Abstract
Objectives:
This study aimed to evaluate the hypoglycemic effects of an ethanol extract of Cassia abbreviata (ECA) bark and the possible mechanisms of its action in diabetic albino rats.
Methods:
ECA was prepared by soaking the powdered plant material in 70% ethanol. It was filtered and made solvent-free by evaporation on a rotary evaporator. Type 2 diabetes was induced in albino rats by injecting 35 mg/kg body weight (bw) of streptozotocin after having fed the rats a high-fat diet for 2 weeks. Diabetic rats were divided into ECA-150, ECA-300 and Metformin (MET)-180 groups, where the numbers are the doses in mg.kg.bw administered to the groups. Normal (NC) and diabetic (DC) controls were given distilled water. The animals had their fasting blood glucose levels and body weights determined every 7 days for 21 days. Oral glucose tolerance tests (OGTTs) were carried out in all animals at the beginning and the end of the experiment. Liver and kidney samples were harvested for glucose 6 phosphatase (G6Pase) and hexokinase activity analyses. Small intestines and diaphragms from normal rats were used for α-glucosidase and glucose uptake studies against the extract.
Results:
Two doses, 150 and 300 mg/kg bw, significantly reduced the fasting blood glucose levels in diabetic rats and helped them maintain normal body weights. The glucose level in DC rats significantly increased while their body weights decreased. The 150 mg/kg bw dose significantly increased hexokinase and decreased G6Pase activities in the liver and the kidneys. ECA inhibited α-glucosidase activity and promoted glucose uptake in the rats’ hemi-diaphragms.
Conclusion:
This study revealed that ECA normalized blood glucose levels and body weights in type 2 diabetic rats. The normalization of the glucose levels may possibly be due to inhibition of α-glucosidase, decreased G6Pase activity, increased hexokinase activity and improved glucose uptake by muscle tissues.
Keywords: α-glucosidase, blood glucose, diabetes mellitus, glucose 6 phosphatase, hexokinase, oral glucose tolerance
1. Introduction
Diabetes mellitus is characterized by chronic hyperglycemia, which may be caused by defects in either insulin secretion by the pancreas or insulin action in target tissues like skeletal muscles. The World Health Organization (WHO) projects that by the year 2030, the global prevalence of diabetes mellitus will have increased to about 366 million people [1]. Therefore, in the fight against type 2 diabetes mellitus, which accounts for about 95% of global diabetic cases, various drugs like sulphonylureas, thiazolidinediones and α-enzyme inhibitors are being used in the management of diabetes [2]. Even though such drugs are clinically used, they are associated with side effects like weight gain, fluid retention in the body, liver toxicity and increased frequency of heart failure [3, 4]. Therefore, a need exists for extensive research to identify bioactive compounds from natural products that can be used in the management of diabetes with holistic effects and very minimal or no side effects.
For the above reasons, pharmaceutical research into anti-diabetic drugs has recently diverted to natural products for either complementary medicine or isolation of bioactive compounds that can be developed into standard drugs. Because botanicals are widely used across many countries, evaluating their anti-diabetic potentials and their toxicity is of paramount importance [5]. Cassia abbreviata (C. abbreviata), known in Setswana as Monepenepe [6], is a plant belonging to the family Caesalpiniaceae and is widely distributed across Southern Africa. The plant has a brown bark, yellowish compound leaves, and long, cylindrical, dark-brown hanging pods [7]. It is extensively used in traditional medicine for the management of diabetes and other diseases [8], and the plant extract was reported to inhibit α-glucosidase activity [9]. Although the plant is used for the management of diabetes, pharmacological findings supporting the safety of its use are lacking. Therefore, the present study was planned with an aim to establish the hypoglycemic effects of C. abbreviata in diabetic albino rats and its possible mechanism of action.
2. Materials and Methods
All chemicals used were purchased from Sigma-Aldrich (USA). Glucose oxidase kits were purchased from Agappe Diagnostics (India). All chemicals and reagents used were of analytical grade. The stem bark of C. abbreviata was collected from Maitengwe village and was authenticated at the Department of Biological Sciences, University of Botswana. The bark was cut into small pieces, air-dried and ground to powder. The powder was soaked in 70% ethanol for 72 hours at 25ºC. The mixture was filtrated using Whatman 0.45-μm filter paper and concentrated in a rotary evaporator to obtain a crude ethanolic extract.
