Abstract
Animals capable of adult regeneration require specific signaling to control injury-induced cell proliferation, specification and patterning, but comparatively little is known about how the regeneration blastema assembles differentiating cells into well-structured functional tissues. Using the planarian Schmidtea mediterranea as a model, we identify β1-integrin as a crucial regulator of blastema architecture. β1-integrin(RNAi) animals formed small head blastemas with severe tissue disorganization, including ectopic neural spheroids containing differentiated neurons normally found in distinct organs. By mimicking aspects of normal brain architecture but without normal cell-type regionalization, these spheroids bore a resemblance to mammalian tissue organoids synthesized in vitro. We identified one of four planarian integrin-alpha subunits inhibition of which phenocopied these effects, suggesting that a specific receptor controls brain organization through regeneration. Neoblast stem cells and progenitor cells were mislocalized in β1-integrin(RNAi) animals without significantly altered body-wide patterning. Furthermore, tissue disorganization phenotypes were most pronounced in animals undergoing brain regeneration and not homeostatic maintenance or regeneration-induced remodeling of the brain. These results suggest that integrin signaling ensures proper progenitor recruitment after injury, enabling the generation of large-scale tissue organization within the regeneration blastema.
KEY WORDS: Brain regeneration, Planaria, Morphogenesis, Organoids, Blastema, Integrin
Highlighted article: Integrins are required for neoblast migration and the formation of organized tissues, and for restricting neurogenesis during planarian regeneration.
INTRODUCTION
A crucial task for adult regeneration is the assembly of tissues from newly differentiating progenitor cells. Injuries variously alter tissue composition, requiring robust mechanisms of regenerative morphogenesis that enable selective construction of appropriate structures and tissue organization. For example, planarians can regenerate an entirely new brain or only part of a brain, depending on the regions removed by injury. Such animals must also rapidly construct complex tissues at length scales relevant to the size of adult organs and interface them with pre-existing tissue. The study of regenerating animals has identified several injury-induced factors required for regulating proliferation to form appropriate numbers of tissue progenitors, differentiating these into the cell types needed in the newly forming tissue, and controlling regional patterning to ensure the appropriate restoration of form (Petersen and Reddien, 2009, 2011; Poss, 2010; Wenemoser et al., 2012; Elliott and Sánchez Alvarado, 2013; Gemberling et al., 2015; Rodrigo Albors et al., 2015; Sugiura et al., 2016). However, little is known about the signaling involved in the construction of new tissues through adult regeneration.
The crucial role of the extracellular matrix (ECM) in embryonic development makes it a likely source of signaling instructing the assembly of complex tissues in regeneration. Components or putative regulators of the ECM have been identified as expressed following injury in several regeneration models, including planarians (Wenemoser et al., 2012; Wurtzel et al., 2015), axolotl (Rao et al., 2009; Knapp et al., 2013) and sea anemone (DuBuc et al., 2014). However, the function of these in tissue assembly is unknown. In axolotl, a transient ECM is formed early in regeneration and some of its components can modify the behavior of isolated axolotl cell types in culture (Calve et al., 2010; Mercer et al., 2013), and in zebrafish cardiac injury causes deposition of fibronectin, which is required for its regenerative repair (Wang et al., 2013). In addition, the injury-induced laminin beta 1a gene is required for zebrafish fin regeneration (Chen et al., 2015). However, among regenerative animals only a limited number of phenotypes have been described in which the architecture of regenerated tissue is specifically disrupted, as contrasted with phenotypes involving a failure to form new tissues or pattern them (Reddien et al., 2005a). Such phenotypes would be instructive for understanding the pathways and processes engaged in tissue assembly within the regeneration blastema.
We investigated integrins, well-characterized transmembrane receptors connecting the ECM environment to the cytoskeleton that can control cell motility, proliferation, survival and polarization (Maartens and Brown, 2015), as potential regulators of blastema organization. Here, we identify a specific integrin alpha/beta complex essential for promoting blastema outgrowth and achieving proper architecture and cell number of the regenerating planarian brain. These results implicate integrin signaling as crucial for regenerative tissue assembly.
RESULTS
β1-integrin is a regulator of brain organization and cell number in regeneration
We cloned and sequenced a gene encoding the single planarian β-integrin (see supplemental Materials and Methods) and used RNAi to examine its requirements in regeneration after decapitation. Normal animals regenerate well-organized cephalic ganglia within 1-2 weeks, as measured by double fluorescence in situ hybridization (FISH) detecting both abundant (chat+ cholinergic neurons) and highly regionalized (cintillo+ chemosensory neurons) cell types of the brain. By contrast, by 2 weeks after decapitation, β1-integrin(RNAi) fragments formed new chat+ and cintillo+ brain tissue that was strongly disorganized and mispositioned (Fig. 1A). qPCR verified β1-integrin RNAi knockdown (Fig. S1A). β1-integrin RNAi caused slow movement and severe effects on other systems, including atrophy of the intestine (as indicated by alterations in porcupine expression) and formation of a smaller pharynx through regeneration (laminin), but without substantial alteration of epidermal progenitors (prog-1) or excretory systems (slc6a-13) (Fig. S1B). Therefore, β1-integrin is required for organization of multiple tissues in planarian regeneration.
Fig. 1.
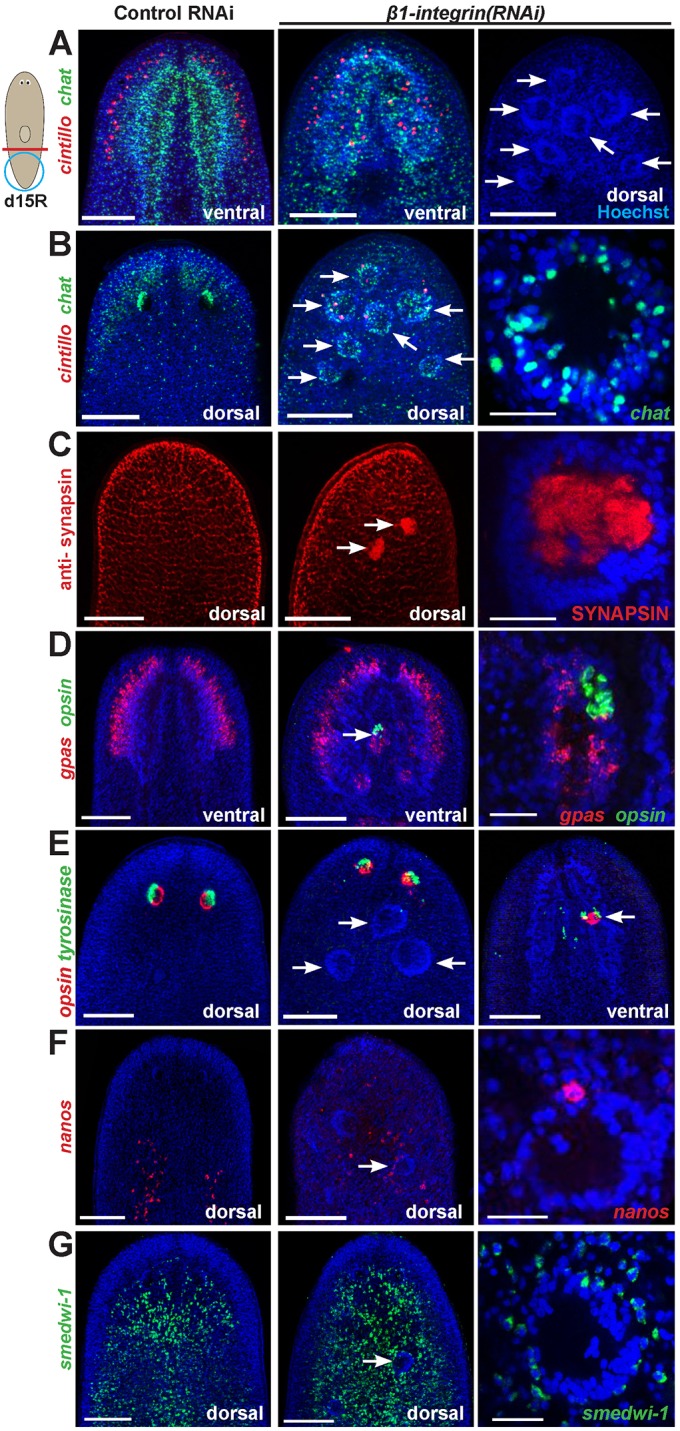
β1-integrin inhibition causes formation of ectopic neurospheroids in planarian head regeneration. (A-G) Control or β1-integrin(RNAi) animals were decapitated as shown (red line), fixed 15 days later (d15R) and stained with the indicated riboprobes or antibodies, then optically sectioned showing either dorsal or ventral views as indicated. Right panels are enlargements, except for A and E. (A,B) β1-integrin inhibition caused regeneration of disorganized CNS (chat, cintillo), including production of ectopic spheroid aggregates identifiable by Hoechst staining (28/35 animals had one to seven aggregates each). (B) Spheroids contained neurons (37/41 Hoechst+ aggregates had chat+ cholinergic neurons, n=24 animals) and (C) were enervated within their interior (9/9 aggregates were labeled with anti-SYNAPSIN antibody, n=10 animals). (D) Spheroids possessed neurons of diverse lineages (3/7 spheroids had gpas+ brain branch neurons and opsin+ photoreceptor neurons, n=7 animals). (E) β1-integrin RNAi produced ectopic eyes ventrally (7/7 animals). (E,F) Disorganized non-neural tissue generally was not abundant within spheroids (E; 0/12 spheroids had tyrosinase+ cells, n=7 animals) but in some cases small numbers of such cells could be detected nearby spheroids (F; 9/14 spheroids had nanos+ primordial germ cells, n=11 animals). (G) Spheroids were surrounded by neoblasts (smedwi-1). Hoechst counterstain shown in blue to mark nuclei. Arrows indicate neural spheroids (A-E,G), ectopic eye tissue (E, right) or disorganized nanos+ cells (F). Anterior, top. Scale bars: 25 µm (right panels B-D,F,G); 150 µm (all others).
