. 9B). Given the observation by flow studies that infiltrating inflammatory CD45-positive cells contributes to complement activation by having increased staining of C3 fragments, we reasoned that this increased C3 fragment expression could represent either increased deposition of C3 fragments vs. increased synthesis. To address this question, we selectively enriched CD45-positive population from single-cell suspensions obtained from sham or UUO mice by using an anti-CD45 antibody column and then performed PCR analysis. CD45-positive cells isolated from UUO kidneys show increased expression of C1r, C1s, C3, and C5 as well as increased expression of the C3a and C5a receptors (Fig. 9C). Interestingly we did not find increased expression of α-SMA in these UUO-derived CD45-positive cells (Fig. 8C). These observations suggest that immune cells, in addition to pericytes, are a major source for complement synthesis in kidney tissue, but unlike PDGFRβ-positive pericytes, CD45-positive cells do not express significant amounts of α-SMA after UUO.
Fig. 9.
Flow cytometry analysis of C3 fragments in kidney cell suspensions of sham and day 7 UUO mice. A: gated population of live singlets positive for C3b (based on isotype control). B: C3b+ population gated for various markers, including CD45 (inflammatory cells), CD31 (endothelial cells), PDGFRβ (pericytes), and collectrin (proximal tubular cells). Bottom: histograms of the %live singlets positive for C3 and other markers (n = 3). C: real-time PCR analysis of kidney cell suspension enriched for CD45-positive population from sham and day 7 UUO mice using an anti-CD45 antibody column. This analysis shows increased mRNA levels for C1r, C1s, C3, C5, and receptors C3aR and C5aR in CD45-positive cells (n = 6). *P < 0.05. NS, not significant.
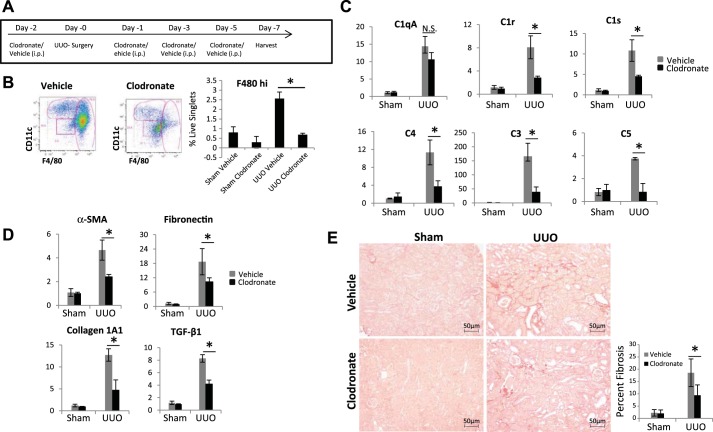
Macrophage depletion reduces complement expression and fibrosis.
Our above results suggest that during UUO, in addition to pericytes, infiltrating immune cells and more specifically macrophages contribute significantly to complement activation. To assess the functional role of infiltrating macrophages in kidney fibrosis, we depleted F480hi macrophages using clodronate. Using the strategy depicted in Fig. 10A, we show that the levels of F4/80hi population were reduced in UUO mice receiving clodronate to levels comparable with sham (Fig. 10B). Macrophage depletion by clodronate was accompanied by significant reduction in C1r and C1s expression as well as C3, C4, and C5 (Fig. 10C). The reduction in complement gene expression was associated with reduced fibrotic gene expression (Fig. 10D) and reduction in tubulointerstitial fibrosis measured by picrosirius red staining (Fig. 10E). Our results further support the pathogenic role of macrophage infiltration, with increased complement activation leading to kidney fibrosis.
Fig. 10.
Macrophage depletion protects against fibrosis. A: scheme of clodronate-mediated macrophage depletion. B: kidney cell suspension of vehicle and clodronate-treated mice gated by CD11b. CD11b-positive cells were then gated for F480 and CD11c. Histogram shows F480hi population in kidneys as %live singlet cells (n = 4). C: real-time PCR analysis for expression of complement genes in vehicle (gray bars) and clodronate-treated (black bars) mice (n = 3). D: quantification of fibrotic gene expression in vehicle (gray bars) and clodronate-treated (black bars) mice (n = 4). E: images showing picrosirius red staining for collagen deposition in vehicle and clodronate-treated mice (original magnification, ×200). Histogram shows quantitation of fibrotic areas. *P < 0.05; NS, not significant.
DISCUSSION
Increased synthesis of complement C1 components by kidney interstitial cells.