The study started with in-vitro studies. In the first experiment, we investigated the effect of different concentrations of an ethanol extract of C. abbreviata (ECA) on crude α-glucosidase from rat intestines according to the method described in Ref [10]. Studies of the enzyme kinetics were also conducted to determine the kinetic constants for and the types of inhibition employed by ECA. The effect of ECA on glucose uptake by muscle tissues was also investigated using the hemi-diaphragms of the rats according to the method proposed in Ref [11]. The amount of glucose absorbed per gram tissue was calculated as the difference between the initial and the final concentrations of glucose in the medium containing Tyrode’s solution.
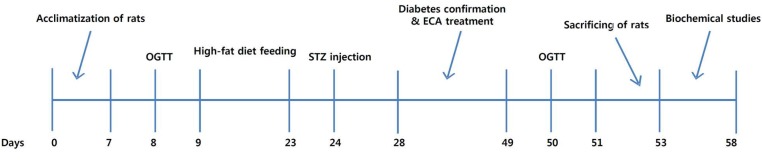
For the in-vivo studies based on the timeline in Fig. 1, male Sprague-Dawley (SD) albino rats with weights from 180 to 210 g were obtained from the University of Botswana’s animal house. They were then housed in the animal house with a 12-h/12-h dark-light cycle and a temperature of 25 ± 2ºC. They were fed normal rodent pellets and distilled water ad libitum. Type 2 diabetes mellitus was induced by feeding the animals a high-fat diet for 14 days, followed by an intra-peritoneal injection of 35 mg/kg body weight (bw) of streptozotocin (Sigma Aldrich) dissolved in freshly prepared 0.1-M citrate buffer (pH 4.5). The diet was prepared by mixing pure white sugar, bovine body fat and powdered normal rat pellets in the ratio 10:16:17, respectively. Animals were closely monitored, and on the 3rd day, blood was drawn from their tails after overnight fasting; their blood glucose levels were determined using a glucometer (Accu-Check Active). Rats with fasting blood glucose levels exceeding 125 mg/dL were considered diabetic and used for the experiment. The experimental protocol was approved by the Institutional Animal Ethics Committee (UB/ RES/ACUC/001).
Fig. 1. Timeline for the in-vivo experiments.
OGTT, oral glucose tolerance tests; ECA, extract of Cassia abbreviata.
Animals were randomly divided into five groups with five animals per group. Group 1 (normal control, NC) received only distilled water. Group 2 (diabetic control, DC) received only the vehicle (distilled water). Group 3 (Met-180) was a diabetic group which received 180 mg/kg bw of metformin (standard drug). Groups 4 (ECA-150) and 5 (ECA-300) received 150 and 300 mg/kg bw of ECA, respectively. Animals were dosed by using oral gavage for 21 days. Their fasting blood glucose levels and body weights were determined at seven-day intervals for 21 days. Oral glucose tolerance tests (OGTTs) were conducted at the beginning and towards the end of the experiment. The glucose levels of rats that had been fasted overnight were monitored at 30-minutes intervals for 3 hours after the animals had been fed a 2 g/kg bw glucose solution.
After the experimental period, animals were sacrificed under diethyl ether to collect the kidneys and the livers for biochemical analyses. The activity of hepatic hexokinase was determined spectrophotometrically at 340 nm in liver homogenates according to the method described in Ref [12], and the enzyme activity was expressed as μmoL/mg/min. Determination of the glucose-6-phosphatase (G6Pase) activity was based on a modified method at 700 nm [12] in a Shimadzu UV-Vis spectrophotometer, and the enzyme activity was expressed as μmoL of inorganic phosphate liberated/mg of protein/minute.
The data obtained were expressed as means ± standard errors of the means (SEMs). The significance of the differences in the means was analyzed using an analysis of variance (ANOVA) with Sigma plot version 11. The level of statistical significance was set as P < 0.05.