β1-integrin(RNAi) animals also formed prominent ectopic cell aggregates readily identifiable by Hoechst staining (Fig. 1A). These aggregates contained abundant chat+ cells on their exterior and high synaptic density in their interior (marked by anti-SYNAPSIN and anti-alpha-TUBULIN staining) and could send projections outward (Fig. 1B,C; Fig. S1C; Movies 1,2). Thus, aggregates had broadly similar architecture to the mature planarian cephalic ganglia (Tazaki et al., 1999; Collins et al., 2010; Nishimura et al., 2010), and we termed them neurospheroids. Unlike the ventral location of normal cephalic ganglia, ectopic neurospheroids from β1-integrin(RNAi) regenerating animals formed both dorsally and ventrally. We further investigated the organization and composition of spheroids histologically. gpas expression normally marks the lateral brain branches, but it evenly surrounded neurospheroids, suggesting that they lack the mediolateral regionalization typical of a mature brain (6/6 animals; Fig. 1D). Additionally, neurospheroids contained serT+ and tph+ serotonergic neurons (Currie and Pearson, 2013; Marz et al., 2013), coe+ neurons (Cowles et al., 2013), as well as cells expressing wntA and wnt5, signaling molecules normally expressed in the brain and CNS (Kobayashi et al., 2007; Adell et al., 2009; Gurley et al., 2010; Hill and Petersen, 2015) (Fig. S1D). Neurospheroids could contain both gpas+ brain neurons as well as opsin+ eye photosensory neurons, indicating that they can be composed of cells from distinct organs and lineages (Lapan and Reddien, 2012) (Fig. 1D). Some β1-integrin(RNAi) animals also had ectopic ventral opsin+ and tyrosinase+ cells localized together but not within spheroids, indicating that some disorganization occurred independently of spheroids (Fig. 1E). We could not detect evidence of non-neuronal cell types populating neurospheroids, such as tyrosinase+ eye pigment cup cells (Fig. 1E), collagen+ muscle cells (6/6 animals), porcupine+ intestinal cells (13/13 animals), laminin+ pharyngeal cells (13/13 animals), prog-1+ epidermal progenitors (10/10 animals) or slc6a-13+ excretory cells (9/9 animals) (Fig. S1B,C). Ectopic germline progenitors (nanos+ cells) (Sato et al., 2006; Handberg-Thorsager and Saló, 2007; Wang et al., 2007) were occasionally found in the vicinity of the spheroids but not clearly located within them (Fig. 1F). smedwi-1+ neoblasts, the source of all differentiated tissue in planarians (Reddien et al., 2005a), surrounded spheroids and did not generally localize on them abundantly (Fig. 1G). Neurospheroids are therefore composed of aggregated differentiated cells lacking proper regionalization, are likely derived from stem cells, and recapitulate aspects of normal organ architecture, similar to in vitro synthesized tissue organoids (Lancaster et al., 2013; Lancaster and Knoblich, 2014). These results suggest that integrin signaling is crucial for generation of higher-order tissue organization formed by the descendants of pluripotent stem cells in vivo.
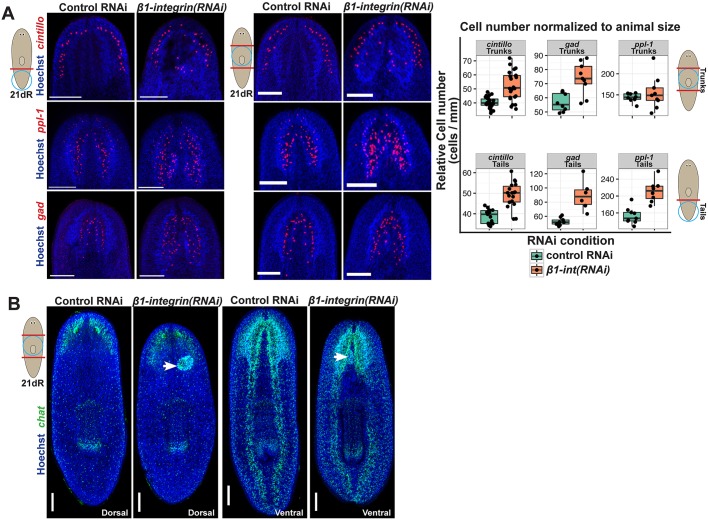
β1-integrin(RNAi) animals appeared to have excess neuronal tissue within their heads, so we investigated this by quantifying three neuron populations in regenerating tail and trunk fragments: cintillo+ chemosensory neurons in the lateral head region, gad+ GABAergic neurons and ppl-1+ interneurons. Regenerating tail fragments often appeared to have more severe brain disorganization following β1-integrin RNAi compared with regenerating trunk fragments, and such animals regenerated excess neurons of each population (Fig. 2A; Fig. S2). Regenerating trunk fragments had on average significantly elevated numbers of cintillo+ and gad+ cells but only rarely produced excess ppl-1+ cells (Fig. 2A; Fig. S2). In addition, these populations of brain neurons were frequently found in abnormal locations within the regenerated head regions of β1-integrin(RNAi) animals. We conclude that β1-integrin suppresses neuron production in addition to enabling proper organization of the regenerating brain.
Fig. 2.
β1-integrin suppresses neurogenesis in regeneration. (A) Day 21 control or β1-integrin(RNAi) regenerating tail fragments fixed and stained for the distribution of three brain neuronal subtypes marked by cintillo, ppl-1 and gad expression, with size-normalized cell numbers plotted (right panels). β1-integrin inhibition produced excess and disorganized tissue. (B) β1-integrin(RNAi) trunk fragments formed neurospheroids only in anterior blastemas (17/28 animals, arrows) without substantially affecting regeneration of ventral nerve cords in posterior blastemas (28/28 animals). Scale bars: 300 µm.
The excess and disorganized brain tissue regenerated in β1-integrin(RNAi) animals was distributed along the dorsoventral axis but restricted to anterior and not posterior blastemas (Fig. 2B), which formed an apparently normal posterior domain of chat+ ventral nerve cords. We therefore examined the possibility that elevated expression of anterior Wnt inhibitors (notum and sFRP-1; Petersen and Reddien, 2011), drivers of head outgrowth in regeneration, and/or modifications to mediolateral patterning (controlled by slit and wnt5; Cebrià et al., 2007; Adell et al., 2009; Gurley et al., 2010) or dorsoventral patterning (controlled by bmp4; Molina et al., 2007; Reddien et al., 2007; Gaviño and Reddien, 2011) might underlie the β1-integrin(RNAi) brain phenotype (Fig. 3). Such animals had elevated expression of the Wnt inhibitor notum within the anterior brain, a signaling region associated with brain size control (Hill and Petersen, 2015), but had normal expression of notum at the anterior pole (Petersen and Reddien, 2011), as well as lack of notum expression at the posterior pole. Likewise, β1-integrin(RNAi) regenerated animals had normal expression of sFRP-1 restricted to the far anterior. Expression of slit was elevated in the pharynx region, and expanded slightly in the anterior of β1-integrin(RNAi) animals but otherwise normally marked the midline. Similarly, wnt5 was expressed in dorsal neurospheroids (Fig. S1D) but normally lined the lateral edge of such animals (Fig. 3). Finally, in such animals, bmp4 expression was localized normally in a graded fashion from the dorsal midline and was absent from the ventral side. Therefore, β1-integrin(RNAi) tissue mislocalization and excess neurogenesis are not likely to be a consequence of global patterning alteration.
Fig. 3.