In the current study, we examine the pathogenic role of increased complement expression and activation in kidney interstitial cells during kidney fibrosis. Our studies are the first to report local synthesis and secretion of C1q by mouse kidney PDGFRβ-positive pericytes isolated from two different animal models of fibrosis. Complement component C1q (460 kDa) is the first subcomponent of the classical pathway with three polypeptide chains (A, B, and C). We find that all three C1q subunits are synthesized during kidney fibrosis by whole kidney tissue and by individual interstitial cells, including PDGFRβ-positive pericytes and CD45-positive inflammatory cells. C1q is involved in innate immunity and several modulatory processes, including pathogen clearance, apoptosis, angiogenesis, chemotaxis, autoimmunity, isotype switching, and tolerance (20, 21, 24). Based on the observation that C1q expression was increased both in kidney tissue as well as in interstitial cells during fibrosis, we were expecting that by reducing the levels of C1q alone using the C1q-null mice we would have protection from fibrosis. This was not the case, suggesting that the activation of C1 complex during fibrosis was more complex. Recent studies suggest the existence of noncomplement functions of C1q (22). For example, elevated levels of serum C1q have been observed during aging, leading to impaired regeneration of skeletal muscle and activation of the Wnt signaling pathway (33). C1q has been shown to promote permeability, proliferation, and migration of endothelial cells. C1q-deficient mice show impaired angiogenesis in a wound-healing model (3). In Alzheimer’s disease, C1q activation by brain microglia triggers amyloid plaque deposition (34). Our data showing C1q synthesis by PDGFRβ-positive pericytes and simultaneous increased Wnt signaling could represent a potential signal to trigger angiogenic responses in vivo in damaged endothelial cells in the interstitium. In future studies, the development of cell type-specific conditional deletions of C1q should help us understand better the contributions of increased C1q expression in fibrosis. With regard to the function of increased C1q expression in kidney interstitial cells isolated from fibrotic kidneys, several important conclusions can be drawn from our studies: 1) increased C1q contributes to complement activation since we also found expression of the proteases C1r and C1s as well as increased expression of C3aR and C5aR (Fig. 9C); 2) increased C1q in PDGFRβ-positive pericytes contributes to increased inflammation via increased cytokine production, and we found that PDGFRβ-positive pericytes isolated from UUO mice secrete increased amounts of IL-6, MCP-1, and MIP1-α (a previous study in endothelial cells in culture showed that exogenous administration of recombinant C1q led to increased cytokine production, including IL-6 and MCP-1 (59); this C1q-dependent cytokine production was abolished when endothelial cells were incubated with C1q in the presence of an inhibitory antibody to C1q receptor, and recent studies have begun to unravel the potential physiological role of the C1q receptor in cancer, but its role in kidney injury is currently unknown) (Fig. 2C) (9); and 3) C1q-mediated increased synthesis of cytokines by PDGFRβ-positive pericytes could further contribute to fibrosis by amplifying the interstitial inflammatory environment via recruitment of macrophages from increased MCP-1 expression.
In addition to C1q, our studies demonstrate that in wild-type mice there was increased expression of C1r and C1s in whole kidney tissue as well as in inflammatory CD45-positive cells isolated from diseased kidneys (Fig. 9C), demonstrating an important contribution to complement activation by immune cells during fibrosis. Within the C1 complex, C1r and C1s are serine proteases responsible for initiating the classical complement pathway. Flow studies of kidney cells confirmed that C1q, C1r, and C1s expression was increased in inflammatory cells isolated from UUO mice compared with sham mice (Figs. 5C and 9C). Interestingly, Ghebrehiwet et al. (8) and Hosszu et al (16) have shown that monocytes/macrophages express C1q, C1r, and C1s components on their surface and are able to initiate the classical complement cascade and regulate dendritic cell maturation. Because of the lack of reliable antibodies to localize the source of increased C1r and C1s protein expression during fibrosis in whole kidney tissue, we performed in situ hybridization studies (data not shown) and found increased expression of C1r and C1s in distal tubular epithelium in UUO mice but could not rule out increased expression in inflammatory cells. The potential mechanisms(s) involved in transcriptional activation of C1r and C1s during kidney fibrosis is not known; however, two previous studies, one in fish, documented increased transcription of C1r and C1s in kidney in response to pathogenic infections (11). A second study in mouse keratinocytes shows increased C1r expression by IFN-γ (5). Of relevance to human studies is that increased plasma levels of C1r and C1s were recently described in the plasma proteome of patients with stage 2–3 CKD and stage 5 CKD on hemodialysis. Molecular analysis done in serum samples of these patients further confirmed changes consistent with increased inflammation in CKD patients (10), lending support to our current findings showing increased expression of C1r and C1s in fibrotic kidney tissue.