3. Results
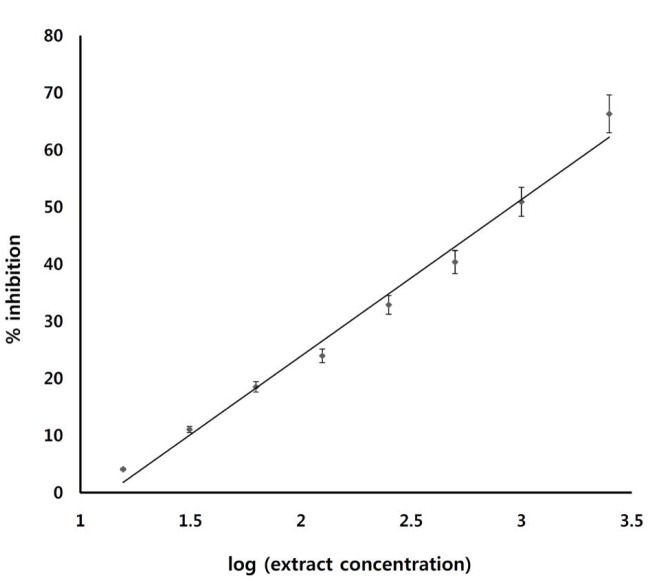
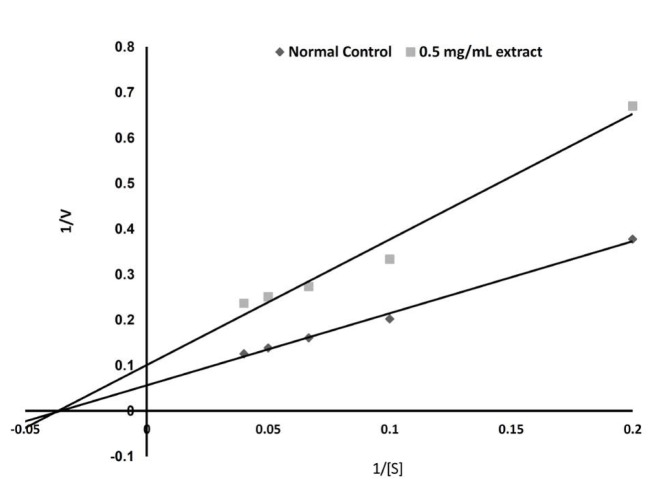
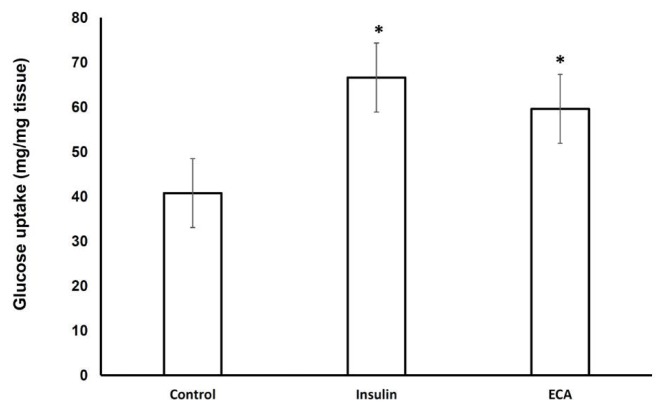
The effect of ECA on α-glucosidase was investigated. As Fig. 2 shows, ECA inhibited the activity of sucrase (α-glucosidase enzyme) in a dose-dependent manner and with an inhibitory concentration giving 50% inhibition (IC50) of 1.4 mg/mL. Fig. 3 and Table 1 show the inhibition of α-glucosidase by ECA. The Michaelis constant (Km) value remained unchanged at 2.86 (mg/mL)-1 while the maximal velocity (Vmax) value was reduced from 16.7 mg/mL/s in the control sample (absence of ECA) to 10.1 mg/mL/s in the presence of ECA. The effect of ECA on the glucose uptake by the isolated hemi-diaphragms of the rats is shown in Fig. 4. Compared to the normal control, the uptake of glucose by the hemi-diaphragms was significantly (P < 0.05) increased by insulin and ECA. However, no significant (P < 0.05) difference was observed between the insulin dose and the ECA dose used.
Fig. 2. Effect of ECA on the activity of the intestinal α-glucosidase in normal rats. Results are represented as means ± SEMs.
ECA, extract of Cassia abbreviata; SEMs, standard errors of the means.
Fig. 3. Type of inhibition exerted by ECA on the activity of intestinal α-glucosidase in NC rats.
ECA, extract of Cassia abbreviata; NC, normal control, [S], substrate concentration.
Table. 1. Kinetic constants determined from the Lineweaver-Burk plot.
| K (mg/mL)-1 | Vmax (mg.mL-1·s-1) | |
|---|---|---|
| Control (without ECA) | 2.86 | 16.7 |
| Experimental (0.5 mg/mL ECA) | 2.86 | 10.1 |
ECA, extract of Cassia abbreviata.