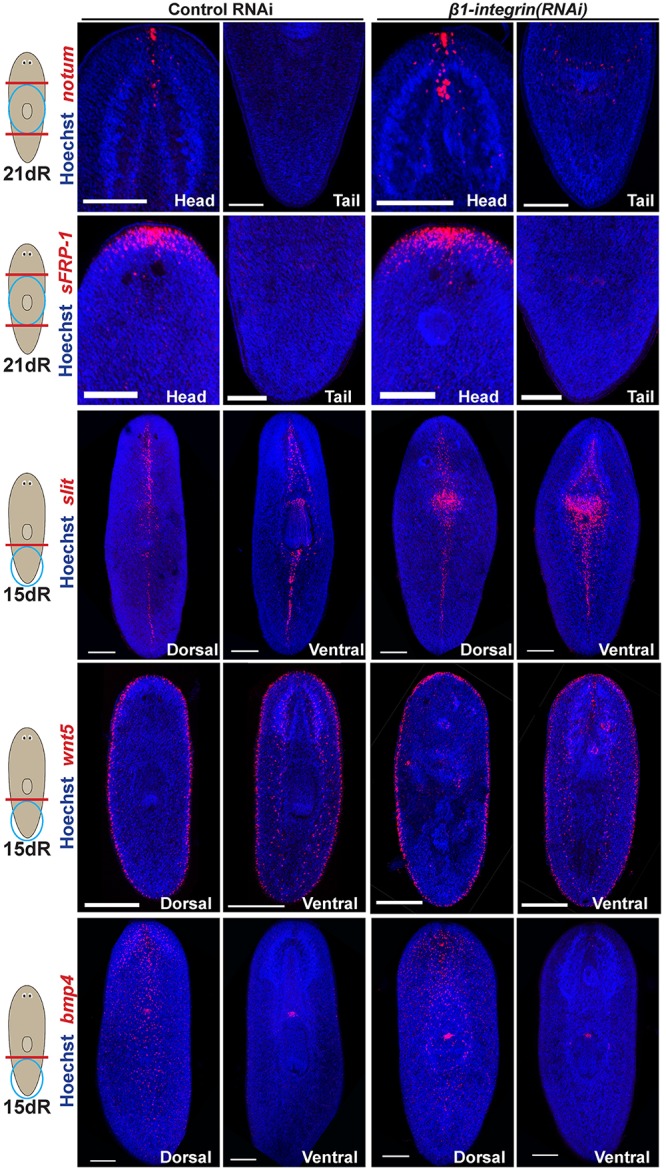
Global axis patterning is retained following β1-integrin RNAi. Regenerating tail fragments were fixed at the indicated times and stained for markers of body-wide patterning of the anteroposterior axis (notum, sFRP-1), the mediolateral axis (wnt5, slit) and the dorsoventral axis (bmp4). β1-integrin RNAi did not disrupt polarized expression of notum at the anterior versus posterior pole (5/5 animals) or of sFRP-1 (8/8 animals). β1-integrin RNAi caused reduced regeneration of the pharynx and excess slit expression in that region, with a slight increase in width along the dorsal anterior midline but otherwise normal medial expression (10/10 animals). β1-integrin RNAi retained expression of wnt5 at the lateral periphery (14/14 animals), with some neurospheroid-related staining evident (6/14 animals). bmp4 expression remained dorsally restricted in β1-integrin(RNAi) animals (5/5 animals). Scale bars: 150 µm.
A specific α/β complex is necessary for normal blastema outgrowth and brain organization
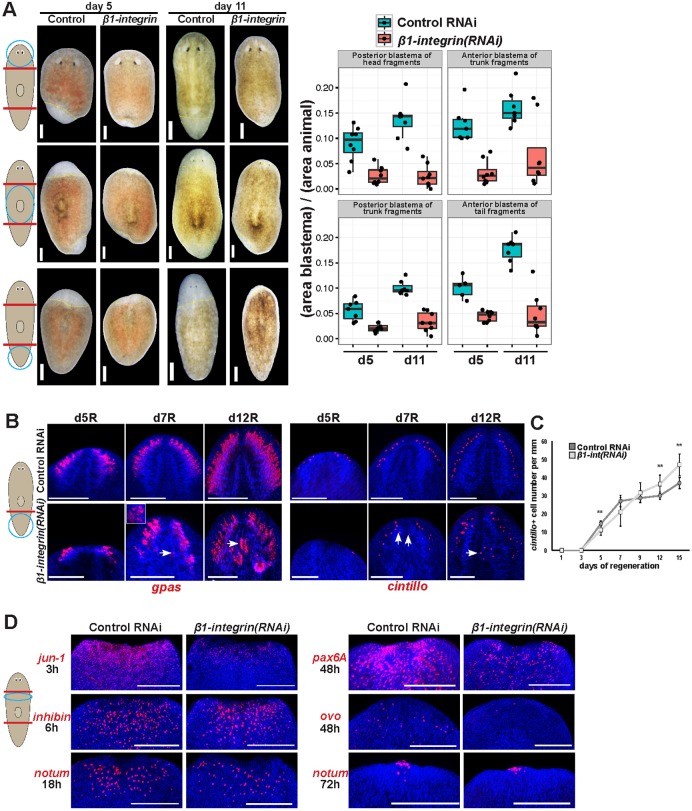
We noticed that β1-integrin inhibition resulted in a reduced size of head and tail blastemas formed through regeneration (Fig. 4A). This appeared to be at odds with the observation that such animals ultimately formed excess brain tissue (Fig. 1A,B; Fig. 2A), and therefore we explored the relationship between these two phenotypes. The interface between blastema and pre-existing tissue regions could not be clearly determined at the relatively late times (2 weeks) at which brain disorganization phenotypes were most apparent. However, the fact that β1-integrin(RNAi) animals regenerated excess brain neurons as well as a small head blastema argues that some brain tissue probably formed within pre-existing tissues near the amputation site. We tested whether β1-integrin constitutively suppressed formation of brain tissues in regeneration by examining expression of gpas and cintillo through a regeneration time series (Fig. 4B). At early times of regeneration (by 5 days), β1-integrin(RNAi) animals had reduced production of gpas+ tissues and numbers of cintillo+ cells, and excess cintillo+ cells were only detected late in regeneration (after 12 days) (Fig. 4B,C). By contrast, disorganized gpas+ tissue could be identified as early as 7 days and abundantly at 12 days (Fig. 4B). Therefore, a delay in regeneration precedes the onset of tissue overproduction and misplacement phenotypes in β1-integrin(RNAi) animals.
Fig. 4.
A regeneration delay precedes integrin RNAi tissue disorganization phenotype. (A) Control and β1-integrin(RNAi) animals were imaged at 5 and 11 days of regeneration after amputation of heads and tails as indicated. β1-integrin RNAi caused a statistically significant reduction to blastema size in each condition (P<0.01 by two-tailed t-test). Right: quantification of blastema size for each treatment normalized to fragment size. Yellow dashed lines indicate boundary between blastema and pre-existing tissue. (B,C) Animals were fixed and stained as indicated to detect cintillo or gpas mRNA (B), and size-normalized cintillo+ cell numbers plotted (mean±s.d.; **P<0.01 by two-tailed t-test) (C). β1-integrin RNAi caused a delay in blastema formation, with ectopic, disorganized brain tissue emerging later (arrows). (D) β1-integrin RNAi reduced amputation-induced gene expression of jun-1 by 3 h, pax6A and ovo by 48 h but did not substantially affect expression of inhibin by 6 h or notum by 18 or 48 h. Inset shows enlargement of indicated region (arrow). Scale bars: 150 µm.
Regeneration initiation proceeds with waves of gene expression induced by injury in differentiated epidermal and muscle cells as well as in neoblasts. Given the phenotypes of reduced blastema size and early delay in production of differentiated brain cells, we tested whether β1-integrin inhibition influenced aspects of the expression of several genes activated by head amputation. Some injury-induced genes were activated normally after amputation of heads and tails of β1-integrin(RNAi) animals: inhibin by 6 h, runt-1 at 12 h and notum at 18 h and 72 h (Fig. 4D; Fig. S3A). However, we detected some abnormalities, including attenuated activation of jun-1 by 3 h after amputation, and subsequent reduced expression of two transcription factors involved in brain and eye differentiation (pax6A and ovo) by 48 h. These observations indicate the importance of β1-integrin in ensuring normal injury responses and are consistent with an early delay in production of brain and eye tissues after β1-integrin RNAi.
Integrins function as αβ heterodimers (Maartens and Brown, 2015), and S. mediterranea possesses four α-integrins, so we reasoned that individual inhibition of these genes could determine functional associations between neuronal disorganization and other phenotypes due to inhibition of the single β-integrin homolog. We found that α-integrin-2 RNAi did not strongly affect intestine formation (porcupine), the excretory system (slc6a-13), musculature (collagen), or epidermal progenitors (prog-1) (Fig. S3B,C) but resulted in reduced blastema formation (Fig. S3D) and production of ectopic chat+ neurospheroids and mispositioned cintillo+, opsin+ and tyrosinase+ cells reminiscent of β1-integrin(RNAi) animals (Fig. S3E). Although these effects occurred at a lower penetrance in α-integrin-2 RNAi (7/25 animals had neurospheroids) compared with β1-integrin RNAi (44/56 animals had neurospheroids), individual inhibition of the other three α-integrins did not cause brain disorganization or spheroid phenotypes, suggesting that β1/α-2 integrin complexes likely promote early blastema formation and CNS organization in brain regeneration.