Importance of detecting C3 fragments as markers of complement activation in kidney interstitial cells.
Previous studies demonstrating activation of complement have used hemolytic assays (50); however, the accumulation of C3 fragments in kidney tissue as markers of complement activation in kidney tissue during fibrosis has not been documented previously. In the present studies, we used a rat monoclonal antibody, clone 2/11, which specifically recognizes cleaved C3 fragments as a result of complement activation and breakdown of complement C3. These antibodies have been well characterized (31) and were used for our flow cytometry analysis, immunohistochemical studies, and Western blot analysis using native gels. We demonstrate clearly the accumulation of C3 fragments in whole kidney tissue homogenates in UUO model of fibrosis as well as accumulation of C3 fragments in CD45-positive inflammatory cells derived from UUO mice. Furthermore, our results using C3-deficient mice support a previous report of reduced fibrosis in these mice (67) but also extend this observation by showing that the lack of C3 expression in kidney tissue was accompanied by reduced deposition of active C3 fragments in the interstitial space as well as reduced infiltration of inflammatory cells, including macrophages. Reduction in macrophage and neutrophilic leukocyte infiltration in C3-deficient mice has been observed previously during development of arthritis in mice (15). Moreover, skin infiltration by eosinophils was also shown to be impaired in C3−/− mice in a model of skin inflammation (62). These observations support our findings that reduced macrophage infiltration plays an important role in the pathogenesis of kidney fibrosis and highlight a potential role of kidney tissue C3 in recruiting inflammatory cells at the site of injury. Although our studies did not examine the potential role of complement regulatory proteins in fibrosis, recent studies also suggest that lack of expression of either factor H or Crry can lead to widespread complement C3 activation in kidney tissue (25). Our studies showing that depletion of macrophages by clodronate reduces not only complement expression and inflammation but also fibrosis in wild-type mice further support the results of the experiments done using C3−/− mice. Bao et al. (1) used kidneys from Crry−/−/C3−/− mice and transplanted them into hosts lacking the C3aR and/or C5aR. Whereas unrestricted complement activation in the tubules was not affected by receptor status in the transplant recipient, C3aR deficiency in the recipients led to significantly reduced renal leukocyte infiltration as manifested in reduced tubulointerstitial inflammation and fibrosis. These authors concluded that manipulating C3a receptor signaling is a possible treatment to reduce inflammation and to preserve renal function.
What activates complement in kidney interstitial cells?
Our studies do not allow us to draw any definite conclusions regarding the mechanisms that led to increased expression of C1q, C1r, and C1s resulting in complement activation in whole kidney tissue; however, our results demonstrate that this increased expression of C1 components on both pericytes and immune cells is intimately linked to C3 complement activation, which is defined by increased accumulation of C3 fragments in kidney tissue during fibrosis. IgM or IgG immune complexes are the best physiological C1 activators identified to date, especially in the presence of the C1 inhibitor. Although it has been known for a long time that C1q binds to the IgG Fc domain and that activation requires multivalent binding, recent studies in this area have begun to describe the details of how the C1 complex is assembled when it is activated (7). To address the issue of immunoglobulins being the potential mechanism for increased complement activation during fibrosis, we performed experiments using the UUO model in Rag1−/− mice that are deficient in immunoglobulins. We did not observe any differences in complement expression or activation during fibrosis, suggesting that the level of immunoglobulins does not affect complement activation during fibrosis. In addition, we performed experiments in isolated PDGFRβ-positive pericytes isolated from sham mice, and we did not see any effect on complement expression levels when these cells were stimulated with TGFβ, indicating that cellular mechanisms other than TGFβ could account for increased complement expression in UUO mice. We postulate that the increased generation of pathogen-associated or danger-associated molecular patters in the interstitium, as well as an increased inflammatory environment, is likely to contribute to complement activation during kidney fibrosis.
