The mind takes years to develop yet can be undone in a matter of hours. Few experiences can be more frightening than watching a loved one disintegrate in a hospital room, either quietly retreating into themselves or becoming aggressive and agitated, unaware of who or where they are. Delirium is defined clinically as a “disturbance of attention,” (1) but in the moment, to the individuals involved, it can seem cataclysmic—a dramatic rending of identity.
Delirium often arises during periods of sickness, which has led researchers to an intuitive belief that there must be a connection with inflammatory states. Yet historically it has been difficult to make any overt connections, in large part because this intuition conflicted with a bedrock theory of the field: for almost a century, the brain has been understood to have a special—and protected—relationship with the immune system. Seminal studies from the 1920s demonstrated that embryonic tissue can be transplanted into the brain without being rejected. This paved the way to discovering how the brain is protected by a near-impermeable blood-brain barrier. This barrier, formed by tight junctions between endothelial cells and astrocytes, prevents most substances from the blood—including immune cells and large macromolecules— from reaching the brain. The brain was deemed “immune privileged”—a separate territory in the body, like a university patrolled by its own security force (microglia) within a city with a larger police force (circulating leukocytes).
However, a strict idea of immune privilege (i.e., that the brain is insulated and protected from the body any time it gets sick) conflicts with the lived, personal experience telling us that some connection must exist. Our behaviors change when we get sick: we sleep more, we may not eat, and we tend to isolate ourselves. We also know from clinical experience that the administration of pegylated interferon, a peripherally produced cytokine, potently induces major depression in patients who receive this treatment for hepatitis C. The peripheral immune system therefore must still be able to signal information to the brain.
Work in the last 15 years has begun to further refine our understanding of the connections between the peripheral immune system and the brain and on the unique role that immune system proteins in the brain play, creating a new understanding of what it means to be immune privileged. Only a few pathways have been identified through which peripheral inflammation can directly impact brain function (Figure 1). One is through activation of the vagus nerve, which itself can detect locally produced cytokines and stimulate primitive areas of the brain, including the nucleus tractus solitarius and lateral medulla. Through projections, these vagal afferents influence firing in the parabrachial nucleus and in limbic regions, including centers in the hypothalamus and amygdala (2). Another pathway is through activation of resident brain immune cells that can detect circulating cytokines in brain regions that are exposed to the periphery, such as in the choroid plexus and circumventricular organs (3). More recently, it has been found that the meninges possess a functional lymphatic system and that cerebrospinal fluid comes into contact with peripheral immune cells in the deep cervical nodes, offering yet another potential point of communication (4). Each of these points of contact between the brain and the peripheral immune system could potentially serve as a link between peripheral inflammation and the development of psychiatric disease.
Figure 1.
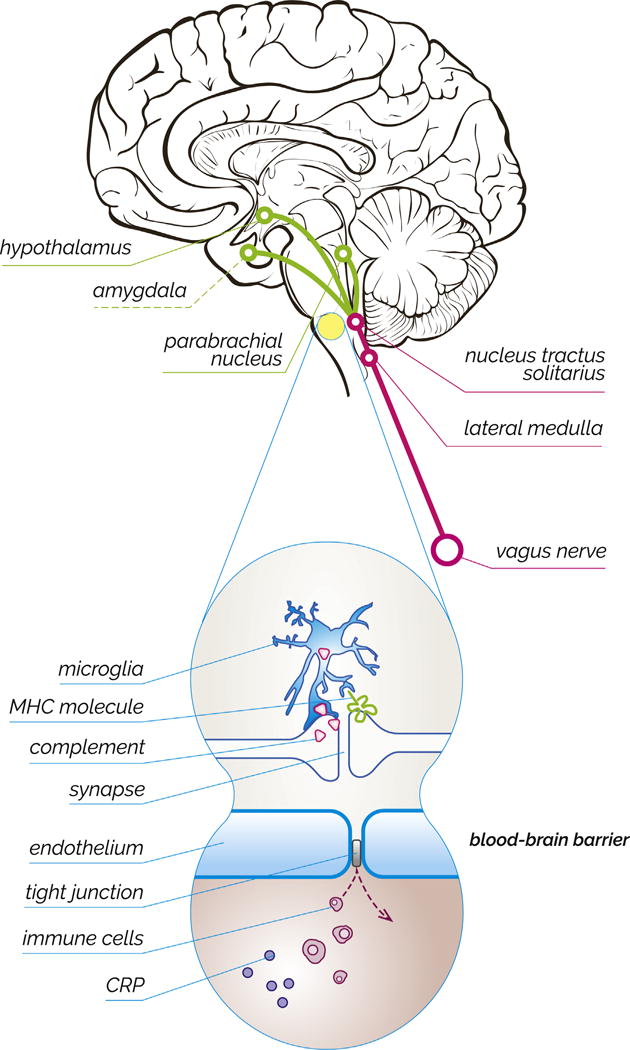