Fig. 4. Effect of ECA on glucose uptake by the hemi-diaphragms of the rats. Results are represented as means (n = 3) + SEMs, and *P < 0.05 in comparison with the normal control.
ECA, extract of Cassia abbreviata; SEMs, standard errors of the means.
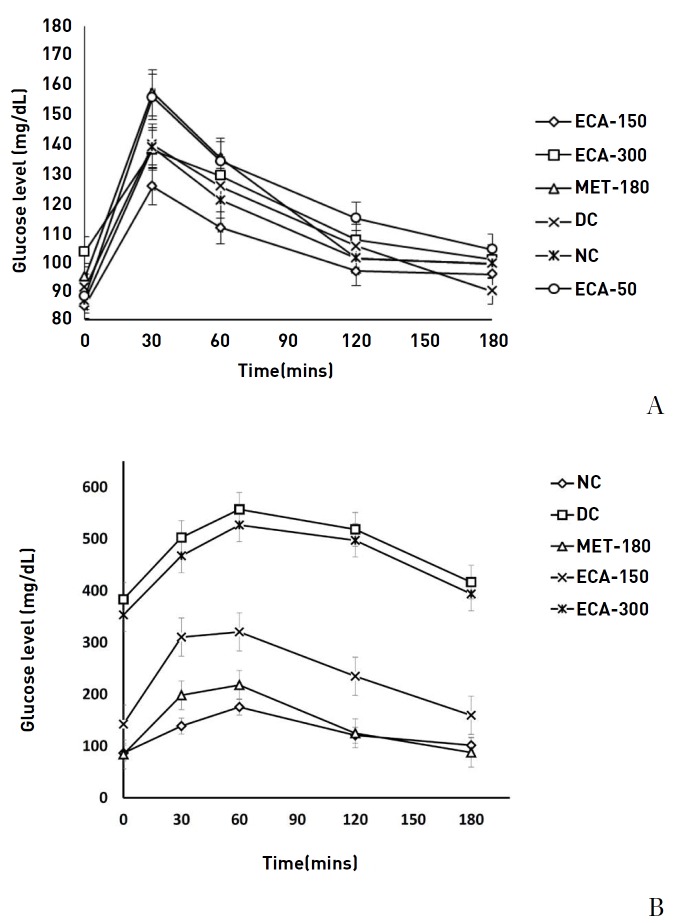
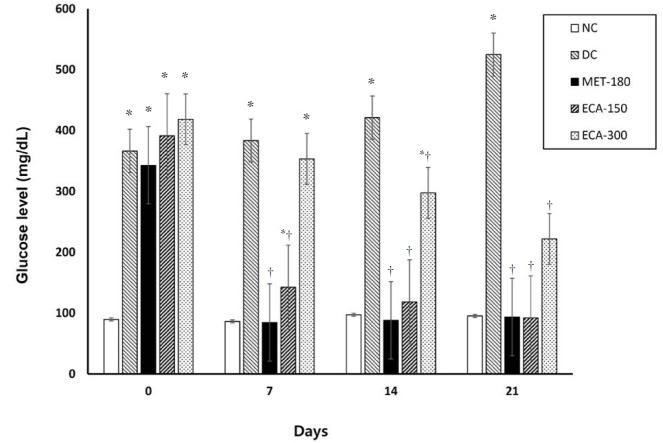
Across all groups, prior to induction of diabetes, the highest blood glucose levels occurred at 30 minutes (Fig. 5) whereas after 21 days of diabetes and treatment, the highest glucose levels occurred mostly at 60 minutes (Fig. 5). Results show that the used doses of ECA had an effect on the OGTT curves. Glucose normalization was observed after 3 hours. Although the glucose levels were reduced after 3 hours, significant and quicker recovery was observed in the NC, ECA-150, ECA-300 and Met-180 groups. The DC and the ECA-50 groups showed slow recovery phases. Moreover, after 21 days of diabetes and treatment, the glucose levels in the DC and the ECA-300 groups remained abnormally high, even after 3 hours. The effects of ECA on the fasting blood glucose levels after 21 days of treatment in diabetic rats are presented in Fig. 6. Results show that ECA significantly reduced the glucose levels in Group 4 (ECA-150) and Group 5 (ECA-300) (P < 0.05) as compared to the results in the DC group. Metformin also showed similar effects. No significant differences (P < 0.05) were observed between the glucose levels of the metformin-treated rats, the ECA-150 treated rats, and the NC rats. The effect of ECA on body weight is presented in Table 2 and shows that the body weights increased significantly (P < 0.05) for the rats in the NC, Met-180 and ECA-150 groups. However, the body weights decreased significantly (P < 0.05) for the rats in the DC group. No significant weight increases were observed for the rats in the ECA-300 group.