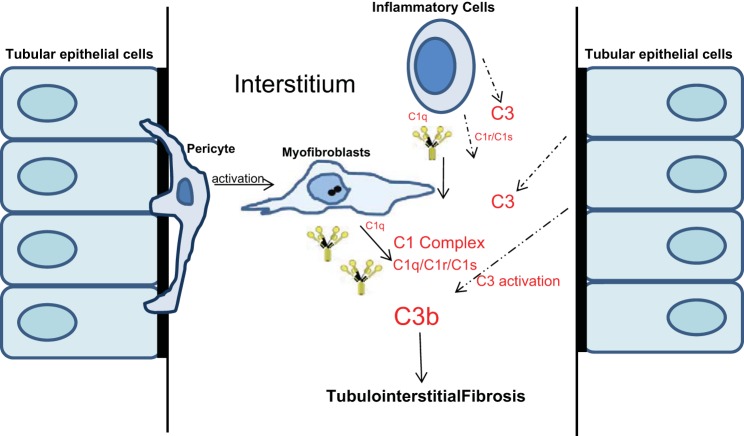
Many of the biological functions of anaphylatoxins C3a and C5a generated during kidney fibrosis are mediated through activation of their receptors C3aR and C5aR. Our studies also demonstrate increased expression of anaphylatoxin receptors C3aR and C5aR in whole kidney tissue as well as in CD45-positive cells (Figs. 7A and 9C). In contrast to our findings, a previous study found C5aR expression to be upregulated in tubular cells during UUO injury and when blocked with an antagonist had minimal effects on reducing renal macrophage influx (2) while affording significant protection against fibrosis. Most recently, in a hypertensive model, expression of C5aR1 on infiltrating and resident renal immune cells was demonstrated, and the loss of C5aR1 did not change the numbers of infiltrating immune cells in the course of hypertension while exhibiting less renal injury (60). Another study by Peng et al. (41) also shows that deficiency of either or both of the receptors protects mice from IRI injury accompanied with the reduced infiltration of immune cells and suggests that C3aR and C5aR on both renal and circulating leukocytes contributes to the pathogenesis of renal ischemia-reperfusion injury. At the moment, we do not know the cellular mechanism(s) that leads to increased expression of C3aR or C5aR expression in kidney interstitial cells but likely depends on the inflammatory environment present during fibrosis. Previous studies also suggest the presence of epigenetic mechanisms at the level of C3 promoter that could contribute to increased transcriptional response of C3 (17, 42). We propose a model by which complement activation could contribute to interstitial fibrosis (Fig. 11). C1q produced by myofibroblasts and immune cells and C1r and C1s produced by immune and epithelial cells allow the formation of the C1 complex in the interstitium. This in turn contributes to complement activation with the breakdown of C3 by a convertase and increased generation of C3 fragments in interstitial cells, which further exacerbates the process of extracellular matrix accumulation and fibrosis. Tubular epithelium could potentially contribute with increased expression of C3a and C5a and activation of C3aR and C5aR.
Fig. 11.
Interstitial cell types that contribute to complement expression and activation during renal fibrosis.
In summary, complement activation during fibrosis offers ample opportunity for targeted therapeutic modulation. Although only two complement-targeted drugs have been used in clinical studies, the therapeutic anti-C5 antibody eculizumab and various preparations of the C1 esterase inhibitor C1-INH, several new candidate drugs targeting various components of the cascade are currently being developed. Among them is a peptide inhibitor, Compstatin, that selectively binds to native C3 and its bioactive fragments C3b and iC3b/C3c, thus blocking C3 activation by all convertases. Future studies aimed at examining the function of complement receptors in kidney interstitial cells as well as the potential mechanisms by which tubular epithelium contributes to inflammation and fibrosis may allow for the identification of well-defined targets to ameliorate progressive kidney disease.
GRANTS
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (RO1-DK-75976) and a Veterans Affairs Merit Award (to D. Portilla).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.X., R.K.S., S.G.L., S.A., J.M., and D.P. performed experiments; S.X., R.K.S., S.G.L., and D.P. analyzed data; S.X., R.K.S., R.P.T., J.S.D., and D.P. interpreted results of experiments; S.X., R.K.S., S.G.L., and D.P. prepared figures; S.X., R.K.S., and D.P. drafted manuscript; S.X., R.K.S., J.Y., R.P.T., W.B.S., J.S.D., and D.P. edited and revised manuscript; S.X., R.K.S., S.G.L., J.Y., R.P.T., S.A., J.M., W.B.S., J.S.D., E.S.R., J.D.L., and D.P. approved final version of manuscript; D.P. conceived and designed research.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Center for Immunity, Inflammation, and Regenerative Medicine at University of Virginia (UVA) for their support during this study. The UVA Flow Cytometry Facility for sample analysis using the Luminex MAGPIX System and cell separation using AutoMACS Pro Separator is gratefully acknowledged. We thank Dr. Marina Botto (Imperial College School of Medicine, London, UK) and Dr. Andrea J. Tenner (University of California at Irvine, Irvine, CA) for the C1qA−/− mice.