Integrin is necessary for neoblast and neuronal progenitor localization in regeneration
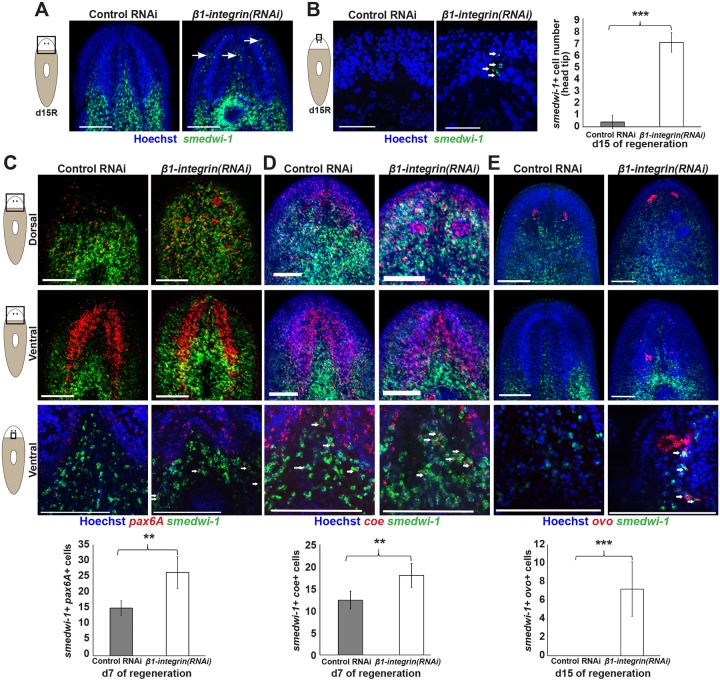
Global patterning did not appear disrupted after β1-integrin inhibition, so we tested whether brain and eye mispositioning and overproduction phenotypes could be due to mislocalization and excess of progenitor cells for these tissues. First, we examined the distribution of neoblasts, which are normally absent from the regenerated head tip and can be recruited to amputation sites in regeneration (Guedelhoefer and Sánchez Alvarado, 2012). By contrast, increased numbers of neoblasts could be identified anterior to the brain after β1-integrin or α-integrin-2 RNAi (Fig. 5A,B; Fig. S4A) without altering the animal-wide abundance of the smedwi-1 transcript as measured by qPCR (Fig. S4B) or significantly altering animal-wide mitotic activity during early regeneration (6 and 48 h post-amputation) (Fig. S4C) (Wenemoser and Reddien, 2010). Therefore, the higher abundance of neoblasts in the head tip is not likely to be due to substantial global increases in neoblast numbers. The fact that integrin inhibition caused reduced size blastemas suggests that neoblasts in the anterior region failed to localize away from regenerating head tip tissue in that context. We reasoned that β1-integrin inhibition could lead to increases in differentiated neuronal cell types through increases in specific progenitor populations as opposed to a broader increase in all neoblast populations. We tested this hypothesis by measuring the localization and numbers of pax6A+smedwi-1+ neuronal progenitors (Scimone et al., 2014a), coe+smedwi-1+ neuronal cell progenitors and ovo+smedwi-1+ eye cell progenitors (Lapan and Reddien, 2012) (Fig. 5C-E). Numbers of pax6A+smedwi-1+ cells and coe+smedwi-1+ cells were elevated in β1-integrin(RNAi) animals at 7 days of regeneration compared with control RNAi animals (Fig. 5C,D). At this time in regeneration, β1-integrin(RNAi) animals had similar numbers of differentiated neurons compared with normal animals (Fig. 4C), indicating that an increase in neuronal progenitors (pax6A+smedwi-1+ cells) preceded the increase in differentiated neurons. We examined the abundance and distribution of migratory ovo+smedwi-1+ eye progenitors (Lapan and Reddien, 2012) to test the generality of these observations (note that pax6A does not participate in planarian eye formation; Pineda et al., 2002). Numbers of ovo+smedwi-1+ cells were elevated in β1-integrin(RNAi) animals (Fig. 5E). Additionally, they were mislocalized, as we could only observe such cells dorsally in fully regenerated animals, but they were readily identifiable in both dorsal and ventral regions of β1-integrin(RNAi) animals (Fig. 5E). Taken together, these results indicate that β1-integrin is required for proper localization of progenitor cells and also limits their numbers in regeneration.
Fig. 5.
Integrin signaling is required for proper localization of neoblasts and progenitor cells in regeneration. (A) Regenerating tail fragments fixed after 15 days of regeneration and stained for smedwi-1 expression marking neoblasts. Neoblasts (arrows) could be identified in greater abundance in the ventral anterior brain region of β1-integrin(RNAi) animals versus control animals. (B) Neoblasts were present in the head tip region anterior to the brain of 15-day regenerated tail fragments, normally devoid of these cells. Right: quantification of smedwi-1+ cell numbers. (C-E) Brain progenitors (C, pax6A+smedwi-1+; D, coe+smedwi-1+) and eye progenitors (E, ovo+smedwi-1+) were detected by double FISH in the head region of day 15 regenerating tail fragments imaged dorsally or ventrally at 10× (upper) or 40× (lower). Quantification is shown below of average numbers of progenitor cell numbers from stacks of 60-70 µm-thick ventromedial regions imaged at 40× with 1-µm slices. Arrows indicate representative double-positive cells. β1-integrin RNAi caused mislocalization of pax6A+ and ovo+ progenitor cells and increased their numbers. Error bars represent s.d.; **P<0.01, ***P<0.001 by two-tailed t-test. Scale bars: 150 µm.
Integrin signaling regulates the organization of newly regenerated CNS tissue
Planarians use neoblasts to undergo constant tissue turnover during homeostasis (Elliott and Sánchez Alvarado, 2013) and also within a process of amputation-induced remodeling of pre-existing tissues during regeneration (Forsthoefel et al., 2011; Hill and Petersen, 2015). We tested the specificity of β1/α-2 integrin functions for brain and eye regeneration versus homeostatic maintenance of those tissues in the absence of injury. Inhibition of β1-integrin for 4 weeks without injury eventually caused tissue lesions and death by animal lysis, indicating that β1-integrin is ultimately essential for survival (Fig. S5A). Such animals had normal numbers of cintillo+ cells in approximately normal organization (Fig. S5B) and only produced neurospheroids at a low penetrance (2/70 animals produced a single neurospheroid each) but their brains otherwise appeared normal (Fig. 6A). By contrast, regenerating tail fragments inhibited for a total of 3 weeks (1 week RNAi followed by 2 weeks of regeneration) produced highly penetrant neurospheroid formation phenotypes (44/56 animals) prior to formation of epidermal lesions. Additionally, β1-integrin(RNAi) homeostasis animals had apparently normal expression of muscle (collagen), neoblast (smedwi-1) excretory (slc6a-13) and epidermal progenitor (prog-1) markers, but had a pronounced loss of intestine organization (10/10 animals), which could be a cause of lethality. By contrast, long-term homeostatic inhibition of α-integrin-2 did not cause lesions, lethality, or neurospheroid formation after 6 weeks (12/12 animals) or 8 weeks of RNAi (9/9 animals). α-integrin-2(RNAi) homeostasis animals had normal intestine, musculature, excretory and early epidermal progenitors and neoblasts (Fig. S5C). These results suggested that tissue disorganization phenotypes from β1/α-2 integrin perturbation are most pronounced in the context of regenerating missing brain tissue after injury.
Fig. 6.
