In depression this faith in deliverance, in ultimate restoration, is absent. The pain is unrelenting, and what makes the condition intolerable is the foreknowledge that no remedy will come—not in a day, an hour, a month, or a minute. If there is mild relief, one knows that it is only temporary; more pain will follow. It is hopelessness even more than pain that crushes the soul.
— William Styron, Darkness Visible: A Memoir of Madness
In his memoir, Styron (1) describes hopelessness as the subjective core of depression. He introduces his experience of depression on a rainy street in Paris in 1985, where he is on the way to receive a prestigious award. Despite expectations of objective success, his mood is despairing and he feels that he will never return to Paris again. As he identifies his malady and seeks treatment, he laments the lack of progress in the study of depression, which has “yielded its secrets far more reluctantly than any of the other major ills.” Styron is disheartened by the lack of a biological explanation for his disorder and by the limited treatment options available at the time. The first selective serotonin reuptake inhibitor, fluoxetine, was introduced during the years following Styron’s episode, ushering in a new era of pharmacological treatments for depression based on the monoaminergic hypothesis. Yet Styron’s critique remains trenchant to the many patients who will not respond to current treatments and in the lack of a cohesive circuit-based model integrating behavioral and learning perspectives on depression. But at that same moment in time, outside of the spotlight of attention, researchers began to ascribe a role for the activation of a tiny brain region, the lateral habenula, in the symptoms Styron experienced (2).
Most clinicians have probably never heard of the habenula. It is a tiny region in the diencephalon, wedged between the stalk of the pineal gland and the thalamus, and it is typically described as having medial and lateral subregions. The lateral habenula, in particular, is notable for receiving inputs from the basal ganglia and limbic system and projecting broadly to dopaminergic and serotonergic neurons in the midbrain. During the past 20 years, a number of studies—as reviewed by Boulos and colleagues in this issue (3)—are increasingly suggesting a novel, circuit-based paradigm through which the habenula may be causally involved in the onset of depression and other psychiatric illnesses.
To understand the model, one needs to first appreciate some basic concepts relating to reward processing. Imagine a rat, walking through a maze for the first time, hoping to find food. When the rat finds food, dopamine neurons in the ventral tegmental area (VTA) have a burst of firing, releasing dopamine in the striatum and cortex. On subsequent runs through the same maze, a very different pattern emerges. As the rat becomes increasingly confident of where the reward will be, dopamine is released in conjunction with cues that will predict where and when the reward will be found rather than as a single burst when the rat finds the actual food (4). Two key points emerge from these studies. First, the dopamine burst can be seen not as indicating reward per se but rather as a learning signal about the environment (what just happened was important and good—remember how you got here!). Second, as the dopamine firing shifts forward to the predictive cues, the signal can again be seen to reflect not the reward itself but rather the expectation of future rewards.
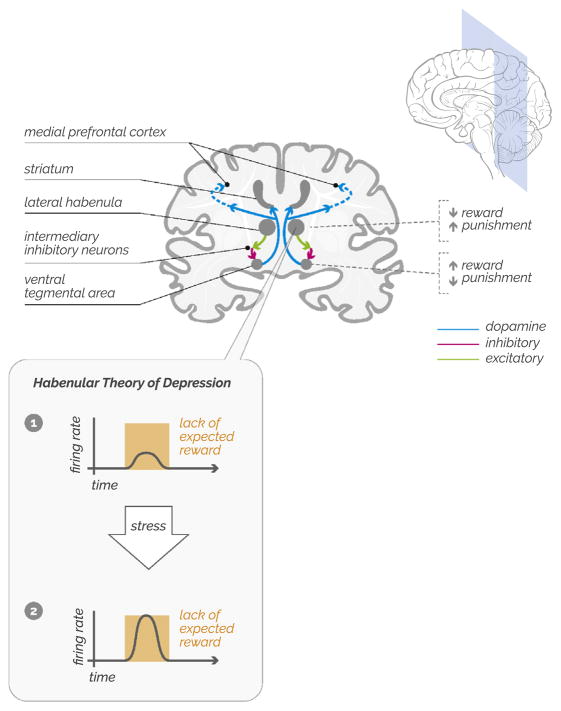
Consider, now, a different scenario. After the rat has learned the maze, it returns to the same space, eager to claim its reward—only to discover that no food is present. In the same way that the VTA previously signaled the presence of an unexpected reward, now the lateral habenula fires—thereby signaling an unexpected setback (5). The firing of the lateral habenula activates intermediary inhibitory neurons that then suppress the VTA dopamine neurons (Figure 1). In this model, the lateral habenula is like Eeyore from A. A. Milne’s classic Winnie-the-Pooh stories—a vocal pessimist attuned to any possibility of misfortune.
Figure 1.
