Abstract
Objective
To determine if time to initial antimicrobial is associated with progression of severe sepsis to septic shock.
Design
Retrospective cohort
Setting
656 bed urban academic medical center
Patients
Emergency department patients ≥18 years of age with severe sepsis and/or septic shock and antimicrobial administration within 24 hours. Patients with shock on presentation were excluded.
Interventions
N/A.
Measurements and Main Results
We identified 3,929 severe sepsis patients, with overall mortality 12.8%. 984 (25.0%) patients progressed to septic shock. The median time to antimicrobial was 3.77 hours (IQR = 1.96 – 6.42) in those who progressed vs 2.76 hours (IQR = 1.60 – 4.82) in those who did not (p < 0.001). Multivariate logistic regression demonstrated that male sex (OR = 1.18; 95% CI, 1.01–1.36), Charlson Comorbidity Index (OR = 1.18; 95% CI, 1.11–1.27), number of infections (OR = 1.05; 95% CI, 1.02–1.08), and time to first antimicrobial (OR = 1.08; 95% CI, 1.06–1.10) were associated with progression. Each hour until initial antimicrobial administration was associated with a 8.0% increase in progression to septic shock. Additionally, time to broad spectrum antimicrobial was associated with progression (OR = 1.06; 95% CI, 1.05–1.08). Time to initial antimicrobial was also associated with in-hospital mortality (OR = 1.05; 95% CI, 1.03–1.07).
Conclusions
This study emphasizes the importance of early, broad spectrum antimicrobial administration in severe sepsis patients admitted through the emergency department, as longer time to initial antimicrobial administration is associated with increased progression of severe sepsis to septic shock and increased mortality.
Indexing keywords: Severe sepsis, septic shock, antibiotics, disease progression, infection, vasopressor agents
Introduction
Severe sepsis and septic shock are deadly conditions with significant morbidity frequently seen in emergency departments (ED) and intensive care units (ICU). Until relatively recently, sepsis, severe sepsis and septic shock were commonly categorized into three stages of progressively higher severity (1,2). However, it is unclear whether patients actually begin in one phase and progress to another, and, if so, how rapidly. The presence of such progression has not been extensively studied, nor has an ability of early antibiotics to interrupt such a progression, although early antibiotics are associated with improved survival in patients with severe sepsis and septic shock (3,4,5).
In one cohort, 20% of ICU patients with infection and Systemic Inflammatory Response Syndrome progressed to severe sepsis or septic shock within 10 days (6). Of patients presenting to the ED with sepsis but without organ dysfunction, progression to severe sepsis or septic shock occurred in 18.6% of patients and independent predictors of early progression to severe sepsis or septic shock included a serum albumin <3.5g/dL or triage diastolic blood pressure <52mmHg (7). Another study found that increased age, female sex, specific comorbid conditions, and vascular access site infections were associated with increased progression to septic shock; however, antimicrobial administration within 24 hours was similar among those who progressed to septic shock and those who did not (8).
To our knowledge, no studies to date have investigated the role of antimicrobial administration in the progression of severe sepsis to septic shock at time intervals < 24 hours, i.e. early in the treatment course. We hypothesized that earlier antimicrobial administration would be associated with decreased progression of severe sepsis to septic shock.