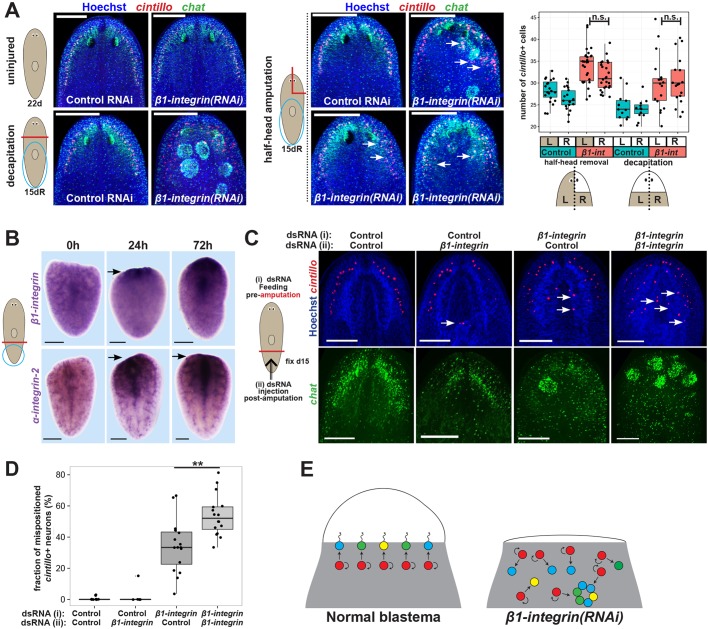
Integrin promotes proper organization of brain tissue formed during regeneration. (A) Animals were fed control or β1-integrin dsRNA for 1 week followed by 15 days of either starvation (for a total of 22 days of that treatment), decapitation and head regeneration, or amputation of the right half of the head and regeneration. Animals were then fixed and stained for chat and cintillo expression to assess brain cell numbers and organization. The majority of homeostatic β1-integrin(RNAi) animals had a normal cintillo distribution (10/10). Regeneration after decapitation produced significant disorganization and neurospheroids (17/28 animals). Half-head removal frequently resulted in more pronounced brain disorganization near the blastema compared with pre-existing regions (14/27 animals, upper right and lower left images). In other cases, mispositioned cintillo+ cells were present in both the newly regenerated and pre-existing sides of the animal's head (10/27 animals). Graph shows quantification of cell numbers after decapitation or half-head removal. cintillo+ cell numbers were increased after β1-integrin inhibition on both left and right sides of the animals in both surgical contexts (P<10−5, two-tailed t-tests for each comparison) but were not significantly different (n.s.) between left and right sides of β1-integrin(RNAi) animals (P>0.05, two-tailed t-tests). Therefore, the excess neurogenesis phenotype equally affected both sides of the head. (B) In situ hybridization that showing β1-integrin and α-integrin-2 are broadly expressed in freshly amputated tail fragments (0 h) but induced in tissue near the amputation site by 24 h (β1-integrin 11/11 animals, α-integrin-2 4/4 animals) and 72 h (β1-integrin 10/10 animals, α-integrin-2 7/7 animals 10/10 animals) (arrows). (C) β1-integrin or control dsRNA was delivered to animals via three feedings prior to amputation and/or two injections on day 3 and day 5 after amputation as indicated and stained for cintillo and chat expression. Arrows indicate mispositioned cintillo+ cells. (D) Additional doses of β1-integrin dsRNA administered during regeneration increased the severity of the tissue disorganization phenotype, quantified as the percentage of total cintillo+ cells per animal located in a non-anterolateral position. **P<0.01 by two-tailed t-test. (E) Model showing normal use of neoblasts (red) and progenitors (blue, green, yellow) that form a well-organized regeneration blastema. β1-integrin RNAi causes impaired blastema formation, mislocalization of progenitor cells, increase in their number, and production of neurospheroids composed of normally separated neuronal tissues. We suggest that this effect arises from a requirement for β1-integrin in the transit of neural progenitor cells through blastema tissue, either by directly enabling them to attach to the ECM for migration or by indirectly integrating them with other tissues. Scale bars: 150 µm.
We used two additional surgeries to examine further the specificity for integrin functions in brain regeneration after amputation. First, we tested regenerating head fragments, which undergo extensive brain remodeling resulting in a net reduction to brain size, likely through a combination of controlling cell death (Pellettieri et al., 2010) and tissue replacement (Forsthoefel and Newmark, 2009; Hill and Petersen, 2015). Compared with control conditions, β1-integrin(RNAi) head fragments remodeling their pre-existing brain did not produce excess or mispositioned cintillo+, gad+ or ppl-1+ cells and did not produce spheroids as revealed by Hoechst staining (32/32 animals) (Fig. S5D), despite regenerating posterior blastemas with a perturbed intestine and smaller pharynx (Fig. S5E). Therefore, generic responses to amputation are not likely to be sufficient to generate disorganized brain tissue in β1-integrin(RNAi) animals. Next, we amputated β1-integrin(RNAi) animals to remove half of the head laterally (Fig. 6A). Whereas control animals produced a well-organized brain lobe through a regeneration blastema, β1-integrin(RNAi) animals produced brain tissue with disorganization, including production of neurospheroids. A majority of such animals (14/27) regenerated to have greater disorganization within the newly formed cephalic ganglion compared with the pre-existing brain tissue. However, several animals also possessed similar tissue disorganization on both sides (10/27). Additionally, we found that such animals produce an equivalent excess of cintillo+ cells on both sides of the brain. These results indicate a propensity for β1-integrin inhibition to more strongly affect tissue organization of entirely newly regenerated tissue and that it can affect the organization of pre-existing tissues in close proximity to an amputation site, which we suggest is due to greater turnover of such tissues. Collectively, these results suggest that the effects of β1-integrin on brain organization are most highly pronounced in tissues normally formed in or near a regeneration blastema rather than undergoing normal homeostatic turnover. Similarly, the mammalian liver has a greater requirement for integrin-beta in regeneration after transection compared with maintenance through short-term tissue homeostasis (Speicher et al., 2014).
These observations prompted us to examine whether the expression of integrins is regulated during regeneration. We found broad expression of β1-integrin and α-integrin-2 in uninjured animals but expression was elevated near the injury site at early times in regeneration (Fig. 6B; Fig. S6A), supporting a role for these integrins in regeneration. β1-integrin and α-integrin-2 expression was reduced after treatment with lethal doses of gamma irradiation that eliminated neoblasts (Fig. S6B). We attempted to determine whether β1-integrin and α-integrin-2 were co-expressed in neoblasts using FISH in uninjured or regenerating animals (Fig. S6C-E). We could identify cells that co-expressed smedwi-1 and either β1-integrin or α-integrin-2, but the majority of FISH signal for these two genes was too disperse and punctate to assign readily to particular cells. Additionally, prior analyses found expression of these genes using RNA-seq to measure the transcriptome of FACS-sorted neoblasts as a bulk population (Labbe et al., 2012) and also in the transcriptomes of neoblasts as measured by single-cell RNA-seq, as well as expression in non-neoblast cells (Wurtzel et al., 2015) (Fig. S6F,G). We conclude that β1-integrin and α-integrin-2 are likely to be expressed broadly in both irradiation-resistant differentiated cells and in neoblasts. These observations are consistent either with a model in which the β1/α-2 complex acts within neoblasts or progenitors to enable ECM attachment necessary for migration and prior localization, or, alternatively, a model in which β1/α-2 complexes act in differentiated cells to anchor and integrate the newly forming brain with other tissues.
The pronounced requirements for β1-integrin in assembly of new brain tissue, along with its injury-induced expression, suggested that β1-integrin might function during regeneration to promote the organization of the regeneration blastema as opposed to acting only prior to injury. To test this, we administered control or β1-integrin dsRNA before amputation by feeding and/or after amputation by injection. Injection of additional β1-integrin dsRNA doses post-amputation increased the severity of brain disorganization phenotypes measured by scoring numbers of medial (disorganized) versus lateral (organized) cintillo+ cells per animal (Fig. 6C,D). Additionally, injection of β1-integrin dsRNA only after amputation could cause brain disorganization at a low frequency (4/25 animals had disorganized chat expression and 1/25 animals formed a Hoechst+ neurospheroid). These results argue against a model in which β1-integrin acts only homeostatically and prior to injury. Instead, we suggest that integrin signaling could act within neoblasts and also during regeneration to ensure the production of normal tissue architecture.
DISCUSSION
Together, these results suggest a model for the function of β1-integrin in regeneration (Fig. 6E). β1-integrin inhibition causes defective blastema formation, misplacement of progenitors and differentiated brain cells, and excess production of brain neurons in regeneration after decapitation. The tissue overproduction phenotype is not constitutive and is preceded by misplacement of newly formed differentiated tissue (Fig. 4B). Therefore, integrin signaling probably has a primary role in organizing the regenerating CNS by enabling proper recruitment of progenitors within the growing blastema. Inhibition of this signaling causes tissue production at inappropriate locations, resulting in aggregation of neural cell types ordinarily found in distinct organs and lineages to form neurospheroids. Neoblasts and their differentiating progeny express β1-integrin, suggesting that they might directly use integrin signaling for ECM attachment and migration to enable blastema outgrowth, proper localization needed for normal tissue assembly, and normal cessation of neurogenesis at the end of regeneration.