REFERENCES
- 1.Bao L, Wang Y, Haas M, Quigg RJ. Distinct roles for C3a and C5a in complement-induced tubulointerstitial injury. Kidney Int 80: 524–534, 2011. doi: 10.1038/ki.2011.158. [DOI] [PubMed] [Google Scholar]
- 2.Boor P, Konieczny A, Villa L, Schult AL, Bücher E, Rong S, Kunter U, van Roeyen CR, Polakowski T, Hawlisch H, Hillebrandt S, Lammert F, Eitner F, Floege J, Ostendorf T. Complement C5 mediates experimental tubulointerstitial fibrosis. J Am Soc Nephrol 18: 1508–1515, 2007. doi: 10.1681/ASN.2006121343. [DOI] [PubMed] [Google Scholar]
- 3.Bossi F, Tripodo C, Rizzi L, Bulla R, Agostinis C, Guarnotta C, Munaut C, Baldassarre G, Papa G, Zorzet S, Ghebrehiwet B, Ling GS, Botto M, Tedesco F. C1q as a unique player in angiogenesis with therapeutic implication in wound healing. Proc Natl Acad Sci U S A 111: 4209–4214, 2014. doi: 10.1073/pnas.1311968111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrum SD, Larson SK, Avaritt NL, Moreland LE, Mackintosh SG, Cheung WL, Tackett AJ. Quantitative proteomics identifies activation of hallmark pathways of cancer in patient melanoma. J Proteomics Bioinform 6: 43–50, 2013. doi: 10.4172/jpb.1000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byun SJ, Jeon IS, Lee H, Kim TY. IFN-gamma upregulates expression of the mouse complement C1rA gene in keratinocytes via IFN-regulatory factor-1. J Invest Dermatol 127: 1187–1196, 2007. doi: 10.1038/sj.jid.5700660. [DOI] [PubMed] [Google Scholar]
- 6.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 7.Gaboriaud C, Ling WL, Thielens NM, Bally I, Rossi V. Deciphering the fine details of c1 assembly and activation mechanisms: “mission impossible”? Front Immunol 5: 565, 2014. doi: 10.3389/fimmu.2014.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghebrehiwet B, Hosszu KK, Valentino A, Ji Y, Peerschke EI. Monocyte expressed macromolecular C1 and C1q receptors as molecular sensors of danger: implications in SLE. Front Immunol 5: 278, 2014. doi: 10.3389/fimmu.2014.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghebrehiwet B, Kaplan AP, Joseph K, Peerschke EI. The complement and contact activation systems: partnership in pathogenesis beyond angioedema. Immunol Rev 274: 281–289, 2016. doi: 10.1111/imr.12469. [DOI] [PubMed] [Google Scholar]
- 10.Glorieux G, Mullen W, Duranton F, Filip S, Gayrard N, Husi H, Schepers E, Neirynck N, Schanstra JP, Jankowski J, Mischak H, Argilés À, Vanholder R, Vlahou A, Klein J. New insights in molecular mechanisms involved in chronic kidney disease using high-resolution plasma proteome analysis. Nephrol Dial Transplant 30: 1842–1852, 2015. doi: 10.1093/ndt/gfv254. [DOI] [PubMed] [Google Scholar]
- 11.Godahewa GI, Bathige SD, Herath HM, Noh JK, Lee J. Characterization of rock bream (Oplegnathus fasciatus) complement components C1r and C1s in terms of molecular aspects, genomic modulation, and immune responsive transcriptional profiles following bacterial and viral pathogen exposure. Fish Shellfish Immunol 46: 656–668, 2015. doi: 10.1016/j.fsi.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 12.Gomez IG, Duffield JS. The FOXD1 lineage of kidney perivascular cells and myofibroblasts: functions and responses to injury. Kidney Int Suppl (2011) 4: 26–33, 2014. doi: 10.1038/kisup.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez IG, MacKenna DA, Johnson BG, Kaimal V, Roach AM, Ren S, Nakagawa N, Xin C, Newitt R, Pandya S, Xia TH, Liu X, Borza DB, Grafals M, Shankland SJ, Himmelfarb J, Portilla D, Liu S, Chau BN, Duffield JS. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J Clin Invest 125: 141–156, 2015. doi: 10.1172/JCI75852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris CL, Heurich M, Rodriguez de Cordoba S, Morgan BP. The complotype: dictating risk for inflammation and infection. Trends Immunol 33: 513–521, 2012. doi: 10.1016/j.it.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hietala MA, Nandakumar KS, Persson L, Fahlén S, Holmdahl R, Pekna M. Complement activation by both classical and alternative pathways is critical for the effector phase of arthritis. Eur J Immunol 34: 1208–1216, 2004. doi: 10.1002/eji.200424895. [DOI] [PubMed] [Google Scholar]