Materials and Methods
This retrospective study was approved by the Institutional Review Board of the University of Kansas with a waiver of informed consent. We identified patients ≥18 years of age who were admitted though the ED at The University of Kansas Hospital (KUH) from 11/01/2007 – 9/31/2015, had an ICD-9 diagnosis code for severe sepsis and/or septic shock (995.92 and/or 785.52), and were administered an antimicrobial agent. We initially evaluated study feasibility and obtained overall patient numbers using Healthcare Enterprise Repository for Ontological Narration (HERON), an i2b2 data repository (9). Subsequently, all data were obtained from the electronic medical record (EMR), Epic (Verona, WI), using the query software Crystal Reports (SAP, Walldorf, Germany) with assistance from the hospital’s Organizational Improvement division. The time of first antimicrobial and first broad spectrum antimicrobial administration times were recorded. Broad spectrum antimicrobials were defined as those covering a broad range of gram-positive and gram-negative organisms, as recommended by the Surviving Sepsis Campaign’s 3-Hour Bundle and by the Infectious Diseases Society of America in various guidelines, and included the following: ampicillin/sulbactam, ceftriaxone, cefepime, ertapenem, levofloxacin, meropenem, and piperacillin/tazobactam (10,11). Imepenem/cilastatin and ticarcilin/clavulanate were also considered broad spectrum antimicrobials; however, neither drug is included on the hospital formulary, and they were therefore not administered during the study period.
ED triage time was used to determine the duration of time to antimicrobial administration. If no ED triage time was available, ED arrival time was used as a surrogate marker. The time from ED triage to antimicrobial administration was calculated for both the first antimicrobial administered and the first broad spectrum antimicrobial administered. If antimicrobial administration time occurred in the EMR before the recorded ED triage time, the following steps were utilized to determine the most appropriate times for each variable using individual record adjudication: 1) use of an alternative/subsequent antimicrobial administration time if initial time detected to be incorrect; 2) arrival time replaced ED triage time if ED triage time detected to be incorrect. If an appropriate time could not be determined, the patient encounter was excluded from analysis. We performed sensitivity analysis to determine if there was a difference when ED arrival was used as a surrogate marker for ED triage time. To identify those patients with an infection on presentation to the ED and in an attempt to eliminate patients who developed infections subsequently during their hospital course, encounters were excluded if initial antibiotic administration time was >24 hours after ED triage. The time to the first administered broad spectrum antimicrobial was determined for those antimicrobials received within 24 hours of ED triage. Broad spectrum antimicrobials administered after 24 hours were excluded from analysis in order to eliminate patients who who received a broad spectrum antimicrobial much later in their hospitalization and clinical course (ex: patient started on a broad spectrum antimicrobial for a subsequent infection rather than for sepsis on presentation to the ED).
Patients were considered to have septic shock on presentation if a vasopressor was administered within 3 hours of ED triage or the patient had a systolic blood pressure (SBP) < 90mmHg or mean arterial pressure (MAP) < 65 mm Hg within the first 3 hours and ultimately required the administration of a vasopressor, i.e. hypotension that was unresponsive to fluid administration. Patients who received the following vasoactive agents within 3 hours of presentation were removed from the analysis: epinephrine, norepinephrine, vasopressin, phenylephrine, dobutamine, or dopamine. Dobutamine is not the vasoactive agent of choice in most cases of septic shock; however, Early Goal Directed Therapy as described by Rivers, et al. was in use in our institution during at least a portion of the time covered by this analysis (12). To determine whether its inclusion as a vasoactive agent altered outcomes of the study, we performed a sensitivity analysis with and without patients who received dobutamine as their initial vasoactive agent. Progression from severe sepsis to septic shock was defined as vasopressor administration during the same hospital encounter > 3 hours after ED triage time.
ICD-9 diagnosis codes were queried to determine infection sources (Supplemental digital content 1). Charlson Comorbidity Index score was determined for all patients (13). Hospital length of stay (LOS), ICU admission, and in-hospital mortality were compared among patient encounters with and without progression to septic shock during their hospitalization.
Data analysis was completed using SAS Studio 3.1 (Copyright 2014). The frequency of progression to septic shock was determined for patients receiving their first antimicrobial agent within each 1-hour time interval up to 24 hours. Chi-square analysis was used to compare proportional data between the patient group who progressed to shock and the group who did not progress. Tests of normality of distribution were applied to all continuous variables. Normally distributed data were compared using t-tests. Non-parametrically distributed data, specifically time to antimicrobial administration, were compared using the Wilcoxon rank-sum test. Statistically significant variables (p-value < 0.05) identified by univariate analyses were included in the final multivariate logistic regression model for progression from severe sepsis to septic shock. Hosmer and Lemeshow Goodness-of-Fit Test was used to assess the overall multivariate logistic regression model fit, where a higher p-value signifies a better overall fit.