It is possible that the functions to control outgrowth, brain organization and neural differentiation represent independent outputs of integrin signaling. First, prior analysis of RNAi phenotypes in planarians indicates that such outputs need not be linked. Brains are the major component of head blastemas, and some treatments jointly reduce both brain and head blastema size, either through reduction of stem cell activity (e.g. smedwi-2 RNAi; Reddien et al., 2005b) or through regulation of patterning (e.g. zic-1 RNAi, notum RNAi, foxD RNAi or prep RNAi; Felix and Aboobaker, 2010; Scimone et al., 2014b; Vásquez-Doorman and Petersen, 2014; Vogg et al., 2014). However, it is also possible to reduce head blastema size without affecting numbers of brain neurons (e.g. pbx RNAi; Blassberg et al., 2013; Chen et al., 2013), and reduce apparent blastema size but increase brain size (e.g. wntA/wnt11-6 RNAi; Hill and Petersen, 2015). Furthermore, phenotypes of excess neurogenesis (e.g. from wntA RNAi or nou darake RNAi; Cebrià et al., 2002; Adell et al., 2009; Hill and Petersen, 2015) can proceed without causing the disorganization and production of neurospheroids seen in β1-integrin RNAi. Second, signs of independence between these outputs can be inferred from our analysis of β1-integrin(RNAi) phenotypes. β1-integrin(RNAi) posterior blastemas were reduced in size without undergoing ectopic neurogenesis or forming disorganized brain tissue. Excess neurogenesis was spatially uncoupled from extent of disorganization in experiments to amputate half of the planarian head. Furthermore, uninjured β1-integrin(RNAi) animals could form neurospheres (albeit at a very low penetrance, 2/70 animals) in the absence of programs to form a blastema. However, disorganization phenotypes were most severe in regeneration suggesting that an inability of progenitors to engage in productive blastema outgrowth, along with the increased proliferation in response to amputation, might exacerbate the production of disorganized tissues. The identification of integrin ligands and/or downstream signaling components specific for these activities will be an important step in further resolving the relationships between β1-integrin signaling outputs in regeneration.
Our studies point to the importance of progenitor localization for regenerative tissue assembly. Pattern disruption, for example by inhibition of Wnt signaling, can cause enhanced neurogenesis to result in an enlarged but well-structured brain (Bartscherer et al., 2006; Kobayashi et al., 2007; Hill and Petersen, 2015). Other perturbations, such as roboA or slit RNAi, can result in ectopic cephalic ganglion tissue, but within tissue outgrowths (Cebrià et al., 2007; Cebrià and Newmark, 2007). By contrast, β1-integrin inhibition caused severe brain disorganization without affecting global patterning or causing significant outgrowth. These observations indicate that patterning and the ability of progenitors to undergo differentiation are likely to be insufficient for appropriate blastema assembly. Integrins can control cell proliferation, survival, migration and adhesion to participate in stem cell/niche interactions, wound healing and formation of tissue barriers among other outputs (Tanentzapf et al., 2007; Goulas et al., 2012; Maartens and Brown, 2015; Wang et al., 2015). Our results suggest that integrins have a prominent role in the organization of regenerative tissue. With a central region of innervation surrounded by neurons, neurospheroids resemble miniature planarian brains but lack normal regionalization and contain both eye and brain neurons. The production of these aggregates suggests that integrin-mediated cell sorting normally enables the generation of higher-order tissue organization by separating cells with intrinsically similar adhesive properties to contribute to distinct organs and regions within the regeneration blastema.
MATERIALS AND METHODS
Planarian culture and irradiation experiments
Asexual strain CIW4 of the planarian Schmidtea mediterranea were maintained in 1× Montjuic salts at 19°C as described (Petersen and Reddien, 2011). Planarians were fed a liver paste and starved for at least 7 days before experiments. Where indicated, animals were gamma-irradiated with a lethal dose of 6000 Rad using a Cesium source irradiator 96 h before experiments (Fig. S6A).
Cloning
β1-integrin and α-integrin-2 sequences were identified by blast using transcriptome assemblies of the planarian genome [respectively, SMU15027599 and SMU15038708 at SmedGD, http://smedgd.stowers.org (Robb et al., 2015); equivalent to dd_Smed_v6_2017_0_1 and dd_Smed_v6_3365_0_1 at PlanMine, http://planmine.mpi-cbg.de (Brandl et al., 2016)].
Riboprobes and double-stranded RNA (dsRNA) for β1-integrin and α-integrin-2 were generated by in vitro transcription (NxGen, Lucigen) as described previously (Petersen and Reddien, 2011). For primer sequences and other riboprobes used, see supplementary Materials and Methods.
RNAi
RNAi was performed either by dsRNA injection or feeding. For RNAi, dsRNA was synthesized from in vitro transcription reactions (NxGen, Lucigen). dsRNA corresponding to Caenorhabditis elegans unc-22, not present in the planarian genome, served as a negative control. Unless noted otherwise, animals were fed a mixture of liver paste and dsRNA three times in 5 days prior to amputation of heads and tails 4 h after the final feeding. For Fig. S5A-C, animals were fed dsRNA 12 times over 4 weeks and starved for 3 days before fixation. In Fig. 6C,D, fragments were injected with dsRNA at day 3 and day 5 post-amputation.
In situ hybridization and immunostaining
Colorimetric (NBT/BCIP) or fluorescence in situ hybridizations were performed as described (Lander and Petersen, 2016) after fixation in 4% formaldehyde and bleaching (Pearson et al., 2009) using blocking solution containing 10% horse serum and western blot blocking reagent (Roche) (King and Newmark, 2013). Digoxigenin- or fluorescein-labeled riboprobes were synthesized as described (Pearson et al., 2009) and detected with anti-digoxigenin-HRP (1:2000, Roche/Sigma-Aldrich 11207733910, lot 10520200), anti-fluorescein-HRP (1:2000, Roche/Sigma-Aldrich 11426346910, lot 11211620) or anti-digoxigenin-AP (1:4000, Roche/Sigma-Aldrich 11093274910, lot 11265026). Hoechst 33342 (Invitrogen) was used at 1:1000 as a counterstain.
For immunostainings, animals were fixed in Carnoy's solution as described (Hill and Petersen, 2015), using tyramide amplification to detect labeling with rabbit anti-phospho-ser10 Histone H3 (1:3000, Cell Signaling D2C8, lot 3377S) or Alexa Fluor 586-conjugated goat anti-mouse IgG (Invitrogen A11031, lot 822389) to detect labeling with mouse anti-synapsin (1:50, anti-SYNORF1, Developmental Studies Hybridoma Bank clone 3C11). For mouse anti-tubulin alpha (1:1000, anti-Tubulin Alpha, Neomarkers, lot 581P1012C) animals were fixed in 4% formaldehyde and tyramide amplification was used to detect labeling (1:150, goat anti-mouse horseradish peroxidase, Life Technologies, T20914, lot 1088037).
Image analysis
Live animals and NBT/BCIP-stained animals were imaged with a Leica M210F dissecting microscope and a Leica DFC295, with adjustments to brightness and contrast using Adobe Photoshop. For Fig. S3D and Fig. 4A, blastema and animal area were determined using Adobe Photoshop and CellProfiler (Lamprecht et al., 2007). Animals were mounted on dark Whatman paper wetted with 1× Montjiuc planaria water and placed on top of a bed of crushed ice. Animals were imaged at 20-25× as they extended. Blastema and total areas were determined from images by selecting regions of interest using a polygon selection tool. The blastema boundary was defined by manual inspection as the region at the location of regenerative outgrowth with reduced pigmentation compared with pre-existing tissue regions away from the wound site. Area was determined as the number of pixels in the cropped regions and measured in CellProfiler. Relative blastema area was calculated normalized to animal fragment size area in pixels. Whole animal fluorescence imaging was performed on either a Leica DM5500B compound microscope with Optigrid structured illumination system or a Leica laser scanning SPE confocal microscope at 40× or 63×, and presented images are maximum projections of a z-series with adjustments to brightness and contrast using ImageJ and Photoshop. Plots were generated in Microsoft Excel or R (ggplot2).
Cell counting
cintillo+, gad+ and ppl-1+ cells in the brain were counted manually and normalized to the square root of the animal area determined using Hoechst staining and CellProfiler (Lamprecht et al., 2007). Cells co-expressing smedwi-1 and pax6A or co-expressing smedwi-1 and ovo were counted manually using ImageJ from 1-µm-thick confocal z-stacks imaged at 40× in the ventral region between the two brain lobes. H3P+ cells were counted manually using ImageJ.
Real-time PCR
Total RNA was extracted by mechanical homogenization in Trizol (Life Technologies), DNase-treated (TURBO DNAse, Ambion), and reverse transcribed with oligo-dT primers (Multiscribe reverse transcriptase, Applied Biosystems), and qPCR was performed using Eva Green PCR Master Mix (Biotium) from nine regenerating fragments in four (Fig. S1A; Fig. S3B) or eight (Fig. S6B) biological replicates. See supplementary Materials and Methods for primer sequences. Relative mRNA abundance was calculated using the delta-Ct method after verification of primer amplification efficiency, normalizing to ubiquilin expression. Reactions producing Ct values flagged by Grubb's outlier test with alpha outlier test with alpha <0.05 were discarded from analysis as described (Burns et al., 2005). P-values below 0.05 by a two-tailed t-test were considered as significant.