- 16.Hosszu KK, Valentino A, Ji Y, Matkovic M, Pednekar L, Rehage N, Tumma N, Peerschke EI, Ghebrehiwet B. Cell surface expression and function of the macromolecular c1 complex on the surface of human monocytes. Front Immunol 3: 38, 2012. doi: 10.3389/fimmu.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang N, Tan L, Xue Z, Cang J, Wang H. Reduction of DNA hydroxymethylation in the mouse kidney insulted by ischemia reperfusion. Biochem Biophys Res Commun 422: 697–702, 2012. doi: 10.1016/j.bbrc.2012.05.061. [DOI] [PubMed] [Google Scholar]
- 18.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, Park AS, Tao J, Sharma K, Pullman J, Bottinger EP, Goldberg IJ, Susztak K. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med 21: 37–46, 2015. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kishore U, Gaboriaud C, Waters P, Shrive AK, Greenhough TJ, Reid KB, Sim RB, Arlaud GJ. C1q and tumor necrosis factor superfamily: modularity and versatility. Trends Immunol 25: 551–561, 2004. doi: 10.1016/j.it.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Kishore U, Ghai R, Greenhough TJ, Shrive AK, Bonifati DM, Gadjeva MG, Waters P, Kojouharova MS, Chakraborty T, Agrawal A. Structural and functional anatomy of the globular domain of complement protein C1q. Immunol Lett 95: 113–128, 2004. doi: 10.1016/j.imlet.2004.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kishore U, Thielens NM, Gaboriaud C. Editorial: state-of-the-art research on C1q and the classical complement pathway. Front Immunol 7: 398, 2016. doi: 10.3389/fimmu.2016.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolev M, Le Friec G, Kemper C. Complement—tapping into new sites and effector systems. Nat Rev Immunol 14: 811–820, 2014. doi: 10.1038/nri3761. [DOI] [PubMed] [Google Scholar]
- 24.Kouser L, Madhukaran SP, Shastri A, Saraon A, Ferluga J, Al-Mozaini M, Kishore U. Emerging and novel functions of complement protein C1q. Front Immunol 6: 317, 2015. doi: 10.3389/fimmu.2015.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laskowski J, Renner B, Le Quintrec M, Panzer S, Hannan JP, Ljubanovic D, Ruseva MM, Borza DB, Antonioli AH, Pickering MC, Holers VM, Thurman JM. Distinct roles for the complement regulators factor H and Crry in protection of the kidney from injury. Kidney Int 90: 109–122, 2016. doi: 10.1016/j.kint.2016.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leelahavanichkul A, Yan Q, Hu X, Eisner C, Huang Y, Chen R, Mizel D, Zhou H, Wright EC, Kopp JB, Schnermann J, Yuen PS, Star RA. Angiotensin II overcomes strain-dependent resistance of rapid CKD progression in a new remnant kidney mouse model. Kidney Int 78: 1136–1153, 2010. doi: 10.1038/ki.2010.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin SL, Chang FC, Schrimpf C, Chen YT, Wu CF, Wu VC, Chiang WC, Kuhnert F, Kuo CJ, Chen YM, Wu KD, Tsai TJ, Duffield JS. Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol 178: 911–923, 2011. doi: 10.1016/j.ajpath.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 173: 1617–1627, 2008. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A 107: 4194–4199, 2010. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maarouf OH, Ikeda Y, Humphreys BD. Wnt signaling in kidney tubulointerstitium during disease. Histol Histopathol 30: 163–171, 2015. doi: 10.14670/HH-30.163. [DOI] [PubMed] [Google Scholar]
- 31.Mastellos D, Prechl J, László G, Papp K, Oláh E, Argyropoulos E, Franchini S, Tudoran R, Markiewski M, Lambris JD, Erdei A. Novel monoclonal antibodies against mouse C3 interfering with complement activation: description of fine specificity and applications to various immunoassays. Mol Immunol 40: 1213–1221, 2004. doi: 10.1016/j.molimm.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 32.Naik A, Sharma S, Quigg RJ. Complement regulation in renal disease models. Semin Nephrol 33: 575–585, 2013. doi: 10.1016/j.semnephrol.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naito AT, Sumida T, Nomura S, Liu ML, Higo T, Nakagawa A, Okada K, Sakai T, Hashimoto A, Hara Y, Shimizu I, Zhu W, Toko H, Katada A, Akazawa H, Oka T, Lee JK, Minamino T, Nagai T, Walsh K, Kikuchi A, Matsumoto M, Botto M, Shiojima I, Komuro I. Complement C1q activates canonical Wnt signaling and promotes aging-related phenotypes. Cell 149: 1298–1313, 2012. doi: 10.1016/j.cell.2012.