In recent years, there has been an explosion of literature published in the area of the microbiome on health and disease. The microbiome has even entered the lexicon of the general public. In 2015, the American Museum of Natural History mounted an exhibition titled “The Secret World Inside You” that aimed to teach children and adults about the importance of the microbiome. Fecal transplantation in the treatment of Clostridium difficile infection has been widely reported in the popular media. There are already online businesses that aim to analyze one’s gut microbiota to formulate a personalized diet. But what do we really know about the microbiome? And what role does it play in psychiatry?
To start with, terminology: microbiome refers to all the microorganisms living in and on the body, or the genome of these microorganisms. On the other hand, microbiota refers to the specific population of microorganisms living in a certain part of the body, such as the gut. These two terms are often used interchangeably. Prebiotics are nondigestible fibers that promote beneficial growth of microorganisms, such as Lacto-bacillus and Bifidobacterium (1). In contrast, probiotics are microorganisms that are administered to directly colonize an organ system. The number of microbes living in the gastrointestinal (GI) tract is ten times the number of human cells in the body, and the total microbial genetic material is 100 times that of the entire human genome (2). In fact, the gut micro-biome has been referred to as the “second genome” of the human body.
A fascinating aspect of the microbiome is its dynamic nature across the lifespan. It was long believed that the colonization of one’s gut microbiota occurs at birth as a neonate passes through the birth canal. However, there is now evidence that the gut microbiota may begin even earlier, as both placenta and meconium have been found to have their own microbiomes, even in premature births (2). The gut microbiota ordinarily reaches maximum diversity during adolescence and then the diversity remains relatively stable until the later stages of life. The decrease in the diversity of the microbiota in older adults may predispose them to conditions such as C. difficile infection. Of course, the microbiome is also impacted by a range of external influences, including diet, antibiotic exposure, and other environmental factors. A major animal model that has been used to study the relative importance of environmental factors on the development of the microbiome—and its impact on all related systems—is raising animals in a germ-free environment, thereby cutting them off from any source of colonizing bacteria. This paradigm allows for close exploration of differences in gene expression, physiology, and myriad other biologic parameters.
As far as healthy functioning, it is not surprising that the gut microbiota has been found to play a key role in a range of normal enteric physiological processes, including digestion, motility, epithelial integrity, and the production of vitamins. Perhaps less intuitively, the gut microbiota has been shown to affect the immune and endocrine systems, and disruption of the gut microbiota has been found in various conditions, including allergies, functional bowel disorders, asthma, obesity, and diabetes (2).
The idea of a connection between gut and brain function is surprisingly old, dating at least to the work of William Beaumont, a U.S. Army surgeon, who wrote about the association between mood and gastric secretions in a patient with a gastrocutaneous fistula in the 1830s (3). Until relatively recently, most work on the gut-brain axis reflected a “top-down” model, with the brain controlling the gut via autonomic systems and hormones. Consistent with this idea, research has shown that the brain can modulate the gut microbiota via catecholamines, serotonin, gamma-aminobutyric acid, and other molecules. More recently, a host of studies have shown that gut-brain connectivity is in fact bidirectional, and these studies are beginning to elucidate the complex ways in which the gut microbiota may affect brain function (Figure 1).