To evaluate factors that may influence the timing of initial antibiotic administration in this cohort, backward elimination stepwise linear regression modeling was used. We used variables in the initial model that are easily accessible to clinicians in the ED, including: age, sex, first white blood cell (WBC) count, first serum lactic acid, presence of hypotension, infection source, and Charlson Comorbidity Index. Although Charlson Comorbidity Index is a value not commonly calculated in the ED, it is likely that ED physicians may incorporate some informal analysis of comorbidities in their decision to order antibiotics. Variables with a p-value > 0.05 were excluded from the final model.
Results
A total of 5,426 patient encounters were identified meeting the inclusion criteria. After data adjudication, a final cohort of 3,929 patient encounters was included in the analysis (Figure 1). Patient demographics for the final cohort are shown in Table 1, and information regarding the infections using grouped ICD-9 diagnosis codes is shown in Table 2. The most common unique ICD-9 infection diagnosis codes included unspecified septicemia, unspecified pneumonia, and urinary tract infection, unspecified site. (Supplemental digital content 2).
Figure 1.
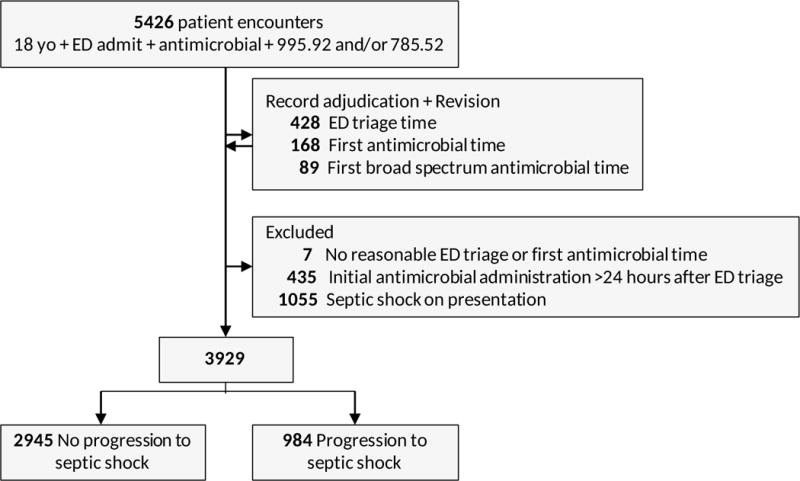
Cohort organization: inclusion and exclusion of patients to identify final cohort for analysis
Table 1.
Characteristics of patients with and without progression of severe sepsis to septic shock
| Variable | All patients | With progression to septic shock | Without progression to septic Shock | p-value (ChiSq or t-test) | |||
|---|---|---|---|---|---|---|---|
| # patients | 3929 | (100%) | 984 | (25.0%) | 2945 | (75.0%) | |
| Age | 58.9 | ± 17.5 | 58.3 | ± 16.7 | 59.1 | ± 17.7 | 0.206 |
| Sex | |||||||
| Female | 1826 | (46.5%) | 427 | (43.4%) | 1399 | (47.5%) | 0.025 |
| Male | 2103 | (53.5%) | 557 | (56.6%) | 1546 | (52.5%) | |
| Ethnicity | |||||||
| Caucasian | 2592 | (66.0%) | 660 | (67.1%) | 1932 | (65.6%) | 0.824 |
| African American | 882 | (22.5%) | 211 | (21.4%) | 671 | (22.8%) | |
| Other | 450 | (11.5%) | 112 | (11.4%) | 338 | (11.5%) | |
| Unknown | 5 | (0.1%) | 1 | (0.1%) | 4 | (0.1%) | |
| Charlson Comorbidity Index | 2.10 | ± 2.40 | 2.34 | ± 2.44 | 2.02 | ± 2.38 | < 0.001 |
| Total # unique infection ICD-9s | 2.47 | ± 1.17 | 2.64 | ± 1.33 | 2.41 | ± 1.11 | < 0.001 |
| Total # infection sites | 1.89 | ± 0.70 | 1.94 | ± 0.77 | 1.88 | ± 0.68 | 0.011 |
| First WBC Count | 13.9 | ± 8.70 | 13.5 | ± 8.95 | 14.1 | ± 8.61 | 0.075 |
| First Lactic Acid | 2.64 | ± 2.01 | 3.05 | ± 2.52 | 2.50 | ± 1.79 | < 0.001 |
Table 2.