Evolving ideas about the brain’s immune privilege. (Top) Schematic drawing illustrating how peripheral inflammation can impact brain regions involved in psychiatric disease. Cytokine release can trigger activation of the vagus nerve, which in turn sends relays to limbic centers, including nuclei within the hypothalamus and amygdala. (Bottom) The blood-brain barrier ordinarily prevents immune cells and immune signaling molecules like C-reactive protein (CRP) from reaching the brain. Neurons require protection from the peripheral immune system in part because they utilize immune signaling proteins, such as major histocompatibility complex (MHC) and complement, for homeostatic processes.
The article by Dillon et al. (5) in this issue of Biological Psychiatry is an example of a study that offers yet another mechanism for connection between peripheral immune mediators and brain dysfunction to offer a hypothesis about the pathogenesis of delirium. The authors show a correlation between levels of C-reactive protein (CRP) in the blood and the risk of developing delirium. CRP is an acute phase reactant that is released by the liver into the blood soon after the development of an infection or bodily injury and can be used as a proxy for inflammation. Under healthy conditions, CRP cannot penetrate the blood-brain barrier, but during periods of high inflammation, there are reports that CRP, along with other cytokines, can weaken and even cross the barrier (1). Several large-scale, prospective, longitudinal studies have shown a correlation between higher levels of inflammatory markers like CRP and the development of neuropsychiatric disease, like depression (6). The article by Dillon et al. builds strongly on these findings: the authors are the first group to prospectively use both pre- and postoperative serum measurements of CRP to show that having higher levels of CRP before entering surgery can increase a patient’s risk of developing delirium. They argue that CRP might be useful as a biomarker to predict which patients are most at risk for developing delirium and, therefore, might best be targeted with prophylactic interventions.
The studies described above have opened researchers’ eyes to other lines of inquiry that challenge the historic idea of immune privilege. In contrast to the idea of the brain being isolated, we are now discovering a range of complex and nuanced connections between the central and peripheral immune systems. For example, one of the bedrock principles of the immune privilege dogma was that neurons, unlike almost every other cell type in the body, were thought not to express major histocompatibility complex (MHC) proteins. In the body, MHC proteins are central players in the adaptive immune system and allow peripheral cells to signal “self” and “nonself” antigens to circulating leukocytes; they serve as little antennae that project whatever is inside the cell to the outside, thereby alerting immune cells when a pathogen is present. In the past decade, despite considerable initial resistance to the idea, Shatz (7) and others have overturned old dogma. Through a series of elegant experiments, they have shown that neurons do indeed express MHC proteins, particularly during periods of brain development. In the brain, however, MHC proteins function differently than they do in the peripheral immune system. They partner with different signaling molecules and on different cell types. While their role is not yet fully understood, MHC proteins appear able to signal the presence of unwanted synapses and to initiate their elimination. They also seem able to influence synaptic plasticity (7).