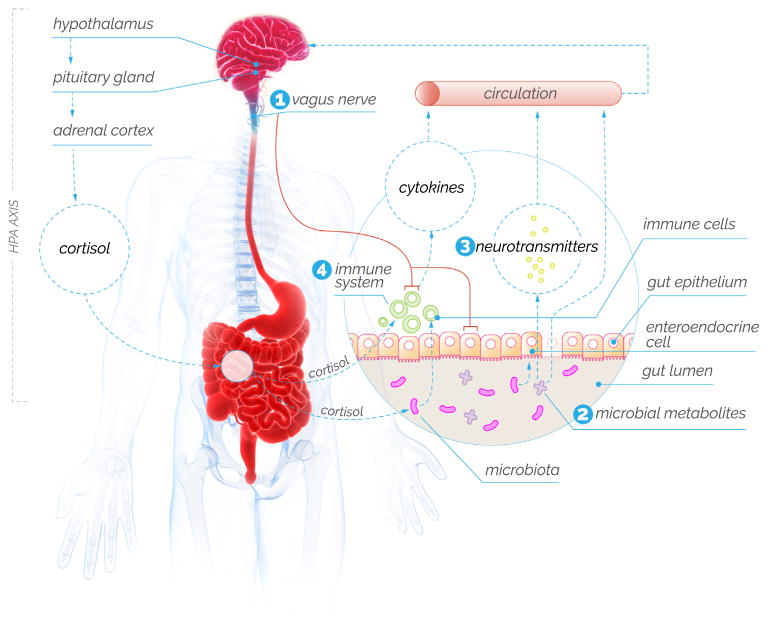
Figure 1.
The gut-brain axis. Gut microbiota communicates with the brain by different mechanisms, including (1) direct activation of the vagus nerve; (2) by producing molecules, such as short-chain fatty acids, that may cause gut epithelium to be “leaky” and then act on a range of other systems; (3) by directly producing or altering the synthesis of neurotransmitters, including serotonin, and; (4) activating immune signaling pathways, including the release of cytokines and other inflammatory molecules. Stress, as signaled via the hypothalamic-pituitary-adrenal (HPA) axis, is one top-down mechanism that may affect gut microbiota. [Based on data from Cryan and Dinan (10).]
One key pathway through which the gut microbiota may affect brain function is modulation of the autonomic nervous system, including via activation of the vagus nerve (4). In one study, mice treated with Lactobacillus rhamnosus produced lower stress-induced corticosterone and exhibited fewer anxiety- and depression-related behaviors. However, in vagotomized mice, anxiety- and depression-related behaviors were not reduced when treated with L. rhamnosus. While gut bacteria can affect vagal firing, the mechanism through which this occurs is not entirely understood.
Another pathway through which the gut microbiota may affect brain function is via microbial signaling molecules, including short-chain fatty acids (SCFAs) that are produced by gut microorganisms (1). Changes in the gut microbiota may cause gut epithelium to become “leaky,” allowing more of these molecules to cross into the blood stream. SCFAs have been shown to inhibit histone deacetylase, an important enzyme involved in epigenetic regulation (including of the hypothalamic-pituitary-adrenal axis), and activate some G protein–coupled receptors in the brain (1). Furthermore, these molecules may also activate inflammatory processes.
The microbiota may also influence the brain through neurotransmitters and neuropeptides. For example, in healthy individuals, the gut microbiota has been shown to facilitate the metabolism of tryptophan by enteroendocrine cells into its metabolites, kynurenine and serotonin (5). This process may occur via SCFAs increasing tryptophan hydroxylase expression (1). However, other microbiota metabolites may be involved, and there is also evidence of de novo production of serotonin by bacteria, at least in culture (5). Of interest, germ-free mice have been shown to have higher levels of tryptophan and lower levels of serotonin when compared to control mice, suggesting impaired conversion in this population.
The gut microbiota can also affect the brain through the immune system. This may occur via the production and release of cytokines (such as interleukins-6 and -1β) by immune cells in the GI system (1). Data from germ-free mice indicate that the gut microbiota also plays a crucial role in regulating microglia in the brain, though the mechanism is unclear. In one study, microglia development was partially restored in germ-free mice when they were colonized with microorganisms (6).
Given the many complex ways in which the microbiome can affect brain function, it is not surprising that data have linked dysregulation of this system to various psychiatric illnesses. In this regard, one of the most interesting lines of inquiry is a series of studies of autism spectrum disorder (ASD). ASD is a neurodevelopment disorder that is characterized by social and communication impairment and is often accompanied by behavioral symptoms, repetitive behaviors, GI disturbance, and immune dysfunction. Despite its prevalence—and the significant burden to patients, families, and society—the pathogenesis of ASD has remained largely a mystery. Moreover, because the underlying neurobiology has remained so elusive, current treatments focus largely on symptom management rather than addressing any core underlying biological process.