Most common infection ICD-9 diagnosis codes by code group for patients without shock on presentation to the emergency department
| Infection Site/Type | Number of patients | Percentage |
|---|---|---|
| Infectious & parasitic (including septicemia) | 3921 | 99.8% |
| Respiratory & Lung | 1500 | 38.2% |
| Genitourinary | 1250 | 31.8% |
| Intra-abdominal | 293 | 7.5% |
| Bone/Joint | 151 | 3.9% |
| Surgical Site, device, implant, graft, or central venous catheter | 146 | 3.7% |
| Central nervous system | 67 | 1.7% |
| Cardiovascular | 57 | 1.5% |
| Skin & Soft Tissue | 36 | 0.9% |
| Bacteremia | 12 | 0.3% |
Of the 3,929 patients who were not in shock at presentation to the ED, 984 (25.0%) progressed to septic shock during their hospitalization. The most commonly administered vasopressor in those who progressed to shock was norepinephrine (707 patients, 71.8%). Patients who progressed to shock had increased hospital LOS (18.7 ± 17.1 days vs. 9.66 ± 9.12 days; p < 0.001), increased ICU admission rates (95.3% vs. 46.3%; p < 0.001), increased ICU LOS (9.73 ± 11.6 days vs. 4.40 ± 4.95 days; p < 0.001), and increased hospital mortality (30.1% vs. 7.00%; p < 0.001), as compared with patients who did not progress to shock (mean ± standard deviation for patients without progression vs patients with progression, respectively).
The most commonly administered initial antimicrobial agent to patients without shock on presentation to the ED was piperacillin-tazobactam, followed by ceftriaxone, and levofloxacin, all of which are broad spectrum antimicrobials (Supplemental Digital Content 3). The most commonly administered narrow spectrum antimicrobial was vancomycin. The median time to initial antimicrobial administration among all patients was 2.95 hours (IQR = 1.67 – 5.26). Median time to initial antimicrobial agent among those with progression to septic shock was 3.77 hours (IQR = 1.96 – 6.42) and among those without progression was 2.76 hours (IQR = 1.60 – 4.82) (p < 0.001). As the time until initial antimicrobial administration increased, the frequency of patient progression to septic shock also increased (Figure 2). In patients who progressed to septic shock, 474 (48.2%) of patients progressed, i.e. received a vasoactive agent, within the first 24 hours after ED triage. Overall, the median time to vasopressor administration in patients who progressed to shock was 26.5 hours (IQR= 8.99 – 106).
Figure 2.