Fig. 5. Effect of ECA on the results of the oral glucose tolerance tests (A) before induction of diabetes and (B) after 21 days of diabetes and various treatments. Results are represented as means ± SEMs.
ECA, extract of Cassia abbreviata; MET, metformin; DC, diabetic; NC, normal; SEMs, standard errors of the means.
Fig. 6. Effect of ECA on fasting blood glucose levels. Results are represented as means ± SEMs. (*) and (†) indicate statistical significance (P < 0.05) in comparison with the NC and the DC, respectively.
NC, normal; DC, diabetic; MET, metformin; ECA, extract of Cassia abbreviata; SEMs, standard errors of the means.
Table. 2. Effect of ECA on the body weights of the rats in the experimental groups.
| Group | Initial body weight (g) | Final body weight (g) | Change (%) |
|---|---|---|---|
| NC | 241.9 ± 0.7 | 263.4 ± 0.9 | 8.9 |
| DC | 242.7 ± 1.4 | 233.8 ± 0.7 | -3.7* |
| MET-180 | 260.0 ± 2.9 | 291.9 ± 8 | 12.3*,† |
| ECA-150 | 264.3 ± 1.3 | 287.9 ± 9.1 | 8.9† |
| ECA-300 | 236.9 ± 8.3 | 237.4 ± 1.4 | 0.2* |
Results are represented as mean ± SEM. (*) and (†) indicate statistical significance (P < 0.05) in comparison with the NC and the DC, respectively. ECA, extract of Cassia abbreviata; NC, normal; DC, diabetic; MET, metformin; SEM, standard errors of the means.
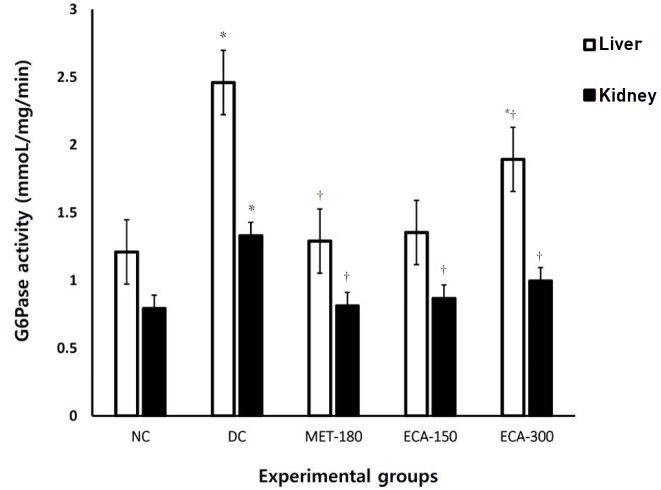
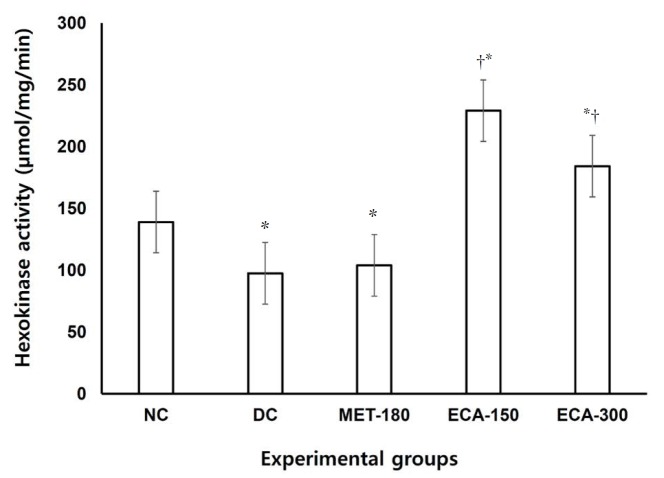
Analyses of tissues from both the livers and the kidneys (Fig. 7) showed that the activities of G6Pase were significantly (P < 0.05) elevated in the diabetic animals in comparison with the normal control animals. Interestingly, the metformin dose of 180 mg/kg bw and the ECA doses of 150 and 300 mg/kg bw were found to lower significantly (P < 0.05) the activities of G6Pase in both kidney and liver tissues in comparison with the rats in the DC group. The study also found that the activities of hexokinase (Fig. 8) in liver homogenates were significantly (P < 0.05) elevated in diabetic animals given a dose of 150 mg/kg bw extract, followed by a dose of 300 mg/kg bw when compared with the activity in the animals in the normal control group. The rats in the MET-180 and the DC groups had the lowest hexokinase activities.