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nayak A, Ferluga J, Tsolaki AG, Kishore U. The non-classical functions of the classical complement pathway recognition subcomponent C1q. Immunol Lett 131: 139–150, 2010. doi: 10.1016/j.imlet.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Neelisetty S, Alford C, Reynolds K, Woodbury L, Nlandu-Khodo S, Yang H, Fogo AB, Hao CM, Harris RC, Zent R, Gewin L. Renal fibrosis is not reduced by blocking transforming growth factor-β signaling in matrix-producing interstitial cells. Kidney Int 88: 503–514, 2015. doi: 10.1038/ki.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noone D, Licht C. Chronic kidney disease: a new look at pathogenetic mechanisms and treatment options. Pediatr Nephrol 29: 779–792, 2014. doi: 10.1007/s00467-013-2436-5. [DOI] [PubMed] [Google Scholar]
- 37.Noone D, Waters A, Pluthero FG, Geary DF, Kirschfink M, Zipfel PF, Licht C. Successful treatment of DEAP-HUS with eculizumab. Pediatr Nephrol 29: 841–851, 2014. doi: 10.1007/s00467-013-2654-x. [DOI] [PubMed] [Google Scholar]
- 38.Noris M, Remuzzi G. Overview of complement activation and regulation. Semin Nephrol 33: 479–492, 2013. doi: 10.1016/j.semnephrol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn 222: 218–227, 2001. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- 40.Park P, Haas M, Cunningham PN, Alexander JJ, Bao L, Guthridge JM, Kraus DM, Holers VM, Quigg RJ. Inhibiting the complement system does not reduce injury in renal ischemia reperfusion. J Am Soc Nephrol 12: 1383–1390, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Peng Q, Li K, Smyth LA, Xing G, Wang N, Meader L, Lu B, Sacks SH, Zhou W. C3a and C5a promote renal ischemia-reperfusion injury. J Am Soc Nephrol 23: 1474–1485, 2012. doi: 10.1681/ASN.2011111072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pratt JR, Parker MD, Affleck LJ, Corps C, Hostert L, Michalak E, Lodge JP. Ischemic epigenetics and the transplanted kidney. Transplant Proc 38: 3344–3346, 2006. doi: 10.1016/j.transproceed.2006.10.112. [DOI] [PubMed] [Google Scholar]
- 43.Ren S, Johnson BG, Kida Y, Ip C, Davidson KC, Lin SL, Kobayashi A, Lang RA, Hadjantonakis AK, Moon RT, Duffield JS. LRP-6 is a coreceptor for multiple fibrogenic signaling pathways in pericytes and myofibroblasts that are inhibited by DKK-1. Proc Natl Acad Sci U S A 110: 1440–1445, 2013. doi: 10.1073/pnas.1211179110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol 11: 785–797, 2010. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: therapeutic interventions. J Immunol 190: 3839–3847, 2013. doi: 10.4049/jimmunol.1203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ricklin D, Reis ES, Lambris JD. Complement in disease: a defence system turning offensive. Nat Rev Nephrol 12: 383–401, 2016. doi: 10.1038/nrneph.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schrimpf C, Teebken OE, Wilhelmi M, Duffield JS. The role of pericyte detachment in vascular rarefaction. J Vasc Res 51: 247–258, 2014. doi: 10.1159/000365149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schrimpf C, Xin C, Campanholle G, Gill SE, Stallcup W, Lin SL, Davis GE, Gharib SA, Humphreys BD, Duffield JS. Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J Am Soc Nephrol 23: 868–883, 2012. doi: 10.1681/ASN.2011080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah D, Romero F, Zhu Y, Duong M, Sun J, Walsh K, Summer R. C1q deficiency promotes pulmonary vascular inflammation and enhances the susceptibility of the lung endothelium to injury. J Biol Chem 290: 29642–29651, 2015. doi: 10.1074/jbc.M115.690784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharp JA, Hair PS, Pallera HK, Kumar PS, Mauriello CT, Nyalwidhe JO, Phelps CA, Park D, Thielens NM, Pascal SM, Chen W, Duffy DM, Lattanzio FA, Cunnion KM, Krishna NK. Peptide inhibitor of complement C1 (PIC1) rapidly inhibits complement activation after intravascular injection in rats. PLoS One 10: e0132446, 2015. doi: 10.1371/journal.pone.0132446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sim RB, Schwaeble W, Fujita T. Complement research in the 18th–21st centuries: progress comes with new technology. Immunobiology 221: 1037–1045, 2016. doi: 10.1016/j.imbio.