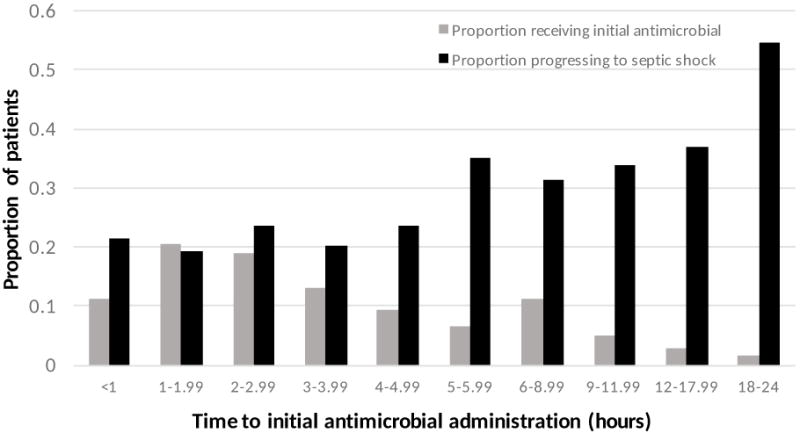
Antimicrobial administration timing and proportion of patients progressing to shock. The gray bars represent the proportion of the total cohort who received their initial antimicrobial during the given time interval. The black bars represent the proportion of patients receiving their initial antimicrobial in the given time interval who progressed to septic shock.
Univariate and multivariate regression analyses to determine predictors of progression are displayed in Table 3. Male sex, increased Charlson Comorbidity Index, increased number of unique infection diagnosis codes, and increased time to initial antimicrobial administration were all associated with increased progression to septic shock in our model. Hosmer and Lemeshow Goodness-of-Fit testing indicated good overall fit of the multivariate logistic regression (p=0.196).
Table 3.
Predictors of progression to septic shock: Univariate and multivariate regression modeling
| Variable | Univariate Model | Multivariate Model | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
|
|
|
|||||
| Sex (Male) | 1.18 | (1.02 – 1.37) | 0.025 | 1.18 | (1.01 – 1.36) | 0.032 |
| Charlson Comorbidity Index | 1.17 | (1.10 – 1.24) | < 0.001 | 1.18 | (1.11 – 1.26) | < 0.001 |
| # Unique Infection Codes | 1.06 | (1.03 – 1.09) | < 0.001 | 1.05 | (1.02 – 1.08) | 0.003 |
| Time to first antimicrobial | 1.08 | (1.06 – 1.10) | < 0.001 | 1.08 | (1.06 – 1.10) | < 0.001 |
| Time to First broad-spectrum antimicrobial | 1.06 | (1.05 – 1.08) | < 0.001 | – | – | – |
Of all initial antimicrobials administered, 2937 (74.8%) were broad spectrum. Broad spectrum antimicrobials were administered at some time during 3819 (97.2%) of all patient encounters. Among all patients who received a broad spectrum antimicrobial, the most common broad spectrum antimicrobial first administered was piperacillin-tazobactam (1343 patients, 35.2%) followed by ceftriaxone (904 patients, 23.7%) and levofloxacin (768 patients, 20.1%) (Supplemental Digital Content 4). Among patients who received a broad spectrum antimicrobial during their hospitalization, 3672 (96.2%) were administered within 24 hours of ED triage and were included in the broad spectrum antimicrobial timing analysis. Of these, the median time to first broad spectrum antimicrobial was 3.13 hours (IQR = 1.79 – 5.53); in patients with progression the median time was 3.77 hours (IQR = 2.77 – 6.47) and in those without progression was 2.97 hours (IQR = 1.72 – 5.11) (p < 0.001). Univariate logistic regression analysis results for time to first broad spectrum antimicrobial administration are also shown in.
Because dobutamine is not a vasopressor, we performed a sensitivity analysis by removing the receipt of dobutamine as a component of our septic shock definition. In our final cohort of 3,929 patients and of the 1,055 patients who were originally considered to be in shock at presentation, 32 patients received only dobutamine and no other vasoactive agents. These patients were reclassified as not progressing to septic shock, and their exclusion did not change outcomes of the model.
Overall, in-hospital mortality in our cohort was 12.8% (502 patients). Patients who progressed to shock had increased in-hospital mortality (30.1% vs. 7.0%; p < 0.001). Increased time to initial antimicrobial administration was associated with in-hospital mortality (OR = 1.05; 95% CI, 1.03 – 1.07).