Acknowledgements
We thank all past and present members of the Petersen lab for helpful discussions of the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceived and designed the experiments: N.A.B., C.P.P.; Performed the experiments: N.A.B.; Analyzed the data: N.A.B., C.P.P.; Contributed reagents/materials/analysis tools: N.A.B., C.P.P.; Wrote the paper: N.A.B., C.P.P.
Funding
This work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) post-graduate scholarship (PGSD3-471547-2015 to N.A.B.); an Ellison Medical Foundation New Scholar in Aging Research Award (AG-NS-0835-11 to C.P.P.); and a National Institutes of Health Director's New Innovator Award (1DP2DE024365-01 to C.P.P.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.139964.supplemental
References
- Adell T., Salo E., Boutros M. and Bartscherer K. (2009). Smed-Evi/Wntless is required for beta-catenin-dependent and -independent processes during planarian regeneration. Development 136, 905-910. 10.1242/dev.033761 [DOI] [PubMed] [Google Scholar]
- Bartscherer K., Pelte N., Ingelfinger D. and Boutros M. (2006). Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell 125, 523-533. 10.1016/j.cell.2006.04.009 [DOI] [PubMed] [Google Scholar]
- Blassberg R. A., Felix D. A., Tejada-Romero B. and Aboobaker A. A. (2013). PBX/extradenticle is required to re-establish axial structures and polarity during planarian regeneration. Development 140, 730-739. 10.1242/dev.082982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl H., Moon H., Vila-Farré M., Liu S. Y., Henry I. and Rink J. C. (2016). PlanMine - a mineable resource of planarian biology and biodiversity. Nucleic Acids Res. 44, D764-D773. 10.1093/nar/gkv1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns M. J., Nixon G. J., Foy C. A. and Harris N. (2005). Standardisation of data from real-time quantitative PCR methods - evaluation of outliers and comparison of calibration curves. BMC Biotechnol. 5, 31 10.1186/1472-6750-5-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calve S., Odelberg S. J. and Simon H.-G. (2010). A transitional extracellular matrix instructs cell behavior during muscle regeneration. Dev. Biol. 344, 259-271. 10.1016/j.ydbio.2010.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebrià F. and Newmark P. A. (2007). Morphogenesis defects are associated with abnormal nervous system regeneration following roboA RNAi in planarians. Development 134, 833-837. 10.1242/dev.02794 [DOI] [PubMed] [Google Scholar]
- Cebrià F., Kobayashi C., Umesono Y., Nakazawa M., Mineta K., Ikeo K., Gojobori T., Itoh M., Taira M., Sánchez Alvarado A. et al. (2002). FGFR-related gene nou-darake restricts brain tissues to the head region of planarians. Nature 419, 620-624. 10.1038/nature01042 [DOI] [PubMed] [Google Scholar]
- Cebrià F., Guo T., Jopek J. and Newmark P. A. (2007). Regeneration and maintenance of the planarian midline is regulated by a slit orthologue. Dev. Biol. 307, 394-406. 10.1016/j.ydbio.2007.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. C. G., Wang I. E. and Reddien P. W. (2013). pbx is required for pole and eye regeneration in planarians. Development 140, 719-729. 10.1242/dev.083741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.-H., Merriman A. F., Savage J., Willer J., Wahlig T., Katsanis N., Yin V. P. and Poss K. D. (2015). Transient laminin beta 1a induction defines the wound epidermis during zebrafish fin regeneration. PLoS Genet. 11, e1005437 10.1371/journal.pgen.1005437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. J. III, Hou X., Romanova E. V., Lambrus B. G., Miller C. M., Saberi A., Sweedler J. V. and Newmark P. A. (2010). Genome-wide analyses reveal a role for peptide hormones in planarian germline development. PLoS Biol. 8, e1000509 10.1371/journal.pbio.1000509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles M. W., Brown D. D. R., Nisperos S. V., Stanley B. N., Pearson B. J. and Zayas R. M. (2013). Genome-wide analysis of the bHLH gene family in planarians identifies factors required for adult neurogenesis and neuronal regeneration. Development 140, 4691-4702. 10.1242/dev.098616 [DOI] [PubMed] [Google Scholar]
- Currie K. W. and Pearson B. J. (2013). Transcription factors lhx1/5-1 and pitx are required for the maintenance and regeneration of serotonergic neurons in planarians. Development 140, 3577-3588. 10.1242/dev.098590 [DOI] [PubMed] [Google Scholar]
- Dubuc T. Q., Traylor-Knowles N. and Martindale M. Q. (2014). Initiating a regenerative response; cellular and molecular features of wound healing in the cnidarian Nematostella vectensis. BMC Biol. 12, 24 10.1186/1741-7007-12-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott S. A. and Sánchez Alvarado A. (2013). The history and enduring contributions of planarians to the study of animal regeneration. Wiley Interdiscip. Rev. Dev. Biol. 2, 301-326. 10.1002/wdev.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix D. A. and Aboobaker A. A. (2010). The TALE class homeobox gene Smed-prep defines the anterior compartment for head regeneration. PLoS Genet. 6, e1000915 10.1371/journal.pgen.1000915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsthoefel D. J. and Newmark P. A. (2009). Emerging patterns in planarian regeneration. Curr. Opin. Genet. Dev. 19, 412-420. 10.1016/j.gde.2009.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsthoefel D. J., Park A. E. and Newmark P. A. (2011). Stem cell-based growth, regeneration, and remodeling of the planarian intestine. Dev. Biol. 356, 445-459. 10.1016/j.ydbio.2011.05.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaviño M. A. and Reddien P. W. (2011). A Bmp/Admp regulatory circuit controls maintenance and regeneration of dorsal-ventral polarity in planarians. Curr. Biol. 21, 294-299. 10.1016/j.cub.2011.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemberling M., Karra R., Dickson A. L. and Poss K. D. (2015). Nrg1 is an injury-induced cardiomyocyte mitogen for the endogenous heart regeneration program in zebrafish. Elife 4, eo5871. 10.7554/eLife.05871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulas S., Conder R. and Knoblich J. A. (2012). The Par complex and integrins direct asymmetric cell division in adult intestinal stem cells. Cell Stem Cell 11, 529-540. 10.1016/j.stem.2012.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedelhoefer O. C. and Sánchez Alvarado A. (2012). Planarian immobilization, partial irradiation, and tissue transplantation. J. Vis. Exp. 66, 4015 10.3791/4015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley K. A., Elliott S. A., Simakov O., Schmidt H. A., Holstein T. W. and Sánchez Alvarado A. (2010). Expression of secreted Wnt pathway components reveals unexpected complexity of the planarian amputation response. Dev. Biol. 347, 24-39. 10.1016/j.ydbio.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handberg-Thorsager M. and Saló E. (2007). The planarian nanos-like gene Smednos is expressed in germline and eye precursor cells during development and regeneration. Dev. Genes Evol. 217, 403-411. 10.1007/s00427-007-0146-3 [DOI] [PubMed] [Google Scholar]
- Hill E. M. and Petersen C. P. (2015). Wnt/Notum spatial feedback inhibition controls neoblast differentiation to regulate reversible growth of the planarian brain. Development 142, 4217-4229. 10.1242/dev.123612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. S. and Newmark P. A. (2013). In situ hybridization protocol for enhanced detection of gene expression in the planarian Schmidtea mediterranea. BMC Dev. Biol. 13, 8 10.1186/1471-213X-13-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp D., Schulz H., Rascon C. A., Volkmer M., Scholz J., Nacu E., Le M., Novozhilov S., Tazaki A., Protze S. et al. (2013). Comparative transcriptional profiling of the axolotl limb identifies a tripartite regeneration-specific gene program. PLoS ONE 8, e61352 10.1371/journal.pone.0061352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi C., Saito Y., Ogawa K. and Agata K. (2007). Wnt signaling is required for antero-posterior patterning of the planarian brain. Dev. Biol. 306, 714-724. 10.1016/j.ydbio.2007.04.010 [DOI] [PubMed] [Google Scholar]
- Labbe R. M., Irimia M., Currie K. W., Lin A., Zhu S. J., Brown D. D. R., Ross E. J., Voisin V., Bader G. D., Blencowe B. J. et al. (2012). A comparative transcriptomic analysis reveals conserved features of stem cell pluripotency in planarians and mammals. Stem Cells 30, 1734-1745. 10.1002/stem.1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht M. R., Sabatini D. M. and Carpenter A. E. (2007). CellProfiler™: free, versatile software for automated biological image analysis. Biotechniques 42, 71 10.2144/000112257 [DOI] [PubMed] [Google Scholar]
- Lancaster M. A. and Knoblich J. A. (2014). Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345, 1247125 10.1126/science.1247125 [DOI] [PubMed] [Google Scholar]