Fig. 7. Effects of ECA on the activity of G6Pase in liver and kidney samples. Results are represented as means ± SEMs. (*) and (†) indicate statistical significance (P < 0.05) in comparison with the NC and the DC, respectively.
G6Pase, glucose 6 phosphatase; NC, normal control; DC, diabetic control; MET, metformin; ECA, extract of Cassia abbreviata; SEMs, standard errors of the means.
Fig. 8. Effect of ECA on the activity of hexokinase in liver samples. Results are represented as means (n = 5) ± SEMs. (*) and (†) indicate statistical significance (P < 0.05) in comparison with the NC and the DC, respectively.
NC, normal control; DC, diabetic control; MET, metformin; ECA, extract of Cassia abbreviata; SEMs, standard errors of the means.
4. Discussion
The anti-diabetic properties of ECA were assessed by using both in-vitro and in-vivo experiments. The effect of ECA on α-glucosidase enzyme was investigated. Alpha-glucosidase is a family of enzymes found on the luminal surfaces of the small intestines where their main function is to breakdown disaccharides, like maltose and sucrose, into monosaccharides, mainly glucose monomers, for easy absorption into the blood stream [13]. ECA was found to inhibit the catalytic activity of sucrase, which is responsible for catalytic breakdown of sucrose into glucose in the small intestines. The extract inhibited the activity of sucrase in a dose-dependent manner, with an inhibitory concentration giving 50% inhibition (IC50) of 1.4 mg/mL. This possibly implies that ECA reduces the rate of absorption of glucose and the amount of glucose absorbed into the blood stream in a manner similar to the commercially available α-glucosidase’ Acarbose [13, 14].
Based on the enzyme kinetics, we found the inhibition of the activity of sucrase (α-glucosidase enzyme) to be non-competitive, which means that the substrate and the inhibitor (ECA) bind at different sites on the enzyme [15]. The inhibitor may bind to either the free site of the enzyme or the enzyme-substrate complex and, hence, prevent the catalytic breakdown of sucrose into glucose. The non-competitive inhibition implies that the inhibition cannot be overcome by increasing the concentration of the substrate and, hence, is likely not be adversely affected by the concentration of the substrate, such as Acarbose, which is a competitive inhibitor [15].
ECA also significantly promoted the uptake of glucose by the rats’ hemi-diaphragms. Estimating the glucose uptake by hemi-diaphragms is a method commonly used in pharmacological research to study the peripheral uptake of glucose by body tissues [13]. The increased uptake of glucose caused by ECA in the hemi-diaphragms implies that ECA promotes the uptake of glucose by muscle tissues in the body, hence helping to reduce glucose in the blood stream. This increased uptake may be due to enhanced insulin sensitivity or increased expression of GLUT4 in muscle tissues [16].
For in-vivo studies, we started with OGTTs in normal rats under different treatment regimens. Results showed that ECA had an effect on the OGTT curves and that the glucose level had returned to normal after 3 hours. However, more significant effects of ECA on the OGTT curves were observed in the ECA-150 and the ECA-300 groups than were observed in the ECA-50 group; hence, doses of 150 and 300 mg/kg bw were chosen for further studies. The normalization of the glucose levels may be due to inhibition of α-amylase and α-glucosidase, enhanced insulin sensitivity, or increased glucose uptake by muscle and adipose tissues [17, 18]. Long-term studies were assessed on the basis of fasting glucose levels in animals fed different concentrations of ECA for 21 days. The results indicated that ECA at a dose of 150 mg/kg bw significantly reduced the fasting glucose levels in diabetic rats, but that reduction was not significantly different (P < 0.05) from the reduction observed for metformin at a dose of 180 mg/kg bw. Significant reductions in the glucose levels were observed in the ECA-300 group (P < 0.05) when compared with the DC group. The reductions in the glucose levels in the ECA-300 group differed significantly from those for the ECA-150 group.