Using backward elimination stepwise linear regression, the variables predictive of decreased time to initial antimicrobial administration included age, WBC count, presence of hypotension at presentation, and a respiratory/lung infection source (Supplemental Digital Content 5). Intra-abdominal infections and Charlson Comorbidity Index were associated with increased time to receipt of antibiotics. The overall model fit was excellent (p < 0.001). While the goal of this model was simply to gain information regarding the influence of such factors on the time-to-treatment, we explored log-transforming the outcome measure in the model, time to first antimicrobial administration; all of these variables remained statistically significant. Although increased initial serum lactic acid was associated with increased progression to septic shock in univariate analysis (OR = 1.13; 95% CI, 1.09 – 1.17), it was not associated with the timing of antimicrobial administration within our cohort (parameter estimate, −0.04; p = 0.15).
Discussion
This is one of the first studies to investigate the association between progression of severe sepsis patients to septic shock and the time to the first antimicrobial. We found that for each hour that passed between ED triage and antimicrobial administration, the risk of progression to septic shock increased by 8.0%, highlighting the importance of early identification and treatment of patients with infection-induced organ dysfunction. The percentage of patients progressing to septic shock in our study was slightly higher than previously reported (6,7,8, 14,15). This is likely because we did not include patients with sepsis, defined as infection + SIRS (ICD-9 diagnosis code 995.91), and because our patients required the presence of an ICD-9 diagnosis code for severe sepsis or septic shock. Therefore, the patients that we identified are likely similar to or identical to patients who would be classified as having sepsis by the new Sepsis-3 criteria, since the diagnosis code 995.92 required the presence of at least one documented organ dysfunction (16). However, the patients we identified as having septic shock may not be identical to patients who would be characterized as having septic shock by Sepsis-3, because we did not require the need for increased serum lactate.
Holder et al. investigated predictors of early progression to severe sepsis or septic shock in patients who presented to the ED with sepsis (infection + SIRS) but without signs of organ dysfunction(7). Their cohort excluded patients with severe sepsis at triage – the specific patient group that we desired to study. Although other studies have investigated predictors of progression to septic shock, we specifically evaluated the role of antimicrobial timing.
The majority of patients received a broad spectrum antimicrobial for their initial antimicrobial administration. Because time to initial antimicrobial and time to first broad spectrum antimicrobial were commonly the same value, it is difficult to distinguish the role of broad vs narrow spectrum coverage antimicrobials in the progression of severe sepsis to septic shock from our data. However, these findings support prompt, broad spectrum antimicrobial administration in patients with severe sepsis.
Our principal finding that timing to antibiotics is an important factor determining progression from severe sepsis to septic shock is underscored by our analysis of factors predictive of receiving earlier antibiotics. Predictors of time to initial antimicrobial administration included age, WBC count, hypotension, and respiratory/lung or intra-abdominal infections. These factors suggest that patients who are perceived as sicker or who are, in fact, more ill on presentation may receive antibiotics faster than other patients. Therefore, in addition to antibiotics inhibiting progression to septic shock, it appears that they do so in the sickest patients of the cohort, principally because they are administered earlier to these patients. Not all factors suggestive of higher illness severity followed suit. Although first serum lactic acid was higher in patients with progression to septic shock, it was not predictive of time to initial antimicrobial administration.
Numerous studies have investigated the role of early interventions, including the role of early antimicrobials, in affecting outcomes of patients with severe sepsis and septic shock. Several studies demonstrated that early antimicrobial administration was associated with decreased mortality in patients with infection as well as in septic patients (3,4,5,17,18). However, others have failed to demonstrate such an association (19,20,21). Our study supports the former finding, as increased time to initial antimicrobial administration was associated with increased in-hospital mortality within our cohort. Our findings are novel because they support a progression from severe sepsis, infection with organ dysfunction, to septic shock and suggest that early interventions can prevent such progression.