- Lancaster M. A., Renner M., Martin C.-A., Wenzel D., Bicknell L. S., Hurles M. E., Homfray T., Penninger J. M., Jackson A. P. and Knoblich J. A. (2013). Cerebral organoids model human brain development and microcephaly. Nature 501, 373-379. 10.1038/nature12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander R. and Petersen C. P. (2016). Wnt, Ptk7, and FGFRL expression gradients control trunk positional identity in planarian regeneration. Elife 5, e12850 10.7554/eLife.12850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapan S. W. and Reddien P. W. (2012). Transcriptome analysis of the planarian eye identifies ovo as a specific regulator of eye regeneration. Cell Rep. 2, 294-307. 10.1016/j.celrep.2012.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maartens A. P. and Brown N. H. (2015). Anchors and signals: the diverse roles of integrins in development. Curr. Top. Dev. Biol. 112, 233-272. 10.1016/bs.ctdb.2014.11.020 [DOI] [PubMed] [Google Scholar]
- Marz M., Seebeck F. and Bartscherer K. (2013). A Pitx transcription factor controls the establishment and maintenance of the serotonergic lineage in planarians. Development 140, 4499-4509. 10.1242/dev.100081 [DOI] [PubMed] [Google Scholar]
- Mercer S. E., Odelberg S. J. and Simon H. G (2013). A dynamic spatiotemporal extracellular matrix facilitates epicardial-mediated vertebrate heart regeneration. Dev. Biol. 382, 457-469. 10.1016/j.ydbio.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina M. D., Saló E. and Cebriá F. (2007). The BMP pathway is essential for re-specification and maintenance of the dorsoventral axis in regenerating and intact planarians. Dev. Biol. 311, 79-94. 10.1016/j.ydbio.2007.08.019 [DOI] [PubMed] [Google Scholar]
- Nishimura K., Kitamura Y., Taniguchi T. and Agata K. (2010). Analysis of motor function modulated by cholinergic neurons in planarian Dugesia japonica. Neuroscience 168, 18-30. 10.1016/j.neuroscience.2010.03.038 [DOI] [PubMed] [Google Scholar]
- Pearson B. J., Eisenhoffer G. T., Gurley K. A., Rink J. C., Miller D. E. and Sánchez Alvarado A. (2009). Formaldehyde-based whole-mount in situ hybridization method for planarians. Dev. Dyn. 238, 443-450. 10.1002/dvdy.21849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellettieri J., Fitzgerald P., Watanabe S., Mancuso J., Green D. R. and Sánchez Alvarado A. (2010). Cell death and tissue remodeling in planarian regeneration. Dev. Biol. 338, 76-85. 10.1016/j.ydbio.2009.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C. P. and Reddien P. W. (2009). A wound-induced Wnt expression program controls planarian regeneration polarity. Proc. Natl. Acad. Sci. USA 106, 17061-17066. 10.1073/pnas.0906823106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C. P. and Reddien P. W. (2011). Polarized notum activation at wounds inhibits Wnt function to promote planarian head regeneration. Science 332, 852-855. 10.1126/science.1202143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda D., Rossi L., Batistoni R., Salvetti A., Marsal M., Gremigni V., Falleni A., Gonzalez-Linares J., Deri P. and Saló E. (2002). The genetic network of prototypic planarian eye regeneration is Pax6 independent. Development 129, 1423-1434. [DOI] [PubMed] [Google Scholar]
- Poss K. D. (2010). Advances in understanding tissue regenerative capacity and mechanisms in animals. Nat. Rev. Genet. 11, 710-722. 10.1038/nrg2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao N., Jhamb D., Milner D. J., Li B., Song F., Wang M., Voss S. R., Palakal M., King M. W., Saranjami B. et al. (2009). Proteomic analysis of blastema formation in regenerating axolotl limbs. BMC Biol. 7, 83 10.1186/1741-7007-7-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien P. W., Bermange A. L., Murfitt K. J., Jennings J. R. and Sánchez Alvarado A. (2005a). Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Dev. Cell 8, 635-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien P. W., Oviedo N. J., Jennings J. R., Jenkin J. C. and Sanchez Alvarado A. (2005b). SMEDWI-2 is a PIWI-like protein that regulates planarian stem cells. Science 310, 1327-1330. 10.1126/science.1116110 [DOI] [PubMed] [Google Scholar]
- Reddien P. W., Bermange A. L., Kicza A. M. and Sanchez Alvarado A. S. (2007). BMP signaling regulates the dorsal planarian midline and is needed for asymmetric regeneration. Development 134, 4043-4051. 10.1242/dev.007138 [DOI] [PubMed] [Google Scholar]
- Robb S. M. C., Gotting K., Ross E. and Sánchez Alvarado A. (2015). SmedGD 2.0: the Schmidtea mediterranea genome database. Genesis 53, 535-546. 10.1002/dvg.22872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo Albors A., Tazaki A., Rost F., Nowoshilow S., Chara O. and Tanaka E. M. (2015). Planar cell polarity-mediated induction of neural stem cell expansion during axolotl spinal cord regeneration. Elife 4, e10230 10.7554/eLife.10230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Shibata N., Orii H., Amikura R., Sakurai T., Agata K., Kobayashi S. and Watanabe K (2006). Identification and origin of the germline stem cells as revealed by the expression of nanos-related gene in planarians . Dev. Growth Differ. 48, 615-628. 10.1111/j.1440-169X.2006.00897.x [DOI] [PubMed] [Google Scholar]
- Scimone M. L., Kravarik K. M., Lapan S. W. and Reddien P. W. (2014a). Neoblast specialization in regeneration of the planarian Schmidtea mediterranea. Stem Cell Rep. 3, 339-352. 10.1016/j.stemcr.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimone M. L., Lapan S. W. and Reddien P. W. (2014b). A forkhead transcription factor is wound-induced at the planarian midline and required for anterior pole regeneration. PLoS Genet. 10, e1003999 10.1371/journal.pgen.1003999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speicher T., Siegenthaler B., Bogorad R. L., Ruppert R., Petzold T., Padrissa-Altes S., Bachofner M., Anderson D. G., Koteliansky V., Fässler R. et al. (2014). Knockdown and knockout of beta1-integrin in hepatocytes impairs liver regeneration through inhibition of growth factor signalling. Nat. Commun. 5, 3862 10.1038/ncomms4862 [DOI] [PubMed] [Google Scholar]
- Sugiura T., Wang H., Barsacchi R., Simon A. and Tanaka E. M. (2016). MARCKS-like protein is an initiating molecule in axolotl appendage regeneration. Nature 531, 237-240. 10.1038/nature16974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanentzapf G., Devenport D., Godt D. and Brown N. H. (2007). Integrin-dependent anchoring of a stem-cell niche. Nat. Cell Biol. 9, 1413-1418. 10.1038/ncb1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazaki A., Gaudieri S., Ikeo K., Gojobori T., Watanabe K. and Agata K. (1999). Neural network in planarian revealed by an antibody against planarian synaptotagmin homologue. Biochem. Biophys. Res. Commun. 260, 426-432. 10.1006/bbrc.1999.0933 [DOI] [PubMed] [Google Scholar]
- Vásquez-Doorman C. and Petersen C. P. (2014). zic-1 Expression in Planarian neoblasts after injury controls anterior pole regeneration. PLoS Genet. 10, e1004452 10.1371/journal.pgen.1004452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogg M. C., Owlarn S., Pérez Rico Y. A., Xie J., Suzuki Y., Gentile L., Wu W. and Bartscherer K (2014). Stem cell-dependent formation of a functional anterior regeneration pole in planarians requires Zic and Forkhead transcription factors. Dev. Biol. 390, 136-148. 10.1016/j.ydbio.2014.03.016 [DOI] [PubMed] [Google Scholar]
- Wang Y., Zayas R. M., Guo T. and Newmark P. A. (2007). Nanos function is essential for development and regeneration of planarian germ cells. Proc. Natl. Acad. Sci. USA 104, 5901-5906. 10.1073/pnas.0609708104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Karra R., Dickson A. L. and Poss K. D. (2013). Fibronectin is deposited by injury-activated epicardial cells and is necessary for zebrafish heart regeneration. Dev. Biol. 382, 427-435. 10.1016/j.ydbio.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Antunes M., Anderson A. E., Kadrmas J. L., Jacinto A. and Galko M. J. (2015). Integrin adhesions suppress syncytium formation in the drosophila larval epidermis. Curr. Biol. 25, 2215-2227. 10.1016/j.cub.2015.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenemoser D. and Reddien P. W. (2010). Planarian regeneration involves distinct stem cell responses to wounds and tissue absence. Dev. Biol. 344, 979-991. 10.1016/j.ydbio.2010.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenemoser D., Lapan S. W., Wilkinson A. W., Bell G. W. and Reddien, P. W. (2012). A molecular wound response program associated with regeneration initiation in planarians. Genes Dev. 26, 988-1002. 10.1101/gad.187377.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzel O., Cote L. E., Poirier A., Satija R., Regev A. and Reddien P. W. (2015). A generic and cell-type-specific wound response precedes regeneration in planarians. Dev. Cell 35, 632-645. 10.1016/j.devcel.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]