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 52.Stallons LJ, Whitaker RM, Schnellmann RG. Suppressed mitochondrial biogenesis in folic acid-induced acute kidney injury and early fibrosis. Toxicol Lett 224: 326–332, 2014. doi: 10.1016/j.toxlet.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun YB, Qu X, Caruana G, Li J. The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differentiation 92: 102–107, 2016. doi: 10.1016/j.diff.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 54.Tampe D, Zeisberg M. Potential approaches to reverse or repair renal fibrosis. Nat Rev Nephrol 10: 226–237, 2014. doi: 10.1038/nrneph.2014.14. [DOI] [PubMed] [Google Scholar]
- 55.Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. J Immunol 176: 1305–1310, 2006. doi: 10.4049/jimmunol.176.3.1305. [DOI] [PubMed] [Google Scholar]
- 56.Thurman JM, Ljubanovic D, Edelstein CL, Gilkeson GS, Holers VM. Lack of a functional alternative complement pathway ameliorates ischemic acute renal failure in mice. J Immunol 170: 1517–1523, 2003. doi: 10.4049/jimmunol.170.3.1517. [DOI] [PubMed] [Google Scholar]
- 57.Thurman JM, Ljubanović D, Royer PA, Kraus DM, Molina H, Barry NP, Proctor G, Levi M, Holers VM. Altered renal tubular expression of the complement inhibitor Crry permits complement activation after ischemia/reperfusion. J Clin Invest 116: 357–368, 2006. doi: 10.1172/JCI24521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thurman JM, Nester CM. All things complement. Clin J Am Soc Nephrol 11: 1856–1866 2016. doi: 10.2215/CJN.01710216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van den Berg RH, Faber-Krol MC, Sim RB, Daha MR. The first subcomponent of complement, C1q, triggers the production of IL-8, IL-6, and monocyte chemoattractant peptide-1 by human umbilical vein endothelial cells. J Immunol 161: 6924–6930, 1998. [PubMed] [Google Scholar]
- 60.Weiss S, Rosendahl A, Czesla D, Meyer-Schwesinger C, Stahl RA, Ehmke H, Kurts C, Zipfel PF, Köhl J, Wenzel UO. The complement receptor C5aR1 contributes to renal damage but protects the heart in angiotensin II-induced hypertension. Am J Physiol Renal Physiol 310: F1356–F1365, 2016. doi: 10.1152/ajprenal.00040.2016. [DOI] [PubMed] [Google Scholar]
- 61.Xiao L, Zhou D, Tan RJ, Fu H, Zhou L, Hou FF, Liu Y. Sustained activation of Wnt/β-catenin signaling drives AKI to CKD progression. J Am Soc Nephrol 27: 1727–1740, 2016. doi: 10.1681/ASN.2015040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yalcindag A, He R, Laouini D, Alenius H, Carroll M, Oettgen HC, Geha RS. The complement component C3 plays a critical role in both Th1 and Th2 responses to antigen. J Allergy Clin Immunol 117: 1455–1461, 2006. doi: 10.1016/j.jaci.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 63.Zhou D, Tan RJ, Fu H, Liu Y. Wnt/β-catenin signaling in kidney injury and repair: a double-edged sword. Lab Invest 96: 156–167, 2016. doi: 10.1038/labinvest.2015.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou D, Tian Y, Sun L, Zhou L, Xiao L, Tan RJ, Tian J, Fu H, Hou FF, Liu Y. Matrix metalloproteinase-7 is a urinary biomarker and pathogenic mediator of kidney fibrosis. J Am Soc Nephrol 28: 598–611, 2017. doi: 10.1681/ASN.2016030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou W, Farrar CA, Abe K, Pratt JR, Marsh JE, Wang Y, Stahl GL, Sacks SH. Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest 105: 1363–1371, 2000. doi: 10.1172/JCI8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou W, Marsh JE, Sacks SH. Intrarenal synthesis of complement. Kidney Int 59: 1227–1235, 2001. doi: 10.1046/j.1523-1755.2001.0590041227.x. [DOI] [PubMed] [Google Scholar]
- 67.Zhou X, Fukuda N, Matsuda H, Endo M, Wang X, Saito K, Ueno T, Matsumoto T, Matsumoto K, Soma M, Kobayashi N, Nishiyama A. Complement 3 activates the renal renin-angiotensin system by induction of epithelial-to-mesenchymal transition of the nephrotubulus in mice. Am J Physiol Renal Physiol 305: F957–F967, 2013. doi: 10.1152/ajprenal.00344.2013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.