Limitations
Previous studies have shown that ICD-9 diagnosis codes for severe sepsis and septic shock provide a lower sensitivity for detection of such conditions (22,23,24). In one study, 48.4% of patients meeting clinical criteria for severe sepsis were not assigned a diagnosis code for severe sepsis (22). Also, patients identified with a diagnosis code tend to be septic patients with more severe illness (23,24). Since our study only addressed encounters with a diagnosis code, some patients who presented with clinical criteria for severe sepsis and/or septic shock are likely missed.
Although patients were diagnosed with severe sepsis or septic shock during their hospitalization, we were unable to determine which patients presented in severe sepsis vs those who presented with a simple infection and subsequently developed severe sepsis. However, we limited our patient cohort to those who had received antibiotics within 24 hours of presentation, making the probability substantially higher that infection was present on presentation. Also, because many patients likely present to the ED already in severe sepsis, the actual duration of severe sepsis is unknown. Similarly, time of onset of septic shock is approximated by time of vasoactive agent administration; however, the time of onset of hypotension unresponsive to fluid resuscitation cannot be discerned from our dataset.
Since our primary goal was to investigate the role of time to initial antimicrobial administration, we did not investigate the appropriateness of antimicrobial coverage for each patient’s specific infectious organism. Although some patients did not receive a broad spectrum antimicrobial or received a narrower spectrum antimicrobial first, the antimicrobial may or may not have provided adequate coverage against their infectious organism. Patients who received a combination of narrower spectrum agents in rapid succession could have achieved the same therapeutic benefit as those receiving an initial broad spectrum antimicrobial.
In this retrospective study, we were also unable to investigate reasons for delay in antimicrobial administration, such as delays due to late clinical recognition by providers or delays due to pharmacy evaluation or delivery. Because our study was limited to the patient’s clinical encounter with ICD-9 diagnosed sepsis, we were unable to identify any pre-admission antimicrobials, such as those administered at home or at a nursing facility. If patients received pre-admission antimicrobial agents, it may confound the analysis and alter both outcomes and antimicrobial administration behaviors by ED personnel.
Conclusions
This study emphasizes the importance of early and broad spectrum antimicrobial administration in patients with severe sepsis presenting to the emergency department. Early intervention may decrease progression to shock in this population, and antimicrobials are given earlier to severe sepsis patients with clinical features suggesting higher risk. Progression itself is associated with adverse clinical and patient-centered outcomes, further emphasizing the need for early antimicrobial administration. This study also defines other risk factors which are identifiable at ED presentation and may allow healthcare providers to give more accurate prognostic information to patients and families.
Supplementary Material
Acknowledgments
Dr. Simpson received funding from Alpha Omega Alpha Honor Medical Society Carolyn L. Kuckein Student Research Fellowship and from CTSA grant from NCATS awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research #TL1TR000120. Dr. Whiles received funding from Alpha Omega Alpha Honor Medical Society Carolyn L. Kuckein Student Research Fellowship.
Financial Support for the study:
This work was supported in part by an Alpha Omega Alpha Honor Medical Society Carolyn L. Kuckein Student Research Fellowship and a CTSA grant from NCATS awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research # TL1TR000120. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCATS.
Footnotes
No reprints will be ordered.
Copyright form disclosure:
Dr. Whiles disclosed: Amanda Deis received a CTSA grant from NCATS awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research #TL1TR000120. Dr. Deis disclosed other support: I received support in the form of tuition while working on this project from a CTSA grant from NCATS awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research # TL1TR000120. None of these funds however were used for the completion of this project.