Ventral tegmental area dopamine neurons convey learning signals related to unexpected rewards throughout the brain, with strong connections to the striatum and medial prefrontal cortex. Conversely, the lateral habenula signals the failure to receive an expected reward by sending excitatory inputs to the rostromedial tegmentum, which in turn suppresses ventral tegmental area dopamine neurons. The habenular model of depression is that chronic stress can lead to increased sensitivity of cells in the lateral habenula, thereby leading to more frequent signaling of disappointment or failure.
This basic computational skill—the ability to update expectations about the world based on setbacks and rewards—reflects a critical survival mechanism integral to an individual’s ability to adapt to a changing environment. This also reflects an especially interesting piece of neuroscience history because the concept of reward prediction error (RPE) was described in the literature on reinforcement learning long before a potential mechanism was identified in the brain. As described above, data now indicate that positive RPE (unexpected reward) may be signaled by the VTA and negative RPE (failure to receive an expected reward) may be signaled by the lateral habenula.
What we have described so far relates largely to individual events—what impact would this type of reinforcement learning framework have on behavior over a longer period of time? Recent work by Niv and colleagues (6) addresses this question. They start from a compellingly straightforward hypothesis: that the average RPE—whether the animal has tended to have more surprises or setbacks lately—could function as something akin to a mood. This quantity provides a useful signal to an individual about the state of the environment as a whole (e.g., allowing an animal to choose to hibernate when there is little value in searching for food). Intrinsic to this model is also the idea that mood, in turn, can alter the perception of new experiences by biasing expectations.
A potential mechanism for depression is immediately apparent: if there was an increased tendency of cells in the lateral habenula to fire, individuals would perceive the world in a systematically negative way. Moreover, animal studies highlight a dangerous vulnerability: stress itself can induce plasticity and learning such that there is a strengthening of inputs to lateral habenula neurons and a corresponding increased reactivity of these cells—essentially, the neurons learn to expect disappointment and thereby signal it more readily (7). Supporting this possibility in humans, both functional magnetic resonance imaging and postmortem anatomical studies have shown abnormalities in the lateral habenula in depression (8,9). A tantalizing case study offered more direct support. An individual with severe depression was treated with a deep brain stimulator that disrupted lateral habenula firing, and the patient experienced a remission of symptoms. Following an accident that led to the device’s being shut off, the patient’s depression returned—and then remitted again when the device was turned back on (10). If validated further, targeted manipulation of the lateral habenula could allow testing of a circuit neuroscience theory of depression.
Styron offered a literary theory of depression in which the core of the disorder was a persistent negative affective state marked by hopelessness regarding future improvements. The great irony of Styron’s work is that as he bemoaned the lack of scientific progress, he was himself offering an elegant computational model of depression. Excessive firing of a region that signals negative reward prediction error would lead to endless disappointment and hopelessness, where reward does not exist and events are always worse than they are expected to be. Recent research on plasticity in the lateral habenula and in the systems that encode reward and failure offers a possible mechanism for Styron’s model. This emerging model of depression raises the possibility that circuit-based interventions in the habenula may offer new therapeutic options for patients with treatment-resistant depression.
Acknowledgments
This clinical commentary was produced in collaboration with the National Neuroscience Curriculum Initiative.
We thank Amanda Wang for her role in developing Figure 1.
AK reports no biomedical financial interests or potential conflicts of interest. DAR, as cochair of the National Neuroscience Curriculum Initiative, receives support from the National Institutes of Health (R25 MH10107602S1 and R25 MH086466 07S1).
Footnotes
Disclosures
DAR reported no other biomedical financial interests or potential conflicts of interest.
References
- 1.Styron W. Darkness Visible: A Memoir of Madness. New York: Vintage; 1992. [Google Scholar]
- 2.Caldecott-Hazard S, Mazziotta J, Phelps M. Cerebral correlates of depressed behavior in rats, visualized using 14C-2-deoxyglucose autoradiography. J Neurosci. 1988;8:1951–1961. doi: 10.1523/JNEUROSCI.08-06-01951.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulos L-J, Darcq E, Kieffer BL. Translating the habenula—From rodents to humans. Biol Psychiatry. 2017;81:296–305. doi: 10.1016/j.biopsych.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howe MW, Tierney PL, Sandberg SG, Phillips PEM, Graybiel AM. Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. Nature. 2013;500:575–579. doi: 10.1038/nature12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 6.Eldar E, Rutledge RB, Dolan RJ, Niv Y. Mood as representation of momentum. Trends Cogn Sci. 2016;20:15–24. doi: 10.1016/j.tics.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furman DJ, Gotlib IH. Habenula responses to potential and actual loss in major depression: Preliminary evidence for lateralized dysfunction. Soc Cogn Affect Neurosci. 2016;11:843–851. doi: 10.1093/scan/nsw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ranft K, Dobrowolny H, Krell D, Bielau H, Bogerts B, Bernstein H-G. Evidence for structural abnormalities of the human habenular complex in affective disorders but not in schizophrenia. Psychol Med. 2010;40:557–567. doi: 10.1017/S0033291709990821. [DOI] [PubMed] [Google Scholar]
- 10.Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]