Significant (P < 0.05) increases in body weights were observed in the NC, Met-180 and ECA-150 groups. Rats in the DC group experienced significant weight reductions. Metformin is a commercially available hypoglycemic drug that works by reducing hepatic glucose output by inhibiting the activity of G6Pase under fasting and diabetic conditions [19]. It also has minimal insulin sensitizing effects and inhibits hepatic lactate uptake, hence its ability to lower basal glucose production and possible fasting blood glucose levels [19].
The lower (150-mg/kg bw) dose of ECA worked more effectively than the higher (300-mg/kg bw) dose, hence, implying some form dose – independent manner. ECA seems to differ from other medicinal plants, which were reported to work in a dose-dependent manner [11, 20]. A hypothesis is that this difference, though not documented, may be due to ECA containing a compound that inhibits the bioactive hypoglycemic compound with increasing concentrations of the extract. The ability of ECA to lower the fasting blood glucose level in experimental diabetes may be linked to the presence of bioactive compounds like polyphenols, anthocyanins, anthranoids, anthraquinones, and tannins, which have been isolated from stem bark [8, 21]. These polyphenols also have been reported to possess anti-oxidant properties [21], which could be implicated in the scavenging of the radicals produced by streptozotocin, hence repairing beta cells or preventing further damage to beta cells.
The enzymatic activities of hepatic hexokinase and G6Pase, which are involved in the glycolysis, gluconeogenesis, and glycogenolysis pathways [22], were also investigated. The use of glucose by the liver depends heavily on the activity of hexokinase, which catalyzes the first step in glycolysis [23], while hepatic glucose production is determined primarily by the activity of G6Pase, which catalyzes the final steps of both gluconeogenesis and glycogenolysis [24] mainly during fasting and diabetic conditions. Therefore, the net hepatic glucose flux is the balance between the rate of glucose phosphorylation catalyzed by hexokinase, the first step of hepatic glucose utilization, and the rate of glucose dephosphorylation catalyzed by glucose-6-phosphatase, the last step of hepatic glucose production [24]. Therefore, the study found that ECA, specifically ECA-150, significantly promoted the enzymatic activity of hexokinase and inhibited the activity of G6Pase in both liver and kidney tissues. These observations mean that ECA-150 accelerates the rate of glycolysis, hence clearing glucose in the blood stream, and limits the rates of gluconeogenesis and glycogenolysis, hence preventing excessive hepatic glucose output and possibly preventing muscle degeneration, which could possibly explain the observed body weight changes in the animals.
5. Conclusion
We conclude that ECA normalized blood glucose levels in diabetic rats. This normalization may be due to non-competitive inhibition of α-glucosidase and to improved glucose uptake and utilization in muscle tissues. The increased activity of hepatic hexokinase and the decreased activity of G6Pase in both kidney and liver tissues may also contribute to the hypoglycemic potential of ECA.
Acknowledgments
We would like to thank Forest Conservation Botswana (FCB) for funding this research.
Footnotes
Conflict of interest The authors declare that there are no conflicts of interest.
ORCID Keagile Bati. http://orcid.org/0000-0003-3562-8730.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes. Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes., Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;33(S1):62–69. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shearer BG, Billin AN. The next generation of PPAR drugs: do we have the tools to find them? Biochim Biophys Acta. 2007;1771(8):1082–1093. doi: 10.1016/j.bbalip.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Noh RM, Graveling AJ, Frier BM. Medically minimising the impact of hypoglycaemia in type 2 diabetes: a review. Expert Opin Pharmacother. 2011;12(14):2161–2175. doi: 10.1517/14656566.2011.589835. [DOI] [PubMed] [Google Scholar]
- 5.WHO Expert Committee on Diabetes Mellitus. The effect of intensive treatment of diabetes on the development and the progression of long term complications in IDDM. Technical Report Series 646. World Health Organization; Geneva: 1980. 123 [Google Scholar]