In this issue of Biological Psychiatry, Vuong and Hsiao (7) review a fascinating range of findings indicating that gut microbiota dysbiosis may be associated with various aspects of ASD—and in ways that reflect the known pathways through which the microbiome may influence brain development and functioning. A core part of the story comes from animal models: rats and mice raised in a germ-free environment have decreased social functioning; additionally, and of particular interest, these impaired social behaviors may be reversed by treating the animals with probiotics or colonizing them with microbes. In humans, differences in both the gut microbiota and in SCFAs, which may serve as a signaling pathway, have been shown between individuals with and without ASD. Presence of Clostridium tetani within the microbiome has been associated with impaired social behaviors in animals; in one study, children with ASD treated with antibiotics targeting Clostridium were reported to have decreased stereotyped behaviors (8). And, of course, given the microbiota’s role in GI and immune function, it is easy to see why individuals with ASD may have a high incidence of abnormalities in these systems.
Critically, this model sets the stage for additional research that could potentially lead to much-needed treatments for ASD. Thus far, case studies, open-label trials, and small randomized trials in humans have been promising. Larger randomized controlled trials are ongoing. For example, one group in Italy is investigating the effects of probiotics on GI symptoms, ASD severity, affective and behavioral symptoms, and biomarkers of GI functions in preschool children (9). While early data are encouraging, Vuong and Hsiao caution that the use of Lactobacillus reuteri has not yet been validated as a safe and effective treatment in humans.
ASD is only one of many clinical areas in which modulation of the gut microbiota holds promising clinical application. Parkinson’s disease, depression, anxiety, and schizophrenia are among the many neuropsychiatric conditions for which the microbiota may play a key role in future treatments. Given the limitations of our current armament of psychotropic medications—including inadequate efficacy and the prevalence of significant side effects —the possibility of relatively low-risk but potentially effective microbiome-modulating treatments is tantalizing.
Acknowledgments
Dr. Ross, as co-chair of the National Neuroscience Curriculum Initiative (NNCI), receives support from the National Institutes of Health (Grant Nos. R25 MH10107602S1 and R25 MH086466 07S1).
This commentary was produced in collaboration with the NNCI.
We thank Ashley Walker for her contribution as NNCI editor and Amanda Wang for her role in developing the figure.
Footnotes
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Sherwin E, Sandhu KV, Dinan TG, Cryan JF. May the force be with you: The light and dark sides of the microbiota–gut–brain axis in neuropsychiatry. CNS Drugs. 2016;30:1019–1041. doi: 10.1007/s40263-016-0370-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slattery J, MacFabe DF, Frye RE. The significance of the enteric microbiome on the development of childhood disease: A review of prebiotic and probiotic therapies in disorders of childhood. Clin Med Insights Pediatr. 2016;10:91–107. doi: 10.4137/CMPed.S38338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaumont W. Experiments and observations on the gastric juice and the physiology of digestion. Plattsburg: F.P. Allen; 1833. [DOI] [PubMed] [Google Scholar]
- 4.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignaca HM, Dinan TC, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;162:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erny DD, de Angelis LA, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vuong HE, Hsiao EY. Emerging roles for the gut microbiome in autism spectrum disorder. Biol Psychiatry. 2017;81:411–423. doi: 10.1016/j.biopsych.2016.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolte ER. Autism and Clostrium tetani. Med Hypotheses. 1998;51:133–144. doi: 10.1016/s0306-9877(98)90107-4. [DOI] [PubMed] [Google Scholar]
- 9.Santocchi E, Guiducci L, Fulceri F, Billeci L, Buzzigoli E, Apicella F, et al. Gut to brain interaction in autism spectrum disorders: A randomized controlled trial on the role of probiotics on clinical, biochemical and neurophysiological parameters. BMC Psychiatry. 2016;16:183–199. doi: 10.1186/s12888-016-0887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cryan JJ, Dinan TG. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]