The complement system is another example of an immune system toolkit that has recently been discovered to play a different role in neurons. The complement system forms a critical part of the innate immune system in the periphery. Through a series of chain reactions, complement proteins cleave each other, become activated, and allow the immune system to target pathogens for destruction. Stephan et al. (8) and others have shown that these same proteins are also expressed by neurons, but rather than serve as weapons against foreign pathogens, these proteins serve as flags or markers for unwanted synapses that are then engulfed by microglia. Dysregulation of complement-mediated synapse elimination may play a role in the pathogenesis of diseases like schizophrenia (9).
These discoveries that neurons repurpose proteins used by the immune system for their own use have reinvigorated research into microglia, the brain’s resident immune cell (10). For many years, the purpose of microglia, the predominant immune cell in the brain, was thought to be limited to the elimination of those rare pathogens that manage to invade the central nervous system. In recent years, however, we have begun to learn about how these cells play a fundamental role in the everyday sculpting of synaptic connections between neurons in the brain. Microglia are likely the effector cell that “reads” these repurposed immune proteins in neurons and helps prune unwanted synaptic connections.
The discovery of the fundamental importance that repurposed immune proteins play in the normal functioning of the nervous system may help explain the need for immune privilege in the first place. If neurons use complement and MHC proteins for their own ends, which are distinct from the uses these molecules have in the periphery, then they may need a barrier to protect them from a peripheral immune cell that could “misread” the neuron’s signals and cause damage. Indeed, these new findings offer novel hypotheses for how brain function might go awry in psychiatric disease.
We can now see that the interplay between the peripheral immune system and immune regulation of the brain is far more complicated than traditionally imagined. While the brain may be “privileged” from cellular aspects of the peripheral immune response, there are multiple pathways through which peripheral inflammation may affect brain function. Moreover, it is increasingly clear that the brain may leverage the same protein building blocks used in the peripheral immune system but for very different purposes—including to guide the strengthening and pruning of synapses. As work in this exciting field continues to grow, we should expect to learn much more about the complex interactions between peripheral and central immune systems and the role of these systems in health and disease. Which is to say: we will continue to gain better clarity on the true nature of immune privilege and a better understanding of how it can be that when the body gets sick, the brain may follow close behind.
Acknowledgments
This work was supported by a collaboration with the National Neuroscience Curriculum Initiative, which receives support from the National Institutes of Health (Grant Nos. R25 MH10107602S1 and R25 MH086466 07S1).
We thank Dr. David Ross for his contributions as National Neuroscience Curriculum Initiative editor and Amanda Wang for her role in developing Figure 1.
Footnotes
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Maldonado JR. Neuropathogenesis of delirium: Review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21:1190–1222. doi: 10.1016/j.jagp.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quan N, Whiteside M, Herkenham M. Time course and localization patterns of interleukin-1beta messenger RNA expression in brain and pituitary after peripheral administration of lipopolysaccharide. Neuroscience. 1998;83:281–293. doi: 10.1016/s0306-4522(97)00350-3. [DOI] [PubMed] [Google Scholar]
- 4.Louveau A, Harris TH, Kipnis J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol. 2015;36:569–577. doi: 10.1016/j.it.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dillon ST, Vasunilashom SM, Ngo L, Otu HH, Inouye SK, Jones RN, et al. Higher C-reactive protein levels predict postoperative delirium in older patients undergoing major elective surgery: A longitudinal nested case-control study. Biol Psychiatry. 2017;81:145–153. doi: 10.1016/j.biopsych.2016.03.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M. Cumulative meta-analysis of interleukins 6 and 1 beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shatz CJ. MHC class I: An unexpected role in neuronal plasticity. Neuron. 2009;64:40–45. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephan AH, Barres BA, Stevens B. The complement system: An unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- 9.Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dwyer JB, Ross DA. Modern microglia: Novel targets in psychiatric neuroscience. Biol Psychiatry. 2016;80:e47–e49. doi: 10.1016/j.biopsych.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