References
- 1.Bone R, Balk R, Cerra F, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 2.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 4.Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38(4):1045–53. doi: 10.1097/CCM.0b013e3181cc4824. [DOI] [PubMed] [Google Scholar]
- 5.Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42(8):1749–55. doi: 10.1097/CCM.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 6.Alberti C, Brun-Buisson C, Chevret S, et al. European Sepsis Study Group Systemic inflammatory response and progression to severe sepsis in critically ill infected patients. Am J Respir Crit Care Med. 2005;171(5):461–8. doi: 10.1164/rccm.200403-324OC. [DOI] [PubMed] [Google Scholar]
- 7.Holder AL, Gupta N, Lulaj E, Furgiuele M, et al. Predictors of early progression to severe sepsis or shock among emergency department patients with nonsevere sepsis. Int J Emerg Med. 2016;9(1):10. doi: 10.1186/s12245-016-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glickman SW, Cairns CB, Otero RM, et al. Disease progression in hemodynamically stable patients presenting to the emergency department with sepsis. Acad Emerg Med. 2010;17(4):383–90. doi: 10.1111/j.1553-2712.2010.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waitman LR, Warren JJ, Manos EL, Connolly DW. Expressing observations from electronic medical record flowsheets in an i2b2 based clinical data repository to support research and quality improvement. AMIA Annu Symp Proc. 2011;2011:1454–63. [PMC free article] [PubMed] [Google Scholar]
- 10.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kollef MH. Broad spectrum antimicrobials and the treatment of serious bacterial infection: getting it right up front. Clin Infect Dis. 2008;47:S3–13. doi: 10.1086/590061. [DOI] [PubMed] [Google Scholar]
- 12.Rivers E, Nguygen B, Havstad S, et al. Early Goal-Directed Therapy in the Treatment of Severe Sepsis and Septic Shock. NEJM. 2001;345(19):1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 13.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 14.Arnold RC, Sherwin R, Shapiro NI, et al. Emergency Medicine Shock Research Network Investigators Multicenter observational study of the development of progressive organ dysfunction and therapeutic interventions in normotensive sepsis patients in the emergency department. Acad Emerg Med. 2013;20(5):433–40. doi: 10.1111/acem.12137. [DOI] [PubMed] [Google Scholar]
- 15.Capp R, Horton CL, Takhar SS, et al. Predictors of patients who present to the emergency department with sepsis and progress to septic shock between 4 and 48 hours of emergency department arrival. Crit Care Med. 2015;43(5):983–8. doi: 10.1097/CCM.0000000000000861. [DOI] [PubMed] [Google Scholar]
- 16.Singer M, Deutschman CS, Seymour C, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meehan TP, Fine MJ, Krumholz HM, et al. Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA. 1997;287(23):2080–4. [PubMed] [Google Scholar]
- 18.Houck PM, Bratzler DW, Nsa W, et al. Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community-acquired pneumonia. Arch Intern Med. 2004;164(6):637–44. doi: 10.1001/archinte.164.6.637. [DOI] [PubMed] [Google Scholar]
- 19.de Groot, Ansems A, Gerling DH, et al. The association between time to antibiotics and relevant clinical outcomes in emergency department patients with various stages of sepsis: a prospective multi-center study. Crit Care. 2015;19:194. doi: 10.1186/s13054-015-0936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puskarich MA, Trzeciak S, Shapiro NI, et al. Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med. 2011;39(9):2066–71. doi: 10.1097/CCM.0b013e31821e87ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterling SA, Miller WR, Pryor J, et al. The impact of timing of antibiotics on outcomes in severe sepsis and septic shock: a systematic review and meta-analysis. Crit Care Med. 2015;43(9):1907–15. doi: 10.1097/CCM.0000000000001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandt BN, Gartner AB, Moncure M, et al. Identifying severe sepsis via electronic surveillance. Am J Med Qual. 2015;30(6):559–65. doi: 10.1177/1062860614541291. [DOI] [PubMed] [Google Scholar]
- 23.Whittaker SA, Mikkelsen ME, Gaieski DF, et al. Severe sepsis cohorts derived from claims-based strategies appear to be biased toward a more severely ill patient population. Crit Care Med. 2013;41(4):945–53. doi: 10.1097/CCM.0b013e31827466f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deis AS, Whiles BB, Miller PJ, Simpson SQ. Diagnosis code vs clinical criteria: variable outcomes in patients with severe sepsis and septic shock. Chest. 2016;149(4S):A187. doi: 10.1016/j.chest.2016.02.194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