- 6.Setshogo MP, Mbereki CM. Floristic diversity and uses of medicinal plants sold by street vendors in Gaborone, Botswana. Afri. J. Plant Sci. Biotech. 2011;5:69–74. [Google Scholar]
- 7.Venter F, Venter J. Making the most of indigenous trees. Briza Publications; Pretoria: 2009. 92 [Google Scholar]
- 8.Leteane MM, Ngwenya BN, Muzila M, Namushe A, Mwinga J, Musonda R et al. Old plant newly discovered: Cassia sieberiana D.C and Cassia abbreviata Oliv. root extracts inhibit in vitro HIV-1c replication in peripheral blood mononuclear cells (PBMCs) by different modes of action. J Ethnopharmacol. 2012;141(1):48–56. doi: 10.1016/j.jep.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 9.Shai LJ, Masoko P, Mokgotho MP, Magano SR, Mogale AM, Boaduo N et al. Yeast alpha glucosidase inhibitory and antioxidant activities of six medicinal plants collected in Phalaborwa, South Africa. S Afr J Bot. 2010;76(3):465–470. [Google Scholar]
- 10.Somani G, Chaudhari RS, Sancheti J, Sathaye S. Inhibition of carbohydrate hydrolyzing enzymes by methanolic extract of Couroupita guianensis leaves. Int J Pharm Bio Sci. 2012;3(4):511–520. [Google Scholar]
- 11.Ahmed F, Urooj A. In vitro studies on the hypoglycemic potential of Ficus racemosa stem bark. J Sci Food Agric. 2010;90(3):397–401. doi: 10.1002/jsfa.3828. [DOI] [PubMed] [Google Scholar]
- 12.Bisswanger H. Practical enzymology. Wiley– VCH; Weinhiem: 2004. 69 [Google Scholar]
- 13.Tiwari AK, Rao JM. Diabetes mellitus and multiple therapeutic approaches of phytochemicals: present status and future prospects. Curr Sci. 2002;83(1):30–38. [Google Scholar]
- 14.Shai LJ, Magano SR, Lebelo SL, Mogale AM. Inhibitory effects of five medicinal plants on rat alpha-glucosidase: comparison with their effects on yeast alpha-glucosidase. J Med Plants Res. 2011;5(13):2863–2867. [Google Scholar]
- 15.Ferrier DR. Lippincott’s illustrated reviews: biochemistry. Lippincott Williams and Wilkins; Philadelphia: 2011. 53 [Google Scholar]
- 16.Richter EA, Hargreaves M. Exercise, GLUT4 and skeletal muscle glucose uptake. Physiol Rev. 2013;93(3):993–1017. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- 17.Erasto P, Majinda RT. Bioactive proanthocyanidins from the root bark of Cassia abbreviata. Int J Biol Chem Sci. 2011;5(5):2170–2179. [Google Scholar]
- 18.Mongalo NI. Antibacterial activities of selected medicinal plants used to treat sexually transmitted infections in Blouberg area, Limpopo Province. University of Zululand; South Africa: 2013. 78 [Google Scholar]
- 19.Mithieux G, Guignot L, Bordet JC, Wiernsperger N. Intrahepatic mechanisms underlying the effect of metformin in decreasing basal glucose production in rats fed a high fat diet. Diabetes. 2002;51(1):139–143. doi: 10.2337/diabetes.51.1.139. [DOI] [PubMed] [Google Scholar]
- 20.Mallick C, Chatterjee K, Guhabiswas M, Ghosh D. Antihyperglycemic effects of separate and composite extract of root of Musa paradisiaca and leaf of Coccinia indica in streptozotocin-induced diabetic male albino rat. Afr J Tradit Complement Altern Med. 2007;4(3):362–371. doi: 10.4314/ajtcam.v4i3.31230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arion JW, Canfield WK, Callaway ES, Burger HJ, Hermmerle H, Schubert G et al. Direct evidence for the involvement of two glucose 6 phosphate binding sites in the glucose 6 phosphatase activity of intact liver microsomes. J Biol Chem. 1998;273(11):6223–6227. doi: 10.1074/jbc.273.11.6223. [DOI] [PubMed] [Google Scholar]
- 22.Bhandari U, Somabhai HC, Khanna G, Najmi AK. Antidiabetic effects of Embelia ribes extract in high fat diet and low dose streptozotocin-induced type 2 diabetic rats. Front Life Sci. 2013;7(3):186–196. [Google Scholar]
- 23.Massa ML, Gagliardino JJ, Francini F. Liver glucokinase: an overview on the regulatory mechanisms of its activity. IUBMB Life. 2011;63(1):1–6. doi: 10.1002/iub.411. [DOI] [PubMed] [Google Scholar]
- 24.Torres TP, Catlin RL, Chan R, Fujimoto Y, Sasaki N, Printz RL et al. Restoration of hepatic glucokinase expression corrects hepatic glucose flux and normalizes plasma glucose in zucker diabetic fatty rats. Diabetes. 2009;58(1):78–86. doi: 10.2337/db08-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]